Summary: A genetic knockout of a pollen-expressed cyclic nucleotide-gated ion channel (cngc16) provides evidence for a signaling pathway that links a cyclic nucleotide signal to changes in gene expression that are critical for heat or drought stress tolerance during reproductive development.
Abstract
Cyclic nucleotide-gated channels (CNGCs) have been implicated in diverse aspects of plant growth and development, including responses to biotic and abiotic stress, as well as pollen tube growth and fertility. Here, genetic evidence identifies CNGC16 in Arabidopsis (Arabidopsis thaliana) as critical for pollen fertility under conditions of heat stress and drought. Two independent transfer DNA disruptions of cngc16 resulted in a greater than 10-fold stress-dependent reduction in pollen fitness and seed set. This phenotype was fully rescued through pollen expression of a CNGC16 transgene, indicating that cngc16-1 and 16-2 were both loss-of-function null alleles. The most stress-sensitive period for cngc16 pollen was during germination and the initiation of pollen tube tip growth. Pollen viability assays indicate that mutant pollen are also hypersensitive to external calcium chloride, a phenomenon analogous to calcium chloride hypersensitivities observed in other cngc mutants. A heat stress was found to increase concentrations of 3′,5′-cyclic guanyl monophosphate in both pollen and leaves, as detected using an antibody-binding assay. A quantitative PCR analysis indicates that cngc16 mutant pollen have attenuated expression of several heat-stress response genes, including two heat shock transcription factor genes, HsfA2 and HsfB1. Together, these results provide evidence for a heat stress response pathway in pollen that connects a cyclic nucleotide signal, a Ca2+-permeable ion channel, and a signaling network that activates a downstream transcriptional heat shock response.
The reproductive phase in flowering plants can be highly sensitive to hot or cold temperature stresses. Even a single hot day or cold night can sometimes be fatal to reproductive success. Pollen development and fertilization are often the most temperature-sensitive part of reproductive development (Zinn et al., 2010). A heat stress response in pollen, like vegetative tissues, involves changes in gene expression, including increased mRNA levels for heat shock transcription factors (e.g. HsfA2) and heat shock proteins (e.g. Heat Shock Protein17-CII and Thermosensitive Male Sterile1; Frank et al., 2009; Yang et al., 2009; Giorno et al., 2010). Nevertheless, the signaling pathways underlying these responses remain poorly understood, especially in pollen development.
Cyclic nucleotide monophosphates (cNMPs) 3′,5′-cyclic guanyl monophosphate (cGMP) and cAMP play key roles in the regulation of diverse cellular processes in eukaryotes and prokaryotes (Jammes et al., 2011), including biotic and abiotic stresses. In plants, cNMPs have been implicated in pathogen responses and salt and osmotic stresses (Jammes et al., 2011; Li et al., 2011; Ma and Berkowitz, 2011; Moeder et al., 2011). Recently, cNMPs have also been linked to heat stress responses in vegetative tissues from Arabidopsis (Arabidopsis thaliana) and the moss Physcomitrella patens (Finka et al., 2012; Gao et al., 2012). In these cases, genetic and electrophysiological evidence suggest that cNMPs activate cyclic nucleotide-gated ion channels (CNGCs).
CNGCs are Ca2+-permeable cation transport channels that are activated by cNMPs and deactivated by binding Ca2+/calmodulin (Cukkemane et al., 2011; Ma and Berkowitz, 2011; Spalding and Harper, 2011). They have been identified in both plant and animal systems (Schuurink et al., 1998; Köhler and Neuhaus, 2000; Becchetti et al., 2009; Zelman et al., 2012). In plants, CNGCs have a binding site for calmodulin that overlaps with a site for cNMP (Köhler et al., 1999). Thus, CNGCs have the potential for integrating signals from cyclic nucleotide and Ca2+ signaling pathways (Newton and Smith, 2004).
Arabidopsis contains 20 CNGC family members (Mäser et al., 2001) that are differentially expressed in all tissues (Talke et al., 2003). CNGC18 has been shown to provide an essential function in pollen tube tip growth (Frietsch et al., 2007). This is consistent with pharmacological evidence that cyclic nucleotide signals in pollen can trigger growth-altering Ca2+ signals (Moutinho et al., 2001; Rato et al., 2004; Wu et al., 2011). Here, we show that CNGC16, another pollen expressing CNGC, is critical for heat stress tolerance, providing a link between a stress-triggered cNMP signal and a downstream transcriptional heat shock response.
RESULTS
cngc16 Disruptions Show a Stress-Dependent Segregation Distortion
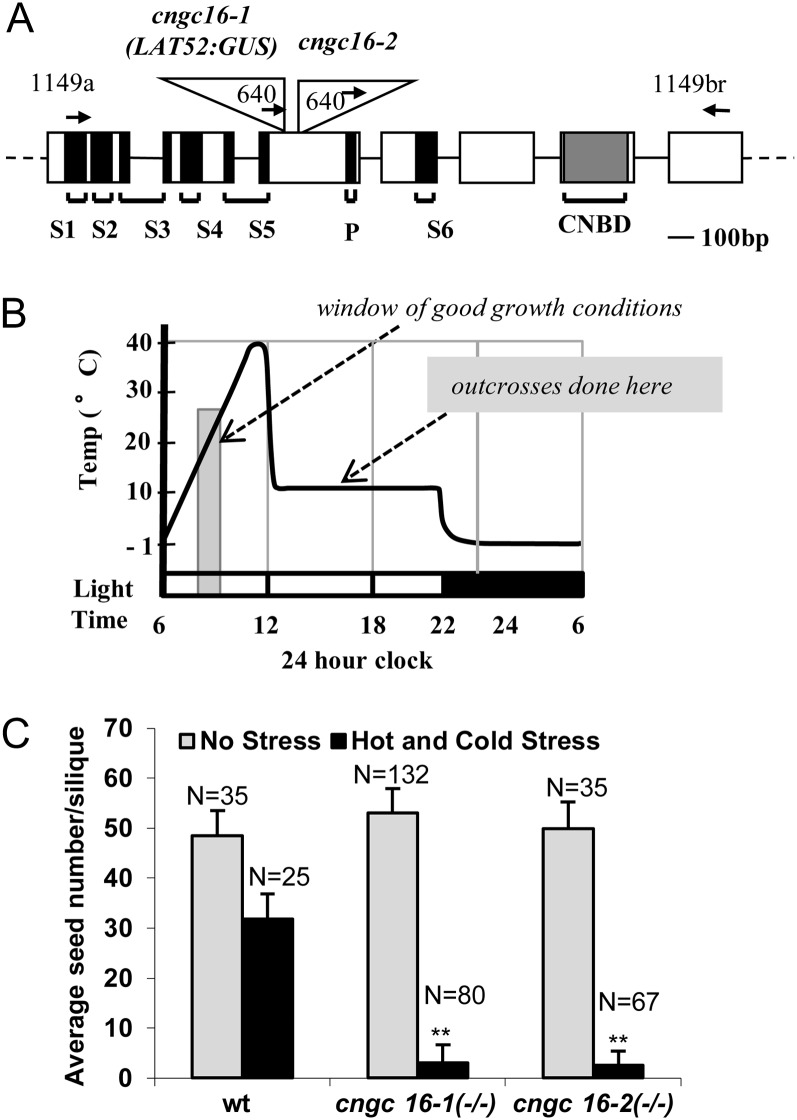
To identify genes involved in stress tolerance, we initiated a screen for transfer DNA (T-DNA) mutations that showed a stress-dependent segregation distortion under conditions of hot days and cold nights (Fig. 1B). For in vivo pollen tube growth in Arabidopsis, approximately 2 h is required for the first pollen tubes to grow through the stigma and enter the ovary and about 4 to 5 h for tubes to traverse 50% of the distance to the bottom of the ovary (Schiøtt et al., 2004; Crawford et al., 2007). The stress conditions chosen here were designed to provide only a small window of optimal growth conditions to force pollen to cope with a stress condition in order to complete a fertilization event. The daily cycling between hot and cold conditions allowed plants to be grown under the same stress regime for their entire life cycle, whereas a continuous exposure to either the hot or cold stress would have been lethal. The stress cycle used was found to reduce seed set in wild-type siliques by nearly 2-fold (Fig. 1C; Supplemental Fig. S1), providing an optimal stress condition in which to screen for mutants that either increased or decreased reproductive stress tolerance.
Figure 1.
