Abstract
Background
Methadone is frequently used in adult anesthesia and pain treatment. Methadone pharmacokinetics in adults are well characterized, including the perioperative period. Methadone is also used in children. There is, however, no information on methadone pharmacokinetics in children of any age. The purpose of this investigation was to determine the pharmacokinetics of intravenous methadone in children undergoing surgery. Perioperative opioid-sparing effects were also assessed.
Methods
Eligible subjects were children 5–18 yr undergoing general anesthesia and surgery, with an anticipated postoperative inpatient stay exceeding 3d. Three groups of 10–11 patients each received intravenous methadone HCl after anesthetic induction in ascending dose groups of 0.1, 0.2, and 0.3 mg/kg (up to 20 mg). Anesthetic care was not otherwise changed. Venous blood was obtained for 4d, for stereoselective determination of methadone and metabolites. Pain assessments were made each morning. Daily and total opioid consumption was determined. Perioperative opioid consumption and pain was determined in a second cohort, which was matched to age, sex, race, ethnicity, surgical procedure, and length of stay, but not receiving methadone.
Results
The final methadone study cohort was 31 adolescents (14 ± 2 yr, range 10–18) undergoing major spine surgery for a diagnosis of scoliosis. Methadone pharmacokinetics were linear over the dose range 0.1–0.3 mg/kg. Disposition was stereoselective. Methadone administration did not dose-dependently affect postoperative pain scores, and did not dose-dependently decrease daily or total postoperative opioid consumption in spinal fusion patients.
Conclusions
Methadone enantiomers disposition in adolescents undergoing surgery was similar to that in healthy adults.
Introduction
Postoperative pediatric pain management remains a considerable challenge. Physicians, nurses and parents under recognize and underestimate the amount of pain experienced by children, often under treat the pain, and may overestimate the risks of pain treatment.1–4 Inadequate pain relief has a significant psychological impact upon children, including alterations in their perceptions of future painful experiences and medical procedures.5 Psychological ramifications of pediatric pain can include lack of adherence to treatment recommendations, poor adjustment and coping skills, long-term changes in willingness and accuracy in self-report of pain, lack of trust in health-care providers, and posttraumatic stress disorder.6 For children, severe pain can occur early in life, and can recur at unpredictable intervals throughout their life. The degree to which this pain is controlled impacts the ability of patients to cope with the next pain episode.
Methadone is a mu opioid agonist and N-methyl-D-aspartate receptor antagonist which is highly efficacious and cost-effective in the treatment of acute, chronic, neuropathic, and cancer pain, as well as substance abuse.7–10 It is used in adults, children and even neonates, and can be administered via oral, intravenous, nasal, and various other parenteral routes. Methadone is particularly useful in the perioperative period.10–13 Methadone is advantageous because it has rapid onset, and slow elimination which results in prolonged effect and diminished need for postoperative analgesics.10 Methadone has a long half-life, averaging 24–36 h in adults. It has no active metabolites or prodrug forms. Methadone is metabolized via N-demethylation to the inactive metabolite 2-ethylidene-1.5-dimethyl-3.3diphenylpyrrolidine (EDDP) in the liver primarily by cytochromes P450 CYP2B6 and CYP3A4,14–18 although it appears that the latter may be less important clinically in determining single dose methadone metabolism and clearance.15,19–22 Methadone is administered clinically as a racemate, however R-methadone is approximately 50-fold more potent than the S-enantiomer.
Methadone use and exposure in children is growing. Clinical applications include acute pain, cancer pain, chronic pain, and palliative care.23–27 Similar to adults, methadone is used to treat opioid-dependent adolescents.28 Oral methadone has also been used to treat neonatal abstinence syndrome resulting from either exposure to opioids in utero or by chronic administration of opioids in neonatal or pediatric intensive care units. Unintended pediatric exposures to methadone have also increased, as prescribing for pain in the adult population has increased over the last decade.27
Despite the increase in methadone use in pediatrics, there is a paucity of clinical data on its pharmacokinetics and pharmacodynamics in children.29–31 The primary purpose of this investigation was to determine the pharmacokinetics of intravenous methadone in children. A secondary purpose was to assess postoperative opioid consumption in pediatric surgical patients who receive methadone.
Materials and Methods
Patients
The investigation was approved by the Washington University in St. Louis Institutional Review Board. Eligible subjects were children 5–18 yr undergoing general anesthesia and surgery, with an anticipated postoperative inpatient stay exceeding 4d. Exclusion criteria were a history of or known liver or kidney disease, and pregnant or nursing females. Patients’ parents or legal guardian provided written informed consent, and patients provided written assent. Patients received standard monitors for anesthesia and postoperative care. Anesthesia and surgical care were not altered for this investigation, except that a second intravenous catheter was placed in the arm or hand immediately after induction of anesthesia for fluid administration and blood sampling, and subjects received methadone as their initial intraoperative opioid after the placement of the second intravenous line. Methadone administration occurred 27 ± 20 min after propofol induction across all dose groups. A dose-escalation protocol was used. Three groups of 10 patients each received intravenous methadone HCl in ascending dose groups of 0.1 mg/kg, 0.2 mg/kg, and 0.3 mg/kg (up to a maximum of 20 mg). Based on prior experience in pediatrics and adults, these doses were conservatively chosen such that additional postoperative pain medication would still be needed, but that increasing methadone doses could result in lessened postoperative opioid use. All other anesthetic care was at the discretion of the anesthesia care team. After induction (typically propofol), anesthesia was maintained with less than 0.5 minimum alveolar concentration of sevoflurane or desflurane in 50:50 oxygen:air, and propofol infusion (50–100 μg/kg/min) used to provide additional anesthesia as needed. Muscle relaxation (typically by rocuronium infusion) was monitored by train of four ratio, and allowed to wear off prior to motor stimulation. For analgesia, methadone was supplemented with opioid infusion and/or bolus, at the discretion of the anesthesia provider.
Postoperatively, patients received standard of care analgesia (patient-controlled analgesia and oral opioids) as determined by their treating surgeon. Postoperative care was not altered for purposes of this study. Venous blood samples were obtained before methadone and at 0.08, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 24, 48, 72, and 96 h after dosing.
