Abstract
Systemic and oral clearances of alfentanil are in vivo probes for hepatic and first-pass CYP3A. Both alfentanil single point plasma concentrations and miosis are surrogates for area under the concentration-time curve (AUC), clearance, and are minimal and non-invasive CYP3A probes. This investigation determined alfentanil sensitivity for detecting graded CYP3A induction, and compared it with that of midazolam Twelve volunteers (sequential crossover) received 0, 5, 10, 25 or 75 mg oral rifampin for 5d. Midazolam and alfentanil were given intravenously and orally on sequential days. Dark-adapted pupil diameters were measured with blood sampling. Graded rifampin decreased plasma midazolam AUCs to 83, 76, 62 and 59% (intravenous) and 78, 66, 39, and 24% (oral) of control. Hepatic and first-pass CYP3A induction were detected comparably by plasma midazolam and alfentanil AUCs. Single alfentanil concentrations detected all CYP3A induction, while midazolam was less sensitive. Alfentanil miosis detected induction of first-pass but not hepatic CYP3A.
Keywords: alfentanil, midazolam, cytochrome P450 3A, CYP3A, in vivo probe, phenotyping
Cytochrome P450 (CYP) 3A subfamily enzymes metabolize >50% of all drugs, are a major determinant of systemic and first-pass drug clearance in adults and children, and exhibit marked inter- and intra-individual differences as a result of variable expression, genetic polymorphism, and sensitivity to drug and dietary interactions, resulting in significant alternations in drug bioavailability, clearance and clinical effect, and challenges to developing and safely using CYP3A drugs.1 Identifying an ideal in vivo probe for assessing (phenotyping) human hepatic and intestinal CYP3A activity, predicting CYP3A-dependent drug metabolism, and individualized dosing of CYP3A drugs with narrow therapeutic indices remains an ongoing effort.2–6 Food and Drug Administration regulations for new drug development require clinical assessment of potential drug interactions, and preferably, their consequences. Additionally, there is clinical interest in using CYP3A activity to predict optimal CYP3A drug dosing, improve therapeutic efficacy, and minimize adverse drug effects.7,8
The CYP3A substrates alfentanil (ALF) and midazolam (MDZ) possess most characteristics of an ideal probe.2,9 Both are “sensitive substrates”,* are metabolized exclusively by CYP3A4 and CYP3A5 (and henceforth will be referred to jointly as CYP3A probes), neither are P-glycoprotein substrates, and they have similar intestinal extraction, although MDZ hepatic extraction is approximately twice that of ALF.10,11 MDZ clearance is the most widely used CYP3A probe. Surrogate approaches for MDZ clearance have also been used, such as limited sampling strategies of reduced or single-point plasma concentrations or metabolite:parent ratios, with widely varying accuracy, success and enthusiasm for their clinical utility.12–16 MDZ clearance, and its surrogates, may be influenced by hepatic blood flow as well as by CYP3A-dependent metabolism,17,18 thus MDZ may not be an optimal CYP3A probe. ALF may have some advantages compared with MDZ, due to a shorter half-life and thus also briefer sampling periods, and ALF clearance is not influenced by hepatic blood flow.19,20 ALF intravenous and oral clearances have been used to probe hepatic, intestinal and first-pass CYP3A activities.10,11,14,21–25 Additionally, single-point ALF plasma concentrations have been used as a surrogate for ALF clearance, and to assess hepatic and first-pass CYP3A activities.14,22 Moreover, the decrease in dark-adapted pupil diameter (miosis) caused by ALF is a surrogate for ALF plasma concentrations, and ALF miosis is a suitable noninvasive in vivo probe for both hepatic and first-pass CYP3A activities.10,11,22
Current recommendations are that inhibitory in vivo probes should be sufficiently sensitive to detect a 20–50% decrease in first-pass CYP3A activity (≥1.25-fold increase in oral MDZ AUC) as the clinically important threshold.8,* A previous investigation demonstrated the sensitivity of ALF and MDZ clearances, single-point concentrations, and ALF miosis, for detecting hepatic and first-pass CYP3A inhibition.22 However, the sensitivity of ALF for detecting CYP3A induction remains unknown. While CYP3A induction by rifampin (600 mg for 5d) significantly increased intravenous and oral MDZ clearances,10,26 and intravenous and oral ALF plasma clearances and oral ALF effect clearance,10 this was nonetheless not a stringent test of a CYP3A induction probe because the induction was so profound. Therefore the primary goal of this investigation was to determine the sensitivity of ALF clearance, single-point plasma concentrations, and miosis in detecting and quantifying CYP3A induction, and in comparison with MDZ clearance and single-point concentrations. This investigation of sensitivity to graded CYP3A induction complements the previous study of sensitivity to graded CYP3A inhibition.22 The target was ~20–80% decreases in the plasma MDZ concentration AUC ratio (rifampin/control) for oral MDZ.
Results
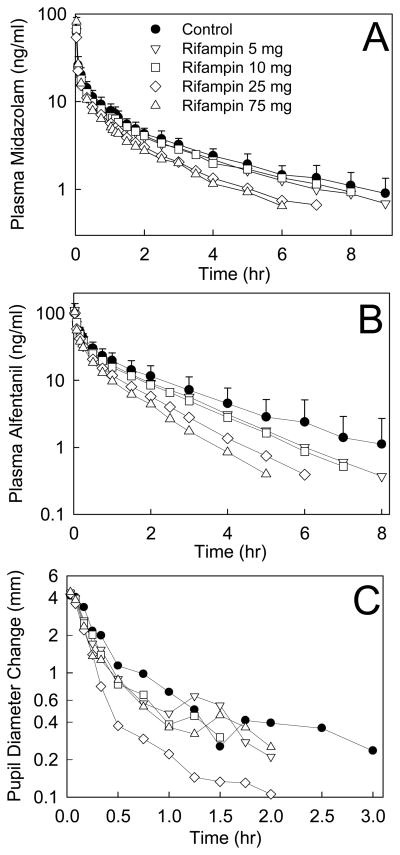
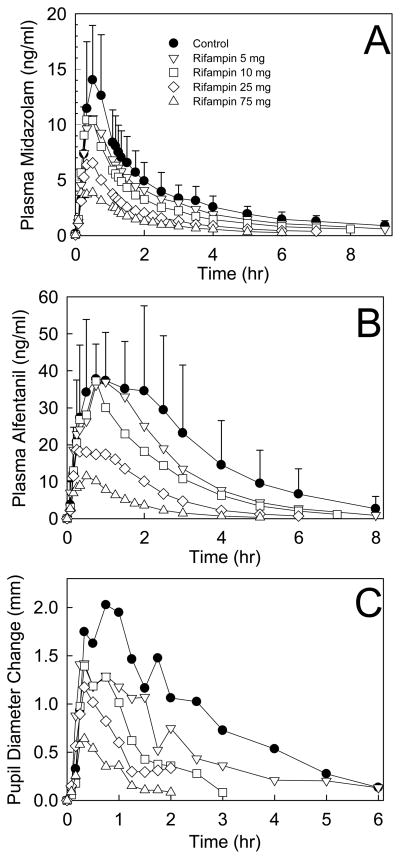
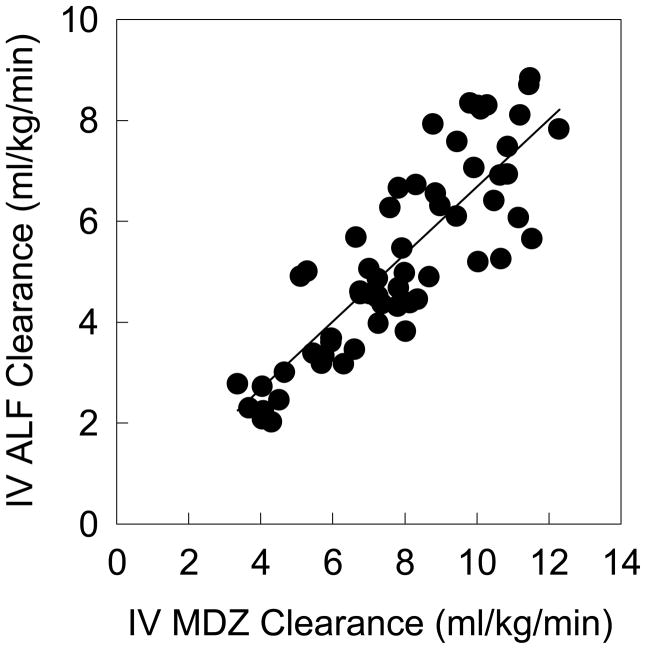
Increasing doses of rifampin progressively reduced MDZ and ALF plasma concentrations and ALF clinical effects (Figure 1 and Table 1; Figure 2 and Table 2, for IV and oral probes, respectively). MDZ systemic clearance and hepatic extraction (EH) were increased 21, 29, 58, and 66% by 5, 10, 25, and 75 mg rifampin, and ALF clearance and EH were increased 19, 32, 70, and 92%. These rifampin doses increased oral MDZ CL/F 23, 45, 246 and 415%, and ALF CL/F 33, 77, 377 and 826%. MDZ intestinal extraction (EG) was only increased (28%) by 75 mg rifampin, and that of ALF by 25 (33%) and 75 mg (63%). Bioavailability (Foral) of ALF was reduced to 78, 51, and 24% of control by 10, 25 and 75 mg rifampin, while that of MDZ was unchanged, and decreased to 62 and 38% of control, respectively. Significant correlations were found between MDZ and ALF disposition, for CLIV, CL/F, EH, Foral, and EG, (shown in Figure 3 for CLIV, and other correlation coefficients provided in the figure legend).
