Summary: Significant interaction between thylakoid twin arginine transport pathway component Tha4 and a twin arginine transport precursor under transport conditions suggests the point of passage across the membrane for the precursor is membrane protein dependent.
Abstract
Proteins destined for the thylakoid lumen of chloroplasts must cross three membranes en route. The chloroplast twin arginine translocation (cpTat) system facilitates the transport of about one-half of all proteins that cross the thylakoid membrane in chloroplasts. Known mechanistic features of the cpTat system are drastically different from other known translocation systems, notably in its formation of a transient complex to transport fully folded proteins utilizing only the protonmotive force generated during photosynthesis for energy. However, key details, such as the structure and composition of the translocation pore, are still unknown. One of the three transmembrane cpTat components, Tha4, is thought to function as the pore by forming an oligomer. Yet, little is known about the topology of Tha4 in thylakoid, and little work has been done to detect precursor-Tha4 interactions, which are expected if Tha4 is the pore. Here, we present evidence of the interaction of the precursor with Tha4 under conditions leading to transport, using cysteine substitutions on the precursor and Tha4 and disulfide bond formation in pea (Pisum sativum). The mature domain of a transport-competent precursor interacts with the amphipathic helix and amino terminus of functional Tha4 under conditions leading to transport. Detergent solubilization of thylakoids post cross linking and blue-native polyacrylamide gel electrophoresis analysis shows that Tha4 is found in a complex containing precursor and Hcf106 (i.e. the cpTat translocase). Affinity precipitation of the cross-linked complex via Tha4 clearly demonstrates that the interaction is with full-length precursor. How these data suggest a role for Tha4 in cpTat transport is discussed.
The thylakoid membrane of plant chloroplasts possesses two systems working in parallel for the transport of soluble proteins across the bilayer and into the lumen, namely the chloroplast secretory system and the chloroplast twin arginine translocation (cpTat) system (Müller and Klösgen, 2005; Cline and Theg, 2007; Cline and Dabney-Smith, 2008; Albiniak et al., 2012). For both systems, proteins destined for the thylakoid lumen are encoded by nuclear genes, cytoplasmically translated as higher molecular mass precursor proteins containing targeting sequences, and imported into the chloroplast. However, lumen-targeting sequences on precursors directed to the cpTat system contain obligate twin Arg residues on the amino-proximal side of the hydrophobic core, and the precursors are transported in folded conformations. Both systems require energy to drive the translocation process, but the cpTat system relies solely on the transmembrane potential generated by the protonmotive force (PMF) of photosynthesis, whereas the secretory system also relies on ATP hydrolysis (Cline and Theg, 2007). It is estimated that roughly one-half of the lumen proteins contain twin-Arg signal peptides (Peltier et al., 2004; Sun et al., 2004), several of which are involved in photosynthetic processes, such as the 23-kD subunit of the oxygen evolving complex of PSII (OE23; Ifuku et al., 2011), OE17 (Yi et al., 2006), and subunit T of PSII (Kapazoglou et al., 1995) and subunit N of PSI (Haldrup et al., 1999), making the cpTat system a vital pathway for higher plants.
Twin arginine translocation (Tat) systems are also found in bacterial plasma membrane, and both thylakoid and bacteria serve as model systems for studies on the Tat pathway mechanism, each providing insight into different aspects of the mechanism and demonstrating important differences between the two. The cpTat machinery contains three membrane-bound components, Tha4, Hcf106, and cpTatC, with homologous proteins TatA, TatB, and TatC in bacteria, respectively. Tha4 and Hcf106 share sequence and structural homology. They both contain an N-terminal single-transmembrane region followed by a hinge region that connects to an amphipathic α-helix and a divergent C-terminal tail. However, they have distinct functions in the cpTat pathway (Sargent et al., 1999; Dabney-Smith et al., 2003). Hcf106 is largely found in complex with cpTatC, together composing the Tat receptor complex in the thylakoid that migrates as an approximately 700-kD complex by blue native (BN)-PAGE, whereas Tha4 is found as a separate homooligomeric complex of approximately 400 kD or less by BN-PAGE. cpTatC contains six transmembrane regions with the N and C termini on the stromal face of the membrane and serves as the initial receptor of the signal peptide in the receptor complex.
Translocation occurs in a cyclical fashion. Precursor binds to the cpTatC-Hcf106 receptor complex in an energy-independent manner. Then, in the presence of the PMF, which mainly consists of ∆pH in isolated thylakoids, Tha4 assembles with the precursor-bound receptor complex to form the active translocase. At this point, transport occurs. After transport, Tha4 dissociates from the receptor complex, thus resetting the system for subsequent rounds of translocation (Mori et al., 1999; Cline and Mori, 2001; Mori and Cline, 2002). This regulated assembly of Tha4 and its tight correlation to transport of the precursor suggests that Tha4 has a critical role in the translocation step.
Several models of the Tat translocase propose that Tha4 (TatA) serves as the protein-conducting channel. Several characteristics support this hypothesis, including a regulated assembly mechanism, the requirement for Tha4 only at the translocation step (Cline and Mori, 2001), the molar excess of Tha4 over cpTatC and Hcf106 (Mori et al., 2001; Celedon and Cline, 2012), oligomerization of Tha4 at the translocase (Dabney-Smith and Cline, 2009), and observations of channel-like structures of the Escherichia coli TatA in detergent extracts or even in vivo in E. coli cells (Gohlke et al., 2005; Sargent et al., 2006; Berthelmann et al., 2008). However, none of these studies demonstrate a direct interaction between precursor and Tha4 (TatA).
Studies on the E. coli Tat system demonstrate weak cross links between TatA and precursor but did not follow the interaction during active transport, as the UV-inducible cross linking occurred after transport (Maurer et al., 2010). The question still remains how Tha4 (TatA) is directly involved in the translocation event itself. If Tha4 serves the role of protein-conducting channel, one would expect that as the precursor passes through the channel it would interact with Tha4. To test this hypothesis, we have employed an alternative cross-linking strategy involving disulfide exchange cross linking by generating Cys-containing variants of both precursor and Tha4 in pea (Pisum sativum). This method allows probing of the interactions between precursor and Tha4 in the steps immediately prior to and during the transport of precursor, unlike other cross-linking methods employed previously. Through one-to-one disulfide bond formation between single Cys residues placed throughout the mature domain of pOE17 and Tha4, we determined that Tha4 is in direct contact with full-length precursor after its binding the receptor and immediately prior to or during transport across the membrane. BN-PAGE demonstrated that Cys-substituted Tha4 was able to relocate into the approximately 700-kD complex in the presence of Cys-substituted precursor, demonstrating that direct interaction between the two occurs as part of the active translocase. Moreover, site-specific Cys mutations allow us to determine, to our knowledge for the first time, the region of Tha4 in contact with precursor during transport. How these data affect current models for protein transport by the cpTat pathway are discussed.
RESULTS
Tha4 is thought to play a critical role in the transport of precursor by the cpTat pathway, but what that role is remains unclear. To investigate Tha4’s role in transport, we took advantage of the ability to functionally replace endogenous Tha4 with the Cys-substituted variants (Dabney-Smith et al., 2003; Dabney-Smith and Cline, 2009). In addition, we generated single Cys substitutions in the maize precursor, pOE17. The wild-type version of this protein lacks Cys residues. Sites for Cys substitution were determined by modeling the amino acid sequence for the maize (Zea mays) protein onto the crystal structure of the highly conserved homolog from spinach (Spinacia oleracea) mature OE17 (Balsera et al., 2005) using Phyre2 (Bennett-Lovsey et al., 2008). This allowed us to place Cys residues in surface-exposed or buried positions (Fig. 1A).
Figure 1.
