Abstract
Background
The abundance of circulating endothelial cells (CECs) and circulating endothelial progenitor cells (CEPs), which serve as surrogate markers for angiogenesis, may be affected by chemotherapy. We studied their dynamic change during consecutive cycles of chemotherapy.
Methods
We collected blood samples from 15 breast cancer patients, who received a total of 56 courses of systemic chemotherapy, and measured the CECs, viable CECs (V-CECs), and CEPs by six-color flow cytometry within the seven days prior to chemotherapy, twice a week during the first and second cycles of chemotherapy, and then once a week during the subsequent cycles.
Results
The CEC, V-CEC, and CEP levels all significantly decreased from day 1 of treatment to the first week of chemotherapy. After one week of chemotherapy, the CEC and V-CEC levels returned to a level similar to day 1. The CEP level remained significantly reduced after the first week of chemotherapy, but gradually rebounded until the next course of chemotherapy. After six cycles of chemotherapy, the total number of CEC and V-CEC cells trended toward a decrease and the CEP cells toward an increase. Clinical factors, including the existence of a tumor, chemotherapy regimens, and the use of granulocyte colony stimulating factor, did not significantly affect these results.
Conclusions
The CEC and CEP counts change dynamically during each course of chemotherapy and after the chemotherapy cycles, providing background data for any future study planning to use CECs and CEPs as surrogate markers of angiogenesis in antiangiogenesis treatments combined with chemotherapy.
Keywords: Circulating endothelial cells, Endothelial progenitor cells, Breast cancer, Angiogenesis, Biomarkers
Background
Antiangiogenic drugs play an important role in the current treatment of cancer. However, single-agent antiangiogenesis therapy has had only a modest effect except in renal cell carcinoma. The combination of antiangiogenesis drugs and cytotoxic chemotherapy has been approved for colon, lung, and breast cancers [1-7]. Chemotherapy with the addition of bevacizumab, an antivascular endothelial growth factor (VEGF) antibody, significantly improves the overall survival of patients with metastatic colorectal cancer [1,3,4,6-8]. Bevacizumab can also improve the objective response rate and progression-free survival of breast and lung cancer patients treated with chemotherapy [9-18].
Although antiangiogenesis treatments are widely used, we still lack a surrogate marker to select the populations for whom the drug will be effective and to define the optimal biological dose and treatment timing for antiangiogenic therapy. Currently, the antiangiogenesis markers that have been extensively studied include functional imaging, serum angiogenesis-related markers, and measuring circulating endothelial cells (CECs).
The level of CECs, which are derived from the turnover of cells in the blood vessel wall, increases in certain cancers [19-25]. Circulating endothelial progenitor cells (CEPs), a subpopulation of CECs, have a progenitor-like phenotype, are derived from bone marrow, and contribute to the vasculogenesis of late-stage cancer [26-30]. Shaked et al. [31] demonstrated that CECs and CEPs could serve as surrogate markers to define the optimal biological dose in antiangiogenesis therapy. The baseline levels of CEC and CEP and the changes in their levels from pretreatment to post-treatment may serve as pharmacodynamic biomarkers to predict responses to antiangiogenesis therapy and to metronomic chemotherapy [31-35]. However, animal studies have shown that CEP mobilization is induced by treatment with the maximal tolerated dose (MTD) of chemotherapy, such as taxane and fluorouracil within a few days after the chemotherapy [36,37]. Preventing CEP mobilization with anti-VEGFR2 blocking antibodies could result in enhanced antitumor effects [37]. Therefore, the timing of the measurement of the CECs and CEPS is important when using them as surrogate markers, especially when antiangiogenesis therapy is combined with MTD chemotherapy. To clarify the dynamic pattern of CECs and CEPs, we designed a study to describe the detailed dynamic change of CECs and CEPs during each cycle of chemotherapy treatment.