Figure 1.
Effect of graded CYP3A induction on (A) MDZ plasma concentration, (B) ALF plasma concentration, and (C) ALF miosis, after 1 mg IV MDZ and 15 μg/kg IV ALF. Results are the mean ± SD (n=12). Some SD are omitted for clarity.
Table 1.
Intravenous midazolam and alfentanil pharmacokinetic and pupil effect parameters
| Control | Rifampin
|
||||
|---|---|---|---|---|---|
| 5 mg | 10 mg | 25 mg | 75 mg | ||
| IV midazolam | |||||
| Cmax (ng/ml) | 62 ± 29 | 64 ± 26 | 66 ± 19 | 52 ± 23 | 82 ± 34 |
| AUC0-∞ (ng •hr •ml−1) | 40.9 ± 7.6 | 34.0 ± 6.5* | 30.7 ± 4.5* | 25.2 ± 3.6* | 24.1 ± 4.5* |
| AUC0-∞ ratio | 0.84± 0.13* | 0.77 ± 0.12* | 0.63 ± 0.11* | 0.60 ± 0.12* | |
| (geometric mean, 90% CI) | 0.83 (0.77, 0.89) | 0.76 (0.71, 0.83) | 0.62 (0.57, 0.68) | 0.59 (0.53,0.65) | |
| CLIV (ml•kg−1•min−1) | 5.9 ± 1.6 | 7.1 ± 2.1* | 7.6 ± 2.0* | 9.3 ± 1.8* | 9.8 ± 1.6* |
| Elimination t1/2 (hr) | 4.0 ± 2.0 | 3.0 ± 0.8* | 2.8 ± 1.0* | 2.6 ± 1.1* | 1.9 ± 0.5* |
| Vss (L/kg) | 1.4 ± 0.5 | 1.3 ± 0.4 | 1.3 ± 0.4 | 1.5 ± 0.6 | 1.1 ± 0.3 |
| EH | 0.38 ± 0.09 | 0.46 ± 0.11* | 0.49 ± 0.10* | 0.60 ± 0.09* | 0.63 ± 0.09* |
| C4 hr (ng/ml) | 2.4 ± 0.5 | 2.2 ± 0.5 | 2.0 ± 1.3 | 1.3 ± 0.4* | 0.2 ± 0.4* |
| C4 hr ratio | 0.88 ± 0.22 | 0.80 ± 0.36 | 0.56 ± 0.13* | 0.48 ± 0.10* | |
| (geometric mean, 90% CI) | 0.86 (0.76,0.96) | 0.75 (0.63,0.89) | 0.55 (0.49,0.61) | 0.47 (0.43,0.52) | |
| C5 hr (ng/ml) | 1.9 ± 0.6 | 1.6 ± 0.4 | 1.7 ±1.1 | 1.0 ± 0.2* | 0.9 ± 0.4* |
| C5 hr ratio | 0.87 ± 0.23 | 0.90 ± 0.48 | 0.55 ± 0.13* | 0.50 ± 0.17* | |
| (geometric mean, 90% CI) | 0.84 (0.74,0.96) | 0.82 (0.68,1.0) | 0.54 (0.48,0.61) | 0.47 (0.41,0.55) | |
| IV alfentanil | |||||
| Cmax (ng/ml) | 102 ± 37 | 106 ± 35 | 107 ± 23 | 102 ± 27 | 105 ± 26 |
| AUC0-∞ (ng •hr •ml−1) | 78.0 ± 30.7 | 61.7 ± 19.4* | 55.5 ± 16.7* | 44.0 ± 15.9* | 37.0 ± 8.5* |
| AUC0-∞ ratio | 0.83 ± 0.16* | 0.75 ± 0.15* | 0.59 ± 0.12* | 0.51 ± 0.12* | |
| (geometric mean, 90% CI) | 0.81 (0.74,0.90) | 0.73 (0.66,0.82) | 0.57 (0.52, 0.63) | 0.50 (0.44,0.56) | |
| CLIV (ml•kg−1•min−1) | 3.7 ± 1.4* | 4.4 ± 1.2* | 4.9 ± 1.4* | 6.3 ± 1.8* | 7.1 ± 1.4* |
| Elimination t1/2 (hr) | 1.4 ± 0.5 | 1.2 ± 0.3* | 1.1 ± 0.2* | 1.0 ± 0.2* | 0.8 ± 0.2* |
| Vss (L/kg) | 0.35 ± 0.06 | 0.35 ± 0.03 | 0.36 ± 0.03 | 0.39 ± 0.09 | 0.38 ± 0.05 |
| EH | 0.24 ± 0.09* | 0.28 ± 0.08* | 0.32 ± 0.09* | 0.40 ± 0.11* | 0.46 ± 0.09* |
| C2 hr (ng/ml) | 11.8 ± 5.1 | 8.9 ± 3.5* | 8.2 ± 3.4* | 5.8 ± 3.4* | 4.5 ± 1.8* |
| C2 hr ratio | 0.82 ± 0.27* | 0.74 ± 0.23* | 0.51 ± 0.17* | 0.42 ± 0.13* | |
| (geometric mean, 90% CI) | 0.78 (0.68,0.91) | 0.71 (0.62,0.82) | 0.48 (0.41,0.57) | 0.40 (0.34,0.46) | |
| C4 hr (ng/ml) | 4.4 ± 3.2 | 3.1 ± 2.2* | 2.7 ± 1.9* | 1.4 ± 1.1* | 0.8 ± 0.4* |
| C4 hr ratio | 0.77 ± 0.28* | 0.72 ± 0.51* | 0.36 ± 0.15* | 0.24 ± 0.15* | |
| (geometric mean, 90% CI) | 0.73 (0.62,0.86) | 0.62 (0.49,0.80) | 0.34 (0.29,0.40) | 0.21 (0.17,0.27) | |
| IV alfentanil pupil miosis | |||||
| Maximum (mm) | 4.3 ± 0.8 | 4.5 ± 0.8 | 4.4 ± 0.8 | 4.4 ± 0.8 | 4.5 ± 0.9 |
| AUEC0-last (mm•hr) | 2.8 ± 2.7 | 2.8 ± 2.3 | 1.7 ± 0.9 | 1.3 ± 1.0 | 2.1 ± 1.4 |
| AUEC0-∞ (mm•hr) | 3.7 ± 5.0 | 3.9 ± 4.0 | 2.0 ± 1.2 | 1.5 ± 1.2 | 2.5 ± 1.8 |
| AUEC0-∞ ratio | 1.6 ± 1.9 | 1.0 ± 0.8 | 1.0 ± 1.0 | 1.2 ± 1.0 | |
| (geometric mean, 90% CI) | 1.1 (0.8, 1.6) | 0.74 (0.51, 1.08) | 0.53 (0.30,0.94) | 0.83 (0.49,1.40) | |
All subjects received 1 mg IV MDZ, followed by 15 μg/kg IV ALF. Results are the arithmetic mean ± SD, except AUC ratios and single-point concentration ratios, which are also shown as the geometric mean and 90% confidence interval. Cmax, maximum concentration; AUC0-∞, area under plasma concentration–time curve extrapolated to infinity; CLIV, systemic clearance; t1/2, half-life; Vss, steady-state volume of distribution; EH, hepatic extraction; C4 hr and C5 hr, plasma concentration 4 or 5 hr after dosing; AUEC0-last, area under effect (miosis) curve to last time point measured; AUEC0-∞, area under effect (miosis) curve extrapolated to infinity.