Transport of precursors used in this study. A, Homology structure of maize OE17 modeled onto the structure of the conserved spinach OE17 (Protein Data Bank ID: 1VYK; Balsera et al., 2005) using PHYRE2 (Kelley and Sternberg, 2009) with Cys substitutions indicated in black. Structure was rendered using MacPyMol (Schrodinger, 2010). B, Transport of precursors used in this study before or after DTNB treatment (+) into isolated thylakoids, lacking exogenously added Tha4, as described in “Materials and Methods.” The wild-type precursor pOE17 was used as a control. The polylinker-containing form, pOE17XnC(G4S)3-His6 (p17GS3), lacks Cys, and the rest of the precursors are Cys substitutions at the indicated positions in p17GS3. Posttransport samples were divided, and one-half of them were treated with thermolysin (T’lysin; +). The precursor (p) and mature (m) forms of OE17 are indicated. Samples reflect equal chlorophyll loading and were analyzed by SDS-PAGE and fluorography. Molecular mass markers are indicated on the left. The image represents at least three separate experiments.
Transport of pOE17XnC
We tested the precursor to see if the Cys substitutions or the polylinker-His6 tag interfered with transport along the cpTat pathway (Fig. 1B). The addition of the polylinker-His6 tag (pOE17GS3) did not inhibit transport as compared with the native precursor pOE17 (lanes 1–3 and 6–8). In addition, pOE17GS3 containing single Cys substitutions at positions Asp-59 (lanes 11–13), Asp-68 (lanes 16–18), Ala-71 (lanes 21–23), Ser-84 (lanes 26–28), Lys-99 (lanes 31–33), Thr-115 (lanes 36–38), and Ala-137 (lanes 41–43) were tested for their ability to transport via the cpTat pathway. Polylinker addition and Cys substitutions did not abolish transport ability for any of the Cys variants tested, although for most precursor variants transport was between 60% and 90% of the wild-type value (Supplemental Fig. S1A).
Previous studies on precursor binding show multiple precursors bound to a Tat receptor (Tarry et al., 2009; Ma and Cline, 2010), so we employed a disulfide-exchange method, similar to that used to detect the presence of Tim11 at the mitochondrial inner membrane import site (Tokatlidis et al., 1996). Thiols on precursors were derivatized by DTNB [for 5,5′-dithiobis(2-nitrobenzoic acid)] to prevent disulfide bond formation between precursor proteins while permitting reaction with unmodified thiols on neighboring proteins (Habeeb, 1972). Transport of precursor after derivatization with DTNB is shown in Figure 1B for the pOE17GS3 Cys variants D59C (lanes 14 and 15), D68C (lanes 19 and 20), A71C (lanes 24 and 25), S84C (lanes 29 and 30), K99C (lanes 34 and 35), T115C (lanes 39 and 40), and A137C (lanes 44 and 45). The DTNB treatment did cause a decrease in transport that was also seen with the pOE17 (lanes 4 and 5) and pOE17GS3 (lanes 9 and 10) wild-type proteins, which lack Cys, suggesting that any DTNB interference with transport was indirect and not due to the derivatization itself (Supplemental Fig. S1A). In general, DTNB-derivatized pOE17GS3 Cys variants maintained transport competency of at least 50% of the wild-type proteins. All subsequent assays used these polylinker-containing, Cys-substituted precursors after DTNB derivatization. For convenience, those precursors are referred to as pOE17XnC, where X is the amino acid that was substituted with Cys at position n.
Affinity Precipitation of Tha4 with Cys Variant pOE17XnC
In order to promote the detection of a cross link between the precursor and Tha4 during the transport process, endogenous Tha4 was inactivated, through antibody pretreatment, followed by integration of recombinant, Cys-substituted Tha4 (Dabney-Smith et al., 2006), allowing functional replacement of the endogenous Tha4 with Tha4 containing single Cys substitutions at various positions throughout the protein (Dabney-Smith et al., 2006). The transport assay was initiated by addition of the precursor. All Cys-substituted Tha4 tested were able to support the transport of precursor (Supplemental Fig. S1B). Thylakoids were recovered and cross linking was determined by nickel affinity precipitation of the precursor and any attached proteins.
Initially, we surveyed potential interactions by generating mixtures of single Cys-substituted proteins to ascertain if the precursor could pull down Tha4, which has an apparent molecular mass of approximately 14 kD. Screens using pOE17K99C showed that the precursor coprecipitated with Tha4 containing Cys substitutions in the N terminus (residues 1–8) and the amphipathic helix (APH; residues 25–54) but not the transmembrane domain (TMD; residues 9–21), the hinge region (residues 22–24), or the C-tail (residues 55–82; Fig. 2A). The presence of the processed mature protein indicated the functionality of the cpTat system for transport, although there was variability in the level of transport when different Cys-substituted Tha4 were included. This variability of transport likely reflects the functional capability of the Cys-substituted Tha4 (Supplemental Fig. S1B). Nonetheless, all Tha4 tested were functional to at least 50% of wild-type Cys-less Tha4. When the Cys substitution was located in the C-tail of Tha4 (e.g. T59C, S63C, A65C, and T78C), no interaction was detected between Tha4 and the His-tagged protein (Fig. 2B, lanes 3 and 4). However, when the Cys substitution was located in the Tha4 APH, interaction with the His-tagged protein occurred, especially in the C-proximal portion of the APH (Fig. 2B, lanes 2, 6, 10, 11, 13, and 14), but not with a Cys that is predicted to be on the hydrophobic side of the APH (Fig. 2B, lane 12). We could not detect an interaction between the His-tagged protein and V13C, I14C, G16C, V17C, A18C, A19C, and P24C of Tha4 encompassing a portion of the TMD and the hinge (Fig. 2B, lanes 1 and 20–25). In addition, we saw reduced interaction between the precursor and the N-proximal portion of the APH (Fig. 2B, lanes 5 and 7–9). However, we did detect an interaction with Tha4 when the Cys was placed in the N terminus at positions Gly-5 and Leu-6 (lanes 15 and 16) or the N-proximal TMD region at positions Pro-9 and Leu-11 (lanes 18 and 19). Taken together, these data suggest precursor interaction with the C-proximal region of the APH and the N-terminal region of the TMD (Fig. 2A).
Figure 2.
pOE17K99C precipitates an extra band when in the presence of Cys-substituted Tha4. A, Amino acid sequence and schematic of Tha4. B, Thylakoids recovered from transport assays containing Cys-substituted precursor and Tha4 were coprecipitated as described in “Materials and Methods.” Single Cys substitutions in Tha4 are indicated across the top of the panels. Those separated by a comma indicate a mixture of two or more single Cys-substituted Tha4 in the assay. The mature (m) form of OE17 and Tha4 (Tha4) bands are indicated. All samples were analyzed by SDS-PAGE and fluorography. Panels in the top row are from gels run in parallel with lanes removed for ease of interpretation. The left panel in the bottom row is a separate experiment from the other two panels. Both rows of panels represent at least three separate experiments.
To confirm the presence of Tha4, we compared coprecipitation in transport assays containing tritiated forms of both precursor and Tha4 to transport assays containing unlabeled precursor and [3H]Tha4. As shown in Figure 3A, affinity precipitation of transport assays containing labeled or unlabeled precursor (pOE17K99C) yielded a radiolabeled band at 14 kD representing [3H]Tha4. In addition, single Cys substitutions at other sites on pOE17, such as pOE17S84C, pOE17D59C, pOE17D68C, and pOE17T115C, also resulted in the precipitation of Cys-substituted Tha4 (Fig. 3B). The interaction was dependent upon disulfide bond formation, because [3H]pOE17K99C was not able to precipitate a 14-kD band when wild-type Tha4 (lacking Cys) was present (Fig. 3C, lane 1), but it did precipitate Tha4 Cys variants Q36C and K46C (lanes 2 and 3). In addition, neither labeled nor unlabeled Cys-less pOE17GS3 was able to precipitate Tha4F48C (lanes 4 and 5), which was precipitated by labeled and unlabeled pOE17K99C (lanes 6 and 7). Taken together, these data indicate that the 14-kD band is Tha4 interacting with the precursor protein in a disulfide bond-dependent manner.