Methods
Patients
Our study was approved by the Research Ethics Committee of National Taiwan University Hospital. We enrolled 15 breast cancer patients who received systemic chemotherapy in the form of neoadjuvant, adjuvant, or palliative chemotherapy. The patients were required to have histologically-confirmed breast carcinoma, to be 18 to 70 years old, to have an Eastern Cooperative Oncology Group (ECOG) performance status of less than 3, and to have hemoglobin levels above 9.0 mg/dL. Exclusion criteria were pregnancy, lactation, and uncontrolled underlying diseases, including active infections, systemic congestive heart failure, unstable angina, arrhythmia, or psychiatric disorders. All patients signed informed consents before beginning the study. Blood samples of 10 mL were drawn within the 7-day period prior to chemotherapy, then twice a week during first and second cycles of chemotherapy, and then once a week during the subsequent cycles of chemotherapy. The blood samples were processed for CEC and CEP analysis within 24 hours of collection (see below). The chemotherapy regimens and dosages depended on the doctors’ discretion.
Biomarker evaluations
Six-color flow cytometry measured the CECs and CEPs using a method from Mancuso et al. [19,38]. Red blood cells were lysed, and then the cell suspensions were evaluated by a BD FACSCanto II cell analyzer (BD Biosciences, San Jose, CA, USA) and FACSDiva software, version 5.0.2 (BD Biosciences), with analysis gates excluding dead cells, platelets, and debris. We acquired 100,000 events per sample to analyze the percentage of CECs and CEPs. The absolute number of CECs and CEPs was then calculated by multiplying the total white cell count by the percentage of events collected in the CEC and CEP enumeration gates. We defined CECs as negative for the hematopoietic marker CD45 (BD Pharmigen, San Diego, CA) and the progenitor marker CD133 (Miltenyi Biotec, Bergisch Glabdach, Germany), and positive for the endothelial markers CD31 and CD146 (BD Pharmigen). We defined CEPs as being negative for CD45 and positive for CD31, CD146, and CD133. We differentiated viable and apoptotic cells by 7-amino actinomycin D staining [39-41]. Representative flow cytometry dot plots demonstrating the negative controls, CEC and CEP gating strategies were provided in our supplementary data. (Additional file 1: Figure S1, Additional file 2: Figure S2, Additional file 3: Figure S3, Additional file 4: Figure S4).
Statistical analysis
Each individual patient received multiple courses of chemotherapy and was followed several times during each course of therapy. The data was two-level (patients and course of chemotherapy) repeated measurement. The changes in the levels of CEC, CEP, and viable-CEC (V-CEC) were analyzed after each course of chemotherapy by a multilevel linear mixed-effects model with random coefficients. Differences in the mean levels were considered patient-level random effects. Each patient’s chemotherapy courses were nested under the patient level as chemotherapy level. Within each chemotherapy course, the mean of the measures during the first week (day 2 to day 7), and the mean of the measures after the first week (day 8 and beyond) were compared to the measurement on the day chemotherapy started (day 1, before chemotherapy started). We analyzed the mean levels of CEC, CEP, and V-CEC for each chemotherapy course. We also analyzed how the existence of a tumor during the course of chemotherapy, the chemotherapy drug, and the use of granulocyte colony-stimulating factor (GCSF) affected these measurements. We modeled the correlation of repeated measurements within the chemotherapy courses of individual patients by first-order autoregressive error terms. Two-sided P values less than 0.05 were considered statistically significant. The “xtmixed” procedure from the Stata statistical package version 11 (StataCorp LP, College Station, TX, USA) analyzed the data.
