Summary: The simple gaseous plant hormone ethylene regulates Arabidopsis flowering via Yin-and-Yang signal transduction pathways.
Abstract
Ethylene is a major plant hormone that plays an important role in regulating bolting, although the underlying molecular mechanism is not well understood. In this study, we report the novel finding that the serine-62 (Ser-62) phosphorylation of Ethylene Response Factor110 (ERF110) is involved in the regulation of bolting time. The gene expression and posttranslational modification (phosphorylation) of ERF110 were analyzed among ethylene-response mutants and ERF110 RNA-interfering knockout lines of Arabidopsis (Arabidopsis thaliana). Physiological and biochemical studies revealed that the Ser-62 phosphorylation of ERF110 was closely related to bolting time, that is, the ethylene-enhanced gene expression of ERF110 and the decreased Ser-62 phosphorylation of the ERF110 protein in Arabidopsis. The expression of a flowering homeotic APETALA1 gene was up-regulated by the Ser-62-phosphorylated isoform of the ERF110 transcription factor, which was necessary but not sufficient for normal bolting. The gene expression and phosphorylation of ERF110 were regulated by ethylene via both Ethylene-Insensitive2-dependent and -independent pathways, which constitute a dual-and-opposing mechanism of action for ethylene in the regulation of Arabidopsis bolting.
Ethylene is known to play an important role in the regulation of plant growth and development, along with the adaptation of plants to both biotic and abiotic stress (Abeles et al., 1992). The role of ethylene in the regulation of flowering, one of the most important developmental events in the angiosperm reproduction of plants, is especially interesting (Koornneef and Peeters, 1997; Levy and Dean, 1998; Weller et al., 2009). In fact, ethylene promotes flowering in some plants but delays it in others (Abeles et al., 1992; Schaller, 2012). For example, it is known to promote flowering in pineapple (Ananas comosus), Guzmania spp., Bougainvillea spp. ’Taipei Red‘, and Arabidopsis (Arabidopsis thaliana; Ogawara et al., 2003; Dukovski et al., 2006; Liu and Chang, 2011), whereas application of ethylene to Arabidopsis and Pharbitis nil delays flowering (Kulikowska-Gulewska and Kopcewicz, 1999; Achard et al., 2007; Tsuchisaka et al., 2009; Wuriyanghan et al., 2009). A puzzling and controversial phenomenon is that ethylene delays bolting in wild-type Arabidopsis, yet both the constitutive triple-response mutant (ctr1-1) and ethylene-insensitive mutants (ethylene response1-1 [etr1-1] and ein2-5) exhibit similar delayed-bolting phenotypes (Hua and Meyerowitz, 1998; Ogawara et al., 2003; Achard et al., 2007).
The seemingly conflicting effects of ethylene on flower bolting time could be viewed as a dual-and-opposing effect (Lu et al., 2001) and might be attributed to a dynamic interplay among multiple ethylene signal transduction pathways (Lu et al., 2001, 2002; Binder et al., 2004; Yoo et al., 2008). According to the current understanding of the molecular mechanism that underlies ethylene signaling, ethylene transduces its signals through membrane-bound ethylene receptor complexes (ETR1, ETR2, ERS1, ERS2, and EIN4) to ethylene response transcription factors (ERFs) via kinase cascades and a putative Natural resistance-associated macrophage protein1 metal ion transporter, EIN2 (Guo and Ecker, 2004; Chen et al., 2005). During the signaling process, EIN2 is considered to be the central regulator of the ethylene signaling pathway (Alonso et al., 1999), in which the C terminus of EIN2 is cleaved after induction by ethylene and translocates into the nucleus to transduce the ethylene signal (Qiao et al., 2012). Immediately downstream of the EIN2 signaling component is a pair of transcriptional regulators, EIN3 and EIL1, the double loss-of-function mutant of which abolishes most ethylene responses observed in Arabidopsis (Alonso et al., 2003), indicating a central role for EIN3/EIL1 in the regulation of nuclear transcriptional events related to ethylene, including most ERFs. The protein level of ethylene signaling components, such as ETR2, EIN2, EIN3, and EIL1, is regulated both by F-box proteins and ethylene-regulated Ubiquitin/26S proteasome-dependent protein degradation systems (Zhao and Guo, 2011).
In combination with the intricate transcriptional regulatory networks, protein phosphorylation has also been reported to be involved in ethylene signaling events (Raz and Fluhr, 1993; Li et al., 2009; Stepanova and Alonso, 2009). Receptors and many of the key regulatory components in ethylene signaling have kinase activities (Gamble et al., 2002; Gao et al., 2003; Wang et al., 2003; Kendrick and Chang, 2008). Although the negative regulator of the ethylene response, CTR1, which is a putative MEK kinase (MAPKKK), directly phosphorylates and inactivates EIN2 in the air, the role of other mitogen-activated protein kinases (MAPKs) in ethylene signaling is still controversial (Ju et al., 2012). MAPK cascades have been reported to be involved in the regulation of ethylene signaling and ethylene-regulated phosphorylation of EIN3 (Ouaked et al., 2003; Yoo et al., 2008) and especially in the control of ethylene biosynthesis (Liu and Zhang, 2004). The phosphorylation of the EIN3 protein appears to play a dual role in the regulation of its stability, which suggests a direct link between the protein phosphorylation regulatory network and the regulatory mechanism of transcription factors in ethylene signaling. Thus, it is speculated that the dual-and-opposing effect of ethylene on flowering might be mediated by both transcriptional and posttranslational levels in Arabidopsis.
To address the complex yet interesting phenomenon via an unbiased approach, label-free quantitative phosphoproteomics was performed on ethylene-regulated protein phosphorylation, identifying ERF110 as one of the putative novel ethylene signaling components (Li et al., 2009). This ERF110 gene encodes a transcriptional factor that belongs to the B4 subfamily of the ERF protein family (Nakano et al., 2006), which was predicted to be phosphorylated and/or dephosphorylated in an EIN2-independent manner. The ERF gene family displays diverse cellular functions, ranging from plant responses to biotic or abiotic stress to hormone treatment (Ohme-Takagi and Shinshi, 1995; Nakano et al., 2006).
We report here the ethylene-regulated phosphorylation of ERF110, the ethylene-regulated Ser-62-phosphorylated isoform of which was found to be required for normal Arabidopsis bolting. Subsequently, a downstream flowering homeotic gene, APETALA1 (AP1), was up-regulated by Ser-62-phosphorylated ERF110 at the transcriptional level, whereas ethylene up-regulated ERF110 gene expression via EIN2 and down-regulated its Ser-62 phosphorylation in an EIN2-independent manner. These results demonstrate, to our knowledge for the first time, that ethylene has a dual-and-opposing effect on the production of the Ser-62-phosphorylated isoform of ERF110 in Arabidopsis and that the level of this specific ERF110 isoform is involved in bolting. This ethylene regulatory model has been proposed as an explanation of why the constitutive ethylene-response mutant (ctr1-1), ERF110-deficient knockout lines (erf110-1 and erf110-2), and ethylene-insensitive mutants (etr1-1 and ein2-5) share similar delayed-bolting phenotypes.
RESULTS
In Vitro and in Vivo Confirmation That ERF110 Is an Ethylene-Regulated Phosphoprotein
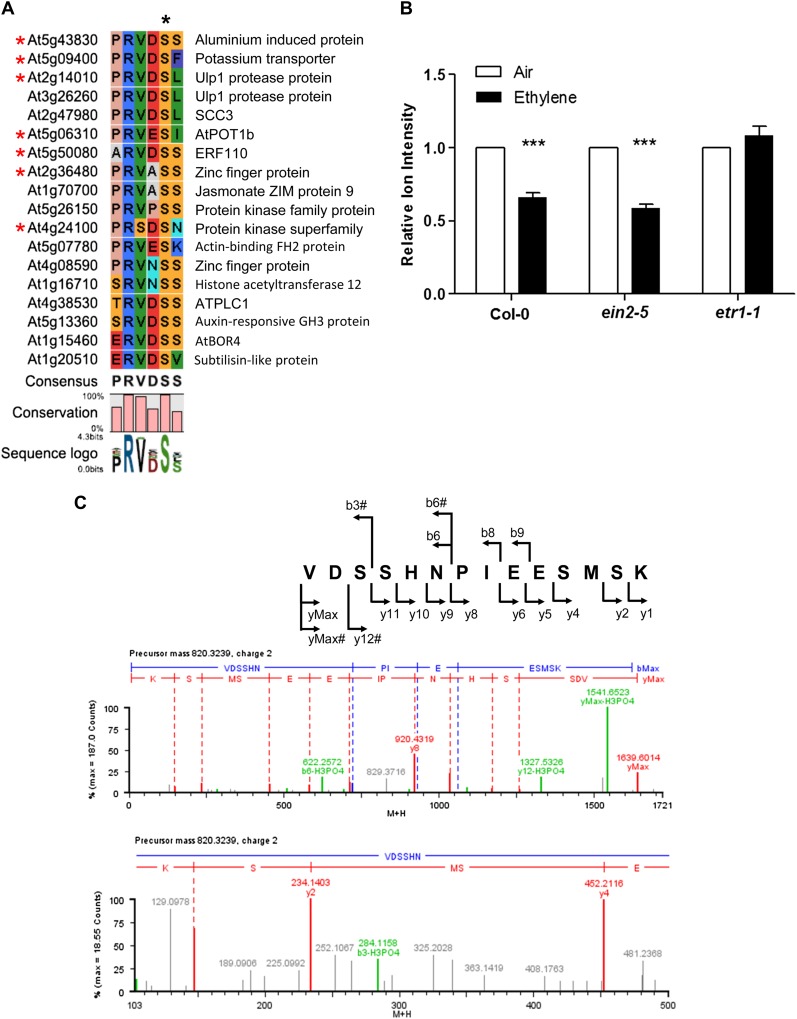
A label-free quantitative phosphoproteomics study was performed on an ethylene-insensitive mutant (ein2-5) in previous experiments (Li et al., 2009). A novel ethylene-regulated and PRVDpSS244-containing phosphopeptide (where boldface S is phosphorylated Ser) of an aluminum-induced protein (At5g43830) was discovered in ethylene-treated etiolated ein2-5 Arabidopsis seedlings. To expand the repertoire of possible phosphoproteins that possess such a phosphorylation motif (Li et al., 2009), nine- to 21-amino acid-long oligopeptide sequences were deduced from the primary sequence of the aluminum-induced protein (At5g43830), which covers the entire phosphorylation site, and were used to search the nonredundant protein sequence database (organism, Arabidopsis; taxid, 3702). Interestingly, 18 predicted putative Arabidopsis phosphoproteins were identified as sharing the conserved phosphosite motif, all of them having a homology of 55.5% or more with that of the query phosphosite motif on an aluminum-induced protein (Fig. 1A). To validate the prediction, the chosen peptides containing the predicted phosphosite motif (Fig. 1A; Supplemental Table S1) were synthesized and used as substrates in the in vitro kinase assay (see “Materials and Methods”) using kinase extracts isolated from wild-type Arabidopsis and ethylene-response mutant (ein2-5 and etr1-1) plant tissues. Six of 18 predicted members were indeed phosphorylated at the predicted phosphosites, as marked by asterisks on the left side in Figure 1A. The tandem mass spectrometry (MS/MS) spectra of neutral loss ions detected from each synthetic substrate peptide are shown in both Figure 1 and Supplemental Figure S1.
Figure 1.
