Abstract
Balkan endemic nephropathy is a chronic tubulointerstitial disease frequently accompanied by urothelial cell carcinomas of the upper urinary tract. This disorder has recently been linked to exposure to aristolochic acid, a powerful nephrotoxin and human carcinogen. Following metabolic activation, aristolochic acid reacts with genomic DNA to form aristolactam-DNA adducts that generate a unique TP53 mutational spectrum in urothelium. The aristolactam-DNA adducts are concentrated in the renal cortex, thus serving as biomarkers of internal exposure to aristolochic acid. Here, we present molecular epidemiologic evidence relating carcinomas of the upper urinary tract to dietary exposure to aristolochic acid. DNA was extracted from the renal cortex and urothelial tumor tissue of 67 patients that underwent nephroureterectomy for carcinomas of the upper urinary tract and resided in regions of known endemic nephropathy. Ten patients from non-endemic regions with carcinomas of the upper urinary tract served as controls. Aristolactam-DNA adducts were quantified by 32P-post-labeling, the adduct was confirmed by mass spectroscopy, and TP53 mutations in tumor tissues were identified by chip-sequencing. Adducts were present in 70% of the endemic cohort and in 94% of patients with specific A:T to T:A mutations in TP53. In contrast, neither aristolactam-DNA adducts nor specific mutations were detected in tissues of patients residing in non-endemic regions. Thus, in genetically susceptible individuals, dietary exposure to aristolochic acid is causally related to endemic nephropathy and carcinomas of the upper urinary tract.
Introduction
Endemic (Balkan) nephropathy (EN) is a chronic, progressive tubulointerstitial disease that affects residents of rural farming villages located along tributaries of the Danube river in Bosnia and Herzegovina, Bulgaria, Croatia, Romania and Serbia (1). An unusual feature of EN is its close association with urothelial (transitional cell) carcinomas of the renal pelvis and ureter (2,3). These upper urinary tract cancers (UUC), which account for only 5% of all urinary tract cancers worldwide, are present in approximately 50% of EN cases (3). Both EN and UUC exhibit a familial, but not inherited association, suggesting the importance of environmental factors as well as genetic determinants in this disease (4–6).
Over the past 50 years, extensive efforts have been made to elucidate the etiology of EN/UUC (1,5,7). The majority of this research focused on the role of environmental agents, including various heavy metals, mycotoxins, trace elements, and organic chemicals (8,9). These investigations, however, fail to account fully for the distinctive pathophysiology and epidemiology of this disease (10).
In 1969, Ivić proposed a role for chronic Aristolochia poisoning in the etiology of EN, based on his observation that seeds from these plants, which grew abundantly in local wheat fields, comingled with wheat grain during the harvesting process (11). Thus, he speculated that human exposure to a toxic component of Aristolochia seeds could occur through ingestion of bread prepared with flour derived from contaminated grain. Ivić also demonstrated that, in animal models, Aristolochia seeds induced nephropathy and sarcomas of the skin (11).
We pursued Ivić’s astute hypothesis, stimulated by reports of end-stage renal disease in a cluster of otherwise healthy Belgian women who ingested Aristolochia fangchi as part of a weight-loss regimen (12). This so-called Chinese herbs nephropathy, later renamed aristolochic acid nephropathy (AAN), bears striking similarities to EN in terms of its pathophysiology and association with UUC (13,14). Importantly, only 5% of the approximately 1800 Belgian women ingesting Aristolochia herbs over the course of approximately one year developed renal disease or UUC, suggesting a role for genetic susceptibility similar to that reported for EN (13).
Previously, we identified aristolactam (AL)-DNA adducts in the renal cortex of four patients with EN and in urothelial tumor tissues of three patients with UUC/EN (15). These adducts were not detected in patients with UUC living outside the endemic region. Importantly, the mutational profile of the TP53 tumor suppressor gene in tumors of patients with EN/UUC was dominated by A:T to T:A transversions and contained several unique hot spots (16), a pattern that differed markedly from TP53 mutational profiles for sporadic cases of UUC reported worldwide (17). Additionally, mutated adenosine residues were located exclusively on the nontranscribed strand. Thus, this TP53 mutational “signature” represents a highly specific bioindicator for the carcinogenicity of AA (15,16).
In this paper, we present molecular and epidemiologic evidence linking both dietary exposure to AA and TP53 mutational spectra to UUC in residents of endemic regions of Bosnia, Croatia and Serbia. Based on these results, we propose the use of these sensitive and specific biomarkers in the diagnosis of AAN and its associated UUC, recently recognized as a global disease (18,19).
Results
Demographics
Of 97 cases screened, 77 subjects fulfilled our inclusion/exclusion criteria; these cases were divided into two groups based on residence histories. The majority (67/77) lived in endemic regions for at least 20 years (endemic cases), whereas ten patients were life-long residents of Zagreb, Belgrade, or other nonendemic sites (nonendemic cases) and, therefore, unlikely to have been exposed to AA-contaminated bread. The key demographic features of these groups are summarized in Table 1. The average age of endemic residents at the time of surgery was 73.4 years while subjects from nonendemic regions were slightly younger with a mean age of 66 years (p=0.01). Females outnumbered males by a ratio of 1.5:1 in endemic regions; the comparable ratio in nonendemic regions was 0.67; this difference was not statistically significant (p=0.311) Seventy percent of tumors were localized exclusively in either the renal pelvis or the ureter, with the majority being found in the renal pelvis. In some cases, bladder cancer accompanied tumors of the upper urinary tract. Tumor from one subject was classified as a (urothelial) carcinoma of the duct of Bellini.
Table 1.
