Abstract
Background
The effect of specific antiretrovirals on inflammation is unclear.
Methods
A5224 s was a substudy of A5202, which randomized HIV-infected treatment-naïve subjects to blinded abacavir/lamivudine (ABC/3TC) or tenofovir/emtricitabine (TDF/FTC) with open-label efavirenz (EFV) or atazanavir/ritonavir (ATV/r) in a factorial design. Our analysis compared changes in inflammation markers from baseline to week 24 between ABC/3TC and TDF/FTC. Secondary analyses included changes at week 96 and comparisons of EFV vs. ATV/r.
Results
Analyses included 244 subjects (85% male, 48% white non-Hispanic), median age 39 years, HIV-1 RNA 4.6 log10 copies/mL, CD4 240 cells/µL. TNF-α, sTNFR-I and -II, sVCAM-1 and sICAM-1 decreased significantly at weeks 24 and 96, without significant differences between components (p ≥ 0.44). At week 24, ABC/3TC had a greater hsCRP mean fold change than TDF/FTC (1.43 vs. 0.88, estimated mean fold change percent difference (Δ) 61.5% [95% CI 13.6%, 129.5%]; p = 0.008). Similar results were seen at week 96 (p = 0.021). At week 24 (but not 96), EFV had a greater hsCRP mean fold change than ATV/r (1.41 vs. 0.88; Δ = 60.2% [12.6%, 127.7%]; p = 0.009). IL-6 decreased significantly at week 24 with TDF/FTC but not with ABC/3TC (between-components p = 0.019). At week 96, IL-6 decreased significantly in both NRTI components (between-components p = 0.11). IL-6 changes were not significantly different between ATV/r and EFV at either time point (p ≥ 0.89).
Conclusions
Soluble TNF-receptors and adhesion molecules decreased following treatment initiation and did not differ by regimens. Differences were seen on hsCRP and IL-6 changes with ABC/3TC vs. TDF/FTC and on hsCRP with EFV vs. ATV/r.
Keywords: abacavir, C-reactive protein, endothelial activation markers, Inflammation markers, interleukin-6, TNF alpha
Introduction
HIV-infected patients experience high rates of non-AIDS complications, including cardiovascular disease (CVD), which has been linked to heightened inflammation. With effective antiretroviral therapy (ART), inflammation markers overall decrease but do not normalize [1–4]. Treatment with abacavir (ABC)-containing regimens has been associated with higher risk of CVD in some studies [5–10] but not others [11–14].
Several attempts have been made to understand this potential association, with one proposed mechanism a deleterious effect of ABC on inflammation. While one observational study found an association between ABC and higher inflammation markers [5], another did not [15]. Prospective randomized studies comparing ABC to non-ABC regimens also have not demonstrated a difference in these markers [16–19]. Because of the remaining uncertainty about the differential effect of ABC versus other nucleoside reverse transcriptase inhibitors (NRTIs) on inflammation, and the potential effect of the concomitant protease inhibitor (PI) or non-nucleoside reverse transcriptase inhibitor (NNRTI) therapy, we sought to compare changes in inflammation markers in the context of a large randomized trial.
Methods
AIDS Clinical Trials Group (ACTG) A5224 s was a metabolic substudy of ACTG A5202 in which ART-naïve subjects ≥16 years old with HIV-1 RNA >1,000 copies/mL were randomized in a double-blinded fashion to co-formulated tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) or ABC/lamivudine (ABC/3TC), along with open-labeled efavirenz (EFV) or atazanavir/ritonavir (ATV/r). Randomization was stratified by screening HIV-1 RNA (< vs. ≥100,000 copies/mL). A secondary biomarker substudy of A5224 s included all subjects with available stored plasma at baseline and week 24 and/or 96, and was designed with the primary objective to compare the effect after 24 weeks of initiating ABC/3TC vs. TDF/FTC on inflammation and endothelial activation markers. Secondary objectives were to compare 24 week biomarkers changes between EFV and ATV/r, and compare ABC/3TC vs. TDF/FTC and EFV vs. ATV/r on 96 week biomarker changes. A5224 s main exclusion criteria were endocrine diseases including diabetes mellitus. The duration of the study was 96 weeks after the last subject enrolled into A5202. Each subject signed an informed consent, which was approved by each participating site’s local IRB.
As previously described [20], the NRTI assignment was prematurely unblinded for subjects with A5202 screening HIV-1 RNA ≥100,000 copies/mL because of higher rates of virologic failure with ABC/3TC-regimens. Baseline smoking status was not collected in A5224 s but was available on a subset of subjects co-enrolled into the observational cohort ACTG A5001.
Biomarker assays
Plasma samples were stored at −80°C without prior thawing until analysis. Assays were performed at Johns Hopkins Bayview Advanced Chemistry Laboratory, Baltimore, MD, USA. We measured high-sensitivity C-reactive protein (hsCRP), interleukin-6 (IL-6), TNF-α, and the soluble receptors of TNF-α, (sTNFR-I,-II), along with the endothelial activation markers soluble vascular cellular and intercellular adhesion molecules (sVCAM-1 &sICAM-1). hsCRP was measured using a highly sensitive ELISA (ALPCO Diagnostics, Windham, NH). Other markers were measured using enzyme-immunosorbent assay (R&D Systems, Minnesota, USA). Markers were measured in duplicate and values averaged for analysis. The intra-assay and inter-assay precisions of these assays were 1.3–7.6% CV (average 3.3%) and 1.83–8.95% CV (average 6.89%), respectively.
Statistical analysis
The primary objective was to compare, between pooled, randomized NRTI components (ABC/3TC vs. TDF/FTC with 3rd drug combined) changes from baseline to week 24 in sVCAM-1 and sTNFR-II. Other objectives were considered secondary. All analyses were initially performed using intent-to-treat principles based on randomized treatment assignment which used all available data and modifications to randomized treatment and missing values were ignored. Supplemental as-treated analyses were performed which censored values after a change in the randomized NRTI (when comparing NRTI components) or NNRTI/PI (when comparing NNRTI/PI components). Comparisons used a factorial analysis approach in which, after assessing for treatment effect modification by the other component, the NRTI effect was assessed by combining EFV and ATV/r arms and vice versa. P-values<0.05 (<0.10 for assessing treatment effect modification) were considered statistically significant, and nominal values are reported without adjustment for multiple comparisons. Analyses were performed using SAS, version 9.2 (SAS Institute).
Within each regimen, 1-sample t-tests were used to assess mean change from baseline, while mean comparisons between regimen components used 2-sample t-tests. Analyses that adjusted for baseline factors and explored associations with biomarkers used linear regression. Due to the highly skewed distribution of the biomarker data, biomarkers were loge transformed prior to analysis. The estimated mean change from baseline of loge-transformed biomarkers was exponentiated to obtain the estimated mean fold change within a component (or arm). The estimated mean difference between components (or arms) of change from baseline in loge-transformed biomarkers were exponentiated, subtracted by 1, and multiplied by 100 to obtain the estimated percent difference between the two mean fold changes(Δ), with TDF/FTC and EFV as reference groups for the comparisons.
The comparison of ABC/3TC and TDF/FTC with EFV and ATV/r combined (factorial analysis) was performed at each timepoint since there was no significant evidence that the NRTI effect differed at 24 or 96 weeks by the NNRTI/PI component. Similarly, the comparison of EFV and ATV/r with ABC/3TC and TDF/FTC combined was performed.
In sensitivity analyses the intent-to-treat analysis on change in biomarkers from entry to weeks 24 and 96 were adjusted for the following pre-specified baseline covariates that could affect inflammation, first individually, then jointly using linear regression: NNRTI/PI (or NRTI components for NNRTI/PI analyses), baseline biomarker level, sex, age, race/ethnicity, log10 HIV-1 RNA, CD4, BMI, smoking status (when available), hypertension, fasting glucose, LDL-cholesterol, and family history of coronary artery disease (CAD)
Results
Subject characteristics
As previously detailed [21,22], 269 subjects were randomized to one of the 4 regimens and were included in A5224 s analysis. Of these 269 subjects, 244(91%) with available stored plasma from baseline and week 24 and/or 96 were included in this biomarker substudy. Among these 244, 61 were randomized to EFV + TDF/FTC, 64 to EFV +ABC/3TC, 57 to ATV/r +TDF/FTC, and 62 to ATV/r +ABC/3TC. Baseline characteristics are summarized in Table 1. Overall, 85% were male, 48% white non-Hispanics, and among 205 with available data, 41% were smokers. Median age was 39 years, CD4 240 cells/µL, and HIV-1 RNA 4.64 log10 copies/mL. None of the subjects had a prior history of myocardial infarction, and only one had a history of stroke. Baseline characteristics were balanced across arms, except that a larger proportion of women were randomized to EFV (18%) than ATV/r (11%), and a larger proportion of hypertensive subjects were randomized to ABC/3TC (21%) than TDF/FTC (11%), and similarly, EFV (22%) than ATV/r (11%). Baseline characteristics were similar between the 244 included in the biomarker substudy and the 25 A5224 s subjects not included (data not shown).
Table 1.
