Abstract
Purified rabies virions, unlabeled or labeled with radioactive amino acids or d-glucosamine, were dissociated into their polypeptides by treatment with sodium dodecyl sulfate in a reducing environment and fractionated by electroiphoresis in sodium dodecyl sulfate-containing polyacrylamide gel. The molecular weights of individual polypeptides were estimated by comparison of their rate of migration with that of protein markers of known molecular weight. Purified viral nucleocapsid and a mixture of envelope components, isolated from virions disrupted by sodium deoxycholate, were analyzed by the same procedure. The number of molecules per virion of each polypeptide was estimated from the proportions of the separated components, the known molecular weight of the viral ribonucleic acid, and the chemical composition of the nucleocapsid. The protein moiety of the nucleocapsid particle was estimated to consist of 1,713 molecules of a major polypeptide (molecular weight, 62,000 daltons) and 76 molecules of a minor polypeptide (molecular weight, 55,000 daltons). In addition to 1,783 molecules of a glycoprotein component (molecular weight, 80,000 daltons), the viral envelope contains 789 and 1,661 molecules, respectively, of two other polypeptides (molecular weight, 40,000 and 25,000 daltons).
Full text
PDF

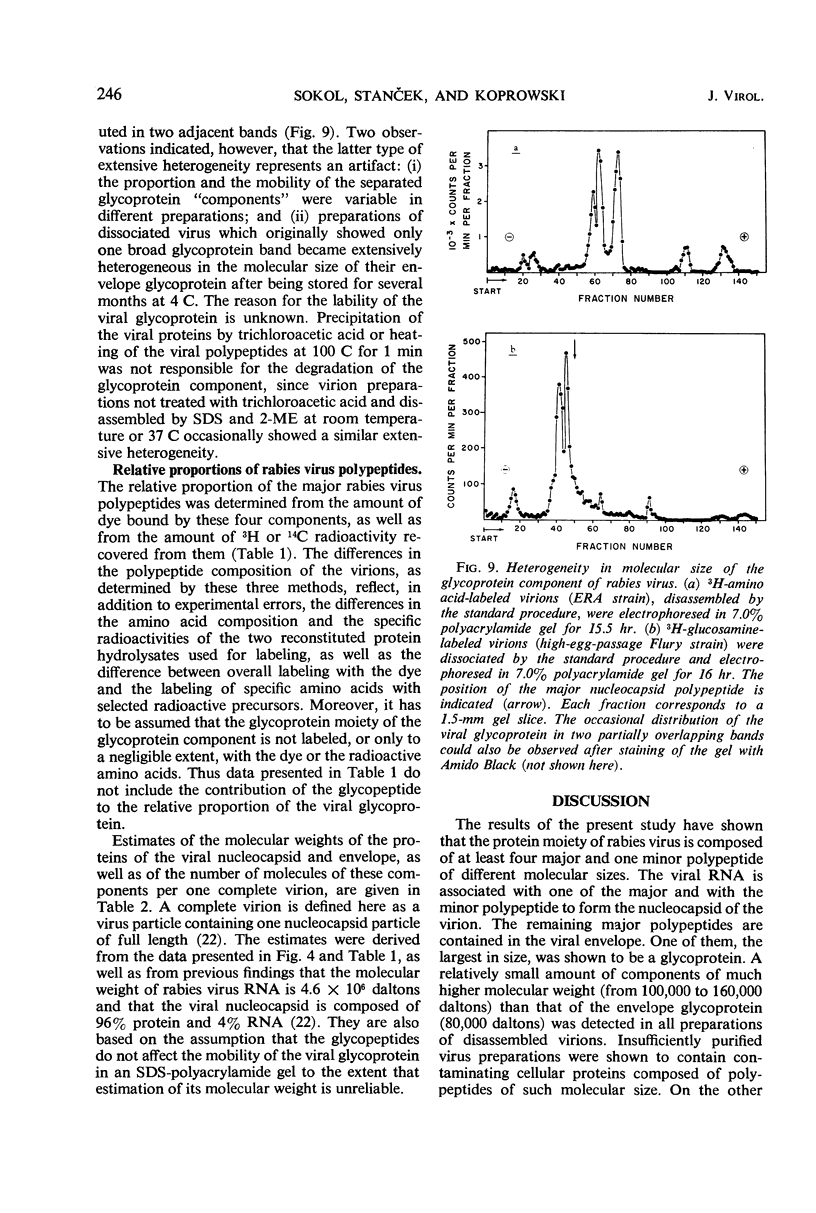
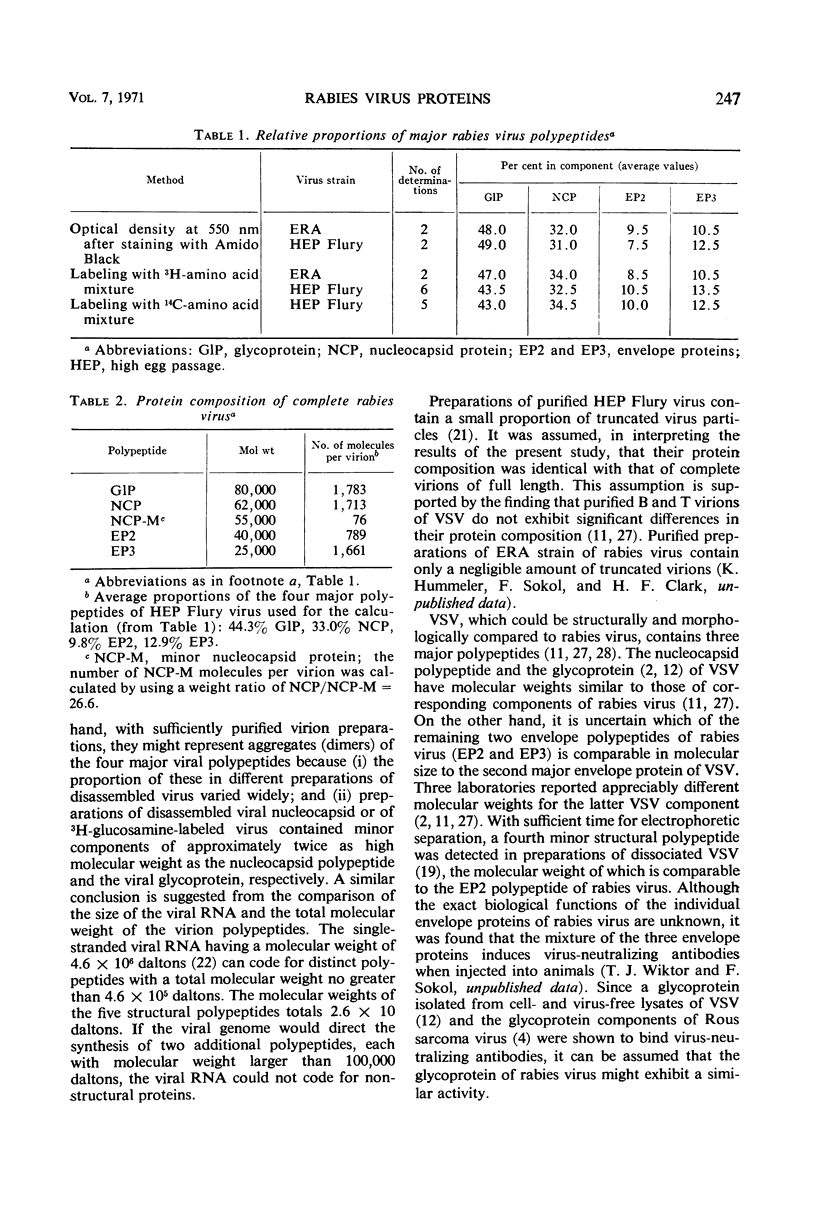


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., Kaplan A. S. Synthesis of proteins in cells infected with herpesvirus. V. Viral glycoproteins. Virology. 1970 Jun;41(2):265–273. doi: 10.1016/0042-6822(70)90078-4. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Huang A. S. Comparison of membrane protein glycopeptides of Sindbis virus and vesicular stomatitis virus. J Virol. 1970 Aug;6(2):176–182. doi: 10.1128/jvi.6.2.176-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge B. W., Strauss J. H., Jr Glycopeptides of the membrane glycoprotein of Sindbis virus. J Mol Biol. 1970 Feb 14;47(3):449–466. doi: 10.1016/0022-2836(70)90314-1. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Martin G. S., Vogt P. K. Glycoprotein components of avian and murine RNA tumor viruses. Virology. 1970 Aug;41(4):631–646. doi: 10.1016/0042-6822(70)90428-9. [DOI] [PubMed] [Google Scholar]
