Abstract
Purpose
SPECT/CT is a hybrid imaging modality, which combines a 3D scintigraphy (SPECT) and a conventional computerised tomography (CT). SPECT/CT allows accurate anatomical localisation of metabolic tracer activity. It allows the correlation of surgical factors such as tunnel position and orientation with mechanical alignment, clinical outcome and biological factors. The purpose of this study was to investigate whether the SPECT/CT tracer uptake (intensity and distribution) correlates with the stability and laxity of the knee joint and the position and orientation of the tibial and femoral tunnels in patients after anterior cruciate ligament (ACL) reconstruction.
Methods
A consecutive series of knees (n = 66), with symptoms of pain and/or instability after ACL reconstruction were prospectively evaluated using clinical examination and 99mTc-HDP-SPECT/CT. Clinical laxity testing was performed using the Rolimeter (Ormed, Freiburg, Germany) including Lachman testing (0–2 mm, 3–5 mm, 6–10 mm, >10 mm), anterior drawer test (0–2 mm, 3–5 mm, 6–10 mm, >10 mm), pivot shift test (positive versus negative) and patient-based subjective instability (yes versus no).
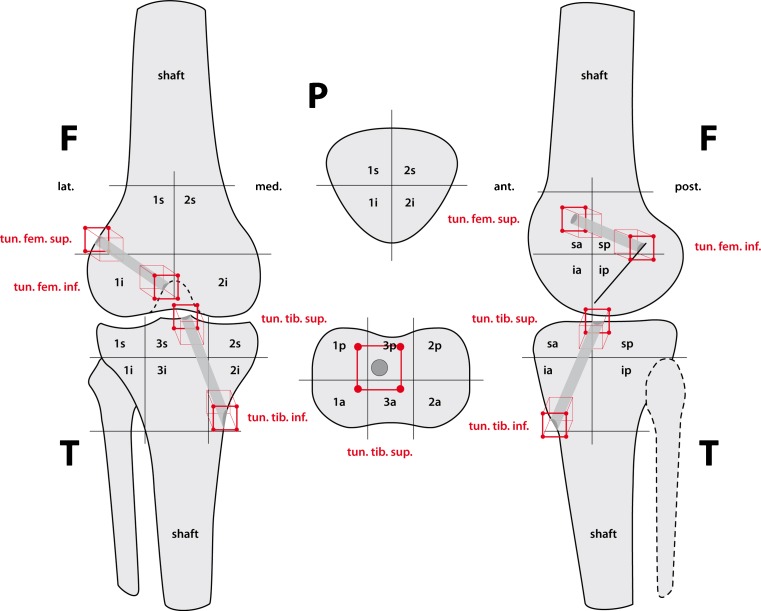
For analysis of SPECT/CT tracer uptake a previously validated SPECT/CT localisation scheme consisting of 17 tibial, nine femoral and four patellar regions on standardised axial, coronal, and sagittal slices was used. The tracer activity on SPECT/CT was localised and recorded using a 3D volumetric and quantitative analysis software.
Mean, standard deviation, minimum and maximum of grading for each area of the localisation scheme were recorded. The position and orientation of the tibial and femoral tunnel was assessed using a previously published method on 3D-CT.
Results
Correlation of instability, pivot shift as well as clinical laxity testing with 99mTc-HDP-SPECT/CT tracer uptake intensity and distribution showed no significant correlation. 99mTc-HDP-SPECT/CT tracer uptake correlated significantly with the position and orientation of the ACL graft. A more horizontal femoral graft position showed significantly increased tracer uptake within the superior and posterior femoral regions. A more posteriorly-placed femoral insertion site showed significantly more tracer uptake within the femoral and tibial tunnel regions. A more vertical or a less medial tibial tunnel orientation showed significant increased uptake within the tibial and femoral tunnel regions. A more anterior tibial tunnel position showed significantly more tracer uptake in the femoral and tibial tunnel regions as well as the entire tibiofemoral joint.
Conclusions
SPECT/CT tracer uptake intensity and distribution showed a significant correlation with the femoral and tibial tunnel position and orientation in patients with symptomatic knees after ACL reconstruction. No correlation was found with stability or clinical laxity. SPECT/CT tracer uptake distribution has the potential to give us important information on joint homeostasis and remodelling after ACL reconstruction. It might help to predict ACL graft failure and improve our surgical ACL reconstruction technique in finding the optimal tunnel and graft position and orientation.
Introduction
For a variety of reasons a considerable number of patients are not satisfied or experience pain after anterior cruciate ligament (ACL) reconstruction [1–5]. Typical reasons include failure of the ACL graft, failure of graft fixation, chronic synovitis due to biodegradable fixation material and missed concomitant injuries [3–6].
Numerous authors have highlighted the clinical value of 3D-CT for the assessment of tunnel position after ACL reconstruction [7–12]. Thus, 3D reconstructed CT images are increasingly used to investigate the femoral and tibial tunnel position after ACL reconstruction [7–9, 11, 12].
SPECT/CT is a hybrid imaging modality, which combines a 3D scintigraphy (SPECT) and a conventional computerised tomography (CT) [13]. SPECT/CT allows accurate anatomical localisation of metabolic tracer activity [9]. Recently, we have introduced a novel diagnostic SPECT/CT algorithm for the challenging group of patients with problems after ACL reconstruction [6, 9, 14, 15]. This standardised algorithm including grading and anatomical localization of SPECT/CT tracer uptake promises the identification of specific pathological tracer uptake patterns and distributions [9]. It now allows the correlation of surgical factors such as tunnel position and orientation with mechanical alignment, clinical outcome and biological factors [9, 16]. It also promises the critical assessment of the osseointegration of the bone-graft-fixation complex.
The intensity and distribution of 99mTc-HDP-SPECT/CT tracer, which as diphosphonate is targeted towards osteoblastic activity, is influenced by mechanical or anatomical alignment, vascular supply, infection, osteoblastic activity or currently unknown other factors [9, 13, 14, 16–19]. In patients after ACL reconstruction it could be hypothesized if the position and orientation of the bone tunnels and the stability and/or laxity of the knee joint are decisive factors.