Bioinformatics prediction and validation of a phosphosite on ERF110. A, Alignment of the phosphosite motif PRVDSS between the authentic mass spectrometry-derived phosphopeptide and those of Arabidopsis gene accessions. The top amino acid sequence motif derives from a phosphopeptide from a segment of aluminum-induced protein (At5g43830). The remaining peptide sequences were retrieved from the Arabidopsis gene database according to the alignment conducted by the BLAST program using the authentic mass spectrometry-derived phosphosite motif PRVDSS. The phosphorylation site (S) is marked with a black asterisk. The synthetic peptides marked with red asterisks on the left were successfully phosphorylated by the in vitro kinase assay. The conserved motif sequence (PRVDSS) among these members is shown at the bottom of the alignment. The conservation score (percentage) and the sequence logo are also shown at the bottom of the alignment, representing frequencies of the conserved amino acids present in the motif. B, Quantitation of the Ser-62 phosphosite of ERF110 using the in vitro kinase assay coupled with iTRAQ. The bars indicate the relative intensities of reporter ions for the phosphorylated ERF110 peptide catalyzed by the in vitro kinase assay (***P < 0.001 by Student’s t test, ethylene versus air treatment). The ion intensity of the air-treated sample was set as 1. For each pair of comparisons, the experiments were repeated with at least two independent biological samples using reciprocal labeling (Supplemental Figs. S2 and S3). Representative MS/MS spectra are presented in Supplemental Figures S2 and S3. The in vitro kinase activity of Ser-62 was ethylene dependent but EIN2 independent. White and black bars represent Arabidopsis kinase extracts prepared from air- and ethylene-treated plants, respectively (for a detailed kinase assay protocol, see “Materials and Methods”). C, MS/MS spectra of the in vivo phosphorylation of ERF110. The precursor ion (VDpSSHNPIEESMSK) was isolated and fragmented by collision-induced dissociation. Both b-type and y-type ions, including the H3PO4 neutral loss ions (indicated as –H3PO4 and # in the spectra), were labeled to determine the peptide sequence of the precursor ion and to locate the sites of phosphorylation. An enlarged portion of the spectrum (mass-to-charge ratio 100–500) that showed the b3 neutral loss ion (284.1158) was put at the bottom of the full MS/MS spectrum obtained from LC-MS/MS (quadrupole time of flight; Waters).
ERF110 is one of six bioinformatics-predicted and in vitro kinase assay-validated phosphoproteins. The biological function of this predicted ERF, particularly its role in the regulation of bolting, has not been previously investigated. Given the availability of quantitative proteomics methodologies, such as isobaric tags for relative and absolute quantitation (iTRAQ), and a large collection of ethylene-response mutants, a combination of a quantitative proteomics protocol with the in vitro kinase assay was adopted here to characterize the ethylene-regulated differential phosphorylation of ERF110 among wild-type Arabidopsis and ethylene-response mutants. A matrix-assisted laser desorption/ionization (MALDI)-MS/MS analysis of the final purified peptides showed a mass peak of 1,927.9042 (Supplemental Fig. S2), which is consistent with the mass prediction of the phosphorylated ERF110 peptides with two isobaric tags (Supplemental Fig. S2). A further MS/MS fragmentation of the peptide confirmed that the peptide sequence was derived from ERF110 protein and that the phosphorylation event occurred at Ser-62, as predicted (Supplemental Fig. S2). The intensity of iTRAQ reporter ions for the mass peak 1,927.9 was obtained using a MALDI-MS/MS analysis to calculate the relative degree of change (Supplemental Fig. S3). As Figure 1B reveals, ethylene down-regulated the Ser-62-phosphorylation 0.66 ± 0.07-fold and 0.59 ± 0.07-fold in wild-type (ecotype Columbia [Col-0]) and ein2-5 plants, respectively. The facts that a similar down-regulation of kinase activity was found in the wild type and the ein2-5 mutant, and that there was no significant difference in ERF110 Ser-62 phosphorylation between air- and ethylene-treated etr1-1 (Fig. 1B), suggest that the signal-elicited alteration in ERF110 Ser-62 phosphorylation is derived from ethylene receptors and ethylene-regulated kinase activity and is independent of the function of the master ethylene-signaling component EIN2. The results, therefore, demonstrate that ERF110 may be a novel signaling component in dual ethylene signal transduction pathways.
To validate that phosphorylation at the Ser-62 position in the ERF110 protein indeed occurs in planta, this putative transcription factor was fused to a His-biotin-His tag at the C terminus and overexpressed in the Arabidopsis Col-0 background. The recombinant ERF110 protein was then isolated using a tandem affinity purification protocol under fully denaturing conditions, followed by mass spectrometry analysis (Guo and Li, 2011; Li et al., 2012). Peptide ions of VDpSSHNPIEESMSK, derived from ERF110, were found using MS/MS spectra (Fig. 1C; Supplemental Fig. S1B). Among the y ion series shown in the MS/MS spectra (Fig. 1C), ymax and y12 ions were found to have a neutral loss of H3PO4 moiety (molecular weight difference between the phosphorylated peptide ion and the peptide ion lack of a phosphate moiety = 98 D), as were both b3 and b6 ions. These four fragmentation ions, especially the b3 neutral loss ion with a mass-to-charge ratio of 284.1158, confirm that Ser-62 is phosphorylated in vivo. Taken together, both in vitro and in vivo proteomics results demonstrated that bioinformatics prediction in combination with the in vitro kinase assay is able to identify authentic in vivo phosphorylation sites related to either an external or internal cue.
ERF110 Is Involved in Ethylene-Regulated Bolting Time
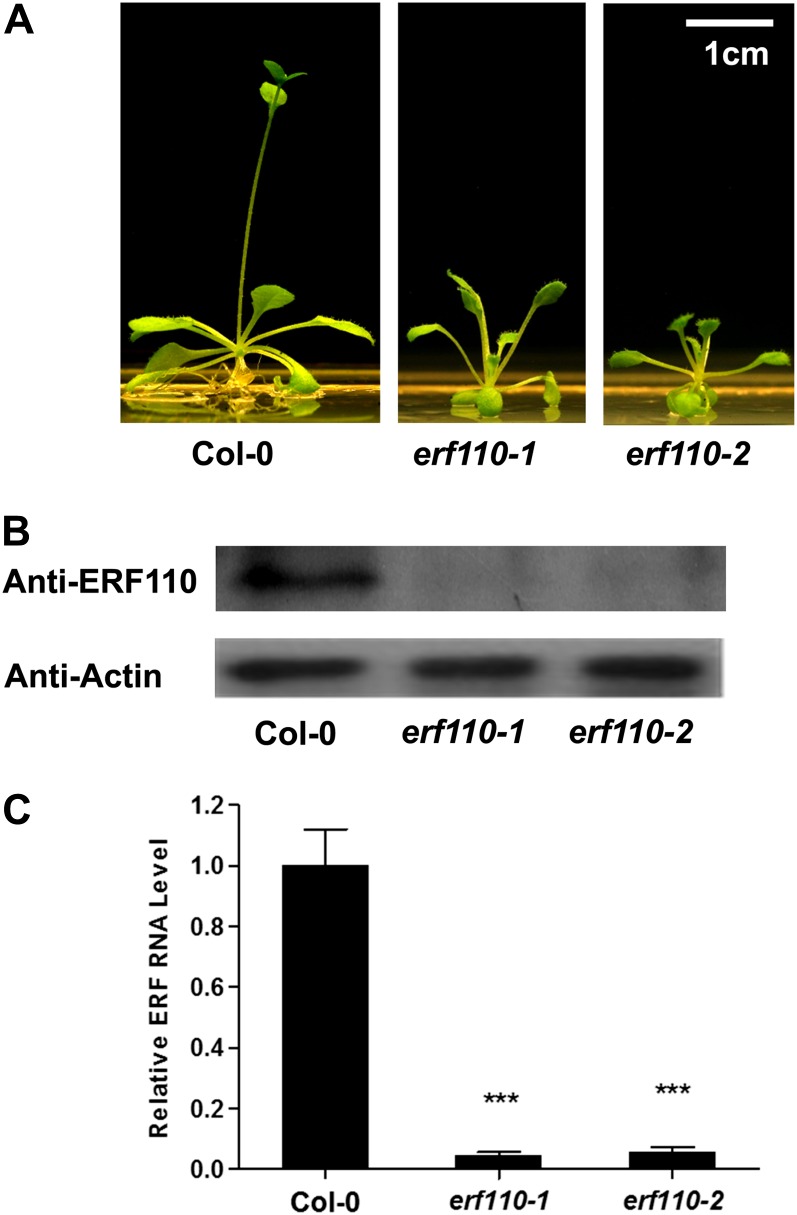
Before an extensive experiment could be performed on the posttranslational modification of the ERF110 protein (i.e. phosphorylation), the biological function of the ERF110 gene needed to be addressed first in Arabidopsis. To that end, we investigated the in planta role of the ERF110 gene. ERF110 RNA-interfering (RNAi) constructs were created to suppress the endogenous ERF110 transcripts in wild-type Arabidopsis. As expected, two ERF110 RNAi lines were found to have severely reduced ERF110 transcripts and protein in T2 plants (Fig. 2, B and C). These transgenic lines are called erf110-1 and erf110-2 knockout lines. When examined in these transgenic plants, it was found that both RNAi lines showed a delayed bolting time (25.8 ± 1.19 and 25.1 ± 1.00 d [P < 0.001] for erf110-1 and erf110-2, respectively; Fig. 2A; Supplemental Table S2) compared with the wild type (Col-0; 19.8 ± 0.18 d). These erf110 mutants also had a greater number of rosette leaves (10.4 ± 0.56 and 10.6 ± 0.62) compared with the wild type (7.1 ± 0.08 leaves per plant [P < 0.001]; Supplemental Table S2), suggesting that ERF110 is involved in the control of the floral transition in Arabidopsis. This interesting phenomenon led us to extend our investigation to incorporate the role of ethylene-regulated ERF110 Ser-62 phosphorylation in bolting.
Figure 2.
Molecular characterization of ERF110 RNAi lines. A, Delayed-bolting phenotype of two ERF110 RNAi lines (erf110-1 and erf110-2) at 23 d of growth; the wild-type (Col-0) was the control. B, Western-blot analysis of the ERF110 protein in both the wild type and mutants was performed using rabbit anti-ERF110 and anti-actin polyclonal antibodies, respectively. Actin signal was used as the sample protein-loading control. C, qRT-PCR analysis of ERF110 mRNA level in transgenic RNAi lines erf110-1 and erf110-2. [See online article for color version of this figure.]
