Summary: Apple is shown to contain only a small number of functional terpene synthase genes whose evolution appears to have been shaped by genome-wide duplication events and commercial breeding strategies.
Abstract
Terpenes are specialized plant metabolites that act as attractants to pollinators and as defensive compounds against pathogens and herbivores, but they also play an important role in determining the quality of horticultural food products. We show that the genome of cultivated apple (Malus domestica) contains 55 putative terpene synthase (TPS) genes, of which only 10 are predicted to be functional. This low number of predicted functional TPS genes compared with other plant species was supported by the identification of only eight potentially functional TPS enzymes in apple ‘Royal Gala’ expressed sequence tag databases, including the previously characterized apple (E,E)-α-farnesene synthase. In planta functional characterization of these TPS enzymes showed that they could account for the majority of terpene volatiles produced in cv Royal Gala, including the sesquiterpenes germacrene-D and (E)-β-caryophyllene, the monoterpenes linalool and α-pinene, and the homoterpene (E)-4,8-dimethyl-1,3,7-nonatriene. Relative expression analysis of the TPS genes indicated that floral and vegetative tissues were the primary sites of terpene production in cv Royal Gala. However, production of cv Royal Gala floral-specific terpenes and TPS genes was observed in the fruit of some heritage apple cultivars. Our results suggest that the apple TPS gene family has been shaped by a combination of ancestral and more recent genome-wide duplication events. The relatively small number of functional enzymes suggests that the remaining terpenes produced in floral and vegetative and fruit tissues are maintained under a positive selective pressure, while the small number of terpenes found in the fruit of modern cultivars may be related to commercial breeding strategies.
Terpenes (also referred to as terpenoids or isoprenoids) constitute a large class of plant natural products with highly diversified functionality. Terpenes serve as precursors for the biosynthesis of essential plant metabolites (Croteau et al., 2000), including those involved in growth regulation (GAs, abscisic acid, and strigolactones), membrane stabilization (sterols), photosynthesis (carotenoids and the phytol side chain of chlorophyll), and electron transport coenzymes (ubiquinone and plastoquinone). Primarily, however, terpenes function as specialized plant metabolites that operate as attractants to pollinators or as defensive compounds against pathogens and herbivores (Kessler and Baldwin, 2001; Pichersky and Gershenzon, 2002). Terpenes are also important in determining the quality of horticultural food products, including the taste and aroma of wine (Styger et al., 2011) and fruit crops such as Citrus spp. (Vora et al., 1983; Maccarone et al., 1998; Aharoni et al., 2004) and strawberry (Fragaria spp.; Aharoni et al., 2004).
Terpenes are derived from linear assemblages of prenyldiphosphates, including the C10 monoterpene precursor geranyl diphosphate (GDP), the C15 sesquiterpene precursor farnesyl diphosphate (FDP), and the C20 diterpene precursor geranylgeranyl diphosphate (GGDP). The catalytic conversion of these relatively simple precursors to the diverse array of terpenes seen in nature is carried out by terpene synthase (TPS) enzymes, which have the capacity to direct myriad precursor-binding conformations through subtle variations in their conserved catalytic fold (Yoshikuni et al., 2006; O’Maille et al., 2008; Miller and Allemann, 2012).
TPS gene families have been explored in Arabidopsis (Arabidopsis thaliana; Aubourg et al., 2002; Lange and Ghassemian, 2003), grape (Vitis vinifera; Martin et al., 2010), poplar (Populus trichocarpa; Tuskan et al., 2006), rice (Oryza sativa; Goff et al., 2002), and sorghum (Sorghum bicolor; Paterson et al., 2009) as well as most recently in cultivated tomato (Solanum lycopersicum; Bleeker et al., 2011; Falara et al., 2011). The identification of 44 tomato TPS genes concurs with the comparative genome analysis work of Chen et al. (2011), indicating that the plant TPS gene family is midsized, with gene numbers ranging from approximately 20 to 150. The only exception so far appears to be the moss Physcomitrella patens, which has a single functional TPS gene (Chen et al., 2011). This analysis has also extended the phylogenetics used to define the initial TPS subfamilies (TPS-a to TPS-g; Bohlmann et al., 1998; Dudareva et al., 2003; Martin et al., 2004), culminating in the merging of the TPS-e and TPS-f subgroups (comprising gymnosperm and angiosperm ent-kaurene genes associated with primary metabolism) and the TPS-h subgroup so far specific to the lycopod Selaginalla moellendorffii.
The domestic apple (Malus domestica), which has long been recognized by consumers for its flavor, health, and nutritional properties (Harker et al., 2003), is one of the most widely cultivated fruit species in the world’s temperate regions. Apple belongs to the Rosaceae family, and while most Rosaceae have a haploid chromosome number of seven, eight, or nine, Malus spp., as the result of a relatively recent genome-wide duplication (GWD) event in the Pyreae, have transitioned from nine ancestral chromosomes to 17 chromosomes (Velasco et al., 2010).
Apple fruit produce more than 300 volatile organic compounds (VOCs), including alcohols, aldehyde esters, and ketones (Dimick and Hoskin, 1983; Paillard, 1990; Dixon and Hewett, 2000). Various terpenes have also been identified, although they only contribute a relatively minor component of total VOCs produced (Rapparini et al., 2001; Fuhrmann and Grosch, 2002; Hern and Dorn, 2003; Rowan et al., 2009a). The specific VOC composition in apple depends on several factors, including cultivar, climacteric ethylene production levels, maturity, and environmental conditions (Loughrin et al., 1990; Dixon and Hewett, 2000; Rapparini et al., 2001; Vallat et al., 2005). While the acyclic branched sesquiterpene (E,E)-α-farnesene appears to be the predominant terpene volatile associated with ripe fruit (Sutherland et al., 1977; Bengtsson et al., 2001; Hern and Dorn, 2003; Ferreira et al., 2009; Rowan et al., 2009b), various monoterpenes, cyclic sesquiterpenes, and terpene derivatives have also been identified, particularly in floral and vegetative tissues (Takabayashi et al., 1991; Bengtsson et al., 2001; Rapparini et al., 2001; Vallat and Dorn, 2005). Although many of these compounds are constitutively produced in relatively low amounts, a subset of common apple terpenes either are induced in response to insect infestation or have been observed to affect apple pest behavior directly. For example, (E)-β-ocimene is induced in immature apple fruit following infestation by codling moth (Cydia pomonella) larvae (Hern and Dorn, 2002), while (E,E)-α-farnesene exerts a concentration-dependent sexually dimorphic response in adult codling moth, attracting mated females at low dosages and repelling them at high dosages, while only attracting mated males at high dosages (Hern and Dorn, 1999). The relative abundance and diversity of terpenes in floral and vegetative tissues compared with ripe fruit suggests that these compounds are more likely to participate in defense and pollinator attraction roles rather than to attract seed dispersers or contribute to fruit flavor and aroma.
Despite numerous studies on apple terpene volatile production, to our knowledge the only reported apple TPS enzyme to be functionally characterized is that of the α-farnesene synthase (AFS1/MdAFS1; Rupasinghe et al., 2000; Pechous and Whitaker, 2004; Green et al., 2007). The recent availability of the draft sequence assembly of the apple genome (Velasco et al., 2010), combined with a large number of accessible apple ESTs, provided an opportunity to extend the current knowledge on the organization and functional annotation of the apple TPS gene family. In this study, we initially survey the volatile terpenes produced in cv Royal Gala and then use available apple genomic and EST information to identify candidate TPS genes for functional characterization and quantitative expression analysis. We show that only a small number of the TPS genes in the apple genome encode functional TPS enzymes, but these enzymes can account for the diversity of terpenes present in apple. Our results also provide insight into how this small but evolutionarily conserved family was shaped by GWD events and how the range of terpenes has been influenced by selective pressures and commercial breeding strategies.
RESULTS
Phenological and Tissue-Specific Terpene Volatile Production in cv Royal Gala
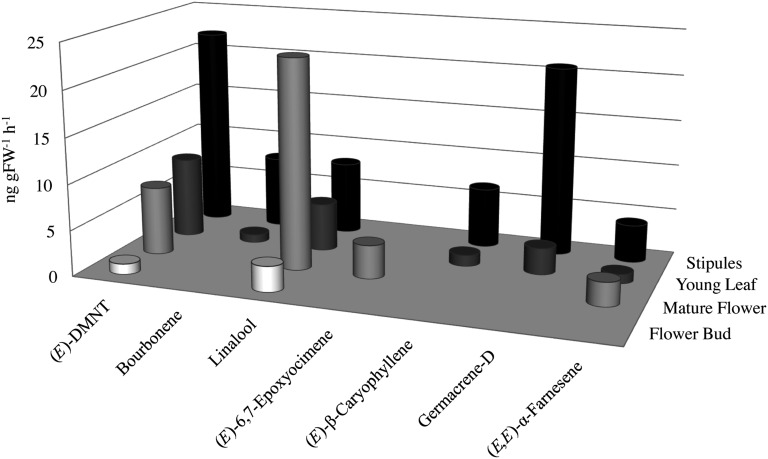
Terpene volatile production in apple ‘Royal Gala’ leaves, flowers, and fruit was investigated at various phenological stages by dynamic headspace trapping and gas chromatography-mass spectrometry (GC-MS). Excluding mature leaves, which showed an almost complete absence of volatile terpene production, floral and vegetative tissues produced a range of monoterpene, sesquiterpene, and homoterpene compounds (Fig. 1). Linalool, germacrene-D, and the C11 homoterpene (E)-4,8-dimethyl-1,3,7-nonatriene [(E)-DMNT], assumed to have derived from nerolidol oxidation (Donath and Boland, 1995; Boland et al., 1998), were the most prevalent nonfruit volatiles in cv Royal Gala and showed the most significant variation in their production. Specifically, germacrene-D, (E)-DMNT, and to a lesser extent bourbonene, (E)-β-caryophyllene, and linalool were the predominant vegetative terpenes, particularly in stipules. Stipules are lateral organs produced in pairs on stem nodes in association with leaves in many angiosperm species (Tyler, 1897), and in cv Royal Gala they have a leaf-like morphology. Linalool and (E)-DMNT were also the predominant terpenes in mature flowers and floral buds. The highest floral terpene production was seen in mature flowers, with linalool accounting for 55% to 58% of the total terpenes.
Figure 1.
Floral and vegetative terpene emission in cv Royal Gala. GC-MS analysis is shown for the predominant terpene volatiles trapped from the headspace of cv Royal Gala vegetative and floral tissues. Averaged data for biologically replicated analyses are shown. Terpenes produced at a rate less than 0.5 ng g−1 fresh weight (FW) h−1 are not included. The complete data set is presented in Supplemental Table S1. The bourbonene isomer was not determined, as the retention indices for α- and β-bourbonene are very similar.
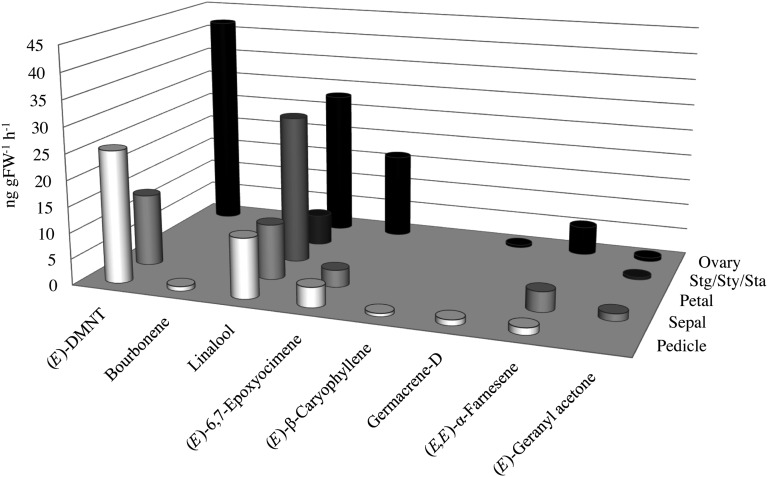
Terpene production in different floral tissues was also investigated in cv Royal Gala (Fig. 2). These results confirmed that linalool and (E)-DMNT were the predominant floral terpenes. Although linalool was produced at relatively high concentrations in all the floral tissues investigated, the highest production of linalool (in ng g−1 fresh weight h−1) was observed from petals and ovaries (accounting for approximately 96% and 28%–41% of the total volatile terpenes, respectively). (E)-DMNT, which was absent from petals, was primarily released from the ovaries and pedicles. Floral (E)-6,7-epoxyocimene [(5E)-2,3-epoxy-2,6-dimethylocta-5,7-diene] and (E,E)-α-farnesene production were also primarily associated with the ovaries. Volatile production from whole apple fruit during the approximately 150-d period from anthesis to harvest ripe (Fig. 3) showed that, with the exception of 30-d after anthesis (DAA) fruit, terpene concentrations were barely detectable (less than 0.005 ng g−1 fresh weight h−1; Supplemental Table S3). In 30-DAA fruit, (E)-DMNT was the predominant terpene, accounting for approximately 89% of the total terpenes identified. (E,E)-α-Farnesene was the only terpene of any significance to be released from intact ripe fruit at 150 DAA, although only at relatively low amounts (approximately 0.046 ng g−1 fresh weight h−1).
Figure 2.