Knockout mutations for cngc16 and corresponding stress-dependent seed set phenotype. A, Schematic diagram of CNGC16 gene model (AT3G48010) and T-DNA insertion sites in cngc16-1 and cngc16-2. Positions are shown for T-DNA insertions (triangles), exons (rectangles), introns (lines), and primers (arrows). Black regions represent transmembranes (S1–S6) and pore (P) domains in the corresponding protein. Gray shading represents the cyclic nucleotide binding domain (CNBD). B, Schematic diagram of the hot day and cold night stress cycling from −1°C to 40°C and forcing the period of pollen tube growth and fertilization to overlap with suboptimal temperatures. C, Seed set analysis of cngc16 shows a near-sterile phenotype under the hot/cold stress conditions diagrammed in B. n, Number of siliques counted. Student’s t test was performed to detect significant differences between cngc16 mutants and the wild type (wt) under hot and cold stress. **Student’s t test significant at P < 0.01.
A stress-dependent decrease in transmission was observed (Table I) for two independent T-DNA insertions in a gene encoding cyclic nucleotide-gated channel16 (CNGC16; Fig. 1A).
Table I. Segregation analysis showing non-Mendelian transmission of the T-DNA in cngc16 mutants under various stresses.
cngc16-1(+/−) and cncg 16-2(+/−) were self-pollinated under no stress, hot/cold (hot days and cold nights; Fig. 1B), 32°C, 37°C, or drought stresses. Statistical significance was determined by Pearson’s χ2 test.
| Segregation of Basta Resistance Marker |
|||||
|---|---|---|---|---|---|
| Allele (−/+) | Selfing Condition | F1Total | Expected | Observed | P Value |
| % | |||||
| cngc16-1 | No Stress | 282 | 75 | 73 | <0.700 |
| cngc16-2 | No Stress | 254 | 75 | 69 | <0.300 |
| cngc16-1 | Hot/Cold | 380 | 75 | 49 | <0.001 |
| cngc16-2 | Hot/Cold | 340 | 75 | 52 | <0.001 |
| cngc16-1 | 32°C | 323 | 75 | 53 | <0.001 |
| cngc16-2 | 32°C | 945 | 75 | 52 | <0.001 |
| cngc16-1 | 37°C | 369 | 75 | 52 | <0.001 |
| cngc16-2 | 37°C | 213 | 75 | 48 | <0.001 |
| cngc16-1 | Drought | 104 | 75 | 48 | <0.001 |
| cngc16-2 | Drought | 390 | 75 | 48 | <0.001 |
Both insertions were associated with a T-DNA harboring a Basta resistance marker (Sessions et al., 2002). When plants heterozygous for cngc16-1(−/+) and 16-2(−/+) were allowed to self-fertilize under conditions of hot days and cold nights, the Basta marker showed an approximately 50% transmission instead of an expected 75% (n = 720, P < 0.001; Table I). This distortion was confirmed in a subset of progeny by PCR genotyping of cngc16-1 and cngc16-2 T-DNA insertion site borders (n = 61, P < 0.01). In contrast, plants grown under standard nonstress conditions showed segregation very close to the expected 75% (e.g. 69%–73%, n = 536).
To determine if a heat stress alone was sufficient to reduce transmission of the cngc16 T-DNA insertions, heterozygous mutants were transferred into a heat stress chamber (32°C or 37°C) for 4 to 6 d while flowering and then returned to normal conditions for seed maturation. Flower meristems were pruned to leave only siliques that resulted from self-fertilization events that occurred during the stress period. Seed from these siliques were then harvested and used to determine the transmission frequency of T-DNA mutations to F1 progeny conceived only during the limited stress period. The heat stress alone resulted in an equivalent segregation distortion to that observed under a hot day/cold night stress regime (Table I).
To determine if a drought stress could also result in a segregation distortion, heterozygous plants were subjected to a period of severe drought and then rescued through restored watering. As the drought stress became more severe, the resulting siliques became shorter and contained fewer seeds. To focus only on F1 progeny whose reproductive origins were actually impacted by the period of drought stress, seeds were selectively harvested from the shortest siliques, which contained progeny conceived during the most severe stages of the drought stress. These progeny showed a segregation distortion equivalent to that observed with a hot day/cold night stress regime (Table I).
cngc16 Mutations Result in a Stress-Dependent Pollen Defect
To determine whether the observed stress-dependent segregation distortion in self-fertilized plants was due to a defect in either the male or female gametophytes, we conducted reciprocal crosses between cngc16(+/−) mutants and plants that were either wild type or male sterile (ms1-1). When cngc16 heterozygotes were used as females, a normal 50% transmission frequency was observed with or without stress conditions (Table II, groups A and E). In contrast, cngc16 pollen transmission under stress conditions was reduced to 1% to 2.4% (Table II, groups B and D). This indicated that cngc16 pollen alone had a stress-dependent defect in transmission.
Table II. Segregation analysis showing a stress-dependent defect in cngc16 pollen transmission.
Transmission efficiencies are shown for reciprocal crosses between cngc16-1(–/+), cngc16-2(–/+), and wild-type (WT) plants or ms1-1 plants under various conditions. All outcrosses in which pollinated plants were moved to a stress chamber were done between 2 and 5 pm (10°C) on the stress cycle shown in Figure 1. “Pre” and “Post” refer to application of stress to the female recipient or pollen before or after the manual cross. All post-cross stress treatments occurred within 30 min of the cross unless otherwise indicated. Statistical significance was determined by Pearson’s χ2 test.
| Hot/Cold Stress Regime |
Segregation of Basta Resistance Marker |
||||||
|---|---|---|---|---|---|---|---|
| Group | Pre | Post | Female × Male | F1 Total | Expected | Observed | P Value |
| % | |||||||
| A | |||||||
| Female | − | − | cngc16-1(–/+) × WT | 285 | 50 | 48 | <0.7 |
| cngc16-2(–/+) × WT | 156 | 50 | 50 | 1 | |||
| Pollen | − | − | WT × cngc16-1(–/+) | 490 | 50 | 33 | <0.001 |
| WT × cngc16-2(–/+) | 792 | 50 | 31 | <0.001 | |||
| B | |||||||
| Female | − | + | ms1-1 × cngc16-1(–/+) | 602 | 50 | 2.4 | <0.001 |
| Pollen | − | + | ms1-1 × cngc16-2(–/+) | 162 | 50 | 1.2 | <0.001 |
| C | |||||||
| Female | − | − | WT × cngc16-1(–/+) | 170 | 50 | 21 | <0.001 |
| Pollen | + | − | WT × cngc16-2(–/+) | 40 | 50 | 20 | <0.01 |
| D | |||||||
| Female | − | + | ms1-1 × cngc16-2(–/+) | 96 | 50 | 1 | <0.001 |
| Pollen | + | + | |||||
| E | |||||||
| Female | + | + | cngc 16-1(–/+) × WT | 97 | 50 | 48.4 | <0.95 |
| Pollen | − | + | |||||
| F | |||||||
| Female | + | + | ms1-1 × cngc16-2(–/+) | 97 | 50 | 2.1 | <0.001 |
| Pollen | + | + | |||||
| G | |||||||
| Female | − | +a | ms1-1 × cngc16-1(–/+) | 458 | 50 | 20.6 | <0.001 |
| Pollen | − | +a | ms1-1 × cngc16-2(–/+) | 427 | 50 | 22.6 | <0.001 |
Indicates a 1-h delay before crossed plants were transferred to the stress chamber.
To determine which aspects of cngc16 pollen are stress sensitive, plants were exposed to stress either before and/or after a cross. When pollen were allowed to develop under a stress of hot days and cold nights and then outcrossed and allowed to fertilize under nonstress conditions, transmission efficiency dropped approximately 1.5-fold from 31% observed for control conditions down to 21% (Table II, group C). For these crosses the expected transmission efficiency was considered to be 31%, based on our nonstress control conditions. This slight decrease in transmission for the nonstress control (i.e. from 50% down to 31%) is likely due to manual pollination resulting in multiple stresses, such as wounding from brushing pollen onto the stigma, in addition to a low humidity stress that is common in dry climates when a growth chamber door is opened to perform a cross.
The largest relative decrease in cngc16 transmission efficiency occurred when pollen from a heterozygous cngc16 mutant (grown without stress) was used to pollinate an ms1-1 female, and the outcrossed plant moved into the stress chamber within 30 min. Under these conditions, the cngc16 transmission efficiency was reduced more than 10-fold, down to 1.2% to 2.4% (31% expected; Table II, group B). However, if the stress exposure was delayed by 1 to 2 h, the transmission efficiency was less severe, with only a 1.5-fold decrease similar to that observed when pollen were stressed only during maturation (Table II, group G). This bracketing indicates that while mutant pollen were still sensitive to stress during grain maturation or tube growth, they were most sensitive during the first 1 to 2 h postpollination, corresponding to germination and early tube growth into the stigma (Crawford et al., 2007).