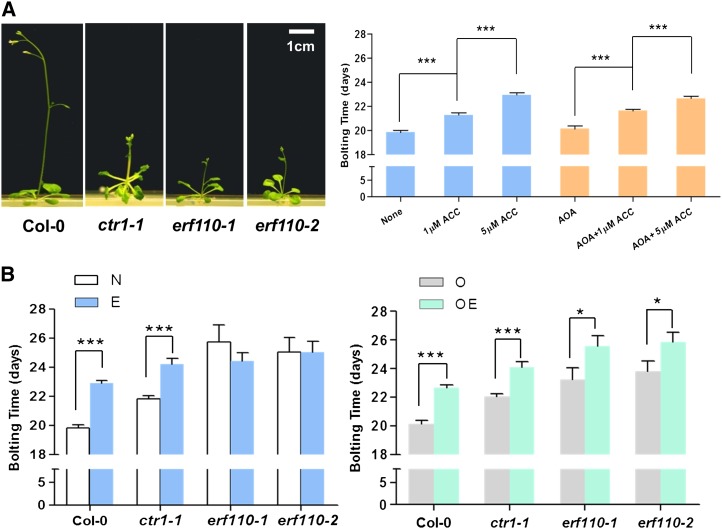
It has been reported that the application of the ethylene biosynthesis precursor 1-aminocyclopropane-1-carboxylic acid (ACC) delays bolting time significantly in wild-type Arabidopsis (Achard et al., 2007), whereas the phenotypic characterization of single or multiple ACC synthase (ACS) mutants also supports an inhibitory role for ACC in bolting (Tsuchisaka et al., 2009). Moreover, the constitutive ethylene-response mutant ctr1-1 is well known to delay bolting (Hua and Meyerowitz, 1998; Achard et al., 2007). To confirm this role of ethylene, the immediate ethylene biosynthesis precursor ACC was used. As Figure 3 shows, the application of ACC delayed the bolting time significantly in a dose-dependent manner in wild-type Arabidopsis. In comparison with the untreated group, 1 µm ACC delayed the bolting time from 19.8 ± 0.18 to 21.3 ± 0.19 d (P < 0.001) in Col-0 and caused a significant increase in the number of leaves, whereas 5 µm ACC further delayed the bolting time to 22.9 ± 0.17 d (P < 0.001) in the Col-0 background with 9.1 ± 0.24 leaves at the time of bolting (P < 0.01).
Figure 3.
Effect of ethylene on bolting time. A, The left panel shows representative images of ethylene-response mutant plants at 25 d of Arabidopsis growth on M/S medium without ACC treatment. The right panel shows that the ethylene production precursor ACC delayed bolting time in wild-type Arabidopsis. None indicates M/S medium only; 1 µm ACC and 5 µm ACC indicate M/S medium supplemented with 1 and 5 µm ACC, respectively; AOA represents M/S medium supplemented with 100 µm AOA; AOA = 1 µm ACC and AOA = 5 µm ACC indicate M/S medium supplemented with 100 µm AOA and 1 and 5 µm ACC, respectively. ***P < 0.001 when compared between treatments. B, Bolting time of mutant plants that were grown on M/S medium with different treatments. N, M/S medium (untreated); E, M/S medium supplemented with 5 µm ACC (ACC treated); O, M/S medium supplemented with 100 µm AOA (AOA treated); OE, M/S medium supplemented with both 100 µm AOA and 5 µm ACC (AOA+ACC treated). *P < 0.05, ***P < 0.001 in comparison with Col-0 by Student’s t test. Both ethylene-response mutant ctr1-1 and ERF110 RNAi lines exhibited a delayed-bolting phenotype after ACC treatment. [See online article for color version of this figure.]
To minimize the effect of endogenous ethylene, an ACC synthase inhibitor, aminooxyacetic acid (AOA), is usually added to the growth medium to reduce the background level of ethylene. ACC is frequently used in combination with AOA to study the effect of ethylene on bolting time among the wild type, erf110 knockout lines, and ethylene-response mutants such as ctr1-1. Because it has been reported that ctr1-1 inflorescence has a faster gravicurvature response to gravistimulation following a long-term ethylene treatment (Li, 2008), we deliberately treated ctr1-1 with ethylene to address whether its bolting time could be altered in response to treatment. Interestingly, treatment with 5 µm ACC delayed the bolting time of ctr1-1 from 21.8 ± 0.24 to 24.3 ± 0.35 d (P < 0.001; Fig. 3B; Supplemental Table S2), whereas, in the presence of AOA, a similar delay in bolting was observed from 22.0 ± 0.21 to 24.1 ± 0.36 d in ACC-treated ctr1-1 (P < 0.001; Fig. 3B; Supplemental Table S2). Similarly, the two ERF110 RNAi lines also exhibited a delayed-bolting phenotype after ACC treatment (from 23.2 ± 0.83 to 25.6 ± 0.75 d [P = 0.044] and from 23.8 ± 0.70 to 25.8 ± 0.69 d [P = 0.045] for erf110-1 and erf110-2, respectively; Fig. 3B; Supplemental Table S2). As the delayed bolting of erf110 knockout lines was more obvious in the presence of AOA, endogenous background ethylene is believed to influence the bolting phenotype measurement, and several other ethylene-regulated flowering regulatory factors may exist in addition to ERF110.
Ethylene Regulates Bolting by Enhancing ERF110 Gene Expression
Initially, the delayed-bolting phenotype of the ERF110-knockout lines erf110-1 and erf110-2 suggested that ethylene (or ACC) may down-regulate ERF110 gene expression, which delays the bolting of Arabidopsis. When the 0.5-kb promoter region of ERF110 (–289 to =226) was fused to a GUS reporter gene and transformed into Arabidopsis Col-0, the application of ethylene actually enhanced rather than inhibited ERF110 promoter activity at the 2- to 3-week-old stage (Supplemental Fig. S4; Supplemental Table S3). ACC-treated seedlings had a GUS activity of 454.1 ± 21.8 pmol 4-methylumbelliferone (4-MU) h−1 µg−1, which was significantly higher than that in the untreated group (372.6 ± 33.4 pmol 4-MU h−1 µg−1; P = 0.03). The ERF110 promoter activity (GUS activities) of AOA-treated background plants was 390.4 ± 26.1 pmol 4-MU h−1 µg−1, whereas plants treated with both AOA and ACC had a GUS activity of 468.8 ± 4.8 pmol 4-MU h−1 µg−1 (P = 0.02). At the 3-week-old stage, ACC-treated Arabidopsis displayed a significantly higher level of GUS activity (658.0 ± 8.7 to 714.3 ± 6.8 pmol 4-MU h−1 µg−1; P < 0.001) compared with untreated plants (574.2 ± 9.3 to 597.0 ± 8.4 pmol 4-MU h−1 µg−1; Supplemental Table S3). These GUS assay results clearly demonstrated that ERF110 promoter activity was increased by ethylene. Furthermore, histochemical GUS staining of ProERF110-GUS transgenics revealed that ERF110 promoter activity was constitutive and especially enhanced in the new leaves and flowers of 2- to 3-week-old plants (Supplemental Fig. S4) and was higher in the shoot apical region throughout plant development. Therefore, it was concluded that ERF110 is involved in bolting and plant reproduction.
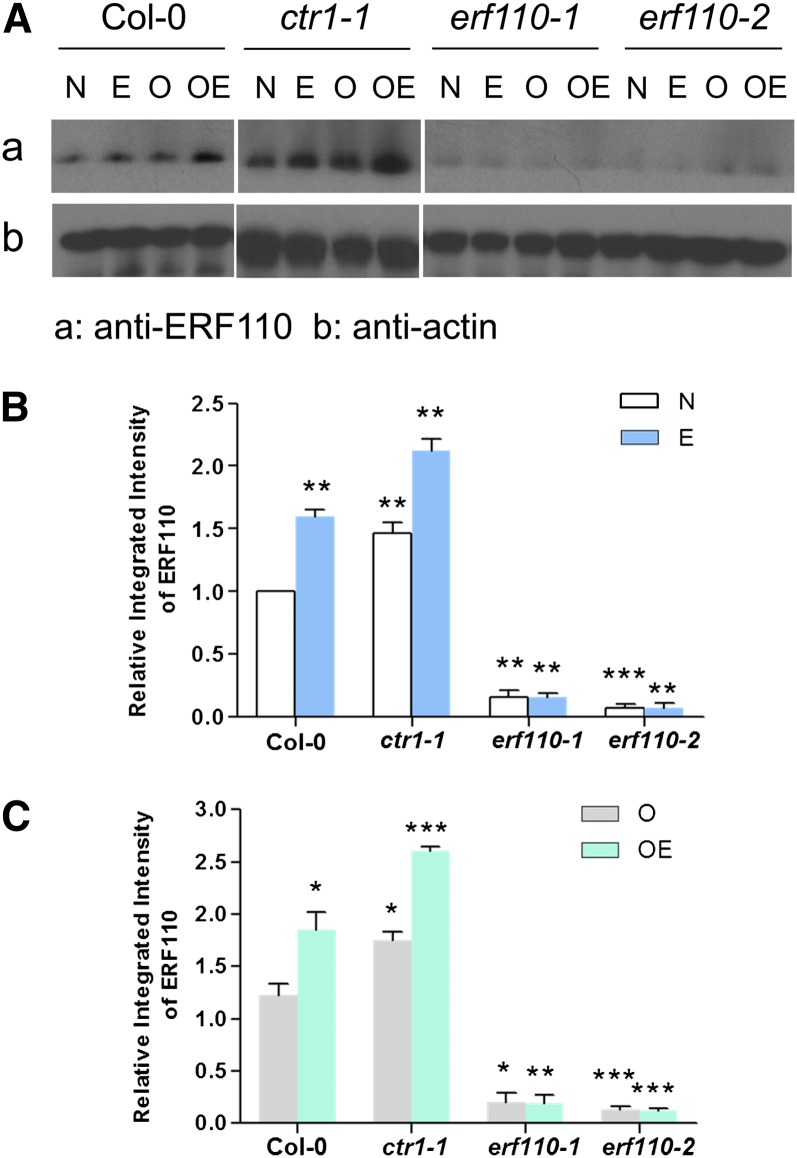
To confirm that the ERF110 protein is also up-regulated by ethylene, 2-week-old light-grown seedlings of both the wild type and mutants were subjected to four types of treatment: (1) control; (2) 5 µm ACC; (3) 100 µm AOA; and (4) 100 µm AOA = 5 µm ACC. The total cellular proteins were extracted from the four groups of plants to examine the endogenous ERF110 protein level (Fig. 4) using western-blot analysis. Overall, ERF110 RNAi lines had five times less ERF110 protein than Col-0 (P < 0.01), regardless of ACC treatment. However, ctr1-1 showed about a 1.5-fold increase in ERF110 protein level compared with Col-0 (P = 0.03; Fig. 4B). Thus, western-blot results on ERF110 protein levels in ctr1-1 were consistent with the histochemical GUS-staining results described above and constituted unequivocal evidence that the level of ERF110 gene expression is enhanced by ethylene via the CTR1-mediated ethylene signaling pathway. However, these two aspects of molecular biology cannot explain the seemingly conflicting phenomenon that ethylene both delays the bolting time and enhances ERF110 expression. The question thus arises of how ERF110 is involved in ethylene-delayed bolting.
Figure 4.
Regulation of ERF110 gene expression in ACC-treated Arabidopsis and mutants. A, Western-blot analysis of endogenous ERF110 protein in the wild-type, ctr1-1, and erf110 transgenic lines. Two-week-old seedlings of Arabidopsis were subjected to various AOA and ACC treatments. N, M/S medium (untreated); E, M/S medium supplemented with 5 µm ACC (ACC treated); O, M/S medium supplemented with 100 µm AOA (AOA treated); OE, M/S medium supplemented with both 100 µm AOA and 5 µm ACC (AOA+ACC treated). Actin was used as the loading control. B, Relative and integrated abundance of endogenous ERF110 protein among mutants with 5 µm ACC (ACC treated). **P < 0.01, ***P < 0.001 according to Student’s t test compared with Col-0 under the same treatment. C, Relative and integrated intensity of endogenous ERF110 protein among mutant plants after AOA and AOA+ACC treatment. *P < 0.05, **P < 0.01, ***P < 0.001 according to Student’s t test compared with Col-0 under the same treatments. [See online article for color version of this figure.]