Volatile terpene emission in cv Royal Gala floral tissues. GC-MS analysis is shown for the predominant terpene volatiles trapped from the headspace of cv Royal Gala floral tissues. Stg/Sty/Sta, Combined sample containing stigma, style, and stamen tissue. Averaged data for biologically replicated analyses are shown. Terpenes produced at less than 0.5 ng g−1 fresh weight (FW) h−1 are not included. The complete data set is presented in Supplemental Table S2.
Figure 3.

cv Royal Gala fruit at various stages of development: 30 DAA (A), 60 DAA (B), 90 DAA (C), 120 DAA (D), and 150 DAA (harvest ripe; E). Bars = 1 cm.
To investigate whether the skin of whole apple fruit was acting as a physical barrier to the release of terpenes derived from internal tissues, separate volatile analyses on skin, cortex, and seed tissues were carried out (Supplemental Table S4). The results indicated that skin, cortex, and seeds all contribute to the total pool of fruit terpenes identified in cv Royal Gala. The highest terpene production on the basis of fresh weight was observed in 60-DAA seeds, with (E)-β-ocimene accounting for approximately 93% of the total terpenes. There was a qualitative increase in the rate of terpene emissions in the separate fruit tissues compared with that in whole fruit, especially over the 60- to 150-DAA period. Notably, the emission rate of (E)-DMNT from 60-DAA skin was approximately 45-fold higher than that observed for 60-DAA whole fruit, while the emission rates of (E,E)-α-farnesene and its oxidation product 6-methyl-5-hepten-2-one (Anet, 1972) in ripe fruit (150 DAA) were approximately 36-fold and between 500- and 900-fold higher, respectively, in the skin than in whole apple fruit. Although a concentration-dependent bias (in g−1 fresh weight) favoring the skin analyses might explain some of the difference, most of the other terpene compounds produced from the 150-DAA skin remained at low concentrations (Supplemental Table S5).
Considering the prevalence of (E)-DMNT in flowers, vegetative tissues, and, to a lesser extent, in unripe fruit, the absence of any detectable (E)-DMNT precursor nerolidol in cv Royal Gala is surprising. Although the presence of (E)-DMNT indicated that a nerolidol synthase was likely to be active in these tissues, the lack of nerolidol suggests that a highly efficient mechanism for converting nerolidol to DMNT is also operating. It is likely that this process will be mediated by a cytochrome P450 monooxygenase similar to that identified in Arabidopsis homoterpene biosynthesis (Lee et al., 2010).
Organization of the TPS Gene Family in the cv Golden Delicious Genome
Interrogation of the genome sequence of apple ‘Golden Delicious’ using previously published TPS sequences identified 55 gene models with significant homology to known TPS enzymes (Supplemental Table S6). While putative TPS genes were located on 11 of the 17 apple linkage groups (chromosomes), a significant proportion of TPS genes were found to colocate on the same chromosome region. However, given the highly heterozygous nature of the apple genome and the challenges this poses for genome sequencing and assembly (Zharkikh et al., 2008), it is difficult to determine accurately what proportion of these clustered genes are the result of gene duplications and what proportion are allelic variants (haplotypic).
A striking feature of the analysis was that only 10 genes were predicted to encode functional TPS enzymes (Table I; Supplemental Fig. S1). This prediction required a TPS open reading frame (ORF) to be of the expected size and organization (i.e. seven exons for TPS-a, TPS-b, and TPS-g and between 13 and 15 exons for TPS-e/TPS-f and TPS-c) and also that it contain structural features such as the Mg2+ binding [DDXX(D/E); Christianson, 2006] and (N,D)Dxx(S,T,G)xxxE (NSE/DTE) regions (Cane et al., 1996; Rynkiewicz et al., 2002) required for TPS activity. The remaining 45 genes were categorized as putative pseudogenes, as they either contained multiple deletions or encoded small TPS fragments (possessing one to two exons only), presumably due to being misspliced and/or containing premature stop codons. Three of the predicted pseudogenes also appeared to contain retrotransposon-like insertions (Supplemental Fig. S2).
Table I. Putative full-length TPS genes in the cv Golden Delicious genome.
Instituto Agrario San Michele all’Adige-annotated gene models (Gene Models 1) encoding potentially functional cv Golden Delicious TPS enzymes were identified following tBLASTn searches of the GDR database with known TPS sequences obtained from the nonredundant protein database at NCBI. For a detailed description of column headings, see Supplemental Table S7.
| Name | Gene Model | Predicted Subgroup | Protein Length | Linkage Group | No. of Exons | RRX8W | DDXXD | NSE/DTE | CTP |
|---|---|---|---|---|---|---|---|---|---|
| MdTPS1-GD | MDP0000120176 | TPS-a | 574 | 7 | 7 | RPX8W | DDVYD | DDxxDxxxE | No |
| MdTPS5-GD | MDP0000161084 | TPS-a | 556 | 12 | 7 | KPX8W | DDVYE | DDxxExxxE | No |
| MdTPS15-GD | MDP0000276976 | TPS-a | 564 | 11 | 7 | RRX8W | DDIYD | NDxxSxxxE | No |
| MdTPS16-GD | MDP0000295452 | TPS-a | 591 | 5 | 7 | No | DDIYD | NDxxSxxxE | Yes |
| MdTPS26-GD | MDP0000297049 | TPS-b | 616 | 17 | 7 | RRX8W | DDMYD | DDxxTxxxE | Yes |
| MdTPS30-GDa | MDP0000147908 | TPS-c | 810 | 11 | 15 | No | GSHFRE | LSSPEHEQL | No |
| MdTPS46-GD | MDP0000828007 | TPS-e/TPS-f | 733 | 15 | 13 | No | DDFFD | NDxxGxxxE | No |
| MdTPS50-GD | MDP0000298903 | TPS-g | 535 | 10 | 7 | No | DDIFD | DDxxSxxxE | No |
| MdTPS52-GD | MDP0000398063 | TPS-g | 554 | 5 | 7 | No | DDIFD | DDxxSxxxE | No |
| MdTPS54-GD | MDP0000562538 | TPS-g | 568 | 5 | 7 | No | DDIFD | DDxxSxxxE | No |
Probable type II diterpene synthase, which uses a protonation-dependent catalytic mechanism (Wendt and Schulz, 1998) and does not require DDXXD or NSE/DTE motifs common to type I TPSs.
On the basis of the current apple genome annotation, the TPS subgroup clustering of the cv Golden Delicious TPS genes (rationalized on the basis of amino acid sequence homology) showed that the TPS-a gene family in apple was the most expanded, with 23 or approximately 40% of the total TPS genes identified (Supplemental Table S6). This is consistent with other plant species, including grape, Arabidopsis, and rice (Chen et al., 2011). For the remaining TPS subgroups, six genes representing the TPS-b subgroup, five representing the TPS-c subgroup, and 12 and nine genes representing the TPS-e/TPS-f and TPS-g subgroups, respectively, were also identified. Not surprisingly, the only subgroup with no representative apple genes was the gymnosperm-specific TPS-d subgroup (Bohlmann et al., 1999).
The largest clusters of TPS genes occurred on chromosomes 17 and 12, with 13 and 12 genes, respectively, eight genes on chromosome 11, seven genes on chromosome 3, and smaller clusters of five genes on chromosomes 10 and four genes each on chromosomes 5 and 15. Single TPS genes were also identified on chromosomes 2, 6, and 7. The identification of TPS-a genes on homeologous chromosomes 3 and 11 and TPS-g genes on homeologous chromosomes 5 and 10 indicates that the expansion of these subgroups can be attributed to the recent GWD in apple (Velasco et al., 2010).
Identification of cv Royal Gala TPSs in EST Databases
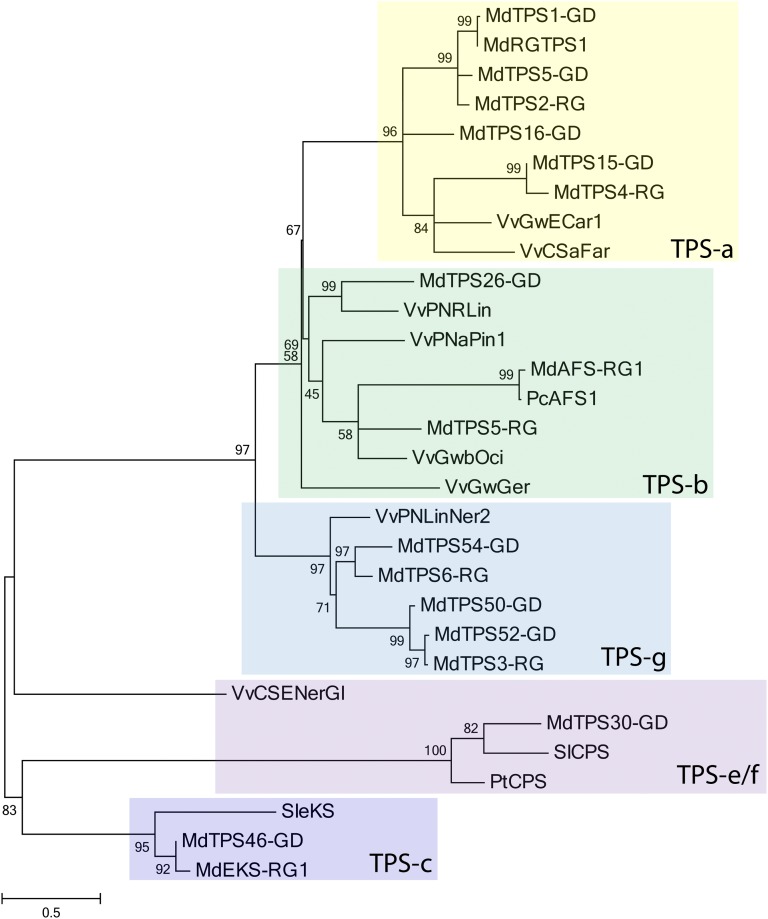
To identify expressed TPSs in apple, BLAST searches of National Center for Biotechnology Information (NCBI) GenBank EST databases and an in-house Malus EST database (Newcomb et al., 2006), which now includes approximately 1.2M cv Royal Gala Next Generation Sequences (R.D. Crowhurst, unpublished data), were carried out. In addition to the previously characterized apple α-farnesene synthase (now termed MdAFS-RG1; accession no. AY787633) and a putative cv Royal Gala ent-kaurene synthase (accession no. JQ281521), six new full-length TPS ORFs were identified (Supplemental Table S7). A partial TPS (termed MdCDS-RG1) encoding a putative copalyl diphosphate (CDP) synthase gene (TPS-c subgroup) was also identified with significant homology to the predicted Golden Delicious TPS-c gene MdTPS30-GD. However, the predicted ORF of MdCDS-RG1 appeared to be missing approximately 80 N-terminal amino acids. Although closer examination of the cv Golden Delicious gene models identified another partial CDS gene (MdTPS33-GD) that included the missing N-terminal amino acids, we have so far been unable to amplify a full-length CDP synthase from cv Royal Gala. Confirmation of the TPS subgroup placement for the cv Royal Gala and Golden Delicious TPS ORFs was also obtained using phylogenetic analysis versus selected nonapple TPS sequences (Fig. 4). This analysis also confirmed that none of the cv Royal Gala TPS enzymes clustered within the TPS-c subgroup, which is specific to CDP synthases.
Figure 4.
Phylogeny of the apple TPS family. Maximum likelihood analysis is shown for the predicted full-length TPSs from cv Royal Gala (RG) and cv Golden Delicious (GD) apple with selected full-length grape, poplar, pear, and tomato TPSs. Bootstrap values are shown as a percentage of 100 replicates. The scale bar represents 0.5 substitutions per site. Abbreviations for previously identified and/or functionally annotated TPS enzymes, including GenBank accession numbers in parentheses, are as follows: grape VvGwECar1 = (E)-β-caryophyllene synthase (ADR74192), VvCSaFar = (E,E)-α-farnesene synthase (ADR74198), VvPNRLin = (3R)-linalool synthase (ADR74209), VvPNaPin1 = pinene synthase (ADR74202), VvGwbOci = (E)-β-ocimene synthase (ADR74204), VvGwGer = geraniol synthase (ADR74217), VvPNLinNer2 = (3S)-linalool/(E)-nerolidol synthase (ADR74211), and VvCSENerGl = (E)-nerolidol/(E,E)-geranyl linalool synthase (ADR74219); apple MdRGAFS1 = (E,E)-α-farnesene synthase (AAX19772) and MdRGEKS = ent-kaurene synthase (AFG18184); pear PcAFS1 = (E,E)-α-farnesene synthase (AAT70237); tomato SleKS = ent-kaurene synthase (AEP82778) and SlCPS = CDP synthase (BAA84918); poplar PtCPS = CDP synthase (EEE81383).
The proportion of pseudogenes to TPS genes predicted to encode functional enzymes in apple (45:10) is higher than expected, considering that plants with comparably sized TPS gene families, including Arabidopsis (Aubourg et al., 2002) and tomato (Falara et al., 2011), possess mostly functional or potentially functional enzymes. However, the eight functional and/or potentially functional TPS genes identified in cv Royal Gala is in agreement with the number predicted from the in silico cv Golden Delicious genome analysis. Given the relatively high degree of amino acid sequence and structural conservation in plant TPS enzymes (Aubourg et al., 2002), we are confident that the majority of full-length apple TPS enzymes have now been identified.