Homozygous cngc16 Mutants Have Reduced Seed Set under Conditions of a Hot/Cold Stress
Two possible types of pollen defects could explain a stress-dependent segregation distortion: (1) A reduction in competitiveness compared with the wild type, for example, from a slightly slower rate of pollen grain germination or tube growth; or (2) complete sterility, for example, from death or the inability to grow or discharge sperm cells. To help distinguish between these two alternatives, we grew homozygous mutants under conditions of hot days and cold nights and quantified the number of seeds per silique (Fig. 1C; Supplemental Fig. S1). For both cngc16-1(−/−) and cngc16-2(−/−) mutants, we observed a stress-dependent 10-fold decrease in seed set. This indicates that there is stress-dependent transmission defect even in the absence of competition with wild-type pollen. This is consistent with a model in which the mutant pollen show a stress-dependent lethality, as opposed to slightly slower rate of pollen grain germination or tube growth.
Expression of GFP-CNGC16 Rescues the cngc16 Stress-Dependent Phenotype
To determine if pollen expression of a GFP-tagged CNGC16 could rescue the stress dependent phenotypes, a transgene encoding a GFP-CNGC16 was stably transformed into homozygous cngc16-1 and cngc16-2 backgrounds. The CNGC18 promoter was used for relatively weak levels of pollen expression (Frietsch et al., 2007), whereas an ACA9 promoter was used for strong expression (Schiøtt et al., 2004). The ACA9 promoter was previously used with a parallel construct to drive the expression of a GFP-CNGC18, which provided sufficiently high expression levels to allow the GFP-GNCG18 to be visualized in pollen (Frietsch et al., 2007). However, for both GFP-CNGC16 rescue constructs, we were unable to detect a GFP signal, suggesting that mRNA or protein expression levels were kept very low, despite the use of a relatively strong ACA9 promoter. Nevertheless, both rescue constructs were observed to restore seed set to levels equivalent to the wild type (approximately 35 seeds) grown under parallel stress conditions (Fig. 2A).
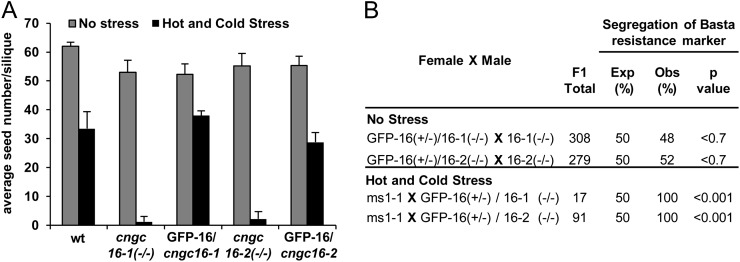
Figure 2.
Seed set and segregation analysis showing the rescue of the male sterile phenotype. A, Seed set analyses of cngc16 knockouts rescued with a CNGC18p(i)-GFP-CNGC16 construct [seed stock nos. 1646 and 1647 for cngc16-1(−/−) and cngc16-2(−/−) backgrounds, respectively] showing seed set levels equivalent to the wild type (wt) under a hot and cold stress regime (siliques counted = 5). B, Outcrosses with representative rescue lines seed stock numbers 1646 and 1647 [for cngc16-1(−/−) and cngc16-2(−/−) backgrounds, respectively]. The rescue construct CNGC18p(i)-GFP-CNGC16 was hemizygous in all crosses. A rescue experiment was repeated with similar results using two additional lines harboring the same GFP-CNGC16 under the control of the ACA9 pollen promoter (seed stock nos. 1648 and 1649; data not shown). For crosses done with a hot/cold stress, the female had a ms1-1 phenotype. After a manual fertilization, the plants were moved to a hot day/cold night stress chamber, with the entry time at approximately 3 pm and temperature at 10°C (see Figure 1B). Statistical significance was determined by Pearson’s χ2 test.
In addition to rescuing the seed set phenotype, the CNGC16 transgene also rescued the stress-dependent segregation distortion phenotype. This was observed in a competition assay in which 50% of the mutant pollen harbored a rescue construct (i.e. cngc16−/− with a hemizygous transgene). The transmission frequency of the rescue construct was assayed using the associated hygromycin marker. In the absence of stress, a normal 50% transmission efficiency was observed for a pollen outcross, indicating that under control conditions, all pollen were equally competitive (Fig. 2B). In contrast, under conditions of a hot/cold stress, the only pollen transmission observed was for mutant pollen harboring GFP-CNGC16 (n = 108, P < 0.001). Together, the observation that the stress-dependent segregation distortion and reduced seed set phenotypes could be rescued using a pollen-expressed GFP-CNGC16 corroborates that cngc16-1 and cngc16-2 represent loss-of-function null mutations.
The Viability of cngc16 Pollen Can Be Reduced by Temperature Stress or Elevated CaCl2
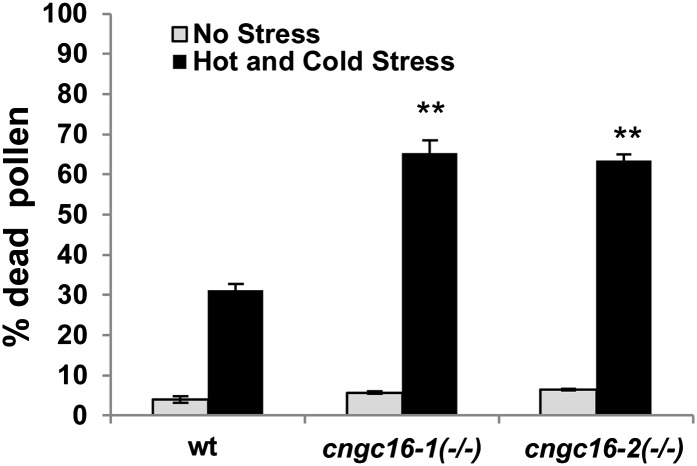
Alexander staining was used as an assay to evaluate pollen viability in response to environmental conditions. In pollen isolated from plants grown under a stress of hot days and cold nights, pollen from homozygous cngc16 mutants were twice as likely to be dead compared with wild-type controls (Fig. 3; Supplemental Fig. S2).
Figure 3.
Viability staining showing cngc16 mutants are hypersensitive to stress. Viability was assayed using Alexander’s reagent. Pollen were harvested from plants growing under conditions of hot days and cold nights (see Figure 1B). Values represent means ± sd of three independent experiments (n = 50–100 pollen grains for each experiment). Student’s t test was done to compare the pollen viability of cngc16 mutants with wild-type (wt) plants grown under a hot and cold stress regime. **Student’s t test significant at P < 0.01.
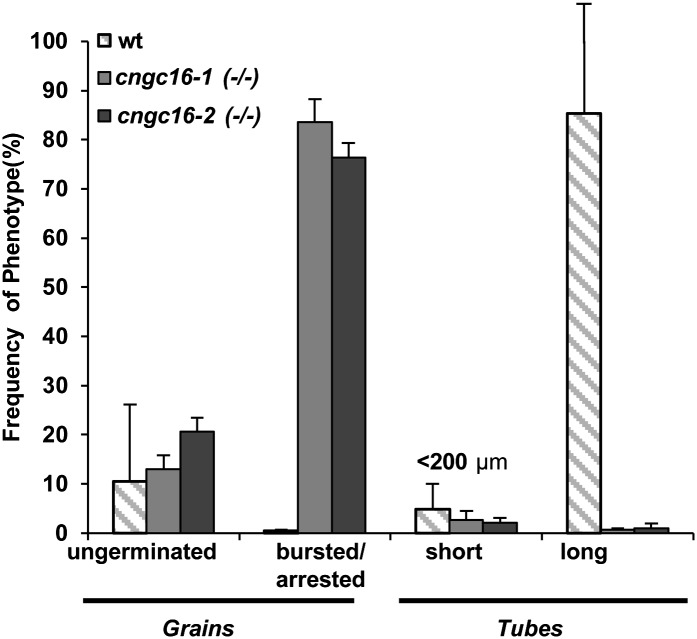
In an attempt to use an in vitro pollen growth assay to study the cngc16 phenotype, we observed that less than 1% of cngc16 mutant pollen grew tubes longer than 200 µm. Instead, more than 80% of the pollen grains either failed to germinate, arrested as short pollen tubes (i.e. <30 µm), or ruptured (Fig. 4).
Figure 4.
cngc16 pollen show poor growth and bursting when germinated in vitro. Pollen grains were harvested from plants grown under normal conditions. Germination and growth were allowed to proceed for approximately 12 h on a standard in vitro agar-based growth medium containing 1 mm CaCl2. Values represent means ± sd of three to five independent experiments, each with approximately 200 pollen grains. wt, Wild type.
