Abstract
Objectives
To test the hypothesis that anemia (hemoglobin <12 g/dL) is associated with a faster rate of 9-year cognitive decline in a community-dwelling sample of women aged 70-80 years at baseline.
Design
A population-based, prospective cohort study
Setting
East Baltimore, Maryland
Participants
436 women sampled to be representative of the 2/3 least disabled women in, aged 70-80 years at baseline (1994-1996).
Measurements
9-year trajectories of cognitive decline, analyzed with linear random effects models, in the domains of immediate verbal recall, delayed verbal recall, psychomotor speed, and executive function.
Results
At baseline and after adjustment for demographic and disease covariates, women with anemia were slower to complete a test of executive function than women without anemia by - 0.43 SD (95% CI: −0.74, −0.13) on the Trail Making Test, Part B (TMTB). During follow-up, anemia was associated with a faster rate of decline in memory. Between baseline and year 3, women with anemia declined at a rate of 0.18 more SD/year (95% CI: −0.29, −0.06) than women without anemia on the Hopkins Verbal Learning Test (HVLT) and by .0.15 more SD/year (95% CI: −0.26. −0.04) on the Hopkins Verbal Learning Test-Delayed (HVLT-Delayed).
Conclusion
Anemia was associated with poorer baseline performance on a test of executive function and with faster rates of decline on tests of immediate and delayed verbal recall. If this relationship is causal, it is possible that treatment of anemia could prevent or postpone cognitive decline.
Keywords: anemia, elderly, executive function, longitudinal study, memory
INTRODUCTION
Cognitive decline is associated with adverse cognitive and physical outcomes, including dementia incidence (1) and difficulty with Instrumental Activities of Daily Living (IADLs) (e.g., shopping, managing finances) (2) and Activities of Daily Living (ADLs) (e.g., dressing, toileting) (3). Although it is generally accepted that cognitive decline occurs in normal aging, this decline is heterogeneous and may represent individual-level factors instead of an inevitable process (4). Observational studies of potential modifiers of cognitive decline are therefore warranted.
Analogous to the documented link between anemia and disability (5), anemia may also be a risk factor for cognitive decline (6) Defined by low hemoglobin concentrations, anemia is potentially modifiable (7) and prevalent in the United States population over age 65, with prevalence increasing with advancing age (8). Preliminary studies have associated anemia with dementia (9,10) and cognitive decline (6) in older adults. Nonetheless, data on the relationship between anemia and cognitive decline in older adults remain scarce, particularly data on the longitudinal relationship between anemia and cognitive decline in specific domains of cognitive functioning.
Although most studies of cognitive function are limited to single measures of global cognitive function (e.g., Mini-Mental State Exam (11)), the assessment of multiple cognitive domains may better identify those at risk for adverse health outcomes. In normative cognitive aging, decline in some cognitive domains is known to precede declines in other domains (12-15); global measures ignore this hierarchy of decline. Additionally, domain-specific cognitive decline has been associated with dementia (16) and disability (2,3).
To our knowledge, only one study has quantified the effect of anemia on a specific cognitive domain; in cross-sectional analysis, low hemoglobin levels were associated with executive function impairment (6)
Previous work has quantified the trajectories of cognitive decline in four domains in a cohort of community-dwelling older women aged 70-80 years at baseline (12). The present study aimed to test the hypothesis that anemia assessed at baseline is an independent risk factor for a faster rate of decline over 9 years on tests of immediate and delayed verbal recall, psychomotor speed, and executive function in this same cohort.
METHODS
Study population
The Women’s Health and Aging Study (WHAS) II is a prospective cohort study of 436 women sampled from among the two-thirds least disabled women living in East Baltimore, Maryland aged between 70 and 80 years at baseline (1994-1996). Selection criteria have been described elsewhere (2)
Data was collected every 1.5 years following the initial visit, excepting a 3-year interval between Rounds 3 and 4 (years 1997-1999). Data through Round 6 are incorporated in this analysis, comprising approximately 9 years of follow-up.
Of 436 women at baseline, 398 had complete cognitive test data for at least one of the follow-up exams. Of these 398 women, 23 were missing baseline hemoglobin values. One additional woman was excluded due to missing data on all confounding variables, yielding a final sample of 374. The missing were less educated, less likely to drink alcohol, more often non-white, and more likely to have interleukin-6 (IL6) levels in the highest tertile (data not shown).
The Johns Hopkins Medical Institutions institutional review board approved the study and informed consent was obtained from all participants at each visit.