Study demographics and tumor sites
| Endemic cases | Nonendemic cases | |
|---|---|---|
| N | 67 | 10 |
| Primary residence | ||
| Bosnia, N (%) | 29 (43.2%) | 0 (0.00%) |
| Croatia, N (%) | 18 (26.9%) | 5 (50.0%) |
| Serbia, N (%) | 20 (29.9%) | 5 (50.0%) |
| Males, N (%) | 27 (40.3%) | 6 (60.0%) |
| Females, N (%) | 40 (59.7%) | 4 (40.0%) |
| Age, median | 73.0 | 65.5 |
| Age, mean ± SD | 73.5 ± 0.8 | 66.1 ± 4.4 |
| Age, range | 57–89 | 43–85 |
| Tumor site, % | ||
| Renal pelvis | 43.3% | 60.0% |
| Ureter | 28.4% | 10.0% |
| Renal pelvis & ureter | 10.4% | 20.0% |
| Renal pelvis & bladder | 4.5% | 0.0% |
| Ureter & bladder | 7.5% | 10.0% |
| Renal pelvis, ureter, & bladder | 6.0% | 0.0% |
DNA adducts in renal cortex
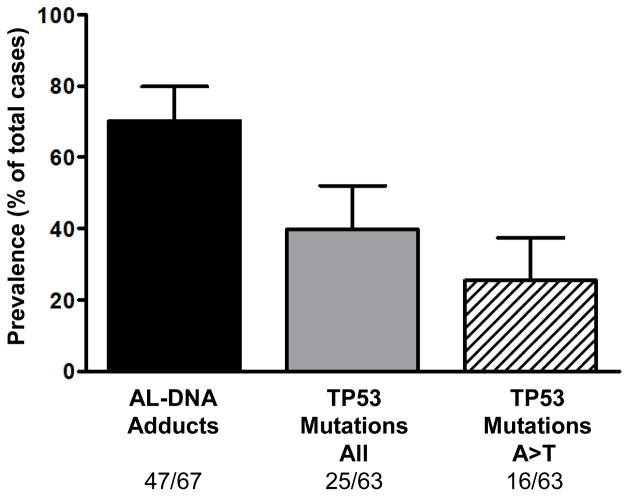
Deoxyadenosine (dA)-AL lesions were present in the renal cortex of 47 (70%) of endemic cases, representing 80% of female subjects and 56% of males (p<0.055; Table 2, Figure 1). dA-AL adduct levels averaged two per 108 nucleotides (range 0.2–19.2); deoxyguanosine (dG)-AL adducts also were detected in two cases. AL-DNA adducts were not detected in five UUC patients residing in nonendemic sites in Serbia; absence of these lesions in five UUC subjects residing in nonendemic sites in Croatia was reported previously (15).
Table 2.
Aristolactam-DNA adducts in renal cortex from endemic cases
| % with adducts | |
|---|---|
| All subjects (N=67) | 70.1% |
| Males (N=27) | 55.6% |
| Females (N=40) | 80.0% |
| Smokers (N=25) | 64.0% |
| Nonsmokers (N=42) | 73.8% |
Figure 1.
Prevalence of biomarkers of aristolochic acid (AA) exposure and TP53 mutations in cases of UUC from endemic villages/regions in Bosnia, Croatia and Serbia. Aristolactam (AL)-DNA adducts, produced during intracellular nitroreduction of AA, were measured in renal cortex by a 32P-postlabeling-PAGE assay (43). Specific mutations in the tumor suppressor gene TP53 were identified in UUC samples using p53 AmpliChip technology. A:T T:A transversions (A>T) are the dominant TP53 mutations associated with AA exposure in UUC (16). Tumor DNA was not available for analysis for four cases. Error bars denote 95% confidence intervals for each value. Cortical adducts and tumor TP53 mutations were not detected in DNA samples obtained from nonendemic cases (n=10; data not shown).
Identification of AL-DNA adducts by mass spectroscopy
The predominant DNA adduct in the renal cortex detected by 32P-postlabelling techniques was identified by mass spectroscopy as 7-(deoxyadenosin-N6-yl) aristolactam-I (dA-AL-I). In the sample analyzed, this lesion was estimated to occur at a level of 1.5 adducts in 108 nucleotides (Figure 2). The exquisite sensitivity of the linear ion trap mass spectrometer permitted acquisition of the product ion spectrum of the dA-AL-I adduct at the MS3 scan stage. The spectrum is in excellent agreement with the spectrum of the reference standard, confirming the identity of the lesion as dA-AL-I. The 7-(deoxyadenosin-N6-yl) aristolactam-II (dA-AL-II) adduct was not detected; the lower limit of detection being approximately 1 adduct per 109 nucleotides (15).
Figure 2.
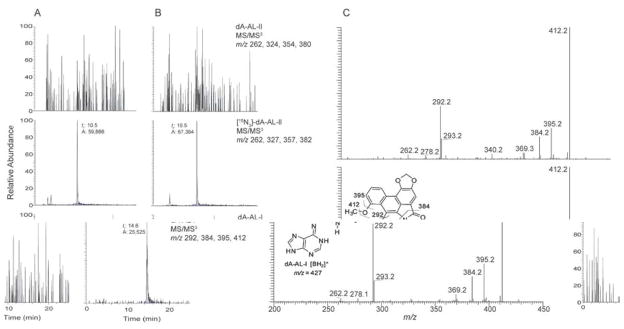
Mass spectroscopic characterization of DNA-aristolactam (AL) adducts in the renal cortex. (A) Reconstructed ion chromatogram of the liquid chromatography electrospray ionization/multistage mass spectrometry (LC-ESI/MS/MS3) analysis of dA-AL adducts. (A) Calf thymus DNA served as the negative control and was spiked with the internal standard [15N3]-dA-AL-II at a level of 5 adducts per 108 DNA bases and (B) DNA sample from a UUC subject from Croatia, the level of the [15N3]-dA-AL-II internal standard was 4.2 adducts per 108 DNA bases. The chromatograms for dA-AL-I, dA-AL- II and [15N3]-dA-AL-II were reconstructed with the four principal fragment ions observed in the spectra of the MS3 stage scan mode. The level of dA-AL-I was estimated at 1.5 adducts/108 bases (based on total ion counts of dA-AL-I to [15N3]-dA-AL-II total ion counts). (C) The product ion spectra of the protonated base adduct [BH2]+ for synthetic dA-AL-I (lower panel) and the DNA adduct found in the human renal cortex (upper panel).
Renal function
Renal function was compromised in the majority of subjects in this study (Table 3; Figure 3). The overall prevalence of chronic kidney disease (CKD) (stage 3 and higher) in endemic and nonendemic cases was similar, affecting 84 and 90% of the subjects, respectively. Severe renal disease (CKD stages 4–5) was evident in the endemic cases, affecting 38.8% of the subjects in this cohort; CKD prevalence was two-times higher in women than in men, a difference of borderline significance after adjusting for age (p=0.068). In comparison, only 20% of nonendemic cases were classified as CKD stages 4–5. There was no association between the presence of AL-DNA adducts and CKD status among subjects from endemic villages, even after adjusting for age and sex. eGFR values were within the normal range for 19% of subjects who tested positive for AL-DNA adducts.