Significantly different from control (p<0.05)
Figure 2.
Effect of graded CYP3A induction on (A) MDZ plasma concentration, (B) ALF plasma concentration, and (C) ALF miosis, after 3 mg oral MDZ and 75 μg/kg oral ALF. Results are the mean ± SD (n=12). Some SD are omitted for clarity.
Table 2.
Oral midazolam and alfentanil pharmacokinetic and pupil effect parameters
| Control | rifampin
|
||||
|---|---|---|---|---|---|
| 5 mg | 10 mg | 25 mg | 75 mg | ||
| Oral midazolam | |||||
| Cmax (ng/ml) | 15 ± 5 | 12 ± 4 | 14 ± 6 | 7.6 ± 3.0* | 5.1 ± 1.9* |
| Tmax (hr) | 0.7 ± 0.4 | 0.6 ± 0.4 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.3 |
| AUC0-∞ (ng•hr •ml−1) | 35.0 ± 12.8 | 26.8 ± 8.4* | 22.7 ± 6.9* | 13.3 ± 3.3* | 8.1 ± 1.9* |
| AUC0-∞ ratio | 0.80 ± 0.19* | 0.68 ± 0.17* | 0.40 ± 0.10* | 0.25 ± 0.09* | |
| (geometric mean, 90% CI) | 0.78 (0.70,0.87) | 0.66 (0.59,0.74) | 0.39 (0.35,0.44) | 0.24 (0.20,0.28) | |
| CL/F (ml•kg−1•min−1) | 23.0 ± 10.0 | 28.4 ± 10.0* | 33.4 ± 12.8* | 56.5 ± 19.4* | 95.5 ± 35.8* |
| Elimination t1/2 (hr) | 3.0 ± 0.9 | 2.7 ± 0.8 | 2.4 ± 1.0* | 2.3 ± 0.9* | 1.7 ± 0.5* |
| Vz/F (L/kg) | 5.6 ± 2.0 | 6.4 ± 2.4 | 6.5 ± 2.4 | 11.1 ± 5.2* | 14.5 ± 8.6* |
| Foral | 0.29 ± 0.10 | 0.26 ± 0.07 | 0.25 ± 0.12 | 0.18 ± 0.05* | 0.11 ± 0.04* |
| EG | 0.53 ± 0.17 | 0.49 ± 0.19 | 0.51 ± 0.22 | 0.54 ± 0.15 | 0.68 ± 0.14* |
| C4 hr (ng/ml) | 2.6 ± 1.0 | 1.9 ± 0.8* | 1.4 ± 0.5* | 0.9 ± 0.3* | 0.5 ± 0.3* |
| C4 hr ratio | 0.73 ± 0.21* | 0.60 ± 0.19* | 0.37 ± 0.13* | 0.21 ± 0.10* | |
| (geometric mean, 90% CI) | 0.74 (0.65,0.84) | 0.57 (0.49,0.66) | 0.35 (0.29,0.41) | 0.19 (0.16,0.24) | |
| C5 hr (ng/ml) | 1.9 ± 0.7 | 1.4 ± 0.6* | 1.1 ± 0.4* | 0.6 ± 0.2* | 0.3 ± 0.1 * |
| C5 h ratio | 0.76 ±0.21* | 0.58 ± 0.16* | 0.32 ± 0.11* | 0.19 ± 0.08* | |
| (geometric mean, 90% CI) | 0.73 (0.65,0.84) | 0.56 (0.48,0.64) | 0.31 (0.27,0.36) | 0.17 (0.14,0.21) | |
| Oral alfentanil | |||||
| Cmax (ng/ml) | 51 ± 21 | 44 ± 15 | 44 ± 17 | 25 ± 12* | 13 ± 10* |
| Tmax (hr) | 1.0 ± 0.6 | 1.0 ± 0.6 | 0.6 ± 0.2 | 0.6 ± 0.5 | 0.6 ± 0.5 |
| AUC0-∞ (ng•hr •ml−1) | 146 ± 84 | 101 ± 42* | 81 ± 41* | 43 ± 27* | 18 ± 13* |
| AUC0-∞ ratio | 0.74 ± 0.16* | 0.61 ± 0.23* | 0.30 ± 0.10* | 0.13 ± 0.06* | |
| (geometric mean, 90% CI) | 0.73 (0.66, 0.80) | 0.57 (0.47,0.69) | 0.28 (0.24, 0.34) | 0.12 (0.10,0.15) | |
| CL/F (ml•kg−1•min−1) | 11.1 ± 5.7 | 14.8 ± 7.2* | 19.7 ± 11.6* | 41.9 ± 28.7* | 91.7 ± 44.6* |
| Elimination t1/2 (hr) | 1.4 ± 0.5 | 1.2 ± 0.3* | 1.1 ± 0.3* | 1.0 ± 0.2* | 0.8 ± 0.2* |
| Vz/F (L/kg) | 1.2 ± 0.4 | 1.4 ± 0.3 | 1.7 ± 0.8* | 3.2 ± 1.9* | 6.1 ± 2.6* |
| Foral | 0.37 ± 0.12 | 0.33 ± 0.09 | 0.29 ± 0.12* | 0.19 ± 0.08* | 0.09 ± 0.04* |
| EG | 0.51 ± 0.15 | 0.54 ± 0.13 | 0.56 ± 0.18 | 0.68 ± 0.14* | 0.83 ± 0.06* |
| C2 hr (ng/ml) | 35 ± 23 | 25 ± 11* | 18 ± 11* | 10 ± 10* | 4 ± 3* |
| C2 hr ratio | 0.78 ± 0.17* | 0.50 ± 0.16* | 0.26 ± 0.11* | 0.10 ± 0.04* | |
| (geometric mean, 90% CI) | 0.76 (0.69,0.85) | 0.47 (0.39,0.58) | 0.24 (0.19,0.30) | 0.09 (0.08,0.11) | |
| C4 hr (ng/ml) | 15 ± 12 | 7 ± 5* | 6 ± 7* | 2 ± 2* | 0.7 ± 0.6* |
| C4 hr ratio | 0.58 ± 0.16* | 0.42 ± 0.25* | 0.16 ± 0.07* | 0.05 ± 0.03* | |
| (geometric mean, 90% CI) | 0.56 (0.49,0.64) | 0.37 (0.29,0.47) | 0.14 (0.12,0.17) | 0.05 (0.03,0.06) | |
| Oral alfentanil pupil miosis | |||||
| maximum (mm) | 3.1 ± 1.3 | 2.5 ± 1.0* | 2.1 ± 1.2* | 1.5 ± 1.3* | 0.9 ± 0.7* |
| AUEC0-last (mm•hr) | 5.0 ± 4.8 | 3.1 ± 1.8* | 2.0 ± 3.0* | 1.8 ± 3.0* | 0.9 ± 1.3* |
| AUEC0-∞ (mm•hr) | 5.4 ± 5.1 | 3.5 ± 2.0* | 2.4 ± 3.5* | 2.6 ± 3.5* | 1.2 ± 1.5* |
| AUEC0-∞ ratio | 0.9 ± 0.5 | 0.4 ± 0.2* | 0.4 ± 0.2* | 0.3 ± 0.4* | |
| (geometric mean, 90% CI) | 0.78 (0.60, 1.00) | 0.32 (0.22,0.46) | 0.30 (0.18,0.50) | 0.10 (0.05,0.22) | |
All subjects received 3 mg oral MDZ, followed by 75 μg/kg oral ALF. Results are the arithmetic mean ± SD, except AUC ratios and single-point concentration ratios, which are also shown as the geometric mean and 90% confidence interval. Cmax, maximum concentration; Tmax, Time of maximum concentration; AUC0-∞, area under plasma concentration–time curve extrapolated to infinity; C2 hr, C4 hr and C5 hr, plasma concentration 2, 4 or 5 hr after dosing; CL/F, apparent oral clearance; t1/2, half-life; Vz/F, apparent volume of distribution based on the terminal phase; Foral, oral bioavailability; EG, intestinal extraction; AUEC0-last, area under effect (miosis) curve to last time point measured; AUEC0-∞, area under effect (miosis) curve extrapolated to infinity.