Cognitive outcomes
All neuropsychological tests were administered by a trained technician and are summarized in Table 1. Memory was assessed with the Hopkins Verbal Learning Test-Revised, which is comprised of tests of both immediate (HVLT) and delayed (HVLT-Delayed) verbal recall (17). Both tests are scored as the number of words correctly recalled. Psychomotor speed was assessed with the Trail Making Test, Part A (TMTA) (18,19). Executive function, a complex set of processes involving the orchestration of goal-directed activities, was assessed using the Trail Making Test, Part B (TMTB) (18,19). Because motor agility is required for completion of both Trail Making Tests, the analysis for the TMTB was repeated, adjusting for TMTA performance.
Table 1.
Description and Distribution of Cognitive Tests
| Cognitive Test (Abbreviation) |
Cognitive Domain |
Test Score (Raw) |
Test Score Used in Analysis† |
Test Description | Baseline Distribution |
|
|---|---|---|---|---|---|---|
| Mean (SD) | Range | |||||
| Hopkins Verbal Learning Test* (HVLT) |
Immediate recall |
# of words correctly recalled |
# of words correctly recalled |
Twelve common nouns are read to participants and verbally recalled in 3 trials. Worst score (minimum): 0; best score (maximum): 36 |
22.6 (±5.1) | 7 - 35 |
| Hopkins Verbal Learning Test– Delayed* (HVLT-Delayed) |
Delayed recall |
# of words correctly recalled |
# of words correctly recalled |
After a filled, 20-minute interval, participants are asked to verbally recall the 12 nouns in one trial. Worst score (minimum): 0; best score (maximum): 12 |
8.1 (±2.7) | 0 - 12 |
| Trail Making Test, Part A (TMTA) |
Psychomotor speed |
Time to task completion (sec) |
Speed to task completion (# of connections made between numbers/min) |
A pen-and-pencil test in which participants are asked to sequentially connect, as quickly as possible and without removing the pencil from paper, the numbers 1-25, which are randomly distributed on the page. Maximum time allowed: 240 seconds. Time (sec): lower scores are better. Speed (connections/min): higher scores are better. |
Time: 48.9 (±30.0) Speed: 35.6 (±13.8) |
Time: 16.8 – 240 Speed: 6.0 - 85.7 |
| Trail Making Test, Part B (TMTB) |
Executive function |
Time to task completion (sec) |
Speed to task completion (# of connections made between numbers and letters /min) |
A pen-and-pencil test in which participants are asked to sequentially connect, as quickly as possible and without removing the pencil from paper, a sequence of alternating letters and numbers, which are randomly distributed on the page. Maximum time allowed: 360 seconds. Time (sec): lower scores are better. Speed (connections/min): higher scores are better. |
Time: 131.8 (±77.2) Speed: 13.9 (±6.2) |
Time: 32.3 – 420 Speed: 3.4 – 44.6 |
Six alternate forms of the Hopkins Verbal Learning Test-Revised were used.
Because of non-normality, scores for both Trail Making Tests were converted from time (seconds) to number of connections made between numbers (TMTA) or numbers and letters (TMTB) per minute.
The Trail Making Tests consist of a series of 25 numbers (TMTA) or letters and numbers (TMTB), which the participant must sequentially connect. In completing the test, therefore, each participant makes 24 connections between numbers or between numbers and letters. Although these tests are typically scored as time to completion, because the test distributions were heavily skewed, scores from both the Trail Making Tests were converted to the number of connections made per minute. This transformation is in keeping with previous work in this cohort (12).
Hemoglobin assessment
Nonfasting blood samples were collected from venipuncture in sterile tubes using ethylenediaminetetraacetic acid (EDTA). Plasma was separated using centrifugation and stored at −70°C until analysis. Hemoglobin was measured by the cyanmethemoglobin method. In accordance with WHO criteria, anemia was defined as hemoglobin <12 g/dL (20).
Additional independent variables
Education was measured as continuous years of schooling. Race was self-reported as White/Caucasian, Black or African-American, Hispanic, Asian or Other. Because of the small number of Hispanics and Asians (N=6) in the study, race was dichotomized as White/Non-white for analysis. Smoking status (current/former/never) was assessed by self-report. Alcohol use was assessed by the question, “Do you usually drink alcoholic beverages, including beer, wine, sherry, or liquor, at least once every week?” (21); data was not available on each type of alcohol independently. Because of the small number of heavy drinkers in this cohort (only 22 participants reported 3 or more drinks per day) alcohol use was coded as a binary variable of yes or no.