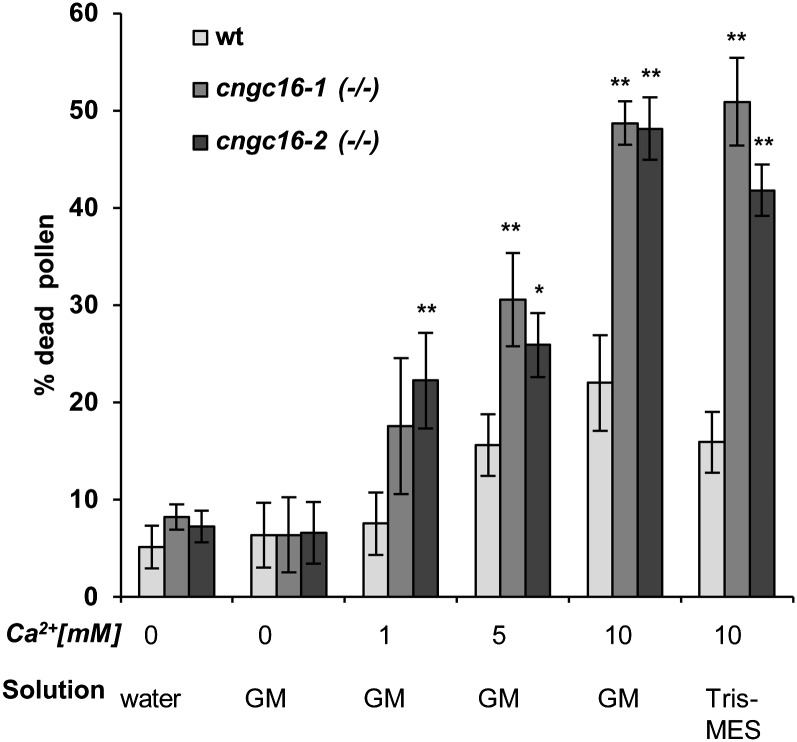
To determine if a specific component of the pollen germination medium was responsible for the observed developmental block, we examined the effects of varying concentrations of CaCl2 from 0 to 10 mm in a standard liquid germination medium. After 3 h, the viability of pollen grains was assayed using Alexander’s stain (Fig. 5). At 10 mm CaCl2, cngc16 pollen showed a more than 2-fold increase in pollen death compared with the wild type. This increase in lethality also occurred with 10 mm CaCl2 in a Tris-MES buffer, suggesting that additional nutrient components normally present in our standard germination medium were not necessary for the hypersensitivity to CaCl2.
Figure 5.
Alexander viability staining demonstrates that cngc16 pollen are hypersensitive to elevated CaCl2 concentrations. Pollen grains were harvested into a water suspension from plants grown under normal conditions. Aliquots were modified as indicated and incubated for 3 h at 20°C in parallel. Incubations were done in solutions corresponding to water only, standard liquid in vitro germination medium, pH 7.5 (GM), or Tris-MES buffer (pH 7.5). Solutions were amended as indicated with Ca2+ using CaCl2. Alexander staining was done after 3 h by pelleting pollen and resuspending pellets in 1 mL of Alexander stain for 30 min. Within the relatively short post hydration time frame assayed, wild-type (wt) controls for each solution showed less than 0.5% pollen grain germination. Viability counts were done with a digital camera mounted on a Leica DM IRE2 microscope. Values represent means ± sd of three independent experiments, each with approximately 50 pollen grains. Student’s t test was done to compare the pollen viability of cngc16 mutants to wild-type plants incubated at the same condition. *Student’s t test significant at P < 0.05. **Student’s t test significant at P < 0.01.
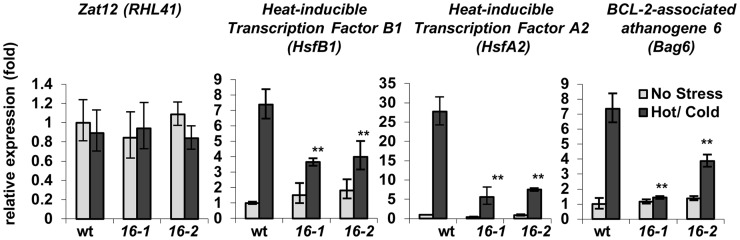
The Induction of Key Thermotolerance Genes Is Impaired in cngc16 Pollen
To elucidate the underlying cause of the cngc16 stress sensitivity, we performed real-time quantitative reverse transcription (RT)-PCR analyses on a subset of genes previously identified as stress markers (Fig. 6). As an example of a nonresponsive marker, Zat12 failed to show any temperature-dependent changes in mutants or the wild type. In contrast, stress-induced changes were observed for two HSF genes, HsfA2 and HsfB1. While both HSFs still showed a weak stress-induced increase in cngc16-1 and cngc16-2 backgrounds, their induction was 2- to 4-fold lower compared with wild-type controls. Similarly, a downstream gene under the control of HsfA2 was also assayed (BCL-2-associated athanogene6 [Bag6], At2g46240; Nishizawa-Yokoi et al., 2009) and found to have a 2- to 7-fold lower induction compared with the wild type. It is noteworthy that the BAG6 induction was more impaired in the cngc16-1 mutant background than cngc16-2, which might indicate that lines harboring cngc16-1 and cngc16-2 have functional differences. At a minimum, the cngc16-1 line includes an additional quartet mutation that was included in subset of lines generated for the Syngenta Arabidopsis Insertion Library T-DNA knockout collection (Alonso et al., 2003). The quartet mutant has a defect that alters the pollen cell wall and keeps the four meiotic products physically linked together in a tetrad (Rhee and Somerville, 1998). Regardless of potential modifiers in cngc16-1, both mutants show an impaired stress-dependent transcriptional response.
Figure 6.
Quantitative PCR indicates cngc16 pollen have attenuated expression of key stress response genes. Four stress-related genes (Zat12 [AT5G59820], HsfB1 [At4g36990], HsfA2 [At2g26150], and Bag6 [At2g46240]) were tested for their steady-state transcript levels in pollen collected after the peak heat stress (Figure 1B) from hot- and cold-stressed Arabidopsis plants. Values represent means ± sd of three to five independent experiments. Student’s t tests were conducted to compare the relative fold change in mRNA abundance of the related gene in cngc16 plants with wild-type (wt) plants. **Student’s t test significant at P < 0.01.
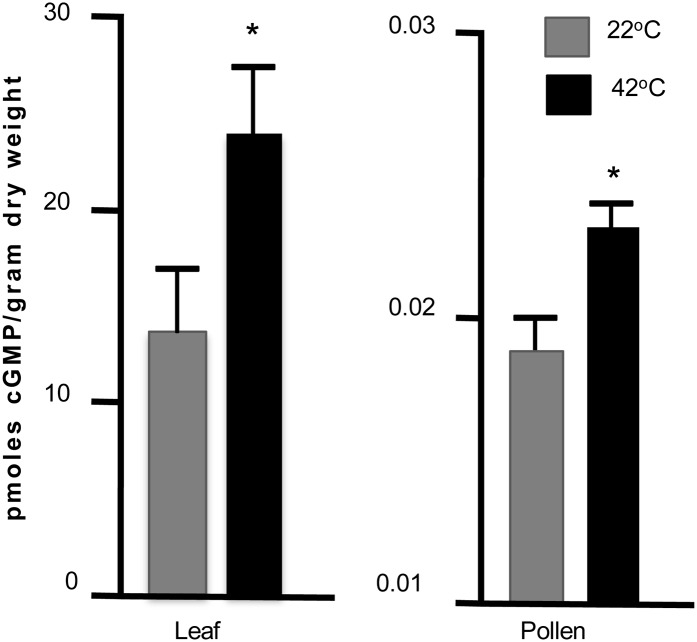
Heat Stress Increases cGMP Levels
To determine if a heat stress can trigger a rise or fall in the level of a cyclic nucleotide, we attempted to quantify levels of cAMP and cGMP in pollen with and without a heat stress. To detect cNMPs, we used antibodies that could distinguish between cAMP and cGMP. Unfortunately, we were unable to detect cAMP above background levels, leaving us to estimate that its concentration in pollen grains and leaves is less than 74 pmol g–1 dry weight. However, we were able to detect cGMP, at 13.65 pmol g–1 dry weight in leaves and 0.019 pmol g–1 dry weight in pollen and observed a small but statistically significant heat stress-dependent increase in both leaves and pollen (Fig. 7).
Figure 7.
Heat stress increases the average cGMP levels in leaves and pollen. Tissues were harvested from plants before or after a 30-min heat stress at 42°C. cGMP concentrations were assayed by competitive binding assay using cGMP-horseradish peroxidase and cGMP-specific antibody. Stress-dependent increases were measured in eight independent experiments for leaf and four independent experiments for pollen. Wilcoxon rank test was used to evaluate significance (asterisk). Means are shown with se.