Table 3.
Characteristics of chronic kidney disease in endemic UUC cases
| Chronic kidney disease | Stages 0–2 | Stage 3 | Stage 4 | Stage 5 |
|---|---|---|---|---|
| N (%) | 11 (16.4%) | 30 (44.8%) | 14 (20.8%) | 12 (17.9%) |
| Males (N=27) | 22.2% | 55.6% | 11.1% | 11.1% |
| Females (N=40) | 12.5% | 37.5% | 27.5% | 22.5% |
| Adductb (+) subjects (N=47) | 19.1% | 40.4% | 23.4% | 17.02% |
| Adductb (−) subjects (N=20) | 10.0% | 55.0% | 15.0% | 20.0% |
| Smokers (N=25) | 24.0% | 64.0% | 0.0% | 12.0% |
| Nonsmokers (N=42) | 11.9% | 33.3% | 33.3% | 21.4% |
| Family history of renal disease (N=18)a | 16.7% | 27.8% | 16.7% | 38.9% |
| No family history of renal disease (N=28)a | 14.3% | 50.0% | 25.0% | 10.7% |
| Age mean (years), N=67 | 72.9 | 72.8 | 75.0 | 73.8 |
| Age range | 65–85 | 57–89 | 69–80 | 63–80 |
Information regarding family history of renal disease was not available for 21 subjects.
Adduct refers to the presence (+) or absence (−) of aristolactam-DNA adducts in renal cortex. UUC, upper urinary tract urothelial cell carcinoma
Figure 3.
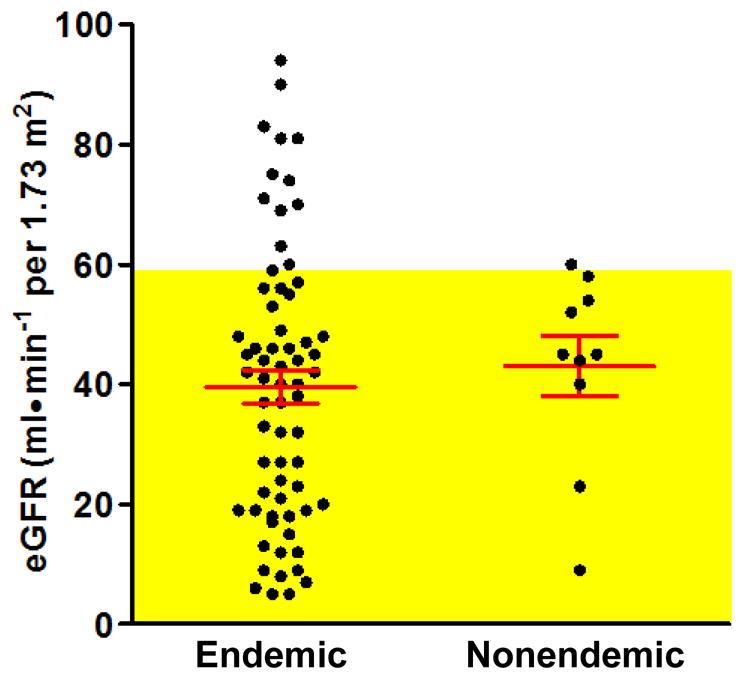
Distribution of estimated glomerular filtration rate (eGFR) values, calculated with the MDRD formula, among UUC cases from endemic and nonendemic villages. Values corresponding to CKD stage 3 or higher are shaded in yellow. Red lines indicate the mean ± SEM of each cohort.
Both EN and UUC exhibit a strong familial association. Among subjects living in endemic regions of Croatia, Bosnia and Herzegovina, 39% reported a family history of renal disease (Table 4). Subjects with a positive family history exhibited more advanced renal disease, such that 39% of these subjects were classified as stage 5 CKD, compared with 11% of subjects with no family history of nephropathy (Table 3) (p=0.033).
Table 4.
Risk factors for urothelial malignancies in endemic cases
| Smokersa, % | 37.3% |
| % of males who smoked | 81.5% |
| % of females who smoked | 7.5% |
| Environmental exposure, % | |
| Cultivated wheat 20–30 years agob | 95.7% |
| Baked bread 20–30 years agob | 97.9% |
| Observed Aristolochia in wheat fields 20–30 years agoc | 86.4% |
| Family history of renal diseased,% | 39.1% |
total 67 subjects;
total 47 subjects;
total 44 subjects;
total 46 subjects
TP53 mutations in UUC
Tumor tissues were available for TP53 mutational analysis in 63 of the 67 endemic cases and five nonendemic residents. TP53 mutations were not detected in the latter group. However, mutations in TP53 were detected in 40% of subjects from endemic areas (Figure 1), including 33% of the males and 44% of the females. Details of the TP53 mutational spectrum for these cases are reported elsewhere (16). A:T→T:A transversion mutations, found exclusively on the nontranscribed strand of TP53, were present in 16/25 (64%) of endemic cases in which TP53 was mutated: this mutation was more frequent in females (12/16) than in males (4/9) but this difference was not significant (p=0.2). Notably, AL-DNA adducts were present in 94% of cases with A:T→T:A mutations, documenting the intimate association of these specific biomarkers of exposure and carcinogenic effect.
Agricultural and dietary practices (Table 4)
All but two subjects from the endemic regions reported cultivating and harvesting their own wheat 20–30 years ago; at that time, the vast majority (98%) of individuals used grain from locally grown wheat to prepare bread for their households. Most subjects (86%) distinctly recalled seeing Aristolochia plants growing in cultivated farm fields. Thus, the probability is high that subjects from the endemic regions were exposed to AA in their diet as a contaminant of flour prepared from locally grown wheat grain (20).
Confounding exposure risks (Table 4)
In addition to aristolochic acid, cigarette smoking, phenacetin abuse, arsenic, cyclophosphamide, and occupational exposure to aromatic amines are recognized as risk factors for UUC (21). Of these potentially confounding factors, only smoking history was associated with UUC in this study. Among endemic subjects, 37% reported tobacco use, as did 50% of the nonendemic subjects. There was a marked gender difference in tobacco use among endemic residents, as 81% of males in this group were smokers compared to only 8% of females (p<0.001). Accordingly, smoking can be eliminated as a confounding variable in the etiology of UUC in women. Notably, among male endemic subjects who tested negative for both biomarkers, 9/12 (75%) were smokers, suggesting a possible causal relationship with UUC.
