Abstract
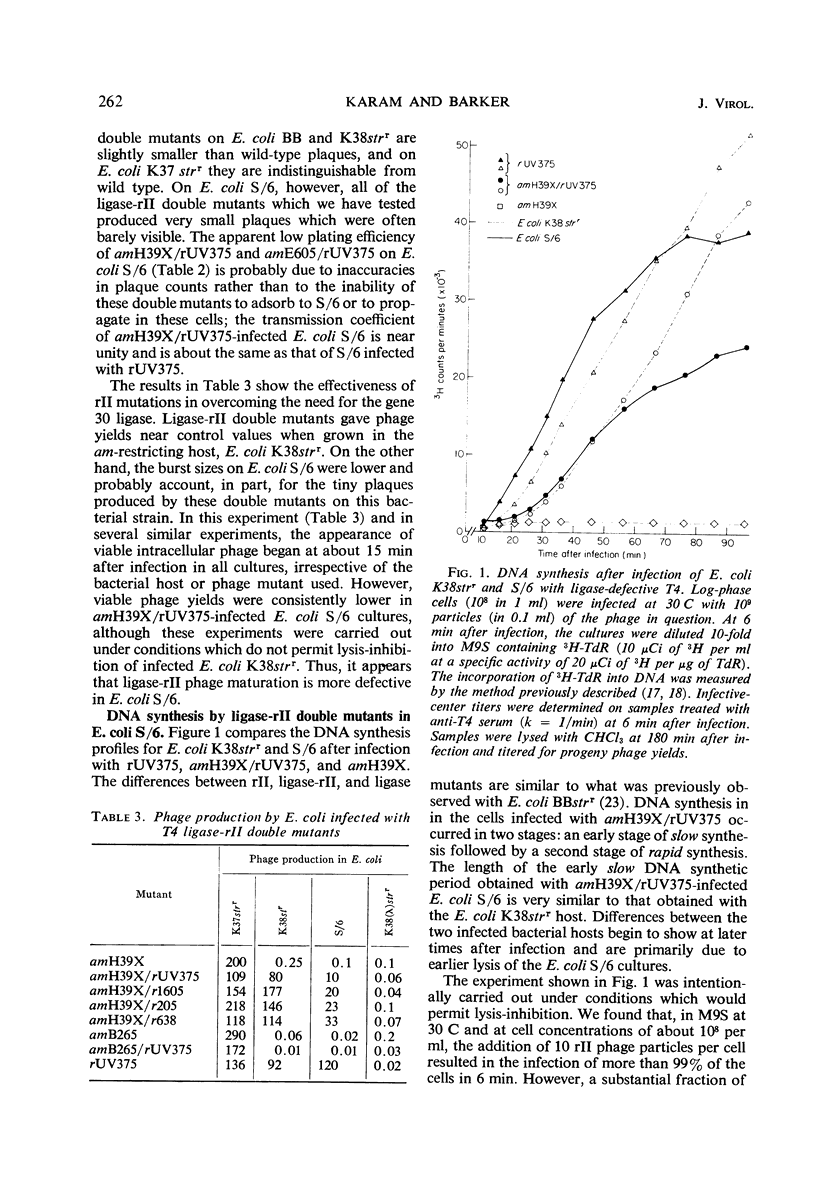
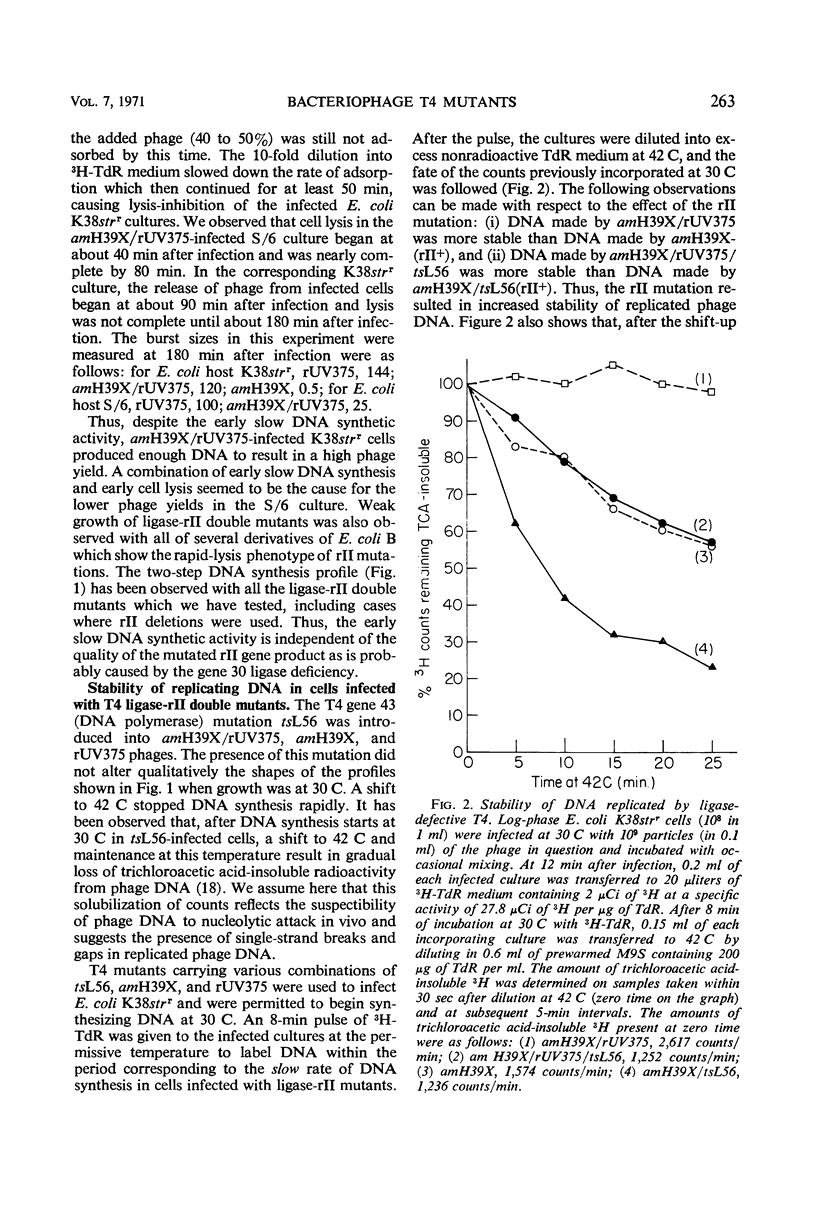
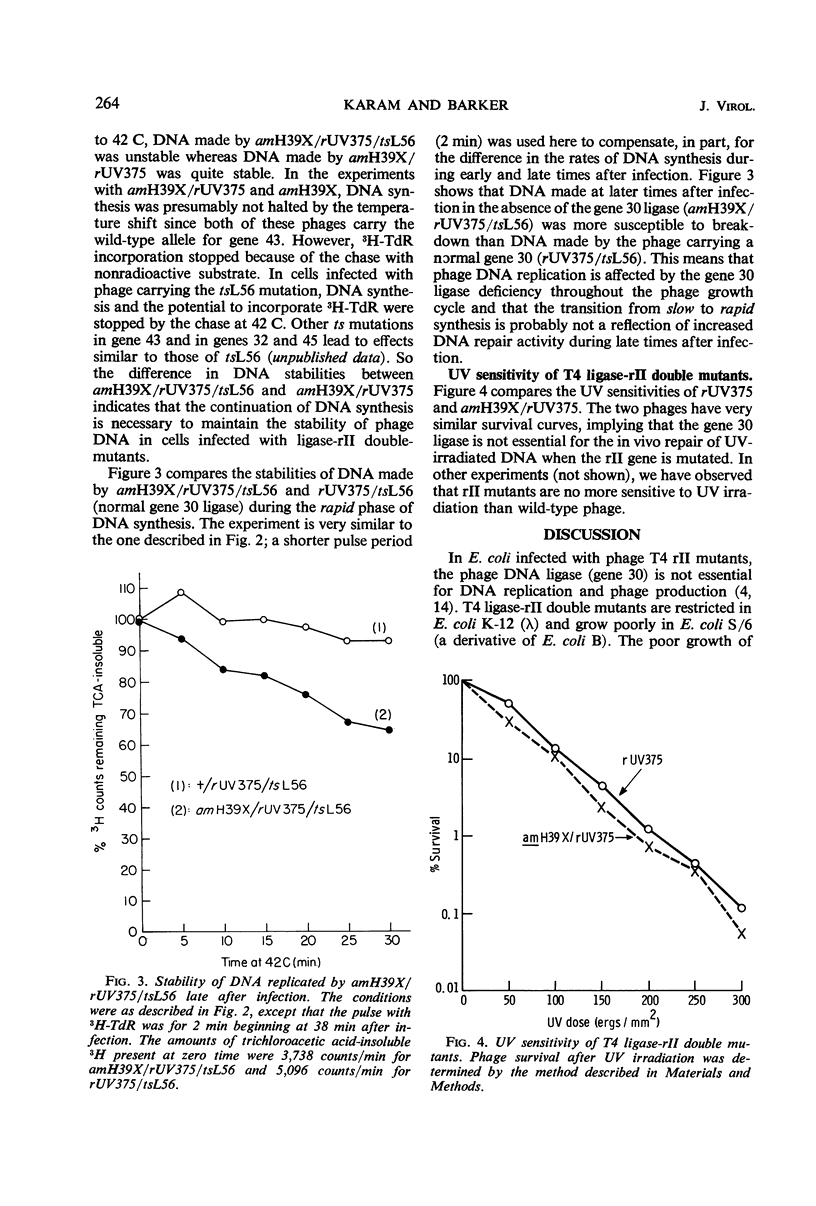
In Escherichia coli K-12 strains infected with phage T4 which is defective in gene 30 [deoxyribonucleic acid (DNA) ligase] and in the rII gene (product unknown), near normal levels of DNA and viable phage were produced. Growth of such T4 ligase-rII double mutants was less efficient in E. coli B strains which show the “rapidlysis” phenotype of rII mutations. In pulse-chase experiments coupled with temperature shifts and with inhibition of DNA synthesis, it was observed that DNA synthesized by gene 30-defective phage is more susceptible to breakdown in vivo when the phage is carrying a wild-type rII gene. Breakdown was delayed or inhibited by continued DNA synthesis. Mutations of the rII gene decreased but did not completely abolish the breakdown. T4 ligase-rII double mutants had normal sensitivity to ultraviolet irradiation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anraku N., Lehman I. R. Enzymic joining of polynucleotides. VII. Role of the T4-induced ligase in the formation of recombinant molecules. J Mol Biol. 1969 Dec 28;46(3):467–479. doi: 10.1016/0022-2836(69)90190-9. [DOI] [PubMed] [Google Scholar]
- Benzer S., Champe S. P. AMBIVALENT rII MUTANTS OF PHAGE T4. Proc Natl Acad Sci U S A. 1961 Jul;47(7):1025–1038. doi: 10.1073/pnas.47.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer S. ON THE TOPOGRAPHY OF THE GENETIC FINE STRUCTURE. Proc Natl Acad Sci U S A. 1961 Mar;47(3):403–415. doi: 10.1073/pnas.47.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H., Kozinski A. W. Suppression of T4D ligase mutations by rIIa and rIIb mutations. Proc Natl Acad Sci U S A. 1969 Nov;64(3):897–904. doi: 10.1073/pnas.64.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Melechen N. E., Jovin T. M., Kornberg A. Polynucleotide cellulose as a substrate for a polynucleotide ligase induced by phage T4. Biochem Biophys Res Commun. 1967 Aug 23;28(4):578–586. doi: 10.1016/0006-291x(67)90353-1. [DOI] [PubMed] [Google Scholar]