- HARBOE A. THE NORMAL ALLANTOIC ANTIGEN WHICH NEUTRALIZES THE INFLUENZA VIRUS HI-ANTIBODY TO HOST MATERIAL. Acta Pathol Microbiol Scand. 1963;57:488–492. doi: 10.1111/j.1699-0463.1963.tb05116.x. [DOI] [PubMed] [Google Scholar]
- HARBOE A. The influenza virus haemagglutinnation inhibition by antibody to host material. Acta Pathol Microbiol Scand. 1963;57:317–330. doi: 10.1111/j.1699-0463.1963.tb05101.x. [DOI] [PubMed] [Google Scholar]
- Haukenes G., Harboe A., Mortensson-Egnund K. A uronic and sialic acid free chick allantoic mucopolysaccharide sulphate which combines with influenza virus hi-antibody to host material. 1. Purification of the substance. Acta Pathol Microbiol Scand. 1965;64(4):534–542. doi: 10.1111/apm.1965.64.4.534. [DOI] [PubMed] [Google Scholar]
- Holowczak J. A. Glycopeptides of vaccinia virus. I. Preliminary characterization and hexosamine content. Virology. 1970 Sep;42(1):87–99. doi: 10.1016/0042-6822(70)90241-2. [DOI] [PubMed] [Google Scholar]
- Hummeler K., Koprowski H., Wiktor T. J. Structure and development of rabies virus in tissue culture. J Virol. 1967 Feb;1(1):152–170. doi: 10.1128/jvi.1.1.152-170.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummeler K., Tomassini N., Sokol F., Kuwert E., Koprowski H. Morphology of the nucleoprotein component of rabies virus. J Virol. 1968 Oct;2(10):1191–1199. doi: 10.1128/jvi.2.10.1191-1199.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. I. Polyacrylamide gel analysis of viral antigens. J Virol. 1969 Apr;3(4):404–413. doi: 10.1128/jvi.3.4.404-413.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. II. Immunological comparisons of viral antigens. J Virol. 1970 Jul;6(1):20–27. doi: 10.1128/jvi.6.1.20-27.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. S., Ben-Porat T. Synthesis of proteins in cells infected with herpesvirus, VI. Characterization of the proteins of the viral membrane. Proc Natl Acad Sci U S A. 1970 Jul;66(3):799–806. doi: 10.1073/pnas.66.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwert E., Wiktor T. J., Sokol F., Koprowski H. Hemagglutination by rabies virus. J Virol. 1968 Dec;2(12):1381–1392. doi: 10.1128/jvi.2.12.1381-1392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. The structure of influenza viruses. IV. Chemical studies of the host antigen. Virology. 1966 Sep;30(1):104–115. doi: 10.1016/s0042-6822(66)81014-0. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Protein synthesis in vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Oct;42(2):328–340. doi: 10.1016/0042-6822(70)90277-1. [DOI] [PubMed] [Google Scholar]