Baseline Characteristics of Study Subjects by Randomized Arms.
| EFV + TDF/FTC (N = 61) |
EFV + ABC/3TC (N = 64) |
ATV/r + TDF/FTC (N = 57) |
ATV/r + ABC/3TC (N = 62) |
Total (N = 244) |
P-Value | ||
|---|---|---|---|---|---|---|---|
| Sex | Male | 50 (82%) | 52 (81%) | 50 (88%) | 56 (90%) | 208 (85%) | 0.41a |
| Female | 11 (18%) | 12 (19%) | 7 (12%) | 6 (10%) | 36 (15%) | ||
| Age (years) | Median (Q1, Q3) | 42 (34, 45) | 39 (31, 45) | 38 (31, 44) | 37 (29, 43) | 39 (31, 44) | 0.24b |
| Race/Ethnicity | White Non-Hispanic | 34 (56%) | 32 (50%) | 24 (42%) | 27 (44%) | 117 (48%) | 0.38a |
| Black Non-Hispanic | 19 (31%) | 17 (27%) | 18 (32%) | 26 (42%) | 80 (33%) | ||
| Hispanic (Regardless of Race) | 7 (11%) | 13 (20%) | 12 (21%) | 8 (13%) | 40 (16%) | ||
| Other | 1 (2%) | 2 (3%) | 3 (5%) | 1 (2%) | 7 (3%) | ||
| BMI (kg/m2) | Median (Q1, Q3) | 24.9 (21.6, 27.1) | 24.9 (22.6, 28.5) | 24.6 (21.7, 28.0) | 25.3 (21.7, 28.9) | 24.8 (21.7, 28.1) | 0.85b |
| CD4 (cells/mm3) | Median (Q1, Q3) | 250 (139, 338) | 225 (124, 360) | 234 (99, 300) | 218 (60, 332) | 240 (106, 335) | 0.70b |
| HIV-1 RNA (log10copies/mL) | Median (Q1, Q3) | 5 (4, 5) | 5 (4, 5) | 5 (4, 5) | 5 (4, 5) | 5 (4, 5) | 0.91b |
| Screening HIV-1 RNA (copies/mL) | < 100,000 | 36 (59%) | 40 (63%) | 32 (56%) | 35 (56%) | 143 (59%) | 0.89a |
| ≥100,000 | 25 (41%) | 24 (38%) | 25 (44%) | 27 (44%) | 101 (41%) | ||
| Fasting glucose (mg/dl) | # missing | 3 | 3 | 0 | 0 | 6 | 0.072b |
| Median (Q1, Q3) | 83 (77, 88) | 85 (78, 90) | 88 (81, 94) | 82 (78, 89) | 84 (78, 90) | ||
| Fasting LDL cholesterol (mg/dl) | # missing | 3 | 4 | 0 | 1 | 8 | 0.23b |
| Median (Q1, Q3) | 101 (83, 121) | 90 (76, 119) | 90 (70, 121) | 89 (67, 105) | 92 (74, 118) | ||
| Current smoker | Yes | 21 (42%) | 24 (44%) | 22 (42%) | 18 (38%) | 85 (41%) | 0.94a |
| No | 29 (58%) | 31 (56%) | 30 (58%) | 30 (63%) | 120 (59%) | ||
| Hypertension | Yes | 10 (16%) | 17 (27%) | 3 (5%) | 10 (16%) | 40 (16%) | 0.015a |
| No | 51 (84%) | 47 (73%) | 54 (95%) | 52 (84%) | 204 (84%) | ||
| Family history of CAD | Yes | 14 (25%) | 20 (36%) | 15 (28%) | 17 (30%) | 66 (30%) | 0.66a |
| No | 41 (75%) | 35 (64%) | 38 (72%) | 40 (70%) | 154 (70%) | ||
| Hepatitis C antibody | Positive | 3 (5%) | 7 (11%) | 3 (5%) | 6 (10%) | 19 (8%) | 0.49a |
| Negative | 58 (95%) | 56 (89%) | 54 (95%) | 54 (90%) | 222 (92%) | ||
| hsCRP (ug/mL) | Median (Q1, Q3) | 1.7 (0.7, 5.5) | 1.7 (0.5, 3.1) | 2.0 (0.8, 4.8) | 1.7 (0.8, 3.2) | 1.7 (0.7, 4.0) | 0.58b |
| IL-6 (pg/mL) | Median (Q1, Q3) | 0.8 (0.5, 1.4) | 0.7 (0.5, 1.3) | 0.9 (0.6, 1.4) | 0.8 (0.5, 1.2) | 0.8 (0.5, 1.4) | 0.44b |
| sICAM-1 (ng/mL) | Median (Q1, Q3) | 323 (270, 374) | 357 (271, 423) | 329 (261, 447) | 329 (269, 395) | 330 (267, 402) | 0.59b |
| sTNF-RI (pg/mL) | # missing | 0 | 0 | 1 | 0 | 1 | 0.68b |
| Median (Q1, Q3) | 1,364 (1,152, 1,502) | 1,294 (1,125, 1,536) | 1,224 (998, 1,681) | 1,230 (1,102, 1,490) | 1,277 (1,105, 1,538) | ||
| sTNF-RII (pg/mL) | Median (Q1, Q3) | 5,147 (4,094, 7,470) | 6,059 (4,594, 7,724) | 5,074 (3,605, 8,351) | 5,251 (3,609, 7,959) | 5,351 (3,965, 7,756) | 0.35b |
| sVCAM-1 (ng/mL) | # missing | 0 | 0 | 1 | 0 | 1 | 0.29b |
| Median (Q1, Q3) | 1,110 (943, 1,511) | 1,291 (996, 1,726) | 1,160 (964, 1,661) | 1,157 (918, 1,508) | 1,187 (939, 1,599) | ||
| TNF-α (pg/mL) | Median (Q1, Q3) | 10.2 (8.0, 14.3) | 11.6 (9.4, 15.5) | 11.3 (8.2, 18.1) | 11.2 (7.5, 13.9) | 11.0 (8.2, 14.7) | 0.43b |
Fishers Exact Test.
Kruskal-Wallis test.
Overall, 26 (10.7%) subjects modified their randomized NRTI (4/118 [3.4%] TDF/FTC; 22/126 [17.5%] ABC/3TC) before week 24 and an additional 39 (16.0%; 13/118 [11.0%] TDF/FTC; 26/126 [20.6%] ABC/3TC) modified between weeks 24 and 96. Also, 22 (9.0%) modified their NNRTI/PI (19/125 [15.2%] EFV; 3/119 [2.5%] ATV/r) before week 24 and an additional 40 (16.4%; 17/125 [13.6%] EFV; 23/119 [19.3%] ATV/r) between weeks 24 and 96.
At week 24, 171 (70%) had HIV-1 RNA<50 copies/mL [70% on TDF/FTC; 70% on ABC/3TC]. Among subjects who had screening HIV-1 RNA ≥100,000 copies/mL, 55% on TDF/FTC and 64% on ABC/3TC had HIV-1 RNA <50 copies/mL. Two subjects experienced a myocardial infarction during the study; one at week 15 and the other at week 164. Both subjects had screening HIV-1 RNA<100,000 copies/mL and were receiving their randomized regimen of TDF/FTC + EFV at the time of the event.
Changes in Plasma TNF-α and Soluble TNF Receptors (sTNFR-II co-Primary Endpoint)
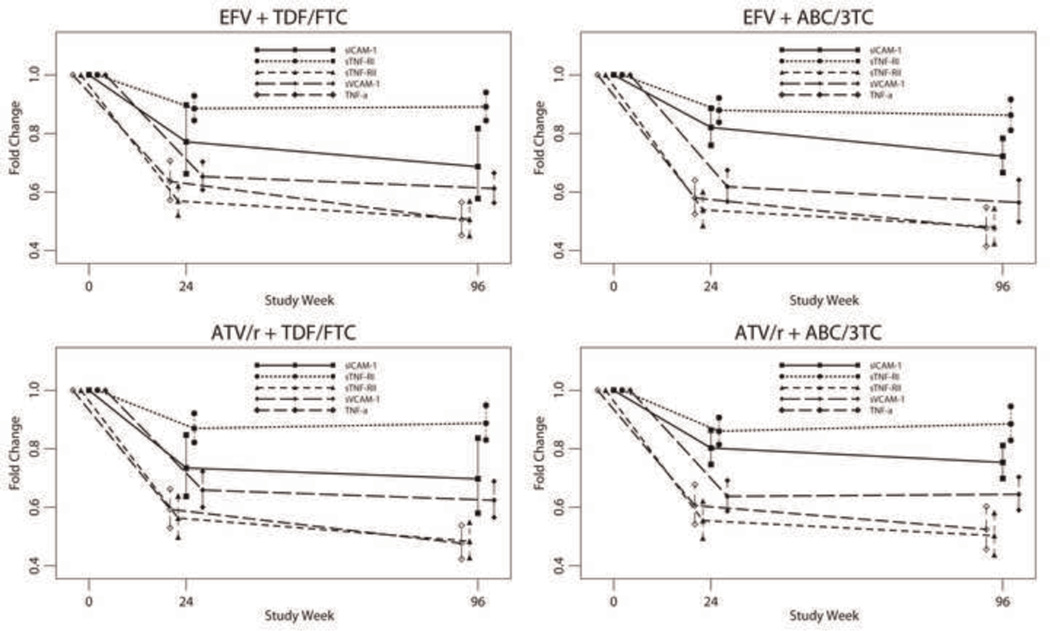
At weeks 24 and 96, there was a statistically significant decrease in TNF-α, sTNFR-I, and sTNFR-II levels within all arms(p<0.001) (Fig. 1 and Table 2), without differences between ABC/3TC and TDF/FTC or ATV/r and EFV by intent-to-treat (p ≥ 0.44) or as-treated analyses.
Fig. 1. Mean fold change in sICAM, sVCAM, TNF-a, sTNFR-I, and sTNFR-II by Intent-to-Treat Analysis with 95% confidence intervals (CI) for the means at weeks 24 and 96 for the four Treatment Arms.
Table 2.