Patient assessments were made each morning by a trained member of the research team. Pain intensity was assessed by patients using the Wong-Baker FACES scale and a laminated card, which provides a rating of 0 (no hurt) – 5 (hurts worst).32 Pain was also assessed by patients using a Colored-Visual Analog Scale, which was then scored with a metric ruler from 0 (no pain relief to 10 (maximum pain relief). The inpatient nursing staff also assessed pain intensity per current institutional practice, using a verbal analog scale (0 being no pain and 10 being the worst pain imaginable). They also assessed sedation using a five-point scale (patient fully alert – not arousable), and itching and nausea using a five-point verbal scale (none, mild, moderate, severe, or excruciating). Information on respiratory depression (<8/min), decreased oxygen saturation (<92% on room air per pulse oximetry), altered mental status (i.e., confusion, hallucinations, disorientation), and excessive somnolence (i.e., arousability, difficulty staying awake, etc.) as observed and documented by inpatient nurses on daily flowsheets was also abstracted from medical records.
The inpatient medical record was used to quantify opiate use by each patient in each 24-h period. Intraoperative opioids, postoperative patient-controlled analgesia use, and oral opioids were quantified separately, and in total. Results are expressed as morphine equivalents.33 Equivalent to intravenous 10 mg morphine was 1.5 mg hydromorphone, 10 mg methadone, 100 μg fentanyl, 10 μg sufentanil, and 20 mg oral oxycodone.
A second cohort of patients, undergoing similar surgical procedures as the methadone cohort, but not receiving methadone, was studied to determine perioperative opioid consumption. Children were selected based on age, sex, race, ethnicity, surgical procedure, and length of stay, to approximate the characteristics of the methadone patients, and medical records reviewed for intraoperative and postoperative opioids and dose, and postoperative pain assessments and complications. These patients received care during the same period (June 2009 to July 2010) as the methadone patients (June 2009 to June 2010).
Analytical
Plasma methadone and EDDP concentrations were determined by liquid chromatography-tandem mass spectrometry, using a significant modification of a previous method.34 Plasma (500 μl of patient plasma, calibrator, or quality control sample) was acidified with freshly prepared 4% phosphoric acid (1 ml, containing the internal standards d9-methadone (5 ng) and d3-EDDP (1 ng)) and vortex mixed. Standards were from Cerilliant (Round Rock, TX). Samples were processed by solid phase extraction. Strata-XC strong cation mixed mode 60 mg plates (Phenomenex, Torrance, CA) were conditioned with 1 ml methanol then 1 ml of 0.1 N HCl. Acidified plasma was loaded, then washed with 1ml 0.1N HCl followed by 1ml methanol. The plate was dried at full vacuum for 2–5 min, then samples were eluted with 0.5 ml of 5% ammonium hydroxide in acetonitrile. Samples were dried under nitrogen stream at 60°C and stored until analysis. Dried samples were reconstituted with 100 μl of 20 mM ammonium formate in water.
Analysis was performed on an API 3200 triple-quadrupole mass spectrometer (Applied Biosystems/MDS Sciex, Foster City, CA) equipped with a Turbo Ion Spray ionization source operated in positive ion mode. The chromatography system was two LC-20AC pumps with a CTO-20A oven, SIL-20A autosampler, DGU-20A3 degasser, FCF-11AL valve and a CBM 20A controller (Shimadzu, Columbia, MD). Chromatographic separation was performed on a chiral AGP analytical column (100 × 2 mm, 5μm) with a chiral AGP guard column (10 × 2 mm) (ChromTech, Apple Valley, MN). The injection volume was 70 μl and the oven temperature was 35°C. Before each injection, the needle was washed with methanol:water (50:50, v:v). Mobile phase (0.22 ml/min) was (A) 20 mM ammonium acetate (pH 5.7) and (B) methanol using the following program: 10% B for 3 min, linear gradient to 20% B at 4 min, held for 3 min, linear gradient to 50% B at 8 min, held for 2 min, linear gradient to 80% B at 10.5 min, held for 2 min, then then reequilibrated to initial conditions between 12.5 and 15 min. Under these conditions, retention times for R- and S-methadone and R-and S-EDDP were 11.5, 12.0, 11.1, and 11.8 min, respectively. Both Q1 and Q3 quadrupoles were optimized to unit mass resolution, and the mass spectrometer conditions were optimized for each analyte. Instrument parameters were: source temperature 600°C, ion spray voltage 5500 V, curtain gas 35, ion source gas 1 at 30, ion source gas 2 at 30, collision gas 5, entrance and exit potentials 5. Multiple reaction monitoring transitions were m/z 310.2>265.2 and 319.3>268.2 for methadone and d9-methadone, and 278.2>234.2 and 281.2>234.2 for EDDP and d3-EDDP, with 250 ms dwell times. For methadone and EDDP, the declustering potential was 40 and 60 V, and the collision energy was 20 and 40 V.
Plasma calibration standards contained 0.1 0.2, 1, 5, 10, 50, 90 and 100 ng/ml RS- methadone, and 0.02, 0.04, 0.2, 1, 10, 18, and 20 ng/ml RS-EDDP. Plasma quality control samples contained 1, 10 and 80 ng/ml RS-methadone and 0.2, 2, and 16 ng/ml RS-EDDP. Interday coefficients of variation were 5, 7, and 7% for 0.5, 5, and 40 ng/ml R-methadone and 4, 5, and 5% for S-methadone, and 6, 6, and 3% for 0.1, 1 and 8 ng/ml R- and S-EDDP.
Pharmacokinetic data were analyzed using noncompartmental methods (WinNonlin 5.3, Pharsight Inc, Sunnyvale, CA), as described previously.15,19,21,22 Dose groups were compared using analysis of variance. Differences in methadone and metabolite enantiomers pharmacokinetics parameters (all subjects) were compared using paired t-tests or Wilcoxon signed rank tests, as appropriate. Opioid use and pain scores were analyzed by 2-way repeated measures analysis of variance. Statistical significance was assigned at p < 0.05.
Results
Patient demographics are provided in table 1. The methadone cohort consisted exclusively of adolescents undergoing major spine surgery, usually for a diagnosis of scoliosis. This was influenced by the study aim of 96 h postoperative blood sampling, which required an inpatient stay of at least 4d, which in our institution is comprised largely of patients undergoing major spine surgery. The age range of the final study population (10–18 yr) was a consequence of the age at which scoliosis patients undergo posterior spinal fusion in our institution. Adolescents undergoing spine surgery was not otherwise the target population.