Significantly different from control (p<0.05)
Figure 3.
Relationship between MDZ and ALF clearances after IV administration. Each data point is an individual subject session. The correlation was significant (Pearson r=0.86, p<0.05). Similar significant correlations were found between MDZ and ALF for CL/F (r=0.72), hepatic extraction (EH, r=0.86), bioavailability (Foral, r=0.87), and intestinal extraction (EG, 0.68).
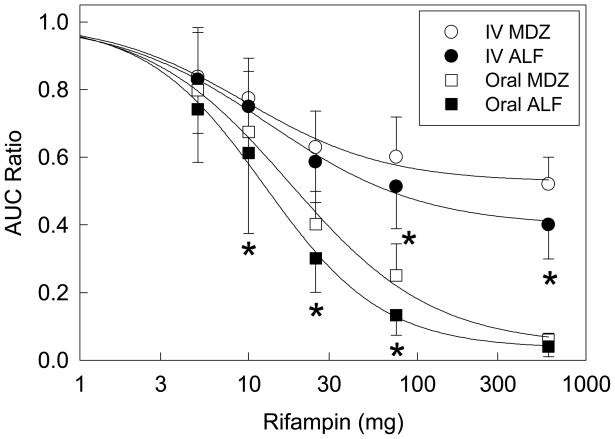
CYP3A induction was assessed by the recommended standard of geometric mean AUC∞ ratios (inducer/control). Ratios for IV and oral ALF and oral MDZ were significantly decreased by all rifampin doses, but those for IV MDZ by only 25 and 75 mg rifampin. Dose-response relationships for rifampin induction, assessed using arithmetic (Figure 4) and geometric means (Tables 1, 2), showed significantly greater magnitude of CYP3A induction effects on ALF than MDZ, except at minimal or full induction.
Figure 4.
Relationship between rifampin dose and CYP3A induction, assessed by the plasma concentration AUC ratio, for both MDZ and ALF. Results are shown as the arithmetic mean ± SD (n=12). Dose-response curves were determined using the Hill equation, and non-linear regression analysis of individual data. Lines represent predicted values based on regression parameters. Significant differences between MDZ and ALF ratios at the same rifampin dose, assessed by 2-way repeated measures analysis of variance, are indicated by asterisks (p<0.05) Results for induction by 600 mg rifampin are from a previous publication, and were also assessed by 2-way repeated measures analysis of variance.10
Rifampin induction of CYP3A was also determined by single point plasma concentrations and concentration ratios (induced/control) for MDZ and ALF. A preliminary analysis showed optimal correlations between plasma AUC and single point concentrations, across all control and rifampin-treated groups, at 4 and 2 hr for MDZ and ALF, respectively (both IV and oral) (not shown). A previous investigation showed that correlations were optimal at 5 and 4 hr for MDZ and ALF, across the full range of CYP3A induction as well as inhibition.14 Therefore, the influence of graded rifampin induction of CYP3A on single point plasma concentration metrics was determined at 4 and 5 hr for MDZ, and 2 and 4 hr for ALF (Tables 1, 2). IV MDZ 4 and 5 hr concentrations and ratios were significantly different from controls in subjects treated with 25 and 75 mg rifampin, while both 2 and 4 hr IV ALF concentrations and ratios were significantly different at all rifampin doses. All oral MDZ and ALF concentrations and ratios were significantly different from controls at all rifampin doses. Ratios (geometric means) for ALF were generally lower than those for MDZ, at both sets of time points.
CYP3A induction was reflected by changes in the time course of ALF dark-adapted pupil diameter change (miosis) for oral ALF, and less so for IV ALF (Figs 1 and 2). Decreases in IV ALF AUEC were not significantly different from control (Table 1). After oral ALF, maximum miosis was significantly reduced at all rifampin doses, as were the AUEC and AUEC ratios (except the latter, at 5 mg rifampin) (Table 2).
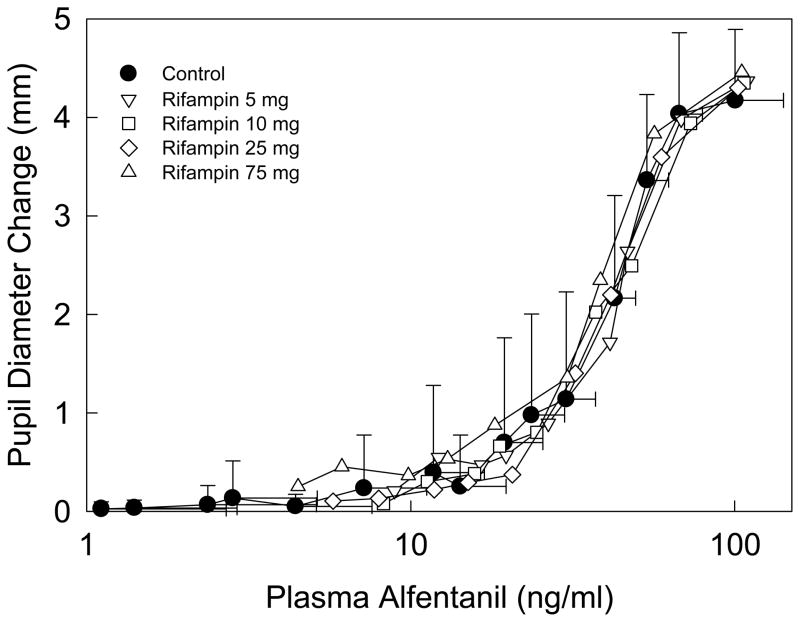
ALF pharmacodynamics were determined by the plasma concentration-miosis relationship (Figure 5). The EC50 for miosis was approximately 40 ng/ml, maximum miosis did not occur at the highest ALF concentrations (100 ng/ml), and little miosis was observed at low ALF concentrations (<10 ng/ml), as reported previously.10,22,27 Rifampin had no influence on ALF concentration-miosis relationships, which were reproducible across all study sessions
Figure 5.
Relationship between alfentanil miosis and plasma concentration after IV administration. Some SD are omitted for clarity. Results are the mean ± SD (n=12).
ALF and MDZ were administered in a total of 120 study sessions. All subjects tolerated MDZ and ALF well and without incident. Side effects were rare. Three subjects received ondansetron for nausea and one experienced emesis, all after IV probe dosing, with no events after oral dosing. No subject had clinically meaningful respiratory side effects. Research nurses were entirely comfortable administering the drugs and monitoring subjects without any physician presence.
Discussion
The purpose of this investigation was to compare responses of the CYP3A probes ALF and MDZ to graded CYP3A induction, and to determine the sensitivity of single point ALF and MDZ concentrations, and ALF miosis, in detecting hepatic and first-pass CYP3A induction. Results were obtained in normal healthy volunteers, and the influence of concomitant disease or other factors was not assessed. The FDA draft guidance on drug interaction studies defines strong, moderate and weak CYP3A inhibitors as causing ≥ 5-fold, ≥ 2-<5-fold, and ≥1.25-<2-fold increases in plasma AUC, or >80%, 50–80%, and 20–50% decreases in clearance, of a sensitive oral CYP3A substrate (e.g. MDZ).* An analogous quantitative classification system for inducers of CYP3A, or other CYPs, is not defined,28–31 however the FDA guidance document lists inducers as those decreasing plasma AUC values by ≥30%* (corresponding to a 43% increase in CL/F). Hence for this investigation, oral CYP3A probe sensitivity was ideally defined as capable of detecting a change equivalent to a 30% decrease in oral MDZ AUC. Although no analogous guidelines exist for hepatic CYP3A and IV dosing, the same criterion of detecting a 30% change in IV MDZ AUC was also applied.