Changes from Baseline to Weeks 24 and 96 in loge Transformed Inflammation and Endothelial Activation Markers for all four Treatment Arms.
| EFV + TDF/ FTC (N = 61) |
EFV + ABC/ 3TC (N = 64) |
ATV/rtv + TDF/FTC (N = 57) |
ATV/rtv + ABC/3TC (N = 62) |
Total (N = 244) | ||
|---|---|---|---|---|---|---|
| Change in hsCRP (loge ug/mL), week 0 to 24 | N | 60 | 63 | 56 | 57 | 236 |
| Mean (s.d.) | 0.11 (1.41) | 0.57 (1.41) | −0.37 (1.40) | 0.12 (1.19) | 0.12 (1.39) | |
| Fold Change (ug/mL) | 1.12 | 1.77 | 0.69 | 1.13 | 1.13 | |
| (95% CI) | (0.78, 1.61) | (1.24, 2.52) | (0.47, 1.00) | (0.82, 1.54) | (0.94, 1.35) | |
| p-valuea | 0.55 | 0.002 | 0.051 | 0.45 | 0.18 | |
| week 0 to 96 | N | 56 | 54 | 46 | 49 | 205 |
| Mean (s.d.) | −0.03 (1.41) | 0.43 (1.37) | −0.23 (1.31) | 0.17 (1.13) | 0.09 (1.33) | |
| Fold Change (ug/mL) | 0.97 | 1.53 | 0.79 | 1.19 | 1.10 | |
| (95% CI) | (0.66, 1.41) | (1.06, 2.23) | (0.54, 1.17) | (0.86, 1.64) | (0.91, 1.32) | |
| p- valuea | 0.86 | 0.026 | 0.24 | 0.29 | 0.32 | |
| Change in IL-6 (loge pg/mL), week 0 to 24 | N | 60 | 63 | 56 | 57 | 236 |
| Mean (s.d.) | −0.30 (0.96) | −0.03 (0.77) | −0.28 (0.72) | −0.05 (0.81) | −0.16 (0.82) | |
| Fold Change (pg/mL) | 0.74 | 0.97 | 0.76 | 0.95 | 0.85 | |
| (95% CI) | (0.58, 0.95) | (0.80, 1.18) | (0.62, 0.92) | (0.77, 1.18) | (0.77, 0.95) | |
| p- valuea | 0.020 | 0.75 | 0.005 | 0.67 | 0.003 | |
| week 0 to 96 | N | 56 | 54 | 46 | 49 | 205 |
| Mean (s.d.) | −0.51 (0.99) | −0.24 (0.78) | −0.46 (0.93) | −0.33 (0.84) | −0.39 (0.89) | |
| Fold Change (pg/mL) | 0.60 | 0.78 | 0.63 | 0.72 | 0.68 | |
| (95% CI) | (0.46, 0.79) | (0.63, 0.97) | (0.48, 0.83) | (0.56, 0.92) | (0.60, 0.77) | |
| p- valuea | <0.001 | 0.026 | 0.002 | 0.008 | <0.001 | |
| Change in sICAM-1 (loge ng/mL), week 0 to 24 | N | 60 | 63 | 56 | 57 | 236 |
| Mean (s.d.) | −0.26 (0.59) | −0.20 (0.31) | −0.31 (0.53) | −0.22 (0.28) | −0.25 (0.44) | |
| Fold Change (ng/mL) | 0.77 | 0.82 | 0.73 | 0.80 | 0.78 | |
| (95% CI) | (0.66, 0.90) | (0.76, 0.89) | (0.64, 0.85) | (0.75, 0.86) | (0.74, 0.83) | |
| p- valuea | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| week 0 to 96 | N | 56 | 53 | 46 | 49 | 204 |
| Mean (s.d.) | −0.38 (0.65) | −0.33 (0.29) | −0.36 (0.62) | −0.28 (0.26) | −0.34 (0.49) | |
| Fold Change (ng/mL) | 0.69 | 0.72 | 0.70 | 0.75 | 0.71 | |
| (95% CI) | (0.58, 0.82) | (0.67, 0.78) | (0.58, 0.84) | (0.70, 0.81) | (0.67, 0.76) | |
| p- valuea | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Change in sTNF-RI (loge pg/mL), week 0 to 24 | N | 60 | 63 | 55 | 57 | 235 |
| Mean (s.d.) | −0.12 (0.18) | −0.13 (0.19) | −0.14 (0.21) | −0.15 (0.20) | −0.13 (0.19) | |
| Fold Change (pg/mL) | 0.89 | 0.88 | 0.87 | 0.86 | 0.87 | |
| (95% CI) | (0.84, 0.93) | (0.84, 0.92) | (0.82, 0.92) | (0.82, 0.91) | (0.85, 0.90) | |
| p- valuea | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| week 0 to 96 | N | 56 | 53 | 46 | 49 | 204 |
| Mean (s.d.) | −0.12 (0.20) | −0.15 (0.22) | −0.12 (0.23) | −0.12 (0.23) | −0.13 (0.22) | |
| Fold Change (pg/mL) | 0.89 | 0.86 | 0.89 | 0.89 | 0.88 | |
| (95% CI) | (0.84, 0.94) | (0.81, 0.92) | (0.83, 0.95) | (0.83, 0.95) | (0.86, 0.91) | |
| p- valuea | <0.001 | <0.001 | 0.001 | 0.001 | <0.001 | |
| Change in sTNF-RII (loge pg/mL), week 0 to 24 | N | 60 | 63 | 56 | 57 | 236 |
| Mean (s.d.) | −0.56 (0.34) | −0.62 (0.42) | −0.57 (0.46) | −0.59 (0.43) | −0.59 (0.41) | |
| Fold Change (pg/mL) | 0.57 | 0.54 | 0.56 | 0.55 | 0.56 | |
| (95% CI) | (0.52, 0.62) | (0.49, 0.60) | (0.50, 0.64) | (0.49, 0.62) | (0.53, 0.59) | |
| p- valuea | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| week 0 to 96 | N | 56 | 54 | 46 | 49 | 205 |
| Mean (s.d.) | −0.68 (0.44) | −0.73 (0.46) | −0.73 (0.42) | −0.69 (0.50) | −0.71 (0.45) | |
| Fold Change (pg/mL) | 0.51 | 0.48 | 0.48 | 0.50 | 0.49 | |
| (95% CI) | (0.45, 0.57) | (0.42, 0.54) | (0.43, 0.55) | (0.44, 0.58) | (0.46, 0.53) | |
| p- valuea | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Change in sVCAM-1 (loge ng/mL), week 0 to 24 | N | 60 | 63 | 55 | 57 | 235 |
| Mean (s.d.) | −0.43 (0.29) | −0.48 (0.34) | −0.42 (0.34) | −0.45 (0.31) | −0.44 (0.32) | |
| Fold Change (ng/mL) | 0.65 | 0.62 | 0.66 | 0.64 | 0.64 | |
| (95% CI) | (0.61, 0.70) | (0.57, 0.67) | (0.60, 0.72) | (0.59, 0.69) | (0.62, 0.67) | |
| p- valuea | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| week 0 to 96 | N | 56 | 53 | 46 | 49 | 204 |
| Mean (s.d.) | −0.49 (0.31) | −0.57 (0.46) | −0.47 (0.33) | −0.44 (0.30) | −0.50 (0.36) | |
| Fold Change (ng/mL) | 0.61 | 0.56 | 0.62 | 0.64 | 0.61 | |
| (95% CI) | (0.56, 0.67) | (0.50, 0.64) | (0.57, 0.69) | (0.59, 0.70) | (0.58, 0.64) | |
| p- valuea | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Change in TNF-α (loge pg/mL), week 0 to 24 | N | 60 | 63 | 56 | 57 | 236 |
| Mean (s.d.) | −0.45 (0.41) | −0.55 (0.39) | −0.52 (0.42) | −0.50 (0.42) | −0.51 (0.41) | |
| Fold Change (pg/mL) | 0.64 | 0.58 | 0.59 | 0.61 | 0.60 | |
| (95% CI) | (0.57, 0.71) | (0.52, 0.64) | (0.53, 0.66) | (0.54, 0.68) | (0.57, 0.64) | |
| p- valuea | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| week 0 to 96 | N | 56 | 54 | 46 | 49 | 205 |
| Mean (s.d.) | −0.68 (0.42) | −0.74 (0.51) | −0.74 (0.40) | −0.65 (0.48) | −0.70 (0.46) | |
| Fold Change (pg/mL) | 0.50 | 0.48 | 0.48 | 0.52 | 0.50 | |
| (95% CI) | 0.45, 0.56) | (0.41, 0.55) | (0.42, 0.54) | (0.46, 0.60) | (0.47, 0.53) | |
| p- valuea | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
1-sample t-test.
There was some evidence that the NRTI effect differed by screening HIV-1 RNA stratum for week 24 sTNFR-II (p = 0.069) and TNF-α (p = 0.093), and that the NNRTI/PI effect differed for week 96 sTNFR-I (p = 0.048), thus analyses were conducted within each stratum for these markers. For sTNFR-II, within the low stratum the ABC/3TC mean fold change was marginally significantly smaller than TDF/FTC at 24 weeks (0.57 vs. 0.65; Δ = −11.4% [95% confidence intervals (CI) −22.3%, 1.2%]; p = 0.073), while within the high stratum it was not significantly different (0.50 vs. 0.47; Δ = 7.4% [−8.6%, 26.1%]; p = 0.38). As-treated analyses showed similar results. For week 24 TNF-α, in both the low stratum and the high stratum, the estimated mean fold change was not significantly different between ABC/3TC and TDF/FTC (0.61 vs. 0.68; Δ = −10.9% [−22.6%, 2.6%]; p = 0.11 within the low stratum and 0.57 vs. 0.53; Δ = 6.6% [−8.1%, 23.8%]; p = 0.39 within the high stratum). As-treated analyses showed similar results. For week 96 sTNFR-I, within the low stratum the ATV/r estimated mean fold change was marginally significantly larger EFV (0.95 vs. 0.89); Δ = 6.5% [−0.6%, 14.2%]; p = 0.074), but similar within the high stratum (0.81 vs. 0.86; Δ = −5.6% [−14.9%, 4.8%]; p = 0.28). As-treated yielded similar results.
Changes in Endothelial Activation Markers (sVCAM-1 co-Primary Endpoint)
At weeks 24 and 96, there was a statistically significant decrease in sVCAM-1 and sICAM-1 within all arms (p ≤ 0.001) (Fig. 1 and Table 2), without differences between ABC/3TC and TDF/FTC or ATV/r and EFV at either time point by intent-to-treat (p ≥ 0.14) or as-treated analyses. There was no significant evidence of an interaction between the NRTI components and the HIV-1 RNA stratum. However, for sVCAM-1 at week 24, there was evidence of an interaction between the NNRTI/PI component and HIV-1 RNA stratum (p = 0.047). Within the low stratum, the ATV/r estimated mean fold change was marginally larger than EFV (0.72 vs. 0.66; Δ = 9.3% [95% CI −0.8%, 20.4%] p = 0.071), while within the high stratum, it was similar (0.55 vs. 0.60; Δ = −7.0% [−18.6%, 6.2%]; p = 0.28). As-treated analysis showed similar results.