The purpose of this study was to investigate whether the SPECT/CT tracer uptake (intensity and distribution) correlates with the stability and laxity of the knee joint and the position and orientation of the tibial and femoral tunnels in patients after ACL reconstruction.
Material and methods
A consecutive series of knees (n = 66, mean age ± SD 28 ± 11 years, m:f = 44:22), with symptoms of pain and/or instability after ACL reconstruction were prospectively collected and evaluated. The study was approved by the local ethical committee. The mean ± standard deviation (SD) time from surgery was 7 ± 6 years.
The following grafts were used in the previous ACL reconstruction: semitendinosus and/or gracilis tendon (n = 25), patellar tendon (n = 35), quadriceps tendon (n = 5), and iliotibial tract (n = 1).
For femoral fixation of the graft a post screw was used in 21 patients, an interference screw in 17 patients, press-fit technique in six patients, endo-button type fixation in 12 patients and others in ten knees. For tibial fixation of the graft a post screw was used in 36 patients, an interference screw in 24 patients, a tibial button in three patients and a staple fixation in three patients.
A detailed clinical examination was performed by an experienced senior orthopaedic surgeon specialised in reconstructive knee surgery. Conventional weightbearing radiographs (anterior-posterior, lateral, patellar skyline view) were performed in each case. In addition, all patients received a commercial 700 MBq, Tc-99m HDP injection (Malinckrodt, Wallerau, Switzerland). 99mTc-HDP-SPECT/CT was performed using a Symbia T16 (Siemens, Erlangen, Germany), which is a system that consists of a pair of low energy, high-resolution collimators, a dual-head gamma camera and an integrated 16-slice CT scanner (collimation of 16x0.75-mm). CT scans of the femoral head (3-mm slices), the knee (0.75-mm slices) and the ankle (3-mm slices) were performed according to the Imperial Knee Protocol [20].
SPECT/CT was performed in the delayed phase three to four hours after tracer injection (matrix size 128 x 128, angle step 32, and time per frame 25 s). Demographic data of all patients were noted including gender, age, date of initial ACL reconstruction, time between surgery and SPECT/CT, type of ACL reconstruction, graft type and tibial and femoral fixation technique.
Clinical stability testing was performed using the Rolimeter (Ormed, Freiburg, Germany) including Lachman testing (0–2 mm, 3–5 mm, 6–10 mm, >10 mm), anterior drawer test (0–2 mm, 3–5 mm, 6–10 mm, >10 mm), pivot shift test (positive versus negative) and patient-based subjective instability (yes versus no).
The tracer activity on SPECT/CT was analysed using a specialised software allowing 3D volumetric quantitative analysis of SPECT data as previously published [21]. A previously published localisation scheme for anatomical localisation of the SPECT/CT tracer activity was used (Fig. 1). Mean, standard deviation, minimum and maximum of maximum tracer activity grading for each area of the localisation scheme were recorded. For analysis the tracer activity was standardised in relation to the tracer uptake in the mid shaft of the femur. The intra- and inter-observer reliability of the localisation scheme and grading of the tracer activity were excellent to good [9].
Fig. 1.
The previously validated localisation scheme for the Tc-99m HDP tracer activity in patients after ACL reconstruction (1 = lateral, 2 = medial, 3 = central). F femur, T tibia, P patella, a anterior, p posterior, i inferior, s superior
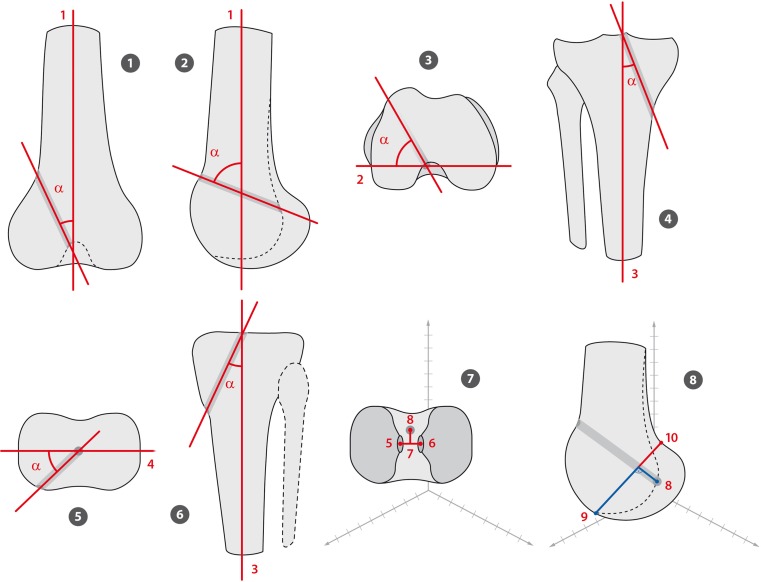
The position and orientation of the tibial and femoral tunnel was assessed using a previously published method on 3D-CT [9]. Orientation of tibial and femoral tunnels were assessed in relation to the anatomical knee axis. The femoral tunnel entry position was determined in mm distance on 3D-CT in relation to the Blumensaat’s line (anterior–posterior and high–low; Fig. 2). The tibial tunnel position was determined in mm distance on 3D-CT in relation to the anterior–posterior tibial plateau length and distance to the medial tibial spine (Fig. 2). The tibial and femoral tunnel length was measured.
Fig. 2.
Illustration of performed measurements indicating tibial and femoral tunnel orientation and position (1 anatomical femoral axis, 2 transepicondylar axis, 3 anatomical tibial axis, 4 tibial condylar axis, 5 and 6 medial and lateral tibial spines, 7 mid-tibial spine axis, 8 tibial and femoral tunnel exit, 9 and 10 Blumensaat’s line)
Statistical analysis
Data were analysed using SPSS 17.0 (SPSS, Chicago, USA). Beneath descriptive statistics, Pearson's correlation of SPECT/CT tracer uptake (intensity and distribution) with the stability and laxity of the knee joint as well as the femoral and tibial tunnel position was performed (p < 0.05).
