Abstract
Binding of activated α2-macroglobulin (α2M) to LDL receptor-related protein-1 (LRP1) in Schwann cells activates ERK/MAP kinase and Akt and thereby promotes cell survival and migration. The goal of this study was to determine whether α2M binding to LRP1 regulates expression of cytokines and chemokines. To assess the LRP1 response selectively, we studied primary cultures of rat Schwann cells. In a screening assay that detects 84 gene products, monocyte chemoattractant protein-1 (MCP-1/CCL2) mRNA expression was increased more than 13-fold in Schwann cells treated with activated α2M. The effects of α2M on MCP-1 expression were selective, because expression of the general proinflammatory cytokine tumor necrosis factor-α (TNF-α) was not induced. We confirmed that α2M selectively induces expression of MCP-1 and not TNF-α in single-target qPCR assays. MCP-1 protein accumulated at increased levels in conditioned medium of α2M-treated cells. LRP1 was necessary for induction of MCP-1 expression, as determined in experiments with the LRP1 antagonist receptor-associated protein, a mutated form of full-length α2M that does not bind LRP1, and in studies with Schwann cells in which LRP1 was silenced. Inhibiting ERK/MAP kinase activation blocked expression of MCP-1. These studies support a model in which LRP1 regulates multiple aspects of Schwann cell physiology in the response to PNS injury.
Keywords: α2-macroglobulin, LDL receptor-related protein-1, monocyte chemoattractant protein-1, TNF-α, Schwann cell, protease
Schwann cells play an important role in injury to the peripheral nervous system (PNS). These cells de-differentiate, migrate, and secrete cytokines and chemokines involved in recruitment of inflammatory cells (Jessen and Mirsky, 1999; Campana, 2007). Recruited macrophages are essential to clear irreversibly damaged debris so that Schwann cells may establish extracellular scaffolds and direct regenerating axons (Stoll and Muller, 1999). Although the molecular mediators that promote macrophage recruitment remain incompletely understood, monocyte chemattractant protein-1 (MCP-1/CCL-2) is known to play a central role (Martini et al., 2008).
Shortly after PNS injury, the type-1 transmembrane protein LDL receptor-related protein-1 (LRP1) is substantially up-regulated in Schwann cells (Campana et al., 2006). LRP1 functions as a broad-spectrum endocytic receptor for diverse ligands and as a phagocytic receptor for apoptotic cells and degenerated myelin debris (Strickland et al., 2002; Kinchen and Ravichandran, 2007; Gaultier et al., 2009). In Schwann cells, LRP1 also functions as a robust cell-signaling receptor. Ligand binding to LRP1 activates ERK/MAP kinase and Akt, promoting Schwann cell survival and migration (Campana et al., 2006; Mantuano et al., 2008a,b).
Tissue-type plasminogen activator (tPA) and matrix metalloprotease-9 (MMP-9) are examples of proteases implicated in the response to PNS injury (La Fleur et al., 1996; Akassoglou et al., 2000; Kobayashi et al., 2008), which bind directly to LRP1 and activate LRP1-dependent cell signaling (Hu et al., 2006; Mantuano et al., 2008b; Shi et al., 2009). Other proteases may bind to the protease inhibitor α2-macroglobulin (α2M) and trigger α2M conformational change so that the complex becomes a high-affinity ligand for LRP1 (Barrett and Starkey, 1973; Barrett et al., 1979; Imber and Pizzo, 1981; Sottrup-Jensen, 1989; Strickland et al., 2002). By this pathway, diverse proteases, including those that do not bind directly to LRP1, may trigger LRP1-dependent Schwann cell signaling.
The goal of this study was to determine whether the cell-signaling pathways activated by ligand binding to LRP1, which are known to promote cell survival and migration, also regulate expression of chemokines and cytokines by Schwann cells. We performed these studies with primary cultures of rat Schwann cells. Our results demonstrate that binding of activated α2M to LRP1 substantially increases expression of MCP-1. This response is selective, because α2M does not induce expression of the potent proinflammatory cytokine tumor necrosis factor-α (TNF-α), which is expressed by Schwann cells in response to other mediators in vivo and in vitro (Campana, 2007). The increase in MCP-1 expression was dependent on ERK/MAP kinase activation downstream of LRP1 (Mantuano et al., 2008a). These results support a model in which LRP1-dependent cell signaling contributes to a wide spectrum of activities demonstrated by Schwann cells in PNS injury.
MATERIALS AND METHODS
Reagents
α2M was purified from human plasma by the method of Imber and Pizzo (1981) and activated for binding to LRP1 by dialysis against 200 mM methylamine HCl, as previously described (Gonias et al., 1982). Modification of α2M by methylamine was confirmed by demonstrating the characteristic increase in α2M electrophoretic mobility by nondenaturing PAGE (Barrett et al., 1979). Recombinant full-length human α2M (rα2M) and a mutated form of rα2M, in which Lys1370 and Lys1374 are converted to alanine (rα2M1370/1374), were expressed in K-562 cells, as previously described (Arandjelovic et al., 2005). α2M-specific polyclonal antibody was from DakoCytomation (Carpinteria, CA). LRP1-specific antibody 11H4 was purified from conditioned medium of a hybriddoma obtained from the American Type Culture Collection. Recombinant rat MCP-1 was from PeproTech. Receptor-associated protein (RAP) was expressed as a GST-fusion protein, as previously described (Herz et al., 1991). Unconjugated GST was expressed as a control. All GST-RAP and GST preparations were subjected to chromatography on Detoxi-gel endotoxin-removing columns (Pierce, Rockford, IL). TaqMan primers and probes for rat MCP-1 were from ABI (Foster City, CA). TNF-α primers were purchased from SA Biosciences. The pharmacological antagonists LY294002 (PI3K inhibitor) and PD098059 (MEK inhibitor) were purchased from Calbiochem (La Jolla, CA). Trypsin was purchased from Worthington. The concentration of active trypsin was determined as previously described (Chase and Shaw, 1969).