Figure 3.
Tha4 affinity precipitation is Cys dependent. A, Affinity precipitation as described in “Materials and Methods.” Even numbered lanes demonstrate affinity precipitation using 3H-labeled precursor as indicated, whereas odd numbered lanes demonstrate affinity precipitation using unlabeled precursor as indicated. The Cys-substituted precursor pOE17K99C was used to precipitate the indicated Cys-substituted [3H]Tha4 (Tha4K46C or Tha4K53C). The processed mature (m) form of OE17 and Tha4 are indicated. Lane 1 represents 2.5% of the Tha4 translation product added to thylakoids. “tp” indicates 0.025% of Tha4 translation product used in the assay. B, Different Cys-substituted precursors (pOE17S84C, pOE17D59C, pOE17D68C, and pOE17T115C) were used to precipitate [3H]Tha4K53C or [3H]Tha4F48C as indicated. C, Left panel, wild-type [3H]Tha4 (WT), lacking Cys residues, was not precipitated by pOE17K99C (lane 1), whereas [3H]Tha4Q36C and [3H]Tha4K46C were (lanes 2 and 3). Right panel, precipitation of [3H]Tha4F48C using pOE17GS3 (lanes 4 and 5) or pOE17K99C (lanes 6 and 7). Images are from gels run in parallel with lanes removed for ease of interpretation. The panels shown are representative of at least three separate experiments.
Precursor Interaction with Tha4 Occurs at the Translocase and Requires the PMF
To determine if the interaction was “on pathway” or a nonspecific interaction, we investigated whether the interaction would occur in the absence of transport. Treatment of the thylakoids with an antibody to Hcf106 strongly inhibits binding, with a concomitant decrease in transport, of the precursor (Ma and Cline, 2000), and disruption of the PMF by addition of the ionophores nigericin and valinomycin prevents transport of the precursor (Cline et al., 1992). Both treatments not only inhibited the transport of precursor but also prevented the affinity precipitation of Tha4 by precursor (Fig. 4). To eliminate a possible nonspecific interaction between precursor and Tha4 during the membrane solubilization and precipitation steps, N-ethylmaleimide was added to quench unreacted free thiols at the end of the transport reaction. The N-ethylmaleimide quench did not interfere with the coprecipitation of Tha4 (Supplemental Fig. S2A). Taken together, these data indicate that the precursor-Tha4 interaction occurs at the translocase and that the interaction is on pathway, occurring after the binding step, and immediately prior to or during the translocation step.
Figure 4.

Tha4 affinity precipitation occurs during active transport. Affinity precipitation is shown for Tha4K48C after inhibition of the transport of precursor using thylakoids that were pretreated with the antibody to Hcf106 (α-Hcf106 [+]; left panel) or the ionophores nigericin and valinomycin (Nig/Val [+]; right panel). The mature form of OE17 (m) and Tha4 are indicated. Molecular mass is indicated on the left. Panels represent three separate experiments and gels run in parallel with lanes removed for ease of interpretation.
To further characterize the precursor-Tha4 interaction, we performed time-course assays for cross linking between Cys-substituted precursor (K99C or T115C) and Tha4K53C (Fig. 5). Previous work in cpTat transport indicates that there is a lag in the transport of precursor of up to 2 min before Tha4 is assembled with the receptor and transport occurs (Mori and Cline, 2002; Celedon and Cline, 2012). As expected, transport of either pOE17K99C (Fig. 5, left panel) or pOE17T115C (Fig. 5, right panel) precursors resulted in an increase of the processed, mature form over time. Both precursors exhibited similar transport kinetics such that the presence of the mature protein increases from approximately 2% to 3% after transport for 2 min to approximately 10% after transport for 10 min (Fig. 5). Interestingly, the amount of Tha4 that is coprecipitated over the same time frame increases as well. When using the pOE17K99C precursor, 5.6% of integrated Tha4 was precipitated after transport for 2 min, increasing to 17% after transport for 10 min; while for the pOE17T115C precursor, 4% of integrated Tha4 was precipitated after transport for 2 min, increasing to 12% after transport for 10 min. Taken together, we conclude from these data that the precursor-Tha4 interaction in thylakoids is time dependent and related to the transport process.
Figure 5.
Tha4 coprecipitation increases as transport proceeds. Samples were removed at 2, 5, and 10 min after the initiation of transport as indicated. Samples were diluted with ice-cold buffer and subjected to immediate centrifugation. Coprecipitation was as described for Figure 2. The percentage added was calculated as the ratio of the radioactivity in the bands after coprecipitation (mOE17GS3 or Tha4) to the radioactivity of added precursor (mature) or integrated Tha4, respectively, by gel band extraction (Cline, 1986) and represents the mean of three separate experiments. Molecular mass markers are to the left and protein indicators (m = mature form; Tha4) are to the right of the images. Panels represent two separate experiments repeated three or more times. Samples were analyzed by SDS-PAGE and fluorography.
Cys-Substituted Precursor Interacts Mainly with the APH of Tha4
Tat pathway precursors are typically transported in a folded conformation, placing the K99C variant in a loop region of the four-helix bundle that is mOE17 (Fig. 1A). Based on our survey with pOE17K99C, we observed that Cys substitutions within the APH of Tha4 had a higher propensity to cross link to the precursor than residues in the C-tail (Fig. 2). To test if this was specific to the Cys variant of precursor used or a general feature of a transporting precursor, we performed transport assays and affinity precipitation using additional single Cys-substitution variants of precursor based on the structure of the folded mature domain, both solvent exposed (pOE17D59C, pOE17D68C, pOE17S84C, and pOE17T115C) and buried (pOE17A71C and pOE17A137C; Fig. 1A). We compared their ability to coprecipitate Tha4 substituted with a Cys in the N terminus (Tha4G5C, Tha4L6C, Tha4P9C), TMD (Tha4G16C), APH (Tha4V30C, Tha4V38C, Tha4K39C, Tha4S40C, Tha4F41C, Tha4K46C, Tha4E47C, Tha4F48C, Tha4T50C, Tha4K53C), and C-tail (Tha4A65C), thus allowing us to map the regions of interaction between the precursor Cys variants and Tha4 Cys variants. When the Cys was located on the solvent-exposed faces of pOE17 (i.e. D59C, D68C, S84C, and T115C), coprecipitation occurred when the Cys on Tha4 was in the APH or the N terminus (Table I). The most consistent interactions were between the precursor and the APH region of Tha4, followed by an interaction with the N terminus of Tha4. By contrast, Cys located in buried areas of the folded precursor (i.e. pOE17A71C and pOE17A137C) were not able to interact with any Cys-substituted Tha4 (Supplemental Fig. S2B), demonstrating the folded nature of the precursor mature domain during transport and the dependence of the interaction upon exposed sulfhydryls.
Table I. Summary of the coprecipitation of Cys-substituted Tha4 by Cys-substituted pOE17.