Clinical cardiovascular disease (CVD) was defined as adjudicated (according to standardized algorithms (21)) definite diagnosis of angina pectoris, myocardial infarction, congestive heart failure, peripheral arterial disease, or adjudicated definite or possible stroke. Subclinical cardiovascular disease was defined as ankle-brachial index <0.9 or presence of left ventricular hypertrophy. Restrictive or obstructive pulmonary disease, diabetes, and cancer were defined according to adjudicated diagnoses at baseline (21) . Hypertensive status was self-reported as a physician-diagnosis of hypertension. Depression was defined as a score of 11 or greater on the 30-item Geriatric Depression Scale (22)
Creatinine clearance was calculated from continuous creatinine using the Cockcroft-Gault equation (23) and categorized based on traditional clinical cutpoints as < 30, 30-60, > 60 mL/min/1.73m2 or missing. Thyroid-stimulating hormone (TSH) was categorized as abnormal if ≤0.40 or ≥ 4.2 mIU/L, or normal if 0.40-4.2 mIU/L; low and high levels of TSH were combined due to the small number of women with abnormal TSH (N=47).
Inflammation was measured using interleukin-6 (IL6). Inflammatory cytokines inhibit erythropoietin, the glycoprotein which regulates red blood cell production, and erythropoietin levels have been shown to differ by inflammation status in older adults (24). Because of the non-linear association between IL6 and cognitive decline, IL6 was categorized based on statistical cutpoints (i.e., tertiles), although alternative cutpoints were explored in sensitivity analyses.
All covariate assessments were from baseline.
Statistical analysis
Baseline covariate distributions were compared between those with and without anemia using Pearson’s chi2 test for categorical values and the Kruskal-Wallace test of equal medians or analysis of variance (ANOVA) test of equal means for continuous variables (Table 2).
Table 2.
Baseline Characteristics of Study Participants by Anemia Status
| Characteristic | Total cohort | With anemia | Without anemia |
P-value* |
|---|---|---|---|---|
| (N=374) | (N=33) | (N=341) | ||
| Age (yrs), median(IQR) | 74 (71-76) | 73 (71-77) | 74 (71-76) | 0.90 |
| Education (yrs), mean(SD) |
12.7 (3.2) | 11.5 (3.4) | 12.8 (3.3) | 0.04 |
| Non-white, N(%) | 64 (17.1) | 16 (48.5) | 48 (14.0) | <0.01 |
| Clinical CVD, N(%)† | 87 (23.3) | 10 (30.3) | 77 (22.6) | 0.32 |
| Diabetes, N(%)‡ | 29 (7.8) | 7 (21.2) | 22 (6.5) | <0.01 |
| Pulmonary disease, N(%)‡ ∥ |
92 (24.6) | 9 (27.3) | 83 (24.3) | 0.71 |
| Cancer, N(%)† | 25 (6.7) | 3 (9.1) | 22 (6.5) | 0.56 |
| Hypertensive, N(%)‡ | 188 (50.3) | 14 (42.4) | 174 (51.0) | 0.35 |
| ABI <0.9, N(%) | 28 (8.1) | 3 (10.7) | 25 (7.8) | 0.59 |
| LVH, N(%) | 45 (12.4) | 3 (9.7) | 42 (12.6) | 0.64 |
| Last study visit attended (of 6 possible), median (IQR) | 6 (5-6) | 6 (5-6) | 6 (5-6) | 0.66 |
Abbreviations: IQR, inter-quartile range (25th -75th percentile); SD, standard deviation; CVD, cardiovascular disease; ABI, ankle-brachial index; LVH, left ventricular hypertrophy;
P-values comparing women with anemia to women without anemia. For continuous variables, p-values from Kruskal-Wallis test of equal medians (non-normal distribution) or from an analysis of variance (ANOVA) test of equal means (normal distribution). P-values for categorical variables from Pearson’s □2 test.
Adjudicated according to standardized algorithms (21).
From the question, “Has a doctor ever told you that you had high blood pressure?” (21).
To increase comparability of results across tests, and to highlight the magnitude of the effect of anemia on cognitive decline over time, test scores were standardized to z-scores according to the corresponding mean test score and standard deviation at baseline. For each cognitive test, a linear random effects model with a random intercept and a random time slope was then used to simultaneously model baseline cognitive test scores and rates of change over time. Interaction terms between anemia and time were included in each model in order to estimate the effect of anemia on cognitive decline over time.
As opposed to marginal, or population-average models, random effects models quantify the heterogeneity of subject-specific baseline performance (i.e. random intercept) and trajectories over time (i.e. random slope). Furthermore, they account for unequal follow-up times, within-subject correlation of repeated measurements over time, and data missing at random (25). The random effects for intercept and slope were allowed to be correlated, and the covariance matrix for the random effects was conservatively assumed to be unstructured.