Results
Lachman and anterior drawer testing of the patients are presented in Table 1. The pivot shift was positive in 29/66 patients and negative in 35/66 patients. Twenty-nine of 66 patients reported subjective instability. In two patients these values were missing. The standardised maximum intensity of 99mTc-HDP-SPECT/CT tracer uptake in each anatomical knee region (mean, standard deviation, minimum, maximum) is shown in Table 2.
Table 1.
Anterior laxity (Lachman and anterior drawer testing) using Rolimeter (Ormed, Freiburg, Germany) in 25° and 70° flexion
| Test | 0–2mm | 3–5mm | 6–10mm | >10mm |
|---|---|---|---|---|
| Lachman testing | 21 (33%) | 16 (25%) | 22 (34%) | 5 (8%) |
| Anterior drawer | 19 (30%) | 18 (28%) | 21 (33%) | 6 (9%) |
Table 2.
Standardised maximum intensity of 99mTc-HDP-SPECT/CT tracer uptake in each anatomical knee region (mean, standard deviation [SD], minimum, maximum)
| Measurement | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| ap.fem.1s | 2.41 | 1.05 | 0.86 | 6.84 |
| ap.fem.2s | 1.95 | 0.83 | 0.61 | 4.96 |
| ap.fem.1i | 3.25 | 2.37 | 0.66 | 15.69 |
| ap.fem.2i | 3.54 | 2.50 | 0.59 | 11.38 |
| ap.tib.1s | 2.62 | 1.51 | 0.60 | 6.76 |
| ap.tib.3s | 3.55 | 2.08 | 0.64 | 10.27 |
| ap.tib.2s | 3.31 | 2.75 | 0.60 | 16.26 |
| ap.tib.1i | 2.40 | 1.26 | 0.51 | 7.51 |
| ap.tib.3i | 2.71 | 1.84 | 0.54 | 9.86 |
| ap.tib.2i | 2.13 | 1.31 | 0.45 | 6.43 |
| ap.pat.1s | 1.76 | 1.00 | 0.43 | 5.51 |
| ap.pat.2s | 1.81 | 1.39 | 0.38 | 9.08 |
| ap.pat.1i | 1.57 | 0.95 | 0.37 | 5.11 |
| ap.pat.2i | 1.64 | 1.06 | 0.36 | 4.82 |
| axial tib.1p | 2.48 | 1.56 | 0.60 | 8.04 |
| axial tib.3p | 3.23 | 1.80 | 0.57 | 9.32 |
| axial tib.2p | 3.08 | 2.55 | 0.67 | 16.26 |
| axial tib.1a | 2.03 | 1.39 | 0.42 | 6.76 |
| axial tib.3a | 3.18 | 2.11 | 0.58 | 10.27 |
| axial tib.2a | 2.85 | 2.22 | 0.51 | 10.14 |
| sag.fem.sa | 2.11 | 0.85 | 0.74 | 4.87 |
| sag.fem.sp | 2.44 | 1.08 | 0.54 | 6.84 |
| sag.fem.ia | 3.79 | 2.77 | 0.75 | 15.69 |
| sag.fem.ip | 3.73 | 2.36 | 0.86 | 10.42 |
| sag.tib.sa | 3.90 | 2.71 | 0.63 | 13.57 |
| sag.tib.sp | 3.87 | 2.55 | 0.84 | 16.26 |
| sag.tib.ia | 2.95 | 1.87 | 0.51 | 9.86 |
| sag.tib.ip | 2.37 | 1.27 | 0.54 | 6.33 |
| tun.fem.points | 2.25 | 1.22 | 0.59 | 8.98 |
| tun.tib.points | 3.19 | 2.05 | 0.48 | 9.22 |
Correlation of instability, pivot shift and clinical laxity testing with 99mTc-HDP-SPECT/CT tracer uptake intensity and distribution showed no significant association of both (Table 3).
Table 3.
Correlation of 99mTc-HDP-SPECT/CT tracer uptake and stability and clinical laxity testing
| Measurement | Standardized maximum 99mTc-HDP tracer uptake values | |||
|---|---|---|---|---|
| Lachmann | Anterior drawer | Pivot-shift testing | Instability | |
| ap.fem.1s | −0.13 | −0.12 | −0.03 | −0.05 |
| ap.fem.2s | −0.11 | −0.12 | −0.06 | −0.05 |
| ap.fem.1i | −0.12 | −0.13 | −0.10 | −0.12 |
| ap.fem.2i | −0.11 | −0.14 | −0.12 | −0.12 |
| ap.tib.1s | −0.17 | −0.17 | −0.04 | −0.05 |
| ap.tib.3s | −0.15 | −0.18 | −0.07 | −0.08 |
| ap.tib.2s | −0.05 | −0.07 | −0.09 | −0.09 |
| ap.tib.1i | −0.27* | −0.25* | −0.12 | −0.14 |
| ap.tib.3i | −0.23 | −0.23 | −0.11 | −0.16 |
| ap.tib.2i | −0.18 | −0.17 | −0.19 | −0.19 |
| ap.pat.1s | −0.07 | −0.09 | −0.01 | 0.02 |
| ap.pat.2s | −0.05 | −0.08 | 0.07 | 0.05 |
| ap.pat.1i | −0.12 | −0.15 | −0.08 | −0.11 |
| ap.pat.2i | −0.16 | −0.19 | −0.06 | −0.1 |
| axial tib.1p | −0.11 | −0.11 | 0.01 | 0.00 |
| axial tib.3p | −0.13 | −0.14 | −0.05 | −0.08 |
| axial tib.2p | −0.06 | −0.08 | −0.12 | −0.11 |
| axial tib.1a | −0.17 | −0.17 | −0.12 | −0.16 |
| axial tib.3a | −0.19 | −0.21 | −0.16 | −0.16 |
| axial tib.2a | −0.14 | −0.16 | −0.16 | −0.15 |
| sag.fem.sa | −0.05 | −0.07 | 0.03 | 0.04 |
| sag.fem.sp | −0.17 | −0.17 | −0.05 | −0.07 |
| sag.fem.ia | −0.14 | −0.16 | −0.15 | −0.16 |
| sag.fem.ip | −0.09 | −0.12 | −0.13 | −0.17 |
| sag.tib.sa | −0.15 | −0.18 | −0.15 | −0.16 |
| sag.tib.sp | −0.01 | −0.04 | −0.04 | −0.05 |
| sag.tib.ia | −0.25 | −0.25* | −0.2 | −0.25 |
| sag.tib.ip | −0.12 | −0.12 | −0.06 | −0.08 |
| tun.fem.points | −0.07 | −0.07 | −0.01 | −0.01 |
| tun.tib.points | −0.15 | −0.18 | −0.07 | −0.09 |
Significant correlations in bold, *p<0.05, **p<0.001
The position and orientation of the tibial and femoral tunnel in relation to the anatomical knee axis is presented in Table 4. Correlation of 99mTc-HDP-SPECT/CT tracer uptake and position and orientation of the ACL graft and tunnel length is shown in Tables 5 and 6. A more horizontal femoral graft position showed significantly increased tracer uptake within the superior and posterior femoral regions. A more posteriorly placed femoral insertion site showed significantly more tracer uptake within the femoral and tibial tunnel regions.