Coprecipitations were conducted as described in “Materials and Methods” and as represented in Figure 2 for pOE17K99C. Results are averages of at least three separate experiments. Coprecipitation is indicated by +, no coprecipitation is indicated by −, and weak coprecipitation is indicated by +/−. n.d., Not determined.
| Precursor |
||||
|---|---|---|---|---|
| Tha4 Residue | pOE17D59C | pOE17D68C | pOE17S84C | pOE17T115C |
| N terminus | ||||
| G5C | n.d. | + | n.d. | + |
| L6C | n.d. | + | n.d. | n.d. |
| P9C | + | + | +/− | − |
| Transmembrane | ||||
| G16C | − | n.d. | − | − |
| APH | ||||
| V30C | + | n.d. | n.d. | n.d. |
| V38C | + | n.d. | + | n.d. |
| K39C | n.d. | + | n.d. | n.d. |
| S40C | n.d. | + | n.d. | n.d. |
| F41C | n.d. | + | n.d. | n.d. |
| K46C | +/− | + | +/− | +/− |
| E47C | n.d. | + | +/− | n.d. |
| F48C | + | + | n.d. | + |
| T50C | + | + | n.d. | + |
| K53C | + | + | + | + |
| C-tail | ||||
| A65C | − | n.d. | +/− | − |
Tha4 Goes into a Large Complex Containing Hcf106 and cpTatC through Direct Interaction with Precursor
So far, we have demonstrated an interaction between the mature domain of precursor and Tha4. However, the affinity precipitation would not distinguish between the full precursor and the cleaved mature domain, as they both contain the His tag. To test if the interaction was between the precursor before cleavage of the signal peptide or after, we reasoned that if the detected interaction was between precursor and Tha4, it would be detectable as part of the translocase, indicating that translocation had not yet occurred or was in the process of occurring; however, if the interaction was between the postcleavage mature domain and Tha4, it should not be detectable as part of the translocase. We analyzed the interaction using BN-PAGE (Fig. 6). Precursor initially recognizes and binds to a receptor complex that can be isolated and migrates at 700 kD by BN-PAGE (Fincher et al., 2003; Gérard and Cline, 2007), but Tha4 is isolated as a separate complex that migrates at less than 400 kD by BN-PAGE (Cline and Mori, 2001). Here, we compared the presence of [3H]pOE17K99C in the 700-kD complex with the presence of Tha4F48C in that same complex. Given the one-to-one nature of the cross linking between precursor and Tha4F48C and the small size of Tha4 (approximately 14 kD), we reasoned that the presence of Tha4 with the receptor complex should not alter the size of the complex appreciably. If Tha4F48C was interacting with the precursor in the translocase, we would expect to trap Tha4F48C in the approximately 700-kD complex. If, however, it was predominantly interacting with the mature form post transport, then we would not expect to trap Tha4 in the 700-kD complex. Integration of in vitro-expressed Hcf106 into thylakoid (Fig. 6A, lane 3, black arrowheads) or immunoblot detection of cpTatC or Hcf106 (Fig. 6B, lanes 1–6, black arrowheads) served as the marker for the receptor complex (Cline and Mori, 2001; Mori and Cline, 2002). During transport, some [3H]pOE17K99C was trapped with the cpTatC-Hcf106 receptor complex (Fig. 6A, lane 1). Interestingly, when unlabeled precursor was used in the presence of [3H]Tha4, Tha4 was also trapped with the receptor complex and precursor (Fig. 6A, lane 2, white arrowhead). The location of Tha4 was also determined by immunoblot (Fig. 6B, lanes 7–9) after digitonin solubilization of thylakoid membranes. At 0.5% digitonin (Fig. 6B, lane 7), the predominant Tha4 complex is around 400 kD, with some Tha4 located in the precursor-bound receptor complex. As the amount of digitonin is increased, the 400-kD complex decreases in size to approximately 300 kD (Fig. 6B, lane 8) and 150 kD (Fig. 6B, lane 9), while some Tha4 remains bound to the precursor in the receptor complex (Fig. 6B, white arrowhead). These data indicate the presence of Tha4 in the translocase through direct interaction with the precursor protein, as analyzed by BN-PAGE.
Figure 6.
Tha4 is found in the 700-kD complex when linked to precursor. A, BN-PAGE of samples cross linked during the transport of 3H-labeled precursor pOE17K99C (lane 1) or unlabeled precursor (lane 2) with [3H]Tha4. In the presence of unlabeled precursor, [3H]Tha4 migrates at approximately 700 kD (white arrowhead). [3H]Hcf106 was included as a marker for the 700-kD complex (lanes 3; black arrowheads). B, Immunodetection of cpTatC, Hcf106, or Tha4 from samples containing [3H]pOE17 (lanes 1, 3, 4, and 6–9) or unlabeled pOE17 (lanes 2 and 5) with [3H]Tha4. BN-PAGE samples were prepared as described in “Materials and Methods,” using the detergent digitonin at the indicated percentage (v/v; % dig). Gels were run in parallel and analyzed by fluorography (A) or immunoblot (B) using the antibodies indicated below each panel. C, FLAG-tagged Tha4 interacts directly with the precursor. Cys-substituted Tha4 containing a FLAG tag at the C terminus was used to affinity precipitate precursor as described in “Materials and Methods.” The indicated Cys-substituted [3H]Tha4-FLAG or unlabeled Tha4-FLAG (Tha4L6C or Tha4K53C) was used to precipitate [3H]pOE17K99C. Panels represent three separate experiments.
To more specifically address the question of whether Tha4 is interacting with the mature domain before signal peptide cleavage or after, we used Cys variants of Tha4 containing the FLAG sequence (DYKDDDDK; mass of 1,012 D) at the C-terminal end to reciprocally precipitate the cross-linked mature domain. This assay would distinguish between Tha4 interaction with full-length precursor (e.g. before the translocation event) and the mature domain after signal peptide cleavage (e.g. after the translocation event). The addition of the FLAG tag to Tha4 did not affect the recombinant Tha4’s ability to functionally replace the endogenous Tha4 protein (Supplemental Fig. S3). Using an anti-FLAG affinity column, we could precipitate Tha4 and anything cross linked to it. When we affinity precipitated either [3H]Tha4L6C-FLAG or [3H]Tha4K53C-FLAG in the presence of the [3H]precursor pOE17K99C, we found that Tha4 specifically precipitated the full-sized precursor form but not the mature form of K99C (Fig. 6C, lanes 1 and 3). This was made clearer when using unlabeled Tha4L6C-FLAG or Tha4K53C-FLAG (Fig. 6C, lanes 2 and 4). Interestingly, Tha4K53C-FLAG was able to precipitate more precursor than Tha4L6C-FLAG. These data strongly suggest that the interaction is on pathway at the translocase but before the completion of transport, as defined by cleavage of the signal peptide by the thylakoid-processing protease.
DISCUSSION
This study shows, to our knowledge for the first time, a direct interaction between the mature domain of a cpTat precursor and Tha4, supporting an active role of Tha4 during the transport event. Multiple lines of evidence suggest Tha4 as the point of passage in the Tat translocase. First, Tha4 is only required for the transport step (Cline and Mori, 2001). Second, Tha4 is found in molar excess over cpTatC and Hcf106 (Celedon and Cline, 2012). Third, Tha4 forms higher order oligomers once assembled into the translocase (Dabney-Smith and Cline, 2009). Fourth, Tha4 undergoes localized conformational changes in direct response to precursor (Aldridge et al., 2012). Furthermore, several models propose that Tha4 or its bacterial ortholog TatA serves as the point of precursor passage during translocation. One model favors a translocation channel composed largely of Tha4 (TatA) providing an aqueous, protein-lined channel for the transporting protein (Müller and Klösgen, 2005; Sargent et al., 2006; Albiniak et al., 2012), while another favors a weakening of the membrane caused by Tha4 (TatA) that likely does not include the formation of a protein-lined channel (Brüser and Sanders, 2003; Dabney-Smith et al., 2006; Dabney-Smith and Cline, 2009). In either case, Tha4 (TatA) plays a pivotal role in the process (Mori and Cline, 2002; Dabney-Smith et al., 2006; Dabney-Smith and Cline, 2009), but the nature of that role has not been fully elucidated.