Sciatic Nerve Crush Injury in Mice
Adult (20-week-old) male C57BL/6 mice (n = 4) were anesthetized by intraperitoneal injection of ketamine/buprenorphine (ketamine 90 mg/kg, buprenorphine 0.05 mg/kg). After shaving and sterilizing the upper thigh, a 1.0-cm incision was made with a scalpel unilaterally on the left side under sterile conditions. The sciatic nerve was gently exposed by blunt dissection using forceps and then crushed for 30 sec at the midthigh level using smooth forceps. The crush site was marked with a 9-0 ethilon suture. The muscle layer was closed using 6-0 silk sutures, followed by the skin. The animals were reanesthetized 24 hr later. The section of sciatic nerve immediately distal to the crush site (0.5 cm) was recovered together with a similar section of the uninjured contralateral nerve. Mice were euthanized by cervical dislocation under anesthesia. This procedure was performed according to protocols approved by the UCSD Committee on Animal Research and conforms to NIH guidelines for animal research.
Immunoblot Analysis
Protein extracts were prepared from sciatic nerve tissue for immunoblot analysis as previously described (Shi et al., 2009). The protein content of each extract was determined by bicinchoninic acid assay (Pierce, Rockville, MD). Equal amounts of protein (10 μg) were subjected to SDS-PAGE and electrotransferred to PVDF membranes.
Cell Culture
Schwann cells were isolated from sciatic nerves of 1-day-old Sprague Dawley rats and further selected from fibroblasts using fibronectin-specific antibody and rabbit complement, as previously described (Campana et al., 1998). The final preparations consisted of >98% Schwann cells, as determined by immunofluorescence for S100, which is a specific Schwann cell marker. Primary cultures of Schwann cells were maintained in DMEM containing 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 21 μg/ml bovine pituitary extract, and 4 μM forskolin (complete medium) at 37°C under humidified 5% CO2. Schwann cell cultures were passaged no more than five times before conducting experiments. For experiments, Schwann cells were transferred to Sato medium (Bottenstein and Sato, 1980) for 1 hr and then treated with various forms of α2M in Sato medium for 6 hr. When indicated, cells were pretreated with either GST-RAP (100 nM) or GST (100 nM) for 1 hr before adding α2M. LY294002 (10 μM) or PD098059 (50 μM) was added for 30 min prior to α2M. This preincubation strategy was designed to ensure blockade of specific steps in the LRP1-initiated cell-signaling pathway, because activated α2M binds rapidly to LRP1 and triggers rapid cell signaling (Mantuano et al., 2008a,b).
qPCR
DNA-free total RNA was extracted from Schwann cells using the Nucleospin RNAII extraction kit (Macherey-Nagel), as directed by the manufacturer. Samples were purified and treated with DNase. cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Expression of MCP-1 and TNF-α were determined by qPCR using a StepOne qPCR machine (Applied Biosystems, Foster City, CA) and a one-step program: 95°C for 10 min followed by: 95°C, 30 sec; 60°C, 1 min for 40 cycles. GAPDH gene expression was measured as a normalizer. Results were analyzed by the relative quantity method (Thellin et al., 1999). All experiments were performed at least in triplicate with internal triplicate determinations.
PCR Array Analysis
Schwann cells in SATO medium were treated with methylamine-activated α2M (α2M-MA) or with vehicle for 6 hr. RNA was extracted and cDNA was synthesized. A screening assay to detect mRNA expression for 84 chemokines/cytokines was performed using the RT2 profiler PCR Array System from SA Biosciences. Results from this study were subjected to subsequent validation by conventional qPCR, as described above.
LRP1 Gene Silencing
The previously described rat LRP1-specific siRNA (CGAGCGACCUCCUAUC-UUUUU; Campana et al., 2006) and nontargeting control (NTC) siRNA were purchased from Dharmacon. Primary cultures of Schwann cells (1 × 106) were transfected with LRP1-specific siRNA (25 nM) or NTC siRNA (25 nM) by electroporation using the Rat Neuron Nucleofector kit (Amaxa). The degree of LRP1 gene silencing was greater than 90% at 24–72 hr, as determined by qPCR and RAP ligand blotting (Campana et al., 2006). Cells were treated with α2M 24–36 hr after introduction of siRNAs.
MCP-1 Protein Determination
Schwann cells in Sato medium were treated with vehicle, 50 nM native α2M, or 50 nM α2M-MA for 12 hr, as previously described (Mantunao et al., 2008a). Conditioned medium (CM) was collected. MCP-1 levels in CM were determined using the rat MCP-1 Instant ELISA kit (Bender Medsystems, Burlingame, CA). Because α2M is known to bind growth factors and cytokines (Crookston et al., 1994; Wolf and Gonias, 1994), we performed control experiments to determine whether the presence of α2M in CM influenced MCP-1 detection by ELISA. Recombinant rat MCP-1 was incubated with 50 nM α2M, α2M-MA, or vehicle for 15 min at 22°C. The reagents were then added together to the ELISA plate wells.
RESULTS
MCP-1 Expression Is Selectively Increased in Schwann Cells Treated With Activated α2M
Schwann cells express LRP1 after PNS injury (Campana et al., 2006). Binding of activated α2M to LRP1 in Schwann cells activates ERK/MAP kinase and Akt (Mantuano et al., 2008a), promoting cell survival and migration (Campana et al., 2006; Mantuano et al., 2008b). We hypothesized that, in PNS injury, damage to the blood–nerve barrier would allow penetration of α2M into the intraneural space, so that α2M is available to interact with LRP1 in vivo. To test this hypothesis, we subjected mouse sciatic nerves to crush injury; 24 hr later, the crushed and contralateral nerves were recovered. Protein extracts from these nerves were subjected to immunoblot analysis to detect α2M. As shown in Figure 1, α2M was detected in four of four injured nerves but barely detectable in the four uninjured, contralateral nerves.