Results
Patient characteristics
Of the 15 patients, 5 were tumor-bearing and 10 were non-tumor-bearing, with 3 (20%) receiving neoadjuvant chemotherapy, 10 (66.7%) receiving adjuvant chemotherapy, and 2 (13.3%) receiving palliative chemotherapy (Table 1). Among these fifteen patients, seven (46.7%) had GCSF administered during their courses of chemotherapy. A total of seven (46.7%) patients received an anthracycline-based chemotherapy regimen, seven (46.7%) received a taxane-based chemotherapy regimen, and two (13.3%) received other chemotherapy regimens that included vinorelbine and liposomal doxorubicin. The 15 patients received a median of four chemotherapy cycles (range: 1–6 cycles), with a total of 56 courses of chemotherapy overall in the study. The average duration between two courses of chemotherapy was 22.2 days.
Table 1.
Patient characteristics
| Characteristic | No. of patients | % | |
|---|---|---|---|
| No. of enrolled |
15 |
100.0% |
|
| Age, y |
|
|
|
| |
Median |
56 |
|
| |
Range |
44-66 |
|
| Chemotherapy | |||
| |
neoadjuvant |
3 |
20.0% |
| |
adjuvant |
10 |
66.7% |
| |
palliative |
2 |
13.3% |
| tumor hormone receptor | |||
| |
ER(+), PR(+) |
5 |
33.3% |
| |
ER(+), PR(-) |
3 |
20.0% |
| |
ER(-), PR(+) |
1 |
6.7% |
| |
ER(-), PR(-) |
5 |
33.3% |
| |
not applicable |
1 |
6.7% |
| Her2/neu | |||
| |
Absent |
5 |
33.3% |
| |
1+ |
3 |
20.0% |
| |
2+ |
4 |
26.7% |
| |
3+ |
2 |
13.3% |
| |
not applicable |
1 |
6.7% |
| GCSF administration | |||
| |
Yes |
7 |
46.7% |
| |
No |
8 |
53.3% |
| pT stage* | |||
| |
pT1 |
3 |
20.0% |
| |
pT2 |
9 |
60.0% |
| |
pT3 |
0 |
0.0% |
| |
pT4 |
3 |
20.0% |
| pN stage* | |||
| |
pN0 |
6 |
40.0% |
| |
pN1 |
4 |
26.7% |
| |
pN2 |
3 |
20.0% |
| |
pN3 |
1 |
6.7% |
| |
not applicable |
1 |
6.7% |
| Tumor grade | |||
| |
grade 1 |
2 |
13.3% |
| |
grade 2 |
6 |
40.0% |
| |
grade 3 |
6 |
40.0% |
| |
not applicable |
1 |
6.7% |
| Distant metastases | |||
| |
Yes |
2 |
13.3% |
| |
No |
13 |
86.7% |
| Chemotherapy regimen | |||
| |
Anthracycline-based |
7 |
46.7% |
| |
Taxane-based |
7 |
46.7% |
| Others** | 2 | 13.3% | |
*pT, pN stage: according to definition of AJCC 7th edition staging system.
**Others include: liposomal doxorubicin, vinorelbine. ER: estrogen receptor; PR: progesterone receptor; GCSF: Granulocyte colony-stimulating factor.