If Tha4 serves as the point of passage as either a channel or a weak patch in the membrane, then as the precursor makes its way across the membrane it would be in close proximity to Tha4 (TatA). Previous work showed an interaction between the signal sequence of a precursor and the cpTatC/Hcf106 receptor (Gérard and Cline, 2006) or the bacterial homolog TatC/TatB receptor (Alami et al., 2003; Maurer et al., 2010). In addition, the N terminus of the bacterial precursor mature domain also interacted with TatA (Alami et al., 2003), albeit weakly. More recently, the bacterial precursor pSufI was shown to be “in the immediate proximity of” TatA when supersaturating conditions of precursor were present (Panahandeh et al., 2008), suggesting the formation of a translocation intermediate. It was also demonstrated that the mature domain of SufI could be cross linked to TatB and TatA (Maurer et al., 2010), and the signal peptides of the precursors of SufI or a chimeric protein, TorA-mCherry, could be linked to TatA (Fröbel et al., 2011). The latter study demonstrated that the interaction was likely not kinetically related to transport: the TatA Cys variants used are largely inactive and the interaction shows no time dependence, occurring faster than the assay could measure (Fröbel et al., 2011). Alternatively, this study demonstrated a functional Tha4 that interacted with the precursor mature domain in a manner dependent upon a functional receptor complex, the presence of the PMF, and the length of the assay, suggesting that the interaction is occurring as part of the transport process. Interestingly, the methods used in both studies are comparable and likely are exposing adaptive differences in mechanisms between the chloroplast and bacterial Tat systems.
In this study, we show the transport of a Cys-substituted pOE17 precursor and its ability to directly coprecipitate Cys-substituted Tha4 during transport through the formation of a one-to-one disulfide bond. Structurally, the mature OE17 protein is composed of a four-helix bundle with a loosely structured N terminus (Balsera et al., 2005). We placed the Cys residues in the four-helix bundle either in a turn between helices (D68C and K99C) or in the solvent-exposed face of a helix (D59C, S84C, and T115C). When the residues were solvent exposed, they formed a disulfide bond with a Cys-substituted Tha4 (Fig. 2) when the Cys was located in the Tha4 APH and N-terminal regions but not the C-tail. However, when the Cys residues on the precursor were not solvent exposed (e.g. A71C or A137C; Supplemental Fig. S2), the precursor was unable to interact with Tha4, demonstrating the folded nature of the substrate immediately prior to and during transport. The hydrophobic transmembrane and hinge regions of Tha4 did not consistently interact with any Cys-variant precursor (Fig. 2; Table I). This can be explained by the nature of the chemistry involved for cross linking. Disulfide exchange requires the presence of a thiolate anion (e.g. Cys on Tha4) to attack the DTNB-derivatized precursor. In the very low polarity of the hydrophobic core of the membrane, formation of the thiolate is unlikely (Bogdanov et al., 2005). Taken together, these data present a region of cross linking at the C-terminal proximal portion of the APH and the N terminus, which may or may not encompass the hinge and the immediately adjacent portion of the transmembrane region but not the C-tail. These data agree with previous oligomerization studies of Tha4, which showed that the C-tail was a key area of contact for oligomerization while transport was occurring, whereas the APH was not (Dabney-Smith et al., 2006; Dabney-Smith and Cline, 2009).
The data presented here clearly show that the precursor-Tha4 interaction in chloroplasts occurs after the initial precursor-binding step, which requires cpTatC and Hcf106, because thylakoid treatment with anti-Hcf106 antibodies prevented the Tha4 interaction, demonstrating that Tha4 was only precipitated by precursor when active translocase was formed. While there may not be a handoff of the precursor signal peptide from cpTatC per se (Gérard and Cline, 2006), the data presented here suggest that translocation of the mature domain requires a direct interaction with Tha4. In addition, the interaction was dependent upon the initial presence of a PMF and increased over time as transport proceeded, indicating a functional translocase. In addition, the interaction resulted in the presence of Tha4 in the same large complex as precursor and the receptor complex (cpTatC and Hcf106) after detergent solubilization and BN-PAGE analysis. Previous analysis by BN-PAGE of detergent-solubilized thylakoids shows that precursor bound to the Tat receptor proteins cpTatC and Hcf106 migrates in an approximately 700-kD complex (Cline and Mori, 2001; Fincher et al., 2003). Furthermore, the interaction is predominantly between the full precursor and Tha4 (Fig. 6C), indicating that the interaction occurs immediately prior to cleavage of the signal peptide by the lumen-localized thylakoid-processing protease. Combining the specific cross-linking strategy and BN-PAGE analysis allowed us to show that Tha4 directly interacts with the precursor in the cpTat translocase. While the bacterial TatBC complex has been shown to contain TatA (Oates et al., 2005), to our knowledge this is the first report of Tha4 being trapped in the thylakoid translocase through direct interaction with the precursor.
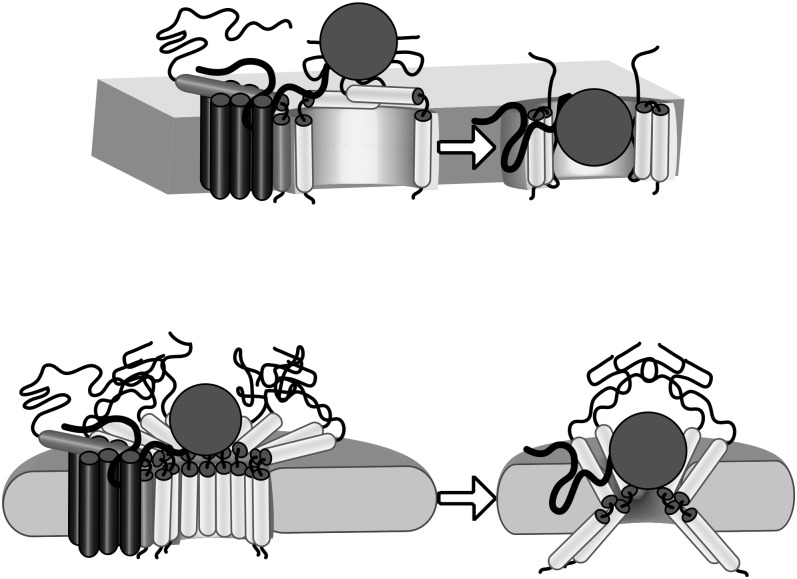
Tha4 contains a single N-terminal TMD followed by an APH and a largely unstructured C-tail. Recent structural data of the bacterial ortholog TatAd from Bacillus subtilis (Walther et al., 2010) and biochemical data from Tha4 and E. coli TatA (Aldridge et al., 2012; Koch et al., 2012) demonstrate a resting topology that is tilted in the membrane such that the transmembrane region and the N-terminal proximal portion of the APH are not solvent exposed. Further biochemical evidence suggests that this global topology does not change during the transport process (Aldridge et al., 2012; Koch et al., 2012). We envision at least two mechanistic models requiring Tha4 for the physical translocation of a precursor during transport (Fig. 7). In both models, the interaction with Tha4 would occur after precursor binding to the receptor complex and assembly of Tha4 to the complex. First, if the precursor is traveling through a channel of Tha4, one might envision a channel-like structure (Fig. 7, top panel) like that observed by cryo-electron microscopy for TatA (Gohlke et al., 2005). In those images, cup-like particles of TatA appeared to include the APH folded into the TMD, with the C-tail likely covering the top of the particle (Gohlke et al., 2005). In this scenario, the TMD would likely be protected from interacting with the precursor by the APH. While this cannot be ruled out, it seems unlikely because we do see interaction between the precursor mature domain and the Tha4 TMD (Figs. 2 and 6C), suggesting that the APH does not fold in to protect the TMD from precursor. In addition, topology studies of Tha4 and E. coli TatA suggest that the folded topology of this first scenario is highly unlikely in situ (Aldridge et al., 2012; Koch et al., 2012) even as transport is occurring (Aldridge et al., 2012; Koch et al., 2012).
Figure 7.
Possible models for a Tha4-mediated precursor translocation point of passage. The top panel shows a channel model of Tha4 exhibiting conformational change such that the APH lines the channel. The bottom panel shows an alternative model whereby the overall topology of Tha4 does not change. For more information, see “Discussion.” In the right part of each panel, the cpTatC-Hcf106 receptor was removed to focus on the contribution of Tha4.