Other risk factors for urothelial cancers are unlikely to play an etiologic role in this series. Only one endemic subject gave a history of chronic excessive analgesic use, and there are no reports of arsenic exposure in the endemic regions. Occupational exposure to carcinogenic aromatic amines occurs primarily in chemical industries, whereas farming is the main occupation of endemic residents.
Renal cortical histopathology
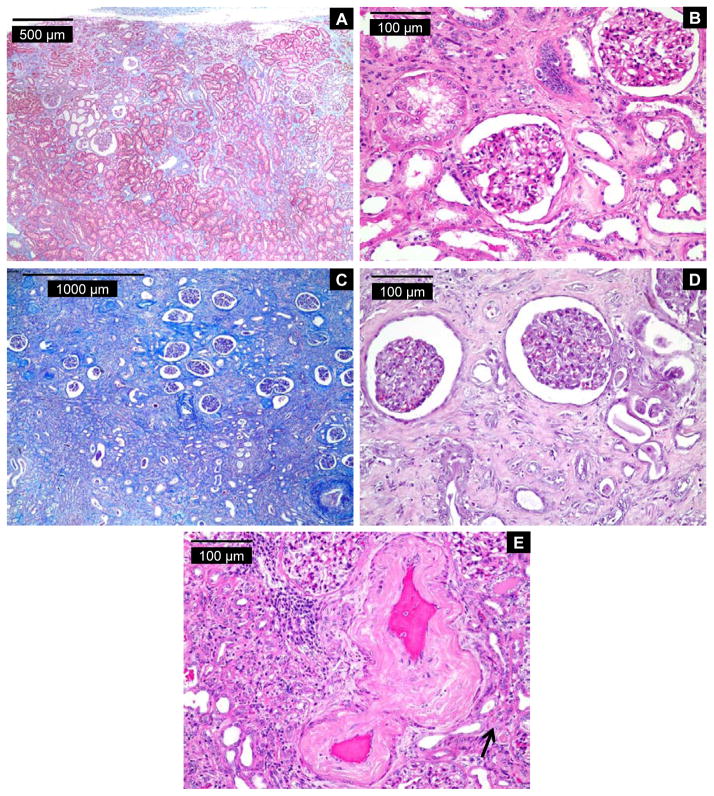
The distinctive renal histopathology in EN is characterized by extensive cortical tubular atrophy and dense interstitial fibrosis, most prominent in the outer area of the cortex, with little evidence of inflammation (22) Glomeruli remain well preserved until late stages of the disease. Of nine residents of nonendemic regions, the histopathologic features of eight were inconsistent with EN as defined in Methods, but, in one case, a contribution of EN could not be ruled out. Of 62 endemic cases, changes were too advanced (end-stage renal disease) in nine to establish a definitive histopathologic diagnosis. In six cases, the changes were highly consistent with EN (Figure 4); most of these cases also displayed fibrosis of the intima and media of the interlobar arteries. In 39 cases, the criteria for histopathologic diagnosis of EN were not satisfied; greatest reliance being placed on the absence of a gradient of fibrosis, the most reliable hallmark for EN. In the remaining cases, a diagnosis of EN could not be excluded because of secondary changes due to severe vascular disease, obstructive and reflux nephropathy, chronic pyelonephritis, and/or diabetic nephropathy. Thus, renal histopathology alone in an older population frequently is insufficient for making a clinical diagnosis of EN.
Figure 4.
Histopathology of renal cortex from three UUC subjects with features highly consistent with endemic (Balkan) nephropathy (EN). Case 1: Trichrome- (A) and hematoxylin- and eosin-stained (H&E; B) sections of renal cortex. (A) Fibrosis is patchy and areas of unaffected tubules are noted in this EN case. Involvement of the labyrinth is evident in this area from a subcapsular zone, but the gradient of fibrosis can just be appreciated. (B) Moderately advanced EN with substantial tubular atrophy and extensive interstitial fibrosis. There is little inflammation. Cortical collecting ducts are seen in the upper center field. Glomeruli are relatively intact. Case 2: Trichrome- (C) and H&E-stained (D) sections of renal cortex. (C) Advanced classic EN with relative glomerular preservation, profound tubular atrophy, and a gradient from superficial (top of image) to deep cortex of extensive interstitial fibrosis. (D) Typical cortex in advanced EN. The most striking alteration is an almost complete atrophy of proximal tubules. There is almost no inflammation. The glomeruli are preserved but show ischemic change (simplification and condensation). An arteriole in the bottom of the image shows nonspecific sclerotic changes. (E) Significant vascular disease involving an interlobular artery is evident in case 3 (H&E). Vasculopathy is very common in EN and has no distinguishing features. Note the adjacent atrophy of proximal convoluted tubules (arrow). DNA-aristolactam adducts were detected in renal cortex for all three cases. eGFR values (ml/min per 1.73m2) were: 61 (case 1), 5 (case 2), and 35 (case 3).
Discussion
This study, designed to confirm and extend observations made in a limited number of patients (15), supports the hypothesis that AA is the causal factor in EN/UUC. Additionally, we demonstrate, in the context of chronic, low-dose exposure to AA, that the presence of AL-DNA adducts in the renal cortex, coupled with TP53 mutational analysis, can serve as a specific biomarker of effect for AA-induced UUC and as a robust biomarker of exposure to AA.
Aristolochic acid is classified formally as a human carcinogen, on the basis of comprehensive and critical reviews of the scientific literature (23,24) that take into consideration established guidelines for causality of disease (25). dA-AL adducts are regarded as precursors for UUC, provided that a causal relationship exists (26,27). In this regard, the recently released National Toxicology Program report concluded that “a causal association between exposure to AA and human cancer is evidenced by the strength of the association, consistency across studies, dose-response effects, detection of AA-DNA adducts in exposed patients, timing of the exposure and disease and specific mutations in the p53 gene similar to the AT to TA transversions seen in rodents and rodent cell cultures exposed to AA.” (24) The present study uses a molecular epidemiologic approach to document the causal relationship between AA and EN/UUC.