Changes in C-reactive protein
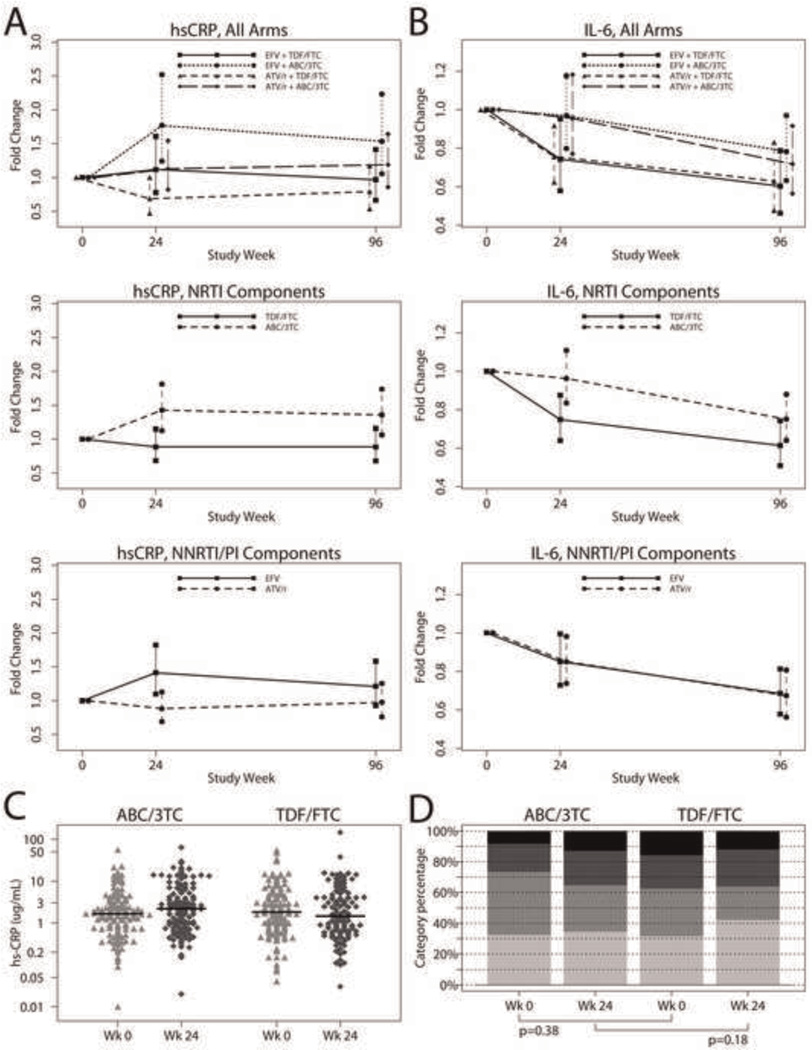
As shown in Table 2 and Fig. 2, at week 24, within the ABC/3TC+EFV arm there was a statistically significant increase in hsCRP (estimated mean fold change 1.77 [95% CI 1.24, 2.52]; p = 0.002) while within the TDF/FTC+ATV/r arm, there was a marginally significant decrease (0.69 [0.47, 1.00]; p = 0.051). In TDF/FTC+EFV and ABC/3TC+ATV/r arms, hsCRP did not change significantly (1.11 [0.78, 1.61]; p = 0.55 and 1.13 [0.82, 1.54]; p = 0.45, respectively). At 96 weeks, hsCRP increased significantly for only ABC/3TC+EFV (1.54 [1.06, 2.23]; p = 0.026).
Fig. 2. Mean fold change in hsCRP (panel a) and IL-6 (panel b) by intent-to-treat analysis with 95% confidence intervals (CI) for the means at weeks 24 and 96 by NRTI and NNRTI/PI components, and for the four treatment arms.
Week 0 and Week 24 hsCRP for ABC/3TC and TDF/FTC are shown in panel c as distribution by hsCRP (mg/liter) values, with black bars representing median values, and in panel d as distribution by American Heart Association Risk categories. From bottom to top: hsCRP <1 mg/liter (low risk), 1–3 mg/liter (average risk), >3 mg/liter (high risk), and >10 mg/liter.
Changes by NRTI components
At week 24 and 96 weeks, in the ABC/3TC arms (3rd drugs combined) there was a statistically significant increase in hsCRP (estimated mean fold change 1.43 [95% CI 1.12, 1.83]; p = 0.004 at 24 weeks and 1.36 [95% CI 1.05, 1.75]; p = 0.016 at 96 weeks) while within the TDF/FTC arms there was no significant change from baseline at either time points (p ≥ 0.35). At week 24 and 96, the change in hsCRP was significantly larger for ABC/3TC than TDF/FTC (1.43 vs. 0.88; Δ = 61.5% [13.6%, 129.5%]; p = 0.008 at week 24 and 1.36 vs. 0.88; Δ = 53.5% [6.9%, 120.4%]; p = 0.021 at week 96). As-treated analyses showed similar results.
At week 24 (but not 96), there was evidence of an interaction between the NRTI component and HIV-1 RNA stratum (p = 0.054). Within the high stratum the ABC/3TC mean fold change was larger than TDF/FTC (1.82 vs. 0.74; Δ = 142.5% [39.8%, 330.3%]; p = 0.002), while within the low stratum no statistically significant difference was detected (1.22 vs. 1.00; Δ = 21.5% [−22.6%, 90.9%]; p = 0.40). As-treated analyses showed similar results.
Changes by NNRTI/PI Components
At week 24, in the EFV arms (NRTIs combined), there was a statistically significant increase in hsCRP (estimated mean fold change 1.41 [95% CI 1.11, 1.80]; p = 0.008) without change in the ATV/r arms (estimated mean fold change 0.88 [0.68, 1.14]; p = 0.31), the difference was significant between the arms (Δ = −37.6% [−56.1%, −11.2%]; p = 0.009). At week 96, hCRP did not change significantly in either the EFV (1.21 [0.95, 1.56]; p = 0.15) or ATV/r (0.98 [0.75, 1.28]; p = 0.85) arms, and no difference was seen between arms (Δ = −19.5% [−44.2%, 16.1%]; p = 0.24). As-treated analyses showed similar results. There was no evidence of an interaction between the NNRTI/PI component and HIV-1 RNA stratum.
Three post-hoc sensitivity analyses were performed; the first excluding subjects with suspected hypersensitivity reaction, the second excluding subjects with HIV-1RNA ≥50 copies/mL at week 24, and the third excluding the 6 subjects with immune reconstitution syndrome. Similar results were seen by intent-to-treat and as-treated analyses for NRTI and NNRTI/PI component comparisons. Among all subjects, no differential NRTI effect was detected between subjects with (n = 168) and without (n = 68) HIV-1 RNA <50 copies/mL at week 24 (p = 0.68). In addition, after adjustment for changes in CD4 counts and in BMI, the results remained unchanged.
Changes in IL-6
At week 24, there was a statistically significant decrease in IL-6 within the TDF/FTC +EFV (estimated mean fold change 0.74 [95% CI 0.58, 0.95]; p = 0.020) and TDF/FTC +ATV/r arm (0.76 [0.62, 0.92]; p = 0.005) (Fig. 2 and Table 2). For ABC/3TC +EFV and ABC/3TC +ATV/r, the estimated mean fold change was 0.97 [0.80, 1.18]; p = 0.75, and 0.95 [0.77, 1.18]; p = 0.67, respectively. At week 96, there was a statistically significant decrease in IL-6 within all arms (p ≤ 0.026).
Changes by NRTI Components
At week 24, there was a statistically significant decrease in IL-6 within the TDF/FTC arms (combining the 3rd drug) (estimated mean fold change 0.75 [95% CI 0.64, 0.87]; p < 0.001) without significant change from baseline in the ABC/3TC arms (0.96 [0.83, 1.11]; p = 0.59). The change in IL-6 was significantly larger for ABC/3TC vs. TDF/FTC (Δ = 28.5% [4.2%, 58.4%]; p = 0.019). As-treated analyses showed similar results. At 96 weeks, both ABC/3TC and TDF/FTC arms (3rd drugs combined) had a statistically significant decrease in IL-6 levels (both p < 0.001), and there was no difference between the NRTI components in IL-6 change by intent-to-treat (0.61 vs. 0.75; Δ = 22.2% [−4.3%, 56.1%]; p = 0.11) but there was by as-treated (0.79 vs. 0.60; Δ = 32.6% [1.2%, 73.6%]; p = 0.040).
At week 24 (but not 96), there was evidence of an interaction between the NRTI component and HIV-1 RNA stratum (p = 0.012).Within the high stratum, there was a significantly different change in IL-6 for ABC/3TC vs. TDF/FTC (1.06 vs. 0.60; Δ = 76.3% [31.3%, 136.8%]; p < 0.001), but not within the low stratum (0.91 vs. 0.88; Δ = 2.8% [−22.9%, 36.9%]; p = 0.85). As-treated analyses showed similar results.
Changes by NNRTI/PI Components
At weeks 24 and 96, by intent-to-treat and as-treated analyses, IL-6 decreased significantly (p ≤ 0.043) in both EFV and ATV/r arms (when NRTIs combined), without significant differences for ATV/r vs. EFV (p ≥ 0.80). There was no interaction between the NNRTI/PI component and HIV-1 RNA stratum at either time point.
Changes in biomarkers adjusted for baseline covariates
The intent-to-treat analysis on change in biomarkers from entry to weeks 24 and 96 were adjusted as detailed in the statistical section. For analyses of the NRTI or NNRTI/PI effect, all the adjusted models, alone and jointly, yielded similar results as the unadjusted analyses for all biomarkers.