A second model invokes the encounter of precursor with the Tha4 APH as an initial step to the translocation event and the interaction with the Tha4 N terminus as the precursor progresses through the translocation process (Fig. 7, bottom panel). This model does not imply a global topology change, such as in the first model, but rather some other mechanism of generating a point of passage for the precursor, such as a weakening of the membrane or formation of a toroidal pore, allowing the global topology of Tha4 to remain unchanged. This model accounts for the interaction detected between the precursor mature domain and the TMD of Tha4. In addition, this model also accounts for the oligomerization of Tha4 through the presence of a mesh or cage-like formation of Tha4 C-tails around the precursor after it engages the Tha4 APH. The presence of such a network would not be required per se, but its presence would increase the efficiency of transport by preventing dissociation of the precursor before translocation could occur. Earlier studies using Tha4 functional replacement assays showed that removal of the C-tails did not abolish but did decrease the transport of precursor (Dabney-Smith et al., 2003). In addition, that region was shown to be very important in Tha4 oligomerization (Dabney-Smith et al., 2006; Dabney-Smith and Cline, 2009). This model is highly speculative, but the methods presented here provide avenues to address the major tenets.
In conclusion, our results strengthen the hypothesis that Tha4 provides the point of passage for the precursor to transport across the membrane through the cpTat system. The data presented here present a method to further explore the nature of the translocase to better elucidate the mechanism of cpTat transport.
MATERIALS AND METHODS
Preparation of Chloroplasts and Thylakoids
Intact chloroplasts were isolated from 10- to 12-d-old pea (Pisum sativum ‘Little Marvel’) seedlings (Cline et al., 1993). Chloroplasts were resuspended to 1 mg chlorophyll mL−1 with import buffer (IB; 50 mm HEPES-KOH, pH 8.0, and 330 mm sorbitol) and kept on ice until used. Chloroplast lysates were prepared from pelleted intact chloroplasts by osmotic lysis at 2 mg chlorophyll mL−1 with 10 mm HEPES-KOH, pH 8.0, and 10 mm MgCl2 (Mori and Cline, 1998). Thylakoids were obtained from lysates by centrifugation at 3,200g for 8 min followed by a wash step of resuspension with IB plus 10 mm MgCl2 and centrifugation at 3,200g, and the supernatant was discarded. Washed thylakoids were resuspended to 1 mg chlorophyll mL−1 with IB plus 10 mm MgCl2. Chlorophyll was measured according to Arnon (1949).
Generation of Precursor and Tha4 Cys Variants
Tha4 proteins with Cys substitutions (Tha4XnC, where X is the amino acid substituted with Cys at position n) were generated by QuikChange mutagenesis (Stratagene) according to the manufacturer’s instructions. The template used for mutagenesis was the coding sequence for mature Tha4 (lacking the transit peptide) from pea as described (Fincher et al., 2003); the coding sequence begins with MAFFGLGVPE. Transcription plasmids for the various precursors were constructed by PCR mutagenesis with the QuikChange mutagenesis kit from Stratagene. Wild-type forms of Tha4 and the precursor pOE17 lack any Cys residues. The clone for pOE17(G4S)3-His6 is a modified version of pOE17 and contains a His6 tag at its C terminus separated by a polylinker consisting of four Gly residues and a Ser repeated three times [(G4S)3] to yield the precursor pOE17XnC(G4S)3-His6 (referred to as pOE17GS3 here) and was a generous gift from Ken Cline. This precursor was used as template DNA to generate Cys substitutions at positions Asp-59, Asp-68, Ala-71, Ser-84, Lys-99, Thr-115, and Ala-137 using the QuikChange method as described. The amino acid positions are designated based on the mature protein after cleavage of the transit peptide. Cys substitutions were verified by sequencing of the DNA on both strands at the Center for Bioinformatics and Functional Genomics at Miami University.
Tha4XnC-FLAG proteins containing the FLAG affinity tag (DYKDDDDK; 1,012 D) were generated by QuikChange mutagenesis (Stratagene) according to the manufacturer’s instructions. The primers used to incorporate the FLAG tag sequence in frame at the 3′ end of tha4 were 5′-GCACAAAGGATAATGTAGACTA-CAAGGACGATGACGACAAGTGATAAGATCTAGAG-3′ and its complement, where the FLAG sequence is underlined and the tha4 sequence is in boldface. The template used for mutagenesis was the coding sequence for the pea mature Tha4 Cys variants indicated in the figure legends. The presence of the FLAG sequence was verified by sequencing of the DNA on both strands at the Center for Bioinformatics and Functional Genomics at Miami University. The coding sequence ends NH3-…V75SSTKDNV81DYKDDDDK-COOH, where the FLAG tag is underlined.
Preparation of Radiolabeled Mature Tha4 and Precursors
Radiolabeled mature Tha4, Tha4 Cys variants, precursor, and precursor Cys variants were prepared by in vitro translation in a wheat germ extract from capped mRNA in the presence of [3H]Leu (Dabney-Smith et al., 2006). The translation products were diluted with an equal volume of 60 mm Leu in 2× IB before use.
DTNB Treatment of Precursor
The precursors were derivatized using DTNB essentially as described previously (Tokatlidis et al., 1996) with the following exceptions. The precursor was incubated with 80 µm DTNB at room temperature for 30 min before use in transports.
Transport Assays
Transport reaction was carried out for 25 min at 25°C under 150 µmol m−2 s−1 light and stopped by transfer to ice and centrifugation at 3,200g to recover thylakoids (Gérard and Cline, 2006). Recovered thylakoids were resuspended in 300 µL of IB plus 10 mm MgCl2 and divided into two aliquots. One half was centrifuged to recover thylakoids, supernatant was removed, and thylakoids were washed with IB containing 5 mm EDTA. Thylakoids in the other half were treated with thermolysin at a final concentration of 0.1 mg mL−1 for 40 min at 4°C. Adding an equal volume of IB containing 14 mm EDTA terminated protease digestion. Thylakoids in all samples were then recovered by centrifugation and were washed with IB plus 5 mm EDTA. Samples were resuspended to approximately 1 mg chlorophyll mL−1 with IB plus 10 mm MgCl2. An equal volume of 2× SSB (100 mm Tris-HCl, pH 6.8, 5 mm dithiothreitol, 5% SDS, 20% glycerol, and 0.2% bromphenol blue) was added to each. Samples were subjected to SDS-PAGE and analyzed by fluorography.
Biochemical Complementation of Transport by Exogenous Tha4
Biochemical complementation was essentially as described (Dabney-Smith et al., 2003). Briefly, isolated thylakoids were incubated with anti-Tha4 IgG for 45 min at 0°C and then washed with 2 volumes of IB plus 10 mm MgCl2. A typical preincubation contained 300 µg of chlorophyll and 75 µg of IgG in 20 mm HEPES-KOH, pH 8.0, and 0.1 m sorbitol. Thylakoids were recovered by centrifugation, washed with IB plus 10 mm MgCl2, and treated with protein A (5 µg per 25 µg of chlorophyll) to clamp the bound IgG. Pretreated thylakoids were incubated with in vitro-translated, Cys-substituted [3H]Tha4, and integration was carried out for 25 min at 20°C. Tha4-integrated thylakoids were assayed for transport of in vitro-translated, Cys-substituted, DTNB-pretreated precursor pOE17. Transport of precursor was as described above without thermolysin posttreatment.