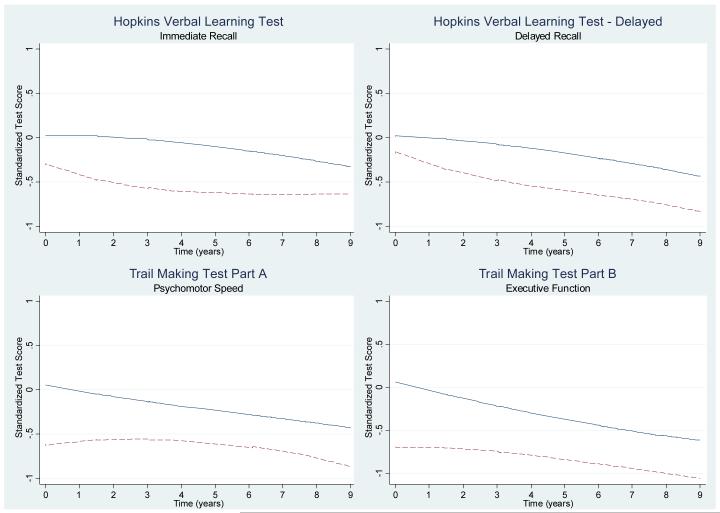
Because a non-linear trend was observed in graphical depictions of cognitive decline in those without anemia over time for the HVLT and HVLT-Delayed, and in those with anemia in all cognitive tests (see Figure 1), a two-piece linear spline was fit in both fixed and random effects at year 3, allowing the slope, or rate, of cognitive decline to differ before and after the third year of follow-up. Trajectories of cognitive decline were estimated for all participants both before and after year 3.
Figure 1.
Lowess-smoothed distribution (locally weighted least squares regression estimate) of standardized cognitive test scores over
The primary adjusted analysis incorporated the covariates age, education, race, cardiovascular disease, pulmonary disease, diabetes, cancer, and hypertension, based on a priori decisions about important confounders of the relationship between anemia and cognitive decline. A secondary analysis incorporating additional hypothesized confounders was conducted to ensure that inferences did not change; these additional covariates included smoking status, drinking status, depression, estimated glomerular filtration rate, thyroid stimulating hormone levels, self-reported health status and inflammation levels.
Model fit was assessed using the Akaike Information Criteria (AIC) and the Schwartz Bayesian Information Criteria (BIC). All analyses were performed in STATA 9.0 (Stata Corp: Stata Statistical Software: Release 9.0, Stata Corporation, College Station, TX, 2005.)
RESULTS
Descriptive analysis
Overall, participants had a median age of 74 years (inter-quartile range (IQR): 71-76), were educated (mean years of schooling 12.7, 95% confidence interval (CI): 12.3, 13.0), and were predominantly white (82.9%) (Table 2). The majority were never smokers (54.6%) and non-drinkers (66.6%). Twenty-three percent had clinically manifested CVD at baseline and 24% had obstructive or restrictive pulmonary disease.
Of 374 women, 8.8% (n=33) met WHO criteria (20) for anemia at baseline. Anemia was mild, with a mean hemoglobin concentration of 11.4 g/dL (± 0.53). Compared to those without anemia, women with anemia were less educated, more likely to be non-white, and more likely to have diabetes (Table 2).
Patterns of cognitive decline over time varied significantly by individual (data not shown). On average, cognitive function declined in all domains during follow-up. Figure 1 displays the lowess-smoothed cognitive test scores over time for those with and without anemia. Lowess smoothing is a locally weighted least squares regression estimate and represents the unadjusted test score over time. Visual inspection of these plots suggested that women with anemia scored worse than those without anemia on all cognitive tests at baseline and continued to perform more poorly during follow-up.
Memory: immediate and delayed verbal recall
After full adjustment for demographic and disease variables, differences in baseline memory function between those with and without anemia were not significant (Table 3).
Table 3.