Functional Characterization of Full-Length TPSs from cv Royal Gala
To investigate their likely in planta activity, the seven full-length TPS ORFs from cv Royal Gala that encoded monoterpene synthase and sesquiterpene synthase enzymes likely to be associated with terpene volatile production were initially expressed in tobacco (Nicotiana benthamiana) leaves and analyzed for terpene production. On the basis that ent-kaurene was not identified as a volatile terpene in any of the cv Royal Gala tissues and that ent-kaurene synthases, along with CDP synthases, have a primary metabolic role in GA biosynthesis (Yamaguchi et al., 1996; Hedden and Thomas, 2012), MdEKS-RG-1 was not characterized in planta.
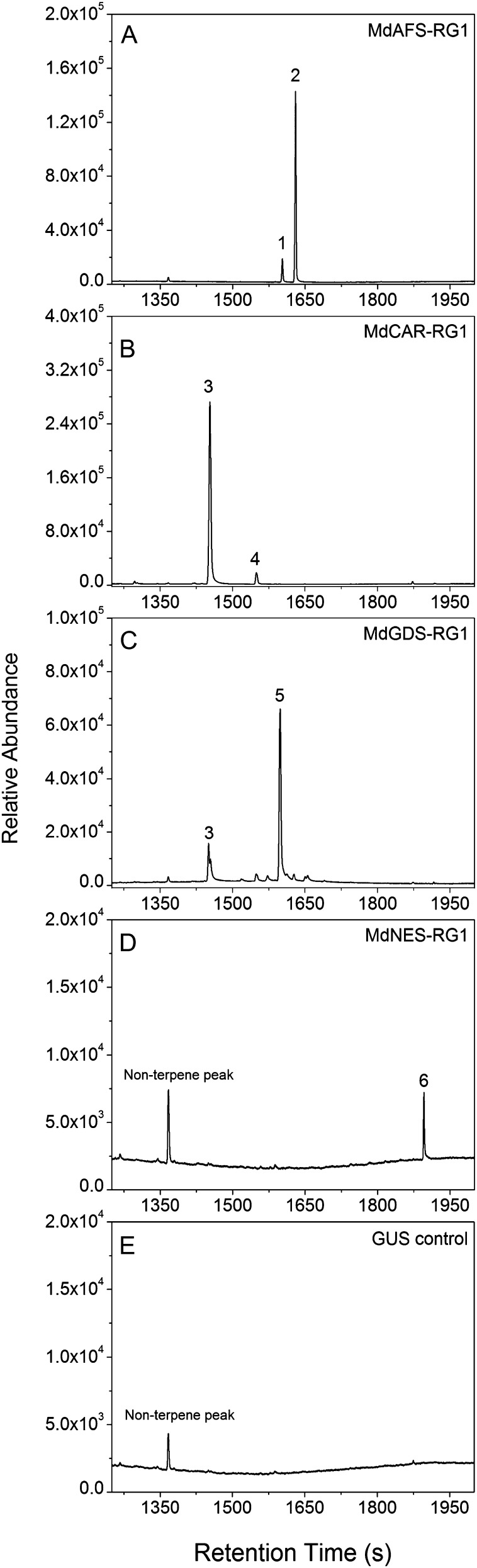
For the TPS enzymes associated with volatile terpene emission in cv Royal Gala, four sesquiterpene synthase enzymes (Fig. 5) and three monoterpene synthase enzymes (Fig. 6) were identified. Consistent with previous experiments conducted in vitro where (E,E)-α-farnesene was the major product, MdAFS-RG1 produced (E,E)-α-farnesene (95.5%) and (Z,E)-α-farnesene (4.5%) in planta (Fig. 5A). Two cyclic sesquiterpene synthases were also identified: MdCAR-RG1 (Fig. 5B), which synthesized (E)-β-caryophyllene (96.3%) with smaller amounts of α-caryophyllene (3.7%), and MdGDS-RG1 (Fig. 5C), which synthesized germacrene-D (85.3%) with smaller quantities of (E)-β-caryophyllene (14.7%). MdNES-RG1 produced a small peak of (E)-nerolidol (Fig. 5D) and is likely to be the source of floral and leaf-derived DMNT in cv Royal Gala resulting from the breakdown of (E)-nerolidol.
Figure 5.
In planta analysis of cv Royal Gala sesquiterpene synthase enzyme activity. Full-length sesquiterpene synthase enzymes were cloned into the pHEX2 plant transformation vector and transiently expressed in tobacco leaves. Selected ion (m/z 93) headspace GC-MS profiles are as follows. A, MdAFS-RG1: peak 1, (Z,E)-α-farnesene; peak 2, (E,E)-α-farnesene. B, MdCAR-RG1: peak 3, (E)-β-caryophyllene; peak 4, α-caryophyllene. C, MdGDS-RG1: peak 3, (E)-β-caryophyllene; peak 5, germacrene-D. D, MdNES-RG1: peak 6, (E)-nerolidol. E, GUS: empty vector control. Products were confirmed by comparison with authentic standards and/or GC-MS retention indices.
Figure 6.
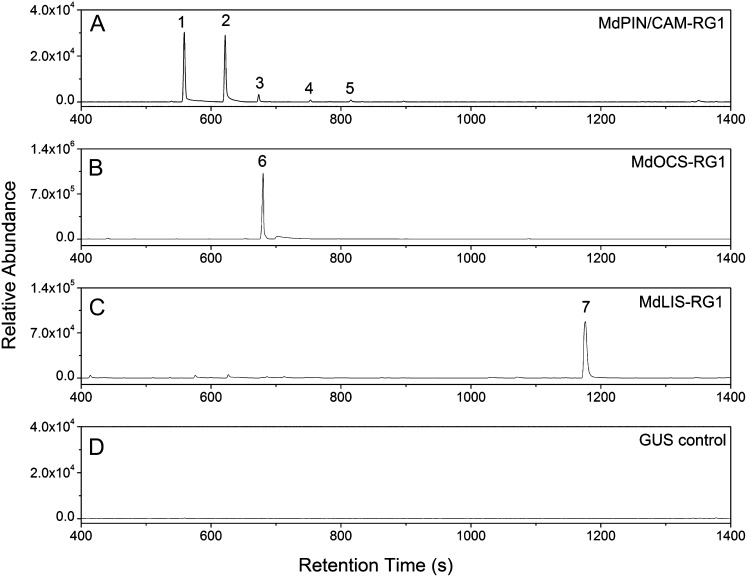
In planta analysis of cv Royal Gala monoterpene synthase enzyme activity. Full-length monoterpene synthase enzymes were cloned into the pHEX2 plant transformation vector and transiently expressed in tobacco leaves. Selected ion (m/z 93) headspace GC-MS profiles are as follows. A, MdPIN/CAM-RG1: peak 1, α-pinene; peak 2, camphene; peak 3, β-pinene; peak 4, β-myrcene; peak 5, limonene. B, MdOCS-RG1: peak 6, (E)-β-ocimene. C, MdLIS-RG1: peak 7, linalool. D, GUS: empty vector control. Products were confirmed by comparison with authentic standards and/or GC-MS retention indices.
For the monoterpene synthase enzymes, MdPIN/CAM-RG1(Fig. 6A) was the only multiproduct monoterpene synthase enzyme, synthesizing camphene (48.5%) and α-pinene (43.8%) as its major products, with smaller amounts of β-pinene (4.8%), limonene (1.5%), and β-myrcene (1.3%); MdOCS-RG1 (Fig. 6B) and MdLIS-RG1 (Fig. 6C) produced (E)-β-ocimene and linalool, respectively, as single products.
No significant amounts of terpenes were identified in vector-only control inoculations, indicating that the terpenes identified in the tobacco leaf analyses were the results of the transiently expressed apple TPS enzymes.
For additional confirmation of cv Royal Gala TPS function, the above enzymes (with the exception of the previously characterized MdAFS-RG1) were also overexpressed in Escherichia coli and the volatiles were solvent extracted and analyzed by GC-MS. Active recombinant proteins were obtained for all the apple TPS enzymes except MdNES-RG1. In agreement with the tobacco transient expression analysis, the recombinant monoterpene synthase enzyme activity results (Supplemental Fig. S3) showed that linalool and (E)-β-ocimene were produced as single products from MdLIS-RG1 and MdOCS-RG1, respectively, while α-pinene (32%) and camphene (26.3%) were the predominant products from MdPIN/CAM-RG. The only notable difference was the identification of several hydroxylated terpenes and terpene derivatives in the MdPIN/CAM-RG1 solvent extractions that were not seen in the headspace analysis, a likely consequence of their decreased volatility. These included borneol (17.4%), a pinene derivative tentatively assigned as 2-pinanol (4.1%), and a probable sabinene derivative, possibly sabinene hydrate (4.1%).
The in vitro results for the two active recombinant sesquiterpene synthase enzymes, MdGDS-RG1 and MdCAR-RG1 (Supplemental Fig. S4), were also very similar to those obtained for the transient expression analysis. MdGDS-RG1 predominantly synthesized germacrene-D (77.7%) with smaller amounts of a number of additional compounds, including (E)-β-caryophyllene (5.9%) and a germacrene-D derivative, possibly germacrene-D-4-ol (5.8%). MdCAR-RG1 predominantly synthesized (E)-β-caryophyllene (91.4%) with smaller amounts of α-caryophyllene (3.6%), α-copaene (2.7%), and germacrene-D (2.3%). Notably, the recombinant linalool synthase (MdLIS-RG1) was also shown to produce nerolidol in the presence of FDP, suggesting that this enzyme could also potentially contribute to nerolidol production in cv Royal Gala. None of the remaining apple TPS enzymes showed any bifunctional FDP/GDP substrate specificity in vitro.
Enantioselective GC-MS analysis was also carried out to establish the main terpene enantiomers produced in cv Royal Gala flowers, stipules, and young leaves and also from the recombinant TPS enzymes. Monoterpene enantiomeric analysis (Supplemental Fig. S5) indicated that MdLIS-RG1 exclusively synthesized the (S)-(+)-linalool enantiomer in vitro, while the predominant enantiomers produced for MdPIN/CAM-RG1 in vitro were (R)-(+)-α-pinene, (R)-(+)-β-pinene, and (R)-(+)-limonene. There was good correlation between the pinene and limonene enantiomers produced from MdPIN/CAN-RG1 and those identified in the cv Royal Gala flowers, young leaves, and stipules (Supplemental Fig. S5, C–E). However, these tissues produced a mix of (S)-(+)-linalool and (S)-(−)-linalool in varying ratios, suggesting the presence of a second linalool synthase in apple capable of producing the (S)-(−)-linalool enantiomer. Sesquiterpene enantiomeric analysis (Supplemental Fig. S6) indicated that (−)-germacrene-D was produced in cv Royal Gala tissues and by the MdGDS-RG1 and MdCAR-RG1 enzymes. However, due to the lack of an appropriate enantiomeric standard, it was also not possible to determine the enantiomeric specificity of (E)-β-caryophyllene in cv Royal Gala. (S)-(E)-Nerolidol was produced by the bifunctional MdLIS-RG1 enzyme. As with the previous cv Royal Gala headspace volatile analyses, nerolidol was not identified in any of the solvent extracts, so further comparisons were not possible.
Together, these results indicate that the above full-length TPS complementary DNAs (cDNAs) can account for the majority of the terpenes produced in cv Royal Gala. The only disparities were the absence of TPS enzymes capable of synthesizing (S)-(−)-linalool and bourbonene. It is likely that bourbonene, which is most abundant in the stipules and to a lesser extent in young leaves (Fig. 1), is derived from an as yet unidentified sesquiterpene synthase that is either specific to these particular tissues in cv Royal Gala or is induced under specific conditions.
The identification of seven functional and one potentially functional (i.e. MdEKS-RG1) TPS genes in cv Royal Gala supports the cv Golden Delicious in silico genome findings, while the above in planta data correlate with the qualitative range of terpenes identified in cv Royal Gala and also in other apple cultivars (Takabayashi et al., 1991; Bengtsson et al., 2001; Rapparini et al., 2001; Vallat and Dorn, 2005). Taken together, these findings support our contention that the number of TPS genes encoding functional enzymes in apple is small compared with that in other plant species (Chen et al., 2011).
TPS Gene Expression in cv Royal Gala
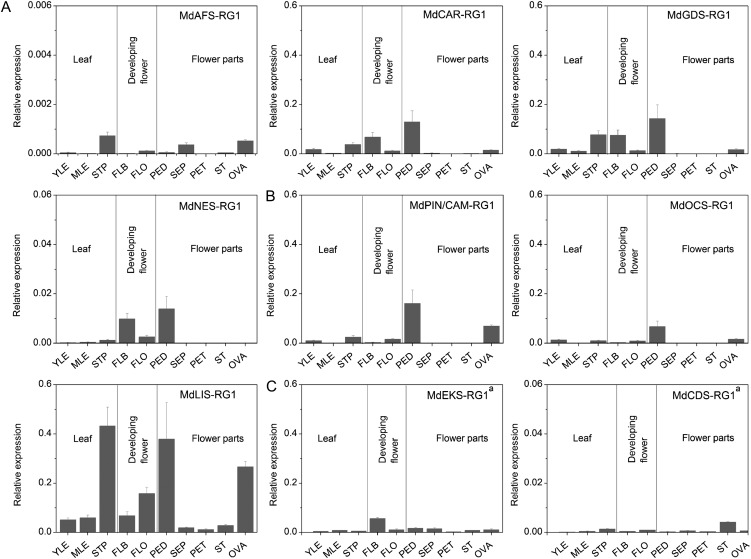
Relative gene expression analysis was conducted on all genes encoding functional TPS enzymes and the two putative cv Royal Gala di-TPS genes (MdEKS-RG1 and MdCDS-RG1) in floral and vegetative tissues (Fig. 7) and fruit (Fig. 8). MdLIS-RG1 was the most highly expressed gene in flowers, leaves, and stipules. In ovaries and pedicels of cv Royal Gala flowers, high rates of MdLIS-RG1 mRNA accumulation correlated with high linalool production (Fig. 2). However, comparatively low levels of MdLIS-RG1 were observed in petals, which are one of the highest linalool-producing tissues in cv Royal Gala (Fig. 2). The expression of an (S)-(−)-linalool synthase in petals might account for this discrepancy. Elevated rates of MdCAR-RG1 and MdGDS-RG1 mRNA accumulation were also observed in stipules, which were the primary sources of (E)-β-caryophyllene and germacrene-D production in cv Royal Gala. Similarly, the higher accumulation of MdNES-RG1 transcript in pedicels correlated with the comparatively high amounts of DMNT (Fig. 2) produced in this tissue. For the di-TPS genes, MdEKS-RG1 transcript was highest in flower buds while MdCDS-RG1 was predominantly expressed in the stamens.