Dynamic change of CEC, V-CEC, and CEP number after chemotherapy
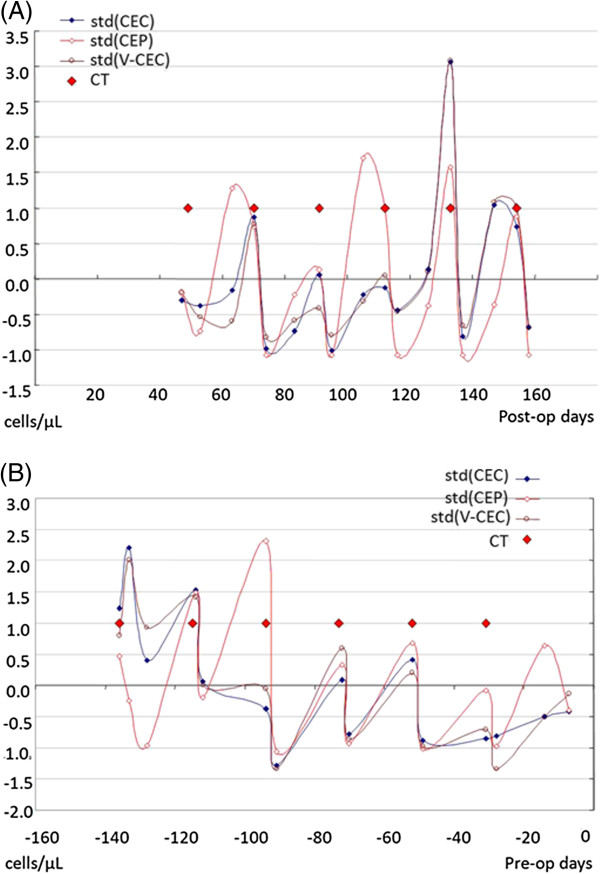
Figure 1 illustrates trends in the levels of CEC, V-CEC, and CEP as a function of days of chemotherapy post-operation in one representative patient who was not tumor-bearing, and as a function of days of chemotherapy prior to operation in one patient who was tumor-bearing. The non-tumor-bearing patient (Figure 1A), a 60-year-old woman diagnosed with pT2N2aM0 stage IIIA breast cancer, received adjuvant TEC chemotherapy (paclitaxel, epirubicin, cyclophosphamide) for a total of six cycles after a modified radical mastectomy. The tumor-bearing patient (Figure 1B), a 56-year-old woman diagnosed with pT4bN2M0 stage IIIB breast cancer, received neoadjuvant TEC chemotherapy for a total of six cycles before a modified radical mastectomy. The changes in the levels of CEC and CEP of both patients had a similar wave pattern, with the chemotherapy immediately reducing the CEC and CEP levels followed by a rebound in their levels. Representative flow cytometry dot plots data of dynamic change of CEC, CEP and viable-CEC levels before and after chemotherapy from one patient was provided in our supplementary data. (Additional file 5: Figure S5).
Figure 1.
Standardized trend of CEC, V-CEC, CEP, as a function of chemotherapy the day before or after tumor resection. (A) Data from a non-tumor-bearing patient who received adjuvant chemotherapy after tumor resection. (B) Data from a tumor-bearing patient who received neoadjuvant chemotherapy before tumor resection. Chemotherapy immediately reduced the CEC, V-CEC, and CEP numbers, followed by a rebound elevation of the CEC and CEP numbers. The “wave” patterns were similar between the non-tumor-bearing and tumor-bearing patients.