Ethylene Suppresses Ser-62 Phosphorylation of ERF110
The Ser-62 phosphosite of ERF110 has been found to be down-regulated by ethylene, as Figure 1 illustrates. To confirm that this phosphorylation also occurs in vivo, a monoclonal antibody was raised against phosphorylated Ser-62 (see “Materials and Methods”). The endogenous ERF110 proteins were first enriched with the total cellular proteins of 2-week-old ethylene-treated wild-type and ctr1-1 seedlings using an immunoaffinity column, which was coated with ERF110 polyclonal antibodies (see “Materials and Methods”). Western-blot analysis was then performed on the protein samples using the Ser-62 phosphosite-specific monoclonal antibody. As a result, it was found that ethylene indeed down-regulated the in planta Ser-62 phosphorylation of ERF110 isolated from Col-0 plants (Fig. 5; Supplemental Figs. S5 and S6). Unexpectedly, the Ser-62-phosphorylated ERF110 was undetectable in ctr1-1 protein extract regardless of whether the plant was exposed to air or ethylene. In fact, the overall ERF110 protein level was actually increased dramatically in ctr1-1 protein samples (Fig. 5), which strongly suggests that the lack of Ser-62 phosphorylation in ERF110 in ctr1-1 is not due to reduced ERF110 protein expression in this mutant. Rather, it may result from reduced or even the lack of kinase activity (or increased phosphatase activity) toward Ser-62.
Figure 5.
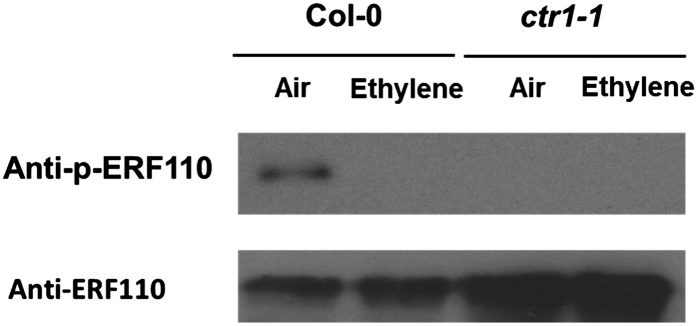
Ethylene represses in vivo Ser-62 phosphorylation of ERF110 protein. Both wild-type Arabidopsis seedlings and constitutive ethylene-response mutant ctr1-1 seedlings were grown to 2 weeks of age on M/S medium supplemented with 100 µm AOA. These two groups of plants were treated either with air (Air) or 10 µL L−1 ethylene (Ethylene) for 12 h. The total cellular proteins were extracted from both groups of plants. The amount of ERF110 protein loaded onto each lane was immunoprecipitated from 10 mg of total cellular proteins. Western-blot analysis was performed using a mouse monoclonal antibody raised against the Ser-62 phosphosite of ERF110 and a rabbit polyclonal antibody raised against ERF110 in order to examine the relative abundance of both endogenous ERF110 and its Ser-62-phosphorylated isoform.
Because a recombinant ERF110 protein can be modified in the same way as an endogenous ERF110 protein (Li et al., 2012), an ERF110-His8-biotin-His8 (HBH) fusion protein transgene (see “Materials and Methods”) was placed under the control of a cauliflower mosaic virus 35S promoter and overexpressed in the wild-type background. Western-blot analysis showed that the relative abundance of the ERF110-HBH fusion protein in ERF110-overexpressing transgenics was 100-fold higher than that in endogenous ERF110 proteins (Supplemental Fig. S5). The successful overexpression of the recombinant ERF110 protein in Pro35S-ERF110::Col-0 transgenics provided sufficient ERF110 protein to study ethylene-regulated phosphorylation events. As expected, more Ser-62-phosphorylated ERF110-HBH isoform was detected using western-blot analysis in the ERF110-overexpressing lines (Supplemental Figs. S5 and S6). Application of 10 µL L−1 ethylene to Pro35S-ERF110::Col-0 transgenics also reduced the Ser-62 phosphorylation of ERF110 by 60% (Supplemental Fig. S6). Western-blot analysis of both endogenous and fusion ERF110 proteins confirmed that Ser-62 phosphorylation of ERF110 is down-regulated by ethylene (Fig. 5). Ethylene also reduced Ser-62 phosphorylation on the overexpressed ERF110 protein in Pro35S-ERF110::ein2-5 transgenic plants (Supplemental Fig. S6). These results suggest that the ethylene-mediated down-regulation of Ser-62 phosphorylation, resulting from either reduced kinase activity or enhanced phosphatase activity, is independent of the biological function of EIN2.
The Ser-62 Phosphorylation of ERF110 Is Associated with Bolting and AP1 Gene Expression
Based on several seemingly conflicting results described above, it was speculated that the Ser-62-phosphorylated isoform of ERF110 would be required for bolting. To examine this hypothesis, Ser-62 of ERF110 was substituted with either Ala (A) or Asp (D) using site-directed mutagenesis (see “Materials and Methods”). The former and latter point mutants are mimetic of the dephosphorylation and phosphorylation isoforms of ERF110, respectively. As Figure 6 reveals, in the presence of higher levels of ethylene (or ACC), the transgenic plants overexpressing the wild-type ERF110 bolted within 23.4 ± 0.17 d, whereas ERF110S62A-overexpressing plants had a delayed bolting (24.3 ± 0.36 d). In contrast, ERF110S62D overexpression transgenic mutants bolted by 22.9 ± 0.22 d (Fig. 6; Table I). These data suggest that Ser-62 phosphorylation status is involved in normal Arabidopsis bolting. Under conditions of exposure to higher levels of ethylene that induce delayed bolting in Arabidopsis, the role of Ser-62-phosphorylated ERF110 becomes significant (Fig. 6; Table I).
Figure 6.
Bolting time for ERF110 overexpression lines after AOA or AOA+ACC treatments. A, Representative photographs of 25-d-old ERF110 overexpression plants. O, 100 µm AOA; OE, 100 µm AOA = 5 µm ACC; WT-OX, ERF110WT overexpression line; WT-OXA, ERF110S62A overexpression line; WT-OXD, ERF110S62D overexpression line. B, Bar chart for bolting time after AOA or AOA+ACC treatment. O, M/S medium supplemented with 100 µm AOA (AOA treated); OE, M/S medium supplemented with both 100 µm AOA and 5 µm ACC (AOA+ACC treated). All three recombinant ERF110 genes were in the Col-0 background. *P < 0.05, **P < 0.01 according to Student’s t test compared with Col-0 under the same treatment. [See online article for color version of this figure.]
Table I. Bolting time of ERF110WT, ERF110S62A, and ERF110S62D overexpression transgenic lines.
Days of bolting and number of leaves are presented as means ± se. O stands for M/S medium = 100 µm AOA, while OE stands for M/S medium = 100 µm AOA = 5 µm ACC. WT-OX, WT-OXA, and WT-OXD are transgenic lines of ERF110WT overexpression, ERF110S62A overexpression, and ERF110S62D overexpression in the Col-0 background, respectively. The number of transgenic plants analyzed is given in parentheses.
| Days of Bolting |
No. of Rosette Leaves at Bolting |
|||
|---|---|---|---|---|
| Line | O | OE | O | OE |
| WT-OX | 19.6 ± 0.22 (n = 44) | 23.4 ± 0.17 (n = 42) | 7.6 ± 0.10 (n = 44) | 10.4 ± 0.16 (n = 42) |
| WT-OXA | 20.2 ± 0.46 (n = 39) | 24.3 ± 0.36 (n = 39) | 7.7 ± 0.14 (n = 39) | 10.1 ± 0.22 (n = 39) |
| WT-OXD | 20.1 ± 0.22 (n = 39) | 22.9 ± 0.22 (n = 35) | 7.7 ± 0.13 (n = 39) | 8.8 ± 0.14 (n = 35) |
To determine how Ser-62-phosphorylated ERF110 affects bolting, quantitative real-time reverse transcription (qRT)-PCR analysis was performed to screen gene expression levels of 23 known flowering homeotic genes in both the wild type and ERF110 knockout lines, erf110-1 and erf110-2 (Supplemental Fig. S7). These homeotic genes are believed to act downstream of ERF110 in the ethylene-delayed bolting process. Interestingly, four of these flowering homeotic genes, APETALA1 (AP1), VERNALIZATION INSENSITIVE3, FLOWERING LOCUS T, and EARLY FLOWERING8, were indeed found to exhibit a significant change in gene expression levels and were selected as downstream putative transcription activation targets for ERF110. The transcript of AP1, a positive integrator of flowering signaling (Irish and Sussex, 1990), showed a consistent association with the abundance of Ser-62-phosphorylated ERF110 protein in Col-0 and the ctr1-1 and erf110 mutant lines (Fig. 7). To confirm the direct link between Ser-62 phosphorylation and AP1 gene expression, ERF110S62A and ERF110S62D transgenic mutants were also included in the investigation. Again, a strong correlation was found between the Ser-62-phosphorylated ERF110 isoform and AP1 gene expression among the ACC-treated transgenic plants (Fig. 7). The AP1 transcript level in Pro35S-ERF110S62A::Col-0 overexpressing plants was 60% of that of the wild-type level, whereas in the Pro35S-ERF110S62D::Col-0 mutant, the abundance of AP1 transcripts was slightly higher than that of the wild-type ERF110 overexpression line (Fig. 7). These molecular genetic results further confirm a positive correlation between the abundance of AP1 transcripts and the level of Ser-62-phosphorylated ERF110 isoform. The finding that treatment with AOA did not alter the AP1 transcript level significantly among any of the three types of transgenic line supports a theory that a lower level of Ser-62-phosphorylated ERF110 isoform expressed from the endogenous ERF110 gene in Col-0 can produce a sufficient level of AP1 transcript that contributes to a normal bolting process in Arabidopsis.
Figure 7.
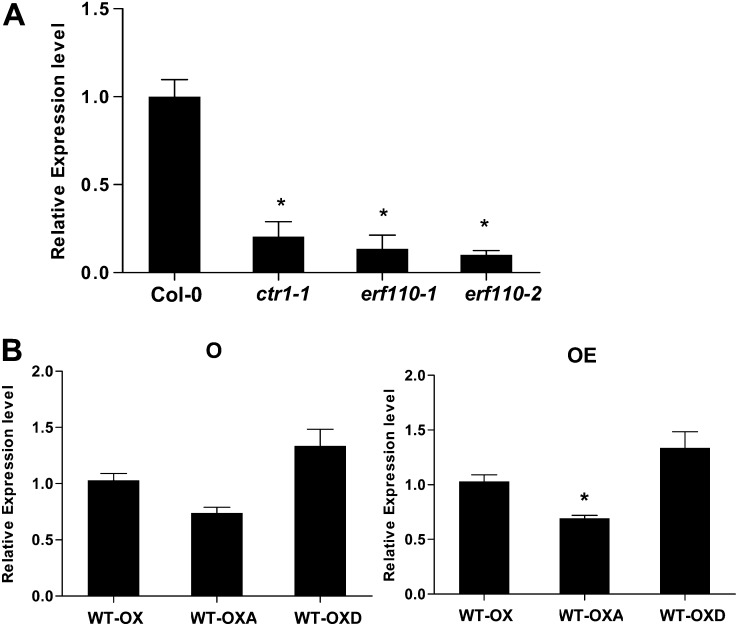
qRT-PCR analysis of the transcript level of the flowering homeotic gene AP1 among ethylene-response mutants and ERF110 overexpression transgenic lines. A, The relative mRNA level of AP1 in both ctr1-1 and erf110 mutants. B, The relative mRNA level of AP1 in ERF110 overexpression lines either under AOA or AOA+ACC treatment. Comparisons were made between AOA and AOA+ACC treatments for each transgenic line. WT-OX, WT-OXA, and WT-OXD represent ERF110WT, ERF110S62A, and ERF110S62D overexpression, respectively, in the Col-0 background. *More than 1.5-fold change compared with the control group, with a significant difference (P < 0.05) according to Student’s t test. O, M/S medium supplemented with 100 µm AOA (AOA treated); OE, M/S medium supplemented with both 100 µm AOA and 5 µm ACC (AOA+ACC treated).
