Abstract
The coordination of subcellular processes during adaptation to environmental change is a key feature of biological systems. Starvation of essential nutrients slows cell cycling and ultimately causes G1 arrest, and nitrogen starvation delays G2/M progression. Here, we show that budding yeast cells can be efficiently returned to the G1 phase under starvation conditions in an autophagy-dependent manner. Starvation attenuates TORC1 activity, causing a G2/M delay in a Swe1-dependent checkpoint mechanism, and starvation-induced autophagy assists in the recovery from a G2/M delay by supplying amino acids required for cell growth. Persistent delay of the cell cycle by a deficiency in autophagy causes aberrant nuclear division without sufficient cell growth, leading to an increased frequency in aneuploidy after refeeding the nitrogen source. Our data establish the role of autophagy in genome stability through modulation of cell division under conditions that repress cell growth.
Author Summary
A nutrient stress such as nitrogen depletion induces pleiotropic responses in eukaryotic cells. For example, nutrient starvation slows cell cycling and ultimately causes G1 arrest. In addition, it is known that nitrogen starvation delays G2/M progression. However, the mechanism as to how G2/M-delayed cells progress through the cell cycle to return to the G1 phase remains unclear. Cells subjected to a nutrient stress induce autophagy, a bulk degradation system within lysosomes/vacuoles, to reconstitute cellular components. In this study, we show that an autophagy-dependent supply of amino acid pools is critical for completion of cell cycle under starvation conditions in the budding yeast Saccharomyces cerevisiae. Autophagy deficiency causes a defect in cell growth and leads to abnormal mitosis associated with a higher incidence of aneuploidy. Thus, our data establish the role of autophagy in genome stability through modulation of cell division under conditions that repress cell growth, which provides a possible mechanism of tumor suppression by autophagy shown in mammalian cells.
Introduction
Autophagy is a protein degradation pathway that is conserved from yeast to mammals. Previous studies have reported the functions and molecular mechanisms of autophagy, including the identification of autophagy-related genes [1], [2], the characterization of the molecular mechanisms of each autophagy stage [3], [4], and the detection of selective autophagy [5], [6]. Autophagy is induced in response to nutrient starvation. In addition, homeostatic autophagy occurs at a low level in mammalian cells under nutrient-rich conditions [7], [8], and is regulated in a cell cycle-dependent manner [9], [10]. Furthermore, autophagy is induced during developmental stages [11].
In budding yeast, cell division and cell growth are precisely regulated by an intrinsic mechanism, and are tightly modulated by nutrient conditions. Target of rapamycin (TOR) is a phosphatidylinositol kinase-related Ser/Thr kinase and a critical regulator of cell growth, which senses the nutrient conditions [12]. TOR forms two distinct complexes, TOR complex 1 (TORC1) and TOR complex 2 (TORC2) [13]. Yeast TORC1 consists of either of the two yeast TOR homologs, Tor1 or Tor2, together with co-factors Kog1, Lst8, and Tco89, which are sensitive to inhibition by rapamycin. Conversely, yeast TORC2, which is produced from Tor2, Avo1–3, and Lst8, is not sensitive to rapamycin. While TORC2 regulates spatial aspects of cell growth, such as the organization of the actin cytoskeleton, TORC1 regulates temporal aspects of cell growth, including protein synthesis, gene transcription, ribosome biogenesis, amino acid uptake, and induction of autophagy [13]–[16]. Under nutrient-rich conditions, TORC1 is active and inhibits the induction of autophagy through inhibitory phosphorylation of Atg13 [17]. In contrast, TORC1 is inactive during starvation, thereby inducing autophagy [18]. Inhibition of TORC1 by rapamycin or by nutrient starvation leads to cell cycle arrest in the G1 phase [19], demonstrating that TORC1 plays a crucial role linking cell growth and cell cycle progression.
In addition to its role in the regulation of cell cycle progression in the G1 phase, we recently found that TORC1 is involved in the G2/M transition in budding yeast [20]. Reduced TORC1 activity, caused by nutrient starvation or by temperature-sensitive mutation in KOG1, which is an essential component of TORC1, leads to cell cycle arrest at G2/M. Two recent reports have shown that nutrient starvation also blocks the onset of mitosis in mammalian cells and the fission yeast Schizosaccharomyces pombe [21], [22]. The regulation is achieved by TORC1-mediated activation of Cdc2/cyclin B in mammalian cells [21], whereas it is suggested to be mediated by TORC2 as well as Sty1 MAPK signaling in the fission yeast [22], [23]. Although the underlying mechanisms may differ among species, the transient suppression of mitotic entry in response to nutrient starvation is conserved throughout evolution. However, it is well-known that cells arrest in the G1 phase and enter G0 during starvation [24]. Thus, the mechanism as to how G2/M-delayed cells progress through the cell cycle to return to the G1 phase remains unclear.
In this study, we elucidated the molecular mechanism underlying cell cycle progression under nutrient-limited conditions. We show that the cell cycle delay at G2/M is rescued in an autophagy-dependent manner. Regulation of the cell cycle by autophagy during starvation is believed to be involved in genome integrity by coupling cell growth with cell division.
Results
Reduced TORC1 activity by nitrogen starvation is partially recovered in an autophagy-dependent manner
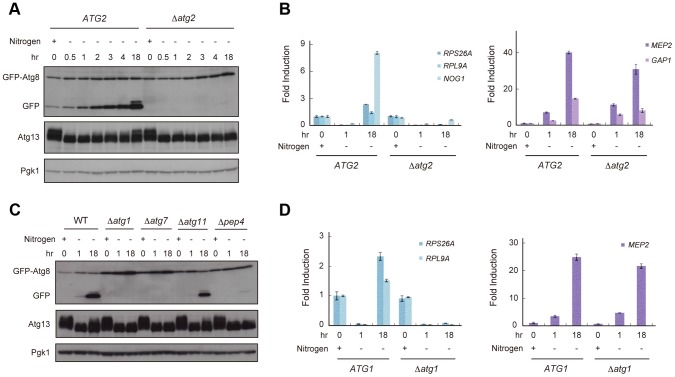
TORC1 is thought to be a nutrient sensor that controls the cell cycle and autophagy directly [12], [18]. We first examined fluctuation in TORC1 activity under starvation conditions by an immunoblot of Atg13, a TORC1 substrate, and RT-qPCR of genes whose expression is a product of TORC1 function. TORC1 directly phosphorylates Atg13 [17], thus, inactivation of TORC1 causes dephosphorylation of Atg13. In addition, TORC1 induces the transcription of RPS26A, RPL9A, and NOG1 but suppresses that of MEP2 and GAP1 [16], [25], [26]. When exponentially growing wild-type (WT) cells were released into nitrogen-depleted medium, TORC1 activity was immediately decreased; Atg13 became dephosphorylated (Figure 1A), resulting in a concomitant decrease in the expression of RPS26A, RPL9A, and NOG1 and an increase in the expression of MEP2 and GAP1 (Figure 1B). We found that phosphorylation of Atg13 gradually recovered after 2–18 h in nitrogen-depleted medium (Figure 1A), suggesting that TORC1 activity was restored in cells. The expressions of RPS26A, RPL9A, and NOG1 were increased, but those of MEP2 and GAP1 remained at high levels 18 h after release into nitrogen-depleted medium (Figure 1B). The lack of a correlation between the recovery of Atg13 phosphorylation and the levels of MEP2 and GAP1 suggests that TORC1 activity did not completely recover during this process.
Figure 1. Reduced TORC1 activity by nitrogen starvation is partially recovered in an autophagy-dependent manner.
(A) ATG2 (AMY182-10C) and Δatg2 (AMY182-10A) cells grown in SCD-Ura-Trp medium at 30°C were transferred to SD-N medium for the indicated times. Cell lysates were prepared using the alkaline-trichloroacetic acid method and analyzed by immunoblot with anti-GFP, anti-Atg13 and anti-Pgk1 antibodies. Progression of autophagy was monitored by accumulation of the free GFP moiety from the GFP-Atg8 fusion protein [65]. Pgk1 was used as a loading control. Uncropped images of blots are shown in Figure S5. Samples taken at 4-h intervals for 20 h demonstrated that the re-phosphorylation of Atg13 increased monotonically (data not shown). (B) ATG2 (AMY182-10C) and Δatg2 (AMY182-10A) cells grown in SCD-Ura-Trp medium at 30°C were transferred to SD-N medium for the indicated times. Total RNA was extracted and analyzed for the expression of RPS26A, RPL9A and NOG1 (left panel), and MEP2 and GAP1 (right panel) by RT-qPCR. Each sample was calibrated by TUB1 (Figure S1). (C) WT (AMY182-10C), Δatg1 (AMY236), Δatg7 (AMY237), Δatg11 (AMY238), and Δpep4 (AMY239) cells grown in SCD-Ura-Trp medium at 30°C were transferred to SD-N medium for the indicated times. Cell lysates were prepared and analyzed by immunoblot as described in (A). Uncropped images of blots are shown in Figure S5. (D) ATG1 (SAY122) and Δatg1 (AMY240) cells grown in SCD-Ura-Trp medium at 30°C were transferred as described in (C). Total RNA was extracted and analyzed for the expression of RPS26A and RPL9A (left panel) and MEP2 (right panel) by RT-qPCR. Each sample was calibrated by TUB1.
Next, we investigated whether autophagy is involved in the recovery of TORC1, and assessed the fluctuation in TORC1 activity in autophagy-deficient Δatg2 cells. When Δatg2 cells were placed in nitrogen-depleted medium, TORC1 activity decreased, similar to WT cells up to 4 h (Figure 1A and 1B). Interestingly, TORC1 activity was not restored in Δatg2 cells 18 h after being released into the starvation medium (Figure 1A and 1B, left panel).