Table 4.
Position and orientation of the tibial and femoral tunnel in relation to the anatomical knee axis assessed using a previously published method on 3D-CT [9]. The femoral tunnel entry position was determined in mm distance on 3D-CT in relation to the Blumensaat’s line (anterior-posterior and high-low). The tibial tunnel position was determined in mm distance on 3D-CT in relation to the anterior-posterior tibial plateau length and distance to the centre point between medial and lateral tibial spine. The tibial and femoral tunnel lengths were measured
| Measurement | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| Fem-Ang-ap (°) | 30.85 | 13.24 | 0.81 | 66.76 |
| Fem-Ang-lat (°) | 36.03 | 14.45 | 6.70 | 74.38 |
| Fem-Ang-axial (°) | 50.02 | 19.33 | 8.52 | 88.42 |
| FemEntry-antBlum-x | −22.94 | 5.79 | −35.14 | −9.17 |
| FemEntry-antBlum-y | −3.21 | 2.83 | −10.89 | 3.34 |
| FemEntry-antBlum-z | −1.36 | 7.95 | −15.09 | 12.33 |
| FemEntry-postBlum-x | 3.15 | 5.22 | −5.22 | 19.74 |
| FemEntry-postBlum-y | −3.20 | 2.83 | −10.89 | 3.34 |
| FemEntry-postBlum-z | −0.54 | 9.42 | −13.40 | 15.20 |
| Fem-Tun-Length | 33.14 | 9.02 | 13.87 | 52.51 |
| Tib-Ang-ap | 16.58 | 9.89 | 0.38 | 44.00 |
| Tib-Ang-lat | 27.54 | 10.28 | 0.54 | 48.25 |
| Tib-Ang-axial | 59.45 | 17.94 | 9.49 | 87.52 |
| Tib-med-lat | 76.47 | 6.51 | 61.55 | 89.56 |
| Tib-ant-post | 49.73 | 4.85 | 38.61 | 66.87 |
| Tib-Tun-Length | 35.36 | 7.57 | 18.26 | 56.33 |
| Tib-TunCentre-MidEmin.-Length | 9.95 | 4.73 | 2.70 | 22.81 |
| ap-TibExit-MidEminentia-x | −0.05 | 2.55 | −6.99 | 7.23 |
| ap-TibExit-MidEminentia-y | 5.10 | 1.87 | 1.95 | 9.56 |
| ap-TibExit-MidEminentia-z | 7.60 | 5.28 | −3.66 | 21.66 |
Table 5.
Correlation of standardised maximum 99mTc-HDP-SPECT/CT tracer uptake and femoral position and orientation of the ACL graft as well as femoral tunnel length evaluated on 3D-CT
| Measurement | Fem-Ang-ap (°) | Fem-Ang-lat (°) | Fem-Ang-axial (°) | FemEntry-antBlum-x | FemEntry-antBlum-y | FemEntry-antBlum-z | FemEntry-postBlum-x | FemEntry-postBlum-y | FemEntry-postBlum-z | Fem-Tun-Length |
|---|---|---|---|---|---|---|---|---|---|---|
| ap.fem.1s | 0.26* | 0.05 | −0.14 | 0.02 | −0.09 | 0.15 | 0.11 | −0.10 | 0.11 | −0.06 |
| ap.fem.2s | 0.34** | 0.07 | −0.20 | 0.29* | 0.03 | 0.2 | 0.34** | 0.03 | 0.09 | 0.08 |
| ap.fem.1i | 0.05 | −0.16 | −0.17 | 0.13 | −0.15 | 0.14 | 0.27* | −0.15 | 0.11 | −0.08 |
| ap.fem.2i | 0.06 | −0.09 | −0.14 | 0.11 | −0.04 | −0.02 | 0.13 | −0.04 | −0.02 | −0.15 |
| ap.tib.1s | 0.11 | −0.09 | −0.17 | 0.11 | −0.04 | 0.20 | 0.22 | −0.04 | 0.16 | 0.07 |
| ap.tib.3s | 0.04 | −0.16 | −0.15 | 0.12 | −0.02 | 0.07 | 0.11 | −0.03 | 0.04 | −0.16 |
| ap.tib.2s | 0.26* | −0.01 | −0.22 | 0.11 | −0.15 | −0.04 | 0.14 | −0.15 | −0.03 | −0.12 |
| ap.tib.1i | 0.11 | −0.21 | −0.29* | 0.05 | −0.05 | 0.2 | 0.17 | −0.06 | 0.11 | 0.04 |
| ap.tib.3i | 0.09 | −0.18 | −0.22 | 0.00 | −0.03 | −0.03 | 0.01 | −0.03 | −0.13 | −0.15 |
| ap.tib.2i | 0.09 | −0.02 | −0.11 | −0.05 | −0.03 | −0.03 | −0.01 | −0.04 | −0.07 | −0.15 |
| ap.pat.1s | 0.18 | −0.12 | −0.20 | 0.18 | −0.10 | 0.14 | 0.34** | −0.11 | 0.06 | −0.11 |
| ap.pat.2s | 0.07 | −0.02 | −0.04 | 0.05 | −0.03 | 0.24 | 0.11 | −0.03 | 0.16 | −0.05 |
| ap.pat.1i | 0.09 | 0.01 | −0.05 | 0.14 | −0.07 | 0.04 | 0.21 | −0.08 | −0.03 | −0.06 |
| ap.pat.2i | 0.00 | −0.09 | −0.05 | 0.1 | −0.05 | 0.09 | 0.15 | −0.06 | 0.03 | −0.14 |