The suggestion that AA is related causally to human cancer raises important issues concerning the role of this powerful nephrotoxin and carcinogen in EN/UUC. For example, is the etiology of EN/UUC similar in all regions that harbor the disease? How does the timing and degree of exposure relate to clinical manifestations of disease? Are other environmental factors, such as ochratoxin A (OTA) involved as co-carcinogens? Are EN and UUC invariably linked? Finally, could AL-DNA adducts, in conjunction with TP53 mutational spectra, be used as prima facie biomarkers to estimate the incidence and prevalence of AAN, now recognized as a global disease? Results of our study are discussed with these questions in mind.
EN was first recognized in the late 1950’s as a novel clinical entity limited to rural farming populations in the Danube river basin (1,5,7). Remarkably, the disease remains confined to several hundred settlements in Bosnia, Bulgaria, Croatia, Romania and Serbia. By 1970, it became apparent that patients with EN were at increased risk of developing UUC. The present study includes subjects living in or near 54 different endemic villages in three countries (2), where past exposure to AA appears to have occurred through contaminated baking flour (11,20). Indeed, as reported in this paper, the presence of AL-DNA adducts in 70% of endemic cases of UUC provides compelling evidence of dietary exposure to AA.
In our study population, exposure to AA occurred decades before the clinical diagnosis of UUC was made. The presence of Aristolochia clematitis in meadows and in cultivated wheat fields in Croatia was documented in early reports of AAN in horses (28) and confirmed by almost all subjects enrolled in the present study. The study cohort lived for at least 20 years and, in most cases, their entire lives in endemic communities where home-baked bread prepared from locally grown wheat grain was a dietary staple. Thus, taking into consideration the long latent period for the development of UUC, residents of endemic villages likely were exposed to low doses of AA since the 1920s. In recent years, however, the same individuals now purchase, for home consumption, flour and bread that rarely is contaminated with Aristolochia seeds. Predictably, as dietary exposure to AA diminishes, the average age of individuals requiring hemodialysis for EN and/or nephroureterectomy for UUC increases, a trend observed in Croatia and Serbia (2,29,30).
OTA, a ubiquitous mycotoxin, has long been hypothesized to play a primary role in the etiology of EN/UUC (31). Indeed, residents in endemic regions are exposed to relatively high concentrations of OTA (32). However, similar high exposure occurs throughout the world in farming communities that are largely free of CKD and UUC (33). Thus, solid epidemiologic evidence supporting an association between exposure to OTA and the prevalence of EN or UUC is lacking. Importantly, acute or chronic nephrotoxicity associated with dietary exposure to OTA has never been observed in humans (34). A comprehensive review concluded that “published epidemiologic studies are inadequate to assess a causal relationship between OTA and human cancer” (35). Additionally, putative DNA adducts identified in patients with EN/UUC appear to represent products of oxidative DNA damage (36,37) or, possibly, covalent modifications of dG (38,39). However, such adducts are unlikely to generate TP53 mutation spectra dominated by A:T→T:A transversions, as reported here for 64% of endemic cases with mutations in TP53.
Bulky DNA damage can be detected by the sensitive, but relatively non-specific 32P-postlabelling assay used to quantify AL-DNA adducts in the renal cortex. In some patients, particularly smokers, a variety of DNA adducts are present, thereby complicating the identification of AL-DNA adducts by thin-layer chromatography (40). In our study, the predominant dA-AL adduct was identified by demonstrating its co-migration on polyacrylamide gel electrophoresis (PAGE) with authentic synthetic standards. In addition, we subjected renal cortical DNA to multistage tandem mass spectroscopic analysis, providing, for the first time, an unequivocal chemical identification of dA-AL adducts in renal tissues of patients from endemic regions with UUC.
The basic pathophysiology of EN/UUC is similar in almost all respects to that associated with so-called Chinese herbs nephropathy, where the amount of AA ingested over 13 months was recorded (41). The slower clinical progression of EN/UUC likely reflects lower levels of dietary exposure to AA ingested over many years. Thus, based on the abundant evidence summarized in (24) and results of the present study, we suggest that endemic (Balkan) nephropathy be referred to as aristolochic acid nephropathy, a designation that carries important connotations for public health authorities in countries harboring this disease, and removes the stigma occurring when the name of a disease is associated with specific countries (42).
Despite being linked to a common etiologic agent, the molecular mechanism of nephrotoxicity in EN/UUC may differ from that leading to urothelial cancer (43). Thus, although hepatic detoxification by CYP1A2 (44,45) reduces both the nephrotoxicity and carcinogenicity of AA, expression of other genes may contribute to the relative susceptibility of individuals to EN and UUC (6,19). In fact, patients with mutations in key tumor suppressor genes and oncogenes are more likely to manifest changes associated with UUC while symptoms of renal tubular dysfunction predominate in patients presenting as EN. A subset of our UUC cohort exhibits the characteristic renal histopathology of EN; however, in many patients, the pathognomonic gradient of fibrosis was likely obscured by secondary changes associated with CKD. This observation underscores the need to utilize more sensitive parameters for evaluating renal tubular function, such as low molecular weight proteinuria, in evaluating nephrotoxicity in AA-induced UUC (46).
Cross-sectional studies can be used to evaluate DNA adduct-exposure relationships in populations exposed to genotoxic agents (26,27). The dose of carcinogen detected in target tissues as DNA adducts reflects the “biologically effective dose”(47). DNA adduct levels represent integration, over time, of exposure to the carcinogen, including inter-individual variation in carcinogen metabolism, DNA repair and other host factors.
Nevertheless, as genetic factors play a significant role in conferring susceptibility (6) the presence of DNA adducts alone is insufficient to predict the development of EN/UUC.
The sensitivity of AL-DNA adduct analysis as a biomarker for exposure is enhanced by the active secretion of AA by the proximal tubule, where the toxin is concentrated 10–20 fold (48). Additionally, dA-AL DNA adducts are resistant to global genomic nucleotide excision repair, as evidenced by their exclusive presence on the nontranscribed strand of DNA (16). As a result, AL-DNA adducts persist in the renal cortex for many years (13, 15); indeed, the last known exposure to AA for one adduct-positive subject in this study occurred 60 years prior to developing symptoms of UUC.