Fig. 1.
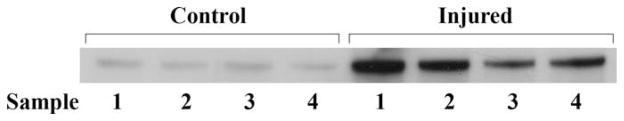
α2M accumulates in the mouse sciatic nerve after crush injury. The left sciatic nerve was subjected to crush injury and harvested 24 hr later together with the uninjured contralateral nerve. Extracts were prepared and subjected to immunoblot analysis to detect α2M. Samples 1–4 were obtained from four separate mice treated equivalently.
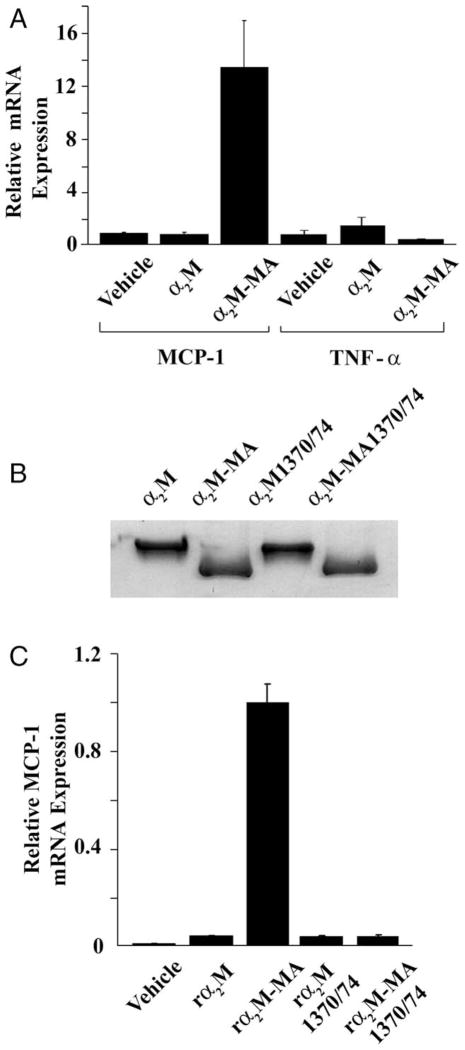
Next, we sought to determine whether ligation of LRP1 by α2M, in addition to its effects on Schwann cell survival and migration, regulates expression of cytokines known to be involved in the response to PNS injury. To begin, we treated Schwann cells in primary culture with α2M (50 nM), which had been purified from plasma and activated to bind to LRP1 by reaction with methylamine (α2M-MA; Gonias et al., 1982; Mantuano et al., 2008a). We then screened for expression of 84 chemokines and cytokines using the RT2 Profiler PCR Array System. Compared with cells that were treated with vehicle, α2M-MA-treated cells demonstrated a 13.4-fold increase in MCP-1 expression; this was the largest change in expression noted in the screening assay. Factors that were regulated 2.5-fold or more are listed in Table I. Importantly, expression of TNF-α, which functions as a central inflammatory mediator in PNS injury (Campana, 2007), was not increased by α2M-MA (expression was decreased by 39% in the screening assay).
TABLE I.
Effects of Activated α2M on Expression of Chemokines/Cytokines and Their Receptors by Rat Schwann Cells in Primary Culture*
| Symbol | Description | Relative mRNA expressiona |
|---|---|---|
| MCP-1/CCL2 | Monocyte chemoattractant protein-1 | 13.4-fold |
| CCL-7 | Chemokine (C-C motif) ligand 7 | 7.3 |
| Inhbb | Inhibin beta-B | 4.7 |
| IL8ra | Interleukin 8 receptor-α | 3.7 |
| LIF | Leukemia inhibitory factor | 3.3 |
| CCR-5 | Chemokine (C-C motif) receptor 5 | 2.8 |
| Rgs3 | Regulator of G-protein signaling 3 | 2.5 |
| CCL-3 | Chemokine (C-C motif) ligand 3 | 0.33 |
| Cx3Cl1 | Chemokine (C-X3-C motif) ligand 1 | 0.33 |
| CSF-2 | Colony-stimulating factor-2 | 0.32 |
| Bmp6 | Bone morphogenetic protein 6 | 0.32 |
Schwann cells were treated with 50 nM activated α2M or vehicle for 6 hr.
Expression is calculated for α2M-treated cells vs. cells that were treated with vehicle. Gene products that were increased or decreased by >2.5-fold are shown.
To validate the results of the screening assay, Schwann cells were treated with α2M-MA and expression of MCP-1 and TNF-α were determined by single-target qPCR. As shown in Figure 2A, α2M-MA increased MCP-1 expression over tenfold. With the identical samples, we determined TNF-α mRNA levels. As suggested by the screening assay, activated α2M failed to regulate expression of TNF-α. Thus, the effects of activated α2M on expression of MCP-1 are selective.
Fig. 2.
Activated α2M induces MCP-1 expression by Schwann cells in culture. A: Schwann cells were treated with vehicle, native α2M (50 nM), or α2M-MA (50 nM) for 6 hr. Levels of mRNA for MCP-1 and TNF-α were determined by qPCR (mean ± SEM). B: rα2M, rα2M-MA, rα2M1370/1374, and rα2M1370/1374-MA were subjected to nondenaturing PAGE. Protein was detected by Coomassie staining. C: Schwann cells were treated with vehicle, rα2M, rα2M-MA, rα2M1370/1374, or rα2M1370/1374-MA (each at 50 nM). MCP-1 mRNA was determined by qPCR (mean ± SEM). Expression is standardized relative to the highest value.