| axial tib.1p | 0.10 | −0.10 | −0.17 | 0.15 | 0.00 | 0.22 | 0.25 | 0.00 | 0.18 | 0.09 |
| axial tib.3p | −0.02 | −0.21 | −0.16 | 0.18 | −0.01 | 0.08 | 0.18 | −0.01 | 0.05 | −0.1 |
| axial tib.2p | 0.25 | 0.06 | −0.15 | 0.06 | −0.12 | −0.07 | 0.07 | −0.12 | −0.04 | −0.11 |
| axial tib.1a | 0.19 | −0.04 | −0.19 | 0.18 | −0.05 | 0.25 | 0.27* | −0.05 | 0.20 | −0.08 |
| axial tib.3a | −0.01 | −0.12 | −0.09 | 0.17 | −0.01 | 0.02 | 0.14 | −0.01 | 0.01 | −0.17 |
| axial tib.2a | 0.16 | −0.05 | −0.18 | 0.15 | −0.03 | 0.01 | 0.15 | −0.04 | 0.02 | −0.15 |
| sag.fem.sa | 0.10 | −0.03 | −0.09 | 0.10 | −0.10 | 0.11 | 0.19 | −0.10 | 0.03 | 0.02 |
| sag.fem.sp | 0.34** | 0.09 | −0.17 | 0.11 | −0.03 | 0.17 | 0.19 | −0.03 | 0.14 | 0.00 |
| sag.fem.ia | 0.08 | −0.12 | −0.17 | 0.11 | −0.01 | 0.07 | 0.18 | −0.02 | 0.04 | −0.17 |
| sag.fem.ip | 0.08 | −0.02 | −0.07 | 0.02 | −0.05 | 0.04 | 0.07 | −0.06 | 0.02 | −0.16 |
| sag.tib.sa | 0.19 | −0.02 | −0.16 | 0.09 | −0.08 | −0.03 | 0.13 | −0.08 | −0.04 | −0.19 |
| sag.tib.sp | 0.24 | −0.01 | −0.18 | 0.05 | −0.14 | −0.03 | 0.08 | −0.14 | −0.04 | −0.12 |
| sag.tib.ia | 0.10 | −0.09 | −0.17 | −0.05 | −0.02 | 0.00 | 0.02 | −0.03 | −0.09 | −0.14 |
| sag.tib.ip | 0.15 | 0.03 | −0.11 | 0.09 | 0.05 | 0.16 | 0.15 | 0.04 | 0.08 | −0.02 |
| tun.fem.points | 0.02 | −0.17 | −0.16 | 0.17 | −0.12 | 0.17 | 0.29* | −0.13 | 0.11 | −0.04 |
| tun.tib.points | 0.00 | −0.07 | −0.06 | 0.12 | 0.00 | 0.03 | 0.11 | −0.01 | 0.00 | −0.16 |
Significant correlations in bold, *p<0.05, **p<0.001
Table 6.
Correlation of 99mTc-HDP-SPECT/CT tracer uptake and tibial position and orientation of the ACL graft and tibial tunnel length evaluated on 3D-CT
| Measurement | Tib-Ang-ap | Tib-Ang-lat | Tib-Ang-axial | Tib-med-lat | Tib-ant-post | Tib-Tun-Length | Tib-TunCentre-MidEmin.-Length | ap-TibExit-MidEminentia-x | ap-TibExit-MidEminentia-y | ap-TibExit-MidEminentia-z |
|---|---|---|---|---|---|---|---|---|---|---|
| ap.fem.1s_stand_max | −0.2 | 0.09 | 0.18 | 0.2 | 0.27* | 0.12 | 0.03 | 0.11 | −0.05 | 0.1 |
| ap.fem.2s_stand_max | −0.02 | 0.03 | 0.02 | 0.16 | 0.27* | −0.07 | 0.19 | 0.15 | −0.04 | 0.22 |
| ap.fem.1i_stand_max | −0.22 | 0.15 | 0.25* | 0.29* | 0.32** | 0.16 | 0 | 0.12 | −0.07 | 0.06 |
| ap.fem.2i_stand_max | −0.2 | −0.11 | 0.09 | 0.25* | 0.19 | −0.01 | 0.05 | 0.11 | −0.07 | 0.1 |
| ap.tib.1s_stand_max | −0.08 | 0.17 | 0.15 | 0.4*** | 0.41*** | 0.05 | 0.11 | −0.01 | 0.03 | 0.14 |
| ap.tib.3s_stand_max | −0.26* | 0.12 | 0.27* | 0.24 | 0.25* | 0.1 | 0.11 | 0.12 | −0.04 | 0.18 |
| ap.tib.2s_stand_max | −0.04 | −0.07 | 0 | 0.28* | 0.26* | −0.16 | 0.19 | −0.01 | −0.03 | 0.24 |
| ap.tib.1i_stand_max | −0.17 | 0.1 | 0.17 | 0.3* | 0.27* | 0.1 | 0.1 | −0.02 | 0.04 | 0.13 |
| ap.tib.3i_stand_max | −0.2 | 0.16 | 0.26* | 0.14 | 0.2 | 0.16 | 0.13 | 0 | −0.05 | 0.19 |
| ap.tib.2i_stand_max | −0.11 | −0.08 | 0.08 | 0.17 | 0.27* | −0.18 | 0.15 | −0.09 | −0.03 | 0.16 |
| ap.pat.1s_stand_max | −0.06 | 0.08 | 0.12 | 0.25* | 0.29* | 0.15 | 0.05 | 0.15 | −0.06 | 0.13 |
| ap.pat.2s_stand_max | −0.12 | 0.18 | 0.21 | 0.21 | 0.3* | 0.09 | 0.01 | 0.12 | 0.06 | 0.06 |
| ap.pat.1i_stand_max | −0.22 | 0.05 | 0.21 | 0.16 | 0.14 | 0.18 | −0.04 | 0.08 | −0.11 | 0.03 |
| ap.pat.2i_stand_max | −0.29* | 0.11 | 0.31* | 0.15 | 0.17 | 0.19 | −0.02 | 0.06 | −0.04 | 0.05 |
| axial tib.1p_stand_max | −0.06 | 0.16 | 0.14 | 0.36** | 0.34** | 0.06 | 0.06 | −0.04 | −0.03 | 0.1 |
| axial tib.3p_stand_max | −0.24 | 0.12 | 0.26* | 0.25* | 0.24 | 0.08 | 0.08 | 0.09 | −0.03 | 0.14 |