Association between baseline factors and changes in biomarkers
Linear regression analyses assessed the association of baseline factors with changes in biomarkers (Table 3). The covariates were the same as those used in adjusted analyses, except for baseline marker level.
Table 3.
Results of the Regression Analyses to Assess the Baseline Factors Associated with 24 and 96 Weeks Changes in all Biomarkers.
| Endpoint | Baseline covariate | 24 Week Change | 96 Week Change | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | ||||||
| Fold Δ% diff. (95% CI) |
p-value | Fold Δ% diff. (95% CI) |
p-value | Fold Δ% diff. (95% CI) |
p-value | Fold Δ% diff. (95% CI) |
p-value | ||
| hsCRP (ug/mL) | ABC/3TC (vs. TDF/FTC) | 61.5 (13.6, 129.5) | 0.008 | 57.8 (9.9, 126.5) | 0.014 | 53.5 (6.9, 120.4) | 0.021 | 48.5 (2.4, 115.3) | 0.037 |
| ATV/r (vs. EFV) | −37.6 (−56.1, −11.2) | 0.009 | −38.3 (−57.1, −11.3) | 0.009 | −19.5 (−44.2, 16.1) | 0.24 | −25.4 (−48.9, 8.8) | 0.13 | |
| Male (vs. female) | −38.6 (−62.5, 0.5) | 0.052 | −24.4 (−55.2, 27.3) | 0.29 | −57.2 (−73.8, −30.1) | 0.001 | −51.3 (−71.8, −16.0) | 0.010 | |
| HIV-1 RNA (log10 copies/mL) | −4.0 (−25.9, 24.4) | 0.76 | −29.6 (−52.0, 3.1) | 0.071 | −0.3 (−24.3, 31.3) | 0.98 | 9.6 (−20.6, 51.5) | 0.58 | |
| CD4 count (50 cells/mm3) | −5.3 (−10.3, −0.1) | 0.046 | −7.1 (−12.7, −1.0) | 0.022 | −3.4 (−8.6, 2.0) | 0.21 | −3.6 (−9.6, 2.9) | 0.27 | |
| ABC/3TC*HIV-1 RNA (log10 copies/mL) | 69.5 (1.7, 182.6) | 0.043 | |||||||
| IL-6 (pg/mL) | ABC/3TC (vs. TDF/FTC) | 28.5 (4.2, 58.4) | 0.019 | 28.6 (2.8, 60.9) | 0.028 | 22.2 (−4.3, 56.1) | 0.11 | 16.3 (−9.2, 49.0) | 0.23 |
| Male (vs. female) | −15.5 (−37.0, 13.4) | 0.26 | −7.7 (−33.2, 27.4) | 0.62 | −44.4 (−60.0, −22.7) | 0.001 | −37.6 (−56.6, −10.3) | 0.011 | |
| HIV-1 RNA (log10 copies/mL) | −18.4 (−29.9, −5.1) | 0.009 | −29.9 (−44.7, −11.2) | 0.003 | −16.1 (−30.2, 0.7) | 0.059 | −1.7 (−20.8, 21.9) | 0.87 | |
| CD4 count (50 cells/mm3) | 1.6 (−1.7, 4.9) | 0.35 | −0.5 (−4.3, 3.5) | 0.81 | 4.9 (1.2, 8.8) | 0.010 | 3.9 (−0.5, 8.5) | 0.082 | |
| BMI (kg/m2) | 0.7 (−1.6, 3.1) | 0.56 | 0.3 (−2.2, 2.9) | 0.83 | 4.4 (1.7, 7.1) | 0.001 | 1.9 (−0.9, 4.8) | 0.19 | |
| Hypertension (vs. none) | 8.2 (−18.9, 44.3) | 0.59 | 4.5 (−23.7, 43.1) | 0.78 | 51.1 (9.5, 108.6) | 0.012 | 32.6 (−5.3, 85.6) | 0.10 | |
| ABC/3TC*HIV-1 RNA (log10 copies/mL) | 36.6 (−0.4, 87.5) | 0.053 | |||||||
| sICAM-1 (ng/mL) | None | None | |||||||
| sTNF-RI (pg/mL) | Race/Ethnicity (vs. white non-Hispanic) | 0.21 | 0.22 | 0.073 | 0.030 | ||||
| Black non-Hispanic | 6.0 (0.2, 12.2) | 0.043 | 5.1 (−0.8, 11.4) | 0.093 | 9.0 (1.9, 16.5) | 0.012 | 9.8 (2.6, 17.5) | 0.007 | |
| Hispanic | 0.9 (−5.9, 8.2) | 0.80 | 4.9 (−2.4, 12.7) | 0.19 | 3.8 (−4.6, 13.0) | 0.38 | 6.9 (−1.8, 16.3) | 0.12 | |
| Other | −1.5 (−15.1, 14.2) | 0.84 | −4.0 (−16.8, 10.8) | 0.58 | −5.4 (−23.7, 17.4) | 0.61 | −5.1 (−22.7, 16.4) | 0.61 | |
| HIV-1 RNA (log10 copies/mL) | −9.5 (−12.5, −6.4) | <0.001 | −7.2 (−11.1, −3.2) | 0.001 | −9.2 (−13.0, −5.2) | <0.001 | −3.9 (−9.7, 2.2) | 0.21 | |
| CD4 count (50 cells/mm3) | 1.5 (0.8, 2.3) | <0.001 | 0.7 (−0.2, 1.6) | 0.12 | 2.0 (1.1, 2.9) | <0.001 | 1.4 (0.4, 2.4) | 0.008 | |
| BMI (kg/m2) | 0.6 (0.1, 1.2) | 0.026 | 0.3 (−0.3, 0.9) | 0.33 | 0.9 (0.2, 1.5) | 0.011 | 0.4 (−0.3, 1.1) | 0.27 | |
| Fasting LDL cholesterol (10 mg/dL) | 1.1 (0.3, 1.9) | 0.008 | 0.7 (−0.1, 1.5) | 0.10 | 1.1 (0.1, 2.1) | 0.028 | 0.7 (−0.3, 1.7) | 0.16 | |
| Hypertension (vs. none) | 3.8 (−2.9, 11.1) | 0.27 | 1.9 (−5.0, 9.3) | 0.59 | 9.6 (1.3, 18.7) | 0.023 | 10.3 (1.9, 19.5) | 0.015 | |
| Family history of CAD (vs. none) | 2.8 (−2.7, 8.7) | 0.32 | 0.7 (−4.7, 6.5) | 0.80 | 7.7 (0.8, 15.2) | 0.029 | 6.3 (−0.6, 13.6) | 0.072 | |
| sTNF-RII (pg/mL) | Age (years) | −0.6 (−1.1, −0.0) | 0.049 | −0.8 (−1.3, −0.2) | 0.008 | −0.8 (−1.4, −0.1) | 0.020 | −1.0 (−1.7, −0.3) | 0.003 |
| HIV-1 RNA (log10 copies/mL) | −22.7 (−27.9, −17.2) | <0.001 | −23.2 (−30.8, −14.8) | <0.001 | −24.1 (−30.3, −17.3) | <0.001 | −18.5 (−26.3, −9.9) | <0.001 | |
| CD4 count (50 cells/mm3) | 3.5 (1.9, 5.1) | <0.001 | 1.0 (−0.8, 2.7) | 0.28 | 4.4 (2.5, 6.2) | <0.001 | 1.6 (−0.4, 3.7) | 0.11 | |
| BMI (kg/m2) | 1.5 (0.3, 2.6) | 0.013 | 1.0 (−0.1, 2.1) | 0.087 | 2.0 (0.7, 3.4) | 0.004 | 1.4 (0.1, 2.8) | 0.037 | |
| Fasting LDL cholesterol (10 mg/dL) | 2.2 (0.5, 4.0) | 0.012 | 1.5 (−0.1, 3.2) | 0.065 | 1.5 (−0.6, 3.6) | 0.15 | 1.0 (−0.9, 2.9) | 0.32 | |
| sVCAM-1 (ng/mL) | ATV/r (vs. EFV) | 1.9 (−6.1, 10.6) | 0.65 | 3.5 (−4.4, 12.0) | 0.40 | 7.8 (−2.3, 19.0) | 0.14 | 10.1 (−0.1, 21.4) | 0.053 |
| HIV-1 RNA (log10 copies/mL) | −15.5 (−20.0, −10.7) | <0.001 | −11.2 (−18.5, −3.2) | 0.007 | −19.1 (−24.4, −13.4) | <0.001 | −19.7 (−26.1, −12.7) | <0.001 | |
| CD4 count (50 cells/mm3) | 2.1 (0.9, 3.4) | 0.001 | 0.2 (−1.1, 1.6) | 0.75 | 2.0 (0.5, 3.5) | 0.009 | −0.5 (−2.2, 1.2) | 0.55 | |
| Fasting LDL cholesterol (10 mg/dL) | 1.4 (0.1, 2.7) | 0.035 | 0.9 (−0.4, 2.2) | 0.17 | 0.7 (−1.0, 2.3) | 0.42 | 0.4 (−1.2, 2.0) | 0.65 | |
| ATV/r* HIV-1 RNA (log10 copies/mL) | −11.0 (−20.4, −0.4) | 0.043 | |||||||
| TNF-α | Age (years) | −0.6 (−1.2, −0.1) | 0.022 | −0.8 (−1.4, −0.2) | 0.006 | −0.5 (−1.2, 0.1) | 0.11 | −0.8 (−1.5, −0.1) | 0.019 |
| HIV-1 RNA (log10 copies/mL) | −13.4 (−19.6, −6.8) | <0.001 | −20.0 (−28.5, −10.4) | <0.001 | −17.5 (−24.6, −9.6) | <0.001 | −20.7 (−28.8, −11.8) | <0.001 | |
| CD4 count (50 cells/mm3) | −0.2 (−1.7, 1.4) | 0.83 | −2.3 (−4.1, −0.5) | 0.015 | 0.6 (−1.3, 2.5) | 0.53 | −2.5 (−4.5, −0.3) | 0.023 | |
| Fasting glucose (10 mg/dL) | 7.3 (1.8, 13.0) | 0.009 | 6.6 (0.8, 12.7) | 0.025 | 9.6 (2.9, 16.7) | 0.004 | 5.4 (−1.5, 12.8) | 0.13 | |
| Hypertension (vs. none) | 5.1 (−8.9, 21.2) | 0.49 | 7.6 (−7.4, 25.1) | 0.34 | 18.4 (0.3, 39.7) | 0.046 | 21.7 (2.9, 43.9) | 0.022 | |
| Family history of CAD (vs. none) | −1.6 (−12.4, 10.6) | 0.79 | 2.7 (−8.9, 15.8) | 0.66 | 10.8 (−3.8, 27.6) | 0.15 | 17.9 (2.2, 35.9) | 0.024 | |
Baseline covariates with p<0.05 at week 24 or 96 in either univariate or multivariable analysis are presented. Interactions with p<0.10 in multivariable analysis are presented. ABC/3TC*HIV-1 RNA interaction was included in the week 24 multivariable model for hsCRP, IL-6, sTNF-RII, and TNF-α. ATV/r*HIV-1 RNA interaction was included in the week 24 multivariable model for sVCAM-1 and the week 96 multivariable model for sTNF-RI.