Figure 7.
TPS gene expression in cv Royal Gala flowers and leaves. Expression profiles are shown for sesquiterpene (A), monoterpene (B), and diterpene (C) synthases in different flower and leaf tissues. aMdEKS-RG1 and MdCDS-RG1 have not been functionally verified but are predicted to encode the respective cv Royal Gala ent-kaurene and CDP synthases. YLE, Young leaf; MLE, mature leaf; STP, stipule; FLB, flower bud; FLO, fully opened flower; PED, pedicel; SEP, sepal; PET, petal; ST, stigma, style, and stamen; OVA, ovary. The data were analyzed using the target-reference ratio calculated with the LightCycler 480 software, enabling a comparison of the level of expression of different gene family members compared with the EF1α reference gene considered stable and unchanging in the different tissues during development. The specific primers used for each gene are detailed in Supplemental Table S7. Data are presented as means ± se (n = 4).
Figure 8.
TPS gene expression in cv Royal Gala fruit tissues. Gene expression profiles are shown for sesquiterpene (A), monoterpene (B), and diterpene (C) synthases in different tissues during apple fruit development. Gene expression data analysis was carried out as for Figure 7. Data are presented as means ± se (n = 4).
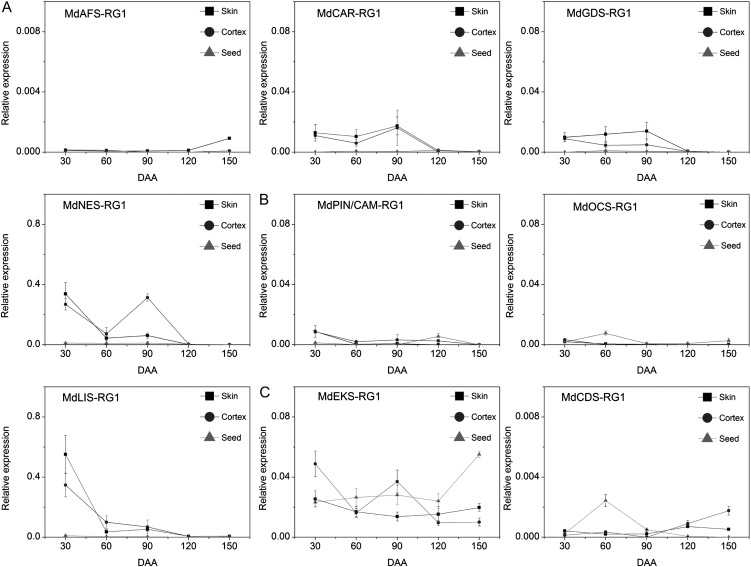
In fruit, mRNA levels for the different TPS genes (Fig. 8) were generally lower than those for the equivalent genes in floral and vegetative tissues. The linalool and nerolidol synthase-encoding genes (MdLIS-RG1 and MdNES-RG1, respectively) were the most highly expressed TPS genes in fruit. Expression of MdLIS-RG1 and MdNES-RG1 was primarily confined to the skin and cortex of young fruit (30 DAA), although a small peak of MdNES-RG1 expression was also observed in 90-DAA skin (Fig. 8). A slight increase in (E,E)-α-farnesene synthase (MdAFS1-RG1) transcript was also observed between 120 and 150 DAA, as was a small peak of (E)-β-ocimene synthase (MdOCS-RG1) expression at 60 DAA. The fruit di-TPS expression analysis showed that MdEKS-RG1 transcript levels peaked at 30 and 90 DAA in the cortex and, with the exception of a peak in seed expression at 150 DAA, remained relatively constant during fruit maturation in both seeds and skin. A peak of MdCDS-RG1 (putative CDP synthase) expression was also observed in 60-DAA seeds, while a gradual increase in MdCDS-RG1 cortex expression between 90 and 150 DAA was also apparent. The lack of detectable ent-kaurene in any of the GC-MS headspace volatile analyses indicates that ent-kaurene flux is predominantly toward GA biosynthesis in apple.
The constitutive low levels of TPS expression in apple are in agreement with the low volatile production from whole apple fruit (Supplemental Table S3). However, increased linalool and nerolidol production in 30-DAA fruit, ocimene production in 60-DAA seeds, and (E,E)-α-farnesene production in harvest-ripe fruit (Fig. 2) all correlate with increases in expression levels of the equivalent TPS genes.
TPS gene expression was also investigated in cv Royal Gala root tissue (Supplemental Fig. S7) and showed that the pinene/camphene synthase (MdPIN/CAM-RG1) gene was the most highly expressed. Lower levels of expression were also observed for the linalool synthase (MdLIS-RG1), the (E,E)-α-farnesene synthase (MdAFS-RG1), and the putative ent-kaurene synthase (MdEKS-RG1).
TPS Expression in the Fruit of Heritage Apple Cultivars
In the ripe fruit of many different apple cultivars including cv Royal Gala, terpene production appears to be restricted to the sesquiterpene α-farnesene (Sutherland et al., 1977; Bengtsson et al., 2001; Hern and Dorn, 2003; Ferreira et al., 2009; Rowan et al., 2009a). The expression of terpene genes typically found in apple flowers and leaves might be expected to introduce new flavor notes into fruit. Five heritage varieties with unusual spicy, nutty, or tangy flavor notes were screened for TPS gene expression and terpene volatile production. Volatile production was assessed in skin from these varieties and cv Royal Gala using solvent extraction to maximize the chances of detecting low-level terpene alcohol production.
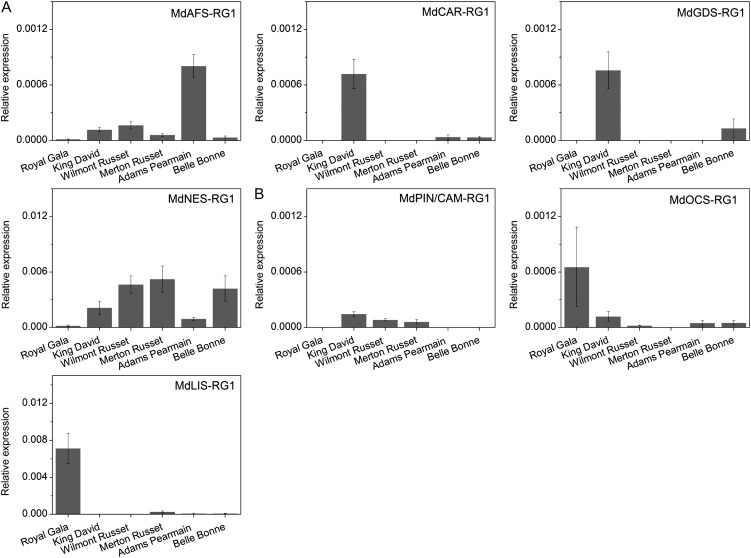
GC-MS analysis (Table II) showed that cv Royal Gala was a comparatively low terpene producer compared with the five heritage cultivars. Interestingly, in cv Royal Gala, solvent extraction analysis showed that (E,E)-farnesol was the predominant accumulated terpene (approximately 84% of total terpenes) and not (E,E)-α-farnesene, which only accounted for approximately 9% of the total terpenes. In the other cultivars, (E,E)-α-farnesene was by far the most prevalent terpene, with concentrations ranging from 220 to 7,500 ng g−1 fresh weight. The relative amounts of AFS mRNA accumulation (Fig. 9) correlated well with (E,E)-α-farnesene production in the heritage apple cultivars. Production of other terpenes in the heritage varieties was generally low, but seven terpenes not detected in the cv Royal Gala control were present, including limonene, which was found in all five heritage varieties. The detection of terpineol in the cv Royal Gala solvent extractions was a little surprising given that it was not detected in any of the fruit headspace analyses (Supplemental Tables S3 and S4). However, the decreased volatility of this terpene alcohol means that it is more likely to be found in the solvent extract than in the headspace. It is also likely that terpineol is a product of one of the existing TPS enzymes, possibly the α-pinene/camphene synthase MdPIN/CAM-RG1.
Table II. Terpene volatiles in five heritage apple cultivars.
Terpene volatiles from five heritage apple cultivars and cv Royal Gala were sampled at approximately 150 DAA by solvent extraction. Compound identification was as described in Table I with the following additions: Additional authentic standards were obtained from: *Aldrich, **Synthesis, ***Givaudan. References for retention indices are listed in the footnotes.
| Compound | Retention Time | Retention Index | cv Royal Gala | cv King David | cv Wilmont Russet | cv Belle Bonne | cv Adam’s Permain | cv Merton Russet |
|---|---|---|---|---|---|---|---|---|
| Limonene | 6.59 | 1,234a | ND | 0.7 | 0.8 | 0.9 | 1.2 | 0.8 |
| 6-Methyl-5-hepten-2-one* | 10.1 | 1,348b | 11 | 35 | 100 | 27 | 24 | 14 |
| Bourbonenei | 14.35 | 1,496c | ND | ND | ND | 2.4 | 11 | ND |
| Menthyl acetate** | 15.53 | 1,541d | ND | 33 | ND | ND | ND | ND |
| (E)-β-Caryophyllene | 16.12 | 1,618e | ND | ND | ND | 3 | 1.4 | ND |
| (E)-β-Farnesene*** | 18.09 | 1,674a | 1.6 | 2.8 | 1.5 | 2.2 | 12 | ND |
| α-Terpineol* | 19.63 | 1,720f | 17 | 0.3 | 5.7 | 6.0 | 7.6 | 8.6 |
| Germacrene-D | 18.66 | 1,722a | ND | ND | ND | 9.9 | 9.9 | ND |
| (Z,E)-α-Farnesene | 19.41 | 1,727d | 2.8 | 47 | 39 | 45 | 61 | 2.9 |
| (E,E)-α-Farnesene | 19.88 | 1,749g | 47 | 2,600 | 2,700 | 1,400 | 7,500 | 220 |
| (E)-Geranyl acetone | 22.04 | 1,840h | ND | 18 | 21 | 15 | 21 | ND |
| (E)-Nerolidol* | 25.87 | 2,054a | ND | 8.6 | ND | 7.2 | 16 | ND |
| (E,E)-Farnesol* | 31.44 | 2,371a | 430 | 900 | 11 | 390 | 440 | 45 |
| Total terpenes (ng g−1 fresh weight) | 509 | 3,645 | 2,879 | 1,909 | 8,105 | 291 |
Choi (2003). bBaşer et al. (2009). cBendiabdellah et al. (2012). dDavies (1990). eHögnadóttir and Rouseff (2003). fCulleré et al. (2004). gWerkhoff et al. (1998). hhttp://www.flavornet.org/d_kovats_c20m.html. iRetention time and distinctive MS (retention index in this sample is 1,498; the compound runs just before pentadecane).
Figure 9.
TPS gene expression in the fruit of five heritage apple cultivars. Fruit were picked at approximately 150 DAA, and gene expression data analysis was carried out using the same conditions and primers as described in Figure 5. The expression of each TPS gene in cv Royal Gala fruit at 150 DAA from Figure 8 has been included for comparison. Data are presented as means ± se (n = 4).
TPS gene expression analysis suggested that a number of the TPS genes expressed in cv Royal Gala flowers and leaves were expressed in the heritage apple cultivar fruit. In particular, nerolidol synthase expression was elevated in all five varieties, and the germacrene-D and (E)-β-caryophyllene synthases were elevated in cv King David. However, there was not a strong correlation between the expression of a particular TPS gene and an elevation in the corresponding terpene volatile. This suggests that other factors including substrate availability may also be important for determining the volatiles produced. Nevertheless, the data indicate the potential for screening germplasm to identify cultivars expressing novel terpenes in the fruit.
DISCUSSION
Volatile Terpene Production in cv Royal Gala Appears To Be Predominantly Defense Associated
Terpene production in cv Royal Gala is primarily associated with mature flowers and vegetative tissues, while fruit terpenes are typically produced at low concentrations. The ovaries were the primary sites of floral terpene production, rather than petals as seen in other plants, including Antirrhinum majus (Dudareva et al., 2003), Clarkia breweri (Pichersky et al., 1994), and Actinidia arguta (Chen et al., 2010). Linalool and (E)-DMNT were the predominant floral terpenes. Although linalool has also been identified as the major floral terpene in other apple cultivars (Rapparini et al., 2001), it appears to be largely sequestered as a nonvolatile glycoside in apple leaves (Wei et al., 2004); hence, its production rate in cv Royal Gala leaves is likely to be higher than indicated in our analyses (Fig. 1).