- Dean C., Pauling C. Properties of a deoxyribonucleic acid ligase mutant of Escherichia coli: x-ray sensitivity. J Bacteriol. 1970 May;102(2):588–589. doi: 10.1128/jb.102.2.588-589.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doermann A H, Hill M B. Genetic Structure of Bacteriophage T4 as Described by Recombination Studies of Factors Influencing Plaque Morphology. Genetics. 1953 Jan;38(1):79–90. doi: 10.1093/genetics/38.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisuzaki K., Campbell L. On the role of ligase in genetic recombination in bacteriophage T4. Virology. 1969 Aug;38(4):701–703. doi: 10.1016/0042-6822(69)90190-1. [DOI] [PubMed] [Google Scholar]
- Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. II. The structural gene for polynucleotide ligase in bacteriophage T4. Proc Natl Acad Sci U S A. 1967 Aug;58(2):665–672. doi: 10.1073/pnas.58.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garen A., Garen S., Wilhelm R. C. Suppressor genes for nonsense mutations. I. The Su-1, Su-2 and Su-3 genes of Escherichia coli. J Mol Biol. 1965 Nov;14(1):167–178. doi: 10.1016/s0022-2836(65)80238-8. [DOI] [PubMed] [Google Scholar]
- Gefter M. L., Becker A., Hurwitz J. The enzymatic repair of DNA. I. Formation of circular lambda-DNA. Proc Natl Acad Sci U S A. 1967 Jul;58(1):240–247. doi: 10.1073/pnas.58.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. Formation of covalent circles of lambda DNA by E. coli extracts. Proc Natl Acad Sci U S A. 1967 Jan;57(1):148–155. doi: 10.1073/pnas.57.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harm W. Recovery of UV-inactivated E. coli cells by the v-gene action of phage T4. Mutat Res. 1968 Jul-Aug;6(1):175–179. doi: 10.1016/0027-5107(68)90115-2. [DOI] [PubMed] [Google Scholar]
- Hosoda J. A mutant of bacteriophage T4 defective in alpha-glucosyl transferase. Biochem Biophys Res Commun. 1967 May 5;27(3):294–298. doi: 10.1016/s0006-291x(67)80095-0. [DOI] [PubMed] [Google Scholar]