Re-phosphorylation of Atg13 and increased expression of RPS26A and RPL9A under starvation conditions required other autophagy-related genes, such as ATG1 and ATG7, which are essential for autophagosome formation, and PEP4 encoding vacuolar proteinase A, which is responsible for autophagic degradation of proteins accompanied by recycling of amino acids (Figure 1C and 1D, left panel). In contrast, recovery of TORC1 activity was not affected by deletion of ATG11, which is essential in selective autophagy and dispensable for starvation-induced autophagy (Figure 1C). Therefore, these results suggest that the partial recovery of TORC1 activity is induced in a non-selective and starvation-induced autophagy-dependent manner.
Autophagy is required for cell cycle progression during starvation
The cell cycle is arrested in the G1 phase under starvation conditions [19], and we previously reported that TORC1 inactivation caused by rapamycin treatment and nitrogen starvation induces a G2/M delay [20]. Therefore, we postulated that an unknown mechanism may exist that regulates cell cycle re-progression from G2/M under nutrient starvation. We carefully examined the cell cycle profiles of nitrogen-starved cells, particularly the relationship between cell cycle progression and TORC1 activity.
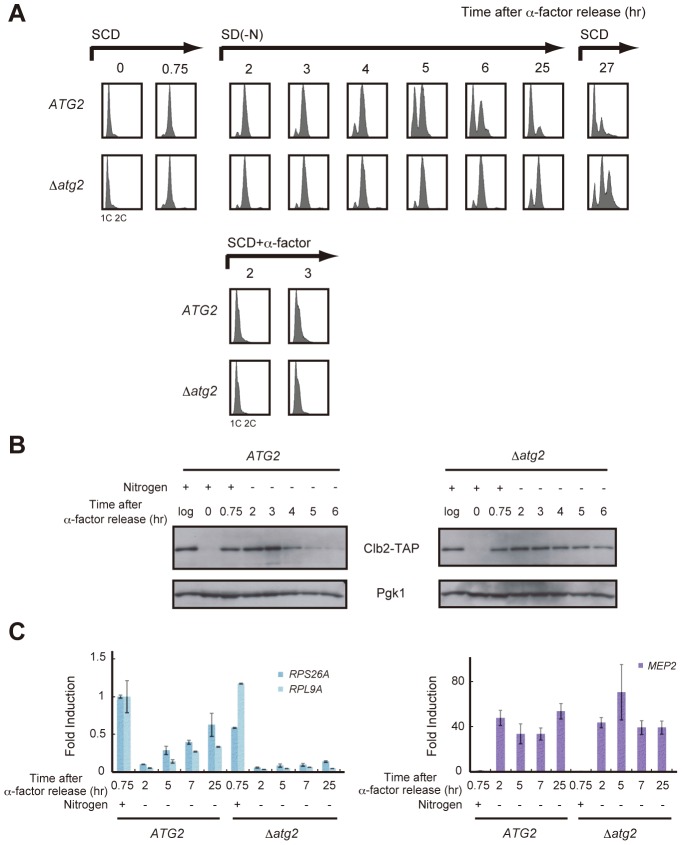
First, WT and Δatg2 cells were synchronized in the G1 phase by treatment with α-factor, and released into SCD medium. When the majority of cells progressed to the S phase, they were released into nitrogen-depleted medium. During this time course, cells were collected at intervals and the DNA content of cells at each time point was examined by FACS analysis. As previously reported, WT and Δatg2 cells remained arrested at 2C DNA content for 2–4 h after α-factor release, demonstrating that cell cycle progression was delayed at G2/M (Figure 2A). In WT cells, the delay in cell cycle progression was overcome after 5 h, and most cells reached the G1 phase after 25 h (Figure 2A). In contrast, a signification portion of Δatg2 cells, as well as Δatg1 cells, remained arrested at 2C DNA content after 25 h (Figure 2A and Figure S2). The difference in the cell cycle profiles between WT and atg2 mutant cells was confirmed by Clb2 levels; G2/M cyclin Clb2 decreased 4–5 h after α-factor release in WT cells, indicating that the cells entered mitosis, whereas Clb2 was consistently present in Δatg2 cells (Figure 2B). These results show that autophagy contributes to re-progression at G2/M during starvation.
Figure 2. Autophagy is required for cell cycle progression during starvation.
(A) ATG2 (SAY122) and Δatg2 (AMY250) cells were arrested at G1 by α-factor and released into SCD medium. Synchronous cultures were collected after 0.75 h and re-released into SD-N medium or SCD medium. To monitor cell cycle progression to G1 under the nutrient-rich condition, SCD medium was supplemented with 6.7 ng/mL α-factor for re-arrest at G1. After 25 h, the cell culture was re-released into SCD medium. DNA content at each time point was measured by FACS analysis. (B) ATG2 (AMY251) and Δatg2 (AMY253) cells were arrested at G1 by α-factor and released into SCD medium. Synchronous cultures were collected after 0.75 h and re-released into SD-N medium. Cell lysates were prepared as described in Figure 1A, and analyzed by immunoblot with anti-PAP and anti-Pgk1 antibodies. Pgk1 was used as a loading control. Uncropped images of blots are shown in Figure S5. (C) ATG2 (SAY122) and Δatg2 (AMY250) cells were grown as described in (A). Total RNA was extracted and analyzed for the expression of RPS26A and RPL9A (left panel) and MEP2 (right panel) by RT-qPCR. Each sample was calibrated by TUB1.
Next, we investigated whether re-activation of TORC1 is correlated with re-progression at G2/M. We analyzed time-dependent changes in gene expression downstream of TORC1. In WT cells, transcription of RPS26A and RPL9A decreased in response to starvation (2 h), but was gradually restored after 5–25 h (Figure 2C). Mitotic entry correlated with the re-activation of TORC1 (Figure 2A and 2C). Conversely, transcription of RPS26A and RPL9A remained low in Δatg2 cells, and the cells remained arrested at G2/M (Figure 2A and 2C). These results show again that TORC1 is partially restored in an autophagy-dependent manner in nitrogen-starved cells, and suggest that a correlation between the partial recovery of TORC1 activity and cell cycle re-progression at G2/M.
A specific amino acid supply is sufficient for cell cycle re-progression after a G2/M delay
To further address the mechanism of autophagy-dependent cell cycle re-progression during starvation, we next focused on the role of amino acid pools. Autophagy contributes to the maintenance of amino acid pools in yeast; during the first two hours of nitrogen starvation, the intracellular amino acid level decreases rapidly, and is then partially recovered in an autophagy-dependent manner [27], [28]. Since the amino acids produced by enhanced autophagy are utilized for new protein synthesis [27], [28], we investigated whether specific amino acids produced by autophagy contribute to cell cycle re-progression after a G2/M delay.
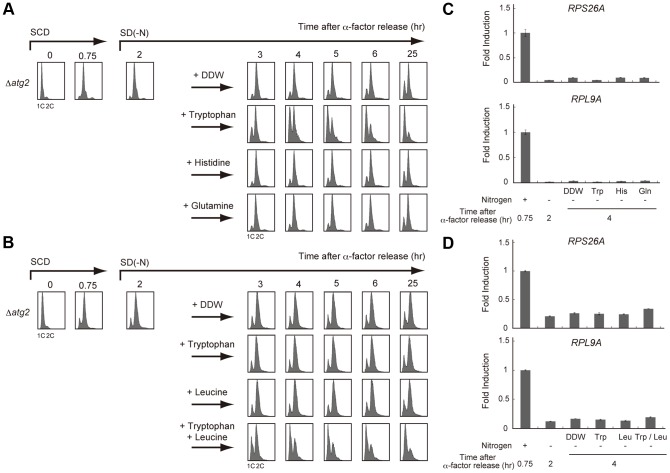
The Δatg2 strain AMY250 possesses his3, trp1, and ura3 mutations causing auxotrophies for histidine, tryptophan, and uracil, respectively. We postulated that these auxotrophic mutations would affect the intracellular pools of the corresponding amino acids especially upon starvation. As hypothesized, the G2/M-delayed Δatg2 mutant returned to G1 phase similarly to wild-type (WT) cells when tryptophan was added to the nitrogen-starved medium (Figure 3A). In contrast, the addition of histidine did not affect the suppression of the prolonged G2/M delay in the Δatg2 mutant, nor did the addition of glutamine, the level of which is believed to be involved in TORC1 activity in yeast [29] (Figure 3A).
Figure 3. Supply of specific amino acids is sufficient for cell cycle re-progression after G2/M delay.
(A) Δatg2 (AMY250) cells were arrested at G1 by α-factor and released into SCD medium. Synchronous cultures were collected after 0.75 h and re-released into SD-N medium. After 2 h from α-factor release, each culture was supplemented with tryptophan, histidine, or glutamine at a final concentration of 25 µg/mL, 10 µg/mL and 25 µg/mL, respectively. Double distilled water (DDW) was used as a control. DNA content at each time point was measured by FACS analysis. (B) Δatg2 (AMY296) cells were arrested at G1 by α-factor and released into SCD medium. Synchronous cultures were collected after 0.75 h and re-released into SD-N medium. After 2 h from α-factor release, each culture was supplemented with tryptophan, leucine, or both tryptophan and leucine. DDW was used as a control. DNA content at each time point was measured by FACS analysis. Tryptophan and leucine were used at a final concentration of 25 µg/mL and 10 µg/mL, respectively. (C) Δatg2 (AMY250) cells were grown as described in (A). Total RNA was extracted and analyzed for the expression of RPS26A (upper panel) and RPL9A (lower panel) by RT-qPCR. Each sample was calibrated by TUB1. (D) Δatg2 (AMY296) cells were grown as described in (B). Total RNA was extracted and analyzed for the expression of RPS26A (upper panel) and RPL9A (lower panel) by RT-qPCR. Each sample was calibrated by TUB1.