DISCUSSION
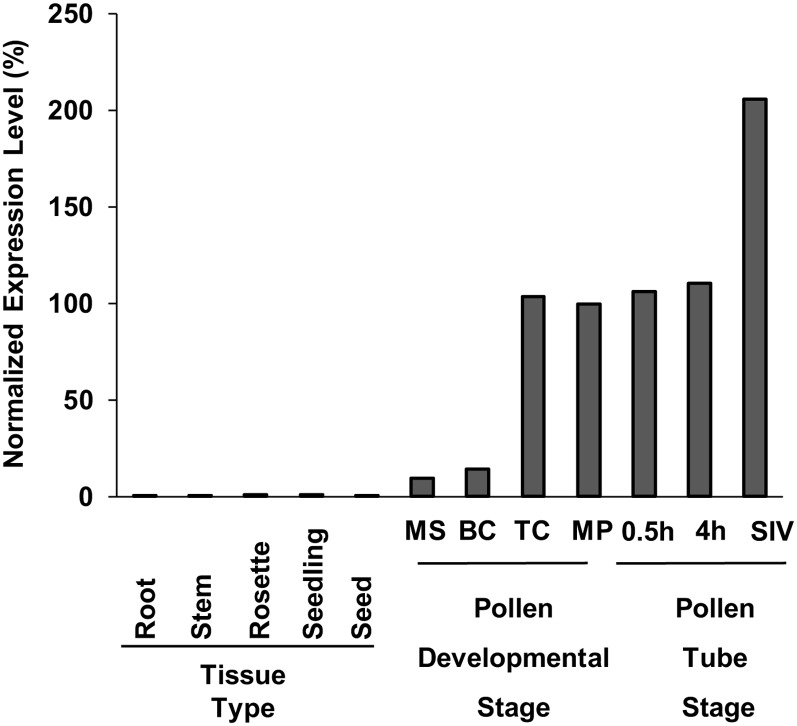
Genetic evidence presented here identifies CNGC16 in Arabidopsis as critical to reproductive success under conditions of heat stress or drought (Table I). Two independent T-DNA disruptions of cngc16 (Fig. 1A) resulted in stress-dependent reductions in pollen fitness (Table II) and seed set (Fig. 1C). This phenotype was fully rescued through pollen expression of a CNGC16 transgene (Fig. 2), indicating that cngc16-1 and cngc16-2 were both loss-of-function null alleles. CNGC16 is expressed primarily in pollen (Fig. 8), which is consistent with a stress-dependent phenotype associated with pollen transmission. In contrast, there was no detectable transmission deficiency through the female gametophyte (Table II, group E).
Figure 8.
The expression profiles of CNGC16 show preferential expression in pollen. Plant tissue types on the graph are as follows: 7-d-old wild-type roots, 7-d-old wild-type stem, 10-d-old wild-type rosette, 7-d-old wild-type seedling and wild-type seeds. Stages of male gametophyte development are as follows: MS, microspore; BC, bicellular; TC, tricellular; MP, mature pollen; 0.5h, pollen tube germinated in vitro for 30 min; 4h, pollen tube germinated in vitro for 4 h; and SIV, pollen tube germinated semi in vivo. The expression level for mature pollen was set to 100%. Expression data were extracted from the AtGenExpress database (http://jsp.weigelworld.org/expviz/expviz.jsp; Schmid et al., 2005) and the Pollen Transcriptome Navigator (http://pollen.umd.edu/), which uses Honys and Twell (2004) data for developmental stages of pollen expression profile and Qin et al. (2009) data for the in vitro- and semi in vivo-grown pollen tubes.
Germination of cngc16 Pollen Grains Is Highly Sensitive to Environmental Conditions
The most stress-sensitive period for cngc16 pollen was observed as a 10-fold decrease in transmission efficiency when the stress environment was introduced just after a manual pollination (Table II). When the stress exposure was limited to prepollination, there was only a 1.5- to 2.5-fold decrease in transmission efficiency. This is consistent with a viability assay showing that when pollen grains developed under stress conditions, the cngc16 pollen were twice as likely to die compared with the wild type (Fig. 3). When the stress environment was applied 2 h postpollination, a similar 1.5- to 2.5-fold decrease in transmission efficiency was observed. This bracketing suggests that cngc16 mutant pollen are most sensitive to stress conditions at the time of germination and/or growth into the stigma surface.
In vitro pollen growth assays corroborate that cngc16 pollen grains are highly sensitive to environmental conditions. In comparison with wild-type pollen germinated under a standard in vitro growth condition (with 1 mm CaCl2), less than 1% of the pollen grains produced tubes and more than 80% arrested as short tubes (<30 μm) or ruptured. This phenotype shows similarities to that observed for knockouts of cngc18 (Frietsch et al., 2007) and double knockouts of cngc7 and cngc8 (J.F. Harper, unpublished data). However, a major difference between cngc18, cngc7, cngc8, and cngc16 was that at least some of cngc16 pollen grains produced long tubes. In addition, cngc16 mutant pollen showed normal Mendelian transmission under nonstress conditions, whereas cngc18, cngc7, and cngc8 are male sterile.
The sensitivity of cngc16 pollen to in vitro growth conditions was at least partially due to an increased sensitivity to external CaCl2 (Fig. 5). Using a viability assay based on Alexander staining, nearly 50% of cngc16 pollen were found to be dead after a 3-h incubation in a buffered solution of 10 mm CaCl2. This level of lethality is 2- to 3-fold higher than a wild-type control. An analogous CaCl2 hypersensitivity has been reported for two other cngc mutant phenotypes (Chaiwongsar et al., 2009; Urquhart et al., 2011). For example, the growth reduction phenotype associated with a knockout of cngc2 in Arabidopsis was further decreased by approximately 2-fold by supplementing soil with 10 mm CaCl2.
While it is not clear why the loss of a Ca2+-permeable CNGC would cause pollen or a whole plant to become more sensitive to CaCl2, a recent patch clamp study on a cngc-b knockout mutant in the moss P. patens showed evidence for an increased open probability for two of three detectable Ca2+ permeable channel activities, consistent with a model in which the loss of one CNGC subunit might leave behind a CNGC complex with altered kinetic properties (Finka et al., 2012).
Heat Stress Can Trigger a Rise in cGMP
Results here provide evidence that a heat stress in either leaf tissue or pollen can trigger an increase in cGMP (Fig. 7). It is not clear if there was an additional increase or decrease in cAMP, as we were unable to detect cAMP using a similar immuno-based assay. In plant tissues, cGMP has been detected at levels ranging from 0.05 to 90 pmol g–1 fresh weight, with various stimuli triggering as much as a 9-fold increase (Penson et al., 1996; Durner et al., 1998). The levels of cGMP detected here lie within this range, with pollen showing approximately 700-fold less than leaves. Despite this variation, the detected concentrations are consistent with the potential for localized levels of cGMP that are sufficient for activating enzymes such as a CNGC (Gehring, 2010).
While a small stress-dependent increase in cGMP was observed here in both leaf and pollen, there are two alternative physiological interpretations to consider. It is possible that the observed increase results from a small percentage of cells with a transient stimulation of cGMP production, consistent with a model in which heat triggers a cNMP signal that activates a CNGC channel. The observed increase in cGMP might appear small because only a small percentage of cells are actively generating a cGMP transient at any given moment. Alternatively, the increase in cGMP might reflect a new steady-state level, generated as a response to a continuous exposure to a cellular stress. In this model, the higher cGMP levels represent an adaptation to the stress response as opposed to being the signal that triggers the response. Regardless, these results indicate that a heat stress can alter the dynamics or steady-state levels of cGMP in pollen.
A CNGC16-Dependent Transcriptional Response
Pollen from cngc16 mutants exposed to a heat stress showed an attenuated expression of three genes known to be associated with a heat stress response (Fig. 6). Two of these marker genes encode heat shock transcription factors HsfA2 and HsfB1, which are required for thermotolerance in vegetative tissues (Charng et al., 2007; Ikeda et al., 2011). The third was Bag6, which is one of the targets under the control of HsfA2 (Nishizawa-Yokoi et al., 2009) and implicated in pollen germination and tube growth in Arabidopsis (Cartagena et al., 2008). The inability to fully activate a transcriptional stress response provides a mechanistic explanation for the stress-sensitive pollen phenotype observed for cngc16 mutants.