Table 1.
Subject Demographics
| Intraoperative Methadone Dose
|
||||
|---|---|---|---|---|
| 0 | 0.1 mg/kg | 0.2 mg/kg | 0.3 mg/kg | |
| N | 30 | 10 | 10 | 11 |
| Age (yr) | 15 ± 2 | 14 ± 2 | 13 ± 2 | 14 ± 2 |
| Sex (M:F) | 9:21 | 2:8 | 6:4 | 2:9 |
| Weight (kg) | 63 ± 26 | 61 ± 13 | 50 ± 8 | 62 ± 14 |
| Diagnosis | ||||
| Scoliosis | 26 | 9 | 9 | 10 |
| Kyphosis | 2 | 0 | 1 | 1 |
| Other | 2 | 1 | 0 | 0 |
| Operation | ||||
| Posterior spinal fusion | 30 | 9 | 10 | 10 |
| Levels fused | 11 ± 3 | 10 ± 5 | 11 ± 2 | 10 ± 3 |
| Other | 0 | 1 | 0 | 0 |
| Anesthesia duration (hr) | 5.6 ± 1.5 | 5.8 ± 1.8 | 5.4 ± 0.7 | 5.8 ± 1.3 |
| Estimated blood loss (ml) | 448 ± 238 | 555 ± 269 | 410 ± 249 | 405 ± 162 |
| Methadone (mg) | 0 | 6.1 ± 1.3 | 9.9 ± 1.4 | 17.4 ± 2.3 |
| Total intraperative nonmethadone opioid (mg morphine equivalents) | 81.0 ± 38.8 | 61.7 ± 25.5 | 76.6 ± 54.0 | 64.6 ± 27.3 |
| Total intraoperative opioid (mg morphine equivalents) | 81.0 ± 38.8 | 67.8 ± 25.5 | 86.5 ± 54.7 | 82.0 ± 26.6 |
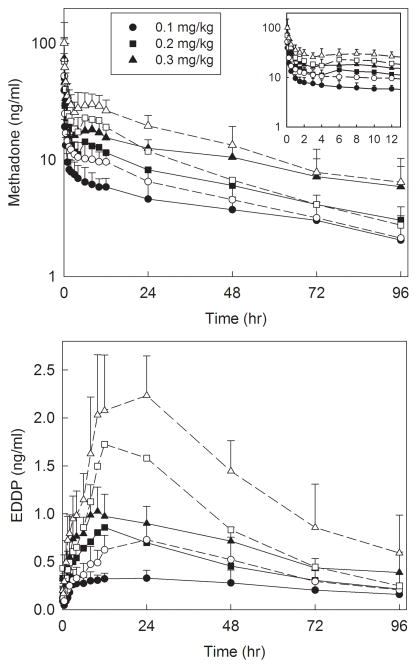
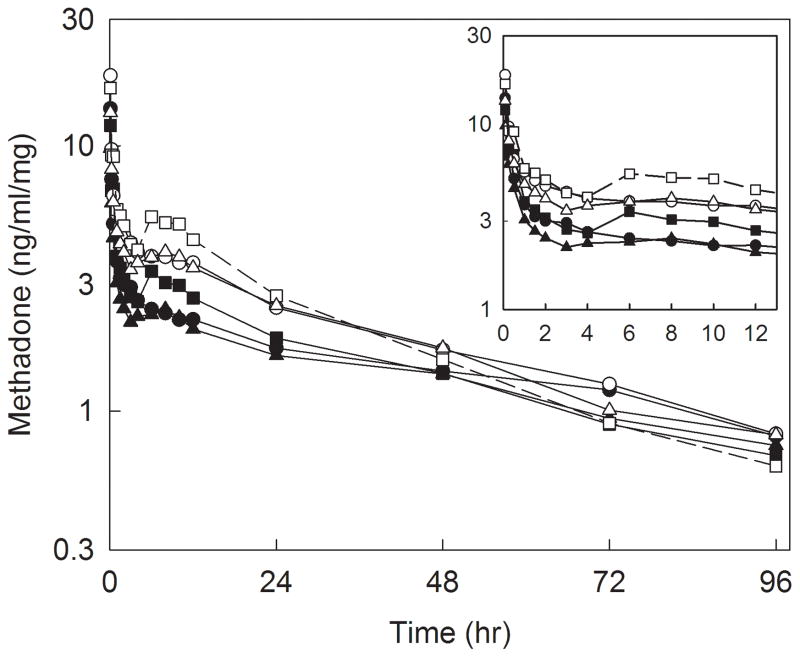
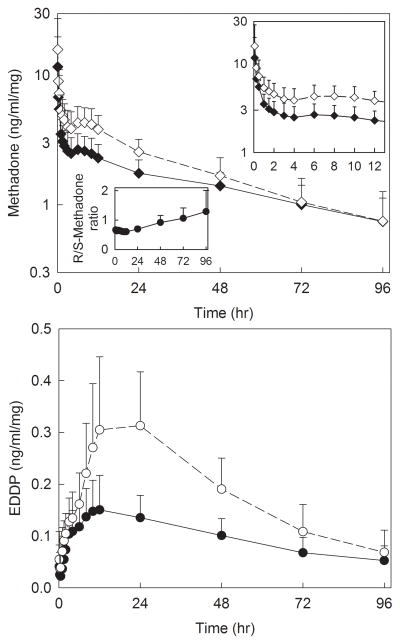
Plasma concentrations of methadone and EDDP enantiomers are shown in figure 1, and pharmacokinetic parameters provided in table 2. There was a secondary peak in methadone plasma concentrations approximately 6 h after dosing, which coincided with the end of surgery and turning supine. Methadone disposition was linear over the dose range 0.1–0.3 mg/kg, with methadone and EDDP Cmax and area under the plasma concentration-time curve (AUC∞) increasing linearly with methadone dose, for both methadone enantiomers. Additionally, methadone Cmax/dose, AUC∞/dose, systemic clearance, elimination t1/2, and Vss, and EDDP Cmax/dose, Tmax, AUC∞/dose, and elimination t1/2 were not significantly different between doses, for both R- and S-methadone. Methadone metabolism also appeared linear with dose, with the EDDP/methadone plasma AUC ratios not significantly different between doses, for both methadone enantiomers. Plasma EDDP concentrations were formation-rate limited, with EDDP enantiomers elimination half-lives not different from those of the corresponding methadone enantiomers. Unit dose (dose-adjusted) methadone and EDDP enantiomer plasma concentrations for dose groups, and all adolescents, are shown in figures 2 and 3. Methadone disposition was stereoselective, with S-enantiomer concentrations greater than those of R-methadone and EDDP, related to the smaller volume of distribution for S-methadone. Nevertheless, S-methadone elimination was more rapid, with a) greater rates of S-methadone N-demethylation (EDDP/methadone ratio), b) a shorter S-methadone elimination half-life, and c) time-dependent increase in the plasma R/S-methadone enantiomer ratio (fig. 3, insert).