Standardized, Multivariate-adjusted* Population-average† Estimates and Difference in Estimates of Baseline Function and Annual Rates of Change in Test Scores by Anemia Status, n=374
| Baseline Function | Annual Rate of Change, Years 1-3 |
Annual Rate of Change, Years 3-9 |
||||
|---|---|---|---|---|---|---|
| Standardized Estimate (95% CI) |
p-value | Standardized Estimate (95% CI) |
p-value | Standardized Estimate (95% CI) |
p-value | |
| HVLT- immediate recall | ||||||
| With anemia | 0.48 (0.01, 0.96) | 0.048 | −0.16 (−0.27, −0.05) | 0.005 | −0.01 (−0.07, 0.05) | 0.768 |
| Without anemia | 0.53 (0.24, 0.84) | <0.001 | 0.02 (−0.02, 0.05) | 0.298 | −0.10 (−0.12, −0.08) | <0.001 |
| Difference | −0.05 (−0.40, 0.29) | 0.752 | −0.18 (−0.29, −0.06) | 0.003 | 0.09 (0.02, 0.15) | 0.011 |
| HVLT-Delayed – delayed recall | ||||||
| With anemia | 0.67 (0.17, 1.17) | 0.009 | −0.16 (−0.27, −0.06) | 0.003 | −0.06 (−0.13, 0.001) | 0.047 |
| Without anemia | 0.60 (0.28, 0.92) | <0.001 | −0.01 (−0.04, 0.02) | 0.619 | −0.11 (−0.13, −0.09) | <0.001 |
| Difference | 0.07 (−0.29, 0.43) | 0.699 | −0.15 (−0.26, −0.04) | 0.007 | 0.05 (−0.02, 0.11) | 0.171 |
| TMTA – psychomotor speed | ||||||
| With anemia | 0.57 (0.11, 1.03) | 0.014 | 0.01 (−0.09, 0.11) | 0.820 | −0.10 (−0.15, −0.04) | 0.001 |
| Without anemia | 0.83 (0.55, 1.11) | <0.001 | −0.07 (−0.10, −0.04) | <0.001 | −0.05 (−0.07, −0.04) | <0.001 |
| Difference | −0.26 (−0.61, 0.09) | 0.141 | 0.08 (−0.02, 0.18) | 0.125 | −0.04 (−0.10, 0.02) | 0.162 |
| TMTB – executive function | ||||||
| With anemia | 0.13 (−0.33, 0.59) | 0.588 | 0.01 (−0.09, 0.11) | 0.832 | −0.10 (−0.15, −0.05) | <0.001 |
| Without anemia | 0.65 (0.37, 0.93) | <0.001 | −0.13 (−0.16, −0.10) | <0.001 | −0.08 (−0.09, −0.06) | <0.001 |
| Difference | −0.52 (−0.87, −0.17) | 0.004 | 0.14 (0.04, 0.24) | 0.006 | −0.02 (−0.08, 0.03) | 0.393 |
| TMTB – executive function, adjusted for TMTA |
||||||
| With anemia | −0.06 (−0.45, 0.33) | 0.778 | 0.01 (−0.09, 0.11) | 0.841 | −0.08 (−0.13, −0.03) | 0.002 |
| Without anemia | 0.38 (0.15, 0.61) | 0.001 | −0.11 (−0.14, −0.08) | <0.001 | −0.06 (−0.08, −0.05) | <0.001 |
| Difference | −0.43 (−0.74, −0.13) | 0.005 | 0.01 (0.02, 0.22) | 0.020 | −0.02 (−0.07, 0.04) | 0.563 |
Full model adjusted for baseline continuous age, continuous years of education, race (white/non-white), CVD [clinical (angina/MI/CHD/PAD/stroke), subclinical (ABI < 0.9 or LVH) or none], obstructive and restrictive pulmonary disease, cancer, diabetes and self-reported hypertension.
Estimated from linear random effects models.
During follow-up, women with anemia showed declines in immediate and delayed recall (Table 3). Between baseline and year 3, memory function in those with anemia declined at an estimated average rate of −0.16 SD/year (95% CI: −0.27, −0.05) on the HVLT. Because the baseline SD of the HVLT was 5.1 (Table 1), a decline of −0.16 SD suggests that each year, women with anemia immediately recalled an average of 0.8 fewer words than the average number of words recalled the year before. Similarly, women with anemia declined at an average rate of −0.16 SD/year (95% CI: −0.27, −0.06) on the HVLT-Delayed. Memory function in women with anemia stabilized in immediate recall after year 3 (estimated rate: −0.01 SD/year, 95% CI: −0.07, 0.05) but continued to decline in delayed recall (estimated rate: −0.06 SD/year, 95% CI: −0.13, 0.001). These results are in contrast to those without anemia, who remained relatively stable in memory function from baseline to year 3 and showed, on average, a significant decline only after year 3.
When comparing rates of decline from baseline to year 3, women with anemia declined by an average of 0.18 more SD/year than women without anemia on the HVLT (immediate recall rate difference: −0.18, 95% CI: −0.29, −0.6). Similarly, the rate difference between those with and without anemia on the HVLT-Delayed was −0.15 SD/year (95% CI: −0.26, −0.06) (Table 3). During years 3-9, the estimated rate differences, comparing those with anemia to those without anemia, are positive for both tests, suggesting that women without anemia began to decline at a faster rate than women with anemia.
Psychomotor speed
Baseline test performance on the TMTA did not differ by anemia status (Table 3).
Women with anemia showed a significant decline in psychomotor speed only after year 3 (Table 3). By contrast, women without anemia showed a significant decline both before and after year 3. However, the difference in rates of decline between women with and without anemia was not significant at either time period (Table 3).
Executive function
At baseline, and after adjustment for performance on the TMTA, women with anemia performed more poorly than women without anemia on the TMTB by −0.43 SD (95% CI: −0.74, −0.13) (Table 3), which corresponds to making an average of approximately 2.6 fewer connections/minute.