Until quite recently, Aristolochia herbal preparations were widely used in the practice of traditional Chinese herbal medicine. Indeed, the production of A. manchuriensis alone in China was estimated at 320,000 kg/year (49). Surprisingly, considering the widespread usage of Aristolochia herbs in certain countries (49–51), only several hundred cases of AAN worldwide have appeared in the literature (summarized in ref 24). Therefore, we posit that AAN/UUC represents a long-overlooked disease and an international public health problem of considerable magnitude. The prevalence of disease in countries where Aristolochia sp have been used for medicinal purposes over the past 50 years could be estimated by applying the molecular epidemiologic approach described in this report, using dA-AL adducts and TP53 mutational spectra as biomarkers of exposure and carcinogenic effect.
In summary, we provide molecular epidemiologic evidence that supports strongly the hypothesis that, in genetically susceptible individuals, dietary exposure to AA is causally related to endemic (Balkan) nephropathy and to the carcinomas of the upper urinary tract associated with this disease.
Methods
Subject selection
All studies involving human subjects were approved by Institutional Review Boards at Stony Brook University and the School of Medicine, University of Zagreb. Subjects were selected from residents of Croatia, Bosnia and Serbia undergoing nephroureterectomy for UUC. The enrollment criteria were: (a) definitive diagnosis of urothelial carcinomas of the renal pelvis and/or ureter, verified by tumor histopathology; (b) known residence history; and (c) availability of fresh, frozen renal cortical tissue for DNA adduct analysis.
Clinical samples and medical histories
Blood samples were obtained prior to surgery. Samples of tumor and renal cortex obtained following surgery were frozen at −80° C, then used for mutational (tumor) or DNA adduct (renal cortex) analyses. Additional tissue samples were fixed in formalin and embedded in paraffin for histopathologic evaluation. Information regarding past health, residency, dietary and agricultural practices was acquired by means of a formal questionnaire, personal interviews and review of medical records.
Renal function
Serum creatinine levels were measured by the modified Jaffe assay and used to calculate estimated glomerular filtration rates (eGFR) (52). One subject was on hemodialysis therapy. KDOQI guidelines were used to classify chronic kidney disease (CKD) by stages, based on eGFR (53).
Molecular biomarkers of exposure
DNA, extracted from renal cortex and tumor tissues, was purified by standard phenol-chloroform extraction techniques. The level of AL-DNA adducts in the renal cortex DNA (10–20 μg) was determined using 32P-postlabeling polyacrylamide gel electrophoresis (PAGE), as previously described (54). Synthetic oligonucleotides containing known quantities of dA-AL and dG-AL adducts were used to identify the position of corresponding adducts on polyacrylamide gels and for quantification. TP53 mutations in DNA isolated from tumor samples were identified with the AmpliChip p53 detection algorithm that detects all single base-pair substitutions and single-base deletions in the TP53 gene (16).
Mass spectroscopic identification of AL-DNA adducts
Renal cortical DNA (62 μg in 5 mM Bis-Tris buffer, pH 7.1) was digested with DNAse I, nuclease P1, alkaline phosphatase and spleen phosphodiesterase for 18 h at 37 °C, followed by solid-phase extraction of the dA-AL adducts (15). Isotopically labeled [15N3]-dA-AL-II was employed as an internal standard to estimate the dA-AL-I and dA-AL-II adduct levels, as both adducts displayed comparable ionization efficiencies. The internal standard was added to DNA prior to enzyme digestion at a level of 4.2 adducts per 108 DNA bases. Untreated calf thymus DNA served as a negative control.
The chemical identities of dA-AL adducts were determined using the Velos linear quadrupole ion trap mass spectrometer (Thermo Fisher Scientific) interfaced with an Agilent capillary 1100 series LC system for separation of DNA adducts. Details of the mass spectroscopic methods used in this study have been reported (15). Mass spectroscopic analysis was conducted by electrospray ionization in the positive ionization mode. The MS/MS scan mode was employed to monitor the loss of deoxyribose from the protonated DNA adducts [M+H −116]+. The aglycone adduct [BH2]+ underwent fragmentation at the MS3 scan stage to obtain full product ion spectra. Analysis involved monitoring the following transitions: dA-AL-I, m/z 543 → 427 → 150 – 500; dA-AL-II, m/z 513 → 397 → 150–500; and [15N3]-dA-AL-II m/z 516 → 400 → 150–500.
Renal histopathology
Renal cortex was available for histopathologic review in 62/67 UUC cases from the endemic regions and 9/10 cases from nonendemic sites. Each case was reviewed by a single pathologist in a blinded fashion using 2-μm sections stained with hematoxylin and eosin and Masson’s trichrome. The criteria required for a positive histopathologic diagnosis of EN were the presence of interstitial fibrosis with a gradient from cortex to medulla, pronounced tubular atrophy with glomerular preservation, and relatively little inflammation.
Acknowledgments
Support:
The research described was supported by award 5 P01 ES04068 (APG) and award R01 ES019564 (RJT) from the National Institute of Environmental Health Sciences, award 1R03TW007042 from the Fogarty International Center of NIH, the Zickler Family Foundation (KGD), and the Croatian Ministry of Science.
We thank Andrea Fernandes and Gyongyi Mihalyne for expert technical assistance, Sava Micic and Cane Tulic for advice and support, and Annette Oestreicher for editorial expertise.
Footnotes
Disclosures
None of the authors has a financial interest in the information contained in this manuscript.