Figure 1.
Methadone and 2-ethylidene-1.5-dimethyl-3.3diphenylpyrrolidine (EDDP) enantiomer plasma concentrations after intravenous methadone. Subjects received 0.1 (circles), 0.2 (squares), or 0.3 (triangles) mg/kg intravenous racemic (RS)-methadone HCl. Solid symbols and lines show R-methadone and R-EDDP, open symbols and dotted lines show S-methadone and S-EDDP. Each data point is the mean ± SD (n=10–11). Some SD are omitted for clarity. The inset shows the period from 0–12 hr.
Table 2.
Intravenous Methadone Pharmacokinetic Parameters
| (RS)-Methadone dose
|
||||||||
|---|---|---|---|---|---|---|---|---|
| 0.1 mg/kg | 0.2 mg/kg | 0.3 mg/kg | All | 0.1 mg/kg | 0.2 mg/kg | 0.3 mg/kg | All | |
|
| ||||||||
| R-methadone | S-methadone | |||||||
|
| ||||||||
| Cmax (ng/ml) | 39 ± 35 | 56 ± 21 | 78 ± 27* | 52 ± 50 | 75 ± 22 | 106 ± 40* | ||
| Cmax/dose (ng/ml/mg) | 14 ± 11 | 13 ± 5 | 10 ± 4 | 12 ± 7 | 19 ± 16 | 17 ± 6 | 14 ± 6 | 17 ± 10** |
| AUC∞ (ng •hr •ml−1) | 594 ± 389 | 837 ± 273 | 1,588 ± 789* | 679 ± 375 | 979 ± 245 | 1,835 ± 757* | ||
| AUC∞/dose (ng •hr•ml−1•mg−1) | 228 ± 164 | 189 ± 54 | 205 ± 102 | 207 ± 113 | 256 ± 151 | 223 ± 58 | 235 ± 88 | 238 ± 103** |
| CLIV (ml•kg−1•min−1) | 1.6 ± 0.7 | 2.0 ± 0.6 | 1.6 ± 0.8 | 1.7 ± 0.7 | 1.4 ± 0.8 | 1.6 ± 0.4 | 1.3 ± 0.5 | 1.4 ± 0.6** |
| Elimination t1/2 (hr) | 55 ± 38 | 43 ± 16 | 59 ± 37 | 52 ± 31 | 40 ± 25 | 28 ± 11 | 37± 17 | 35 ± 18 ** |
| Vss (L/kg) | 6.4 ± 1.4 | 6.6 ± 1.7 | 6.6 ± 1.5 | 6.5 ± 1.5 | 4.0 ± 1.0 | 3.8 ± 1.1 | 3.8± 0.9 | 3.8 ± 0.9** |
|
|
||||||||
| R-EDDP | S-EDDP | |||||||
|
|
||||||||
| Cmax (ng/ml) | 0.38 ± 0.11 | 0.88 ± 0.24* | 1.14 ± 0.30* | 0.76 ± 0.18* | 1.79 ± 0.52* | 2.35 ± 0.54* | ||
| Cmax/dose (ng/ml/mg) | 0.06 ± 0.01 | 0.09 ± 0.04 | 0.07 ± 0.03 | 0.07 ± 0.03 | 0.13 ± 0.03 | 0.19 ± 0.08 | 0.14 ± 0.05 | 0.15 ± 0.06** |
| Tmax (hr) | 14 ± 14 | 12 ± 6 | 11 ± 6 | 13 ± 9 | 24 ± 9 | 17 ± 7 | 14 ± 6 | 18 ± 8** |
| AUC0–96 (ng •hr •ml−1) | 23 ± 10 | 42 ± 11 | 55 ± 15 | 42 ± 16 | 78 ± 22 | 111 ± 32* | ||
| AUC∞ (ng •hr •ml−1) | 46 ± 28 | 59 ± 20 | 106 ± 65* | 60 ± 26 | 107 ± 49 | 192 ± 93* | ||
| AUC∞/dose (ng •hr•ml−1•mg−1) | 7.4 ± 4.0 | 6.0 ± 1.9 | 6.1 ± 3.7 | 6.5 ± 3.3 | 9.8 ± 3.4 | 11.4 ± 7.1 | 11.2 ± 5.5 | 10.8 ± 5.4** |
| Elimination t1/2 (hr) | 72 ± 42 | 41 ± 17 | 68 ± 57 | 61 ± 43 | 41 ± 20 | 31 ± 14 | 49 ± 36 | 41 ± 25** |
| AUC0–96 (EDDP/methadone) | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.09 ± 0.03 | 0.09 ± 0.02 | 0.09 ± 0.02 | 0.09 ± 0.02** |
| AUC∞ (EDDP/methadone) | 0.08 ± 0.03 | 0.07 ± 0.02 | 0.07 ± 0.02 | 0.07 ± 0.02 | 0.10 ± 0.03 | 0.12 ± 0.07 | 0.10 ± 0.04 | 0.11 ± 0.05** |
AUC = area under the plasma concentration-time curve; Cmax = peak plasma concentration; Cmax/dose = dose-adjusted peak plasma concentration; CLIV = systemic clearance; Tmax = time to peak plasma concentration EDDP = 2-ethyl-1,5-dimethyl-3,3-diphenylpyrrolidine; Vs = steady-state volume of distribution.
Significantly different compared with 0.1 mg/kg (p < 0.05)
Significantly different between enantiomers (p < 0.05)
Figure 2.