This investigation is the first to use lesser, graded CYP3A induction with low-dose rifampin in contrast to more profound CYP3A induction with full therapeutic antitubercular (600mg) doses, to evaluate either MDZ or ALF as CYP3A probes. Previously, hepatic and intestinal CYP3A induction by 5–7d full-dose rifampin increased MDZ CL, EH, CL/F, and EG 2.2-, 2-, 22-, and 1.6-fold, and decreased IV and oral AUC ratios to 45–52% and 5–10% of control.10,26,32 Presently, graded low-dose rifampin (5, 10, 25, and 75 mg) decreased the IV MDZ AUC ratios to 83, 76, 62, and 50% of control, corresponding to 1.2-, 1.3-, 1.6-, and 1.7-fold increases in hepatic CL and EH. Oral MDZ AUC ratios were reduced to 78, 66, 39 and 24% of control, corresponding to 1.2-, 1.4-, 2.5-, and 4.2-fold increases in CL/F. Thus, the investigational target of graded hepatic and first-pass CYP3A induction, using a range within which a 30% decrease in oral MDZ AUC (1.4-fold increase in CL/F) would occur, was achieved.
This investigation provides rifampin dose-response data for CYP3A induction, a previously identified unmet need,30 Most rifampin induction studies evaluate full antitubercular doses for 5–7d, although some evaluated longer.26 CYP3A induction was maximal after 8d.26 Maximal CYP3A induction, based on IV and oral MDZ AUC ratios, is approximately 2- and 25-fold, respectively.10,26,32 The first major finding of this (crossover) investigation was that the 5d rifampin dose to achieve 50% of maximal induction (IV and oral ALF AUC ratios of 0.7 and 0.52, based on AUC ratios of ~0.4 and 0.04 at full induction10) was between 10 and 25 mg. In a previous (parallel group) study, using 20, 100 or 500 mg rifampin for 14d, and CYP3A assessment by plasma single point oral quinine:3-hydroxyquinine ratios and 4β-hydroxycholesterol concentrations, induction ratios after 20/100/500mg rifampin were 1.6/3.0/4.2 and 1.5/2.5/4.0 for the two probes, respectively.33 Assuming maximal induction at the highest rifampin dose, this also suggested 50% of maximal induction at approximately 20 mg rifampin.
The second major finding was that both MDZ and ALF could detect induction of hepatic and first-pass clearances, using the conventional metric of AUC ratios (≥30% decrease in plasma AUC, corresponding to ≥1.4-fold induction of clearance). Indeed, even at the lowest rifampin dose (5mg), which decreased mean IV and oral MDZ AUCs 16 and 20% (corresponding to 1.2-fold increases in clearance), ALF IV and oral AUC ratios were also significantly decreased. Ability to detect smaller degrees of induction was not evaluated. Thus both IV and oral MDZ and ALF have requisite and ample sensitivity to detect at least a 30% decrease (and in actuality a 20% decrease) in plasma AUC with 12 subjects.
The third finding of this investigation was a greater effect of rifampin on ALF compared with MDZ disposition. For example, after 75 mg rifampin, the increase in IV and oral ALF clearances was 16 and 200% greater than that of MDZ. This may simply reflect the lower EH of ALF, although both probes have similar EG, and that ALF metabolism was induced more and at lower rifampin doses than that of MDZ. Another potential explanation is that ALF could be a better substrate than MDZ for a rifampin-inducible efflux transporter, although neither drug has been identified to date as a substrate for hepatic or intestinal transporters such as P-glycoprotein,34,35 making this explanation unlikely. Greater induction of ALF than MDZ clearances resulted in greater sensitivity of ALF to detect CYP3A induction (vide infra). Consensus documents recommend that the most sensitive CYP probe substrates be used to assess drug interactions.8,31,*, therefore ALF may be considered a more sensitive and therefore preferable CYP3A probe than the current standard MDZ. Other more sensitive CYP3A probes have also been identified.36,37
This investigation is the first to evaluate the sensitivity of single point MDZ and ALF concentrations for assessing graded CYP3A induction. Both 2 and 4 hr ALF concentrations could detect 1.2-fold induction of hepatic and first-pass CYP3A, which occurred at the lowest rifampin dose tested (5 mg). Thus single point ALF met the criteria for CYP3A probe sensitivity and appears suitable as a minimally invasive (single blood sample) probe. Greater sensitivity may be achievable, but an additional investigation using lower rifampin doses would be required to test this hypothesis. Results for 4 and 5 hr single point MDZ were similar to those for single point ALF for oral dosing, however for IV dosing, single point MDZ concentrations (both 4 and 5 hr) were unable to detect small degrees of induction (1.2 to 1.3-fold). Thus the fourth major finding is that single point ALF is a valid probe (and more sensitive than MDZ) for CYP3A induction. Although both 4 hr (optimal time to evaluate a full spectrum of CYP3A induction and inhibition)14,22 and 2 hr (optimal time for specifically evaluating CYP3A induction) times were equally sensitive, the latter is more time-efficient (and assuming that induction is anticipated).
This is the first investigation to evaluate the sensitivity of ALF miosis in detecting CYP3A induction. Miosis was sufficiently sensitive, based on geometric mean AUEC ratios, to detect a 40% decrease in oral ALF or MDZ plasma AUC ratios (with 10 mg rifampin). It was not sensitive enough to detect 22–28% decreases in oral ALF or MDZ plasma AUC ratios (with 5 mg rifampin). Maximum miosis, in addition to AUEC, could also detect first-pass CYP3A induction. More intermediate degrees of CYP3A induction were not assessed, so the sensitivity of ALF miosis (to detect a 30% decrease in oral MDZ AUC) cannot be estimated from the present data. ALF miosis (AUEC ratio) was not sufficiently sensitive to detect changes in hepatic CYP3A activity at the threshold of a 30% decrease in IV MDZ AUC. Thus the fifth major finding of this investigation is that ALF miosis can detect significant induction of first-pass CYP3A activity, and can serve as a noninvasive first-pass CYP3A probe. It could not, however, under the conditions used in this investigation (ALF dose, pupillometer used, cut-off criterion of a 30% decrease in probe concentration AUC, and group size of 12 subjects), and due in part to the interindividual variability in the miotic response, detect hepatic CYP3A induction. Therefore the sensitivity of IV ALF miosis is less than that of ALF plasma AUCs or single-point measurements for detecting hepatic CYP3A induction. It is possible that larger group sizes or other technological improvements might overcome this limitation.