Recent studies have shown that TORC1 activity is regulated by the availability of some amino acid species including leucine; this is dependent on the editing function of aminoacyl-tRNA transferase [30], [31]. Using another Δatg2 mutant (AMY296), which is congenic to AMY250 with adenine and leucine auxotrophies, we further examined the effect of amino acid supplementation to a starvation medium on autophagy-deficient cells. As shown in Figure 3B, the addition of tryptophan alone was insufficient for the cell cycle progression of AMY296. However, the simultaneous addition of tryptophan and leucine efficiently rendered the cells to a G1 arrest. These results show that cell cycle perturbations caused by a deficiency in autophagy may be suppressed by the addition of amino acids specific to the strains, suggesting that autophagy contributes to cell cycle progression by allocating some, but not all, amino acids that are present in a limited intracellular amount.
Next, we examined whether the addition of specific amino acids would upregulate the starvation-repressed TORC1 activity. Interestingly, while the addition of amino acids mimicked cell cycle progression in the autophagy-proficient cells, it was not associated with an increase in transcription of TORC1 downstream RPS26A and RPL9A (Figure 3C and 3D). This result raises the possibility that TORC1 recovery is not essential for cell cycle re-progression after a G2/M delay, although TORC1 re-activation correlates with re-progression.
The recovery of TORC1 activity may be dispensable for cell cycle progression under starvation conditions
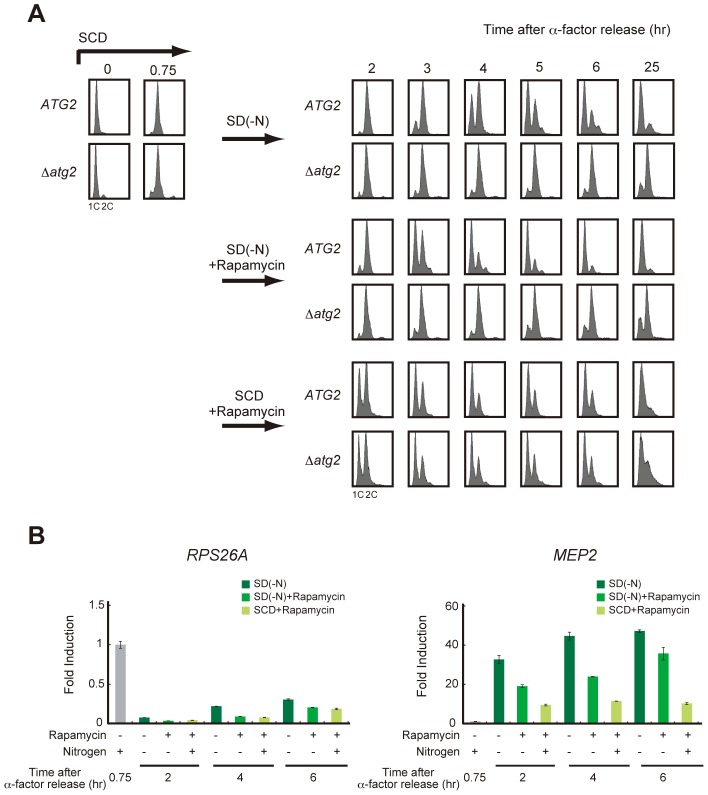
To examine the causal relationship between the recovery of TORC1 activity and cell cycle re-progression, we examined cell cycle progression during starvation when TORC1 activity was inhibited by the addition of rapamycin. As reported previously [20], rapamycin treatment transiently delayed cell cycle progression at G2/M in nutrient-rich SCD medium, and most cells returned to G1 irrespective of their autophagic competency (Figure 4A). In contrast, rapamycin-treated WT cells showed a G2/M delay and proceeded to G1 when rapamycin was added to the nitrogen-starved medium (Figure 4A). TORC1 activity, monitored by RPS26A expression, was persistently lower in rapamycin-treated cells than that in those without rapamycin treatment during starvation (Figure 4B), supporting the hypothesis that TORC1 recovery is dispensable for cell cycle re-progression after a G2/M delay. Cell cycle progression to G1 phase in rapamycin-treated starved cells was significantly faster than in those that without rapamycin treatment (Figure 4A). However, the effect of rapamycin was not observed in Δatg2 cells, in which the cell cycle remained arrested in the G2/M transition in nitrogen-starved medium, even with the addition of rapamycin (Figure 4A). Again, these results show that autophagy is critical for cell cycle progression from G2/M to G1 under starvation conditions, and suggest the involvement of autophagy-dependent supply of amino acid pools in the accelerated cell cycle progression by rapamycin treatment.
Figure 4. Recovery of TORC1 activity may be dispensable for cell cycle progression under starvation conditions.
(A) ATG2 (SAY122) and Δatg2 (AMY250) cells were arrested at G1 by α-factor and released into SCD medium. Synchronous cultures were collected after 0.75 h and re-released into SD-N medium, SD-N medium containing rapamycin, or SCD medium containing rapamycin. DNA content was measured by FACS analysis. Rapamycin was used at a final concentration of 200 ng/mL. (B) ATG2 (SAY122) cells were grown as described in (A). Total RNA was extracted and analyzed for the expression of RPS26A (left panel) and MEP2 (right panel) by RT-qPCR. Each sample was calibrated by TUB1.
We further examined the role of TORC1 activity in cell cycle re-progression using a temperature-sensitive KOG1mutant (kog1-105) [20], encoding an essential component of TORC1. Since the kog1-105 mutant showed a G2/M arrest phenotype at the restrictive temperature, the experiment was performed at 25°C, a permissive temperature at which the mutant is able to proliferate. When cells were cultured under nitrogen-starved conditions, the G2/M delay was prolonged in kog1-105 cells compared to WT cells (Figure S3A). During the course of the experiment, TORC1 activity monitored by RPS26A expression was slightly lower in the kog1-105 mutant than in WT cells, both of which were exposed to nutrient-rich condition and nitrogen-starved conditions (Figure S3B). Moreover, the delay in cell cycle re-progression in the kog1-105 cells was relieved by the addition of rapamycin (Figure S3C). This result shows that further inhibition of TORC1 activity in kog1-105 cells by rapamycin is sufficient for cell cycle re-progression at G2/M, suggesting that the delay in cell cycle re-progression of the kog1-105 mutant is not likely due to the reduction in global TORC1 activity. Rather, kog1-105 may cause a defect in specific pathways downstream of TORC1, and the residual activity of TORC1 in the kog1-105 mutant, which can be inhibited by rapamycin, may prevent cell cycle recovery after a G2/M delay.
Autophagy is important for normal cell division and cell growth during nutrient starvation
We investigated how amino acids produced by autophagy contribute to re-progression of the cell cycle from a G2/M delay under starvation conditions. We first observed the morphology of WT and Δatg2 cells cultured under starvation conditions. As shown in Figure 5, the daughter cell of Δatg2 cells was smaller than that of WT cells 4 h after α-factor release, suggesting that amino acids produced by autophagy are important for sufficient cell growth during nutrient starvation.
Figure 5. Autophagy is important for cell growth under nutrient starvation conditions.
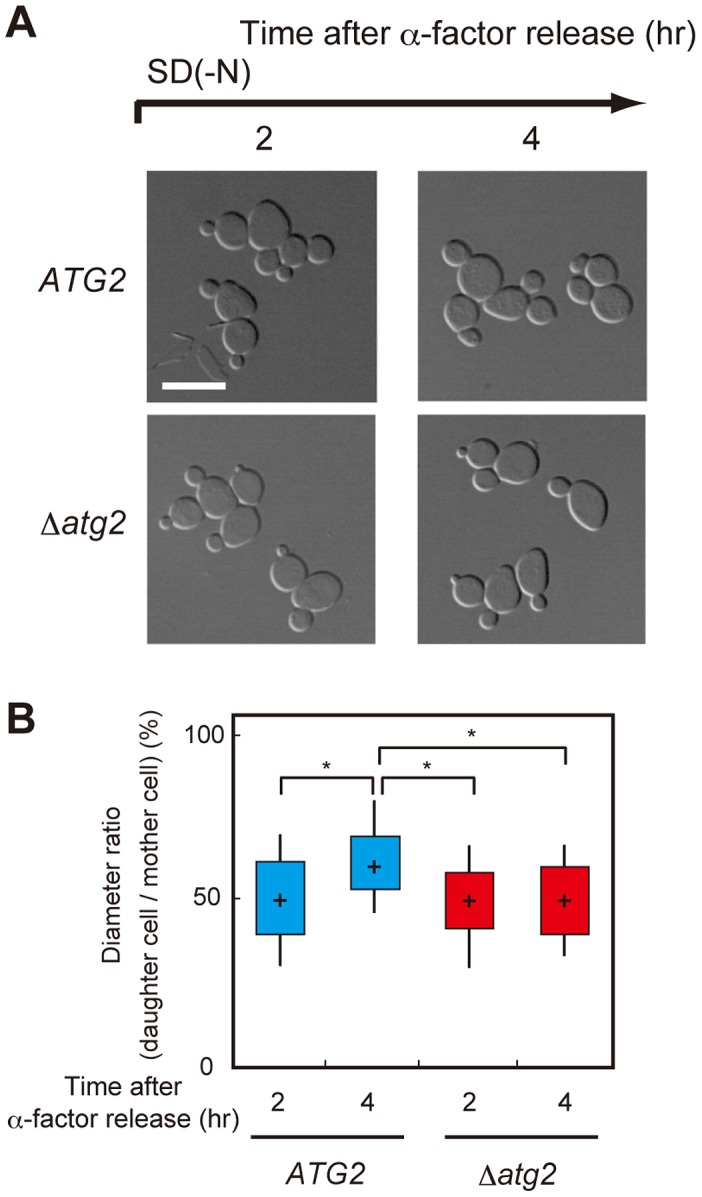
(A) ATG2 (SAY122), Δatg2 (AMY255) cells were arrested at G1 by α-factor and released into SCD medium. Synchronous cultures were collected after 0.75 h and re-released into SD-N medium. The fixed cells were observed by differential interference contrast microscopy. Bar, 10 µm. (B) ATG2 (SAY122) and Δatg2 (AMY255) cells were grown as described in (A). The ratio of the diameter of a bud or daughter cell to that of the mother was calculated 2 h and 4 h after α-factor release. The box plots represent the distribution of the ratios. The plot shows the medians (central cross figure) with the 25th and 75th percentiles (box). The lower line protruding from the box ends at the 10th percentiles, whereas the upper line ends at the 90th percentile. Single asterisks designate significant differences (P<0.01); unstarred comparisons are not significantly different (P>0.05).