Unlike other LRP1 ligands, activated α2M does not bind to functionally redundant members of the LDL receptor gene family, such as the VLDL receptor (Strickland et al., 2002). As an alternative to plasma-purified α2M, we studied recombinant α2M (rα2M), which was expressed in K-562 cells (Arandjelovic et al., 2005). Rα2M1370/1374 is a derivative of full-length rα2M in which Lys1370 and Lys1374 are mutated to alanine so that the protein is not recognized by LRP1, even after activation (Arandjelovic et al., 2005). Both forms of rα2M undergo conformational change when reacted with methylamine, which is accompanied by an increase in mobility when the proteins are subjected to nondenaturing PAGE (Fig. 2B). Methylamine-activated rα2M significantly increased MCP-1 expression by Schwann cells, whereas the native form of rα2M did not (Fig. 2C). Thus, rα2M and plasma-purified α2M behaved equivalently. rα2M1370/1374 failed to induce expression of MCP-1, even after activation with methylamine. Failure of rα2M1370/1374 to induce MCP-1 expression provided evidence that LRP1 is required for this response.
The Effects of α2M on MCP-1 Expression by Schwann Cells Are Dependent on LRP1
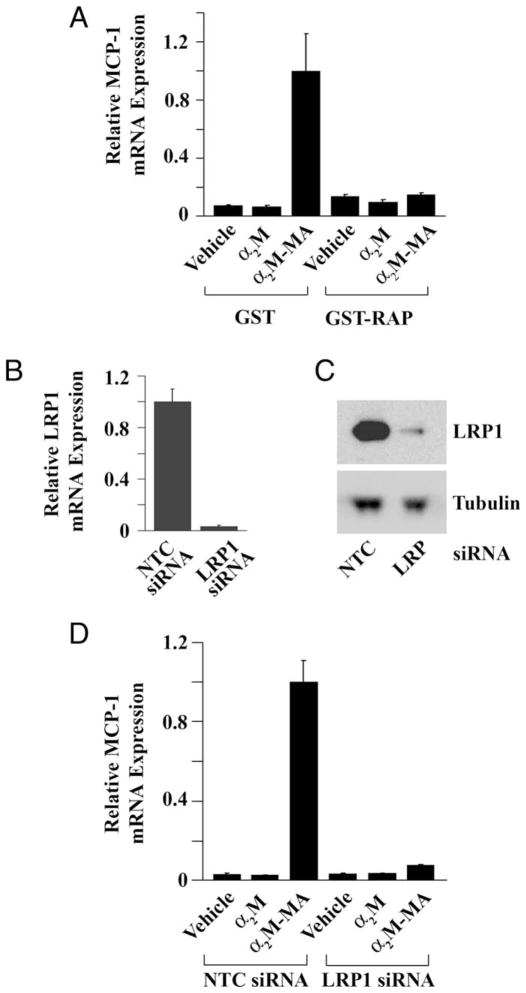
To confirm that binding to LRP1 is necessary for α2M-induced MCP-1 expression, we treated cells with α2M-MA after pretreating with RAP, which binds to LRP1 and inhibits binding of all other ligands (Herz et al., 1991; Strickland et al., 2002). RAP does not trigger cell signaling and blocks cell signaling initiated by other LRP1 ligands, including activated α2M (Mantuano et al., 2008a; Shi et al., 2009). Figure 3A shows that RAP completely blocked MCP-1 expression in response to α2M-MA. Because RAP is expressed as a GST-fusion protein, we treated cells with GST as a control. GST had no effect on α2M-MA-induced MCP-1 expression.
Fig. 3.
Increased MCP-1 expression requires LRP1. A: Schwann cells were pretreated with GST-RAP (100 nM) or GST and then with α2M or α2M-MA for 6 hr, and MCP-1 mRNA was determined. B: LRP1 mRNA was determined by qPCR 30 hr after introducing LRP1-specific siRNA or NTC siRNA. C: LRP1 gene silencing also was assessed by immunoblot analysis using antibody 11H4. D: Schwann cells transfected with LRP1-specific or NTC siRNA were treated with native α2M or α2M-MA, and MCP-1 mRNA was determined.
RAP antagonizes ligand binding to multiple receptors in the LDL receptor gene family (Strickland et al., 2002). Thus, as a second method to assess the role of LRP1, we applied gene silencing, using a previously described LRP1-specific siRNA (Campana et al., 2006). Figure 3B shows that, 30 hr after introducing siRNA, the level of LRP1 mRNA was decreased by greater than 95% in gene-silenced cells compared with cells that were transfected with NTC siRNA. LRP1 protein expression also was substantially decreased, as determined by immunoblot analysis (Fig. 3C). Figure 3D shows that LRP1 gene silencing almost completely neutralized the effects of α2M-MA on MCP-1 expression.