Cross Linking between Precursor and Tha4 or Tha4-FLAG
In vitro-translated [3H]Tha4XnC was integrated into isolated anti-Tha4 antibody-treated thylakoids for 25 min at 20°C as described above. Recovered thylakoids (100 µg of chlorophyll) were incubated with in vitro-translated, Cys-substituted, DTNB-activated [3H]pOE17GS3 under 150 µmol m−2 s−1 light for transport. Cross linking was initiated when the DTNB-activated Cys residue of precursor came into close contact with the Cys-substituted Tha4 under transport conditions. Transfer to ice and centrifugation to recover thylakoids stopped the transport reactions. Thylakoids were washed and recovered by centrifugation and suspended in one-half volume of Tris-buffered saline (TBS; 50 mm Tris-HCl, pH 7.5, and 150 mm NaCl). An equal volume of TBS containing 2% SDS and 1 mm EDTA was added to solubilize the thylakoids. Thylakoids were incubated at 37°C for 10 min and spun down at 14,000g for 10 min at 4°C. For affinity precipitation using His-tagged precursor, equal volumes of sample and precharged immobilized metal affinity chromatography resin (GE Healthcare) were transferred to the same 1.5-mL tube and combined with 500 µL of binding buffer (20 mm Tris-HCl, pH 7.9, 0.5 m NaCl, 5 mm imidazole, 1% Triton X-100, and 0.5% deoxycholate). The samples were incubated end-over-end for 1 h at 4°C. Samples were centrifuged at 500g for 2 min, and the supernatant (i.e. unbound fraction) was transferred to a new tube. The pellet was washed three times with wash buffer (20 mm Tris-HCl, pH 7.9, 0.5 m NaCl, 20 mm imidazole, 1% Triton X-100, and 0.5% deoxycholate) and one time with final wash buffer (20 mm Tris-HCl, pH 7.9, 0.5 m NaCl, 20 mm imidazole, and 0.05% Triton X-100) by suspension in the appropriate buffer and centrifugation at 500g for 2 min, discarding the supernatants. Finally, 60 µL of elution buffer (20 mm Tris-HCl, pH 6.8, 0.5 m NaCl, 0.5 m imidazole, 6 m urea, and 5% SDS) was added and incubated overnight at 4°C. The samples were centrifuged at 17,000g, and the supernatants were removed to new tubes. An equal volume of 2× SSB was added to each supernatant and subjected to SDS-PAGE and analysis by fluorography.
For affinity precipitation using Tha4-FLAG, equal volumes of sample and precharged anti-FLAG M2 magnetic beads (Sigma-Aldrich) were transferred to the same 1.5-mL tube and combined with 500 µL of 1× TBS (50 mm Tris-HCl, 150 mm NaCl, pH 7.4). The samples were incubated end-over-end overnight at 4°C. Samples were placed on a magnetic separator, and the unbound fraction was removed to a new tube. The beads were washed four times with 1× TBS, collecting the beads with the magnetic separator and discarding the supernatant after each wash. Finally, samples were eluted with 10 bead volumes of elution buffer (0.1 m Gly-HCl, pH 3.0) at room temperature, and the beads were separated from the elution with the magnetic separator. The elution samples were precipitated with TCA following standard protocols (Sambrook and Russell, 2001), and the pellet was suspended in a volume of 2× SSB equal to the starting material and subjected to SDS-PAGE and analysis by fluorography.
BN Gel Electrophoresis
Cross-linking experiments between precursor and Tha4 were performed as described above. Recovered thylakoids from assays were washed, solubilized with 1% digitonin (Cline and Mori, 2001), and subjected to BN-PAGE as described (Von Jagow and Schagger, 1994). Gels were analyzed by fluorography or subjected to immunodetection as described (Cline and Mori, 2001). Molecular mass markers used for BN gels were ferritin (880 and 440 kD) and bovine serum albumin (132 and 66 kD).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AF144708 (tha4) and NM_001111878 (psbq).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Functionality of precursors and Tha4 used in this study.
Supplemental Figure S2. The precursor-Tha4 interaction occurs during transport conditions and requires an exposed Cys on the precursor.
Supplemental Figure S3. Tha4-FLAG is transport competent.
Acknowledgments
We thank Ann Hagerman (Miami University) and members of the Dabney-Smith laboratory for critical review of the manuscript. We also thank Ken Cline (University of Florida) for the precursor.
Glossary
- cpTat
chloroplast twin arginine translocation
- BN
blue native
- DTNB
5,5′-dithiobis(2-nitrobenzoic acid)
- APH
amphipathic helix
- TMD
transmembrane domain
- PMF
protonmotive force
- IB
import buffer
- 2× SSB
100 mm Tris-HCl, pH 6.8, 5 mm dithiothreitol, 5% SDS, 20% glycerol, and 0.2% bromphenol blue
- TBS
Tris-buffered saline
References
- Alami M, Lüke I, Deitermann S, Eisner G, Koch HG, Brunner J, Müller M. (2003) Differential interactions between a twin-arginine signal peptide and its translocase in Escherichia coli. Mol Cell 12: 937–946 [DOI] [PubMed] [Google Scholar]
- Albiniak AM, Baglieri J, Robinson C. (2012) Targeting of lumenal proteins across the thylakoid membrane. J Exp Bot 63: 1689–1698 [DOI] [PubMed] [Google Scholar]
- Aldridge C, Storm A, Cline K, Dabney-Smith C. (2012) The chloroplast twin arginine transport (tat) component, tha4, undergoes conformational changes leading to tat protein transport. J Biol Chem 287: 34752–34763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon DI. (1949) Copper enzymes in isolated chloroplasts: polyphenol oxidase in Beta vulgaris. Plant Physiol 24: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsera M, Arellano JB, Revuelta JL, de las Rivas J, Hermoso JA. (2005) The 1.49 A resolution crystal structure of PsbQ from photosystem II of Spinacia oleracea reveals a PPII structure in the N-terminal region. J Mol Biol 350: 1051–1060 [DOI] [PubMed] [Google Scholar]
- Bennett-Lovsey RM, Herbert AD, Sternberg MJ, Kelley LA. (2008) Exploring the extremes of sequence/structure space with ensemble fold recognition in the program Phyre. Proteins 70: 611–625 [DOI] [PubMed] [Google Scholar]
- Berthelmann F, Mehner D, Richter S, Lindenstrauss U, Lünsdorf H, Hause G, Brüser T. (2008) Recombinant expression of tatABC and tatAC results in the formation of interacting cytoplasmic TatA tubes in Escherichia coli. J Biol Chem 283: 25281–25289 [DOI] [PubMed] [Google Scholar]
- Bogdanov M, Zhang W, Xie J, Dowhan W. (2005) Transmembrane protein topology mapping by the substituted cysteine accessibility method (SCAM(TM)): application to lipid-specific membrane protein topogenesis. Methods 36: 148–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüser T, Sanders C. (2003) An alternative model of the twin arginine translocation system. Microbiol Res 158: 7–17 [DOI] [PubMed] [Google Scholar]
- Celedon JM, Cline K. (2012) Stoichiometry for binding and transport by the twin arginine translocation system. J Cell Biol 197: 523–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K. (1986) Import of proteins into chloroplasts: membrane integration of a thylakoid precursor protein reconstituted in chloroplast lysates. J Biol Chem 261: 14804–14810 [PubMed] [Google Scholar]
- Cline K, Dabney-Smith C. (2008) Plastid protein import and sorting: different paths to the same compartments. Curr Opin Plant Biol 11: 585–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K, Ettinger WF, Theg SM. (1992) Protein-specific energy requirements for protein transport across or into thylakoid membranes: two lumenal proteins are transported in the absence of ATP. J Biol Chem 267: 2688–2696 [PubMed] [Google Scholar]