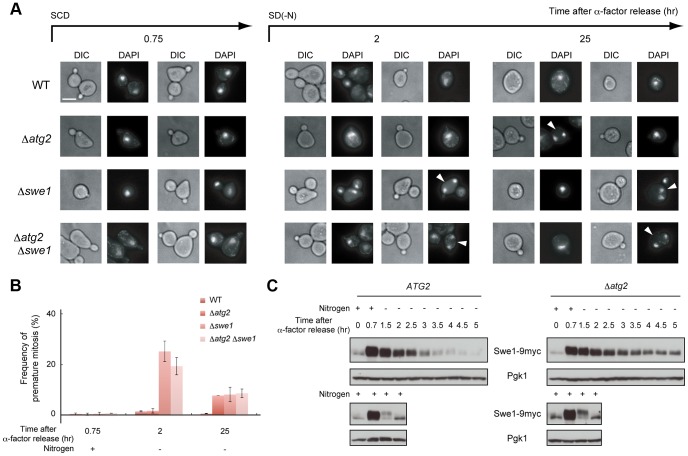
The status of the bud (daughter cell) and timing of nuclear division are strictly regulated by the Swe1-dependent checkpoint mechanism in budding yeast [32]–[35]. Therefore, we further analyzed cell division using Δswe1 and Δatg2 Δswe1 cells by DAPI staining to examine the potential relationship between the checkpoint mechanism and autophagy. As shown in Figure 6A and 6B, loss of the Swe1 function in both WT and Δatg2 cells caused an increase in premature mitosis at early time points (2–3 h after α-factor release; type 4 in Figure 7A). Interestingly, some of the Δswe1 cells arrested at 2C DNA content underwent normal cell division and returned to the G1 phase after 25 h (Figure S4), suggesting that premature mitosis caused by Swe1 dysfunction was rescued. This result is likely due to the spindle orientation checkpoint [36], which can correct an abnormal nuclear position at later time points.
Figure 6. Autophagy is important for normal cell division under nutrient starvation conditions.
(A) WT (SAY122), Δatg2 (AMY255), Δswe1 (AMY260), and Δatg2 Δswe1 (AMY261) cells were arrested at G1 by α-factor and released into SCD medium. Synchronous cultures were collected after 0.75 h and re-released into SD-N medium. The fixed cells were stained with DAPI and observed by differential interference contrast microscopy (DIC) or by fluorescence microscopy (DAPI). The arrowheads indicate cells undergoing premature mitosis (type 4 in Figure 7A). Bar, 5 µm. (B) WT (SAY122), Δatg2 (AMY255), Δswe1 (AMY260), and Δatg2 Δswe1 (AMY261) cells were grown as described in (A). The frequency of premature mitosis (type 4 in Figure 7A) was calculated. (C) ATG2 (YYK536) [66] and Δatg2 (AMY330) cells were arrested at G1 by α-factor and released into SCD medium. Synchronous cultures were collected after 0.7 h and re-released into SD-N medium or SCD medium. To monitor cell cycle progression to G1 under the nutrient-rich condition, SCD medium was supplemented with 6.7 ng/mL α-factor for re-arrest at G1. Cell lysates were prepared as described in Figure 1A, and analyzed by immunoblot with anti-myc and anti-Pgk1 antibodies. Pgk1 was used as a loading control. Uncropped images of blots are shown in Figure S5.
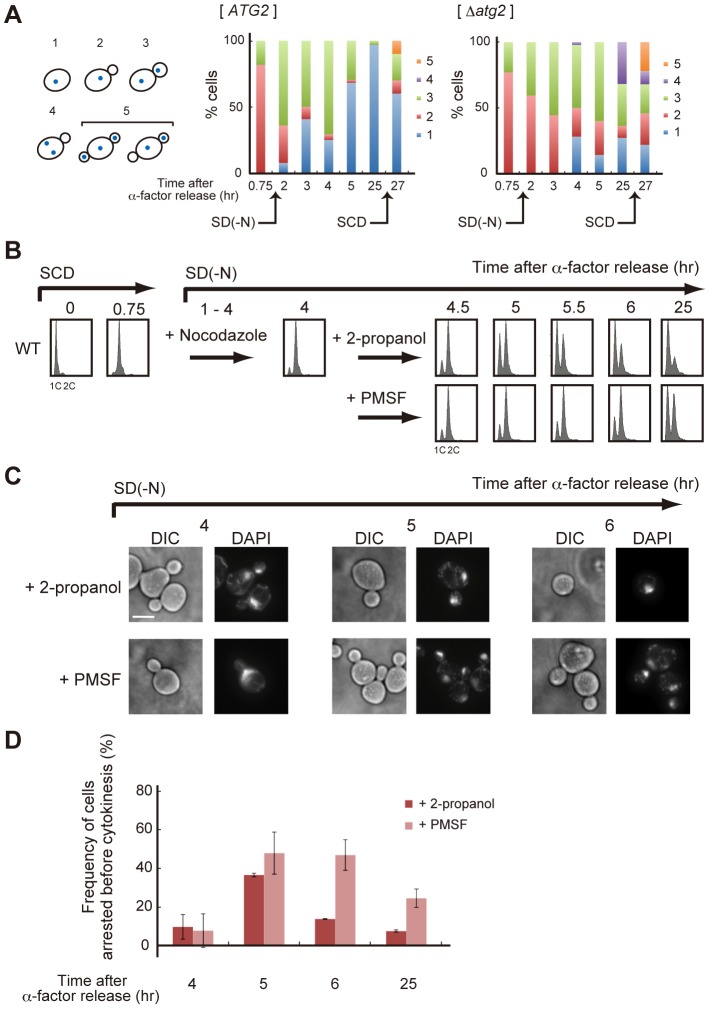
Figure 7. Autophagy is required for completion of cytokinesis.
(A) ATG2 (SAY122) and Δatg2 (AMY250) cells were arrested at G1 with α-factor and released into SCD medium. Synchronous cultures were collected after 0.75 h and re-released into SD-N medium. After 25 h, cell cultures were re-released into SCD medium. Cells were observed by differential interference contrast microscopy, and Hoechst 33342-stained nuclei and the tubulin-GFP of each cell were observed by fluorescence microscopy. The rates of cells with the following morphologies were calculated: 1) unbudded cells with a nucleus, 2) small-budded cells with a nucleus, 3) large-budded cells with one nucleus each in the mother and the daughter, 4) small-budded cells with two nuclei in the mother, and 5) cells harboring two small buds, with one nucleus each in the mother cells and at least one daughter cell. (B) WT (SAY122) cells were arrested at G1 by α-factor and released into SCD medium. Synchronous cultures were collected after 0.75 h and re-released into SD-N medium containing 5 µg/mL nocodazole. After 3 h, synchronous cultures arrested at metaphase by nocodazole were collected and re-released into SD-N medium containing 1 mM PMSF. 2-propanol was used as a control. DNA content at each time point was measured by FACS analysis. (C) WT (SAY122) cells were grown as described in (B). The fixed cells were stained with DAPI and observed by differential interference contrast microscopy (DIC) or by fluorescence microscopy (DAPI). Bar, 5 µm. (D) WT (SAY122) cells were grown as described in (B). The frequency of cells arrested before cytokinesis (type 3 in A) was calculated.
Since the Swe1-dependent checkpoint delays entry into mitosis by regulating Swe1 degradation [37], we examined the amount of Swe1 in response to nitrogen starvation. During normal cell cycling under nutrient-rich conditions, Swe1 was accumulated 40 min after α-factor release, and degraded after 1.5 h (Figure 6C). Under starvation conditions, Swe1 was more stable, and degraded only after 3 h (Figure 6C). These results support the hypothesis that the starvation-induced delay of mitosis is mediated by Swe1. This notion is consistent with our previous result that TORC1 regulates G2/M progression through budding yeast polo-like kinase Cdc5, a negative regulator of Swe1 in the initiation of mitosis [20].