α2M-protease Complexes Induce Expression of MCP-1
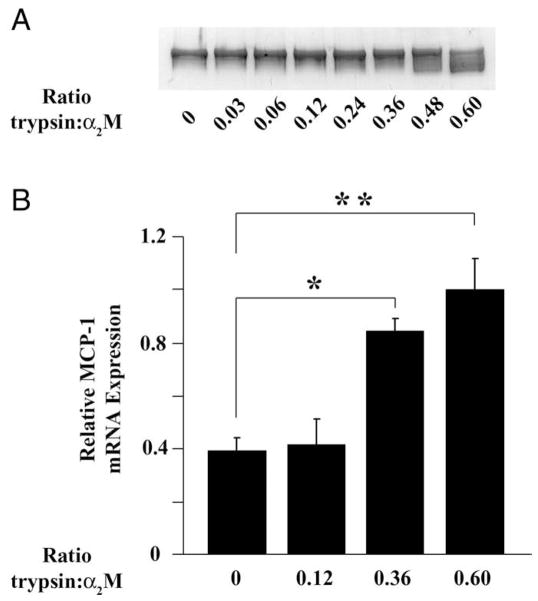
The major form of activated α2M generated in vivo is α2M-protease complex and not α2M-MA; however, the two forms of activated α2M have equivalent conformations (Gonias et al., 1982) and bind to LRP1 with equivalent affinity (Imber and Pizzo, 1981). Proteases that bind to α2M retain enzymatic activity (Sottrup-Jensen, 1989), introducing complexities, which frequently make α2M-MA the preferred ligand for studying LRP1 activity. To test whether α2M-protease complexes induce MCP-1 expression, we treated α2M with increasing molar ratios (r) of trypsin. The α2M was always in excess to avoid free protease. Because α2M has two distinct protease-binding sites, the concentration of α2M that is activated for LRP1 binding by trypsin follows a nonlinear mathematical relationship (Gonias and Pizzo, 1983). For example, if 50 nM α2M is reacted with trypsin at a ratio of 0.5 (r = 0.5), the concentration of activated α2M is predicted to be 22 nM (Gonias et al., 1983). Figure 4A shows that, as the r value was increased, α2M was progressively converted into the more rapidly migrating species by nondenaturing PAGE, indicative of conformational change and activation for LRP1 binding (Barrett et al., 1979; Gonias and Pizzo, 1983). Figure 4B shows that α2M-trypsin complex formed at r = 0.36 or 0.6 significantly increased MCP-1 expression (P < 0.05 for r = 0.36 and < 0.01 for r = 0.6).
Fig. 4.
α2M-protease complexes induce MCP-1 expression by Schwann cells. A: α2M was reacted with increasing concentrations of active trypsin. The products were subjected to nondenaturing PAGE and Coomassie staining. The ratio (r) of active trypsin to α2M is shown. B: α2M-trypsin complex formed at the listed molar ratios was added to Schwann cell cultures for 6 hr. The total concentration of α2M (trypsin-activated and unactivated) was 50 nM. MCP-1 mRNA was determined (mean ± SEM, *P < 0.05, **P < 0.01).
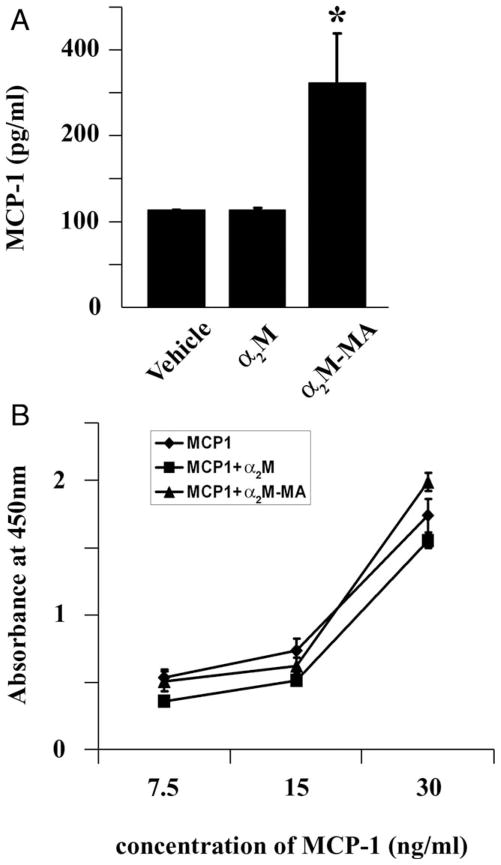
To confirm that activated α2M induces expression of MCP-1 protein as well as mRNA, cells were treated with α2M-MA (50 nM), native α2M, or vehicle. Conditioned medium (CM) was collected, and the level of MCP-1 was determined by ELISA. Figure 5A shows that α2M-MA significantly increased MCP-1 recovery in CM. Native α2M had no effect on MCP-1 protein in CM. Because α2M is known to bind growth factors and cytokines and influence recognition of these proteins by antibodies, in control experiments, we preincubated purified recombinant MCP-1 with native α2M or α2M-MA and determined the effects on MCP-1 detection by ELISA. Figure 5B shows that neither form of α2M significantly affected MCP-1 detection.
Fig. 5.
Activated α2M induces expression of MCP-1 protein by Schwann cells. A: Schwann cells were treated with 50 nM native α2M, α2M-MA, or vehicle for 12 hr. CM was collected, and MCP-1 in the CM was determined by ELISA. B: Different concentrations of recombinant MCP-1 were incubated with 50 nM native α2M or α2M-MA for 15 min. The incubation mixture was added directly to ELISA plates, and MCP-1 protein was determined (mean ± SEM).
Inhibiting ERK/MAP Kinase Activation Blocks Activated α2M-Induced MCP-1 Expression
To test whether increased MCP-1 expression results from LRP1-initiated cell signaling in α2M-MA-treated cells, we treated Schwann cells with a pharmacologic inhibitor of ERK/MAP kinase activation (PD098059). Figure 6 shows that PD098059 significantly inhibited α2M-MA-induced MCP-1 expression. The cells also were treated with 10 μM LY294002; however, Schwann cell viability was affected by this inhibitor, precluding analysis of the effects of LY294002 on MCP-1 expression. These results are consistent with our previous studies demonstrating activation of ERK/MAP kinase by α2M-MA and the work of others showing that MCP-1 expression is regulated by ERK/MAP kinase in Schwann cells (Martini et al., 2008; Mantuano et al., 2008a; Fischer et al., 2008).
Fig. 6.