Between years 1-3, women with anemia remained relatively stable in TMTB performance but women without anemia declined at an average rate of −0.11 SD/year (95% CI: −0.14, −0.08) (Table 3). Both groups declined from years 3-9.
The differences in rates comparing those with anemia to those without are positive prior to year 3 (0.01 SD/year, 95% CI: 0.02, 0.22) (Table 3). These results suggest that women with anemia maintained a level of functioning more similar to (their initially lower) baseline levels than did those without anemia during this time interval.
Random effects
There was significant heterogeneity of baseline performance (random intercept) and rates of decline (random slope) among individuals in all tests (data not shown). Correlations between the random effects (or between baseline function and rate of decline) were negative for the TMTA and TMTB, consistent with potential floor effects. That is, for both components of the Trail Making Test, the poorer the baseline function, the slower the subsequent rate of decline over time. There were no correlations between the random effects for the HVLT or HVLT-Delayed.
DISCUSSION
In a cohort of community-dwelling older women, this study found a weak to moderate association between anemia and cognitive decline in the domains of verbal memory and executive function. After adjustment for demographic and disease variables, and as estimated by linear random effects models, anemia was associated with poorer baseline performance in executive function but not in immediate or delayed recall. By contrast, anemia was associated with a faster rate of decline over time in memory, but not in executive function.
Most studies demonstrating an association between anemia and cognitive impairment have done so in a clinical setting, specifically in patients with congestive heart failure (26), chronic kidney disease (27) and cancer (28). Of those few studies that have examined the relationship between anemia and cognition in community-dwelling populations, most have been limited to a single index of global cognitive function (29). To our knowledge, only one study has assessed the relationship between anemia and a specific domain of cognitive function in a community-based sample. In a cross-sectional study in the Women’s Health and Aging Study II, anemia was associated with executive function performance in the worst (as opposed to best) tertile of the TMTB (6). Our work adds to the literature in that it is prospective with a lengthy follow-up (9 years), assesses multiple domains of cognitive functioning in a community-dwelling sample, and utilizes statistical methods that account for the heterogeneity of cognitive decline within this population.
Our results could suggest that the association between anemia and cognitive decline over time is differential by domain; in our study, anemia was associated with a faster rate of decline in memory, but not in executive function. We did find, however, a significant difference between women with and without anemia in baseline executive function. It could be, therefore, that anemia also acts as a modifier of the rate of decline in executive functioning, and that the observed discrepancies may be explained by the hierarchy of cognitive decline and the timing of the study window.
Declines in executive function precede those in memory by approximately 3 years in this cohort (12). In our study, we observed this relationship for women without anemia; executive function began to decline at baseline while memory remained stable until year 3. However, women with anemia declined in memory during the first 3 years (Table 3). The established hierarchy of decline, coupled with the observed, significant difference in baseline executive function performance between women with and without anemia, could suggest that women with anemia had declined in executive function prior to the start of the study.
We hypothesize that the rate of decline in executive function in those without anemia seems to be accelerated during years 1-3 (as compared to those with anemia) because, at the time of study entry, women with anemia had already declined and stabilized, perhaps due to floor effects, but those without anemia were just beginning to exhibit a normative, age-related decline. We were therefore able to detect the differences in baseline function, but not in rates of decline. The differential impact of anemia on the rate of decline in memory function was captured because, at study entry, normative decline in memory function had not yet accelerated. Although those with and without anemia began the study at approximately the same level of functioning, decline was accelerated in those with anemia during years 1-3. After year 3, normal memory decline began (12), so that the impact of anemia on the rate of decline appeared to lessen.
This hypothesis is consistent with the observed negative correlation between random effects (i.e. the correlation between the random intercept and random slope for each participant) for the TMTB. Such a correlation could suggest that those with poorer baseline function (e.g. women with anemia) declined at a slower rate over time. Furthermore, this pattern of decline and stabilization is mirrored on the HVLT. Women with anemia declined at a significantly faster rate than women without anemia on the HVLT during years 1-3. After this time, women without anemia began to decline at a faster rate. Had we begun the study at year 3, we hypothesize that our results on the HVLT would have been similar to what we observed for the TMTB.
Within an observational context, our study yields evidence of a weak to moderate association between anemia and cognitive decline. We cannot discount, however, that our results could be due to non-causal factors, such as residual confounding or reverse causality. Although an attempt was made to adjust for comorbidity in our study, categorization of some variables could have resulted in residual confounding. Additionally, more refined measures of subclinical cardiovascular disease, such as magnetic resonance imaging (MRI) for the identification of potential silent infarcts in the brain, could help to alleviate the concern of residual confounding by cardiovascular disease. Additionally, our results could potentially be explained by reverse causation. For example, changes in cognition could lead to poorer eating habits; as one cause of anemia is nutritional deficiency in iron, folate or Vitamin B12, poor diet could then cause anemia. However, in our study, the majority of women had serum Vitamin B12 levels within the normal range at baseline (6).