In Δatg2 cells, degradation of Swe1 occurred normally in nutrient-rich medium, and was delayed under starvation conditions (Figure 6C). Indeed, in Δatg2 cells, cells that underwent premature mitosis were scarcely observed 2 h after α-factor release (Figure 6A and 6B). Degradation of Swe1 was delayed in Δatg2 cells, and a portion of Swe1 still remained 5 h after α-factor release (Figure 6C), showing that autophagy is important for Swe1 degradation in nutrient-starved cells. However, we noted that Swe1 decreased gradually in Δatg2 cells under starvation conditions. Consistently, after 2–3 h, approximately half of the Δatg2 cells were arrested before mitosis, but at later time points, the majority of the cells appeared to enter mitosis, as indicated by a reduction in the population of cells with one nucleus (type 2 in Figure 7A). Thus, autophagy is important for efficient recovery from the Swe1-dependent checkpoint under starvation conditions, although autophagy-deficient cells might, at least partly, execute nuclear division after a prolonged cell cycle delay. Nonetheless, cells cannot complete cell division without autophagy under starvation conditions because, unlike Δswe1 cells, the majority of Δatg2 Δswe1 cells remained arrested at 2C DNA content even 25 h after α-factor release (Figure S4). Indeed, cells that passed nuclear division but not cytokinesis (type 3 in Figure 7A) also accumulated transiently in WT cells 2–4 h after α-factor release, and such cells were consistently observed even at later time points in Δatg2 cells (Figure 7A).
To examine whether autophagy is involved in cell cycle progression after nuclear division, we used an anti-microtubule drug nocodazole to synchronize cell cycle at metaphase. WT cells were first synchronized in the G1 phase by treatment with α-factor, released into SCD medium, and then transferred into nitrogen-depleted medium containing nocodazole. After 3 h when the majority of cells was arrested at metaphase, cells were collected and re-released into SD-N medium containing 1 mM PMSF that specifically inhibited autophagic degradation under nutrient-starved conditions [38]. As shown in Figure 7B–7D, nocodazole-arrested cells showed a delay in completion of cytokinesis when autophagy was inhibited by the addition of PMSF. Therefore, autophagy contributes not only to nuclear division, but also to cytokinesis, or cell separation, under nutrient-starved conditions.
Autophagy deficiency results in an increased frequency of aneuploidy after the addition of a nitrogen source
Finally, we investigated the physiological significance of autophagy-dependent cell cycle re-progression from G2/M to G1 during starvation conditions. Δatg2 cells cultured in nitrogen-starved conditions for 24 h showed an increased frequency of aberrant mitosis, in which two nuclei were present in a mother cell (type 4 in Figure 7A; Figure 6A and 6B). This was likely due to the leaky recovery from the Swe1-dependent mitotic delay. Moreover, when cells were replenished with a nitrogen source and cultured for 2 h, cells with unusually high DNA content (3C DNA content) appeared in Δatg2 and Δatg1 mutants but not in WT cells (Figure 2A and Figure S2). In addition, analysis by a genetic system using intrachromosomal recombination [39] revealed that Δatg2 cells cultured in starvation medium displayed an increased frequency of aneuploidy (2.3-fold and 6.6-fold higher than that of WT cells after starvation for 24 and 48 h, respectively; Figure 8). These results indicate that cell cycle arrest in the G1 phase under starvation conditions is critical for the normal progression of mitosis after restoration of nutrient conditions, and highlight the importance of autophagy-dependent cell cycle re-progression in genome stability.
Figure 8. Autophagy deficiency results in an increased frequency of aneuploidy after the addition of a nitrogen source.
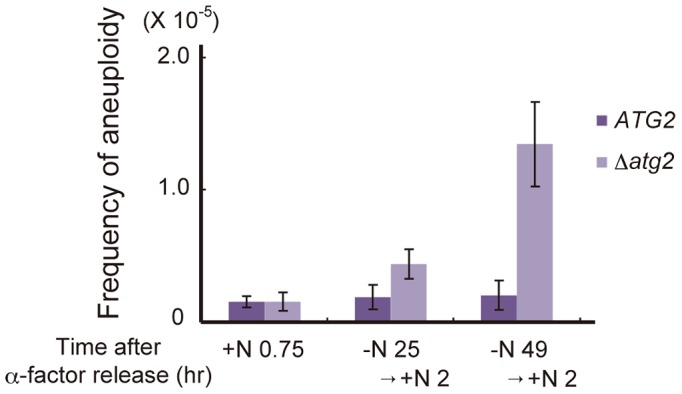
ATG2 (DBY4962) and Δatg2 (AMY262) cells arrested at G1 by α-factor were released into SC-Ura medium. Synchronous cultures were collected after 0.75 h and re-released into SD-N medium. After 25 h and 49 h, cell cultures were re-released into SCD medium. Appropriately diluted 0.75, 25+2 and 49+2 h aliquots were plated onto YEPD plates and selective medium lacking both leucine and uracil. Colonies were scored after 4 days at 30°C.
Discussion
Although nutrient starvation reduces TORC1 activity and subsequently induces cell cycle arrest in the G1 phase [19], cell cycle progression at the G2/M boundary was blocked under starvation conditions [20]–[23]. Autophagy has been implicated in cellular physiologies under starvation conditions, and the data presented herein uncover another aspect of autophagic functions, namely, its contribution to cell cycle regulation and genome integrity in nutrient-starved yeast cells through the regulation of mitosis progression.
Nuclear division and cytokinesis are two critical events during the cell cycle, both of which require protein synthesis [40]. Here we showed that, in addition to nuclear division, cytokinesis is another step of the cell cycle with limitations, whose entry is blocked or slowed by nutrient starvation, and that starvation-induced autophagy is required to overcome the cell cycle delay at both steps (Figure 6 and Figure 7). Previous reports have revealed that amino acids produced by autophagy are used for protein synthesis [27], [28]. In the present study, we showed that the defects in cell cycle re-progression caused by a lack of autophagy was suppressed by the addition of particular amino acids (Figure 3), and that autophagy promoted the growth of daughter cells in starvation medium (Figure 5). These results clearly show that the amino acid supply through autophagy contributes to sufficient cell growth during nutrient starvation. We found that this phenotype was associated with auxotrophies to specific amino acids, including leucine and tryptophan, but not histidine or nucleobases, including uracil and adenine (Figure 3). The composition of the amino acid pool in budding yeast cells is influenced by nutrient conditions; cells using NH4 + as the sole nitrogen source accumulate glutamic acid and arginine, but contain amino acids such as tyrosine, leucine, tryptophan, and phenylalanine at low levels [41]. Thus, we assume that, at least under our experimental conditions, leucine and tryptophan are limiting amino acids whose pools cannot support protein synthesis without autophagy upon nitrogen starvation. It will be interesting to examine the potential difference in amino acid requirements for the suppression of autophagy deficiency by varying the composition of amino acid pools using alternate nitrogen sources. In cultured human cells, the cellular pools of glutamine and glutamic acid are maintained at high levels, whereas those of tryptophan, cysteine, and arginine are maintained at low levels [42]. It is important to note that the latter three are essential amino acids that cannot be synthesized de novo in humans; thus, autophagy may be involved in maintaining the pools of these amino acids in human cells.
A recent paper reported that TORC1 activity was partially reactivated in an autophagy-dependent manner in ongoing starvation conditions, which played a role in the attenuation of autophagy [43]. Consistent with their findings, we demonstrated that starvation-induced autophagy leads to partial re-activation of TORC1 activity and that the timing of the re-activation correlates with that of cell cycle re-progression at G2/M (Figure 2). Although a reduction in TORC1 activity appears to contribute to the transient cell cycle delay at G2/M, we showed that the cell cycle delay was relieved under TORC1-repressed conditions (Figure 4). These results argue against the positive role of TORC1 re-activation during a rescue from the delay. Although TORC1 activity is involved in translation [19], previous studies have shown that the synthesis of specific proteins continues under TORC1-inhibited conditions [44]. Thus, even in case that TORC1 activity is not re-activated, protein synthesis supported by the autophagy-mediated amino acid pools may be sufficient for starvation-adapted cells to complete a final round of the cell cycle.
Although the inhibition of TORC1 by rapamycin did not abolish cell cycle re-progression from a G2/M delay, the temperature-sensitive kog1-105 mutation did affect this process (Figure 4 and Figure S3). There are three possible models to explain the cell cycle-specific phenotype of kog1-105 cells. In the first model, kog1-105 cells may be defective in only a part of multiple TORC1 functions, and the function affected by kog1-105 is necessary for cell cycle progression after a G2/M delay. In the second model, TORC1 may be significantly affected in kog1-105 and the level of TORC1 activity in the mutant is below that caused by nutrient starvation or rapamycin treatment in WT cells. The last model, based on the possibility that kog1-105 is a gain-of-function mutation, suggests that Kog1-105 protein fulfills a function in addition to the TORC1 function, which is not performed by the normal Kog1 protein. Our results showing that rapamycin suppresses the kog1-105 defect contradict the latter two models; therefore, it is likely that the kog1-105 mutation blocks only a portion of the pathways downstream of TORC1. This scenario is consistent with our previous findings that kog1-105 is not defective in the progression of G1 and does not induce autophagy under nutrient-rich conditions at a restrictive temperature [20]. We also found that the kog1-105 mutation affected the interaction between Kog1 and TORC1 [20]. Raptor, the mammalian ortholog of Kog1, contributes to the inhibition of mTOR activity upon nutrient depletion through stabilization of the mTOR-Raptor complex [45]. Although it is unknown whether Kog1 inhibits TORC1 under starvation conditions, a decreased interaction with TORC1 could contribute to the phenotypes observed in kog1-105, which are distinct from those caused by rapamycin treatment.