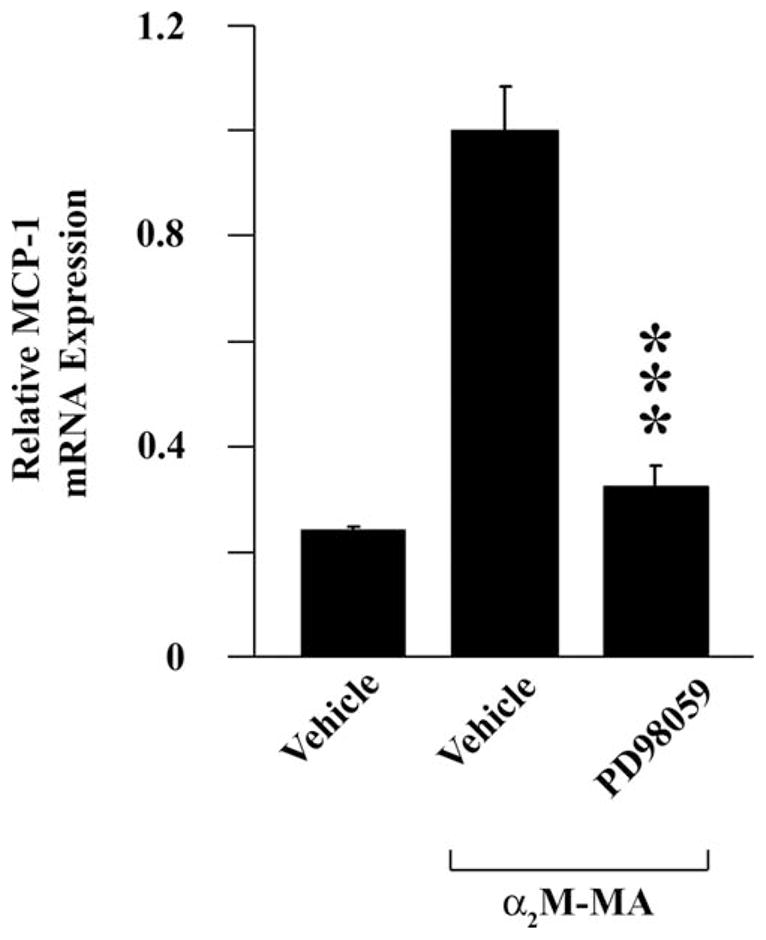
Expression of MCP-1 in response to activated α2M is inhibited by ERK/MAP kinase activation inhibitor. Schwann cells were pretreated with 50 μM PD98059 or vehicle and then with 50 nm α2M-MA for 6 hr, and MCP-1 expression was determined (mean ± SEM; ***P < 0.001 compared with respective vehicle control).
DISCUSSION
Schwann cells in vivo express LRP1 selectively after PNS injury (Campana et al., 2006), and we have shown that Schwann cell LRP1 functions as a cell-signaling receptor in vitro and in vivo (Campana et al., 2006; Mantuano et al., 2008a,b). Over 40 ligands bind to LRP1 (Strickland et al., 2002), many of which may be present in the injured PNS. Although it is not yet clear whether all ligands that bind to LRP1 trigger cell signaling, ligands that have been implicated include α2M, tPA, MMP-9, apolipoprotein E, lactoferrin, thrombospondin-1, and connective tissue growth factor (Orr et al., 2003; Qui et al, 2004; Grey et al., 2004; Yang et al., 2004; Hu et al., 2006; Mantuano et al., 2008b; Shi et al., 2009). α2M provides a novel pathway for converting a large spectrum of proteases into LRP1 ligands based on its ability to react with endopeptidases from all four major mechanistic classes and with diverse substrate specificities (Barrett and Starkey, 1973; Barrett et al., 1979; Sottrup-Jensen, 1989). Irrespective of the reacting protease, the resulting α2M conformational change reveals LRP1-binding sites that are cryptic in the structure of the native form of the protein (Imber and Pizzo, 1981; Sottrup-Jensen, 1989). By this pathway, multiple proteases may be converted into LRP1 ligands capable of triggering cell signaling in Schwann cells.
LRP1-initiated cell signaling has been previously implicated in Schwann cell survival (Campana et al., 2006) and migration (Mantuano et al., 2008b). These are fundamental processes in the Schwann cell response to PNS injury and axonal damage (Jessen and Mirsky, 1999; Campana, 2007). In this study, we have shown that binding of activated α2M to LRP1 regulates expression of MCP-1 and possibly other key extracellular mediators by Schwann cells. Although some variability was noted in the degree of regulation of MCP-1 expression with different preparations of Schwann cells for primary culture, in response to α2M-MA, MCP-1 mRNA expression was typically increased by more than 10-fold. Regulation of MCP-1 expression downstream of LRP1 ligation resulted from activation of ERK/MAP kinase. MCP-1 expression is known to be induced in Schwann cells in the injured PNS and to play a major role in macrophage recruitment (Toews et al., 1998; Taskinen and Röyttä, 2000; Tofaris et al., 2002; Martini et al., 2008). In mice that are deficient in the major MCP-1 receptor, CCR2, macrophage recruitment into the injured PNS is significantly delayed (Siebert et al., 2000). A second cytokine produced by Schwann cells in vivo, which has been identified as a major macrophage chemotactic factor, is leukemia inhibitory factor (LIF; Tofaris et al., 2002). Although we did not validate this result, in our screening assay, LIF mRNA expression was increased more than threefold by activated α2M. Similarly, MCP-3/CCL-7, which was up-regulated 7.3-fold in our screening assay, is known to regulate chemotaxis and activation of diverse inflammatory cells (Okada et al., 2009).