As described previously,22 one major factor affecting the sensitivity of ALF miosis is the sigmoidal character of plasma concentration-effect relationships. Current and previous10,22 results show that the optimal (linear) range for measuring miosis is ~20–100 ng/ml ALF, and that changes in miosis will underestimate those in plasma ALF at lower concentrations. In this protocol, 15 μg/kg IV ALF resulted in plasma concentrations >20 ng/ml only briefly, and particularly after CYP3A induction. This contrasts with CYP3A inhibition, where plasma ALF concentrations (and miosis) were increased and prolonged.10,22 Other considerations affecting the sensitivity of ALF miosis include interindividual variability in ALF pharmacodynamics, hippus or pupillary unrest, pupillary noise, and differences between studies in the hand-held pupillometers used, as previously summarized.22
The sixth major finding of this investigation was that rifampin had no effect on ALF concentration-miosis relationships. Using clinical effect as a surrogate for plasma concentrations, in general, requires minimal delay, or hysteresis, in the plasma concentration-effect relationship. Moreover, when using an effect surrogate for plasma concentrations to probe a pharmacokinetic interaction, lack of active transport at the blood-brain barrier (uptake or efflux) is a desirable characteristic to ensure that the effect vs time relationship is not confounded by a blood-brain barrier interaction in addition to a pharmacokinetic interaction. Thus, ideally, to use ALF miosis as a concentration surrogate, ALF should have rapid brain access and equilibration, and undergo negligible active transport at the blood-brain barrier. This is particularly important, because drugs which might induce CYP3A activity might also induce one or more transporters, through effects on pregnane X or constitutive androstane receptors.38
ALF blood-brain barrier permeability and brain uptake have been studied in rodents and humans. In mice, among a physicochemically diverse set of clinically-used drugs, ALF had the largest permeability surface coefficient, and one of the highest brain uptake clearances and rates of brain equilibration.39 In rats, similar near-instantaneous ALF brain distribution kinetics were also observed, and ALF transport to an effect site was considered not to be rate-limiting, due to high passive permeability.35 Clinically, ALF has extremely fast onset, rapidly equilibration (1 min half-life for equilibration between plasma concentrations and effect, t½,ke0) and no hysteresis.10,40 Thus, ALF has rapid brain access. ALF transport by uptake and efflux proteins is less well studied. In cells overexpressing human P-glycoprotein (P-gp), transcellular ALF movement was not affected by P-gp inhibition and ALF was a poor P-gp inhibitor, suggesting that ALF was not a P-gp substrate.34,35 In mice, ALF was considered a weak P-gp substrate, with brain uptake clearance increased only 1.4-fold in P-gp knock-outs,39 although a prior investigation unambiguously classified ALF as a P-gp substrate, with P-gp knock-outs having a 3-fold greater steady-state brain-to-serum concentration ratio, and P-gp efflux significantly altering ALF antinociception.41 That report suggested that ALF pupillometry might have the potential to detect alterations in P-gp activity, and that future ALF drug interaction studies should assess whether any observed effect interactions are caused by P-gp inhibition.
In the present investigation, rifampin had no effect on ALF concentration-effect relationships. This recapitulates a previous finding that 600 mg rifampin also had no influence on ALF pharmacodynamics.10 One interpretation is that ALF is not a substrate for rifampin-inducible transporters at the blood-brain barrier. Another is that rifampin does not induce blood-brain barrier transporters. Although clinical effects of rifampin on human brain P-gp are not known, rifampin did upregulate P-gp expression in vivo in transgenic mice expressing human pregnane X receptor42 and in vitro in a human brain microvessel endothelial cell line43 and porcine brain capillaries.44 Since rifampin appears to upregulate P-gp at the blood-brain barrier, while ALF pharmacodynamics are unchanged by rifampin, ALF therefore appears to undergo little or no active transport at the blood-brain barrier by rifampin-inducible transporters. Thus, ALF has rapid brain access and equilibration, and there is no evidence for active transport at the blood-brain barrier. This further supports ALF miosis as a surrogate for plasma concentrations and noninvasive CYP3A assessment.
This investigation used measured hematocrits for calculating hepatic plasma flow. Previous ALF studies used population hematocrits (36 in women and 40 in men).11,22,27 Present hematocrits overall were 33±3 and 42±2 in females and males, respectively, and were unchanged across sessions (35±2, 34±3, 34±4, 32±3, and 33±3 in females, 43±2, 41±2, 42±1, 42±3, and 42±3 in males), in controls and ascending rifampin dose groups, respectively. Interday variations, and differences between measured and population hematocrits, had little influence on pharmacokinetic parameter estimates. Therefore, using actual vs population hematocrit values had little effect on the use of these CYP3A probes.
Although MDZ is the standard oral CYP3A probe,8,31,36,37,* discussion continues about the optimal in vivo probe for assessing CYP3A and drug interactions, predicting CYP3A-dependent drug metabolism, and individualized dosing of CYP3A drugs with narrow therapeutic indices.2,7–9,36,37 MDZ is probably the most widely used CYP3A probe, albeit possibly not ideal, because MDZ clearance may be influenced by hepatic blood flow as well as by CYP3A-dependent metabolism,17,18 and variability in hepatic blood flow may contribute to variability in MDZ clearance,17,45,46 and other substrates, including buspirone, sildenafil, simvastatin, and ALF, appear more sensitive.36,37
In summary, this investigation assessed the sensitivity of IV and oral ALF AUCs and clearances, single-point plasma concentrations, and miosis, in detecting and quantifying CYP3A induction, and in comparison with MDZ AUCs and clearance and single-point concentrations. ALF was a more sensitive substrate for detecting CYP3A induction, and 2 hr single-point ALF plasma concentrations were excellent surrogates for formal ALF clearance determinations and detecting CYP3A induction. ALF miosis may be a useful surrogate for ALF plasma concentrations and a probe for first-pass, but not hepatic CYP3A induction. ALF miosis confers significant advantages of noninvasiveness and real-time availability of results.
Methods
Study Population and Protocol
Twelve volunteers (8 male, 4 female) participated in the investigation, which was approved by the Washington University Institutional Review Board, after obtaining written informed consent. Subjects (25 ± 6 yr, range 18–38, smokers and nonsmokers) were healthy, within 30% of ideal body weight (74 ± 16 kg, range 55–108), had no remarkable medical problems, history of hepatic or renal disease, and were taking no drugs or natural products known to alter CYP3A activity. Subjects using oral or implanted contraceptives were excluded, due to potential rifampin effects on their effectiveness. Subjects were instructed to consume no grapefruit-containing foods 5d before and during each study day, abstain from alcohol for 24 hr prior to and during study days, and from caffeine on study days. The study period was approximately 2 mo per subject, and 9 mo overall.
The protocol was a 5-way sequential crossover. Each 5-session pair was separated by at least 2 weeks. Subjects were studied in the clinical research center. Catheters were placed in an arm vein for drug administration and blood sampling. For hepatic and intestinal CYP3A induction, subjects received nothing or rifampin (5, 10, 25 or 75 mg capsules orally) at bedtime for 5 days. The next day they received 1 mg intravenous (IV) MDZ followed 1 hr later by 15 μg/kg IV ALF. That night, subjects received an additional rifampin dose. The next day they received 3 mg oral MDZ followed 1 hr later by 75 μg/kg oral ALF (both injectable formulations, used without modification) with 100cc water. Subjects were monitored with pulse oximetry for routine safety, and received supplemental oxygen for saturation <94%. They received a standard breakfast 2 hr after ALF, with free access to food and water thereafter. Blood samples were obtained for up to 9 hr after MDZ (8 hr after ALF). Plasma was stored at −20°C for later analysis. Coincident with blood sampling, and at intermediate times, dark-adapted pupil diameter was measured using a PLR-200™ Pupillometer (NeurOptics, Irvine, CA) as described previously.10,11 Pupil diameter change (miosis) at each time point was the pre-drug baseline diameter minus the diameter at each time point.
Analytical Methods
Plasma MDZ and ALF concentrations were measured by HPLC-tandem mass spectrometry with multiple reaction monitoring as previously reported.47 Interday coefficients of variation were 7, 8, and 7% at 1.4, 12 and 100 ng/ml MDZ, and 9, 7, and 7% at 1, 10, and 100 ng/ml ALF.