If our results represent a true causal relationship, the mechanism by which anemia acts on the brain to reduce cognitive function remains to be elucidated. Two hypotheses have been previously proposed. First, anemia leads to brain hypo-oxygenation and consequently to cognitive decline (10). Second, aerobic capacity, a basic measure of physical functioning, mediates the relationship between low hemoglobin levels and decreased cognitive capacity (6).
The current study is limited in that numbers of women with anemia were too small to permit any stratification by race. Forty-nine percent of women with anemia were non-white, as compared to 14% of women without anemia. WHO-defined anemia is more common in African Americans than whites, and has been shown to differentially predict mortality and mobility disability by race (30). If possible, future studies should address potential differential effects of anemia on cognitive decline by race. Additionally, we were unable to stratify by anemia type (e.g. nutritional deficiency, anemia of chronic disease or unexplained anemia). Future studies incorporating information on anemia type may yield insight into potential mechanisms by which anemia may act to cause cognitive decline.
Anemia in this study was defined by baseline hemoglobin levels. Because prevalence of anemia increases with older age (8), it is likely that measuring anemia at a single time point resulted in an underestimate of the risk of cognitive decline associated with anemia. In future studies, we will incorporate anemia as a time-dependent covariate.
In summary, our findings support the hypothesis that anemia may be a modifier of cognitive decline over time in multiple cognitive domains in a cohort of high-functioning older women. If this relationship is causal, it is possible that treatment of anemia could prevent or postpone cognitive decline and its associated sequelae. The association between anemia and cognitive decline was weak to moderate in this study. However, because of the high prevalence of cognitive decline in older adults, any intervention that could improve cognition, even modestly at the individual level, could have a large public health impact at the population level. Because little is known about how to prevent cognitive decline in older adults, future studies of anemia and cognitive decline are warranted. Our findings are relevant to the planning of such studies, as they may help inform decisions about which instruments should be used to measure cognition; anemia was associated with poorer cognitive function in the domains of immediate recall, delayed recall and executive function. Additionally, our results could suggest that, depending on the length of the study and the age of participants, future investigators may expect to see differential relationships between anemia and specific cognitive domains.
ACKNOWLEDGMENTS
We thank Jonathan Samet, MD, MS, Department of Preventive Medicine, Institute for Global Health, University of Southern California Keck School of Medicine, and Kala Visvanathan, MBBS, MHS, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, for editorial review of a previous version of this manuscript.
Support: Research supported by the National Institute on Aging (NIA), Claude D. Pepper Older Americans Independence Centers Grant P30 AG021334 and National Institutes of Health (NIH)-NIA Grant R37 AG19905.
Research supported by National Institutes of Health (NIH)-National Institute on Aging (NIA) Grants R01 AG19825-02, R01 AG11703-10, T32 AG00247 and R37 AG19905, by the NIA, Claude D. Pepper Older Americans Independence Centers Grant P30 AG021334, and by NIH-NCRR OPD-GCRC Grant RR00722.
Footnotes
Previous presentation. Preliminary results of this study have been accepted for presentation at the 2008 Gerontological Society of America Annual Scientific Meeting, National Harbor, MD.
Author Contributions: All authors have equally contributed to the concept and design, acquisition of subjects and/or data, analysis and interpretation of data, and manuscript preparation
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Dr. Chaves has been the recipient of a research grant from Amgen, Inc., and has served as a consultant for FibroGen, Inc. None of the products developed and/or marketed from these companies are discussed in the paper.
Sponsor’s Role: None.