- STRANDLI O. K., MORTENSSON-EGNUND K., HARBOE A. PURIFICATION OF THE NORMAL ALLANTOIC ANTIGEN WHICH REACTS WITH INFLUENZA VIRUS HI-ANTIBODY TO HOST MATERIAL. Acta Pathol Microbiol Scand. 1964;60:265–270. doi: 10.1111/apm.1964.60.2.265. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Sokol F., Kuwert E., Wiktor T. J., Hummeler K., Koprowski H. Purification of rabies virus grown in tissue culture. J Virol. 1968 Aug;2(8):836–849. doi: 10.1128/jvi.2.8.836-849.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol F., Schlumberger H. D., Wiktor T. J., Koprowski H. Biochemical and biophysical studies on the nucleocapsid and on the RNA of rabies virus. Virology. 1969 Aug;38(4):651–665. doi: 10.1016/0042-6822(69)90184-6. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Kellejmroian B. Proteins spcified by herpes simplex virus. II. Viral glycoprotins associated with cellular membranes. J Virol. 1970 Feb;5(2):123–131. doi: 10.1128/jvi.5.2.123-131.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollar V. Studies on the nature of dengue viruses. IV. The structural proteins of type 2 dengue virus. Virology. 1969 Nov;39(3):426–438. doi: 10.1016/0042-6822(69)90091-9. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Carbohydrate content of the membrane protein of Sindbis virus. J Mol Biol. 1970 Feb 14;47(3):437–448. doi: 10.1016/0022-2836(70)90313-x. [DOI] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. A., Snyder R. M. Structural proteins of vesicular stomatitis viruses. J Virol. 1969 Apr;3(4):395–403. doi: 10.1128/jvi.3.4.395-403.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. C., Snyder R. M., Schnaitman C. A. Protein composition of the structural components of vesicular stomatitis virus. J Virol. 1969 Jun;3(6):611–618. doi: 10.1128/jvi.3.6.611-618.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Kuwert E., Koprowski H. Immune lysis of rabies virus-infected cells. J Immunol. 1968 Dec;101(6):1271–1282. [PubMed] [Google Scholar]