REFERENCES CITED
- 1.Djukanović L, Radovanović Z. Balkan endemic nephropathy. In: De Broe ME, Porter GA, Bennett WM, Verpooten GA, editors. Clinical Nephrotoxins. 2. Dordrecht: Kluwer; 2003. pp. 588–601. [Google Scholar]
- 2.Nikolić J. Izdavaćko Preduzeće. Belgrade, Serbia: Beograd AD; 2006. Epidemic nephropathy and upper urothelial tumors. [Google Scholar]
- 3.Petronić V. Tumors of the upper urothelium and endemic nephropathy. In: Radovanović Z, Sindić M, Polenaković M, Djukanović L, Petronić V, editors. Endemic Nephropathy. Belgrade, Serbia: Institute for Textbook Publishing; 2000. pp. 350–439. [Google Scholar]
- 4.Čeović S, Hrabar A, Radonić M. An etiological approach to Balkan endemic nephropathy based on the investigation of two genetically different populations. Nephron. 1985;40:175–179. doi: 10.1159/000183456. [DOI] [PubMed] [Google Scholar]
- 5.Radovanović Z. Epidemiology and aetiology of endemic nephropathy. In: Radovanović Z, Sindić M, Polenaković M, Djukanović L, Petronić V, editors. Endemic Nephropathy. Belgrade, Serbia: Institute for Textbook Publishing; 2000. pp. 22–152. [Google Scholar]
- 6.Toncheva D, Dimitrov T, Stojanova S. Etiology of Balkan endemic nephropathy: A multifactorial disease? Eur J Epidemiol. 1998;14:389–394. doi: 10.1023/a:1007445120729. [DOI] [PubMed] [Google Scholar]
- 7.Bamias G, Boletis J. Balkan nephropathy: Evolution of our knowledge. Am J Kidney Dis. 2008;52:606–616. doi: 10.1053/j.ajkd.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voice TC, Long DT, Radovanović Z, et al. Critical evaluation of environmental exposure agents suspected in the etiology of Balkan endemic nephropathy. Int J Occup Environ Health. 2006;12:369–376. doi: 10.1179/oeh.2006.12.4.369. [DOI] [PubMed] [Google Scholar]
- 9.Pfohl-Leszkowicz A, Tozlovanu M, Manderville R, et al. New molecular and field evidences for the implication of mycotoxins but not aristolochic acid in human nephropathy and urinary tract tumor. Mol Nutr Food Res. 2007;51:1131–1146. doi: 10.1002/mnfr.200700045. [DOI] [PubMed] [Google Scholar]
- 10.Batuman V. Fifty years of Balkan endemic nephropathy: Daunting questions, elusive answers. Kidney Int. 2006;69:644–646. doi: 10.1038/sj.ki.5000231. [DOI] [PubMed] [Google Scholar]
- 11.Ivić M. The problem of etiology of endemic nephropathy. Lije Vjesć. 1969;91:1273–1281. [PubMed] [Google Scholar]
- 12.Vanherweghem JL, Depierreux M, Tielemans C, et al. Rapidly progressive interstitial renal fibrosis in young women: Association with slimming regimen including Chinese herbs. Lancet. 1993;341:387–391. doi: 10.1016/0140-6736(93)92984-2. [DOI] [PubMed] [Google Scholar]
- 13.Nortier JL, Martinez MC, Schmeiser HH, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi) N Engl J Med. 2000;342:1686–1692. doi: 10.1056/NEJM200006083422301. [DOI] [PubMed] [Google Scholar]
- 14.Cosyns JP, Jadoul M, Squifflet JP, et al. Urothelial lesions in Chinese-herb nephropathy. Am J Kidney Dis. 1999;33:1011–1017. doi: 10.1016/S0272-6386(99)70136-8. [DOI] [PubMed] [Google Scholar]
- 15.Grollman AP, Shibutani S, Moriya M, et al. Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc Natl Acad Sci USA. 2007;104:12129–12134. doi: 10.1073/pnas.0701248104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moriya M, Slade N, Brdar B, et al. TP53 mutational signature for aristolochic acid: An environmental carcinogen. Int J Cancer. 2011;129:1532–1536. doi: 10.1002/ijc.26077. [DOI] [PubMed] [Google Scholar]
- 17.Olivier M, Eeles R, Hollstein M, et al. The IARC TP53 database: New online mutation analysis and recommendations to users. Hum Mutat. 2002;19:607–614. doi: 10.1002/humu.10081. [DOI] [PubMed] [Google Scholar]
- 18.Debelle FD, Vanherweghem JL, Nortier JL. Aristolochic acid nephropathy: A worldwide problem. Kidney Int. 2008;74:158–169. doi: 10.1038/ki.2008.129. [DOI] [PubMed] [Google Scholar]
- 19.Grollman AP, Scarborough J, Jelaković B. Aristolochic acid nephropathy: An environmental and iatrogenic disease. In: Fishbein J, editor. Advances in Molecular Toxicology. Vol. 3. Amsterdam: Elsevier; 2009. pp. 211–222. [Google Scholar]
- 20.Hranjec T, Kovac A, Kos J, et al. Endemic nephropathy: The case for chronic poisoning by Aristolochia. Croat Med J. 2005;46:116–125. [PubMed] [Google Scholar]
- 21.Colin P, Koenig P, Ouzzane A, et al. Environmental factors involved in carcinogenesis of urothelial cell carcinomas of the upper urinary tract. BJU Int. 2009;104:1436–1440. doi: 10.1111/j.1464-410X.2009.08838.x. [DOI] [PubMed] [Google Scholar]
- 22.Cosyns JP, Jadoul M, Squifflet JP, et al. Chinese herbs nephropathy: A clue to Balkan endemic nephropathy? Kidney Int. 1994;45:1680–1688. doi: 10.1038/ki.1994.220. [DOI] [PubMed] [Google Scholar]
- 23.Some traditional herbal medicines, some mycotoxins, naphthalene and styrene: Aristolochia species and aristolochic acids. IARC Monogr Eval Carcinog Risks Hum. 2002;82:69–128. [PMC free article] [PubMed] [Google Scholar]
- 24.National Toxicology Program. Aristolochic acids. Rep Carcinog. 2011;12:45–49. [PubMed] [Google Scholar]
- 25.Hill AB. The environment and disease: Association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarabek AM, Pottenger LH, Andrews LS, et al. Creating context for the use of DNA adduct data in cancer risk assessment: I. Data organization. Crit Rev Toxicol. 2009;39:659–678. doi: 10.1080/10408440903164155. [DOI] [PubMed] [Google Scholar]
- 27.Swenberg JA, Fryar-Tita E, Jeong YC, et al. Biomarkers in toxicology and risk assessment: Informing critical dose-response relationships. Chem Res Toxicol. 2008;21:253–265. doi: 10.1021/tx700408t. [DOI] [PubMed] [Google Scholar]
- 28.Martincić M. Toxische einwirkungen der Aristolochia clematitis auf die Niere des Pferdes. Veterinarski Archiv. 1958;27:51–59. [Google Scholar]