Dose-adjusted methadone plasma concentrations after intravenous methadone. Subjects received 0.1 (circles), 0.2 (squares), or 0.3 (triangles) mg/kg intravenous racemic (RS)-methadone HCl. Solid symbols and lines show R-methadone, open symbols and dotted lines show S-methadone. Each data point is the mean. The inset shows the period from 0–12 hr.
Figure 3.
Dose-adjusted methadone and 2-ethylidene-1.5-dimethyl-3.3diphenylpyrrolidine (EDDP) enantiomer plasma concentrations after intravenous methadone in all subjects. Solid symbols and lines show R-methadone and R-EDDP, open symbols and dotted lines show S-methadone and S-EDDP. Each data pint is the mean ± SD (n=31). The insets show dose-adjusted methadone and EDDP enantiomer plasma concentrations from 0–12 hr, and plasma R/S-methadone concentration ratios.
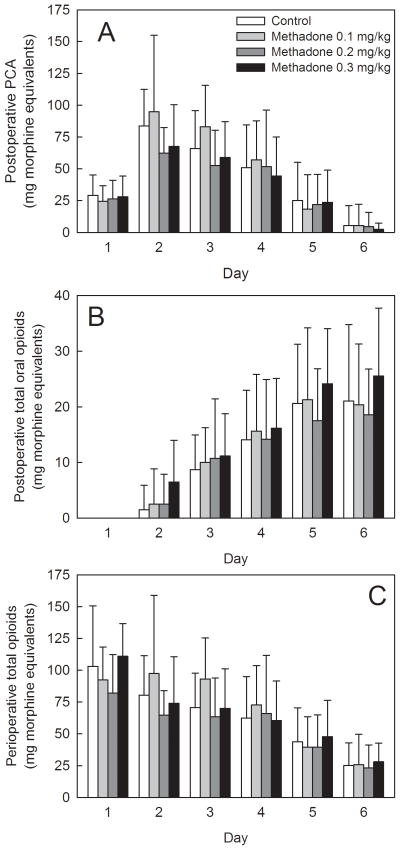
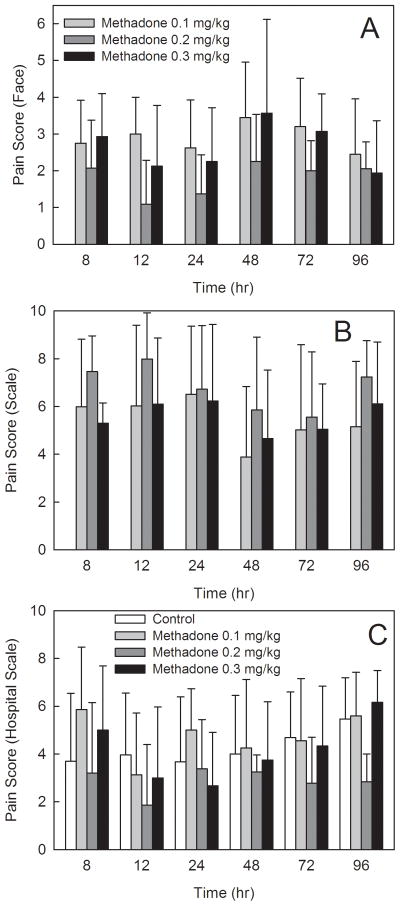
Perioperative opioid consumption and postoperative pain scores are shown in figures 4 and 5. There were no significant differences between controls (not receiving methadone) and adolescents receiving 0.1, 0.2, or 0.3 mg/kg methadone, in postoperative opioid consumption by patient controlled analgesia, oral opioid administration, or total postoperative opioid consumption. Excluding the dose of methadone received intraoperatively, cumulative 0–72 h morphine equivalents (patient controlled analgesia plus oral opiods) was 274 ± 82, 277 ± 92, 215 ± 76 and 221 ± 80 mg in patients receiving 0, 0.1, 0.2, or 0.3 mg/kg methadone, respectively. Differences between groups were not statistically significant.
Figure 4.
Influence of intraoperative methadone on analgesic requirements. Subjects received 0.1, 0.2, or 0.3 mg/kg intravenous racemic (RS)-methadone HCl. Controls received no intraoperative methadone. Day 1 was the day of surgery. Results are shown for (A) postoperative patient-controlled analgesia use, (B) postoperative oral opioid use, and (C) total (day 1–6) perioperative opioid use. Intraoperative opioids, patient controlled analgesia (morphine or hydromorphone), oral opioids (typically oxycodone) were converted to morphine equivalents. There were no significant differences between controls and patients receiving methadone.
Figure 5.
Influence of intraoperative methadone on pain scores. Time zero was the beginning of surgery. Results are shown for (A) pain scores based on patients facial expression using the Wong-Baker FACES scale (0–5), (B) pain scores based on patient report using a Colored-Visual Analog Scale (0–10), and (C) ward nurse assessment of pain scores using a verbal analogue scale (0–10). Only nurse assessment of pain scores were determined for all groups. There were no significant differences between controls and patients receiving methadone.
There were no significant differences between methadone groups in research staff assessments of patient pain, based either on FACES scores or patient self-reports of pain based on visual analog scales. There were no significant differences between controls (not receiving methadone) and adolescents receiving 0.1, 0.2, or 0.3 mg/kg methadone, in pain scores based on standard assessments of ward nurses and recorded in the medical record.
There were no serious adverse events, or adverse events specifically associated with the use of methadone. Table 3 summarizes the incidence of respiratory depression, decreased oxygen saturation, or altered mental status as observed and documented by inpatient nurses. There were no differences between methadone dose groups, therefore data were combined for ease of reporting. The incidence of adverse events was not different between controls and subjects receiving methadone.
Table 3.
Perioperative Opioid-related Adverse Events
| Controls (n = 31) | Methadone (all doses, n = 31) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Day | Respiratory depression | Decreased oxygen saturation | Altered mental status | Respiratory depression | Decreased oxygen saturation | Altered Mental Status |
| 1 | 5 | 8 | 1 | 5 | 4 | 0 |
| 2 | 3 | 11 | 0 | 2 | 10 | 0 |
| 3 | 0 | 3 | 0 | 0 | 3 | 0 |
| 4 | 2 | 7 | 0 | 0 | 1 | 0 |
| 5 | 0 | 2 | 0 | 0 | 0 | 0 |
| 6 | 0 | 1 | 0 | 0 | 0 | 0 |
Results are the number of patient episodes in each group. Information on respiratory depression (<8/min), decreased oxygen saturation (<92% on room air), and altered mental status (i.e., confusion, hallucinations, disorientation) as observed and documented by inpatient nurses on daily flowsheets was abstracted from medical records. Day 1 was the day of surgery.