There are several examples of rapamycin-insensitive, TORC1-dependent processes in mammalian cells [46]. Although the presence of such processes is elusive in yeast, it is possible that rapamycin-insensitive TORC1 activity is involved in the recovery from a G2/M delay. However, under these circumstances, it is difficult to explain why the addition of rapamycin to nitrogen-depleted medium leads to an early recovery from a cell cycle delay (Figure 4A). It is interesting to note that the addition of rapamycin to fission yeast cells leads to early mitotic onset in nutrient-rich medium, resembling the cell cycle behavior caused by a reduction in the quality of the nutrient source [47]. In budding yeast, cellular responses to rapamycin are not identical to those induced by nitrogen starvation [48], [49]; rapamycin treatment rapidly activates the quality-sensitive nitrogen discrimination pathway, which is distinct from the nitrogen starvation pathway, to facilitate use of poor nitrogen sources. Such a difference may induce rapid adaptation to nitrogen starvation in rapamycin-treated cells. Indeed, the addition of rapamycin facilitates adaptation to an environment containing a low-quality nitrogen source in budding yeast [50]. We tested the involvement of the nitrogen discrimination pathway in the rapamycin-induced early cell cycle recovery by deleting GLN3, which encodes the key transcriptional regulator in the nitrogen discrimination pathway, and found that rapamycin treatment accelerated recovery from the G2/M delay even in Δgln3 mutants (data not shown). Therefore, we can at least conclude that rapamycin-induced transcription through Gln3 is not essential for this phenotype. It is known that TORC1 regulates a variety of cellular events including transcription, translation, and post-translation [51]. Note that autophagy is essential for rapamycin-mediated early cell cycle recovery (Figure 4A) and that protein synthesis supported by the amino acid pool appears to be involved in this mechanism. Further studies are required to specify the pathway responsible for the early recovery of cell cycle by rapamycin. In addition, it would be interesting to determine physiological conditions that induce the early cell cycle recovery phenotype in a similar manner to that caused by rapamycin treatment; such approaches will help clarify the significance of cell cycle regulation in response to acute inhibition of TORC1 activity.
We have shown that the G2/M specific function of TORC1 is mediated by the polo-like kinase Cdc5 [20], an upstream regulator of Swe1 [52]. Swe1 is involved in a checkpoint mechanism to ensure accuracy of cell division by monitoring daughter cells [32]–[35]. When this checkpoint mechanism is inhibited by the swe1 mutation, or overexpression of its negative regulators, HSL1 and HSL7, nuclear division occurs prematurely, even if the growth of the bud is suppressed by a mutation in CDC24 [53]. We observed a similar premature mitosis of Δswe1 cells under nitrogen starvation (Figure 5A and 5C), indicating that the Swe1-dependent checkpoint, probably activated by insufficient bud growth, contributes to the G2/M delay phenotype induced by starvation. We found that a deficiency in SWE1 also increased the fraction of cells that cannot return to the G1 phase normally (Figure S4), indicating that both the Swe1-dependent cell cycle delay and the autophagy-dependent recovery are critical for the integrity of mitosis. Thus, timely regulation of cell cycle progression is of significance under starvation conditions.
Although autophagy is required for the growth of daughter cells during starvation conditions (Figure 5), in our experimental conditions, more than 80% of the autophagy-deficient cells ultimately proceeded to nuclear division after delay at the G2/M boundary. This may be because cells can produce a limited pool of amino acids that are independent of autophagy that support maturation of the bud to overcome the Swe1-dependent checkpoint. Otherwise, long-term starvation might cause an imbalance in the amount of proteins regulating M phase entry, thereby initiating nuclear division, since the Swe1-dependent checkpoint does not appear to monitor bud size per se, but detects an accumulation of mitotic cyclins in the bud [54]. In either case, cell division is not completed before cytokinesis without autophagy. Cytokinesis may serve as an additional control gate for the fulfillment of daughter cell maturation under nutrient-limited conditions.
Cell cycle-dependent regulation of constitutive autophagy has been shown in mammalian cells [7]–[10]. However, this study established the direct involvement of autophagy in cell cycle regulation under starvation conditions; autophagy ensures the accomplishment of the final round of cell cycle progression to nutritionally starved cells. Without autophagy, prolonged treatment with nitrogen starvation caused perturbation of the cell cycle, including premature mitosis, and caused an increased frequency of aneuploidy in budding yeast (Figure 6A, 6B and Figure 8). Thus, in addition to the developmental significance of returning to the G1 phase under starvation conditions (i.e., meiotic division is only initiated from the G1 phase in diploid yeast cells), our results indicate that returning to the G1 phase is critical for maintaining genome integrity after restoration of the nutrient condition from starvation. Notably, a previous study reported that compromised autophagy promotes genomic instability, such as increased DNA damage, gene amplification, and aneuploidy in mammalian cells [55], consistent with the tumor-suppressive activity of autophagy that was previously reported [56]–[59].
Our results demonstrate that autophagy allows a final round of cell cycle progression in budding yeast cells by supplying amino acids during nutrient starvation. This type of regulation can be considered part of the feedback system that maintains the minimum cell volume after division. Asymmetric division, the manner of cell division in the budding yeast, would require sufficient de novo protein synthesis for producing a daughter cell different from the mother, and autophagy may support this under nutrient-starved conditions. It will be interesting to determine whether autophagy-dependent regulation is implicated in genetically determined asymmetric division in mammalian development, such as differentiation and maintenance of stem cells. Further studies will shed light on the physiological roles of autophagy, including cell cycle regulation and development.
Materials and Methods
Strains, plasmids, media, and genetics
The yeast strains used in this study are listed in Table S1. Unless otherwise noted, all strains were derived from W303-1A and W303-1B. DBY4962 was a gift from Dr. Botstein (Princeton University, USA) [39]. Standard genetic techniques including growth media, cell growth conditions, and transformations were performed as described previously [60], [61].
The plasmid, pRS316-GFP-ATG8, was a gift from Dr. Suzuki (University of Tokyo, Japan). It harbors the ATG8 sequence with an N-terminal GFP tag in pRS316 [62]. The GFP-ATG8 fragment was integrated into pRS314 to construct pRS314-GFP-ATG8. YEp352-ATG13, pRS313-KOG1, and pRS313-kog1-105 were described previously [20].
Immunoblotting
Samples corresponding to 0.2 OD600 (anti-GFP, anti-Pgk1, PAP, and anti-myc antibodies) or 0.1 OD600 (anti-Atg13 antibody) units of cells were separated by SDS-PAGE followed by Western blotting and immunodecoration. Signal detection was performed using an enhanced chemiluminescent (ECL) detection system (GE Healthcare) or Immunostar Zeta (Wako). The anti-Atg13 antibody was a gift from Dr. Ohsumi (Tokyo Institute of Technology, Japan). Anti-GFP (NACALAI TESQUE), anti-Pgk1 (Invitrogen), PAP (Sigma Aldrich), and anti-myc (Cell Signaling Technology) antibodies were used to detect GFP, Pgk1, TAP, and myc, respectively.
Real-time quantitative RT–PCR
RNA samples were prepared by the hot-phenol assay using phenol∶chloroform (5∶1; pH 4.7) (Sigma Aldrich) and the Qiagen RNeasy Mini Kit (Qiagen). The reverse transcriptase reaction was performed using the ReverTra Ace qPCR RT Kit (TOYOBO) or the ReverTra Ace qPCR RT Master Mix with gDNA Remover (TOYOBO). Real-time PCR was performed using SYBR Green Realtime PCR Master Mix Plus (TOYOBO) in duplicate. The mRNA levels of RPS26A, RPL9A, MEP2, GAP1, and NOG1 were quantified using corresponding primer sets purchased from OPERON. TUB1 and ACT1 were used as controls for quantification.
Cell synchrony and fluorescence-activated cell sorter (FACS) analysis
Cell synchronization in G1 was obtained by treating exponentially growing MAT a cells with 4 ng/mL α-factor mating pheromone for ∼2 h. G1-arrested cells were washed with distilled water twice and conditioned medium once, and released into fresh SCD medium. After 0.75 h, synchronized cells were washed with distilled water three times, released into fresh SD-N and SCD medium with or without 200 ng/mL rapamycin (Sigma Aldrich) and 6.7 ng/mL α-factor, and collected at the indicated times. For cell synchronization at metaphase, G1-synchronized cells were washed with distilled water three times, released into fresh SD-N medium containing 5 µg/mL nocodazole (Sigma Aldrich) after 0.75 h from α-factor release. After 3 h, synchronous cultures arrested at metaphase by nocodazole were collected and re-released into SD-N medium with or without 1 mM PMSF (Wako), and collected at the indicated times.
For FACS analysis, cells were fixed with 70% ethanol at 4°C overnight. Then the cells were washed and suspended in 50 mM sodium citrate, treated with 250 µg/mL RNase A at 50°C for 1 h, and then treated with 1 mg/mL proteinase K at 50°C for 1 h. The resuspended cells were stained with 16 µg/mL propidium iodide at room temperature for 30 min. The DNA content of cells was measured on a Beckman-Coulter flow cytometer.
Examination of nuclear DNA
Nuclear DNA was visualized by the addition of 1 µg/mL Hoechst 33342 or 50 ng/mL DAPI after cells were fixed with 3% formaldehyde at 4°C overnight. For staining by DAPI, cells fixed with formaldehyde were washed with PBS twice, and suspended in PBS with 0.1% Triton-X100. After 1 h, cells were washed with PBS 5 times, and suspended in PBS. Cells were observed using an inverted microscope (Delta Vision or Leica DMI 4000B). Images were captured using image acquisition and analysis software.