For CRP and IL-6, by multivariable analysis, in addition to the ABC/3TC and ATV/r effects, lower CD4 count was independently associated with increased 24-week hsCRP change while greater HIV-1 RNA was associated with decreased IL-6. Additionally, a significant interaction between the NRTI component and HIV-1 RNA was seen for hsCRP and IL-6. Specifically, per log10 copies/mL, HIV-1 RNA was associated with a 69.5% (1.7%, 182.6%) and 36.6% (−0.4%, 87.5%) larger mean fold change in ABC/3TC for hsCRP (1.19 vs. 0.70) and IL-6 (0.96 vs. 0.70), respectively. At 96 weeks, for both hsCRP and IL-6, male sex was associated with decreases. For the subset with smoking data (n = 205), smoking was associated with 24 week increases in hsCRP, IL-6.
DISCUSSION
This report details changes in inflammation and endothelial activation markers among subjects randomized to one of four commonly-used ART regimens. As seen in prior studies, we demonstrated that ART initiation led to a decrease in markers of TNF-α activation and endothelial activation, but not hsCRP. We also found that on average with ABC/3TC there were short-term (24 weeks) and longer-term (96 weeks) increases in hsCRP and short term differences in IL-6 compared to TDF/FTC, and that EFV induced increases in hsCRP compared to ATV/r at week 24. Adjusting for potential confounders/imbalances, including virologic efficacy, did not change these results and as-treated analyses yielded similar results to intent-to-treat.
Cardiovascular disease recently emerged as a major cause of morbidity and mortality in HIV. In addition to traditional CVD risk factors, inflammation appears to be a major component of this enhanced risk. So far, this has been shown in mostly cross sectional studies where higher antigen-specific T-cells responses [23], CRP [4,24,25], TNF-α4 and IL-6 [26] have been associated with carotid IMT, and higher CD4+CD38+HLA-DR+ T-cells% with arterial stiffness [27].
Our study supports the findings of earlier studies that initiation of effective ART results in an overall decrease in inflammation markers, with the exception of hsCRP, which remains unchanged or even increases [2,17,28,29]. The reason hsCRP behaves differently from other markers remains elusive, with one potential explanation being HIV-associated subclinical hepatocyte dysfunction, as CRP is mainly produced by hepatocytes in response to IL-6. Results were unchanged after adjusting for several factors known to affect CRP, including hepatitis C [30]. Oral contraceptives have been shown to increase hsCRP [31,32], but excluding the 4 women who were on oral contraceptives (all on EFV; 3 on ABC/3TC) did not modify the results. Likewise, statins were shown to decrease hsCRP [33], but excluding the 23 statin users did not affect hsCRP or IL-6 results. Regardless, hsCRP appears to be an important marker, independently associated with CVD [4,24], HIV progression [34], and mortality [35], even after adjusting for CD4 and HIV-1 RNA. However, it is unclear whether the AHA CVD risk stratification cut-offs established for HIV-uninfected populations [36] are valid in HIV. Fig. 2 (c, d) shows the baseline and week 24 AHA hsCRP risk categories, without apparent shift to higher risk categories in either group.
Only limited data exist regarding the effect of specific ART on inflammation, and most focus on ABC, because of its potential link to increased CVD in some studies [5–9]. Others [11–14] did not find such link, including A5001 [11] that included data from A5202. Studies investigating the effect of ABC on inflammation yielded conflicting results. The INSIGHT group found higher IL-6 and hsCRP, but similar TNF-α, with ABC regimens [5], while the MACS cohort found similar biomarker levels, including IL-6 and hsCRP, in ABC- vs. non-ABC-treated subjects [15]. Moreover, studies of virologically-suppressed individuals who switched their NRTIs to ABC or TDF found no differences in inflammation markers after switching [18,19]. Furthermore, the HEAT study, which prior to the current study was the only randomized study [16] that measured inflammation markers in subjects initiating their first ART regimen with ABC- versus TDF-based therapy, found no difference in these markers between the regimens. However, HEAT investigated only lopinavir/ritonavir-based therapies, whereas our study had both EFV and ATV/r as 3rd drugs, and EFV (but not ATV/r) was found to be associated with hsCRP increase.
We found an interaction between screening HIV-1 RNA stratum and the NRTI effect so that the differences seen between ABC/3TC and TDF/FTC were larger in the high screening viral load stratum than in the lower. This observation may explain the discrepancies seen in prior biomarker studies, and specifically in prior randomized studies of virologically-suppressed subjects where no difference in markers were found between ABC- and TDF-regimens [18,19].
The mechanism by which ABC affects inflammatory pathways is not clear. In one study, ABC induced dose-dependent increases in neutrophil adhesion through the activation of Mac-1, which interacts with its endothelial ligand ICAM-1 [37]. Some have suggested that analogous to other drug toxicity [38], ABC may interfere with purine signaling pathways leading to impairment of lymphocyte activation [39]. A recent study found that ABC up-regulates pro-inflammatory cytokine mRNA transcription by stimulation of Toll-like receptor 8 signaling [40]. Others have suggested that ABC can induce a low-level hypercoagulable state by increasing platelets aggregation due to hyperactive platelets [41], or increase fibrinolytic capacity by raising PAI-1 levels [42].
Although several studies have shown that CD4 cell recovery is similar after initiation of PI vs. NNRTI regimens [43,44], only one study found similar decreases in sTNFR-I and –II [45] with PI vs. NNRTI-regimens, but hsCRP and IL-6 were not measured. Our finding of higher CRP with EFV vs. ATV/r is consistent with a study showing that in adipocytes, EFV induces the release of higher levels of cytokines than PIs [46].
Our study has several limitations, including decreased sample size at week 96, possible selection bias of A5224 s subjects who have available week 24 and/or 96 samples (possibly healthier subjects with lower inflammation), and the large number of analysis performed without adjustment for multiple comparisons, which may increase the risk of a type I error. Although the co-primary endpoints (sTNFR-II and VCAM-1) were chosen because our prior studies showed them to be associated with clinically-relevant outcomes, such as incident diabetes and carotid IMT [4,25,28], other markers chosen as secondary endpoints (e.g. IL-6 and hsCRP) have also been associated with mortality and CVD [24,26].
We have shown in a randomized study a differential treatment effect on some inflammation markers, with differences in hsCRP and IL-6 in those receiving ABC-compared to TDF- and EFV- compared to ATV/r-containing regimens. Further investigations are needed to duplicate these findings and clarify their clinical significance.
Acknowledgements
The project described was supported by Award Numbers U01AI068636, AI068634, AI38855, AI065348 from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. GlaxoSmithKline and Gilead funded the cost of the inflammation marker assays. Study medications were provided by Abbott Pharmaceuticals, Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline.