- Cline K, Henry R, Li C, Yuan J. (1993) Multiple pathways for protein transport into or across the thylakoid membrane. EMBO J 12: 4105–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K, Mori H. (2001) Thylakoid DeltapH-dependent precursor proteins bind to a cpTatC-Hcf106 complex before Tha4-dependent transport. J Cell Biol 154: 719–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K, Theg S. (2007) The Sec and Tat protein translocation pathways in chloroplasts. In RE Dalbey, CM Koehler, F Tamanoi, eds, The Enzymes: Molecular Machines Involved in Protein Transport across Cellular Membranes. Academic Press, New York, pp 463–492
- Dabney-Smith C, Cline K. (2009) Clustering of C-terminal stromal domains of Tha4 homo-oligomers during translocation by the Tat protein transport system. Mol Biol Cell 20: 2060–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabney-Smith C, Mori H, Cline K. (2003) Requirement of a Tha4-conserved transmembrane glutamate in thylakoid Tat translocase assembly revealed by biochemical complementation. J Biol Chem 278: 43027–43033 [DOI] [PubMed] [Google Scholar]
- Dabney-Smith C, Mori H, Cline K. (2006) Oligomers of Tha4 organize at the thylakoid Tat translocase during protein transport. J Biol Chem 281: 5476–5483 [DOI] [PubMed] [Google Scholar]
- Fincher V, Dabney-Smith C, Cline K. (2003) Functional assembly of thylakoid deltapH-dependent/Tat protein transport pathway components in vitro. Eur J Biochem 270: 4930–4941 [DOI] [PubMed] [Google Scholar]
- Fröbel J, Rose P, Müller M. (2011) Early contacts between substrate proteins and TatA translocase component in twin-arginine translocation. J Biol Chem 286: 43679–43689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard F, Cline K. (2006) Efficient twin arginine translocation (Tat) pathway transport of a precursor protein covalently anchored to its initial cpTatC binding site. J Biol Chem 281: 6130–6135 [DOI] [PubMed] [Google Scholar]
- Gérard F, Cline K. (2007) The thylakoid proton gradient promotes an advanced stage of signal peptide binding deep within the Tat pathway receptor complex. J Biol Chem 282: 5263–5272 [DOI] [PubMed] [Google Scholar]
- Gohlke U, Pullan L, McDevitt CA, Porcelli I, de Leeuw E, Palmer T, Saibil HR, Berks BC. (2005) The TatA component of the twin-arginine protein transport system forms channel complexes of variable diameter. Proc Natl Acad Sci USA 102: 10482–10486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeeb AFSA. (1972) Reaction of protein sulfhydryl groups with Ellman's reagent. In CHW Hirs, SN Timasheff, eds, Enzyme Structure Part B. Academic Press, New York, pp 457–464
- Haldrup A, Naver H, Scheller HV. (1999) The interaction between plastocyanin and photosystem I is inefficient in transgenic Arabidopsis plants lacking the PSI-N subunit of photosystem I. Plant J 17: 689–698 [DOI] [PubMed] [Google Scholar]
- Ifuku K, Ido K, Sato F. (2011) Molecular functions of PsbP and PsbQ proteins in the photosystem II supercomplex. J Photochem Photobiol B 104: 158–164 [DOI] [PubMed] [Google Scholar]
- Kapazoglou A, Sagliocco F, Dure L., III (1995) PSII-T, a new nuclear encoded lumenal protein from photosystem II: targeting and processing in isolated chloroplasts. J Biol Chem 270: 12197–12202 [DOI] [PubMed] [Google Scholar]
- Kelley LA, Sternberg MJ. (2009) Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc 4: 363–371 [DOI] [PubMed] [Google Scholar]
- Koch S, Fritsch MJ, Buchanan G, Palmer T. (2012) Escherichia coli TatA and TatB proteins have N-out, C-in topology in intact cells. J Biol Chem 287: 14420–14431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Cline K. (2000) Precursors bind to specific sites on thylakoid membranes prior to transport on the delta pH protein translocation system. J Biol Chem 275: 10016–10022 [DOI] [PubMed] [Google Scholar]
- Ma X, Cline K. (2010) Multiple precursor proteins bind individual Tat receptor complexes and are collectively transported. EMBO J 29: 1477–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer C, Panahandeh S, Jungkamp AC, Moser M, Müller M. (2010) TatB functions as an oligomeric binding site for folded Tat precursor proteins. Mol Biol Cell 21: 4151–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Cline K. (1998) A signal peptide that directs non-Sec transport in bacteria also directs efficient and exclusive transport on the thylakoid Delta pH pathway. J Biol Chem 273: 11405–11408 [DOI] [PubMed] [Google Scholar]
- Mori H, Cline K. (2002) A twin arginine signal peptide and the pH gradient trigger reversible assembly of the thylakoid [Delta]pH/Tat translocase. J Cell Biol 157: 205–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Summer EJ, Cline K. (2001) Chloroplast TatC plays a direct role in thylakoid (Delta)pH-dependent protein transport. FEBS Lett 501: 65–68 [DOI] [PubMed] [Google Scholar]
- Mori H, Summer EJ, Ma X, Cline K. (1999) Component specificity for the thylakoidal Sec and Delta pH-dependent protein transport pathways. J Cell Biol 146: 45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Klösgen RB. (2005) The Tat pathway in bacteria and chloroplasts (review). Mol Membr Biol 22: 113–121 [DOI] [PubMed] [Google Scholar]
- Oates J, Barrett CM, Barnett JP, Byrne KG, Bolhuis A, Robinson C. (2005) The Escherichia coli twin-arginine translocation apparatus incorporates a distinct form of TatABC complex, spectrum of modular TatA complexes and minor TatAB complex. J Mol Biol 346: 295–305 [DOI] [PubMed] [Google Scholar]
- Panahandeh S, Maurer C, Moser M, DeLisa MP, Müller M. (2008) Following the path of a twin-arginine precursor along the TatABC translocase of Escherichia coli. J Biol Chem 283: 33267–33275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier JB, Ytterberg AJ, Sun Q, van Wijk KJ. (2004) New functions of the thylakoid membrane proteome of Arabidopsis thaliana revealed by a simple, fast, and versatile fractionation strategy. J Biol Chem 279: 49367–49383 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. (2001) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Sargent F, Berks BC, Palmer T. (2006) Pathfinders and trailblazers: a prokaryotic targeting system for transport of folded proteins. FEMS Microbiol Lett 254: 198–207 [DOI] [PubMed] [Google Scholar]
- Sargent F, Stanley NR, Berks BC, Palmer T. (1999) Sec-independent protein translocation in Escherichia coli: a distinct and pivotal role for the TatB protein. J Biol Chem 274: 36073–36082 [DOI] [PubMed] [Google Scholar]
- Schrodinger LLC. (2010) The PyMOL Molecular Graphics System, v1.3r1. www.pymol.org (April 29, 2012)
- Sun Q, Emanuelsson O, van Wijk KJ. (2004) Analysis of curated and predicted plastid subproteomes of Arabidopsis: subcellular compartmentalization leads to distinctive proteome properties. Plant Physiol 135: 723–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarry MJ, Schäfer E, Chen S, Buchanan G, Greene NP, Lea SM, Palmer T, Saibil HR, Berks BC. (2009) Structural analysis of substrate binding by the TatBC component of the twin-arginine protein transport system. Proc Natl Acad Sci USA 106: 13284–13289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokatlidis K, Junne T, Moes S, Schatz G, Glick BS, Kronidou N. (1996) Translocation arrest of an intramitochondrial sorting signal next to Tim11 at the inner-membrane import site. Nature 384: 585–588 [DOI] [PubMed] [Google Scholar]
- Von Jagow G, Schagger H. (1994) A Practical Guide to Membrane Protein Purification. Academic Press, New York [Google Scholar]
- Walther TH, Grage SL, Roth N, Ulrich AS. (2010) Membrane alignment of the pore-forming component TatA(d) of the twin-arginine translocase from Bacillus subtilis resolved by solid-state NMR spectroscopy. J Am Chem Soc 132: 15945–15956 [DOI] [PubMed] [Google Scholar]
- Yi X, Hargett SR, Frankel LK, Bricker TM. (2006) The PsbQ protein is required in Arabidopsis for photosystem II assembly/stability and photoautotrophy under low light conditions. J Biol Chem 281: 26260–26267 [DOI] [PubMed] [Google Scholar]