Quantification of aneuploidy formation
Quantification of aneuploidy formation was performed using the system developed by Chan and Botstein [39]. This assay is based on a genetic system that detects yeast cells with extra copies of a genetically marked chromosome. To monitor the frequency of aneuploidy, appropriately diluted cell cultures were plated onto yeast extract peptone dextrose (YEPD) plates and selective medium lacking both leucine and uracil. The number of colonies was scored after incubation for 4 days at 30°C.
Statistic analysis of diameter ratios
Data sets of diameter ratios were analyzed as described previously [63]. First, the data were subjected to the Kruskal-Wallis rank sum test, with P<0.05 considered significant. Then, the significance of differences between WT and Δatg2 cells was determined using Sokal and Rohlf's test of nonparametric multiple comparisons by STP [64]. Equal-sized subsets were obtained from the larger data subsets using a random selection algorithm.
Supporting Information
Validation of TUB1 as a control for quantification of RT-qPCR under starvation conditions. ATG2 (SAY122) and Δatg2 (AMY250) cells were grown as described in Figure 2A. Total RNA was extracted and analyzed for the expression of ACT1 (left panel) and TUB1 (right panel) by RT-qPCR. Each sample was calibrated by TUB1 and ACT1, respectively.
(PDF)
Atg1 is required for cell cycle progression during starvation. ATG1 (SAY122) and Δatg1 (AMY240) cells were arrested at G1 by α-factor and released into SCD medium. Synchronous cultures were collected at 0.75 h and re-released into SD-N medium or SCD medium. To monitor cell cycle progression to G1 under the nutrient-rich condition, SCD medium was supplemented with 6.7 ng/mL α-factor for re-arrest at G1. After 25 h, the cell cultures were re-released into SCD medium. DNA content at each time point was measured by FACS analysis.
(PDF)
Recovery of TORC1 activity may be dispensable for cell cycle progression during starvation conditions. (A) KOG1 (YYK409) and kog1-105 (YYK410) cells were arrested at G1 by α-factor and released into SCD medium at 25°C. Synchronous cultures were collected after 0.75 h and re-released into SD-N medium or SCD medium at 25°C. To monitor cell cycle progression to G1 under the nutrient-rich condition, SCD medium was supplemented with 6.7 ng/mL α-factor for re-arrest at G1. DNA content was measured by FACS analysis. (B) KOG1 (YYK409) and kog1-105 (YYK410) cells were grown as described in (A). Total RNA was extracted and analyzed for the expression of RPS26A (upper panel) and MEP2 (lower panel) by RT-qPCR. Each sample was calibrated by TUB1. Values represent the mean ± SEM (n = 3; *p<0.001, Student's t test). (C) KOG1 (YYK409) and kog1-105 (YYK410) cells were arrested at G1 by α-factor and released into SCD medium at 25°C. Synchronous cultures were collected after 0.75 h and re-released into SD-N medium or SD-N medium containing rapamycin. DNA content was measured by FACS analysis. Rapamycin was used at a final concentration of 200 ng/mL.
(PDF)
Autophagy is important for efficient recovery from the Swe1-dependent checkpoint under starvation conditions. Δswe1 (AMY260) and Δatg2 Δswe1 (AMY261) cells were arrested at G1 with α-factor and released into SCD medium. Synchronous cultures were collected after 0.75 h and re-released into SD-N medium or SCD medium. To monitor cell cycle progression to G1 under the nutrient-rich condition, SCD medium was supplemented with 6.7 ng/mL α-factor for re-arrest at G1. DNA content was measured by FACS analysis.
(PDF)
Strains used in this study.
(PDF)
Acknowledgments
This study was carried out under the NIBB Cooperative Research Program (12–323). We thank Y. Ohsumi (Tokyo Institute of Technology), K. Suzuki (University of Tokyo), K. Fujimura-Kamada (Hokkaido University), and D. Botstein (Princeton University) for providing materials; T. Maeda (University of Tokyo) for valuable discussions; and Textcheck for editing the manuscript.
Funding Statement
This work was supported in part by Grants-in-Aid for Scientific Research from JSPS and the MEXT of Japan to A Matsuura. A Matsui was supported by a JSPS Research Fellowship for Young Scientists. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Tsukada M, Ohsumi Y (1993) Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae . FEBS Lett 333: 169–174. [DOI] [PubMed] [Google Scholar]
- 2. Thumm M, Egner R, Koch B, Schlumpberger M, Straub M, et al. (1994) Isolation of autophagocytosis mutants of Saccharomyces cerevisiae . FEBS Lett 349: 275–280. [DOI] [PubMed] [Google Scholar]
- 3. Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y (2009) Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol 10: 458–467. [DOI] [PubMed] [Google Scholar]
- 4. Mizushima N (2007) Autophagy: process and function. Genes Dev 21: 2861–2873. [DOI] [PubMed] [Google Scholar]
- 5. Oku M, Sakai Y (2008) Pexophagy in Pichia pastoris. Methods Enzymol 451: 217–228. [DOI] [PubMed] [Google Scholar]
- 6. Kraft C, Deplazes A, Sohrmann M, Peter M (2008) Mature ribosomes are selectively degraded upon starvation by an autophagy pathway requiring the Ubp3p/Bre5p ubiquitin protease. Nat Cell Biol 10: 602–610. [DOI] [PubMed] [Google Scholar]
- 7. Mizushima N (2005) The pleiotropic role of autophagy: from protein metabolism to bactericide. Cell Death Differ 12 Suppl 2: 1535–1541. [DOI] [PubMed] [Google Scholar]
- 8. Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, et al. (2005) Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol 169: 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Furuya T, Kim M, Lipinski M, Li J, Kim D, et al. (2010) Negative regulation of Vps34 by Cdk mediated phosphorylation. Mol Cell 38: 500–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eskelinen EL, Prescott AR, Cooper J, Brachmann SM, Wang L, et al. (2002) Inhibition of autophagy in mitotic animal cells. Traffic 3: 878–893. [DOI] [PubMed] [Google Scholar]
- 11. Tsukamoto S, Kuma A, Murakami M, Kishi C, Yamamoto A, et al. (2008) Autophagy is essential for preimplantation development of mouse embryos. Science 321: 117–120. [DOI] [PubMed] [Google Scholar]
- 12. Jacinto E, Hall MN (2003) Tor signalling in bugs, brain and brawn. Nat Rev Mol Cell Biol 4: 117–126. [DOI] [PubMed] [Google Scholar]
- 13. Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, et al. (2002) Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 10: 457–468. [DOI] [PubMed] [Google Scholar]
- 14. Powers T, Walter P (1999) Regulation of ribosome biogenesis by the rapamycin-sensitive TOR-signaling pathway in Saccharomyces cerevisiae . Mol Biol Cell 10: 987–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martin DE, Soulard A, Hall MN (2004) TOR regulates ribosomal protein gene expression via PKA and the Forkhead transcription factor FHL1. Cell 119: 969–979. [DOI] [PubMed] [Google Scholar]
- 16. Honma Y, Kitamura A, Shioda R, Maruyama H, Ozaki K, et al. (2006) TOR regulates late steps of ribosome maturation in the nucleoplasm via Nog1 in response to nutrients. EMBO J 25: 3832–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamada Y, Yoshino K, Kondo C, Kawamata T, Oshiro N, et al. (2010) Tor directly controls the Atg1 kinase complex to regulate autophagy. Mol Cell Biol 30: 1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124: 471–484. [DOI] [PubMed] [Google Scholar]
- 19. Barbet NC, Schneider U, Helliwell SB, Stansfield I, Tuite MF, et al. (1996) TOR controls translation initiation and early G1 progression in yeast. Mol Biol Cell 7: 25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakashima A, Maruki Y, Imamura Y, Kondo C, Kawamata T, et al. (2008) The yeast Tor signaling pathway is involved in G2/M transition via polo-kinase. PLoS ONE 3: e2223 doi:10.1371/journal.pone.0002223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith EM, Proud CG (2008) cdc2-cyclin B regulates eEF2 kinase activity in a cell cycle- and amino acid-dependent manner. EMBO J 27: 1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hartmuth S, Petersen J (2009) Fission yeast Tor1 functions as part of TORC1 to control mitotic entry through the stress MAPK pathway following nutrient stress. J Cell Sci 122: 1737–1746. [DOI] [PubMed] [Google Scholar]
- 23. Wilkinson MG, Pino TS, Tournier S, Buck V, Martin H, et al. (1999) Sin1: an evolutionarily conserved component of the eukaryotic SAPK pathway. EMBO J 18: 4210–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnston GC, Pringle JR, Hartwell LH (1977) Coordination of growth with cell division in the yeast Saccharomyces cerevisiae . Exp Cell Res 105: 79–98. [DOI] [PubMed] [Google Scholar]
- 25. Beck T, Hall MN (1999) The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature 402: 689–692. [DOI] [PubMed] [Google Scholar]
- 26. Cardenas ME, Cutler NS, Lorenz MC, Di Como CJ, Heitman J (1999) The TOR signaling cascade regulates gene expression in response to nutrients. Genes Dev 13: 3271–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Onodera J, Ohsumi Y (2005) Autophagy is required for maintenance of amino acid levels and protein synthesis under nitrogen starvation. J Biol Chem 280: 31582–31586. [DOI] [PubMed] [Google Scholar]
- 28. Yang Z, Huang J, Geng J, Nair U, Klionsky DJ (2006) Atg22 recycles amino acids to link the degradative and recycling functions of autophagy. Mol Biol Cell 17: 5094–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crespo JL, Powers T, Fowler B, Hall MN (2002) The TOR-controlled transcription activators GLN3, RTG1, and RTG3 are regulated in response to intracellular levels of glutamine. Proc Natl Acad Sci U S A 99: 6784–6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bonfils G, Jaquenoud M, Bontron S, Ostrowicz C, Ungermann C, et al. (2012) Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol Cell 46: 105–110. [DOI] [PubMed] [Google Scholar]