Discussion
This investigation is the first to systematically evaluate methadone pharmacokinetics and perioperative opioid-sparing effects in children 10–18 yr. The protocol used a conservative dose-escalation design, and the 0.1–0.3 mg dose range was chosen, because a) this was to be the first investigation of methadone pharmacokinetics in children, b) previous reports of methadone use in children used doses of 0.1 or 0.2 mg/kg,30,31 and previous perioperative investigations of methadone in adults had not used doses higher than 20 mg (nominally 0.3 mg/kg, assuming 70 kg patients).11–13 Due to the goal of blood sampling for 96 h, and our hospital surgical demographics, the study population unintentionally consisted almost exclusively of scoliosis patients undergoing major spinal surgery. Since the most common age for this procedure is adolescence, the final study population unintentionally consisted of adolescents.
Results of this investigation provide several novel findings about methadone disposition. One major finding was that intravenous methadone pharmacokinetics in adolescents in the perioperative period were linear over the dose range 0.1–0.3 mg/kg. For both methadone enantiomers, dose-adjusted methadone Cmax and AUC, and systemic clearance, Vss, and elimination half-life, were constant over the dose range, as were dose-adjusted EDDP Cmax and AUC, and the EDDP/methadone AUC ratio. Therefore subsequent discussion refers to parameters averaged across the entire study population. A second major finding was that methadone disposition in adolescents was stereoselective. The initial S-methadone concentrations were greater than those of R-methadone. Systemic clearance, elimination t1/2, and Vss were significantly greater for R- than S-methadone. S-methadone N-demethylation was greater than that of R-methadone, evidenced by greater EDDP/methadone AUC ratios for the R- than S-enantiomers, and a time-dependent increase in the plasma R/S-methadone concentration ratio. A third major finding was that perioperative methadone disposition in adolescents was similar to that in adults. While there are numerous studies of methadone pharmacokinetics in adults, most single-dose intravenous studies have evaluated racemic methadone concentrations. Several recent investigations evaluated methadone and EDDP enantiomers concentrations after intravenous dosing in healthy adult volunteers.19,21,22 In this investigation, the time-dependent increase in plasma R/S-methadone ratio was similar to that in the healthy adult volunteers, R- and S-methadone clearances (1.7 and 1.4 ml/kg/min) were approximately 15% lower than the averages in the healthy adults (2.0 and 1.6 ml/kg/min), Vss (6.5 and 3.8 L/kg) were similar to those in adults (6.0 and 3.3 L/kg), the elimination t1/2 (52 and 35 h) was longer than the average in adults (39 and 27 h) but consistent with the slightly lower clearances. Methadone N-demethylation (based on the R- and S-EDDP/methadone AUC ratios) was similar in the adolescents (0.07 and 0.11) and adults (0.07 and 0.09). Reasons for the slightly lower methadone clearance in adolescents are not apparent, but the differences are not considered clinically significant. The present results are consistent with the predictions of Yang et al, who used a physiologically based pharmacokinetic model to predict that methadone enantiomer clearances would increase with age in infants but reach plateau values by age 2 yr.29 Additional studies are needed to characterize methadone pharmacokinetics in younger children and infants.
Methadone effects on analgesia and opioid consumption were somewhat unexpected. Methadone administration did not affect postoperative pain scores, and did not decrease daily or total postoperative opioid consumption. The former result could be explained on the basis of access to patient controlled analgesia throughout the perioperative period, although previous studies did report slightly lower pain scores in adults undergoing abdominal hysterectomy35 and in children30 receiving methadone compared with morphine, although there was no major difference in pain scores between methadone- and sufentanil-treated adults undergoing complex spine surgery.36 The lack of opioid-sparing with methadone, however, was initially more surprising. Previous studies of perioperative methadone in adults and children found longer analgesia, fewer postoperative opioid doses, and lower cumulative postoperative opioid use compared with morphine or other opioids.30,31,35,36 For example, adults receiving 0.2 mg/kg intravenous methadone at induction had significantly lower postoperative opioid requirements (median 98 vs. 219 mg morphine equivalents 0–72 h postoperatively) than those receiving sufentanil.36 In this investigation, patient controlled analgesia use on days 2 and 3 appeared numerically lower, but this did not achieve statistical significance. Nonetheless, the investigation was not powered specifically to evaluate opioid consumption, which was a secondary outcome. In addition, as identified previously, the type of surgical procedure and associated severity of pain likely influence the opioid-sparing effect of methadone.35 For example, after methadone, adults undergoing inguinal hernoirrhaphy received no additional opioids,37 those undergoing orthopedic (typically anterior spinal fusion) or general surgery (typically open cholecystectomy) often needed no or minimal postoperative opioids,11, while opioid-sparing was less in adults undergoing more painful upper abdominal 12 or complex spine surgery.36 Indeed, in the present population undergoing posterior spinal fusion, the cumulative 0–72 h postoperative morphine equivalent use was 274 ± 82, 277 ± 92, 215 ± 76 and 221 ± 80 mg in adolescents receiving 0, 0.1, 0.2, or 0.3 mg/kg methadone, respectively, where opioid-sparing was not statistically significant, while that in adults undergoing multilevel thoracolumbar surgery was a median of 219 mg morphine equivalents, where opioid-sparing occurred,36 suggesting that the present population had more pain compared with other studies. Finally, there was considerable use of other intraoperative opioids (mainly fentanyl and sufentanil), and even at the highest methadone dose, methadone constituted only a small fraction of total perioperative opioid on the day of surgery. Thus, using fixed-doses of methadone across the full spectrum of surgical procedures and associated pain may not be optimal; rather, higher doses may be needed for more painful procedures. Use of shorter-duration supplemental opioids (i.e., remifentanil), use of methadone rather than other opioids in the postanesthesia care unit, or use of higher methadone doses may have decreased daily or total postoperative opioid consumption and demonstrated an opioid-sparing effect of methadone in this investigation.