DISCUSSION
Phosphoproteomics has provided a unique means to understand cellular signaling networks in various physiological events in an organism (Morandell et al., 2006; de la Fuente van Bentem et al., 2008). The recent developments in high-throughput phosphopeptide isolation and MS/MS-based identification have further increased the phosphosite discovery rate. According to the repertoire of phosphopeptides collected by the PhosPhAt 3.0 database, more than 10,000 unique phosphopeptides have been identified from Arabidopsis to date (Durek et al., 2010). Even with the addition of those phosphopeptides collected from the label-free quantitative proteomic approach (Li et al., 2009; Chen et al., 2011), the number of hormone-regulated phosphopeptides is still much smaller than that of theoretically predicted phosphosites found from a plant (de la Fuente van Bentem et al., 2008). Therefore, bioinformatics-based phosphosite prediction and motif analysis, coupled with in vitro validation, play an indispensable role in compensating for the current deficiency in high-throughput MS analysis. For example, Hernández Sebastià et al. (2004) have demonstrated that the in vitro kinase assay is useful in establishing a highly conserved phosphosite motif on an ACC synthase, the key enzyme in the conversion of S-adenosyl-Met to the ethylene biosynthesis precursor ACC, and that some ACC synthase isomers might serve as substrates for calmodulin-dependent protein kinases. In another example, both MS-identified and bioinformatics-predicted BAK1 and BRI1 phosphorylation sites were validated both in vitro and in vivo. These findings provided some novel insights into the function of kinase in brassinosteroid signaling (Wang et al., 2005b). In this study, we used the peptide sequence alignment to identify a putative Ser-62 phosphosite on a putative bioinformatics-predicted transcription factor, ERF110 (Li et al., 2009), delineate a functional role for the Ser-62-phosphorylated isomer of the ERF110 transcription factor, and provide a demonstration of functional phosphoproteomics by integrating quantitative posttranslation modification (PTM) proteomics with bioinformatics prediction and in vitro and in vivo validations.
Kinase- and phosphatase-mediated phosphorylation is known to be an important molecular mechanism that regulates ethylene responses. CTR1 has been predicted to encode a MAPKKK. A loss-of-function mutation (ctr1-1) in the CTR1 gene leads to a strong constitutive triple-response phenotype, which acts as if the plant had constantly been exposed to ethylene. However, in this ctr1-1 Arabidopsis mutant, a higher level of ERF110 protein was found (Fig. 5). The enhanced accumulation of the ERF110 protein in ctr1-1 may result from the suppression of Ubiquitin/26S proteasome-mediated protein degradation by constitutive ethylene signaling. This speculation is supported by western-blot analysis of an ethylene-insensitive mutant, etr1-1, in which the ERF110 protein appears to be dramatically decreased (data not shown) compared with that of the wild type. An unexpected finding from western-blot analysis of ctr1-1 was that Ser-62 phosphorylation of ERF110 could not be detected (Figs. 4 and 5). The diminished Ser-62-phosphorylated isoform in ctr1-1 might be attributed to two factors: it may result from an increase in phosphatase activities or a reduction in kinase activities or from the combined effects of both enzymes on the Ser-62 phosphosite that are indirectly activated by the ctr1-1 mutation. Alternatively, the Ser-62 phosphosite motif of ERF110 may serve as a direct substrate for CTR1 kinase. The in vitro kinase assays that include an excess of synthetic substrate, as shown in Figure 1, suggest that a functional ethylene perception is involved in the regulation of Ser-62-specific kinase/phosphatase activities (Fig. 1).
Because ctr1-1 has an enhanced level of the ERF110 protein and its bolting time is also delayed, it was speculated that an ERF110 overexpression transgenic plant, Pro35S-ERF110WT::Col-0, may confer delayed bolting. The ERF110 protein was overexpressed 100-fold or more (Supplemental Fig. S5) in this transgenic plant; however, it did not produce a significant difference in the bolting time compared with that of the wild type (19.8 ± 0.18 versus 19.6 ± 0.22 d; Table I; Supplemental Table S2) in the presence of AOA, which indicates that the overexpression of ERF110 alone does not delay bolting time. In contrast, physiological studies have shown that ethylene (or ACC) delays the bolting of Pro35S-ERF110WT::Col-0 transgenic and wild-type plants for 3 d (Fig. 6; Table I; Supplemental Table S2). Together with ethylene-reduced Ser-62 phosphorylation, the phosphorylated ERF110 isoform and other flowering homeotic regulators are postulated to act together to control the bolting phenotype (Figs. 1, 5, and 8). Therefore, it is proposed that, under ambient air conditions (or at the basal level of ethylene), ethylene signaling produces sufficient Ser-62-phosphorylated ERF110 isoform (Supplemental Fig. S5) to maintain a normal bolting time, whereas the application of a higher level of ethylene to wild-type, ctr1-1, and erf110 plants leads to reduced Ser-62-phosphorylated ERF110 isoform and the repression of the bolting-stimulatory function of other regulators, which results in delayed bolting. The finding that both the ctr1-1 mutant and the Pro35S-ERF110WT::Col-0 transgenic plant exhibit delayed bolting after ethylene treatment strongly suggests that other CTR1- and ERF110-independent ethylene signaling pathway(s) may participate in the delayed-bolting response. Thus, the Ser-62-phosphrylated isoform of ERF110 is necessary but not sufficient for the normal bolting phenotype.
Figure 8.
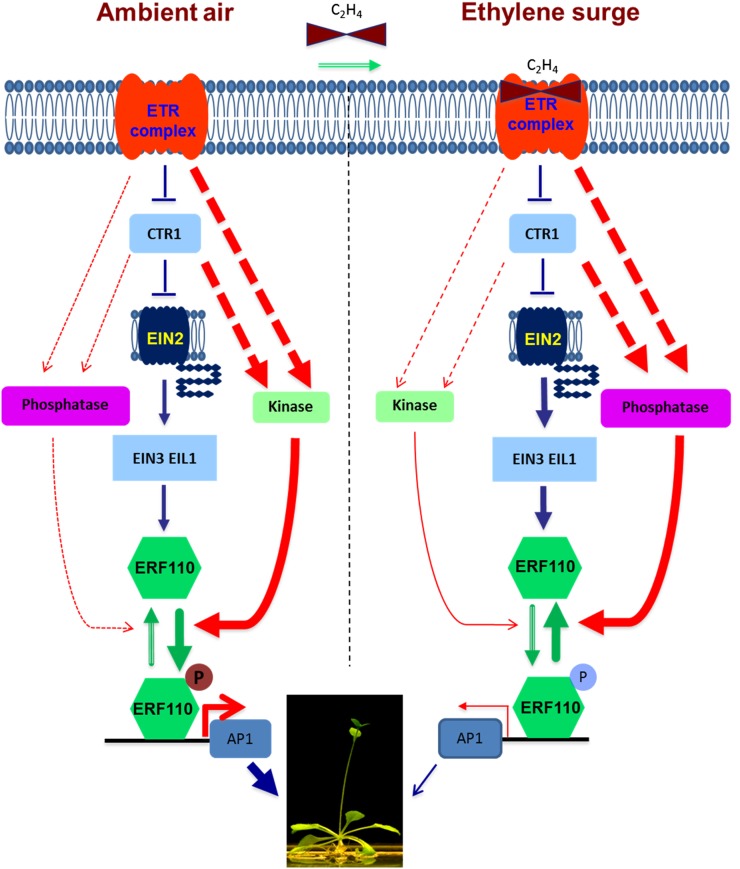
The mode of action for ethylene in the regulation of a Ser-62-phosphorylated ERF110 isoform during Arabidopsis bolting. Arrows represent positive effects, whereas stop signs represents inhibitory effects. Red dashed lines represent regulation by kinase or phosphatase enzymatic activity, whereas blue lines represent the regulation of gene expression. Green lines stand for a conformational change due to a PTM isoform or ligand binding. The relative strength of a particular regulation, either negative or positive, is indicated by the thickness of the line. Under ambient air conditions, CTR1-driven kinase activity dominated over phosphatase activity, leading to an accumulation of Ser-62-phosphorylated ERF110 isomer produced from the basal-level expression of ERF110 protein and thus maintaining a normal bolting time. In contrast, phosphatase activity dominated over kinase activities under higher levels of ethylene exposure when ethylene surges caused stress induction or developmental induction, leading to an overall decrease in the phosphorylation level on ERF110, and the dephosphorylation of ERF110 delays the bolting time in Arabidopsis. In both situations, kinase and phosphatase counteract each other in the regulation of Ser-62 phosphorylation of ERF110 and mediate a dual-and-opposing effect on bolting time under ethylene exposure.
To demonstrate how ethylene delays bolting by down-regulating the level of the Ser-62-phosphorylated isoform of ERF110, both phosphorylation-mimetic (S62D) and dephosphorylation-mimetic (S62A) mutants of ERF110 were overexpressed in wild-type Arabidopsis (Supplemental Fig. S9). Because the expression level of endogenous ERF110 is not affected by AOA treatment (Fig. 4), it is expected that a certain amount of endogenous phosphorylated ERF110 present in plant cells in the presence of AOA is sufficient to initiate normal bolting. Therefore, no significant difference in bolting was observed between S62A and S62D overexpression transgenic mutant plants treated with AOA (Table I). However, after AOA+ACC treatment, the endogenous phosphorylated ERF110 was reduced dramatically by a higher dose of ethylene (Fig. 5). The S62D mutant, therefore, provided sufficient phosphorylation-mimetic ERF110 to promote the bolting process and to rescue the ethylene-delayed bolting phenotype (Fig. 6).
Because AP1 interacts with LEAFY (Putterill et al., 2004; Quesada et al., 2005) and ERF and AP2 genes mutually regulate each other (Ogawa et al., 2007), it was believed that ERF110 acts upstream of AP1. qRT-PCR results supported this linear epistatic relationship between AP1 gene expression and the phosphorylated isoform of ERF110 (Figs. 7 and 8; Supplemental Fig. S7). Because AP1 is a positive regulator of flowering time (Irish and Sussex, 1990), the positive correlation between AP1 mRNA and the Ser-62-phosphorylated ERF110 suggests that the latter regulates bolting time by up-regulating the former (Fig. 7). Overexpression of the positive flowering regulator CONSTANS (CO) partially rescued the delayed-bolting phenotype in the ein2 mutant background (Samach et al., 2000), suggesting that EIN2 acts upstream of CO to control flowering gene expression. The ethylene-responsive element-binding protein (AtEBP) is positively involved in flower development (Ogawa et al., 2007). It has been reported that AtEBP transcripts are up-regulated by ethylene treatment and down-regulated in the ein2-1 mutant (Ogawa et al., 2005). Therefore, it is reasonable that overexpression of the ERF110 transcription factor alone without the participation of CO and AtEBP flower homeotic regulatory factors is unable to promote normal bolting in the EIN2-deficient background. Thus, overexpression of both wild-type ERF110 and the S62D mutant in ein2-5 cannot rescue its severely delayed bolting phenotype.