- 31. Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, et al. (2012) Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 149: 410–424. [DOI] [PubMed] [Google Scholar]
- 32. McMillan JN, Sia RA, Lew DJ (1998) A morphogenesis checkpoint monitors the actin cytoskeleton in yeast. J Cell Biol 142: 1487–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barral Y, Parra M, Bidlingmaier S, Snyder M (1999) Nim1-related kinases coordinate cell cycle progression with the organization of the peripheral cytoskeleton in yeast. Genes Dev 13: 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harvey SL, Kellogg DR (2003) Conservation of mechanisms controlling entry into mitosis: budding yeast wee1 delays entry into mitosis and is required for cell size control. Curr Biol 13: 264–275. [DOI] [PubMed] [Google Scholar]
- 35. Theesfeld CL, Zyla TR, Bardes EG, Lew DJ (2003) A monitor for bud emergence in the yeast morphogenesis checkpoint. Mol Biol Cell 14: 3280–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Siller KH, Doe CQ (2009) Spindle orientation during asymmetric cell division. Nat Cell Biol 11: 365–374. [DOI] [PubMed] [Google Scholar]
- 37. Sia RA, Bardes ES, Lew DJ (1998) Control of Swe1p degradation by the morphogenesis checkpoint. EMBO J 17: 6678–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takeshige K, Baba M, Tsuboi S, Noda T, Ohsumi Y (1992) Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol 119: 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chan CS, Botstein D (1993) Isolation and characterization of chromosome-gain and increase-in-ploidy mutants in yeast. Genetics 135: 677–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burke DJ, Church D (1991) Protein synthesis requirements for nuclear division, cytokinesis, and cell separation in Saccharomyces cerevisiae . Mol Cell Biol 11: 3691–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Watson TG (1976) Amino-acid pool composition of Saccharomyces cerevisiae as a function of growth rate and amino-acid nitrogen source. J Gen Microbiol 96: 263–268. [DOI] [PubMed] [Google Scholar]
- 42. Piez KA, Eagle H (1958) The free amino acid pool of cultured human cells. J Biol Chem 231: 533–545. [PubMed] [Google Scholar]
- 43. Shin CS, Huh WK (2011) Bidirectional regulation between TORC1 and autophagy in Saccharomyces cerevisiae . Autophagy 7: 854–862. [DOI] [PubMed] [Google Scholar]
- 44. Fournier ML, Paulson A, Pavelka N, Mosley AL, Gaudenz K, et al. (2010) Delayed correlation of mRNA and protein expression in rapamycin-treated cells and a role for Ggc1 in cellular sensitivity to rapamycin. Mol Cell Proteomics 9: 271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, et al. (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110: 163–175. [DOI] [PubMed] [Google Scholar]
- 46. Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, et al. (2009) An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 284: 8023–8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Petersen J, Nurse P (2007) TOR signalling regulates mitotic commitment through the stress MAP kinase pathway and the Polo and Cdc2 kinases. Nat Cell Biol 9: 1263–1272. [DOI] [PubMed] [Google Scholar]
- 48. Hardwick JS, Kuruvilla FG, Tong JK, Shamji AF, Schreiber SL (1999) Rapamycin-modulated transcription defines the subset of nutrient-sensitive signaling pathways directly controlled by the Tor proteins. Proc Natl Acad Sci U S A 96: 14866–14870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cox KH, Kulkarni A, Tate JJ, Cooper TG (2004) Gln3 phosphorylation and intracellular localization in nutrient limitation and starvation differ from those generated by rapamycin inhibition of Tor1/2 in Saccharomyces cerevisiae . J Biol Chem 279: 10270–10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Neklesa TK, Davis RW (2009) A genome-wide screen for regulators of TORC1 in response to amino acid starvation reveals a conserved Npr2/3 complex. PLoS Genet 5: e1000515 doi:10.1371/journal.pgen.1000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Loewith R, Hall MN (2011) Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics 189: 1177–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bartholomew CR, Woo SH, Chung YS, Jones C, Hardy CF (2001) Cdc5 interacts with the Wee1 kinase in budding yeast. Mol Cell Biol 21: 4949–4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McMillan JN, Longtine MS, Sia RA, Theesfeld CL, Bardes ES, et al. (1999) The morphogenesis checkpoint in Saccharomyces cerevisiae: cell cycle control of Swe1p degradation by Hsl1p and Hsl7p. Mol Cell Biol 19: 6929–6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McNulty JJ, Lew DJ (2005) Swe1p responds to cytoskeletal perturbation, not bud size, in S. cerevisiae . Curr Biol 15: 2190–2198. [DOI] [PubMed] [Google Scholar]
- 55. Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, et al. (2007) Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev 21: 1367–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aita VM, Liang XH, Murty VV, Pincus DL, Yu W, et al. (1999) Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics 59: 59–65. [DOI] [PubMed] [Google Scholar]
- 57. Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, et al. (2003) Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest 112: 1809–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yue Z, Jin S, Yang C, Levine AJ, Heintz N (2003) Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A 100: 15077–15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, et al. (2011) Autophagy-deficient mice develop multiple liver tumors. Genes Dev 25: 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amberg DC, Burke DJ, Strathern JN, editors (2005) Methods In Yeast Genetics: COLD SPRING HARBOR LABORATORY PRESS.
- 61.Sambrook J, Russell DW, editors (2001) Molecular Cloning: COLD SPRING HARBOR LABORATORY PRESS.
- 62. Suzuki K, Kirisako T, Kamada Y, Mizushima N, Noda T, et al. (2001) The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J 20: 5971–5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hood-DeGrenier JK, Boulton CN, Lyo V (2007) Cytoplasmic Clb2 is required for timely inactivation of the mitotic inhibitor Swe1 and normal bud morphogenesis in Saccharomyces cerevisiae . Curr Genet 51: 1–18. [DOI] [PubMed] [Google Scholar]
- 64.Sokal RR, Rohlf J (1995) Biometry: the principles and practice of statistics in biological research, 3rd edition: W.H. Freeman and Company, New York
- 65. Shintani T, Klionsky DJ (2004) Cargo proteins facilitate the formation of transport vesicles in the cytoplasm to vacuole targeting pathway. J Biol Chem 279: 29889–29894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shitamukai A, Hirata D, Sonobe S, Miyakawa T (2004) Evidence for antagonistic regulation of cell growth by the calcineurin and high osmolarity glycerol pathways in Saccharomyces cerevisiae . J Biol Chem 279: 3651–3661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Validation of TUB1 as a control for quantification of RT-qPCR under starvation conditions. ATG2 (SAY122) and Δatg2 (AMY250) cells were grown as described in Figure 2A. Total RNA was extracted and analyzed for the expression of ACT1 (left panel) and TUB1 (right panel) by RT-qPCR. Each sample was calibrated by TUB1 and ACT1, respectively.
(PDF)
Atg1 is required for cell cycle progression during starvation. ATG1 (SAY122) and Δatg1 (AMY240) cells were arrested at G1 by α-factor and released into SCD medium. Synchronous cultures were collected at 0.75 h and re-released into SD-N medium or SCD medium. To monitor cell cycle progression to G1 under the nutrient-rich condition, SCD medium was supplemented with 6.7 ng/mL α-factor for re-arrest at G1. After 25 h, the cell cultures were re-released into SCD medium. DNA content at each time point was measured by FACS analysis.
(PDF)
Recovery of TORC1 activity may be dispensable for cell cycle progression during starvation conditions. (A) KOG1 (YYK409) and kog1-105 (YYK410) cells were arrested at G1 by α-factor and released into SCD medium at 25°C. Synchronous cultures were collected after 0.75 h and re-released into SD-N medium or SCD medium at 25°C. To monitor cell cycle progression to G1 under the nutrient-rich condition, SCD medium was supplemented with 6.7 ng/mL α-factor for re-arrest at G1. DNA content was measured by FACS analysis. (B) KOG1 (YYK409) and kog1-105 (YYK410) cells were grown as described in (A). Total RNA was extracted and analyzed for the expression of RPS26A (upper panel) and MEP2 (lower panel) by RT-qPCR. Each sample was calibrated by TUB1. Values represent the mean ± SEM (n = 3; *p<0.001, Student's t test). (C) KOG1 (YYK409) and kog1-105 (YYK410) cells were arrested at G1 by α-factor and released into SCD medium at 25°C. Synchronous cultures were collected after 0.75 h and re-released into SD-N medium or SD-N medium containing rapamycin. DNA content was measured by FACS analysis. Rapamycin was used at a final concentration of 200 ng/mL.
(PDF)
Autophagy is important for efficient recovery from the Swe1-dependent checkpoint under starvation conditions. Δswe1 (AMY260) and Δatg2 Δswe1 (AMY261) cells were arrested at G1 with α-factor and released into SCD medium. Synchronous cultures were collected after 0.75 h and re-released into SD-N medium or SCD medium. To monitor cell cycle progression to G1 under the nutrient-rich condition, SCD medium was supplemented with 6.7 ng/mL α-factor for re-arrest at G1. DNA content was measured by FACS analysis.
(PDF)
Strains used in this study.
(PDF)