| axial tib.2p_stand_max | 0 | −0.07 | −0.05 | 0.27* | 0.29* | −0.23 | 0.24 | −0.02 | −0.01 | 0.28* |
| axial tib.1a_stand_max | −0.2 | 0.12 | 0.22 | 0.27* | 0.36** | 0.01 | 0.17 | 0.13 | 0.09 | 0.2 |
| axial tib.3a_stand_max | −0.27* | 0.13 | 0.27* | 0.16 | 0.21 | 0.09 | 0.11 | 0.13 | −0.06 | 0.18 |
| axial tib.2a_stand_max | −0.16 | −0.13 | 0.06 | 0.23 | 0.18 | −0.13 | 0.16 | 0.08 | −0.02 | 0.21 |
| sag.fem.sa_stand_max | −0.11 | −0.03 | 0.06 | 0.06 | 0.1 | 0.1 | −0.16 | 0.17 | −0.1 | −0.09 |
| sag.fem.sp_stand_max | −0.19 | 0.07 | 0.16 | 0.22 | 0.27* | 0.02 | 0.18 | 0.14 | −0.02 | 0.24 |
| sag.fem.ia_stand_max | −0.22 | 0.07 | 0.21 | 0.31* | 0.35** | 0.08 | 0.07 | 0.1 | −0.07 | 0.14 |
| sag.fem.ip_stand_max | −0.27* | −0.08 | 0.15 | 0.29* | 0.28* | 0.01 | 0.06 | 0.16 | −0.04 | 0.11 |
| sag.tib.sa_stand_max | −0.24 | −0.04 | 0.16 | 0.29* | 0.3* | 0.05 | 0.13 | 0.14 | −0.03 | 0.2 |
| sag.tib.sp_stand_max | −0.1 | −0.07 | 0.03 | 0.31* | 0.28* | −0.09 | 0.1 | 0 | −0.1 | 0.17 |
| sag.tib.ia_stand_max | −0.27* | 0 | 0.21 | 0.22 | 0.29* | 0.1 | 0.09 | 0.03 | −0.08 | 0.14 |
| sag.tib.ip_stand_max | −0.15 | −0.12 | 0.08 | 0.15 | 0.14 | −0.08 | 0.03 | −0.03 | −0.13 | 0.06 |
| tun.fem.points_stand_max | −0.18 | 0 | 0.13 | 0.08 | 0.01 | 0.27* | −0.16 | 0.13 | −0.16 | −0.09 |
| tun.tib.points_stand_max | −0.29* | 0.1 | 0.27nn | 0.15 | 0.17 | 0.14 | 0.05 | 0.14 | −0.1 | 0.11 |
Significant correlations in bold, *p<0.05, **p<0.001
A more vertical or a less medial tibial tunnel orientation showed significantly increased uptake within the tibial and femoral tunnel regions. A more anterior tibial tunnel position showed significantly more tracer uptake in the femoral and tibial tunnel regions as well as the entire tibiofemoral joint.
Discussion
SPECT/CT is a new and interesting imaging modality which offers the combined assessment of anatomical 3D-CT assessment of the tunnel placement and orientation of the graft with information on joint biology. In particular, information on the loading history of the joint can be obtained [21].
However, it is currently unclear whether SPECT/CT tracer uptake shows a significant correlation with stability and/or laxity of the knee joint or the position of the femoral or tibial tunnels. The findings of our study will facilitate analysis of SPECT/CT imaging in patients after ACL reconstruction. Only when the influence of mechanical axis, tunnel position, biological factors such as bone remodelling and laxity and stability of the knee joint on SPECT/CT tracer uptake are identified, will the intensity and distribution of SPECT/CT tracer uptake be understood. This is the first study investigating the potential value of SPECT/CT in assessment of in-vivo loading history of the knee joint after ACL reconstruction.
The most important findings and implications of this study are threefold.
First, this is the first study investigating the relationship of stability or clinical laxity in combination with the intensity and distribution of SPECT tracer uptake. Interestingly, it seems that the stability or laxity of the knee joint is not a contributing factor to the increased SPECT tracer uptake in the group investigated. It has to be further studied to determine whether these findings could also be confirmed in patients with normal ACL function (natural history group). In addition, the time from surgery might have a significant impact on tracer uptake.