The role of ethylene in the regulation of Arabidopsis bolting is a controversial issue. The constitutive ethylene-response mutant ctr1-1 and the ethylene-insensitive mutants etr1-1, ein2-5, and ein2-T exhibit delayed bolting, as do the loss-of-function erf110-1 and erf110-2 mutant lines (Fig. 3; Supplemental Table S2). The increase in the number of rosette leaves at the time of bolting, which serves as an indicator of floral transition, suggests that all of these mutants delayed bolting via floral transition rather than a slower growth rate (Supplemental Table S2). The delayed-bolting phenotype of etr1-1 and ein2-5 mutants may be partially attributed to the suppressed gene expression of ERF110 and other flowering homeotic genes by the ethylene insensitivity of mutants (Supplemental Fig. S8). Any mutation that affects the gene expression of ERF110 will eventually affect the level of Ser-62 phosphorylation, leading to delayed bolting. This conclusion is consistent with the delayed-bolting phenotype of ERF110-deficient transgenics, erf110-1 and erf110-2 (Fig. 3). The triple-response phenotype of ethylene-treated etiolated seedlings of erf110-1 and erf110-2 indicates that the Ser-62-phosphorylated ERF110 isoform plays a different role in the regulation of bolting and the etiolated hypocotyl elongation (Supplemental Fig. S10).
The immediate ethylene biosynthesis precursor ACC has recently been suggested to play an ethylene-independent biological role in plants (Tsang et al., 2011). However, the direct application of ethylene gas to wild-type and ein2-5 mutant plants in our experiments still resulted in a decrease in both the Ser-62-dephosphorylated isoform of ERF110 (Li et al., 2012) and kinase activity (or increased phosphatase activity) toward Ser-62 of ERF110 (Fig. 1), which is consistent with the effect of ACC on the Ser-62 dephosphorylation of this transcription factor in Arabidopsis (Supplemental Fig. S6). Thus, the ACC-delayed bolting phenotype is believed to result from the ethylene-dependent signaling pathways rather than ACC-dependent signaling pathways. It is possible that the delayed bolting reported here may actually result from both signaling pathways. Treatment of a complete ACC oxidase loss-of-function mutant with ACC will help differentiate the possible dual roles of ACC between its direct influence on Arabidopsis bolting and its indirect effect through ethylene.
The dual-and-opposing effect of ethylene has been reported previously in shoot negative gravitropism (Lu et al., 2001; Li, 2008). The dual effect of ethylene on the production of the Ser-62-phosphorylated ERF110 isomer is the second example of a “yin and yang effect” of ethylene on the regulation of growth and development. Abscisic acid also plays an inhibitory role in the flowering of the short-day plant P. nil after long-term treatment, whereas it promotes the flowering of P. nil after short-term treatment (Takeno and Maeda, 1996). Our study indicates that the effect of ethylene on bolting appears to be achieved by the dynamic interplay between two separate pathways: EIN2-dependent gene expression and EIN2-independent PTM (Fig. 8). Ethylene also represses Ser-62 phosphorylation in ein2-5, which further confirms that ethylene-repressed phosphorylation is independent of the function of EIN2. This is consistent with the finding that long-term ethylene treatment decreases overall protein phosphorylation in ein2-5 (Li et al., 2009). The kinase activity that phosphorylates the Ser-62 phosphosite might act similarly to the MKK9-MPK3/6 cascade, which phosphorylates the EIN3 protein. The dephosphorylation of the Ser-62 phosphosite, elicited by a higher dose of ethylene, might result from ethylene-enhanced phosphatase activity. As protein phosphatase 2A is known to destabilize ACS6 via dephosphorylation of the ACC synthase C terminus (Skottke et al., 2011), this complex may be involved in the dephosphorylation of ERF110.
By integrating these molecular genetic and biochemical results, we hereby propose a molecular model for the mechanistic pathways of signaling that underlie the dual-and-opposing effect of ethylene on Arabidopsis bolting (Fig. 8). Under a low level of endogenous ethylene or ambient air conditions, the kinase cascades may promote the production of Ser-62-phosphorylated ERF110, which consequently increases the gene expression of AP1 to promote bolting. In contrast, a higher level of ethylene reduces the overall molar amount of the Ser-62-phosphorylated isoform of ERF110, although it increases the overall ERF110 transcript and protein levels. Thus, the final outcome leads to a decrease in AP1 gene expression, which then delays the bolting. Generally speaking, the dual-and-opposing effect of ethylene on bolting is a consequence of dynamic balancing between ethylene down- and up-regulated PTM and transcription, respectively, in the production of the Ser-62-phosphorylated ERF110 isoform that controls the abundance of AP1 mRNA. In particular, the effect of the phosphorylation-mimetic ERF110 gene observed after treatment with ACC suggests an involvement of multiple regulatory factors in bolting. The Ser-62-phosphorylated isoform of ERF110 is necessary but not sufficient for the regulation of bolting. Based on these findings, it is anticipated that a cyclically periodic application or in vivo production of ethylene might promote bolting in plants, because the ERF110 protein could be induced first by a surge of ethylene exposure and then phosphorylated under ambient air conditions (lower level of ethylene). Because ethylene delays bolting similarly in both Murashige and Skoog (M/S)-salt medium and soil among ethylene-response mutants (Hua and Meyerowitz, 1998; Ogawara et al., 2003; Achard et al., 2007), such a periodic and cyclic exposure to ethylene in some crops may help increase agricultural productivity.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
etr1-1 is a dominant negative ethylene-insensitive mutant and encodes a mutated ethylene receptor that blocks ethylene perception, whereas ctr1-1 is a constitutive triple-response mutant that acts as if it were constantly exposed to ethylene. ein2-5 and ein2-T have a 7-bp deletion and a transfer DNA insertion in the master ethylene-signaling gene, EIN2, respectively. The wild-type Arabidopsis (Arabidopsis thaliana), ein2-T, and ctr1-1 mutants were obtained from the Arabidopsis Biological Resource Center. ein2-5 and etr1-1 were gifts from Dr. Joseph Ecker and Dr. Elliot Meyerowitz, respectively. Arabidopsis seedlings were grown on M/S basal medium (Sigma) plus Suc at 23°C ± 1°C with a 14/10-h light/dark regime under a light intensity of 150 to 250 µmol photons m−2 s−1. The plants subjected to qRT-PCR analysis and/or western-blot analysis were 2-week-old seedlings.
Phenotypic Analysis
Transgenic or nontransgenic seeds were sterilized and grown on M/S plus Suc medium in a glass jar. ACC, the immediate ethylene precursor, was added to the M/S medium to provide continuous exposure to ethylene. AOA is an ACC biosynthesis inhibitor, which was used to suppress the majority of endogenous ethylene production. Bolting time was defined as the number of days after sewing until the first inflorescence stem reached 1 cm in height, and the number of rosette leaves was counted at the time of bolting. To perform statistical analysis, more than 15 individual plants from each mutant line were measured for each treatment. Student’s t test was used as the standard statistical method for analysis. To exclude the positional effect of insertion, at least two independent transformants from either ERF110 RNAi or ERF110 overexpression transgenic plants were included in the phenotypic characterization.
Chemicals and Reagents
Trypsin was purchased from Amersham Bioscience. Ammonium hydroxide, HPLC-grade methanol, and acetonitrile were purchased from Thermo Fisher Scientific. Dithiothreitol (DTT), acrylamide, and bis-acrylamide were purchased from Bio-Rad. C18 Zip-tips were purchased from Millipore. Synthetic peptides were purchased from GL Biochem. Nickel-nitrilotriacetic acid (Ni2+-NTA) agarose beads were purchased from Qiagen. Dynabeads M-280 streptavidin, Trizol, and the SuperScriptIII First Strand synthesis kit were from Invitrogen. The GoTaq Real-Time PCR System for qRT-PCR was purchased from Promega. M/S basal salt mixture, Suc, trifluoroacetic acid (TFA), and other chemicals were purchased from Sigma-Aldrich.
In Vitro Kinase Assay and PTM Site Quantification Using iTRAQ Labeling and MS/MS Analysis
Both the in vitro kinase assay and synthetic peptide purification were performed according to a previously described method (Li et al., 2009). Total cellular proteins were extracted from the frozen fine powders of Arabidopsis tissues using a kinase extraction buffer containing 20 mm HEPES (pH 7.5), 150 mm NaCl, 1% Triton X-100, 2.5 mm sodium pyrophosphate, 1 mm sodium fluoride, 1 mm sodium orthovanadate, 1 mm sodium molybdate, 1 mm glycerol 2-phosphate, and 1 mm phenylmethanesulfonyl fluoride (PMSF) protease inhibitors mix (Roche). Plant cells were mixed with the kinase buffer at a ratio of 1:3 (w/v), which was incubated on ice for an additional 10 min. The plant kinase extract was then centrifuged for 10 min at 14,000g at 4°C to remove cell debris. Plant kinase extracts were activated by adding a one-fourth volume of kinase assay buffer (45% glycerol, 2.5 mm ATP, 50 mm MgCl2, and 125 µg mL−1 bovine serum albumin). Substrate peptides were added at a concentration of 10 µm. The kinase-substrate mixture was incubated at 30°C for 1 h. Peptides were purified using Ni2+-NTA beads (Qiagen) according to the manufacturer’s instructions. Following overnight digestion using trypsin at 37°C, the purified peptides were desalted with C18 Zip-tip columns and resuspended in 0.1% TFA for liquid chromatography (LC)-MS/MS analysis.
In the iTRAQ experiment, the purified ERF110 peptides containing the Ser-62 phosphosite (Supplemental Table S1) were first labeled with iTRAQ 4-Plex labeling reagent (Applied Biosystems) following the kinase assay. The isotope-labeled phosphopeptides were purified with titanium oxide beads according to the manufacturer’s instructions, desalted with C18 Zip-tip columns, and resuspended in 0.1% TFA for MALDI-MS/MS analysis using a Bruker Autoflex III MALDI-TOF/TOF MS/Dionex device. The iTRAQ experiment was repeated on the same plant at least twice with a reciprocal labeling of iTRAQ chemicals to exclude the difference brought about by the differential labeling process. The ion intensity of the reporter ion of the air-treated sample was set as 1, whereas the relative degree of change in the ethylene-treated sample was calculated against the intensity of its reporter ion from the air-treated peptide sample.
Overexpression of the Wild-Type and Mutated ERF110 Proteins
A 0.9-kb full-length genomic DNA encoding Arabidopsis ERF110 (GenBank accession no. JN819205) was amplified by PCR using the following primers: gERF-F, 5′-CATAGTCGACTCTGCCATGGTCTCGGCC-3′ (SalI underlined); gERF-R, 5′-TCATGGCGCGCCTGTATTAGGTAGAGAAGG-3′ (AscI underlined). Point mutations from Ser-62 to Ala-62 or Asp-62 were introduced into the ERF110 genomic DNA fragment using the following primers: gERF-A-F, 5′-CGTGTAGACGCTTCACATAATC-3′; gERF-A-R, 5′-GATTATGTGAAGCGTCTACACG-3′; gERF-D-F, 5′-CGTGTAGACGATTCACATAATC-3′; gERF-D-R, 5′-GATTATGTGAATCGTCTACACG-3′. Following AscI and SalI double digestion, all three DNA fragments were inserted into a modified binary vector pBI121 between a double cauliflower mosaic virus 35S promoter and HBH tag (The Arabidopsis Information Resource accession no. At5G16390) as described previously (Li et al., 2012). The HBH polypeptide tag was generated by one-step PCR using the primers HBH-F (5′-AAAGTCGACGGCGCGCCTCATCATCATCACCACCATCATCATCCAGCCAAATCGTCA-3′) and HBH-R (5′-AAAGAGCTCCTACTTAATTAAGGTACCATGATGATGGTGGTGATGATGATGCGGTTGAACCACAA-3′). The resulting recombinant binary vectors harboring ERF110WT-HBH, ERF110S62A-HBH, and ERF110S62D-HBH fusion genes were transformed into Arabidopsis wild-type background Col-0 via a routine floral dip protocol. Transgenic plants were selected from M/S basal medium supplemented with Suc and 50 mg L−1 hygromycin (Invitrogen).