- 29.Cukuranović R, Jovanović I, Miljković S, et al. Hemodialysis treatment in patients with Balkan endemic nephropathy: An epidemiological study. Ren Fail. 2007;29:805–810. doi: 10.1080/08860220701573475. [DOI] [PubMed] [Google Scholar]
- 30.Belicza M, Demirović A, Tomić K, et al. Comparison of occurrence of upper urinary tract carcinomas in the region with endemic villages and non-endemic nephropathy region in Croatia. Coll Antropol. 2008;32:1203–1207. [PubMed] [Google Scholar]
- 31.Krogh P. Mycotoxin porcine nephropathy: A possible model for Balkan endemic nephropathy. In: Puchlev A, editor. Endemic Nephropathy. Sofia, Bulgaria: Bulgarian Academy of Science; 1974. pp. 266–270. [Google Scholar]
- 32.Radić B, Fuchs R, Peraica M, Lucić A. Ochratoxin A in human sera in the area with endemic nephropathy in Croatia. Toxicol Lett. 1997;91:105–109. doi: 10.1016/s0378-4274(97)03877-0. [DOI] [PubMed] [Google Scholar]
- 33.Clark HA, Snedeker SM. Ochratoxin A: Its cancer risk and potential for exposure. J Toxicol Environ Health B Crit Rev. 2006;9:265–296. doi: 10.1080/15287390500195570. [DOI] [PubMed] [Google Scholar]
- 34.Godin M, Fillastre JP, Simon P, et al. Is ochratoxin A nephrotoxic in human beings? Adv Nephrol Necker Hosp. 1997;26:181–206. [PubMed] [Google Scholar]
- 35.Fink-Gremmels J. Ochratoxin A in food: Recent developments and significance. Food Addit Contam. 2005;22(Suppl 1):1–5. doi: 10.1080/02652030500358415. [DOI] [PubMed] [Google Scholar]
- 36.Cavin C, Delatour T, Marin-Kuan M, et al. Ochratoxin A-mediated DNA and protein damage: Roles of nitrosative and oxidative stresses. Toxicol Sci. 2009;110:84–94. doi: 10.1093/toxsci/kfp090. [DOI] [PubMed] [Google Scholar]
- 37.Palma N, Cinelli S, Sapora O, et al. Ochratoxin A-induced mutagenesis in mammalian cells is consistent with the production of oxidative stress. Chem Res Toxicol. 2007;20:1031–1037. doi: 10.1021/tx700027j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mantle PG, Faucet-Marquis V, Manderville RA, et al. Structures of covalent adducts between DNA and ochratoxin A: A new factor in debate about genotoxicity and human risk assessment. Chem Res Toxicol. 2010;23:89–98. doi: 10.1021/tx900295a. [DOI] [PubMed] [Google Scholar]
- 39.Delatour T, Mally A, Richoz J, et al. Absence of 2′-deoxyguanosine-carbon 8-bound ochratoxin A adduct in rat kidney DNA monitored by isotope dilution LC-MS/MS. Mol Nutr Food Res. 2008;52:472–482. doi: 10.1002/mnfr.200700276. [DOI] [PubMed] [Google Scholar]
- 40.Peluso M, Castegnaro M, Malaveille C, et al. 32P-postlabelling analysis of DNA adducted with urinary mutagens from smokers of black tobacco. Carcinogenesis. 1990;11:1307–1311. doi: 10.1093/carcin/11.8.1307. [DOI] [PubMed] [Google Scholar]
- 41.Cosyns JP. Aristolochic acid and ‘Chinese herbs nephropathy’: A review of the evidence to date. Drug Saf. 2003;26:33–48. doi: 10.2165/00002018-200326010-00004. [DOI] [PubMed] [Google Scholar]
- 42.Chen HY, Ma BY, Grant A, Lampert N. Time to abandon the term “Chinese herbs nephropathy”. Kidney Int. 2001;60:2039–2040. doi: 10.1046/j.1523-1755.2001.00034.x. [DOI] [PubMed] [Google Scholar]
- 43.Shibutani S, Dong H, Suzuki N, et al. Selective toxicity of aristolochic acids I and II. Drug Metab Dispos. 2007;35:1217–1222. doi: 10.1124/dmd.107.014688. [DOI] [PubMed] [Google Scholar]
- 44.Shibutani S, Bonala RR, Rosenquist T, et al. Detoxification of aristolochic acid I by O-demethylation: Less nephrotoxicity and genotoxicity of aristolochic acid Ia in rodents. Int J Cancer. 2010;127:1021–1027. doi: 10.1002/ijc.25141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenquist TA, Einolf HJ, Dickman KG, et al. Cytochrome P450 1A2 detoxicates aristolochic acid in the mouse. Drug Metab Dispos. 2010;38:761–768. doi: 10.1124/dmd.110.032201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kabanda A, Jadoul M, Lauwerys R, et al. Low molecular weight proteinuria in Chinese herbs nephropathy. Kidney Int. 1995;48:1571–1576. doi: 10.1038/ki.1995.449. [DOI] [PubMed] [Google Scholar]
- 47.Poirier MC, Santella RM, Weston A. Carcinogen macromolecular adducts and their measurement. Carcinogenesis. 2000;21:353–359. doi: 10.1093/carcin/21.3.353. [DOI] [PubMed] [Google Scholar]
- 48.Dickman KG, Sweet DH, Bonala R, et al. Physiological and molecular characterization of aristolochic acid transport by the kidney. J Pharmacol Exp Ther. 2011;338:588–597. doi: 10.1124/jpet.111.180984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu SL, Zhang HQ, Chan K, Mei QX. Studies on the toxicity of Aristolochia manshuriensis (Guanmuton) Toxicology. 2004;198:195–201. doi: 10.1016/j.tox.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 50.Hsieh SC, Lin IH, Tseng WL, et al. Prescription profile of potentially aristolochic acid containing Chinese herbal products: An analysis of national health insurance data in Taiwan between 1997 and 2003. Chin Med. 2008;3:13. doi: 10.1186/1749-8546-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lai MN, Wang SM, Chen PC, et al. Population-based case-control study of Chinese herbal products containing aristolochic acid and urinary tract cancer risk. J Natl Cancer Inst. 2010;102:179–186. doi: 10.1093/jnci/djp467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 53.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: A position statement from kidney disease: Improving global outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 54.Dong H, Suzuki N, Torres MC, et al. Quantitative determination of aristolochic acid-derived DNA adducts in rats using 32P-postlabeling/polyacrylamide gel electrophoresis analysis. Drug Metab Dispos. 2006;34:1122–1127. doi: 10.1124/dmd.105.008706. [DOI] [PubMed] [Google Scholar]