In a previous study we have shown that a non-ideal mechanical axis such as varus or valgus aligned knee leads to increased SPECT tracer uptake in the stressed compartment [16]. However, the altered tracer uptake in patients with leg deformity is caused by mechanical stress, which is not the case in these patients after ACL reconstruction. An increased anterior translation or instability leads to increased anterior shear forces and not inevitably higher joint loading forces. Furthermore, it could be that only if instability or increased anterior laxity persists over a longer time that it leads to detectable changes of SPECT tracer uptake.
Second, SPECT/CT tracer uptake intensity and distribution showed significant correlation with the femoral and tibial tunnel position and orientation in patients with problems after ACL reconstruction.
Although controversy exists regarding the effect of graft position and orientation on knee stability after ACL reconstruction it is well accepted that a lower femoral tunnel position in ACL reconstruction leads to a more stable knee in rotation and anterior tibial translation when compared to a high femoral graft placement [22]. Consequently, higher forces within the ACL graft were reported [22]. These higher forces could explain why we found that a more horizontal femoral graft position showed significantly increased tracer uptake within the superior and posterior femoral regions. In particular, the area around the articular femoral tunnel entry and exit showed increased tracer uptake, which is in line with the findings of others describing high stress at these insertions points [23]. Smolinski et al. used a finite element model developed from a CT scan of a cadaveric knee to compute the stress change in the distal femur caused by graft tunnels in ACL reconstruction [23]. In this study the position and the geometry of the ACL graft tunnel exits were varied and the change in stress on the distal femur was computed [23]. They found that the highest stress was found at the tunnel exits [23]. The position of the tunnel exit significantly influenced the stress in the distal femur, which they explained by increased condylar loads [23]. Stress decreased when the distance from the tunnel exits to the condyle decreased [23]. These findings were consistent with ours.
In addition to the results of Smolinski et al. we also investigated the tibiofemoral joint as well as the tibia. A more anterior or more vertical tibial tunnel position showed significantly more tracer uptake in the femoral and tibial tunnel regions as well as in the entire tibiofemoral joint. The causes of this are not yet fully determined, but it seems most likely to be related to altered bone remodelling processes. Another reason could be that due to anterior tunnel placement there is an increase in forces within the ACL graft [24].
Limitations
The following limitations have to be considered when interpreting the study results.
First, the patients investigated in our study were all patients with symptoms after ACL reconstruction. It could be speculated that the same findings might be found in patients without any problems after ACL reconstruction. In patients with problems after ACL reconstruction the SPECT/CT tracer uptake could be influenced by a variety of factors such as a malposition of the graft, which does not inevitably lead to an altered laxity, but a changed joint kinematics and loading. However, in clinical practice SPECT/CT imaging would be used in patients with problems after ACL reconstruction and that is why we chose this study cohort.
Second, the patients investigated here did undergo ACL reconstruction using a variety of different grafts and graft fixation techniques. It is possible that the difference here influences the correlation of stability and laxity and SPECT/CT tracer uptake. Further studies should investigate a more homogenous patient cohorts.
Conclusion
SPECT/CT tracer uptake intensity and distribution showed a significant correlation with the femoral and tibial tunnel position and orientation in patients with relevant symptoms after ACL reconstruction. No correlation was found with stability or clinical laxity. SPECT/CT tracer uptake distribution has the potential to provide important information on joint homeostasis and remodelling after ACL reconstruction. It might help to predict ACL graft failure and improve our surgical ACL reconstruction technique in finding the optimal tunnel and graft position and orientation.
Acknowledgments
We greatly thank the Deutsche Arthrose Hilfe e.V, Saarlouis, Germany and the Alwin-Jäger-Foundation, Aschaffenburg, Germany for funding of the study.
Footnotes
Michael T. Hirschmann and Dominic Mathis contributed equally to this paper.
References
- 1.Arnold MP, Friederich NF, Muller W, Hirschmann MT (2012) From open to arthroscopic anatomical ACL-reconstructions: the long way round. A statement paper. Knee Surg Sports Traumatol Arthrosc. May 9 [Epub ahead of print] [DOI] [PubMed]
- 2.Gerhard P, Bolt R, Duck K, Mayer R, Friederich NF, Hirschmann MT (2012) Long-term results of arthroscopically assisted anatomical single-bundle anterior cruciate ligament reconstruction using patellar tendon autograft: are there any predictors for the development of osteoarthritis? Knee Surg Sports Traumatol Arthrosc. Apr 10 [Epub ahead of print] [DOI] [PubMed]
- 3.Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39:199–217. doi: 10.1177/0363546510370929. [DOI] [PubMed] [Google Scholar]
- 4.Trojani C, Sbihi A, Djian P, Potel JF, Hulet C, Jouve F, Bussiere C, Ehkirch FP, Burdin G, Dubrana F, Beaufils P, Franceschi JP, Chassaing V, Colombet P, Neyret P. Causes for failure of ACL reconstruction and influence of meniscectomies after revision. Knee Surg Sports Traumatol Arthrosc. 2011;19:196–201. doi: 10.1007/s00167-010-1201-6. [DOI] [PubMed] [Google Scholar]