REFERENCES
- 1.Saxton J, Lopez OL, Ratcliff G, et al. Preclinical Alzheimer disease: Neuropsychological test performance 1.5 to 8 years prior to onset. Neurology. 2004;63:2341–2347. doi: 10.1212/01.wnl.0000147470.58328.50. [DOI] [PubMed] [Google Scholar]
- 2.Carlson MC, Fried LP, Xue QL, et al. Association between executive attention and physical functional performance in community-dwelling older women. J Gerontol B Psychol Sci Soc Sci. 1999;54:S262–S270. doi: 10.1093/geronb/54b.5.s262. [DOI] [PubMed] [Google Scholar]
- 3.Johnson JK, Lui LY, Yaffe K. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. J Gerontol A Biol Sci Med Sci. 2007;62:1134–1141. doi: 10.1093/gerona/62.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 5.Chaves PH, Ashar B, Guralnik JM, et al. Looking at the relationship between hemoglobin concentration and prevalent mobility difficulty in older women. Should the criteria currently used to define anemia in older people be reevaluated? J Am Geriatr Soc. 2002;50:1257–1264. doi: 10.1046/j.1532-5415.2002.50313.x. [DOI] [PubMed] [Google Scholar]
- 6.Chaves PH, Carlson MC, Ferrucci L, et al. Association between mild anemia and executive function impairment in community-dwelling older women: The Women’s Health and Aging Study II. J Am Geriatr Soc. 2006;54:1429–1435. doi: 10.1111/j.1532-5415.2006.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agnihotri P, Telfer M, Butt Z, et al. Chronic anemia and fatigue in elderly patients: Results of a randomized, double-blind, placebo-controlled, crossover exploratory study with epoetin alfa. J Am Geriatr Soc. 2007;55:1557–1565. doi: 10.1111/j.1532-5415.2007.01357.x. [DOI] [PubMed] [Google Scholar]
- 8.Guralnik JM, Eisenstaedt RS, Ferrucci L, et al. Prevalence of anemia in persons 65 years and older in the United States: Evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 9.Beard CM, Kokmen E, O’Brien PC, et al. Risk of Alzheimer’s disease among elderly patients with anemia: population-based investigations in Olmsted county, Minnesota. Ann Epidemiol. 1997;7:219–224. doi: 10.1016/s1047-2797(97)00015-x. [DOI] [PubMed] [Google Scholar]
- 10.Atti AR, Palmer K, Volpato S, et al. Anaemia increases the risk of dementia in cognitively intact elderly. Neurobiol Aging. 2006;27:278–284. doi: 10.1016/j.neurobiolaging.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Carlson M, Xue QL, Zhou J, et al. Declines and deficits in executive function precede those in memory in high-functioning older women: The Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci. In press. [Google Scholar]
- 13.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 14.Schaie KW. The Seattle Longitudinal Study: A thirty-five-year inquiry of adult intellectual development. Z Gerontol. 1993;26:129–137. [PubMed] [Google Scholar]
- 15.Giambra LM, Arenberg D, Kawas C, et al. Adult life span changes in immediate visual memory and verbal intelligence. Psychol Aging. 1995;10:123–139. doi: 10.1037//0882-7974.10.1.123. [DOI] [PubMed] [Google Scholar]
- 16.Backman L, Jones S, Berger AK, et al. Cognitive impairment in preclinical Alzheimer’s disease: A meta-analysis. Neuropsychology. 2005;19:520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- 17.Brandt J. The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. Clin Neuropsychologist. 1991;5:125–142. [Google Scholar]
- 18.Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 19.Spreen O, Strauss E. A compendium of neuropsychological tests: administration, norms, and commentary. 2nd ed Oxford University Press; New York, NY: 1991. [Google Scholar]
- 20.World Health Organization . Nutritional anemias: Reports of a WHO scientific group. World Health Organization; Geneva, Switzerland: 1968. [Google Scholar]
- 21.Guralnik JM, Fried LP, Simonsick EM, et al. The Women’s Health and Aging Study: Health and social characteristics of older women with disability. National Institute on Aging; Bethesda, MD: 1995. [Google Scholar]
- 22.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 23.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 24.Eisenstaedt R, Penninx BW, Woodman RC. Anemia in the elderly: Current understanding and emerging concepts. Blood Rev. 2006;20:213–226. doi: 10.1016/j.blre.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Rubin D. Estimating causal effects of treatments in randomized and non-randomized studies. J Ed Psychol. 1974;66:688–701. [Google Scholar]
- 26.Zuccala G, Marzetti E, Cesari M, et al. Correlates of cognitive impairment among patients with heart failure: Results of a multicenter survey. Am J Med. 2005;118:496–502. doi: 10.1016/j.amjmed.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 27.Grimm G, Stockenhuber F, Schneeweiss B, et al. Improvement of brain function in hemodialysis patients treated with erythropoietin. Kidney Int. 1990;38:480–486. doi: 10.1038/ki.1990.229. [DOI] [PubMed] [Google Scholar]
- 28.Massa E, Madeddu C, Lusso MR, et al. Evaluation of the effectiveness of treatment with erythropoietin on anemia, cognitive functioning and functions studied by comprehensive geriatric assessment in elderly cancer patients with anemia related to cancer chemotherapy. Crit Rev Oncol Hematol. 2006;57:175–182. doi: 10.1016/j.critrevonc.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Denny SD, Kuchibhatla MN, Cohen HJ. Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am J Med. 2006;119:327–334. doi: 10.1016/j.amjmed.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 30.Patel KV, Harris TB, Faulhaber M, et al. Racial variation in the relationship of anemia with mortality and mobility disability among older adults. Blood. 2007;109:4663–4670. doi: 10.1182/blood-2006-10-055384. [DOI] [PMC free article] [PubMed] [Google Scholar]