Abstract
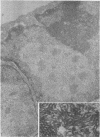
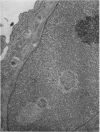
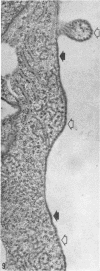
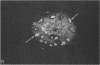
The ultrastructure of CV-1 cells infected with subacute sclerosing panencephalitis (SSPE) viruses was compared with that of CV-1 cells infected with the wild or Edmonston strain of measles virus. Both SSPE viruses and the measles viruses produced two types of nucleocapsid structures: smooth filaments, 15 to 17 nm in diameter, and granular filaments, 22 to 25 nm. The smooth and granular filaments produced by SSPE and measles virus did not differ in appearance. In CV-1 cells infected with SSPE viruses, smooth filaments formed large intranuclear inclusions and granular filaments occupied a large area of the cytoplasm, but always spared the area under the cell membrane. Particles budding from the surface of these cells contained no nucleocapsids. In CV-1 cells infected with measles virus, only small aggregates of smooth filaments were seen in the nuclei. Granular filaments in the cytoplasm predominantly occupied the area under the cell membrane, and were aligned beneath the cell membrane in a parallel fashion and assembled into budding particles. These differences between SSPE and measles virus may be regarded as quantitative, but they do distinguish SSPE viruses from measles virus. Moreover, the formation of large nuclear inclusions filled with smooth filaments appears to be a characteristic process of SSPE, but not of measles, since this type of inclusion is invariably seen in SSPE brain tissues, brain cultures derived from them, and CV-1 cells infected with SSPE viruses.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbanti-Brodano G., Oyanagi S., Katz M., Koprowski H. Presence of 2 different viral agents in brain cells of patients with subacute sclerosing panencephalitis. Proc Soc Exp Biol Med. 1970 May;134(1):230–236. doi: 10.3181/00379727-134-34765. [DOI] [PubMed] [Google Scholar]
- Chen T. T., Watanabe I., Zeman W., Mealey J., Jr Subacute sclerosing panencephalitis: propagation of measles virus from brain biopsy in tissue culture. Science. 1969 Mar 14;163(3872):1193–1194. doi: 10.1126/science.163.3872.1193. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Holmes K. V., Dales S., Choppin P. W. An electron microscopic study of moderate and virulent virus-cell interactions of the parainfluenza virus SV5. Virology. 1966 Nov;30(3):411–426. doi: 10.1016/0042-6822(66)90119-x. [DOI] [PubMed] [Google Scholar]
- Dawson J. R. Cellular Inclusions in Cerebral Lesions of Lethargic Encephalitis. Am J Pathol. 1933 Jan;9(1):7–16.3. [PMC free article] [PubMed] [Google Scholar]
- Feller U., Dougherty R. M., Di Stefano H. S. Morphogenesis of Newcastle disease virus in chorioallantoic membrane. J Virol. 1969 Nov;4(5):753–762. doi: 10.1128/jvi.4.5.753-762.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon R. M., Rubinstein L. J. Light and electron microscopy observations on the development of viral particles in the inclusions of Dawson's encephalitis (subacute sclerosing panencephalitis). Neurology. 1968 Jan;18(1 Pt 2):8–20. doi: 10.1212/wnl.18.1_part_2.008. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., London W. T., Jabbour J. T., Zeman W., Sever J. L. Isolation of measles virus from brain cell cultures of two patients with subacute sclerosing panencephalitis. Proc Soc Exp Biol Med. 1969 Oct;132(1):272–277. doi: 10.3181/00379727-132-34196. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., Sever J. L., Zeman W. Subacute sclerosing panencephalitis: isolation of measles virus from a brain biopsy. Nature. 1969 Mar 8;221(5184):974–974. doi: 10.1038/221974a0. [DOI] [PubMed] [Google Scholar]
- Howe C., Morgan C., de Vaux St Cyr C., Hsu K. C., Rose H. M. Morphogenesis of type 2 parainfluenza virus examined by light and electron microscopy. J Virol. 1967 Feb;1(1):215–237. doi: 10.1128/jvi.1.1.215-237.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- KALLMAN F., ADAMS J. M., WILLIAMS R. C., IMAGAWA D. T. Fine structure of cellular inclusions in measles virus infections. J Biophys Biochem Cytol. 1959 Dec;6:379–382. doi: 10.1083/jcb.6.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M., Koprowski H., Moorhead P. Transformation of cells cultured from human brain tissue. Exp Cell Res. 1969 Sep;57(1):149–153. doi: 10.1016/0014-4827(69)90381-4. [DOI] [PubMed] [Google Scholar]
- Katz M., Oyanagi S., Koprowski H. Subacute sclerosing panencephalitis: structures resembling myxovirus nucleocapsids in cells cultured from brain. Nature. 1969 May 31;222(5196):888–890. doi: 10.1038/222888a0. [DOI] [PubMed] [Google Scholar]
- Nakai T., Shand F. L., Howatson A. F. Development of measles virus in vitro. Virology. 1969 May;38(1):50–67. doi: 10.1016/0042-6822(69)90127-5. [DOI] [PubMed] [Google Scholar]
- Norrby E. C., Magnusson P. Some morphological characteristics of the internal component of measles virus. Arch Gesamte Virusforsch. 1965;17(3):443–447. doi: 10.1007/BF01241199. [DOI] [PubMed] [Google Scholar]
- Oyanagi S., ter Meulen V., Müller D., Katz M., Koprowski H. Electron microscopic observations in subacute sclerosing panencephalitis brain cell cultures: their correlation with cytochemical and immunocytological findings. J Virol. 1970 Sep;6(3):370–379. doi: 10.1128/jvi.6.3.370-379.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne F. E., Baublis J. V., Itabashi H. H. Isolation of measles virus from cell cultures of brain from a patient with subacute sclerosing panencephalitis. N Engl J Med. 1969 Sep 11;281(11):585–589. doi: 10.1056/NEJM196909112811103. [DOI] [PubMed] [Google Scholar]
- Prose P. H., Balk S. D., Liebhaber H., Krugman S. Studies of a myxovirus recovered from patients with infectious hepatitis. II. Fine structure and electron microscopic demonstration of intracytoplasmic internal component and viral filament formation. J Exp Med. 1965 Dec 1;122(6):1151–1160. doi: 10.1084/jem.122.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périer O., Vanderhaeghen J. J., Pelc S. Subacute sclerosing leuco-encephalitis. Electron microscopic finding in two cases with inclusion bodies. Acta Neuropathol. 1967 Jul 5;8(4):362–380. doi: 10.1007/BF00696673. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Feldman L. A., Sheppard R. D., Bornstein M. B. Ultrastructure of measles virus in cultures of hamster cerebellum. J Virol. 1969 Aug;4(2):169–181. doi: 10.1128/jvi.4.2.169-181.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Negal I., Harter D. H. Subacute sclerosing leukoencephalitis: ultrastructure of intranuclear and intracytoplasmic inclusions. Science. 1966 Nov 18;154(3751):899–901. doi: 10.1126/science.154.3751.899. [DOI] [PubMed] [Google Scholar]
- ter Meulen V., Müller D., Katz M., Käckell M. Y., Joppich G. Immunohistological, microscical and neurochemical studies on encephalitides. IV. Subacute sclerosing (progressive) panencephalitis. Histochemical and immunohistological findings in tissue cultures derived from SSPE brain biopsies. Acta Neuropathol. 1970;15(1):1–10. doi: 10.1007/BF00690684. [DOI] [PubMed] [Google Scholar]