A General Model for a cNMP-Triggered Heat Stress Response
There are two types of CNGC signaling pathways that are well characterized in animal systems (Kaupp and Seifert, 2002; Pifferi et al., 2006). In the first, the channel is gated open when a cNMP signal is produced, as occurs in the olfactory pathway. In the second, the channel is normally open due to a steady basal level of cNMP and closes when a phosphodiesterase is activated to reduce cNMP levels below the activation threshold, as occurs during vision. It is not clear if plants utilize one or both of these different pathways. The observation here that heat stress increases in cGMP can currently be explained in the context of both paradigms. In one model, heat stress triggers a cGMP signal, which activates CNGC16, similar to what happens in the animal olfactory response pathway (Leinders-Zufall et al., 1995). However, as discussed above, an increase in cGMP under stress conditions might alternatively reflect a new steady-state basal level, similar to what happens in animal vision when rod cells adapt to darkness (Fain, 2011).
There are several examples in animal systems for a potential role of cNMP in a heat stress response. In mammalian cells, a heat stress has been reported to increase cNMP levels (Kornbluth et al., 1977; Ewing et al., 1994; Zensho et al., 1998). Increases in cNMP concentrations have also been linked to the expression of heat stress response genes, such as those encoding heat shock proteins such as HSP-70 and HSP-90 in capillary endothelial cells (Martínez et al., 2006) or neuroblastoma cells (Zensho et al., 1998). However, the role of a CNGC in these pathways has not been established, and it should be noted that animals (and plants) have other potential cNMP-activated enzymes (Bridges et al., 2005). Nevertheless, a genetic knockout of a CNGC in Caenorhabditis elegans provides evidence for an animal CNGC pathway being involved in thermosensing (Cho et al., 2004; Hellman and Shen, 2011; Liu et al., 2012).
In plants, there are three recent examples linking CNGCs to a heat stress response. In the first, a knockout of AtCNGC6 in Arabidopsis resulted in plants with vegetative tissues showing a decreased tolerance to heat stress (Gao et al., 2012). This appears analogous to the increased heat stress sensitivity observed here for cngc16 pollen. However, a comparable disruption of a heat stress response was not observed in knockouts of a CNGC-b in the moss P. patens and its putative ortholog in Arabidopsis (CNGC2; Finka et al., 2012). Rather, these mutant plants showed a hyperactivation of a heat stress response at lower temperatures. One possible explanation is that the plant CNGCs form heteromultimers, and the loss of one subunit may leave behind a new CNGC complex with altered channel properties, potentially making some channel activities hyperresponsive and others nonresponsive. In Arabidopsis pollen, it is not clear which of the other five pollen expressed CNGCs might have functional interactions with CNGC16. Regardless, a knockout of cngc16 alone results in a disrupted stress response, providing genetic evidence for a signaling pathway that links cNMP signals to a transcriptional response that is critical for heat or drought stress tolerance during reproductive development.
MATERIALS AND METHODS
Plant Material and Control Growth Conditions
Metadata for CNGC16 can be found at The Arabidopsis Information Resource (http://www.arabidopsis.org/) under Arabidopsis Genome Initiative accession number At3g48010. Both cngc16-1 and cngc16-2 alleles are in the background of Arabidopsis (Arabidopsis thaliana) accession Columbia and were derived from the Syngenta Arabidopsis Insertion Library collection (Sessions et al., 2002). The cngc16-1 (SAIL_232_B12, seed stock no. 95) and cngc16-2 (SAIL_726_B04, seed stock no. 91) mutants carry a glufosinate (Basta) resistance marker in the T-DNA. In addition, the cngc16-1 T-DNA insertion has a GUS marker under the control of the pollen-specific LAT52 promoter. PCR-based genotyping of cngc16 mutants was done using the following primers. To detect the wild-type gene, primers used were 1149a, 5′-GGACTAGTCGGCGCGCCCGGCGGAATGAGCAACCTCCACCTCTACACCTC-3′, and 1149b, 5′-CCCCGCGGCCGCGCCTAGGCCTCAGAAGAATCCTGGATCCTCTGGTTTAA-3′. To detect the T-DNA borders, primers 1149a and 1149b were used in combination with 640 (LB3), 5′-TAGCATCTGAATTTCATAACCAATCTCGATACAC-3′.
Seeds were germinated and grown on 0.5× Murashige and Skoog medium containing 1% agar with or without Basta (10 μg mL−1). Two-week-old seedlings were transferred to soil (MetroMix200; Hummert). Unless otherwise stated, all plants were grown under control conditions (8 h of light and 16 h of darkness, a light intensity of approximately 110 μmol m−2 s−1, 40% humidity, and 22°C temperature) in growth chambers (Percival Scientific).
For experiments involving a hot/cold stress regime, flowering plants were grown for 2 to 4 weeks under conditions of hot days and cold nights (Zinn et al., 2010; Fig. 1B). Plants were grown in a growth chamber with 16 h of light, with the daybreak starting temperature at 10°C, rising to a peak 40°C for 1 h, and falling back to 10°C. For the 8-h night, the temperature was reduced to −1°C. For the heat-stress-only assays, cngc16 heterozygous mutants were transferred into chamber at 32°C or 37°C for 4 to 6 d while flowering and then returned to normal conditions for seed maturation. For drought-stress experiments, plants were grown in a greenhouse with limited watering.
Plasmid Constructs
Plant expression constructs were made in a modified pGreenII vector system (Hellens et al., 2000) with a kanamycin selection marker for bacteria and a hygromycin marker for plants. The DNA sequence of each construct is provided as a supplemental file (Supplemental Data S1). CNGC16 is encoded by a complementary DNA (cDNA) that was PCR amplified and sequenced to ensure the absence of PCR mistakes. Plasmid 1610, CNGC18p(i)-GFP-CNGC16, encodes a GFP-tagged CNGC16 under the control of a CNGC18 promoter (Frietsch et al., 2007). Representative transgenic plants rescued with plasmid construct 1610 are seed stock numbers 1646 and 1647 [cngc16-1(−/−) and cngc16-2(−/−) backgrounds, respectively]. Plasmid 1183, ACA9p(i)-GFP-CNGC16, is the same except the promoter is from ACA9 (Schiøtt et al., 2004). Representative transgenic plants rescued with this construct were seed stock numbers 1648 and 1649 [cngc16-1(−/−) and cngc16-2(−/−) backgrounds, respectively].
In Vitro Germination of Pollen
In vitro germination of pollen was done with a slightly modified protocol from Boavida and McCormick (2007). In brief, pollen from open flowers was spread on the surface of a slide with a solid medium containing 1% low-melting agarose with 0.01% H3BO3, 1 mm CaCl2, 5 mm KCl, and 10% Suc, pH 7.5. Slides were placed into a petri dish with a wet paper towel to humidify the chamber. Pollen were incubated at 22°C.
RNA Isolation and Real-Time Quantitative RT-PCR
For gene expression analysis, pollen from open flowers (stage 14; Nasrallah et al., 2002) were collected by vortexing cut flowers in water for approximately 10 s, allowing pollen to pass through a cell strainer with a 70-µm nylon mesh (Becton Dickinson and Company) and centrifuging pollen into a pellet at 14,000 rpm in a microfuge for 30 s. The supernatant was removed and pollen pellets frozen with liquid nitrogen. Pollen were harvested at midday for all experiments. For stress treatments, the midday harvest was preceded by 40°C for 1 h.
Total RNA was isolated from a pollen pellet (about 100 μL) using the RNeasy Plant Mini Kit (Qiagen). RNase-free DNase was used to eliminate genomic DNA. First-strand cDNA was synthesized using 2 μg of total RNA, GeneRacer oligo(dT) primer, and Superscript II RNase H– reverse transcriptase (Invitrogen). A fraction (0.14 µg) of the cDNA was used as the template in 20-µL reaction mixture per well in real-time PCR. TaqMan assays were purchased for HsfA2, HSF4, Bag6, and Zat12 (assay ID nos. At02302989_g1, At02332920_g1, At02256657_g1 At02263674_g1, and At02269070_s1, respectively) from Applied Biosystems. Target gene data were normalized against elongation factor1-α (EF1-α) RNA levels (assay ID no. At02337969_g1; Applied Biosystems; Fig. 6). Supplemental Figure S3 includes results for five additional genes that showed similar patterns in the wild type and cngc16 mutants: Cation/H+ exchanger9 (AT5G22910), Catalase2 (AT4G35090), GRAM domain-containing protein/ABA-responsive protein-related (AT5G13200), l-ascorbate peroxidase6 (AT4G32320), and Cold Regulated78 (AT5G52310; assay ID nos. At02302989_g1, At02255530_g1, At02198567_g1, At02248433_g1, At02320471_g1, and At02320471_g1, respectively, from Applied Biosystems). Gene expression levels were quantified by real-time quantitative RT-PCR ( CFX96; Bio-Rad). The thermal profile used for amplification was 95°C for 2 min followed by 45 cycles of 95°C for 15 s and 60°C for 1 min. Data were analyzed by using the comparative cycle threshold method. All quantitative PCR assays were validated in compliance with the “minimum information for the publication of real-time quantitative PCR experiments” guidelines (Bustin et al., 2009). This included validating probe efficiencies with a standard curve method (Larionov et al., 2005). Amplification efficiencies, which were calculated from the slope of the standard curve done with serial dilutions of cDNA (ranging from 0.1 to 1 µg), were 106.7%, 103%, 100%, 99.1%, and 90.6%, for EF-1-α, HSF4, HsfA2, Bag6, and Zat12, respectively. In addition, a “no template control” reaction was included to rule out cross contamination of reagents and surfaces. For each sample, a control was done without reverse transcriptase to test for genomic DNA contamination.