Pharmacokinetic Analysis
Data were analyzed using noncompartmental methods (WinNonlin, Pharsight, Cary, NC) as described previously.10,11 Systemic clearance was (CLIV)=DoseIV,/AUCIV,, apparent oral clearance was (CL/F)=Doseoral/AUCoral,, bioavailability was (Foral)=(AUCoral/Doseoral)x(DoseIV/AUCIV), steady-state volume of distribution was (Vss)=mean residence time x CL. Hepatic extraction (EH) was (CLIV/Qp), where hepatic plasma flow (Qp) was estimated as the product of hepatic blood flow (25.3 and 25.5 ml/kg in males and females)32 and hematocrit (determined on the morning of the oral ALF study day), and negligible extrahepatic ALF metabolism was assumed. Gastrointestinal extraction (EG) was 1−(Foral/(FH × Fabs)), where oral absorption was assumed to be complete and thus Fabs considered to be unity. Rifampin was assumed not to influence hepatic blood flow.10 In addition to formal clearance determinations, single point plasma concentrations were also assessed as potential surrogates for AUC. Concentrations used were 2 and 4 hr for IV and oral ALF and 5 hr for IV and oral MDZ, respectively.14 ALF effect (miois) disposition curves were analyzed using noncompartmental analysis, analogously to conventional plasma concentration curves, to yield effect parameters (area under the effect curve, AUEC) similar to conventional pharmacokinetic parameters, as described previously.10,11
Statistical Analysis
Results are expressed as the arithmetic mean ± SD, unless indicated otherwise. One-way (rifampin dose) or two-way (rifampin dose and substrate) repeated measures analysis of variance (with log transformation of non-normal data) followed by the Student-Newman-Keuls test was used to assess the significance (assigned at p<0.05) of differences between groups (SigmaPlot, Systat Corp., San Jose, CA) AUC, AUEC and single-point concentration ratios with and without the interacting drug (rifampin/control) were used to assess the differences between groups using geometric mean ratios and 90% confidence intervals. Relationships between MDZ and ALF pharmacokinetic parameters were analyzed using Pearson product moment correlations. Correlations between MDZ or ALF AUC and plasma concentrations at each time point were evaluated by weighted (1/y2) linear regression, and goodness of fit determined by comparing the coefficients of determination (r2) as described previously.14 Dose-response relationships for CYP3A induction were analyzed by nonlinear regression using the Hill equation.
Sample size was based on ALF clearance. Based on 13% intraindividual variability in ALF clearance,48,49 11 subjects per group were required to detect a 20% difference in clearance (α=0.05, β=0.8). Twelve subjects were enrolled.
Acknowledgments
Supported by National Institutes of Health Grants R01-GM63674, R01-DA14211, and K24-DA00417 (to EDK), and NCRR grant UL1 RR024992.
Footnotes
Food and Drug Administration Center for Drug Evaluation and Research (FDA-CDER) Guidance for Industry: Drug Interaction Studies-Study Design, Data Analysis, and Implications for Dosing and Labeling, (http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072101.pdf). Last accessed December 28, 2010.
This investigation was conducted before the requirement for clinical trials registration
Conflict of Interest/Disclosure
No author has any conflict of interest
References
- 1.Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352:2211–21. doi: 10.1056/NEJMra032424. [DOI] [PubMed] [Google Scholar]
- 2.Watkins PB. Noninvasive tests of CYP3A enzymes. Pharmacogenetics. 1994;4:171–84. doi: 10.1097/00008571-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Benet LZ. There are no useful CYP3A probes that quantitatively predict the in vivo kinetics of other CYP3A substrates and no expectation that one will be found. Mol Intervent. 2005;5:79–83. doi: 10.1124/mi.5.2.5. [DOI] [PubMed] [Google Scholar]
- 4.Kharasch ED, Thummel KE, Watkins PB. CYP3A probes can quantitatively predict the in vivo kinetics of other CYP3A substrates and can accurately assess CYP3A induction and inhibition. Mol Interv. 2005;5:151–3. doi: 10.1124/mi.5.3.3. [DOI] [PubMed] [Google Scholar]
- 5.Liu YT, Hao HP, Liu CX, Wang GJ, Xie HG. Drugs as CYP3A probes, inducers, and inhibitors. Drug Metab Rev. 2007;39:699–721. doi: 10.1080/03602530701690374. [DOI] [PubMed] [Google Scholar]
- 6.Kirwan C, Macphee I, Philips B. Using drug probes to monitor hepatic drug metabolism in critically ill patients: midazolam, a flawed but useful tool for clinical investigation of CYP3A activity? Expert Opin Drug Metab Toxicol. 2010;6:761–71. doi: 10.1517/17425255.2010.482929. [DOI] [PubMed] [Google Scholar]
- 7.Zaigler M, Tantcheva-Poor I, Fuhr U. Problems and perspectives of phenotyping for drug-metabolizing enzymes in man. Int J Clin Pharmacol Ther. 2000;38:1–9. doi: 10.5414/cpp38001. [DOI] [PubMed] [Google Scholar]
- 8.Bjornsson TD, et al. The conduct of in vitro and in vivo drug-drug interaction studies: a pharmaceutical research and manufacturers of America (PhRMA) perspective. Drug Metab Dispos. 2003;31:815–32. doi: 10.1124/dmd.31.7.815. [DOI] [PubMed] [Google Scholar]
- 9.Streetman DS, Bertino JS, Jr, Nafziger AN. Phenotyping of drug-metabolizing enzymes in adults: a review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics. 2000;10:187–216. doi: 10.1097/00008571-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Kharasch ED, Walker A, Hoffer C, Sheffels P. Intravenous and oral alfentanil as in vivo probes for hepatic and first-pass cytochrome P450 3A activity: Noninvasive assessment using pupillary miosis. Clin Pharmacol Ther. 2004;76:452–66. doi: 10.1016/j.clpt.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Kharasch ED, et al. Influence of CYP3A5 genotype on the pharmacokinetics and pharmacodynamics of the cytochrome P4503A probes alfentanil and midazolam. Clin Pharmacol Ther. 2007;82:410–26. doi: 10.1038/sj.clpt.6100237. [DOI] [PubMed] [Google Scholar]
- 12.Lin YS, et al. In-vivo phenotyping for CYP3A by a single-point determination of midazolam plasma concentration. Pharmacogenetics. 2001;11:781–91. doi: 10.1097/00008571-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Rogers JF, et al. Single plasma concentrations of 1′-hydroxymidazolam or the ratio of 1′-hydroxymidazolam:midazolam do not predict midazolam clearance in healthy subjects. J Clin Pharmacol. 2002;42:1079–82. doi: 10.1177/009127002401382614. [DOI] [PubMed] [Google Scholar]
- 14.Chaobal HN, Kharasch ED. Single point sampling for assessment of constitutive, induced and inhibited cytochrome P450 3A activity with alfentanil or midazolam. Clin Pharmacol Ther. 2005;78:529–39. doi: 10.1016/j.clpt.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Lee LS, Bertino JS, Jr, Nafziger AN. Limited sampling models for oral midazolam: midazolam plasma concentrations not the ratio of 1-hydroxymidazolam to midazolam plasma concentrations accurately predicts AUC as a biomarker of CYP3A activity. J Clin Pharmacol. 2006;46:229–34. doi: 10.1177/0091270005283466. [DOI] [PubMed] [Google Scholar]
- 16.Penzak SR, Busse KH, Robertson SM, Formentini E, Alfaro RM, Davey RT., Jr Limitations of using a single postdose midazolam concentration to predict CYP3A-mediated drug interactions. J Clin Pharmacol. 2008;48:671–80. doi: 10.1177/0091270008317305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klotz U, Ziegler G. Physiologic and temporal variation in hepatic elimination of midazolam. Clin Pharmacol Ther. 1982;32:107–12. doi: 10.1038/clpt.1982.133. [DOI] [PubMed] [Google Scholar]
- 18.Rogers JF, Rocci ML, Jr, Haughey DB, Bertino JS., Jr An evaluation of the suitability of intravenous midazolam as an in vivo marker for hepatic cytochrome P4503A activity. Clin Pharmacol Ther. 2003;73:153–8. doi: 10.1067/mcp.2003.23. [DOI] [PubMed] [Google Scholar]