Despite the absence of specific quantitative analgesic and opioid-sparing effects of methadone, there were clearly observable, albeit anecdotal, differences in patients receiving methadone. Recovery room nurses commented spontaneously on greater comfort in those patients enrolled in the investigation. They requested that whatever had changed be implemented for all patients. Similarly, many pediatric anesthesiologists, who had not previously used methadone, have implemented its use intraoperatively for other for other painful operations like pectus excavatum repair. Whether the qualitative differences in the adolescents treated with methadone compared with other opioids are related to the unique pharmacology of methadone (i.e., N-methyl-D-aspartate receptor antagonism), as speculated previously,36 remains unknown and merits further investigation.
There was no difference in the frequency of adverse effects associated with the use of methadone in this investigation, consistent with previous report. Indeed, the use of substantial additional intraoperative opioids, and the requirement for substantial postoperative opioids, suggests that even the highest dose used (0.3 mg/kg) was well below the threshold for significant opioid-related adverse events.
There are several potential limitations to the present investigation. The need for several days of venous sampling, and hence inpatient stay, resulted in a patient population undergoing major surgery (posterior spinal fusion), which consequently resulted also in an adolescent study cohort. Characterization of methadone pharmacokinetics and perioperative opioid-sparing effects in younger children would be desirable. Similarly, evaluation of appropriate dosing, analgesia and opioid-sparing in less extensive and painful operations is also desirable. This investigation did not use methadone in the postoperative period (either the postanesthesia care unit or the ward), and additional benefit might be gained by this approach. Similarly, only single-dose methadone pharmacokinetics and perioperative opioid-sparing effects were evaluated. Anesthetic care was not otherwise changed for this investigation, specifically, a proscription against other intraoperative opioids,10 which might have masked an opioid-sparing effect from methadone. Two cohorts were studied, a methadone group in a dose-escalation protocol, and a non-methadone group, rather than a single cohort with full randomization across all groups. This was because a conservative, dose-escalation approach was required for safety considerations. The non-methadone controls were not randomized, but rather taken from a contemporaneous group of adolescents having surgery. They were carefully matched demographically to the adolescents receiving methadone, as evidenced by the similarity of pain scores and opioid use to the lowest dose methadone group.
In summary, this investigation is the first to evaluate the pharmacokinetics of methadone enantiomers in children, specifically adolescents. Methadone disposition in this population was similar to that in adults.
Final box summary.
What we already know about this topic
Methadone may be a useful perioperative opioid analgesic because of its rapid onset of effect and its prolonged duration of action due to its long elimination half-life.
Methadone pharmacokinetics have been characterized in adults.
What this article tells us that is new
Methadone pharmacokinetics in adolescents are similar to those in adults.
A single intraoperative dose of methadone did not decrease postoperative opioid consumption in adolescents undergoing major spine surgery
Acknowledgments
Supported by grants R01-DA14211 and K24-DA00417 (to Dr. Kharasch) and UL1-RR024992 (to the Washington University Institute for Clinical and Translational Research) from the National Institutes of Health, Bethesda, Maryland
Footnotes
ClinicalTrials.gov Identifier: NCT00921843
References
- 1.Kokki H. Current management of pediatric postoperative pain. Expert Rev Neurother. 2004;4:295–306. doi: 10.1586/14737175.4.2.295. [DOI] [PubMed] [Google Scholar]
- 2.Fortier MA, MacLaren JE, Martin SR, Perret-Karimi D, Kain ZN. Pediatric pain after ambulatory surgery: Where’s the medication? Pediatrics. 2009;124:e588–95. doi: 10.1542/peds.2008-3529. [DOI] [PubMed] [Google Scholar]
- 3.Rony RY, Fortier MA, Chorney JM, Perret D, Kain ZN. Parental postoperative pain management: Attitudes, assessment, and management. Pediatrics. 2010;125:e1372–8. doi: 10.1542/peds.2009-2632. [DOI] [PubMed] [Google Scholar]
- 4.Monitto CL, Kost-Byerly S, Yaster M. In: Pain management, Anesthesia for infants and children. Davis PJ, Cladis FP, Motoyama EK, editors. Philadelphia: Elsevier-Mosby; 2011. pp. 418–51. [Google Scholar]
- 5.Zempsky WT, Cravero JP. Relief of pain and anxiety in pediatric patients in emergency medical systems. Pediatrics. 2004;114:1348–56. doi: 10.1542/peds.2004-1752. [DOI] [PubMed] [Google Scholar]
- 6.Stearns SD, Smith CA, Carter BD. Psychlogical ramifications of pediatric pain. Clin Ped Emerg Med. 2000;1:299–305. [Google Scholar]
- 7.Nicholson AB. Methadone for cancer pain. Cochrane Database Syst Rev. 2007:CD003971. doi: 10.1002/14651858.CD003971.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O’Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts RG, Todd KH, Miaskowski C. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–30. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lobmaier P, Gossop M, Waal H, Bramness J. The pharmacological treatment of opioid addiction--a clinical perspective. Eur J Clin Pharmacol. 2010;66:537–45. doi: 10.1007/s00228-010-0793-6. [DOI] [PubMed] [Google Scholar]
- 10.Kharasch ED. Intraoperative methadone: Rediscovery, reappraisal, and reinvigoration? Anesth Analg. 2011;112:13–6. doi: 10.1213/ANE.0b013e3181fec9a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gourlay GK, Wilson PR, Glynn CJ. Pharmacodynamics and pharmacokinetics of methadone during the perioperative period. Anesthesiology. 1982;57:458–67. doi: 10.1097/00000542-198212000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Gourlay GK, Willis RJ, Wilson PR. Postoperative pain control with methadone: Influence of supplementary methadone doses and blood concentration-response relationships. Anesthesiology. 1984;61:19–26. [PubMed] [Google Scholar]