(E)-DMNT is a common constitutively produced floral terpene (Kaiser, 1991; Azuma et al., 1997) and is also induced in response to herbivore damage of foliage in gymnosperms (Su et al., 2009) and in many monocot and dicot angiosperm plant species (Bouwmeester et al., 1999; Arimura et al., 2000, 2008; Degenhardt and Gershenzon, 2000; Kant et al., 2004; Lee et al., 2010). However, (E)-DMNT is not generally detected from undamaged or mechanically damaged foliage. It is interesting, therefore, that the primary source of DMNT production in cv Royal Gala is stipules rather than flowers (Fig. 1). Although very little is known about the function of these organs in plants, a possible role in protecting differentiating leaf and axillary bud tissues against pests, pathogens, and/or extreme heat or cold conditions has been put forward (Kumar et al., 2012). Moreover, stipule-derived production of DMNT and germacrene-D, which have also been shown to deter insect herbivores (Arimura et al., 2004; Ghirardo et al., 2012), would support an insect defense-associated role for these leaf-like structures in apple.
Although petal-associated linalool production in cv Royal Gala points to a role in pollinator attraction, especially considering that apples rely on cross pollination by insects (Thomson and Goodell, 2001; Katoh et al., 2002), the remaining floral terpenes, which are released from nonpetal tissues (particularly ovaries and pedicels; Fig. 2), are likely to have different ecological roles. For example, as proposed for floral terpenes in Arabidopsis (Chen et al., 2003), an important role for the non-petal-associated floral terpenes in apple and other plants, including grapevine (Martin et al., 2009) and Magnolia spp. (Lee and Chappell, 2008), could be to provide protection for the reproductive organs, and more specifically, the germ line cells, against oxidative stress and pathogenic microbes. Terpenes, including β-myrcene, (E)-β-ocimene, linalool, and α-caryophyllene, have been shown to react readily with reactive oxygen species (Calogirou et al., 1999; Loreto and Velikova, 2001), while a variety of monoterpenes and sesquiterpenes are also reported to have antimicrobial activity (Cowan, 1999).
Although terpene production in apple fruit was generally low, several features were noteworthy. For example, (E,E)-α-farnesene, which is the terpene most associated with ripe apple fruit (Sutherland et al., 1977; Bengtsson et al., 2001; Hern and Dorn, 2003; Ferreira et al., 2009; Rowan et al., 2009a), was only produced at very low concentrations in undamaged harvest-ripe (150-DAA) fruit in our analysis. (E,E)-α-Farnesene has previously been shown to increase significantly in ripe apple following exposure to exogenous ethylene (Johnston et al., 2009), with the greatest increase in (E,E)-α-farnesene production (approximately 0.1–1.3 ng g−1 fresh weight h−1) occurring as ethylene concentrations were increased from 0.1 to 10 μg mL−1. Ethylene concentrations in 150-DAA cv Royal Gala fruit occur at approximately 1 μg mL−1 (Johnston et al., 2002); hence, the low (E,E)-α-farnesene emission we observed in 150-DAA whole fruit (approximately 0.046 ng g−1 fresh weight h−1; Supplemental Table S3) are consistent with internal ethylene below 1 μg mL−1.
(E,E)-α-Farnesene also appears to be induced in ripe apple skin in response to mechanical wounding, in this case during sample preparation to peel the skin (Supplemental Table S3). Similar induction of (E)-DMNT was also noted in 60-DAA skin. The peak of (E)-β-ocimene production observed in maturing apple seeds (60 DAA; Supplemental Table S2), however, does not appear to be produced in response to wounding. Instead, it may be related to increased plastidial 2-C-methyl-d-erythritol 4-phosphate pathway flux (responsible for both GDP and GGDP isoprenoid precursors) toward GA biosynthesis, which is 15 to 500 times higher in apple seeds than in leaves and shoots (Luckwill, 1974). Although previous studies have shown that GDP and GGDP levels can be modulated in planta by the GDP synthase small subunit, leading to decreased GGDP-derived metabolites (including GA) and increased monoterpene production (Orlova et al., 2009), the (E)-β-ocimene peak in apple seeds coincides with maximal GA biosynthesis at 9 weeks (i.e. approximately 60 DAA; Luckwill et al., 1969). The concomitant increase in GDP- and GGDP-derived metabolites, namely (E)-β-ocimene and GA, respectively, suggests that GDP synthase small subunit-directed effects are either absent or suppressed in cv Royal Gala seeds.
On the basis that GAs are biosynthesized from GGDP via CDP and ent-kaurene (Yamaguchi, 2008; Hedden and Thomas, 2012), the peak of MdCDS-RG1 seed expression at 60 DAA (Fig. 8) also supports the assumption that this gene encodes the functional CDP synthase in cv Royal Gala.
The Apple TPS Gene Family
The genome duplication events that have shaped the overall organization of the apple genome (Velasco et al., 2010) also appear to have significantly affected the organization and diversity of the apple TPS gene family. However, unlike grape, where duplication events have culminated in a TPS gene family encoding a large number of functionally diversified enzymes (Martin et al., 2010), our in silico analysis of the apple ‘Golden Delicious’ genome identified only 10 potentially functional TPS enzymes. Importantly, our in silico findings are also strongly supported by the identification and functional annotation of an equivalent number of cv Royal Gala TPS enzymes. This equivalency not only gives us confidence that we have identified the majority of TPS enzymes in apple but also indicates that the preservation of TPS enzymes since the GWD is of physiological importance.
It is possible that the high proportion of TPS pseudogenes in the cv Golden Delicious genome compared with other plant species (Chen et al., 2011) arose after the ancestral duplication events in the Malus genome (Velasco et al., 2010), allowing for the acquisition of deleterious mutations in one of the duplicated genes (Walsh, 1995; Lynch and Conery, 2003). Alternatively, some of the pseudogenes may have arisen from retrotransposition. Evidence for this process can be seen in a number of the predicted TPS gene models that contain retrotransposon-like insertions (Supplemental Fig. S2). Specifically, the expansion of the TPS-a subgroup in apple appears to have been driven by a combination of the recent GWD and more recent gene duplication events. The GWD-associated gene duplications are inferred from the identification of equivalent TPS-a genes on homeologous chromosomes 3 and 11, while more recent duplications are inferred from an additional TPS-a gene cluster located on chromosome 12, with the corresponding absence of equivalent genes on the homeologous chromosome 4 (Supplemental Table S6). Although it is conceivable that a TPS-a gene cluster on chromosome 4 may have been lost after the GWD, the complete absence of TPS-like genes anywhere on this chromosome suggests that this is unlikely. Gene duplications occurring after the GWD could also account for the expansion of the TPS-e/TPS-f family. Gene clusters for this family are primarily located on the nonhomeologous chromosomes 15 and 17, while the approximately 50:50 split of TPS-g genes on homeologous chromosomes 5 and 10 (Supplemental Table S6) is again indicative of a GWD-associated expansion.
TPS Regulation and Conservation
It has previously been shown that volatile production in apple fruit is primarily associated with ripening and that ethylene modulates the biosynthesis of pathways leading to ester, phenylpropanoid, and sesquiterpene [specifically (E,E)-α-farnesene] production by up-regulating the expression of end-point genes in these pathways (Defilippi et al., 2005; Schaffer et al., 2007). Our work supports the notion that in cv Royal Gala, only (E,E)-α-farnesene production is ethylene responsive and that terpene metabolism is predominantly associated with unripe fruit in the absence of climacteric ethylene production (Reid et al., 1973; Lay-Yee et al., 1990; Volz et al., 2003; Schaffer et al., 2007). The higher amounts of monoterpenes in early fruit development are more likely to be related to the transition of flowers (which have comparatively high TPS expression) to fruit. Although cv Royal Gala ripe fruit produce relatively small amounts of terpenes compared with other volatiles such as esters (Souleyre et al., 2005), the conservation and phenological variation in terpene production indicate that there is selective pressure to maintain functional TPS enzymes in cv Royal Gala fruit. It is also reasonable to assume that the conservation of fruit terpene production is directly related to the specialized “fitness” roles to which they contribute. Specifically, the primary function of terpenes in flowers appears to relate to both the attraction of pollinators and the protection of reproductive structures. In immature fruit, this function appears to transition to a defense role against herbivores and pathogens, while changes in volatile compounds that occur during fruit maturation, in combination with changes in texture, taste, and color, are necessary to attract legitimate seed-dispersal agents (Jensen, 1976; Herrera, 1982; Sallabanks and Courtney, 1992). The conservation of (E,E)-α-farnesene in particular points to a role in the attraction of frugivorous animals to apple fruit as they transition from harvest ripe (approximately 150 DAA) to fully ripe.
The Effect of Commercial Breeding Strategies on Terpene Production
The presence of additional terpenes in the five heritage apple varieties tested compared with cv Royal Gala suggests that modern breeding strategies might be reducing the range of terpenes in ripe fruit. In apple, modern breeding strategies have placed the emphasis on the selection of sweet, dessert varieties with superior texture and “fruity” ester flavor/aroma notes (Volz et al., 2004). This focus would select against the terpenes associated with nutty/aromatic notes in the heritage varieties and consequently reduce the range of aromas/flavors in modern apple cultivars such as cv Royal Gala. Alternatively, the lack of terpenes could be linked to producing apples with longer storage qualities, which can be associated with reduced climacteric ethylene production. The effects of breeding practices on fruit flavor have previously been observed in modern tomato varieties, which have desirable production traits, including yield and uniform ripening, but appear to have been negatively affected in terms of fruit quality traits, particularly fruit flavor (Klee, 2010; Powell et al., 2012).
The conservation of (E,E)-α-farnesene production in ripe fruit suggests that (unlike other terpenes) there must be a specific selective pressure to maintain the production of this simple acyclic sesquiterpene in apple fruit. The amino acid sequence identity of functional and potentially functional (E,E)-α-farnesene synthase-encoding genes in different apple and pear (Pyrus communis) cultivars (Supplemental Fig. S8), combined with the fact that only a single predicted apple (E,E)-α-farnesene synthase-like gene model (MdTPS25-GD; Supplemental Table S6) was identified, supports this assumption.
In conclusion, this work brings together, to our knowledge for the first time, an overall picture of the organization and expression of the apple TPS gene family and provides a reference point to initiate future studies into the evolution of TPS enzymes in other important Rosaceous plant species. This work also highlights the different biological roles these compounds are likely to fulfill in apple and will help with the breeding of new apple varieties with novel and interesting flavors.
MATERIALS AND METHODS
Headspace Volatile Trapping
Apple (Malus domestica ‘Royal Gala’) flowers, flower parts (approximately 5 g), fruit parts (approximately 5 g), and leaves were harvested and placed in 50-mL Quickfit tubes, and volatiles were trapped for 4 h in direct thermal desorption tubes (ATAS GL International) packed with 80 mg of Chromosorb 105 absorbent (Shimadzu) using purified air at a flow rate of 25 mL min−1. For the separate analyses of whole fruit harvested at various stages of maturity, five cv Royal Gala apples were placed in 5-L wide-necked sampling vessels that were each attached to a gas line and a sorbent cartridge containing 100 mg of Chromosorb 105. The headspace in the flask was allowed to equilibrate at 23°C for 1 h, after which the headspace was purged with air (25 mL min−1, 15 min).
Solvent Extraction of Fruit Terpene Volatiles
Solvent extractions were carried out on pooled samples (approximately 5 g) of apple skin taken from 10 apples harvested at approximately 150 DAA from heritage varieties grown under research orchard conditions. Tissue was ground to a fine powder in liquid N2 in 50-mL Quickfit conical flasks and extracted twice with 10 mL of diethyl ether for 30 min with gentle shaking. The two extractions were combined and stored overnight at −20°C. The following day, the upper solvent layer was carefully removed from the lower frozen water layer using a glass pipette and reduced to 2 mL under a gentle stream of nitrogen. The concentrated extract was then dried by passage through a column of anhydrous MgSO4.
GC-MS Analysis
Headspace volatiles were desorbed directly from the direct thermal desorption tubes with a temperature ramp of 45°C to 175°C at 16°C s−1 and cryofocused on the front of the capillary column by a liquid nitrogen-cooled cryogenic trap at −110°C. After cryofocusing, the trap temperature was ramped to 175°C at 50°C min−1 (Optic 3 thermal desorption system; ATAS GL). A 15:1 split was employed while the volatiles were transferred into the capillary column at a column flow of 1 mL min−1. The GC oven ramp was 35°C for 2 min, 3°C min−1 to 60°C, 5°C min−1 to 100°C, 8°C min−1 to 170°C, 10°C min−1 to 200°C, and then held for 13 min. GC separations were on a 30-m × 0.25-mm (i.d.) × 0.25-μm film thickness DB-Wax (J&W Scientific) capillary column in an HP6890 GC apparatus (Agilent Technologies) with helium as the carrier gas. The GC device was coupled to a time of flight-mass spectrometer (Leco Pegasus III). The ion source temperature was kept at 200°C, and ionization energy of 70 eV was used for electron-impact ionization. The detector voltage was 1,700 V, and ion spectra from 33 to 320 atomic mass units were collected with a data acquisition rate of 20 Hz. The total ion chromatograms were processed using the LECO ChromaTOF software.