Pollen Viability Assay
Alexander’s stain (Alexander, 1969), modified by Johnson-Brousseau and McCormick (2004), was used to score pollen viability. Pollen grains were incubated in 1-mL Alexander stain buffer diluted 1:50 in water (stock solution: 10 mL 95% ethanol, 5 mL 1% malachite green in 95% ethanol, 5 g of phenol, 5 mL 1% acid fuschin in water, 0.5 mL 1% orange G dye in water, 2 mL glacial acetic acid, 25 mL glycerol, and 50 mL of water) for 30 to 40 min. Stained pollen grains were imaged with a digital camera mounted on a Leica DM IRE2 microscope. Viable pollen grains were identified with a reddish color, while dead pollen grains were a pale turquoise blue (Supplemental Fig. S2).
ELISA Assay for Quantification of cGMP
Leaves (approximately 1.5 cm across) were collected from young plants before bolting and immediately pulverized in 500 μL extraction buffer (1 m HCl in 100% ethanol). Samples were collected before and after a 30-min heat stress of 42°C. After spinning at 14,000 rpm in a microfuge for 10 min, the supernatant was transferred to a new tube, dried overnight, and resuspended in 250 μL of water. The debris pellet was dried and weighed and used for normalization of cNMP concentrations.
Pollen was collected at midday (as described above for RNA extractions) from approximately 200 mature wild-type plants. Each technical replicate had an approximately 0.2-mg wet weight of pollen (approximately 22,600 grains) in 0.5 mm 3-isobutyl-1-methylxanthine, a competitive nonselective phosphodiesterase inhibitor (Sigma-Aldrich), in 100 μL of water. Pollen samples were incubated for 30 min at room temperature (control conditions) or at 42°C (heat stress). After the incubation period, 500 µL extraction buffer was added and the samples were pulverized, vortexed, and spun at 14,000 rpm in a microfuge for 10 min. The supernatant was transferred to a new tube, dried overnight, and resuspended in 250 μL of water.
Plates were prepared and processed with a GenScript ELISA kit as described by the manufacturer (catalog no. L00253). Anti-cGMP (GenScript; catalog no. A01508) was used as the antigen specific antibody and was diluted to 0.05 µg mL–1 with binding buffer. The antigen (20 μL), which was either the extraction sample or a cGMP standard (Sigma-Aldrich), was loaded into each well and incubated 30 min at room temperature. The enzyme-linked secondary antibody cGMP-horseradish peroxidase (GenScript; M01058) was added to each well and incubated at room temperature for 1 h or 4°C overnight and then washed with 1× wash solution. The plate was developed with 100 μL of SureBlue Reserve TMB peroxidase substrate (Kirkegaard & Perry Laboratories; catalog no. 120283) and incubated at room temperature for 15 min with gentle agitation. The absorbance was measured at 640 nm. In this competitive ELISA assay, the lower the optical density, the higher the cGMP concentration. The concentration of extraction samples was determined using a standard curve of cGMP (0.061 pm/mL to 400 pmol mL–1). A linear function was used to determine the concentration of samples. A Wilcoxon two-sample test was done to determine the statistical significance of the data.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. The near-sterile phenotype of homozygous cngc16 mutants under hot/cold stress regime.
Supplemental Figure S2. Viability of pollen as detected by Alexander’s stain and light microscopy.
Supplemental Figure S3. Control quantitative PCR reactions showing examples of gene expression patterns that are similar between the wild type and cngc16 mutant.
Supplemental Data S1. DNA sequences of plasmid constructs 1610, CNGC18p(i)::GFP-CNGC16, and 1183, ACA9p(i)::GFP-CNGC16.
Acknowledgments
We thank Jason Stubrich for technical assistance.
Glossary
- CNGC
cyclic nucleotide-gated ion channel
- cNMP
cyclic nucleotide monophosphate
- cGMP
3′,5′-cyclic guanyl monophosphate
- RT
reverse transcription
- cDNA
complementary DNA
- T-DNA
transfer DNA
References
- Alexander MP. (1969) Differential staining of aborted and nonaborted pollen. Stain Technol 44: 117–122 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Becchetti A, Gamel K, Torre V. (2009) Cyclic nucleotide-gated channels. J Gen Physiol 114: S136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boavida LC, McCormick S. (2007) Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J 52: 570–582 [DOI] [PubMed] [Google Scholar]
- Bridges D, Fraser ME, Moorhead GBG. (2005) Cyclic nucleotide binding proteins in the Arabidopsis thaliana and Oryza sativa genomes. BMC Bioinformatics 6: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, et al. (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55: 611–622 [DOI] [PubMed] [Google Scholar]
- Cartagena JA, Matsunaga S, Seki M, Kurihara D, Yokoyama M, Shinozaki K, Fujimoto S, Azumi Y, Uchiyama S, Fukui K. (2008) The Arabidopsis SDG4 contributes to the regulation of pollen tube growth by methylation of histone H3 lysines 4 and 36 in mature pollen. Dev Biol 315: 355–368 [DOI] [PubMed] [Google Scholar]
- Chaiwongsar S, Strohm AK, Roe JR, Godiwalla RY, Chan CWM. (2009) A cyclic nucleotide-gated channel is necessary for optimum fertility in high-calcium environments. New Phytol 183: 76–87 [DOI] [PubMed] [Google Scholar]
- Charng YY, Liu HC, Liu NY, Chi WT, Wang CN, Chang SH, Wang TT. (2007) A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol 143: 251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S-W, Choi KY, Park C-S. (2004) A new putative cyclic nucleotide-gated channel gene, cng-3, is critical for thermotolerance in Caenorhabditis elegans. Biochem Biophys Res Commun 325: 525–531 [DOI] [PubMed] [Google Scholar]
- Crawford BCW, Ditta G, Yanofsky MF. (2007) The NTT gene is required for transmitting-tract development in carpels of Arabidopsis thaliana. Curr Biol 17: 1101–1108 [DOI] [PubMed] [Google Scholar]
- Cukkemane A, Seifert R, Kaupp UB. (2011) Cooperative and uncooperative cyclic-nucleotide-gated ion channels. Trends Biochem Sci 36: 55–64 [DOI] [PubMed] [Google Scholar]
- Durner J, Wendehenne D, Klessig DF. (1998) Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci USA 95: 10328–10333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing JF, Raju VS, Maines MD. (1994) Induction of heart heme oxygenase-1 (HSP32) by hyperthermia: possible role in stress-mediated elevation of cyclic 3′:5′-guanosine monophosphate. J Pharmacol Exp Ther 271: 408–414 [PubMed] [Google Scholar]
- Fain GL. (2011) Adaptation of mammalian photoreceptors to background light: putative role for direct modulation of phosphodiesterase. Mol Neurobiol 44: 374–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finka A, Cuendet AF, Maathuis FJM, Saidi Y, Goloubinoff P. (2012) Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 24: 3333–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G, Pressman E, Ophir R, Althan L, Shaked R, Freedman M, Shen S, Firon N. (2009) Transcriptional profiling of maturing tomato (Solanum lycopersicum L.) microspores reveals the involvement of heat shock proteins, ROS scavengers, hormones, and sugars in the heat stress response. J Exp Bot 60: 3891–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frietsch S, Wang Y-F, Sladek C, Poulsen LR, Romanowsky SM, Schroeder JI, Harper JF. (2007) A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc Natl Acad Sci USA 104: 14531–14536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Han X, Wu J, Zheng S, Shang Z, Sun D, Zhou R, Li B. (2012) A heat-activated calcium-permeable channel—Arabidopsis cyclic nucleotide-gated ion channel 6—is involved in heat shock responses. Plant J 70: 1056–1069 [DOI] [PubMed] [Google Scholar]
- Gehring C. (2010) Adenyl cyclases and cAMP in plant signaling: past and present. Cell Commun Signal 8: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorno F, Wolters-Arts M, Grillo S, Scharf K-D, Vriezen WH, Mariani C. (2010) Developmental and heat stress-regulated expression of HsfA2 and small heat shock proteins in tomato anthers. J Exp Bot 61: 453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM. (2000) pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol 42: 819–832 [DOI] [PubMed] [Google Scholar]