Acknowledgement Appendix for A5224 s
Sadia Shaik, M.D. and Ruben Lopez, M.D.- Harbor-UCLA Medical Center (Site 603) CTU Grant #:AI0694241,UL1-RR033176
Susan L. Koletar, MD and Diane Gochnour, RN- The Ohio State University Medical Center (Site 2301) CTU Grant # AI069474
Geyoul Kim, RN and Mark Rodriguez, RN- Washington University (Site 2101) CTU Grant #:U01AI069495; GCRC Grant: UL1 RR024992
Elizabeth Lindsey, RN and Tamara James, BS - Alabama Therapeutics CRS (Site 5801) CTU Grant #: U01 AI069452
Ann C. Collier, MD and Jeffrey Schouten, MD, JD-University of Washington (Site 1401) CTU Grant #: AI069434; UL1 RR025014
Jorge L. Santana Bagur,MD and Santiago Marrero, MD-Puerto Rico-AIDS Clinical Trials Unit (Site 5401) CTU Grant # 5 U0I AI069415-03
Jenifer Baer, RN, BSN and Carl Fichtenbaum, MD-University of Cincinnati (Site 2401) CTU Grant # AI069513
Patricia Walton BSN RN and Barbara Philpotts BSN RN- Case Western Reserve (Site 2501) CTU Grant #: AI69501
Princy Kumar, M.D. and Joseph Timpone, M.D.-Georgetown University (Site 1008) CTU Grant#: ACTG grant # 5U01AI069494
Donna Pittard RN BSN and David Currin RN-University of North Carolina (Site 3201) CTU Grant #: 5-U01 AI069423-03; UNC CFAR #: P30 AI050410(-11); UNC CTRC #: UL 1RR 025747
Julie Hoffman, R.N. and Edward Seefried, R.N.- San Diego Medical Center UC (Site 701) CTU Grant # AI69432
Susan Swindells MBBS and Frances Van Meter APRN-University of Nebraska (Site 1505) CTU Grant #: AI 27661
Deborah McMahon, MD and Barbara Rutecki, MSN, MPH, CRNP- University of Pittsburgh (Site 1001) CTU Grant #: 1 U01 AI069494-01
Michael P. Dube, M.D. and Martha Greenwald, R.N., M.S.N- Indiana University (Site 2601) CTU Grant #: 5U01AI025859; GCRC #: M01 RR00750
Ilene Wiggins, RN, and Eric Zimmerman, RN- Johns Hopkins University (Site 201) CTU Grant #: AI27668; CTSA Grant # UL1 RR025005
Judith. Aberg, M.D. and Margarita Vasquez R.N.- New York University/NYC HHC at Bellevue Hospital Center (Site 401) CTU Grant #: AI27665, New grant number: AI069532
Martin McCarter and M. Graham Ray, R.N., M.S.N. - Colorado AIDS Clinical Trials Unit, (Site 6101) CTU Grant # AI69450; RR025780
Mamta Jain, MD -PI and Tianna Petersen, MS-University of Texas Southwestern Medical Center (Site 3751) CTU Grant #: 3U01AI046376-05S4
Emily Stumm, BS and Pablo Tebas MD- University of Pennsylvania, Philadelphia (Site 6201)CTUGrant #: P30-AI0450008-11; CFAR Grant #: UO1-AI069467-04
Mary Albrecht, MD and Neah Kim, NP- Beth Israel Deaconess (Partners/Harvard) CRS (Site 103) CTU Grant # U01 AI069472-04
Paul Edward Sax, M.D. and Joanne Delaney RN-Brigham andWomen’s Hospital (Site 107) CTU Grant # UOI AI 069472
Christine Hurley, RN and Roberto Corales, DO- AIDS Care (Site 1108) CTU Grant #: U01AI069511-02 (as of 2/12/08); GCRC: UL1 RR 024160
Keith Henry, MD and Bette Bordenave, RN- Hennepin County Medical Center (Site 1502) CTU Grant #: N01 AI72626
Wendy Armstrong, MD and Ericka R. Patrick, RN, MSN, CCRC- Emory University HIV/AIDS Clinical Trails Unit (Site 5802) CTU Grant #: UO1Al69418-01/CFAR Grant Number: P30Al050409
Jane Reid RNC MS and Mary Adams RN MPh-University of Rochester (Site 1101) CTU Grant #: U01AI069511-02 (as of 2/12/08); GCRC: UL1 RR 024160
Gene D. Morse, Pharm.D., FCCP, BCPS- SUNY - Buffalo, Erie County Medical Ctr. (Site 1102) CTU Grant # AI27658
Michael P. Dube, M.D. and Martha Greenwald, R.N., M.S.N- Wishard Memorial Hospital Indiana University (Site 2603) CTU Grant #: 5U01AI025859; GCRC #: M01 RR00750
Kimberly Y. Smith, MD, MPH and Joan A. Swiatek, APN- Rush University Medical Center (Site 2702) CTU Grant #: U01 AI069471
Nancy Hanks, RN, and Debra Ogata-Arakaki, RN, - University of Hawaii at Manoa, Leahi Hospital (Site 5201) CTU Grant # AI34853
Ardis Moe, MD and Maria Palmer PA-C- UCLA Medical Center (Site 601) CTU Grant
1U01AI069424-01
Jeffery Meier, M.D. and Jack T. Stapleton, M.D. - University of Iowa Hospitals and Clinics (Site 1504) CTU Grant #: UL1RR024979
Gary Matthew Cox, MD and Martha Silberman, RN-Duke University Medical Center Adult CRS (Site 1601) CTU Grant # 5U01 AI069 484-02
2705 - Cook County Hospital
Gerianne Casey, RN and William O’Brien MD-University of Texas, Galveston (Site 6301) CTU Grant # AI32782
Valery Hughes, FNP and Todd Stroberg, RN- Cornell CRS (Site 7803, 7804) – CTU Grant#: U01 AI069419; CTSC #: UL1 RR024996
Nyef El-Daher MD -McCree McCuller Wellness Center at the Connection (Site 1107) CTU Grant #: U01AI069511-02 (as of 2/12/08); GCRC: UL1 RR 024160
Rebecca J. Basham, B.S. and Husamettin Erdem, M.D.-Vanderbilt Therapeutics CRS (Site 3652) CTU Grant #: AI46339-01; MO1 RR 00095
Footnotes
This work was presented at the at the 19th Conference on Retroviruses and Opportunistic Infections, March 5–8, Seattle, WA
Role of authors: All authors played a role in editing the manuscript and approved the text as submitted. Grace A McComsey designed the study and wrote the manuscript. Paul Sax and Eric Daar assisted in the design of the study, reviewed and edited the manuscript. Douglas Kitch and Camlin Tierney performed the data analysis and assisted in the interpretation of statistical data. Kathleen Melbourne, Nasreen Jahed, Anthony Bloom, Belinda Ha, and Todd Brown reviewed and edited the manuscript. Neal Fedarko ran the biomarker assays, and reviewed and edited the manuscript. Data in this manuscript were collected by AIDS Clinical Trials Group. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest
Potential conflicts: Grace A McComsey has served as a scientific advisor or speaker for Bristol Myers Squibb, GlaxoSmithKline, Tibotec, and Gilead Sciences, has received research grants from Bristol Myers Squibb, GlaxoSmithKline, and Gilead Sciences, and is currently serving as the DSMB Chair for a Pfizer-sponsored study. Douglas Kitch has no conflict. Eric S. Daar serves as a consultant for Bristol Myers Squibb, Gilead, GlaxoSmithKline, Merck, ViiV and receives research grant support from Abbott Laboratories, Merck, Gilead, ViiV, and Pfizer. Camlin Tierney is a member of a DSMB for Tibotec. Nasreen Jahed has no conflict. Kathleen Melbourne is an employee of Gilead Sciences and owns stock in Gilead Sciences. Belinda Ha is an employee of GlaxoSmithKline. Todd Brown has served as a scientific consultant for Bristol Myers Squibb, GlaxoSmithKline, Abbott, Tibotec, and Gilead Sciences, has received research grants from GlaxoSmithKline and Merck. Neal Fedarko has no conflict. Anthony Bloom has no conflict. Paul Sax serves as a consultant for Abbott, BMS, Gilead, GSK, Merck, Tibotec, and ViiV and receives grant Support from Gilead, Merck, and Tibotec.
References
- 1.Aukrust P, Muller F, Lien E, Nordoy I, Liabakk NB, Kvale D, et al. Tumor necrosis factor (TNF) system levels in human immunodeficiency virus-infected patients during highly active antiretroviral therapy: persistent TNF activation is associated with virologic and immunologic treatment failure. J Infect Dis. 1999;179:74–82. doi: 10.1086/314572. [DOI] [PubMed] [Google Scholar]
- 2.Henry K, Kitch D, Dube M, Zackin R, Parker RA, Sprecher D, et al. C-Reactive protein levels over time and cardiovascular risk in HIV-infected individuals suppressed on an indinavir-based regimen: AIDS Clinical Trials Group 5056 s. AIDS. 2004;18:2434–2437. [PubMed] [Google Scholar]
- 3.Ross AC, Armentrout R, O’Riordan MA, Storer N, Rizk N, Harrill D, et al. Endothelial activation markers are linked to HIV status and are independent of antiretroviral therapy and lipoatrophy. J Acquir Immune Defic Syndr. 2008;49:499–506. doi: 10.1097/QAI.0b013e318189a794. PMCID:2778267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross AC, Rizk N, O’Riordan MA, Dogra V, El-Bejjani D, Storer N, et al. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis. 2009;49:1119–1127. doi: 10.1086/605578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lundgren J, Neuhaus J, Babiker A, Cooper DA, Duprez D, El-Sadr W, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients; Strategies for Management of Anti-Retroviral Therapy/INSIGHT; DAD Study Study Groups. AIDS. 2008;22:F12–F24. doi: 10.1097/QAD.0b013e32830fe35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Worm SW, Sabin C, Weber R, Reiss P, El-Sadr W, Dabis F, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201:318–330. doi: 10.1086/649897. [DOI] [PubMed] [Google Scholar]
- 7.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec’s public health insurance database (RAMQ) J Acquir Immune Defic Syndr. 2011 doi: 10.1097/QAI.0b013e31821d33a5. [DOI] [PubMed] [Google Scholar]
- 8.Choi AI, Vittinghoff E, Deeks SG, Weekley CC, Li Y, Shlipak MG. Cardiovascular risks associated with abacavir and tenofovir exposure in HIV-infected persons. AIDS. 2011;25:1289–1298. doi: 10.1097/QAD.0b013e328347fa16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obel N, Farkas DK, Kronborg G, Larsen CS, Pedersen G, Riis A, et al. Abacavir and risk of myocardial infarction in HIV-infected patients on highly active antiretroviral therapy: a population-based nationwide cohort study. HIV Med. 2010;11:130–136. doi: 10.1111/j.1468-1293.2009.00751.x. [DOI] [PubMed] [Google Scholar]