Terpenes were identified using the following reference compounds: α-pinene, linalool, β-myrcene, and α-terpineol (Aldrich), limonene (BDH), β-pinene (K&K Laboratories), (E)-β-caryophyllene (Koch-Light and Givaudan), (E)-β-farnesene (Givaudan), and germacrene-D (RC Treatt). (E,E)-α-Farnesene and (Z,E)-α-farnesene were obtained by pentane extraction of Granny Smith apple skin (Anet, 1970). Other compounds for which we did not have authentic standards were identified by the ChromTOF software (version 2.3; Pegasus; Leco Australia) using the National Institute of Standards and Technology (version 2.0d, 2005) mass-spectral database, in combination with comparison of analyte retention indices with those of a series of straight-chain hydrocarbon standards (C8–C23, 0.005 μL mL−1 for each hydrocarbon). Peaks were selected and integrated manually using the molecular ion and/or specific diagnostic ions of each compound. The terpenes were quantified by measuring mass-to-charge ratio (m/z) 93 peak areas against an average response factor for the m/z 93 peak areas of 1,8-cineole (eucalyptol; 0.0366 μL mL−1), linalool (0.0263 μL mL−1), and β-caryophyllene (0.03 μL mL−1), contained in an external standard. Calibration curves determined that the GC-MS system gave a linear response, with respect to analyte concentration, over the range of concentrations for which they were measured in the headspace and in the solvent extracts.
GC-MS analysis of solvent extraction samples from the heritage apple fruit was performed on an Agilent 6890N GC device coupled to a Waters GCT time of flight-mass spectrometer with an electron-impact energy of 70 eV and a scan time of 0.4 s. One-microliter splitless (30-s) injections were made at 220°C onto a 20-m × 0.18-mm (i.d.) × 0.18-μm film thickness DB-Wax (J&W Scientific) capillary column with a helium flow of 0.9 mL min−1. The oven temperature program was 1 min at 35°C, 5°C min−1 to 230°C, and hold for 5 min.
Terpene quantitation standards for the heritage apple fruit analysis were as follows: linalool, β-myrcene, α-terpineol, 6-methyl-5-hepten-2-one, (E)-nerolidol, and (E,E)-farnesol (Aldrich), limonene (also for menthyl acetate; BDH), β-caryophyllene (Koch-Light), and germacrene-D (also for α- and β-farnesene; RC Treatt).
Enantioselective GC-MS
A 1-μL splitless sample injection (30 s) was made onto a 30-m × 0.25-mm (i.d.) × 0.25-μm film thickness β-Dex 325 (Supelco) enantioselective capillary column. The phase on this column was 25% 2,3-di-O-methyl-6-O-tertbutyldimethylsilyl-β-cyclodextrin in SPB-20 (poly-[20% dimethylsiloxane/80% dimethylsiloxane]). The flow rate of helium was 0.9 mL min−1, and the injection port was at 220°C. The oven temperature program was 1 min at 35°C, increasing by 5°C min−1 to 230°C, and hold for 5 min.
(+/−)-Germacrene-D [mainly the (−)-enantiomer] was obtained from goldenrod (Solidago canadensis) plants, and the enantiomers were assigned as described previously (Nieuwenhuizen et al., 2009). (S)-(E)-Nerolidol was obtained from neroli essential oil (Neroli; Citrus aurantium; Lotus Essential Oils). The essential oil (0.25 mL) was partially purified by flash chromatography on silica gel (pentane:dichloromethane:methanol, 100:99:1), and (E)-nerolidol was identified by GC-MS comparison with (E/Z)-nerolidol (Aldrich). Neroli contains exclusively (E)-nerolidol and predominantly (S)-(E)-nerolidol (Mondello et al., 2002). Enantioselective GC-MS of the partially purified neroli essential oil determined it to be 14:86 (R):(S)-(E)-nerolidol. (S)-(−)-Limonene and (R)-(+)-limonene, rac-linalool, and (+)- and (−)-α-pinene were obtained from Aldrich, and (+)- and (−)-β-pinene and (R)-(−)-linalool were from Fluka.
Identification of cv Golden Delicious and Royal Gala TPS Genes
The release of the draft apple ‘Golden Delicious’ genome from the Genome Database for Rosaceae (GDR; http://www.rosaceae.org/) facilitated the screening of the Instituto Agrario San Michele all’Adige-annotated gene models (Gene Models 1) with known TPS sequences obtained from the nonredundant protein database at NCBI. The previously identified TPS sequences were used as query sequences for tBLASTn searches of the GDR database. The genomic data presented here are based on the genome assembly as annotated on July 30, 2012, and no additional curation of putative cv Golden Delicious TPS gene models was carried out.
Putative full-length cv Royal Gala TPS ORFs were identified from BLAST searches (tBLASTn and BLASTp) of an in-house EST database (primarily containing cv Royal Gala sequences; Newcomb et al., 2006) and nonredundant protein, nucleotide, and apple-specific EST sequence databases available at NCBI. EST sequences from the in-house and publicly accessible EST databases were derived from a wide variety of apple tissues, including flower, vegetative, and root tissues collected at various developmental stages, as well as from tissues subjected to conditions that would be expected to induce terpene production.
For simplification, the “MDP” (for Malus domestica protein) gene model annotations are given corresponding MdTPS numbers for TPS-specific gene models and, where relevant, a full-length cDNA identifier is given to previously predicted full-length TPS cDNAs and functionally characterized TPS enzymes. In accordance with the grape (Vitis vinifera) TPS gene annotation (Martin et al., 2010), this identifier includes a “GD” or “RG” notation to differentiate between TPS enzymes identified or cloned from the cv Golden Delicious and Royal Gala apple cultivars, respectively. For example, the previously characterized cv Royal Gala (E,E)-α-farnesene synthase (MdAFS1) will now be termed MdAFS-RG1.
Sequence Analysis
Multiple amino acid sequence alignments of TPS genes were performed with ClustalX (Thompson et al., 1997) using default parameters and were manually adjusted in GeneDoc (www.nrbsc.org/gfx/genedoc/). Prediction of the protein ORF was carried out using the EMBOSS (http://www.ch.embnet.org/EMBOSS/index.html) translation tool Transeq (Rice et al., 2000). The bioinformatics tool ChloroP (Emanuelsson et al., 1999), available at http://www.cbs.dtu.dk/services/, was used to predict the intracellular targeting of full-length TPS ORFs.
The evolutionary history was inferred by using the maximum likelihood method based on the Dayhoff matrix-based model (Schwarz and Dayhoff, 1979). The tree with the highest log likelihood is shown. Initial tree(s) for the heuristic search were obtained automatically as follows. When the number of common sites was less than 100, or less than one-quarter of the total number of sites, the maximum parsimony method was used; otherwise, the BIONJ method (Gascuel, 1997) with the Maximum Composite Likelihood matrix (Tamura et al., 2004) was used. A discrete γ-distribution was used to model evolutionary rate differences among sites (five categories; +G, parameter = 5.1574). The analysis involved 30 TPS amino acid sequences. All positions containing gaps and missing data were eliminated. Evolutionary analyses were conducted in MEGA5 (Tamura et al., 2007).
Transient Expression in Tobacco Leaves
In planta transient expression in tobacco (Nicotiana benthamiana) leaves was carried out as described previously (Nieuwenhuizen et al., 2009; Green et al., 2012a) utilizing the Agrobacterium tumefaciens strain GV3101. For the construction of cv Royal Gala TPS plant transformation vectors, full-length TPS ORFs were amplified by PCR, using the primers listed in Supplemental Table S8, and cloned into either pENTR/D-TOPO (Invitrogen) or pDONR221. The resulting clones were transferred by Gateway LR reactions as recommended by the manufacturer (Invitrogen) into the binary destination vector pHEX2 (Hellens et al., 2005) that contains the cauliflower mosaic virus 35S promoter and octopine synthase terminator.
In Vitro Analysis of Recombinant TPS Enzymes
TPS genes were cloned into pET200/D-TOP0 or pET300/NT-DEST (Invitrogen) for bacterial overexpression according to the manufacturer’s recommendations. Details of the primers are given in Supplemental Table S8. Recombinant TPS enzymes were expressed under autoinducible conditions and purified by immobilized affinity metal chromatography according to previous methods (Green et al., 2007, 2012b). Solvent extraction assays for terpene identification and enantiomeric determinations were carried out as described previously (Green et al., 2012b). Terpene reference compounds used for the in vitro analysis were as for the headspace analysis but also included α-copaene and α-caryophyllene (Givaudan) and borneol (Aldrich).
Real-Time Gene Expression Analysis
RNA was extracted from apple flowers, flower parts, fruit, and leaves according to Nieuwenhuizen et al. (2009) and treated with 10 units of DNaseI (Roche Applied Science) before cDNA synthesis. First-strand cDNA was synthesized using the Transcriptor First Strand cDNA Synthesis Kit (Roche) according to the manufacturer’s instructions and diluted 50-fold before use. Relative quantitative real-time gene expression analyses of targets and the reference gene ELONGATION FACTOR1α (EF1α) were performed (four technical replicates) on a LightCycler 480 platform using the LightCycler 480 SYBR Green master mix, and the results were analyzed using the LightCycler 480 software (Roche). To determine the level of expression, the differences (Δ) between the threshold cycle (Ct) or crossing points (CP) were measured (i.e. −ΔCp method) according to Montefiori et al. (2011), enabling a comparison of the level of expression of multiple target genes normalized to a common reference gene considered stable and unchanging in the different samples. The program was as follows: 5 min at 95°C; 40 cycles of 10 s at 95°C, 10 s at 60°C, and 20 s at 72°C; followed by melting curve analysis: 95°C for 5 s, 65°C for 60 s, then ramping at 0.18°C s−1 to 95°C. Primers for real-time gene expression analysis were designed using Vector NTI software (Invitrogen) based on a melting temperature of 60°C and amplicon length of 70 to 120 bp and are listed in Supplemental Table S9.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers JX848729, JX848730, JX848731, JX848732, X848733, and JX848734.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Genomic, predicted cDNA, and protein sequences for the functional cv Royal Gala sesquiterpene synthase and monoterpene synthases and the putatively functional diterpene synthases.
Supplemental Figure S2. TPSs in the cv Golden Delicious genome with predicted retrotransposon insertions.
Supplemental Figure S3. In vitro analysis of cv Royal Gala monoterpene synthase enzyme activity.
Supplemental Figure S4. In vitro analysis of cv Royal Gala sesquiterpene synthase enzyme activity.
Supplemental Figure S5. Monoterpene enantiomeric determinations.
Supplemental Figure S6. Sesquiterpene enantiomeric determinations.
Supplemental Figure S7. TPS gene expression in cv Royal Gala roots.
Supplemental Figure S8. Alignment of apple and pear (E,E)-α-farnesene synthases.
Supplemental Table S1. Volatile terpenes released from cv Royal Gala leaves and flowers.
Supplemental Table S2. Volatile terpene production from cv Royal Gala floral tissues.
Supplemental Table S3. Volatile terpenes released from whole cv Royal Gala fruit.
Supplemental Table S4. Phenological terpene emission in cv Royal Gala fruit tissues.
Supplemental Table S5. Fold difference in skin and whole apple fruit terpene production at 150 DAA.
Supplemental Table S6. Tabulated information on the 55 TPS gene models identified in the cv Golden Delicious genome.
Supplemental Table S7. Full-length TPSs in cv Royal Gala EST collections.
Supplemental Table S8. TPS gene-specific cloning primers.
Supplemental Table S9. Gene-specific TPS primers used for expression analysis.
Acknowledgments
We thank Rosemary Weskett for fruit collection, Tim Holmes and Johanna John for photography and help with figure preparation, Andrew Gleave and team for EST clone retrieval and help with binary vector construction, Monica Dragulescu and Gnanaseela Wadasinghe for plant care, Miva Splawinski for contributing to preliminary TPS studies, Roy Storey for advice on bioinformatics, and Robert Schaffer and Richard Espley for reviewing the manuscript. This research underpins long-range targets in a commercial apple breeding program undertaken in partnership between Plant & Food Research and Prevar, Ltd.