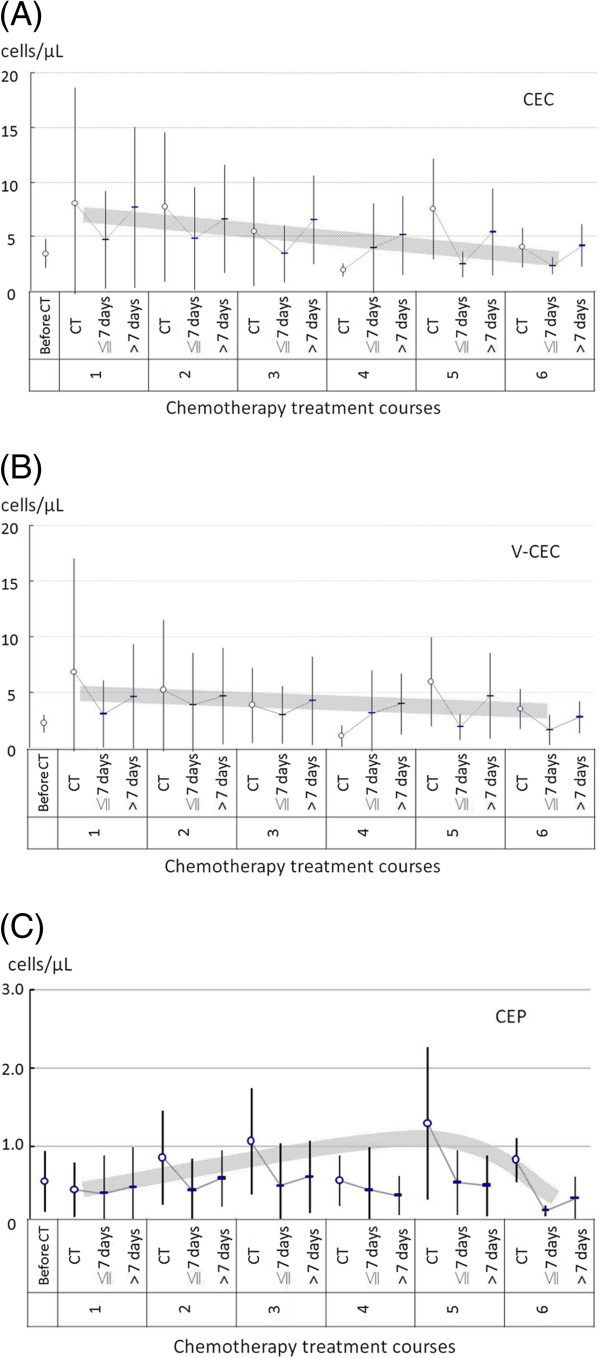
During the first week of chemotherapy, the mean CEC levels decreased by -2.92/μL (95% CI = -4.93, -0.92), V-CEC by -2.29/μL (95% CI = -3.86, -0.72), and CEP by -0.37/μL (95% CI = -0.58, -0.15) compared to the day that chemotherapy started (Figure 2 and Table 2). After the first week of treatment, their levels of CEC and V-CEC returned to levels not significantly different from their levels on the first day of chemotherapy. However, the level of CEP remained significantly reduced after the first week of chemotherapy. On the first day of subsequent courses of chemotherapy, the CEP level gradually rebounded back to a level similar to or even higher than the first day of the previous cycle of chemotherapy (Figure 2C).
Figure 2.
The means and standard deviations of (A) CEC, (B) V-CEC, and (C) CEP along the course of treatment. Within a chemotherapy course, the means of the CEC, V-CEC, and CEP were all significantly decreased in the 1st week of chemotherapy as compared to the day chemotherapy started. After the 1st week of treatment, the means of the CEC and V-CEC returned to a level close to that of the starting day of chemotherapy (Figure 2A, 2B). However, the mean of the CEP remained significantly reduced after the 1st week of chemotherapy (Figure 2C). Note that the CEP level gradually rebounded back to a level similar to or even higher than the day 1 count of the previous chemotherapy cycle (Figure 2C). The gray shadow denotes the trends of CEC, V-CEC, and CEP among the courses of chemotherapy
Table 2.