- Hosoda J., Mathews E. DNA replication in vivo by a temperature-sensitive polynucleotide ligase mutant of T4. Proc Natl Acad Sci U S A. 1968 Nov;61(3):997–1004. doi: 10.1073/pnas.61.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N., Okazaki R. Mechanism of DNA chain growth. V. Effect of chloramphenicol on the formation of T4 nascent short DNA chains. J Mol Biol. 1970 Aug 28;52(1):37–44. doi: 10.1016/0022-2836(70)90175-0. [DOI] [PubMed] [Google Scholar]
- Karam J. D. DNA replication of phage T4 rII mutants without polynucleotide ligase (gene 30). Biochem Biophys Res Commun. 1969 Oct 22;37(3):416–422. doi: 10.1016/0006-291x(69)90931-0. [DOI] [PubMed] [Google Scholar]
- Karam J. D., Speyer J. F. Reversible inactivation of T4 ts DNA polymerase mutants in vivo. Virology. 1970 Sep;42(1):196–203. doi: 10.1016/0042-6822(70)90252-7. [DOI] [PubMed] [Google Scholar]
- Kozinski A. W., Kozinski P. B., James R. Molecular recombination in T4 bacteriophage deoxyribonucleic acid. I. Tertiary structure of early replicative and recombining deoxyribonucleic acid. J Virol. 1967 Aug;1(4):758–770. doi: 10.1128/jvi.1.4.758-770.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W. Molecular recombination in the ligase negative T4 amber mutant. Cold Spring Harb Symp Quant Biol. 1968;33:375–391. doi: 10.1101/sqb.1968.033.01.044. [DOI] [PubMed] [Google Scholar]
- Masamune Y., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. IV. DNA synthesis in E. coli infected with ligase-negative mutants of phage T4. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1328–1335. doi: 10.1073/pnas.61.4.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J., Hanawalt P. Intermediates in T4 DNA replication in a T4 ligase deficient strain. Cold Spring Harb Symp Quant Biol. 1968;33:145–150. doi: 10.1101/sqb.1968.033.01.018. [DOI] [PubMed] [Google Scholar]