Protein Extraction, Quantitation, and Determination of the in Vivo Phosphorylation Site
The frozen Arabidopsis plant tissues (4 g) were ground to a fine powder with an ice-cold mortar and pestle. The fine tissue powder was extracted with 12 mL of urea extraction buffer (Guo and Li, 2011) containing 150 mm Tris-HCl, pH 7.6, 8 m urea, 0.1% SDS, 1.2% Triton X-100, 5 mm ascorbic acid, 50 mm DTT, 20 mm EDTA, 20 mm EGTA, 50 mm NaF, 1% glycerol 2-phosphate, 1 mm PMSF, 0.5% phosphatase inhibitor cocktail 2 (Sigma P5726), 0.5% protease inhibitor (complete EDTA free; Roche), and 2% polyvinylpolypyrrolidone (Guo and Li, 2011). The extract was centrifuged at 110,000g for 2 h at 10°C to remove cell debris. The total protein supernatant fraction was precipitated with 3 volumes of prechilled acetone:methanol (12:1). The protein pellet was collected by centrifugation and resuspended in protein resuspension buffer (50 mm Tris-HCl, pH 6.8, 8 m urea, 50 mm DTT, 20 mm EDTA, and 2% SDS). The resulting protein extract was then used either for western-blot analysis or purification of overexpressed ERF110 protein from plant cells to determine in vivo phosphorylation sites and phosphorylation occupancy (Li et al., 2012).
The overexpressed ERF110 was purified by tandem affinity purification as described previously (Li et al., 2012). The protein extract underwent a standard Ni2+-NTA beads (Qiagen) purification procedure. Proteins were eluted three times with 1 mL of buffer B (8 m urea, 200 mm NaCl, 10 mm sodium phosphate, 0.2% SDS, 100 mm Tris, and 250 mm imidazole) and loaded onto immobilized streptavidin magnetic beads (Invitrogen). The protein-beads mixture was incubated overnight at room temperature and washed three times with 1 mL of buffer C (8 m urea, 200 mm NaCl, 0.2% SDS, and 100 mm Tris, pH 8.0). Biotin-labeled protein was eluted using 1× SDS loading buffer containing 30 mm d-biotin at 96°C for 15 min and chilled on ice quickly. The resulting ERF110 fusion proteins were separated by SDS-PAGE, and the corresponding band of ERF110 was sliced out, followed by a standard in-gel trypsin digestion protocol. The digested peptides were desalted with C18 Zip-tip columns and resuspended in 0.1% TFA for later LC-MS/MS analysis (Li et al., 2009).
To compare the relative abundance of endogenous ERF110 protein among different treatments and mutants, 100 µg of total cellular protein from each sample was loaded onto a 12% SDS-PAGE gel followed by western-blot analysis. The experiments were repeated on three independent biological samples. Both ERF110 and actin were detected either by homemade anti-ERF110 polyclonal antibodies or commercially available monoclonal antibody raised against actin (Sigma; catalog no. A0480). The exposed films were scanned using a densitometer, and the relative gray intensities of the target bands were read by ImageJ software. Within each experiment, the relative ERF110 abundance of the untreated wild-type sample was set as 1, and others were calculated by normalizing them against the amount of actin. To compare the relative abundance of phosphorylated ERF110, the targeted bands were detected with monoclonal antibody raised against the Ser-62-phosphorylated peptide. The integrated intensity of the protein bands of interest was measured using ImageJ. The relative abundance of phosphorylation was calculated following normalization against ERF110.
Construction of ERF110 RNAi Knockout Lines
To make ERF110 RNAi knockout lines, complementary DNA (cDNA) fragments encoding the ERF110 transcription factor were amplified by two sets of primers as follows: ERF110-BamHI-F (26–45 cDNA), 5′-CGCGGATCCCACAGGTGGTTTCTGCTCGC-3′; ERF110-BspHI-R (201–221), 5′-CGCTCATGAGCCTTGCTCATGGATTCTTCG-3′; ERF110-SacI-F, 5′-AACGAGCTCCACAGTGGTTTCTGCTCGC-3′; ERF110-PvuII-R, 5′-AACCAGCTGGCCTTGCTCATGGATTCTTCG-3′. The cDNA fragments were double digested with either the NcoI and BamHI or PvuII and SacI restriction endonucleases. The resulting fragments were then fused together in a head-to-head manner to generate a hairpin structure, which is driven by a constitutive cauliflower mosaic virus 35S promoter in a modified binary pBI121 vector (Guo et al., 2011). The binary construct was transformed into wild-type Col-0 Arabidopsis by Agrobacterium tumefaciens-mediated transfer during the floral dip. Transgenic plants were selected on M/S medium supplemented with 50 mg L−1 hygromycin.
Histochemical Analysis of the Promoter Activity of ERF110
A 0.5-kb ERF110 promoter region (–289 to =226 of the ERF110 genomic sequence) was amplified by PCR using primers 5′-CCCAAGCTTAAACGAAAGTGATAACATATATCA-3′ and 5′-CTAGTCCCAGGCCGTGCCAAACCTATT-3′. Following digestion by KpnI and NcoI, PCR products were inserted into a modified pCAMBIA1301 binary vector (digested by HindIII and NcoI) and fused with a GUS reporter gene. ProERF110-GUS was introduced into wild-type Col-0 Arabidopsis by floral dip. Transgenic plants were selected on an M/S agar plate with 25 mg L−1 hygromycin. More than 15 seedlings of the T2 generation propagated from three independent transgenic lines were grown for further analysis. They showed identical GUS stain patterns for each time point of the treatments. The GUS assay was performed to quantify GUS activity after different treatments on three sets of independent biological samples. The histochemical GUS staining and assay followed a previously described method (Wang et al., 2005a).
Immunoprecipitation of the Endogenous ERF110
Frozen Arabidopsis plant tissue (1 g) was ground to a fine powder with an ice-cold mortar and pestle. The fine tissue powder was extracted with 3 mL of extraction buffer containing 50 mm sodium phosphate, pH 7.6, 1% Nonidet P-40, 5 mm EDTA, 5 mm EGTA, 50 mm NaF, 1% glycerol 2-phosphate, 20 mm sodium pyrophosphate, 10 mm sodium vanadate, 10 mm sodium molybdate, 10 mm sodium tartrate, 1 mm PMSF, 0.5% phosphatase inhibitor cocktail 2 (Sigma), 0.5% protease inhibitor (complete EDTA free; Roche), and 2% polyvinylpolypyrrolidone. The extract was centrifuged at maximum speed in a bench-top centrifuge for 10 min at 4°C to remove cell debris. The lysate was then incubated with 60 μL of N-hydroxysuccinimide-activated beads that had been conjugated with homemade anti-ERF110 polyclonal antibodies for 2 h at 4°C. After a brief washing with the extraction buffer three times, the endogenous ERF110 was eluted with 100 μL of SDS-PAGE loading buffer and loaded onto an SDS-PAGE gel to perform western-blot analysis. Anti-ERF110 antibodies were raised in rabbits using His6-ERF110 as the antigen. Custom-made monoclonal antibody against the phosphopeptide ARVDpSSHNPIEESM was purchased from GenScript and diluted at a ratio of 1:200 (Kim et al., 2009; Oh et al., 2009). The specificity of this monoclonal antibody was determined using dot-blot analysis on 100 ng each of both phosphorylated and nonphosphorylated synthetic peptides.
Molecular Biological Analysis of the Expression of ERF110 and Other Flowering-Related Genes
Two-week-old plant tissues were selected for qRT-PCR analysis. RNA samples were extracted by Trizol (Invitrogen). qRT-PCR was performed using the SuperScriptIII First Strand synthesis kit (Invitrogen). GoTaq qPCR master mix was purchased from Promega for qRT-PCR under the following conditions: 95°C for 2 min; 60 cycles of 95°C for 3 s and 59°C for 30 s; then 60°C to 95°C. Gene-specific primers were used. The Actin gene was used as an internal control, and sequences of primers were as follows: ERF110-F, 5′-ACGGTGGCGAATAAAGCAGAAGAG-3′; ERF110-R, 5′-GGCAGAGGTTGTTCCATTGGTGAA-3′; Actin-F, 5′-GACCAGCTCTTCCATCGAGAA-3′; Actin-R, 5′-TCTCGTGGATTCCAGCAGC-3′; AP1-F, 5′-CAGCATAACCAAGGCCACAA-3′; AP1-R, 5′-CATTCCTCCTCATTGCCATTG-3′.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. MS/MS spectra of phosphorylated peptides.
Supplemental Figure S2. MS spectra of iTRAQ-labeled ERF110 phosphopeptides.
Supplemental Figure S3. Spectra of iTRAQ reporter ions.
Supplemental Figure S4. GUS histochemical staining of ProERF110-GUS transgenic plants.
Supplemental Figure S5. Fold increase in ERF110 protein level in transgenic lines.
Supplemental Figure S6. In vivo phosphorylation status of ectopically overexpressed ERF110.
Supplemental Figure S7. Screening for downstream flowering gene for ERF110.
Supplemental Figure S8. Levels of endogenous ERF110 protein among ein mutants.
Supplemental Figure S9. Ectopic expression of ERF110 mutants.
Supplemental Figure S10. Triple response of ERF110-RNAi lines.
Supplemental Table S1. Sequences of peptides used for in vitro kinase assay.
Supplemental Table S2. Bolting time of the wild type and etr mutants upon AOA and ACC treatments.
Supplemental Table S3. GUS activity.