- 19.Chauvin M, Bonnet F, Montembault C, Levron JC, Viars P. The influence of hepatic plasma flow on alfentanil plasma concentration plateaus achieved with an infusion model in humans: measurement of alfentanil hepatic extraction coefficient. Anesth Analg. 1986;65:999–1003. [PubMed] [Google Scholar]
- 20.Henthorn TK, Krejcie TC, Avram MJ. The relationship between alfentanil distribution kinetics and cardiac output. Clin Pharmacol Ther. 1992;52:190–6. doi: 10.1038/clpt.1992.129. [DOI] [PubMed] [Google Scholar]
- 21.Kharasch ED, et al. The role of cytochrome P450 3A4 in alfentanil clearance. Implications for interindividual variability in disposition and perioperative drug interactions. Anesthesiology. 1997;87:36–50. doi: 10.1097/00000542-199707000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Kharasch ED, Walker A, Hoffer C, Sheffels P. Sensitivity of intravenous and oral alfentanil and pupillary miosis as minimally invasive and noninvasive probes for hepatic and first-pass CYP3A activity. J Clin Pharmacol. 2005;45:1187–97. doi: 10.1177/0091270005280077. [DOI] [PubMed] [Google Scholar]
- 23.Kharasch ED, Walker A, Whittington D, Hoffer C, Bedynek PS. Methadone metabolism and clearance are induced by nelfinavir despite inhibition of cytochrome P4503A (CYP3A) activity. Drug Alcohol Depend. 2009;101:158–68. doi: 10.1016/j.drugalcdep.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kharasch ED, Hoffer C, Whittington D, Walker A, Bedynek PS. Methadone pharmacokinetics are independent of cytochrome P4503A (CYP3A) activity and gastrointestinal drug transport: Insights from methadone interactions with ritonavir/indinavir. Anesthesiology. 2009;110:660–72. doi: 10.1097/ALN.0b013e3181986a9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kharasch ED, et al. Concurrent assessment of hepatic and intestinal cytochrome P450 3A activities using deuterated alfentanil. Clin Pharmacol Ther. 2011 doi: 10.1038/clpt.2010.313. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin JH. CYP induction-mediated drug interactions: in vitro assessment and clinical implications. Pharm Res. 2006;23:1089–116. doi: 10.1007/s11095-006-0277-7. [DOI] [PubMed] [Google Scholar]
- 27.Kharasch ED, Hoffer C, Walker A, Sheffels P. Disposition and miotic effects of oral alfentanil: a potential noninvasive probe for first-pass cytochrome P4503A activity. Clin Pharmacol Ther. 2003;73:199–208. doi: 10.1067/mcp.2003.30. [DOI] [PubMed] [Google Scholar]
- 28.Smith DA, et al. The time to move cytochrome P450 induction into mainstream pharmacology is long overdue. Drug Metab Dispos. 2007;35:697–8. doi: 10.1124/dmd.106.013284. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Zhang Y, Huang SM. Scientific and regulatory perspectives on metabolizing enzyme-transporter interplay and its role in drug interactions: challenges in predicting drug interactions. Mol Pharm. 2009;6:1766–74. doi: 10.1021/mp900132e. [DOI] [PubMed] [Google Scholar]
- 30.Almond LM, Yang J, Jamei M, Tucker GT, Rostami-Hodjegan A. Towards a quantitative framework for the prediction of DDIs arising from cytochrome P450 induction. Curr Drug Metab. 2009;10:420–32. doi: 10.2174/138920009788498978. [DOI] [PubMed] [Google Scholar]
- 31.Chu V, et al. In vitro and in vivo induction of cytochrome P450: A survey of the current practices and recommendations: A Pharmaceutical Research and Manufacturers of America perspective. Drug Metab Dispos. 2009;37:1339–54. doi: 10.1124/dmd.109.027029. [DOI] [PubMed] [Google Scholar]
- 32.Gorski JC, et al. The effect of age, sex, and rifampin administration on intestinal and hepatic cytochrome P450 3A activity. Clin Pharmacol Ther. 2003;74:275–87. doi: 10.1016/S0009-9236(03)00187-5. [DOI] [PubMed] [Google Scholar]
- 33.Kanebratt KP, et al. Cytochrome P450 induction by rifampicin in healthy subjects: determination using the Karolinska cocktail and the endogenous CYP3A4 marker 4β-hydroxycholesterol. Clin Pharmacol Ther. 2008;84:589–94. doi: 10.1038/clpt.2008.132. [DOI] [PubMed] [Google Scholar]
- 34.Wandel C, Kim R, Wood M, Wood A. Interaction of morphine, fentanyl, sufentanil, alfentanil, and loperamide with the efflux drug transporter P-glycoprotein. Anesthesiology. 2002;96:913–20. doi: 10.1097/00000542-200204000-00019. [DOI] [PubMed] [Google Scholar]
- 35.Groenendaal D, et al. Pharmacokinetic/pharmacodynamic modelling of the EEG effects of opioids: the role of complex biophase distribution kinetics. Eur J Pharm Sci. 2008;34:149–63. doi: 10.1016/j.ejps.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Ragueneau-Majlessi I, Boulenc X, Rauch C, Hachad H, Levy RH. Quantitative correlations among CYP3A sensitive substrates and inhibitors: literature analysis. Curr Drug Metab. 2007;8:810–4. doi: 10.2174/138920007782798135. [DOI] [PubMed] [Google Scholar]
- 37.Foti RS, Rock DA, Wienkers LC, Wahlstrom JL. Selection of alternative CYP3A4 probe substrates for clinical drug interaction studies using in vitro data and in vivo simulation. Drug Metab Dispos. 2010;38:981–7. doi: 10.1124/dmd.110.032094. [DOI] [PubMed] [Google Scholar]
- 38.Tolson AH, Wang H. Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Adv Drug Deliv Rev. 2010;62:1238–49. doi: 10.1016/j.addr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao R, Kalvass JC, Pollack GM. Assessment of blood-brain barrier permeability using the in situ mouse brain perfusion technique. Pharm Res. 2009;26:1657–64. doi: 10.1007/s11095-009-9876-4. [DOI] [PubMed] [Google Scholar]
- 40.Scott JC, Ponganis KV, Stanski DR. EEG quantitation of narcotic effect: The comparative pharmacodynamics of fentanyl and alfentanil. Anesthesiology. 1985;62:234–41. doi: 10.1097/00000542-198503000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Kalvass JC, Olson ER, Pollack GM. Pharmacokinetics and pharmacodynamics of alfentanil in P-glycoprotein-competent and P-glycoprotein-deficient mice: P-glycoprotein efflux alters alfentanil brain disposition and antinociception. Drug Metab Dispos. 2007;35:455–59. doi: 10.1124/dmd.106.011445. [DOI] [PubMed] [Google Scholar]
- 42.Bauer B, et al. In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol Pharmacol. 2006;70:1212–9. doi: 10.1124/mol.106.023796. [DOI] [PubMed] [Google Scholar]
- 43.Zastre JA, et al. Up-regulation of P-glycoprotein by HIV protease inhibitors in a human brain microvessel endothelial cell line. J Neurosci Res. 2009;87:1023–36. doi: 10.1002/jnr.21898. [DOI] [PubMed] [Google Scholar]
- 44.Ott M, Fricker G, Bauer B. Pregnane X receptor (PXR) regulates P-glycoprotein at the blood-brain barrier: Functional similarities between pig and human PXR. J Pharmacol Exp Ther. 2009;329:141–9. doi: 10.1124/jpet.108.149690. [DOI] [PubMed] [Google Scholar]
- 45.Bornemann LD, Crews T, Chen SS, Twardak S, Patel IH. Influence of food on midazolam absorption. J Clin Pharmacol. 1986;26:55–9. doi: 10.1002/j.1552-4604.1986.tb02903.x. [DOI] [PubMed] [Google Scholar]
- 46.Strömberg C, Vanakoski J, Olkkola KT, Lindqvist A, Seppälä T, Laitinen LA. Exercise alters the pharmacokinetics of midazolam. Clin Pharmacol Ther. 1992;51:527–32. doi: 10.1038/clpt.1992.58. [DOI] [PubMed] [Google Scholar]
- 47.Kim T, London A, Kharasch ED. Simultaneous determination of alfentanil and midazolam in human plasma using liquid chromatography and tandem mass spectrometry. J Pharm Biomed Anal. 2011 doi: 10.1016/j.jpba.2011.01.040. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kharasch ED, Russell M, Garton K, Lentz G, Bowdle TA, Cox K. Assessment of cytochrome P450 3A4 activity during the menstrual cycle using alfentanil as a noninvasive probe. Anesthesiology. 1997;87:26–35. doi: 10.1097/00000542-199707000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Kharasch ED, Jubert C, Senn T, Bowdle TA, Thummel KT. Intraindividual variability in male hepatic CYP3A4 activity assessed by alfentanil and midazolam clearance. J Clin Pharmacol. 1999;39:664–9. doi: 10.1177/00912709922008290. [DOI] [PubMed] [Google Scholar]