- Newman J., Hanawalt P. Role of polynucleotide ligase in T4 DNA replication. J Mol Biol. 1968 Aug 14;35(3):639–642. doi: 10.1016/s0022-2836(68)80020-8. [DOI] [PubMed] [Google Scholar]
- Oishi M. Studies of DNA replication in vivo. I. Isolation of the first intermediate of DNA replication in bacteria as single-stranded DNA. Proc Natl Acad Sci U S A. 1968 May;60(1):329–336. doi: 10.1073/pnas.60.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K., Sugimoto K., Sugino A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc Natl Acad Sci U S A. 1968 Feb;59(2):598–605. doi: 10.1073/pnas.59.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M., Lehman I. R. Linkage of polynucleotides through phosphodiester bonds by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1967 May;57(5):1426–1433. doi: 10.1073/pnas.57.5.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling C., Hamm L. Properties of a temperature-sensitive radiation-sensitive mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1495–1502. doi: 10.1073/pnas.60.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINBERG C. M., EDGAR R. S. A critical test of a current theory of genetic recombination in bacteriophage. Genetics. 1962 Feb;47:187–208. doi: 10.1093/genetics/47.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakabe K., Okazaki R. A unique property of the replicating region of chromosomal DNA. Biochim Biophys Acta. 1966 Dec 21;129(3):651–654. doi: 10.1016/0005-2787(66)90088-8. [DOI] [PubMed] [Google Scholar]
- Speyer J. F., Rosenberg D. The function of T4 DNA polymerase. Cold Spring Harb Symp Quant Biol. 1968;33:345–350. doi: 10.1101/sqb.1968.033.01.040. [DOI] [PubMed] [Google Scholar]
- Weiss B., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid, I. Repair of single-strand breaks in DNA by an enzyme system from Escherichia coli infected with T4 bacteriophage. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1021–1028. doi: 10.1073/pnas.57.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]