Glossary
- MAPKKK
mitogen-activated protein kinase
- MS/MS
tandem mass spectrometry
- iTRAQ
isobaric tags for relative and absolute quantitation
- MALDI
matrix-assisted laser desorption/ionization
- Col-0
ecotype Columbia
- RNAi
RNA-interfering
- ACC
1-aminocyclopropane-1-carboxylic acid
- AOA
aminooxyacetic acid
- 4-MU
4-methylumbelliferone
- qRT
quantitative real-time reverse transcription
- PTM
posttranslation modification
- M/S
Murashige and Skoog
- DTT
dithiothreitol
- Ni2+-NTA
nickel-nitrilotriacetic acid agarose
- TFA
trifluoroacetic acid
- PMSF
phenylmethanesulfonyl fluoride
- LC
liquid chromatography
- cDNA
complementary DNA
- HBH
His8-biotin-His8
References
- Abeles FB, Morgan PW, Saltveit ME., Jr (1992) Ethylene in Plant Biology. Academic Press, New York [Google Scholar]
- Achard P, Baghour M, Chapple A, Hedden P, Van Der Straeten D, Genschik P, Moritz T, Harberd NP. (2007) The plant stress hormone ethylene controls floral transition via DELLA-dependent regulation of floral meristem-identity genes. Proc Natl Acad Sci USA 104: 6484–6489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Solano R, Wisman E, Ferrari S, Ausubel FM, Ecker JR. (2003) Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proc Natl Acad Sci USA 100: 2992–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM, Mortimore LA, Stepanova AN, Ecker JR, Bleecker AB. (2004) Short-term growth responses to ethylene in Arabidopsis seedlings are EIN3/EIL1 independent. Plant Physiol 136: 2921–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Binder BM, Garrett WM, Tucker ML, Chang C, Cooper B. (2011) Proteomic responses in Arabidopsis thaliana seedlings treated with ethylene. Mol Biosyst 7: 2637–2650 [DOI] [PubMed] [Google Scholar]
- Chen YF, Etheridge N, Schaller GE. (2005) Ethylene signal transduction. Ann Bot (Lond) 95: 901–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente van Bentem S, Mentzen WI, de la Fuente A, Hirt H. (2008) Towards functional phosphoproteomics by mapping differential phosphorylation events in signaling networks. Proteomics 8: 4453–4465 [DOI] [PubMed] [Google Scholar]
- Dukovski D, Bernatzky R, Han S. (2006) Flowering induction of Guzmania by ethylene. Sci Hortic (Amsterdam) 110: 104–108 [Google Scholar]
- Durek P, Schmidt R, Heazlewood JL, Jones A, MacLean D, Nagel A, Kersten B, Schulze WX. (2010) PhosPhAt: the Arabidopsis thaliana phosphorylation site database. An update. Nucleic Acids Res 38: D828–D834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble RL, Qu X, Schaller GE. (2002) Mutational analysis of the ethylene receptor ETR1: role of the histidine kinase domain in dominant ethylene insensitivity. Plant Physiol 128: 1428–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZY, Chen YF, Randlett MD, Zhao XC, Findell JL, Kieber JJ, Schaller GE. (2003) Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J Biol Chem 278: 34725–34732 [DOI] [PubMed] [Google Scholar]
- Guo D, Wong WS, Xu WZ, Sun FF, Qing DJ, Li N. (2011) Cis-cinnamic acid-enhanced 1 gene plays a role in regulation of Arabidopsis bolting. Plant Mol Biol 75: 481–495 [DOI] [PubMed] [Google Scholar]
- Guo GY, Li N. (2011) Relative and accurate measurement of protein abundance using 15N stable isotope labeling in Arabidopsis (SILIA). Phytochemistry 72: 1028–1039 [DOI] [PubMed] [Google Scholar]
- Guo HW, Ecker JR. (2004) The ethylene signaling pathway: new insights. Curr Opin Plant Biol 7: 40–49 [DOI] [PubMed] [Google Scholar]
- Hernández Sebastià C, Hardin SC, Clouse SD, Kieber JJ, Huber SC. (2004) Identification of a new motif for CDPK phosphorylation in vitro that suggests ACC synthase may be a CDPK substrate. Arch Biochem Biophys 428: 81–91 [DOI] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM. (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94: 261–271 [DOI] [PubMed] [Google Scholar]
- Irish VF, Sussex IM. (1990) Function of the apetala-1 gene during Arabidopsis floral development. Plant Cell 2: 741–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju C, Yoon GM, Shemansky JM, Lin DY, Ying ZI, Chang J, Garrett WM, Kessenbrock M, Groth G, Tucker ML, et al. (2012) CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc Natl Acad Sci USA 109: 19486–19491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick MD, Chang C. (2008) Ethylene signaling: new levels of complexity and regulation. Curr Opin Plant Biol 11: 479–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW, Guan SH, Sun Y, Deng ZP, Tang WQ, Shang JX, Sun Y, Burlingame AL, Wang ZY. (2009) Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat Cell Biol 11: 1254–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Peeters AJM. (1997) Floral transition mutants in Arabidopsis. Plant Cell Environ 20: 779–784 [Google Scholar]
- Kulikowska-Gulewska H, Kopcewicz J. (1999) Ethylene in the control of photoperiodic flower induction in Pharbitis nil Chois. Acta Soc Bot Pol 68: 33–37 [Google Scholar]
- Levy YY, Dean C. (1998) The transition to flowering. Plant Cell 10: 1973–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wong WS, Zhu L, Guo HW, Ecker J, Li N. (2009) Phosphoproteomic analysis of ethylene-regulated protein phosphorylation in etiolated seedlings of Arabidopsis mutant ein2 using two-dimensional separations coupled with a hybrid quadrupole time-of-flight mass spectrometer. Proteomics 9: 1646–1661 [DOI] [PubMed] [Google Scholar]
- Li N. (2008) The dual-and-opposing-effect of ethylene on the negative gravitropism of Arabidopsis inflorescence stem and light-grown hypocotyls. Plant Sci 175: 71–86 [Google Scholar]
- Li Y, Shu Y, Peng C, Zhu L, Guo G, Li N. (2012) Absolute quantitation of isoforms of post-translationally modified proteins in transgenic organism. Mol Cell Proteomics 11: 272–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FY, Chang YS. (2011) Effects of shoot bending on ACC content, ethylene production, growth and flowering of bougainvillea. Plant Growth Regul 63: 37–44 [Google Scholar]
- Liu YD, Zhang SQ. (2004) Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 16: 3386–3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu BW, Pei LK, Chan WK, Zhang H, Zhu G, Li JY, Li N. (2001) The dual effects of ethylene on the negative gravicurvature of Arabidopsis inflorescence, an intriguing action model for the plant hormone ethylene. Chin Sci Bull 46: 279–283 [Google Scholar]
- Lu BW, Yu HY, Pei LK, Wong MY, Li N. (2002) Prolonged exposure to ethylene stimulates the negative gravitropic responses of Arabidopsis inflorescence stems and hypocotyls. Funct Plant Biol 29: 987–997 [DOI] [PubMed] [Google Scholar]
- Morandell S, Stasyk T, Grosstessner-Hain K, Roitinger E, Mechtler K, Bonn GK, Huber LA. (2006) Phosphoproteomics strategies for the functional analysis of signal transduction. Proteomics 6: 4047–4056 [DOI] [PubMed] [Google Scholar]
- Nakano T, Suzuki K, Fujimura T, Shinshi H. (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140: 411–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Pan L, Kawai-Yamada M, Yu LH, Yamamura S, Koyama T, Kitajima S, Ohme-Takagi M, Sato F, Uchimiya H. (2005) Functional analysis of Arabidopsis ethylene-responsive element binding protein conferring resistance to Bax and abiotic stress-induced plant cell death. Plant Physiol 138: 1436–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Uchimiya H, Kawai-Yamada M. (2007) Mutual regulation of Arabidopsis thaliana ethylene-responsive element binding protein and a plant floral homeotic gene, APETALA2. Ann Bot (Lond) 99: 239–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawara T, Higashi K, Kamada H, Ezura H. (2003) Ethylene advances the transition from vegetative growth to flowering in Arabidopsis thaliana. J Plant Physiol 160: 1335–1340 [DOI] [PubMed] [Google Scholar]
- Oh MH, Wang XF, Kota U, Goshe MB, Clouse SD, Huber SC. (2009) Tyrosine phosphorylation of the BRI1 receptor kinase emerges as a component of brassinosteroid signaling in Arabidopsis. Proc Natl Acad Sci USA 106: 658–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohme-Takagi M, Shinshi H. (1995) Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell 7: 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouaked F, Rozhon W, Lecourieux D, Hirt H. (2003) A MAPK pathway mediates ethylene signaling in plants. EMBO J 22: 1282–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putterill J, Laurie R, Macknight R. (2004) It’s time to flower: the genetic control of flowering time. Bioessays 26: 363–373 [DOI] [PubMed] [Google Scholar]
- Qiao H, Shen Z, Huang SS, Schmitz RJ, Urich MA, Briggs SP, Ecker JR. (2012) Processing and subcellular trafficking of ER-tethered EIN2 control response to ethylene gas. Science 338: 390–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada V, Dean C, Simpson GG. (2005) Regulated RNA processing in the control of Arabidopsis flowering. Int J Dev Biol 49: 773–780 [DOI] [PubMed] [Google Scholar]
- Raz V, Fluhr R. (1993) Ethylene signal is transduced via protein phosphorylation events in plants. Plant Cell 5: 523–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G. (2000) Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288: 1613–1616 [DOI] [PubMed] [Google Scholar]
- Schaller GE. (2012) Ethylene and the regulation of plant development. BMC Biol 10: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skottke KR, Yoon GM, Kieber JJ, DeLong A. (2011) Protein phosphatase 2A controls ethylene biosynthesis by differentially regulating the turnover of ACC synthase isoforms. PLoS Genet 7: e1001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Alonso JM. (2009) Ethylene signaling and response: where different regulatory modules meet. Curr Opin Plant Biol 12: 548–555 [DOI] [PubMed] [Google Scholar]
- Takeno K, Maeda T. (1996) Abscisic acid both promotes and inhibits photoperiodic flowering of Pharbitis nil. Physiol Plant 98: 467–470 [Google Scholar]
- Tsang DL, Edmond C, Harrington JL, Nühse TS. (2011) Cell wall integrity controls root elongation via a general 1-aminocyclopropane-1-carboxylic acid-dependent, ethylene-independent pathway. Plant Physiol 156: 596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchisaka A, Yu GX, Jin HL, Alonso JM, Ecker JR, Zhang XM, Gao S, Theologis A. (2009) A combinatorial interplay among the 1-aminocyclopropane-1-carboxylate isoforms regulates ethylene biosynthesis in Arabidopsis thaliana. Genetics 183: 979–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang NN, Shih MC, Li N. (2005a) The GUS reporter-aided analysis of the promoter activities of Arabidopsis ACC synthase genes AtACS4, AtACS5, and AtACS7 induced by hormones and stresses. J Exp Bot 56: 909–920 [DOI] [PubMed] [Google Scholar]
- Wang WY, Hall AE, O’Malley R, Bleecker AB. (2003) Canonical histidine kinase activity of the transmitter domain of the ETR1 ethylene receptor from Arabidopsis is not required for signal transmission. Proc Natl Acad Sci USA 100: 352–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Goshe MB, Soderblom EJ, Phinney BS, Kuchar JA, Li J, Asami T, Yoshida S, Huber SC, Clouse SD. (2005b) Identification and functional analysis of in vivo phosphorylation sites of the Arabidopsis BRASSINOSTEROID-INSENSITIVE1 receptor kinase. Plant Cell 17: 1685–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller JL, Hecht V, Liew LC, Sussmilch FC, Wenden B, Knowles CL, Vander Schoor JK. (2009) Update on the genetic control of flowering in garden pea. J Exp Bot 60: 2493–2499 [DOI] [PubMed] [Google Scholar]
- Wuriyanghan H, Zhang B, Cao WH, Ma B, Lei G, Liu YF, Wei W, Wu HJ, Chen LJ, Chen HW, et al. (2009) The ethylene receptor ETR2 delays floral transition and affects starch accumulation in rice. Plant Cell 21: 1473–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Tena G, Xiong Y, Sheen J. (2008) Dual control of nuclear EIN3 by bifurcate MAPK cascades in C2H4 signalling. Nature 451: 789–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Guo HW. (2011) Paradigms and paradox in the ethylene signaling pathway and interaction network. Mol Plant 4: 626–634 [DOI] [PubMed] [Google Scholar]