- 13.Gourlay GK, Willis RJ, Lamberty J. A double-blind comparison of the efficacy of methadone and morphine in postoperative pain control. Anesthesiology. 1986;64:322–7. doi: 10.1097/00000542-198603000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Gerber JG, Rhodes RJ, Gal J. Stereoselective metabolism of methadone N-demethylation by cytochrome P4502B6 and 2C19. Chirality. 2004;16:36–44. doi: 10.1002/chir.10303. [DOI] [PubMed] [Google Scholar]
- 15.Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of hepatic and intestinal cytochrome P450 3A and 2B6 in the metabolism, disposition and miotic effects of methadone. Clin Pharmacol Ther. 2004;76:250–69. doi: 10.1016/j.clpt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Totah RA, Allen KE, Sheffels P, Whittington D, Kharasch ED. Enantiomeric metabolic interactions and stereoselective human methadone metabolism. J Pharmacol Exp Ther. 2007;321:389–99. doi: 10.1124/jpet.106.117580. [DOI] [PubMed] [Google Scholar]
- 17.Totah RA, Sheffels P, Roberts T, Whittington D, Thummel K, Kharasch ED. Role of CYP2B6 in stereoselective human methadone metabolism. Anesthesiology. 2008;108:363–74. doi: 10.1097/ALN.0b013e3181642938. [DOI] [PubMed] [Google Scholar]
- 18.Chang Y, Fang WB, Lin SN, Moody DE. Stereo-selective metabolism of methadone by human liver microsomes and cDNA-expressed cytochrome P450s: A reconciliation. Basic Clin Pharmacol Toxicol. 2010;108:55–62. doi: 10.1111/j.1742-7843.2010.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kharasch ED, Bedynek PS, Park S, Whittington D, Walker A, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics. I. Evidence against CYP3A mediation of methadone clearance. Clin Pharmacol Ther. 2008;84:497–505. doi: 10.1038/clpt.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kharasch ED, Bedynek PS, Walker A, Whittington D, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics. II. Ritonavir effects on CYP3A and P-glycoprotein activities. Clin Pharmacol Ther. 2008;84:506–12. doi: 10.1038/clpt.2008.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kharasch ED, Walker A, Whittington D, Hoffer C, Bedynek PS. Methadone metabolism and clearance are induced by nelfinavir despite inhibition of cytochrome P4503A (CYP3A) activity. Drug Alcohol Depend. 2009;101:158–68. doi: 10.1016/j.drugalcdep.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kharasch ED, Hoffer C, Whittington D, Walker A, Bedynek PS. Methadone pharmacokinetics are independent of cytochrome P4503A (CYP3A) activity and gastrointestinal drug transport: Insights from methadone interactions with ritonavir/indinavir. Anesthesiology. 2009;110:660–72. doi: 10.1097/ALN.0b013e3181986a9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shir Y, Rosen G, Zeldin A, Davidson EM. Methadone is safe for treating hospitalized patients with severe pain. Can J Anaesth. 2001;48:1109–13. doi: 10.1007/BF03020377. [DOI] [PubMed] [Google Scholar]
- 24.Berde CB, Sethna NF. Analgesics for the treatment of pain in children. N Engl J Med. 2002;347:1094–103. doi: 10.1056/NEJMra012626. [DOI] [PubMed] [Google Scholar]
- 25.Mercadante S. Cancer pain management in children. Palliat Med. 2004;18:654–62. doi: 10.1191/0269216304pm945rr. [DOI] [PubMed] [Google Scholar]
- 26.Friedrichsdorf SJ, Kang TI. The management of pain in children with life-limiting illnesses. Pediatr Clin North Am. 2007;54:645–72. doi: 10.1016/j.pcl.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Boyer EW, McCance-Katz EF, Marcus S. Methadone and buprenorphine toxicity in children. Am J Addict. 2009;19:89–95. doi: 10.1111/j.1521-0391.2009.00002.x. [DOI] [PubMed] [Google Scholar]
- 28.Simkin DR, Grenoble S. Pharmacotherapies for adolescent substance use disorders. Child Adolesc Psychiatr Clin N Am. 2010;19:591–608. doi: 10.1016/j.chc.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Yang F, Tong X, McCarver DG, Hines RN, Beard DA. Population-based analysis of methadone distribution and metabolism using an age-dependent physiologically based pharmacokinetic model. J Pharmacokinet Pharmacodyn. 2006;33:485–518. doi: 10.1007/s10928-006-9018-0. [DOI] [PubMed] [Google Scholar]
- 30.Berde CB, Beyer JE, Bournaki M-C, Levin CR, Sethna NF. Comparison of morphine and methadone for prevention of postoperative pain in 3- to 7-year-old children. J Pediatr. 1991;119:136–41. doi: 10.1016/s0022-3476(05)81054-6. [DOI] [PubMed] [Google Scholar]
- 31.Hamunen K. Ventilatory effects of morphine, pethidine and methadone in children. Br J Anaesth. 1993;70:414–8. doi: 10.1093/bja/70.4.414. [DOI] [PubMed] [Google Scholar]
- 32.Luffy R, Grove SK. Examining the validity, reliability, and preference of three pediatric pain measurement tools in African-American children. Pediatr Nurs. 2003;29:54–9. [PubMed] [Google Scholar]
- 33.Shaheen PE, Walsh D, Lasheen W, Davis MP, Lagman RL. Opioid equianalgesic tables: Are they all equally dangerous? J Pain Symptom Manage. 2009;38:409–17. doi: 10.1016/j.jpainsymman.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Whittington D, Sheffels P, Kharasch ED. Stereoselective determination of methadone and the primary metabolite EDDP in human plasma by automated on-line extraction and liquid chromatography mass spectrometry. J Chrom B. 2004;809:313–21. doi: 10.1016/j.jchromb.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 35.Richlin DM, Reuben SS. Postoperative pain control with methadone following lower abdominal surgery. J Clin Anesth. 1991;3:112–6. doi: 10.1016/0952-8180(91)90007-a. [DOI] [PubMed] [Google Scholar]
- 36.Gottschalk A, Durieux ME, Nemergut EC. Intraoperative methadone improves postoperative pain control in patients undergoing complex spine surgery. Anesth Analg. 2011;112:218–23. doi: 10.1213/ANE.0b013e3181d8a095. [DOI] [PubMed] [Google Scholar]
- 37.Callesen T, Bech K, Andersen J, Nielsen R, Roikjaer O, Kehlet H. Pain after primary inguinal herniorrhaphy: Influence of surgical technique. J Am Coll Surg. 1999;188:355–9. doi: 10.1016/s1072-7515(98)00316-0. [DOI] [PubMed] [Google Scholar]