By preactivating α2M with methylamine, we generated a derivative that binds directly to LRP1 but lacks further protease inhibitory activity. This allowed us to focus on the α2M–LRP1 interaction. As a control, we studied rα2M1370/1374, which is incapable of binding to LRP1 even after conformational change (Arandjelovic et al., 2005). Although our results provide a model of how LRP1 ligation may regulate cytokine expression in vivo, the overall function of α2M in PNS injury may be influenced by its protease-inhibitory activity and its function as a major carrier of growth factors (Crookston et al., 1994; Wolf and Gonias, 1994) in addition to its role as an LRP1 ligand. Because our sciatic nerve crush injury experiments confirmed that α2M is indeed present in vivo following injury, understanding the integrated function of α2M in PNS injury remains a goal for future investigation.
Binding of α2M to LRP1 did not induce expression of TNF-α, which functions as a major inflammatory agent in PNS injury (Campana, 2007). Thus, the transcriptional response to activated α2M was selective. Overall, LRP1 and its ligands emerge as an intriguing system capable of orchestrating a variety of phenotypic changes required in Schwann cells during PNS injury, including survival, migration, and transcriptional activation of gene products that are necessary for recruitment of inflammatory cells.
Acknowledgments
Contract grant sponsor: National Institutes of Health; Contract grant numbers: R01 NS054571; NS057456.
References
- Akassoglou K, Kombrinck K, Degen J, Strickland S. Tissue plasminogen activator-mediated fibrinolysis protects against axonal degeneration and demyelination after sciatic nerve injury. J Cell Biol. 2000;149:1157–1166. doi: 10.1083/jcb.149.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arandjelovic S, Hall BD, Gonias SL. Mutation of lysine 1370 in full-length human alpha2-macroglobulin blocks binding to the low density lipoprotein receptor-related protein-1. Arch Biochem Biophys. 2005;438:29–35. doi: 10.1016/j.abb.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Barrett A, Starkey P. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973;133:709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A, Brown M, Sayers C. The electrophoretically “slow” and “fast” forms of the alpha 2-macroglobulin molecule. Biochem J. 1979;181:401–408. doi: 10.1042/bj1810401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenstein J, Sato G. Fibronectin and polylysine requirement for proliferation of neuroblastoma cells in defined medium. Exp Cell Res. 1980;129:361–366. doi: 10.1016/0014-4827(80)90504-2. [DOI] [PubMed] [Google Scholar]
- Campana WM. Schwann cells: activated peripheral glia and their role in neuropathic pain. Brain Behav Immun. 2007;21:522–527. doi: 10.1016/j.bbi.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana W, Hiraiwa M, O’Brien J. Prosaptide activates the MAPK pathway by a G-protein-dependent mechanism essential for enhanced sulfatide synthesis by Schwann cells. FASEB J. 1998;12:307–314. doi: 10.1096/fasebj.12.3.307. [DOI] [PubMed] [Google Scholar]
- Campana WM, Li X, Dragojlovic N, Janes J, Gaultier A, Gonias SL. The low density lipoprotein receptor-related protein (LRP-1) is a pro-survival receptor in Schwann cells: possible implications in peripheral nerve injury. J Neurosci. 2006;26:11197–11207. doi: 10.1523/JNEUROSCI.2709-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase T, Jr, Shaw E. Comparison of the esterase activities of trypsin, plasmin, and thrombin on guanidinobenzoate esters. Titration of the enzymes. Biochemistry. 1969;8:2212–2224. doi: 10.1021/bi00833a063. [DOI] [PubMed] [Google Scholar]
- Crookston K, Webb D, Wolf B, Gonias S. Classification of alpha 2-macroglobulin-cytokine interactions based on affinity of noncovalent association in solution under apparent equilibrium conditions. J Biol Chem. 1994;269:1533–1540. [PubMed] [Google Scholar]
- Fischer S, Weishaupt A, Troppmair J, Martini R. Increase of MCP-1 (CCL2) in myelin mutant Schwann cells is mediated by MEK-ERK signaling pathway. Glia. 2008;56:836–843. doi: 10.1002/glia.20657. [DOI] [PubMed] [Google Scholar]
- Gaultier A, Wu X, Le Moan N, Takimoto S, Mukandala G, Akassoglou K, Campana W, Gonias S. Low-density lipoprotein receptor-related protein 1 is an essential receptor for myelin phagocytosis. J Cell Sci. 2009;122:1155–1162. doi: 10.1242/jcs.040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonias S, Pizzo S. Conformation and protease binding activity of binary and ternary human alpha 2-macroglobulin-protease complexes. J Biol Chem. 1983;258:14682–14685. [PubMed] [Google Scholar]
- Gonias S, Reynolds J, Pizzo S. Physical properties of human alpha 2-macroglobulin following reaction with methylamine and trypsin. Biochem Biophys Acta. 1982;705:306–314. doi: 10.1016/0167-4838(82)90252-7. [DOI] [PubMed] [Google Scholar]
- Grey A, Banovic T, Zhu Q, Watson M, Callon K, Palmano K, Ross J, Naot D, Reid I, Cornish J. The low-density lipoprotein receptor-related protein 1 is a mitogenic receptor for lactoferrin in osteoblastic cells. Mol Endocrinol. 2004;18:2268–2278. doi: 10.1210/me.2003-0456. [DOI] [PubMed] [Google Scholar]
- Herz J, Goldstein JL, Strickland DK, Ho YK, Brown MS. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J Biol Chem. 1991;266:21232–21238. [PubMed] [Google Scholar]
- Hu K, Yang J, Tanaka S, Gonias SL, Mars WM, Liu Y. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem. 2006;281:2120–2127. doi: 10.1074/jbc.M504988200. [DOI] [PubMed] [Google Scholar]