- 10.Sabin CA, Worm SW, Weber R, Reiss P, El-Sadr W, Dabis F, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet. 2008;371:1417–1426. doi: 10.1016/S0140-6736(08)60423-7. PMCID:2688660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribaudo HJ, Benson CA, Zheng Y, Koletar SL, Collier AC, Lok JJ, et al. No risk of myocardial infarction associated with initial antiretroviral treatment containing abacavir: short and long-term results from ACTG A5001/ALLRT. Clin Infect Dis. 2011;52:929–940. doi: 10.1093/cid/ciq244. PMCID:3062545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brothers CH, Hernandez JE, Cutrell AG, Curtis L, Ait-Khaled M, Bowlin SJ, et al. Risk of myocardial infarction and abacavir therapy: no increased risk across 52 GlaxoSmithKline-spon-sored clinical trials in adult subjects. J Acquir Immune Defic Syndr. 2009;51:20–28. doi: 10.1097/QAI.0b013e31819ff0e6. [DOI] [PubMed] [Google Scholar]
- 13.Lang S, Mary-Krause M, Cotte L, Gilquin J, Partisani M, Simon A, et al. Impact of individual antiretroviral drugs on the risk of myocardial infarction in human immunodeficiency virus-infected patients: a case-control study nested within the French Hospital Database on HIV ANRS cohort CO4. Arch Intern Med. 2010;170:1228–1238. doi: 10.1001/archinternmed.2010.197. [DOI] [PubMed] [Google Scholar]
- 14.Cruciani M, Zanichelli V, Serpelloni G, Bosco O, Malena M, Mazzi R, et al. ABACAVIR use and cardiovascular disease events: a meta-analysis of published and unpublished data. AIDS. 2011 doi: 10.1097/QAD.0b013e328349c6ee. [DOI] [PubMed] [Google Scholar]
- 15.Palella FJ, Jr, Gange SJ, Benning L, Jacobson L, Kaplan RC, Landay AL, et al. Inflammatory biomarkers and abacavir use in the Women’s Interagency HIV Study and the Multicenter AIDS Cohort Study. AIDS. 2010;24:1657–1665. doi: 10.1097/QAD.0b013e3283389dfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McComsey G, Smith K, Patel P, Bellos N, Sloan L, Lackey P, et al., editors. Similar Reductions in Markers of Inflammation and Endothelial Activation after Initiation of Abacavir/Lamivudine or Tenofovir/Emtricitabine: The HEAT Study. 16th Conference on Retroviruses and Opportunistic Infections; 2009 February; Montreal, Canada. [Google Scholar]
- 17.Shikuma CM, Ribaudo HJ, Zheng Y, Gulick RM, Meyer WA, Tashima KT, et al. Change in high-sensitivity c-reactive protein levels following initiation of efavirenz-based antiretroviral regimens in HIV-infected individuals. AIDS Res Hum Retroviruses. 2011;27:461–468. doi: 10.1089/aid.2010.0154. PMCID:3083724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin A, Amin J, Cooper DA, Carr A, Kelleher AD, Bloch M, et al. Abacavir does not affect circulating levels of inflammatory or coagulopathic biomarkers in suppressed HIV: a randomized clinical trial. AIDS. 2010;24:2657–2663. doi: 10.1097/QAD.0b013e32833f147f. [DOI] [PubMed] [Google Scholar]
- 19.Martinez E, Larrousse M, Podzamczer D, Perez I, Gutierrez F, Lonca M, et al. Abacavir-based therapy does not affect biological mechanisms associated with cardiovascular dysfunction. AIDS. 2010;24:F1–F9. doi: 10.1097/QAD.0b013e32833562c5. [DOI] [PubMed] [Google Scholar]
- 20.Sax PE, Tierney C, Collier AC, Fischl MA, Mollan K, Peeples L, et al. Abacavir-lamivudine versus tenofovir-emtricitabine for initial HIV-1 therapy. N Engl J Med. 2009;361:2230–2240. doi: 10.1056/NEJMoa0906768. PMCID:2800041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McComsey G, Kitch D, Daar E, Tierney C, Jahed N, Tebas P, et al. Bone mineral density and fractures in antiretroviral-naïve subjects randomized to abacavir/lamivudine or tenofovir disoproxil fumarate /emtricitabine along with efavirenz or atazanavir/ritonavir: AIDS Clinical Trials Group A5224 s, a substudy of ACTG A5202. Journal of Infectious Diseases. 2011 doi: 10.1093/infdis/jir188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McComsey G, Kitch D, Sax PE, Tebas P, Tierney C, Jahed N, et al. Peripheral and central fat Changes in Subjects Randomized to Abacavir/Lamivudine or Tenofovir/Emtricitabine with Atazanavir/Ritonavir or Efavirenz: ACTG study A5224 s. CID. 2011 doi: 10.1093/cid/cir324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsue PY, Hunt PW, Sinclair E, Bredt B, Franklin A, Killian M, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS. 2006;20:2275–2283. doi: 10.1097/QAD.0b013e3280108704. [DOI] [PubMed] [Google Scholar]
- 24.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51:268–273. doi: 10.1097/QAI.0b013e3181a9992c. PMCID:2763381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross AC, O’Riordan MA, Storer N, Dogra V, McComsey GA. Heightened inflammation is linked to carotid intima-media thickness and endothelial activation in HIV-infected children. Atherosclerosis. 2010;211:492–498. doi: 10.1016/j.atherosclerosis.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. PMCID:2570418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 2011;217:207–213. doi: 10.1016/j.atherosclerosis.2011.03.011. PMCID:3139014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care. 2010;33:2244–2249. doi: 10.2337/dc10-0633. PMCID:2945167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar P, De Jesus E, Huhn G, et al., editors. Abacavir/lamivudine with fosamprenavir/ritonavir or efavirenz in underrepresented, antiretroviral-naïve HIV–infected subjects (SUPPORT): Tolerability, safety, and efficacy after 24 weeks. 18th International AIDS Conference; 2010 July 18–23; Vienna, Austria. [Google Scholar]
- 30.Reingold J, Wanke C, Kotler D, Lewis C, Tracy R, Heymsfield S, et al. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. J Acquir Immune Defic Syndr. 2008;48:142–148. doi: 10.1097/QAI.0b013e3181685727. PMCID:2561207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buchbinder S, Kratzsch J, Fiedler GM, Yar V, Brugel M, Leichtle A, et al. Body weight and oral contraceptives are the most important modulators of serum CRP levels. Scand J Clin Lab Invest. 2008;68:140–144. doi: 10.1080/00365510701487727. [DOI] [PubMed] [Google Scholar]
- 32.Cauci S, Di Santolo M, Culhane JF, Stel G, Gonano F, Guaschino S. Effects of third-generation oral contraceptives on high-sensitivity C-reactive protein and homocysteine in young women. Obstet Gynecol. 2008;111:857–864. doi: 10.1097/AOG.0b013e31816a2476. [DOI] [PubMed] [Google Scholar]
- 33.Aslangul E, Fellahi S, Assoumou LK, Bastard JP, Capeau J, Costagliola D. High-sensitivity C-reactive protein levels fall during statin therapy in HIV-infected patients receiving ritonavir-boosted protease inhibitors. AIDS. 2011;25:1128–1131. doi: 10.1097/QAD.0b013e328346be29. [DOI] [PubMed] [Google Scholar]
- 34.Lau B, Sharrett AR, Kingsley LA, Post W, Palella FJ, Visscher B, et al. C-reactive protein is a marker for human immunodeficiency virus disease progression. Arch Intern Med. 2006;166:64–70. doi: 10.1001/archinte.166.1.64. [DOI] [PubMed] [Google Scholar]
- 35.Feldman JG, Goldwasser P, Holman S, DeHovitz J, Minkoff H. C-reactive protein is an independent predictor of mortality in women with HIV-1 infection. J Acquir Immune Defic Syndr. 2003;32:210–214. doi: 10.1097/00126334-200302010-00014. [DOI] [PubMed] [Google Scholar]
- 36.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 37.De Pablo C, Orden S, Apostolova N, Blanquer A, Esplugues JV, Alvarez A. Abacavir and didanosine induce the interaction between human leukocytes and endothelial cells through Mac-1 upregulation. AIDS. 2010;24:1259–1266. doi: 10.1097/QAD.0b013e32833a2b02. [DOI] [PubMed] [Google Scholar]
- 38.Morabito L, Montesinos MC, Schreibman DM, Balter L, Thompson LF, Resta R, et al. Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5’-nucleotidase-mediated conversion of adenine nucleotides. J Clin Invest. 1998;101:295–300. doi: 10.1172/JCI1554. PMCID:508567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marchetti G, Casana M, Tincati C, Bellistri GM, Monforte A. Abacavir and cardiovascular risk in HIV-infected patients: does T lymphocyte hyperactivation exert a pathogenic role? Clin Infect Dis. 2008;47:1495–1496. doi: 10.1086/593110. [DOI] [PubMed] [Google Scholar]
- 40.MacLeod I, Rowley C, Essex M, editors. Molecular Basis for the Immunostimulatory Activity of Abacavir via TLR-8. The 19th Conference on Retroviruses and Opportunistic Infections; 2012 March 5–8; Seattle, WA. [Google Scholar]
- 41.Satchell CS, Cotter AG, O’Connor EF, Peace AJ, Tedesco AF, Clare A, et al. Platelet function and HIV: a case-control study. AIDS. 2010;24:649–657. doi: 10.1097/QAD.0b013e328336098c. [DOI] [PubMed] [Google Scholar]
- 42.Padilla S, Masia M, Garcia N, Jarrin I, Tormo C, Gutierrez F. Early changes in inflammatory and pro-thrombotic biomarkers in patients initiating antiretroviral therapy with abacavir or tenofovir. BMC Infect Dis. 2011;11:40. doi: 10.1186/1471-2334-11-40. PMCID:3042932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Landay AL, Spritzler J, Kessler H, Mildvan D, Pu M, Fox L, et al. Immune reconstitution is comparable in antiretroviral-naive subjects after 1 year of successful therapy with a nucleoside reverse-transcriptase inhibitor- or protease inhibitor-containing antiretroviral regimen. J Infect Dis. 2003;188:1444–1454. doi: 10.1086/379041. [DOI] [PubMed] [Google Scholar]
- 44.Samri A, Goodall R, Burton C, Imami N, Pantaleo G, Kelleher A, et al. Three-year immune reconstitution in PI-sparing and PI-containing antiretroviral regimens in advanced HIV-1 disease. Antivir Ther. 2007;12:553–558. [PubMed] [Google Scholar]
- 45.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51:554–561. doi: 10.1097/QAI.0b013e3181adce44. [DOI] [PubMed] [Google Scholar]
- 46.Gallego-Escuredo JM, Del Mar Gutierrez M, Diaz-Delfin J, Domingo JC, Mateo MG, Domingo P, et al. Differential effects of efavirenz and lopinavir/ritonavir on human adipocyte differentiation, gene expression and release of adipokines and pro-inflammatory cytokines. Curr HIV Res. 2010;8:545–553. doi: 10.2174/157016210793499222. [DOI] [PubMed] [Google Scholar]