- Hellman AB, Shen K. (2011) Sensory transduction channel subunits, tax-4 and tax-2, modify presynaptic molecular architecture in C. elegans. PLoS ONE 6: e24562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honys D, Twell D. (2004) Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol 5: R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Mitsuda N, Ohme-Takagi M. (2011) Arabidopsis HsfB1 and HsfB2b act as repressors of the expression of heat-inducible Hsfs but positively regulate the acquired thermotolerance. Plant Physiol 157: 1243–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jammes F, Hu H-C, Villiers F, Bouten R, Kwak JM. (2011) Calcium-permeable channels in plant cells. FEBS J 278: 4262–4276 [DOI] [PubMed] [Google Scholar]
- Johnson-Brousseau SA, McCormick S. (2004) A compendium of methods useful for characterizing Arabidopsis pollen mutants and gametophytically-expressed genes. Plant J 39: 761–775 [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Seifert R. (2002) Cyclic nucleotide-gated ion channels. Physiol Rev 82: 769–824 [DOI] [PubMed] [Google Scholar]
- Kornbluth I, Siegel RA, Conforti N, Chowers I. (1977) cAMP in temperature- and ADH-regulating centers after thermal stress. J Appl Physiol 42: 257–261 [DOI] [PubMed] [Google Scholar]
- Köhler C, Merkle T, Neuhaus G. (1999) Characterisation of a novel gene family of putative cyclic nucleotide- and calmodulin-regulated ion channels in Arabidopsis thaliana. Plant J 18: 97–104 [DOI] [PubMed] [Google Scholar]
- Köhler C, Neuhaus G. (2000) Characterisation of calmodulin binding to cyclic nucleotide-gated ion channels from Arabidopsis thaliana. FEBS Lett 471: 133–136 [DOI] [PubMed] [Google Scholar]
- Larionov A, Krause A, Miller W. (2005) A standard curve based method for relative real time PCR data processing. BMC Bioinformatics 6: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T, Shepherd GM, Zufall F. (1995) Regulation of cyclic nucleotide-gated channels and membrane excitability in olfactory receptor cells by carbon monoxide. J Neurophysiol 74: 1498–1508 [DOI] [PubMed] [Google Scholar]
- Li J, Wang X, Zhang Y, Jia H, Bi Y. (2011) cGMP regulates hydrogen peroxide accumulation in calcium-dependent salt resistance pathway in Arabidopsis thaliana roots. Planta 234: 709–722 [DOI] [PubMed] [Google Scholar]
- Liu S, Schulze E, Baumeister R. (2012) Temperature- and touch-sensitive neurons couple CNG and TRPV channel activities to control heat avoidance in Caenorhabditis elegans. PLoS ONE 7: e32360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Berkowitz GA. (2011) Ca2+ conduction by plant cyclic nucleotide gated channels and associated signaling components in pathogen defense signal transduction cascades. New Phytol 190: 566–572 [DOI] [PubMed] [Google Scholar]
- Martínez JA, Tavárez JJ, Oliveira CM, Banerjee DK. (2006) Potentiation of angiogenic switch in capillary endothelial cells by cAMP: a cross-talk between up-regulated LLO biosynthesis and the HSP-70 expression. Glycoconj J 23: 209–220 [DOI] [PubMed] [Google Scholar]
- Moeder W, Urquhart W, Ung H, Yoshioka K. (2011) The role of cyclic nucleotide-gated ion channels in plant immunity. Mol Plant 4: 442–452 [DOI] [PubMed] [Google Scholar]
- Moutinho A, Hussey PJ, Trewavas AJ, Malhó R. (2001) cAMP acts as a second messenger in pollen tube growth and reorientation. Proc Natl Acad Sci USA 98: 10481–10486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJ, Sanders D, et al. (2001) Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol 126: 1646–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah ME, Liu P, Nasrallah JB. (2002) Generation of self-incompatible Arabidopsis thaliana by transfer of two S locus genes from A. lyrata. Science 297: 247–249 [DOI] [PubMed] [Google Scholar]
- Newton RP, Smith CJ. (2004) Cyclic nucleotides. Phytochemistry 65: 2423–2437 [DOI] [PubMed] [Google Scholar]
- Nishizawa-Yokoi A, Yoshida E, Yabuta Y, Shigeoka S. (2009) Analysis of the regulation of target genes by an Arabidopsis heat shock transcription factor, HsfA2. Biosci Biotechnol Biochem 73: 890–895 [DOI] [PubMed] [Google Scholar]
- Penson SP, Schuurink RC, Fath A, Gubler F, Jacobsen JV, Jones RL. (1996) cGMP is required for gibberellic acid-induced gene expression in barley aleurone. Plant Cell 8: 2325–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifferi S, Boccaccio A, Menini A. (2006) Cyclic nucleotide-gated ion channels in sensory transduction. FEBS Lett 580: 2853–2859 [DOI] [PubMed] [Google Scholar]
- Qin Y, Leydon AR, Manziello A, Pandey R, Mount D, Denic S, Vasic B, Johnson MA, Palanivelu R. (2009) Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genet 5: e1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rato C, Monteiro D, Hepler PK, Malhó R. (2004) Calmodulin activity and cAMP signalling modulate growth and apical secretion in pollen tubes. Plant J 38: 887–897 [DOI] [PubMed] [Google Scholar]
- Rhee SY, Somerville CR. (1998) Tetrad pollen formation in quartet mutants of Arabidopsis thaliana is associated with persistence of pectic polysaccharides of the pollen mother cell wall. Plant J 15: 79–88 [DOI] [PubMed] [Google Scholar]
- Schiøtt M, Romanowsky SM, Baekgaard L, Jakobsen MK, Palmgren MG, Harper JF. (2004) A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc Natl Acad Sci USA 101: 9502–9507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Schölkopf B, Weigel D, Lohmann JU. (2005) A gene expression map of Arabidopsis thaliana development. Nat Genet 37: 501–506 [DOI] [PubMed] [Google Scholar]
- Schuurink RC, Shartzer SF, Fath A, Jones RL. (1998) Characterization of a calmodulin-binding transporter from the plasma membrane of barley aleurone. Proc Natl Acad Sci USA 95: 1944–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions A, Burke E, Presting G, Aux G, McElver J, Patton D, Dietrich B, Ho P, Bacwaden J, Ko C, et al. (2002) A high-throughput Arabidopsis reverse genetics system. Plant Cell 14: 2985–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding EP, Harper JF. (2011) The ins and outs of cellular Ca2+ transport. Curr Opin Plant Biol 14: 715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talke IN, Blaudez D, Maathuis FJM, Sanders D. (2003) CNGCs: prime targets of plant cyclic nucleotide signalling? Trends Plant Sci 8: 286–293 [DOI] [PubMed] [Google Scholar]
- Urquhart W, Chin K, Ung H, Moeder W, Yoshioka K. (2011) The cyclic nucleotide-gated channels AtCNGC11 and 12 are involved in multiple Ca2+-dependent physiological responses and act in a synergistic manner. J Exp Bot 62: 3671–3682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Qu H, Jin C, Shang Z, Wu J, Xu G, Gao Y, Zhang S. (2011) cAMP activates hyperpolarization-activated Ca2+ channels in the pollen of Pyrus pyrifolia. Plant Cell Rep 30: 1193–1200 [DOI] [PubMed] [Google Scholar]
- Yang K-Z, Xia C, Liu X-L, Dou X-Y, Wang W, Chen L-Q, Zhang X-Q, Xie L-F, He L, Ma X, et al. (2009) A mutation in Thermosensitive Male Sterile 1, encoding a heat shock protein with DnaJ and PDI domains, leads to thermosensitive gametophytic male sterility in Arabidopsis. Plant J 57: 870–882 [DOI] [PubMed] [Google Scholar]
- Zelman AK, Dawe A, Gehring C, Berkowitz GA. (2012) Evolutionary and structural perspectives of plant cyclic nucleotide-gated cation channels. Front Plant Sci 3: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zensho H, Nishida A, Shimizu M, Uchitomi Y, Yamawaki S. (1998) Heat shock protein 72 restores cyclic AMP accumulation after heat shock in N18TG2 cells. Brain Res 790: 278–283 [DOI] [PubMed] [Google Scholar]
- Zinn KE, Tunc-Ozdemir M, Harper JF. (2010) Temperature stress and plant sexual reproduction: uncovering the weakest links. J Exp Bot 61: 1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]