Glossary
- GDP
geranyl diphosphate
- GGDP
geranylgeranyl diphosphate
- TPS
terpene synthase
- GWD
genome-wide duplication
- VOC
volatile organic compound
- GC
gas chromatography
- MS
mass spectrometry
- (E)-DMNT
(E)-4,8-dimethyl-1,3,7-nonatriene
- DMNT
4,8-dimethyl-1,3,7-nonatriene
- DAA
d after anthesis
- ORF
open reading frame
- NCBI
National Center for Biotechnology Information
- CDP
copalyl diphosphate
- m/z
mass-to-charge ratio
- GDR
Genome Database for Rosaceae
- cDNA
complementary DNA
References
- Aharoni A, Giri AP, Verstappen FW, Bertea CM, Sevenier R, Sun Z, Jongsma MA, Schwab W, Bouwmeester HJ. (2004) Gain and loss of fruit flavor compounds produced by wild and cultivated strawberry species. Plant Cell 16: 3110–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anet EFLJ. (1970) Synthesis of (E,Z)-α-, (Z,Z)-α-, and (Z)-β-farnesene. Aust J Chem 23: 2101–2108 [Google Scholar]
- Anet EFLJ. (1972) Superficial scald, a functional disorder of stored apples. VIII. Volatile products from the autoxidation of alpha-farnesene. J Sci Food Agric 23: 605–608 [Google Scholar]
- Arimura G, Garms S, Maffei M, Bossi S, Schulze B, Leitner M, Mithöfer A, Boland W. (2008) Herbivore-induced terpenoid emission in Medicago truncatula: concerted action of jasmonate, ethylene and calcium signaling. Planta 227: 453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura G, Huber DP, Bohlmann J. (2004) Forest tent caterpillars (Malacosoma disstria) induce local and systemic diurnal emissions of terpenoid volatiles in hybrid poplar (Populus trichocarpa × deltoides): cDNA cloning, functional characterization, and patterns of gene expression of (−)-germacrene D synthase, PtdTPS1. Plant J 37: 603–616 [DOI] [PubMed] [Google Scholar]
- Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J. (2000) Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 406: 512–515 [DOI] [PubMed] [Google Scholar]
- Aubourg S, Lecharny A, Bohlmann J. (2002) Genomic analysis of the terpenoid synthase (AtTPS) gene family of Arabidopsis thaliana. Mol Genet Genomics 267: 730–745 [DOI] [PubMed] [Google Scholar]
- Azuma H, Thien LB, Toyota M, Asakawa Y, Kawano S. (1997) Distribution and differential expression of (E)-4,8-dimethyl-1,3,7-nonatriene in leaf and floral volatiles of Magnolia and Liriodendron taxa. J Chem Ecol 23: 2467–2478 [Google Scholar]
- Başer KHC, Demirci B, Kürkçüoğlu M, Satil F, Tümen G. (2009) Comparative morphological and photochemical characterisation of Salvia cadmica and S. smyrnaea. Pak J Bot 41: 1545–1555 [Google Scholar]
- Bendiabdellah A, El Amine Dib M, Djabou N, Allali H, Tabti B, Muselli A, Costa J. (2012) Biological activities and volatile constituents of Daucus muricatus L. from Algeria. Chem Cent J 6: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson M, Bäckman AC, Liblikas I, Ramirez MI, Borg-Karlson AK, Ansebo L, Anderson P, Löfqvist J, Witzgall P. (2001) Plant odor analysis of apple: antennal response of codling moth females to apple volatiles during phenological development. J Agric Food Chem 49: 3736–3741 [DOI] [PubMed] [Google Scholar]
- Bleeker PM, Spyropoulou EA, Diergaarde PJ, Volpin H, De Both MT, Zerbe P, Bohlmann J, Falara V, Matsuba Y, Pichersky E, et al. (2011) RNA-seq discovery, functional characterization, and comparison of sesquiterpene synthases from Solanum lycopersicum and Solanum habrochaites trichomes. Plant Mol Biol 77: 323–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlmann J, Meyer-Gauen G, Croteau R. (1998) Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci USA 95: 4126–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlmann J, Phillips M, Ramachandiran V, Katoh S, Croteau R. (1999) cDNA cloning, characterization, and functional expression of four new monoterpene synthase members of the Tpsd gene family from grand fir (Abies grandis). Arch Biochem Biophys 368: 232–243 [DOI] [PubMed] [Google Scholar]
- Boland W, Gäbler A, Gilbert M, Feng Z. (1998) Biosynthesis of C11 and C16 homoterpenes in higher plants: stereochemistry of the C-C-bond cleavage reaction. Tetrahedron 54: 14725–14736 [Google Scholar]
- Bouwmeester HJ, Verstappen FW, Posthumus MA, Dicke M. (1999) Spider mite-induced (3S)-(E)-nerolidol synthase activity in cucumber and lima bean: the first dedicated step in acyclic C11-homoterpene biosynthesis. Plant Physiol 121: 173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calogirou A, Larsen BR, Kotzias D. (1999) Gas-phase terpene oxidation products: a review. Atmos Environ 33: 1423–1439 [Google Scholar]
- Cane DE, Xue Q, Fitzsimons BC. (1996) Trichodiene synthase: probing the role of the highly conserved aspartate-rich region by site-directed mutagenesis. Biochemistry 35: 12369–12376 [DOI] [PubMed] [Google Scholar]
- Chen F, Tholl D, Bohlmann J, Pichersky E. (2011) The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J 66: 212–229 [DOI] [PubMed] [Google Scholar]
- Chen F, Tholl D, D’Auria JC, Farooq A, Pichersky E, Gershenzon J. (2003) Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. Plant Cell 15: 481–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XY, Yauk YK, Nieuwenhuizen NJ, Matich AJ, Wang MY, Perez RL, Atkinson RG, Beuning LL. (2010) Characterisation of an (S)-linalool synthase from kiwifruit (Actinidia arguta) that catalyses the first committed step in the production of floral lilac compounds. Funct Plant Biol 37: 232–243 [Google Scholar]
- Choi HS. (2003) Character impact odorants of Citrus Hallabong [(C. unshiu Marcov × C. sinensis Osbeck) × C. reticulata Blanco] cold-pressed peel oil. J Agric Food Chem 51: 2687–2692 [DOI] [PubMed] [Google Scholar]
- Christianson DW. (2006) Structural biology and chemistry of the terpenoid cyclases. Chem Rev 106: 3412–3442 [DOI] [PubMed] [Google Scholar]
- Cowan MM. (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12: 564–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R, Kutchan TM, Lewis NG. (2000) Natural products (secondary metabolites). In BB Buchanan, W Gruissem, RL Jones, eds, Biochemistry and Molecular Biology of Plants. American Society of Plant Physiology, Rockville, MD, pp 1250–1318
- Culleré L, Escudero A, Cacho J, Ferreira V. (2004) Gas chromatography-olfactometry and chemical quantitative study of the aroma of six premium quality Spanish aged red wines. J Agric Food Chem 52: 1653–1660 [DOI] [PubMed] [Google Scholar]
- Davies NW. (1990) Gas-chromatographic retention indexes of monoterpenes and sesquiterpenes on methyl silicone and carbowax 20m phases. J Chromatogr A 503: 1–24 [Google Scholar]
- Defilippi BG, Dandekar AM, Kader AA. (2005) Relationship of ethylene biosynthesis to volatile production, related enzymes, and precursor availability in apple peel and flesh tissues. J Agric Food Chem 53: 3133–3141 [DOI] [PubMed] [Google Scholar]
- Degenhardt J, Gershenzon J. (2000) Demonstration and characterization of (E)-nerolidol synthase from maize: a herbivore-inducible terpene synthase participating in (3E)-4,8-dimethyl-1,3,7-nonatriene biosynthesis. Planta 210: 815–822 [DOI] [PubMed] [Google Scholar]
- Dimick PS, Hoskin JC. (1983) Review of apple flavor: state of the art. Crit Rev Food Sci Nutr 18: 387–409 [DOI] [PubMed] [Google Scholar]
- Dixon J, Hewett EW. (2000) Factors affecting apple aroma/flavour volatile concentration: a review. N Z J Crop Hortic Sci 28: 155–173 [Google Scholar]
- Donath J, Boland W. (1995) Biosynthesis of acyclic homoterpenes: enzyme selectivity and absolute-configuration of the nerolidol precursor. Phytochemistry 39: 785–790 [Google Scholar]
- Dudareva N, Martin D, Kish CM, Kolosova N, Gorenstein N, Fäldt J, Miller B, Bohlmann J. (2003) (E)-β-Ocimene and myrcene synthase genes of floral scent biosynthesis in snapdragon: function and expression of three terpene synthase genes of a new terpene synthase subfamily. Plant Cell 15: 1227–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, von Heijne G. (1999) ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci 8: 978–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falara V, Akhtar TA, Nguyen TT, Spyropoulou EA, Bleeker PM, Schauvinhold I, Matsuba Y, Bonini ME, Schilmiller AL, Last RL, et al. (2011) The tomato terpene synthase gene family. Plant Physiol 157: 770–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira L, Perestrelo R, Caldeira M, Câmara JS. (2009) Characterization of volatile substances in apples from Rosaceae family by headspace solid-phase microextraction followed by GC-qMS. J Sep Sci 32: 1875–1888 [DOI] [PubMed] [Google Scholar]
- Fuhrmann E, Grosch W. (2002) Character impact odorants of the apple cultivars Elstar and Cox Orange. Nahrung 46: 187–193 [DOI] [PubMed] [Google Scholar]
- Gascuel O. (1997) BIONJ: an improved version of the NJ algorithm based on a simple model of sequence data. Mol Biol Evol 14: 685–695 [DOI] [PubMed] [Google Scholar]
- Ghirardo A, Heller W, Fladung M, Schnitzler JP, Schroeder H. (2012) Function of defensive volatiles in pedunculate oak (Quercus robur) is tricked by the moth Tortrix viridana. Plant Cell Environ 35: 2192–2207 [DOI] [PubMed] [Google Scholar]
- Goff SA, Ricke D, Lan TH, Presting G, Wang R, Dunn M, Glazebrook J, Sessions A, Oeller P, Varma H, et al. (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science 296: 92–100 [DOI] [PubMed] [Google Scholar]
- Green S, Friel EN, Matich A, Beuning LL, Cooney JM, Rowan DD, MacRae E. (2007) Unusual features of a recombinant apple α-farnesene synthase. Phytochemistry 68: 176–188 [DOI] [PubMed] [Google Scholar]
- Green SA, Chen X, Matich AJ. (2012a) In planta transient expression analysis of monoterpene synthases. Methods Enzymol 515: 43–61 [DOI] [PubMed] [Google Scholar]
- Green SA, Chen X, Nieuwenhuizen NJ, Matich AJ, Wang MY, Bunn BJ, Yauk YK, Atkinson RG. (2012b) Identification, functional characterization, and regulation of the enzyme responsible for floral (E)-nerolidol biosynthesis in kiwifruit (Actinidia chinensis). J Exp Bot 63: 1951–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker FR, Gunson FA, Jaeger SR. (2003) The case for fruit quality: an interpretive review of consumer attitudes, and preferences for apples. Postharvest Biol Technol 28: 333–347 [Google Scholar]
- Hedden P, Thomas SG. (2012) Gibberellin biosynthesis and its regulation. Biochem J 444: 11–25 [DOI] [PubMed] [Google Scholar]
- Hellens RP, Allan AC, Friel EN, Bolitho K, Grafton K, Templeton MD, Karunairetnam S, Gleave AP, Laing WA. (2005) Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hern A, Dorn S. (1999) Sexual dimorphism in the olfactory orientation of adult Cydia pomonella in response to a alpha-farnesene. Entomol Exp Appl 92: 63–72 [Google Scholar]
- Hern A, Dorn S. (2002) Induction of volatile emissions from ripening apple fruits infested with Cydia pomonella and the attraction of adult females. Entomol Exp Appl 102: 145–151 [Google Scholar]
- Hern A, Dorn S. (2003) Monitoring seasonal variation in apple fruit volatile emissions in situ using solid-phase microextraction. Phytochem Anal 14: 232–240 [DOI] [PubMed] [Google Scholar]
- Herrera C. (1982) Defense of ripe fruit from pests: its significance in relation to plant-disperser interactions. Am Nat 120: 218–241 [Google Scholar]
- Högnadóttir A, Rouseff RL. (2003) Identification of aroma active compounds in orange essence oil using gas chromatography-olfactometry and gas chromatography-mass spectrometry. J Chromatogr A 998: 201–211 [DOI] [PubMed] [Google Scholar]
- Jensen RA. (1976) Enzyme recruitment in evolution of new function. Annu Rev Microbiol 30: 409–425 [DOI] [PubMed] [Google Scholar]
- Johnston JW, Gunaseelan K, Pidakala P, Wang M, Schaffer RJ. (2009) Co-ordination of early and late ripening events in apples is regulated through differential sensitivities to ethylene. J Exp Bot 60: 2689–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JW, Hewett EW, Hertog MLATM, Harker FR. (2002) Harvest date and fruit size affect postharvest softening of apple fruit. J Hortic Sci Biotechnol 77: 355–360 [Google Scholar]
- Kaiser R. (1991) Trapping, investigation and reconstitution of flower scents. In P Muller, D Lamparsky, eds, Perfume: Art, Science and Technology. Elsevier Applied Sciences, New York, pp 213–248
- Kant MR, Ament K, Sabelis MW, Haring MA, Schuurink RC. (2004) Differential timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiol 135: 483–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh N, Goto K, Asano J, Fukushima K, Yamada K, Kasai A, Li TZ, Takanoha M, Miyairi K, Okuno T. (2002) S-RNases from self-incompatible and -compatible apple cultivars: purification, cloning, enzymic properties, and pollen tube growth inhibitory activity. Biosci Biotechnol Biochem 66: 1185–1195 [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291: 2141–2144 [DOI] [PubMed] [Google Scholar]
- Klee HJ. (2010) Improving the flavor of fresh fruits: genomics, biochemistry, and biotechnology. New Phytol 187: 44–56 [DOI] [PubMed] [Google Scholar]