- Imber MJ, Pizzo SV. Clearance and binding of two electrophoretic “fast” forms of human alpha 2-macroglobulin. J Biol Chem. 1981;256:8134–8139. [PubMed] [Google Scholar]
- Jessen K, Mirsky R. Why do Schwann cells survive in the absence of axons? Ann N Y Acad Sci. 1999;883:109–115. [PubMed] [Google Scholar]
- Kinchen J, Ravichandran K. Journey to the grave: signaling events regulating removal of apoptotic cells. J Cell Sci. 2007;120:2143–2149. doi: 10.1242/jcs.03463. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Chattopadhyay S, Kato K, Dolkas J, Kikuchi S, Myers R, Shubayev V. MMPs initiate Schwann cell-mediated MBP degradation and mechanical nociception after nerve damage. Mol Cell Neurosci. 2008;39:619–627. doi: 10.1016/j.mcn.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fleur M, Underwood J, Rappolee D, Werb Z. Basement membrane and repair of injury to peripheral nerve: defining a potential role for macrophages, matrix metalloproteinases, and tissue inhibitor of metalloproteinases-1. J Exp Med. 1996;184:2311–2326. doi: 10.1084/jem.184.6.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantuano E, Mukandala G, Li X, Campana WM, Gonias SL. Molecular dissection of the human alpha2-macroglobulin subunit reveals domains with antagonistic activities in cell signaling. J Biol Chem. 2008a;283:19904–19911. doi: 10.1074/jbc.M801762200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantuano E, Inoue G, Li X, Takahashi K, Gaultier A, Gonias SL, Campana WM. The hemopexin domain of matrix metalloproteinase-9 activates cell signaling and promotes migration of Schwann cells by binding to low-density lipoprotein receptor-related protein. J Neurosci. 2008b;28:11571–11582. doi: 10.1523/JNEUROSCI.3053-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R, Fischer S, López-Vales R, David S. Interactions between Schwann cells and macrophages in injury and inherited demyelinating disease. Glia. 2008;56:1566–1577. doi: 10.1002/glia.20766. [DOI] [PubMed] [Google Scholar]
- Okada M, Saio M, Kito Y, Ohe N, Yano H, Yoshimura S, Iwama T, Takami T. Tumor-associated macrophage/microglia infiltration in gliomas is correlated with MCP-3, but not MCP-1. Int J Oncol. 2009;34:1621–1627. doi: 10.3892/ijo_00000292. [DOI] [PubMed] [Google Scholar]
- Orr A, Elzie C, Kucik D, Murphy-Ullrich J. Thrombospondin signaling through the calreticulin/LDL receptor-related protein co-complex stimulates random and directed cell migration. J Cell Sci. 2003;116:2917–2927. doi: 10.1242/jcs.00600. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Hyman B, Rebeck G. Apolipoprotein E receptors mediate neurite outgrowth through activation of p44/42 mitogen-activated protein kinase in primary neurons. J Biol Chem. 2004;279:34948–34956. doi: 10.1074/jbc.M401055200. [DOI] [PubMed] [Google Scholar]
- Siebert H, Sachse A, Kuziel WA, Maeda N, Bruck W. The chemokine receptor CCR2 is involved in macrophage recruitment to the injured peripheral nervous system. J Neuroimmunol. 2000;110:177–185. doi: 10.1016/s0165-5728(00)00343-x. [DOI] [PubMed] [Google Scholar]
- Shi Y, Mantuano E, Inoue G, Campana W, Gonias S. Ligand binding to LRP1 transactivates Trk receptors by a Src family kinase-dependent pathway. Sci Signal. 2009;2:ra18. doi: 10.1126/scisignal.2000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottrup-Jensen L. Alpha-macroglobulins: structure, shape, and mechanism of proteinase complex formation. J Biol Chem. 1989;264:11539–11542. [PubMed] [Google Scholar]
- Stoll G, Muller HW. Nerve injury, axonal degeneration and neural regeneration: basic insights. Brain Pathol. 1999;9:313–25. doi: 10.1111/j.1750-3639.1999.tb00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland DK, Gonias SL, Argraves WS. Diverse roles for the LDL receptor family. Trends Endocrinol Metab. 2002;13:66–74. doi: 10.1016/s1043-2760(01)00526-4. [DOI] [PubMed] [Google Scholar]
- Taskinen H, Röyttä M. Increased expression of chemokines (MCP-1, MIP-1alpha, RANTES) after peripheral nerve transection. J Peripher Nerv Syst. 2000;5:75–81. doi: 10.1046/j.1529-8027.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- Thellin O, Zorzi W, Lakayem B, De Borman B, Coumans B, Hennen G, Grisar T, Igout A, Heinen E. Housekeeping genes as internal standards: use and limits. J Biotechnol. 1999;75:291–295. doi: 10.1016/s0168-1656(99)00163-7. [DOI] [PubMed] [Google Scholar]
- Toews A, Barrett C, Morell P. Monocyte chemoattractant protein 1 is responsible for macrophage recruitment following injury to sciatic nerve. J Neurosci Res. 1998;53:260–267. doi: 10.1002/(SICI)1097-4547(19980715)53:2<260::AID-JNR15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Tofaris G, Patterson P, Jessen K, Mirsky R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci. 2002;22:6696–6703. doi: 10.1523/JNEUROSCI.22-15-06696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf B, Gonias S. Neurotrophin binding to human alpha 2-macroglobulin under apparent equilibrium conditions. Biochemistry. 1994;33:11270–11277. doi: 10.1021/bi00203a024. [DOI] [PubMed] [Google Scholar]
- Yang M, Huang H, Li J, Li D, Wang H. Tyrosine phosphorylation of the LDL receptor-related protein (LRP) and activation of the ERK pathway are required for connective tissue growth factor to potentiate myofibroblast differentiation. FASEB J. 2004;18:1920–1921. doi: 10.1096/fj.04-2357fje. [DOI] [PubMed] [Google Scholar]