- 5.van Eck CF, Kropf EJ, Romanowski JR, Lesniak BP, Tranovich MJ, van Dijk CN, Fu FH. ACL graft re-rupture after double-bundle reconstruction: factors that influence the intra-articular pattern of injury. Knee Surg Sports Traumatol Arthrosc. 2011;19:340–346. doi: 10.1007/s00167-010-1297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirschmann MT, Adler T, Rasch H, Hugli RW, Friederich NF, Arnold MP. Painful knee joint after ACL reconstruction using biodegradable interference screws- SPECT/CT a valuable diagnostic tool? A case report. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:24. doi: 10.1186/1758-2555-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basdekis G, Christel P, Anne F. Validation of the position of the femoral tunnels in anatomic double-bundle ACL reconstruction with 3-D CT scan. Knee Surg Sports Traumatol Arthrosc. 2009;17:1089–1094. doi: 10.1007/s00167-009-0829-6. [DOI] [PubMed] [Google Scholar]
- 8.Forsythe B, Kopf S, Wong AK, Martins CA, Anderst W, Tashman S, Fu FH. The location of femoral and tibial tunnels in anatomic double-bundle anterior cruciate ligament reconstruction analyzed by three-dimensional computed tomography models. J Bone Joint Surg Am. 2010;92:1418–1426. doi: 10.2106/JBJS.I.00654. [DOI] [PubMed] [Google Scholar]
- 9.Hirschmann MT, Mathis D, Afifi FK, Rasch H, Henckel J, Amsler F, Wagner CR, Friederich NF, Arnold MP (2012) Single photon emission computerized tomography and conventional computerized tomography (SPECT/CT) for evaluation of patients after anterior cruciate ligament reconstruction: a novel standardized algorithm combining mechanical and metabolic information. Knee Surg Sports Traumatol Arthrosc. Jun 13 [Epub ahead of print] [DOI] [PubMed]
- 10.Kopf S, Forsythe B, Wong AK, Tashman S, Irrgang JJ, Fu FH. Transtibial ACL reconstruction technique fails to position drill tunnels anatomically in vivo 3D CT study. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2200–2207. doi: 10.1007/s00167-011-1851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Purnell ML, Larson AI, Clancy W. Anterior cruciate ligament insertions on the tibia and femur and their relationships to critical bony landmarks using high-resolution volume-rendering computed tomography. Am J Sports Med. 2008;36:2083–2090. doi: 10.1177/0363546508319896. [DOI] [PubMed] [Google Scholar]
- 12.Sadoghi P, Kropfl A, Jansson V, Muller PE, Pietschmann MF, Fischmeister MF. Impact of tibial and femoral tunnel position on clinical results after anterior cruciate ligament reconstruction. Arthroscopy. 2011;27:355–364. doi: 10.1016/j.arthro.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Hirschmann MT, Iranpour F, Davda K, Rasch H, Hugli R, Friederich NF. Combined single-photon emission computerized tomography and conventional computerized tomography (SPECT/CT): clinical value for the knee surgeons? Knee Surg Sports Traumatol Arthrosc. 2010;18:341–345. doi: 10.1007/s00167-009-0879-9. [DOI] [PubMed] [Google Scholar]
- 14.Hirschmann MT, Davda K, Rasch H, Arnold MP, Friederich NF. Clinical value of combined single photon emission computerized tomography and conventional computer tomography (SPECT/CT) in sports medicine. Sports Med Arthrosc. 2011;19:174–181. doi: 10.1097/JSA.0b013e3181ec8707. [DOI] [PubMed] [Google Scholar]
- 15.Hirschmann MT, Wagner CR, Rasch H, Henckel J. Standardized volumetric 3D-analysis of SPECT/CT imaging in orthopaedics: overcoming the limitations of qualitative 2D analysis. BMC Med Imaging. 2012;12:5. doi: 10.1186/1471-2342-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirschmann MT, Schon S, Afifi FK, Amsler F, Rasch H, Friederich NF, Arnold MP (2012) Assessment of loading history of compartments in the knee using bone SPECT/CT: A study combining alignment and 99mTc-HDP tracer uptake/distribution patterns. J Orthop Res. doi:10.1002/jor.22206 [DOI] [PubMed]
- 17.Hirschmann MT, Davda K, Iranpour F, Rasch H, Friederich NF. Combined single photon emission computerised tomography and conventional computerised tomography (SPECT/CT) in patellofemoral disorders: a clinical review. Int Orthop. 2011;35:675–680. doi: 10.1007/s00264-010-1049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirschmann MT, Iranpour F, Konala P, Kerner A, Rasch H, Cobb JP, Friederich NF. A novel standardized algorithm for evaluating patients with painful total knee arthroplasty using combined single photon emission tomography and conventional computerized tomography. Knee Surg Sports Traumatol Arthrosc. 2010;18:939–944. doi: 10.1007/s00167-010-1070-z. [DOI] [PubMed] [Google Scholar]
- 19.Hirschmann MT, Konala P, Iranpour F, Kerner A, Rasch H, Friederich NF. Clinical value of SPECT/CT for evaluation of patients with painful knees after total knee arthroplasty—a new dimension of diagnostics? BMC Musculoskelet Disord. 2011;12:36. doi: 10.1186/1471-2474-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henckel J, Richards R, Lozhkin K, Harris S, Baena FM, Barrett AR, Cobb JP. Very low-dose computed tomography for planning and outcome measurement in knee replacement. The imperial knee protocol. J Bone Joint Surg Br. 2006;88:1513–1518. doi: 10.1302/0301-620X.88B11.17986. [DOI] [PubMed] [Google Scholar]
- 21.Hirschmann MT, Wagner CR, Rasch H, Henckel J. Standardized volumetric 3D analysis of SPECT/CT imaging in orthopaedics: overcoming the limitations of qualitative 2D analysis. BMC Med Imaging. 2011;12:5. doi: 10.1186/1471-2342-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loh JC, Fukuda Y, Tsuda E, Steadman RJ, Fu FH, Woo SL. Knee stability and graft function following anterior cruciate ligament reconstruction: Comparison between 11 o’clock and 10 o’clock femoral tunnel placement. 2002 Richard O’Connor Award paper. Arthroscopy. 2003;19:297–304. doi: 10.1053/jars.2003.50084. [DOI] [PubMed] [Google Scholar]
- 23.Smolinski P, O’Farrell M, Bell K, Gilbertson L, Fu FH (2012) Effect of ACL reconstruction tunnels on stress in the distal femur. Knee Surg Sports Traumatol Arthrosc. Apr 18 [Epub ahead of print] [DOI] [PubMed]
- 24.Markolf KL, Hame S, Hunter DM, Oakes DA, Zoric B, Gause P, Finerman GA. Effects of femoral tunnel placement on knee laxity and forces in an anterior cruciate ligament graft. J Orthop Res. 2002;20:1016–1024. doi: 10.1016/S0736-0266(02)00035-9. [DOI] [PubMed] [Google Scholar]