- Kumar A, Sharma V, Khan M, Tripathi BN, Kumar S. (2012) Pisum sativum wild-type and mutant stipules and those induced by an auxin transport inhibitor demonstrate the entire diversity of laminated stipules observed in angiosperms. Protoplasma (in press) [DOI] [PubMed] [Google Scholar]
- Lange BM, Ghassemian M. (2003) Genome organization in Arabidopsis thaliana: a survey for genes involved in isoprenoid and chlorophyll metabolism. Plant Mol Biol 51: 925–948 [DOI] [PubMed] [Google Scholar]
- Lay-Yee M, Dellapenna D, Ross GS. (1990) Changes in mRNA and protein during ripening in apple fruit (Malus domestica Borkh. cv Golden Delicious). Plant Physiol 94: 850–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Badieyan S, Bevan DR, Herde M, Gatz C, Tholl D. (2010) Herbivore-induced and floral homoterpene volatiles are biosynthesized by a single P450 enzyme (CYP82G1) in Arabidopsis. Proc Natl Acad Sci USA 107: 21205–21210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Chappell J. (2008) Biochemical and genomic characterization of terpene synthases in Magnolia grandiflora. Plant Physiol 147: 1017–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Velikova V. (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127: 1781–1787 [PMC free article] [PubMed] [Google Scholar]
- Loughrin JN, Hamilton-Kemp TR, Andersen RA, Hildebrand DF. (1990) Volatiles from flowers of Nicotiana sylvestris, N. otophora and Malus × domestica: headspace components and day/night changes in their relative concentrations. Phytochemistry 29: 2473–2477 [Google Scholar]
- Luckwill LC. (1974) A new look at the process of fruit bud formation in apple. In Proceedings of the XIX International Horticultural Congress, Vol 3. International Society for Horticultural Science, Warsaw, Poland, pp 237–245
- Luckwill LC, Weaver P, Macmillan J. (1969) Gibberellins and other growth hormones in apple seeds. J Hortic Sci Biotechnol 44: 413–424 [Google Scholar]
- Lynch M, Conery JS. (2003) The evolutionary demography of duplicate genes. J Struct Funct Genomics 3: 35–44 [PubMed] [Google Scholar]
- Maccarone E, Campisi S, Fallico B, Rapisarda P, Sgarlata R. (1998) Flavor components of Italian orange juices. J Agric Food Chem 46: 2293–2298 [DOI] [PubMed] [Google Scholar]
- Martin DM, Aubourg S, Schouwey MB, Daviet L, Schalk M, Toub O, Lund ST, Bohlmann J. (2010) Functional annotation, genome organization and phylogeny of the grapevine (Vitis vinifera) terpene synthase gene family based on genome assembly, FLcDNA cloning, and enzyme assays. BMC Plant Biol 10: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DM, Fäldt J, Bohlmann J. (2004) Functional characterization of nine Norway spruce TPS genes and evolution of gymnosperm terpene synthases of the TPS-d subfamily. Plant Physiol 135: 1908–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DM, Toub O, Chiang A, Lo BC, Ohse S, Lund ST, Bohlmann J. (2009) The bouquet of grapevine (Vitis vinifera L. cv. Cabernet Sauvignon) flowers arises from the biosynthesis of sesquiterpene volatiles in pollen grains. Proc Natl Acad Sci USA 106: 7245–7250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DJ, Allemann RK. (2012) Sesquiterpene synthases: passive catalysts or active players? Nat Prod Rep 29: 60–71 [DOI] [PubMed] [Google Scholar]
- Mondello L, Dugo P, Dugo G. (2002) The chiral compounds of citrus essential oils. In G Dugo, A Di Giacomo, eds, Citrus: The Genus Citrus. Taylor and Francis, London and New York, pp 461–495
- Montefiori M, Espley RV, Stevenson D, Cooney J, Datson PM, Saiz A, Atkinson RG, Hellens RP, Allan AC. (2011) Identification and characterisation of F3GT1 and F3GGT1, two glycosyltransferases responsible for anthocyanin biosynthesis in red-fleshed kiwifruit (Actinidia chinensis). Plant J 65: 106–118 [DOI] [PubMed] [Google Scholar]
- Newcomb RD, Crowhurst RN, Gleave AP, Rikkerink EH, Allan AC, Beuning LL, Bowen JH, Gera E, Jamieson KR, Janssen BJ, et al. (2006) Analyses of expressed sequence tags from apple. Plant Physiol 141: 147–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuizen NJ, Wang MY, Matich AJ, Green SA, Chen X, Yauk YK, Beuning LL, Nagegowda DA, Dudareva N, Atkinson RG. (2009) Two terpene synthases are responsible for the major sesquiterpenes emitted from the flowers of kiwifruit (Actinidia deliciosa). J Exp Bot 60: 3203–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Maille PE, Malone A, Dellas N, Andes Hess B, Jr, Smentek L, Sheehan I, Greenhagen BT, Chappell J, Manning G, Noel JP. (2008) Quantitative exploration of the catalytic landscape separating divergent plant sesquiterpene synthases. Nat Chem Biol 4: 617–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova I, Nagegowda DA, Kish CM, Gutensohn M, Maeda H, Varbanova M, Fridman E, Yamaguchi S, Hanada A, Kamiya Y, et al. (2009) The small subunit of snapdragon geranyl diphosphate synthase modifies the chain length specificity of tobacco geranylgeranyl diphosphate synthase in planta. Plant Cell 21: 4002–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillard NMM. (1990) The flavour of apples, pears and quinces. In ID Morton, AJ Macheod, eds, Food Flavours. Part C. The Flavour of Fruits. Elsevier Science, Amsterdam, pp 1–41
- Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, Haberer G, Hellsten U, Mitros T, Poliakov A, et al. (2009) The Sorghum bicolor genome and the diversification of grasses. Nature 457: 551–556 [DOI] [PubMed] [Google Scholar]
- Pechous SW, Whitaker BD. (2004) Cloning and functional expression of an (E,E)-α-farnesene synthase cDNA from peel tissue of apple fruit. Planta 219: 84–94 [DOI] [PubMed] [Google Scholar]
- Pichersky E, Gershenzon J. (2002) The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol 5: 237–243 [DOI] [PubMed] [Google Scholar]
- Pichersky E, Raguso RA, Lewinsohn E, Croteau R. (1994) Floral scent production in Clarkia (Onagraceae). I. Localization and developmental modulation of monoterpene emission and linalool synthase activity. Plant Physiol 106: 1533–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell AL, Nguyen CV, Hill T, Cheng KL, Figueroa-Balderas R, Aktas H, Ashrafi H, Pons C, Fernández-Muñoz R, Vicente A, et al. (2012) Uniform ripening encodes a Golden 2-like transcription factor regulating tomato fruit chloroplast development. Science 336: 1711–1715 [DOI] [PubMed] [Google Scholar]
- Rapparini F, Baraldi R, Facini O. (2001) Seasonal variation of monoterpene emission from Malus domestica and Prunus avium. Phytochemistry 57: 681–687 [DOI] [PubMed] [Google Scholar]
- Reid M, Rhodes M, Hulme A. (1973) Changes in ethylene and CO2 during the ripening of apples. J Sci Food Agric 24: 971–979 [Google Scholar]
- Rice P, Longden I, Bleasby A. (2000) EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet 16: 276–277 [DOI] [PubMed] [Google Scholar]
- Rowan DD, Hunt MB, Alspach PA, Whitworth CJ, Oraguzie NC. (2009a) Heritability and genetic and phenotypic correlations of apple (Malus × domestica) fruit volatiles in a genetically diverse breeding population. J Agric Food Chem 57: 7944–7952 [DOI] [PubMed] [Google Scholar]
- Rowan DD, Hunt MB, Dimouro A, Alspach PA, Weskett R, Volz RK, Gardiner SE, Chagné D. (2009b) Profiling fruit volatiles in the progeny of a ‘Royal Gala’ × ‘Granny Smith’ apple (Malus × domestica) cross. J Agric Food Chem 57: 7953–7961 [DOI] [PubMed] [Google Scholar]
- Rupasinghe HPV, Paliyath G, Murr DP. (2000) Sesquiterpene alpha-farnesene synthase: partial purification, characterization, and activity in relation to superficial scald development in apples. J Am Soc Hortic Sci 125: 111–119 [Google Scholar]
- Rynkiewicz MJ, Cane DE, Christianson DW. (2002) X-ray crystal structures of D100E trichodiene synthase and its pyrophosphate complex reveal the basis for terpene product diversity. Biochemistry 41: 1732–1741 [DOI] [PubMed] [Google Scholar]
- Sallabanks R, Courtney SP. (1992) Frugivory, seed predation, and insect-vertebrate interactions. Annu Rev Entomol 37: 377–400 [DOI] [PubMed] [Google Scholar]
- Schaffer RJ, Friel EN, Souleyre EJ, Bolitho K, Thodey K, Ledger S, Bowen JH, Ma JH, Nain B, Cohen D, et al. (2007) A genomics approach reveals that aroma production in apple is controlled by ethylene predominantly at the final step in each biosynthetic pathway. Plant Physiol 144: 1899–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R, Dayhoff M. (1979) Matrices for detecting distant relationships. In MO Dayhoff, ed, Atlas of Protein Sequences, Vol 5. National Biomedical Research Foundation, Washington, DC, pp 353–358
- Souleyre EJF, Greenwood DR, Friel EN, Karunairetnam S, Newcomb RD. (2005) An alcohol acyl transferase from apple (cv. Royal Gala), MpAAT1, produces esters involved in apple fruit flavor. FEBS J 272: 3132–3144 [DOI] [PubMed] [Google Scholar]
- Styger G, Prior B, Bauer FF. (2011) Wine flavor and aroma. J Ind Microbiol Biotechnol 38: 1145–1159 [DOI] [PubMed] [Google Scholar]
- Su JW, Zeng JP, Qin XW, Ge F. (2009) Effect of needle damage on the release rate of Masson pine (Pinus massoniana) volatiles. J Plant Res 122: 193–200 [DOI] [PubMed] [Google Scholar]
- Sutherland ORW, Wearing CH, Hutchins RFN. (1977) Production of α-farnesene, an attractant and oviposition stimulant for codling moth, by developing fruit of ten varieties of apple. J Chem Ecol 3: 625–631 [Google Scholar]
- Takabayashi J, Dicke M, Posthumus MA. (1991) Variation in composition of predator-attracting allelochemicals emitted by herbivore-infested plants: relative influence of plant and herbivore. Chemoecology 2: 1–6 [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24: 1596–1599 [DOI] [PubMed] [Google Scholar]
- Tamura K, Nei M, Kumar S. (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA 101: 11030–11035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. (1997) The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25: 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JD, Goodell K. (2001) Pollen removal and deposition by honeybee and bumblebee visitors to apple and almond flowers. J Appl Ecol 38: 1032–1044 [Google Scholar]
- Tuskan GA, Difazio S, Jansson S, Bohlmann J, Grigoriev I, Hellsten U, Putnam N, Ralph S, Rombauts S, Salamov A, et al. (2006) The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313: 1596–1604 [DOI] [PubMed] [Google Scholar]
- Tyler AA. (1897) The nature and origin of stipules. Ann N Y Acad Sci 10: 1–49 [Google Scholar]
- Vallat A, Dorn S. (2005) Changes in volatile emissions from apple trees and associated response of adult female codling moths over the fruit-growing season. J Agric Food Chem 53: 4083–4090 [DOI] [PubMed] [Google Scholar]
- Vallat A, Gu H, Dorn S. (2005) How rainfall, relative humidity and temperature influence volatile emissions from apple trees in situ. Phytochemistry 66: 1540–1550 [DOI] [PubMed] [Google Scholar]
- Velasco R, Zharkikh A, Affourtit J, Dhingra A, Cestaro A, Kalyanaraman A, Fontana P, Bhatnagar SK, Troggio M, Pruss D, et al. (2010) The genome of the domesticated apple (Malus × domestica Borkh.). Nat Genet 42: 833–839 [DOI] [PubMed] [Google Scholar]
- Volz RK, Harker FR, Lang S. (2003) Firmness decline in ‘Gala’ apple during fruit development. J Am Soc Hortic Sci 128: 797–802 [Google Scholar]
- Volz RK, White AG, Brookfield PL, Weskett R, Legg S, Koorey R, Tustin DS, Hughes J, White M. (2004) Breeding and developement of the new apple cultivar ‘Jazz’. Acta Hortic 663: 891–894 [Google Scholar]
- Vora JD, Matthews RF, Crandall PG, Cook R. (1983) Preparation and chemical-composition of orange oil concentrates. J Food Sci 48: 1197–1199 [Google Scholar]
- Walsh JB. (1995) How often do duplicated genes evolve new functions? Genetics 139: 421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Reuveny H, Bravdo BA, Shoseyov O. (2004) Hydrolysis of glycosidically bound volatiles from apple leaves (cv. Anna) by Aspergillus niger β-glucosidase affects the behavior of codling moth (Cydia pomonella L.). J Agric Food Chem 52: 6212–6216 [DOI] [PubMed] [Google Scholar]
- Wendt KU, Schulz GE. (1998) Isoprenoid biosynthesis: manifold chemistry catalyzed by similar enzymes. Structure 6: 127–133 [DOI] [PubMed] [Google Scholar]
- Werkhoff P, Guntert M, Krammer G, Sommer H, Kaulen J. (1998) Vacuum headspace method in aroma research: flavor chemistry of yellow passion fruits. J Agric Food Chem 46: 1076–1093 [Google Scholar]
- Yamaguchi S. (2008) Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59: 225–251 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Saito T, Abe H, Yamane H, Murofushi N, Kamiya Y. (1996) Molecular cloning and characterization of a cDNA encoding the gibberellin biosynthetic enzyme ent-kaurene synthase B from pumpkin (Cucurbita maxima L.). Plant J 10: 203–213 [DOI] [PubMed] [Google Scholar]
- Yoshikuni Y, Ferrin TE, Keasling JD. (2006) Designed divergent evolution of enzyme function. Nature 440: 1078–1082 [DOI] [PubMed] [Google Scholar]
- Zharkikh A, Troggio M, Pruss D, Cestaro A, Eldrdge G, Pindo M, Mitchell JT, Vezzulli S, Bhatnagar S, Fontana P, et al. (2008) Sequencing and assembly of highly heterozygous genome of Vitis vinifera L. cv Pinot Noir: problems and solutions. J Biotechnol 136: 38–43 [DOI] [PubMed] [Google Scholar]