CEC, V-CEC, CEP trends within and among the chemotherapy courses
|
Trends within chemotherapy course |
|||||
|---|---|---|---|---|---|
| Outcome measurement | Time point | Coefficient (cells/μL) | 95% CI | p value | |
| CEC |
start of CT |
reference |
|
|
|
| |
1st wk of CT |
−2.924 |
(-4.93,−0.92) |
< 0.01 |
|
| |
after 1st wk of CT |
−0.909 |
(-2.80,0.98) |
0.35 |
|
| |
before 1st CT |
−5.848 |
(-9.83,−1.86) |
< 0.01 |
|
| V-CEC |
start of CT |
reference |
|
|
|
| |
1st wk of CT |
−2.289 |
(-3.86,−0.72) |
< 0.01 |
|
| |
after 1st wk of CT |
−0.996 |
(-2.56,0.57) |
0.21 |
|
| |
before 1st CT |
−4.014 |
(-7.29,−0.73) |
0.02 |
|
| CEP |
start of CT |
reference |
|
|
|
| |
1st wk of CT |
−0.366 |
(-0.58,−0.15) |
< 0.01 |
|
| |
after 1st wk of CT |
−0.295 |
(-0.47,−0.12) |
< 0.01 |
|
| Trends among courses of chemotherapy |
|||||
| Measurement |
Terms |
Coefficient (cells/μL) |
95% CI |
p value |
|
| CEC |
Linear |
−0.609 |
(-1.12,-0.10) |
0.02 |
|
| V-CEC |
Linear |
−0.288 |
(-0.72,0.15) |
0.19 |
|
| CEP |
Linear |
0.194 |
(0.03,0.36) |
0.02 |
|
| Quadratic | −0.03 | (-0.06,0.01) | 0.02 | ||
Before chemotherapy was first initiated, the mean levels CEC and V-CEC were significantly lower as compared to the initiation day of the 1st course of chemotherapy (P < 0.01 and P < 0.02, respectively; Table 2). The level of CEC significantly declined (P = 0.02) and the V-CEC trended toward a decline over the courses of chemotherapy (gray shadows in Figure 2). The level of CEP gradually increased during the first three courses of chemotherapy and declined during the later treatment courses (Figure 2C). This “n”-shaped, convex trend was represented by a set of statistically significant positive linear (P = 0.02) and negative quadratic (P = 0.02) terms (Table 2).
The measurements were not significantly associated with factors including the presence of a tumor, the operation status, the drug used, and the use of GCSF (all P values > 0.1; Table 3).
Table 3.
CEC, V-CEC, and CEP levels among different clinical conditions
| |
CEC |
V-CEC |
CEP |
|||
|---|---|---|---|---|---|---|
| Variable | Coef* (cells/μL) | p value | Coef (cells/μL) | p value | Coef (cells/μL) | p value |
| Tumor bearing |
−0.57 |
0.74 |
−1.08 |
0.46 |
0.10 |
0.44 |
| Chemotherapy regimen* | ||||||
| CEF |
0.04 |
0.99 |
0.65 |
0.82 |
0.12 |
0.64 |
| Liposomal doxorubicin |
−3.00 |
0.58 |
−0.43 |
0.92 |
−0.03 |
0.93 |
| N-HDFL |
1.79 |
0.70 |
3.22 |
0.40 |
0.20 |
0.55 |
| TCH |
−1.42 |
0.63 |
−0.55 |
0.82 |
0.01 |
0.98 |
| TEC |
1.08 |
0.79 |
2.50 |
0.45 |
0.27 |
0.32 |
| TH |
(Ref) |
|
(Ref) |
|
(Ref) |
|
| G-CSF use | 0.03 | 0.85 | −0.02 | 0.86 | −0.004 | 0.78 |
*Coef = adjusted regression coefficient.
*CEF: cyclophosphamide, epirubicin, fluorouracil.
N-HDFL: vinorelbine, continuous infusion fluorouracil and leucovorin.
TCH: docetaxel, carboplatin, herceptin.
TEC: docetaxel, epirubicin, cyclophosphamide.
TH: docetaxel, herceptin.
Discussion
We have clinically demonstrated the dynamic pattern of CEC and CEP during the course of chemotherapy treatment. The mean levels of CEC, V-CEC, and CEP all significantly decreased during the first week of chemotherapy. Then the levels of CEC and V-CEC returned gradually after one week whereas the level of CEP remained significantly reduced. After six cycles of chemotherapy, the level of CEC decreased significantly, V-CEC trended toward a decrease, and CEP increased significantly. These results were not significantly affected by clinical factors including the existence of a tumor, the chemotherapy regimen, and the use of GCSF.
The sole antigen to distinct CEPs with CECs is CD133 at present. However, human CD133 is not only expressed in endothelial progenitor cells but also in haematopoietic progenitor cells and bi-potential haemangioblasts. Currently, a phenotypic distinction between these three kinds of cells is not feasible and controversial [42]. In our six-color flow cytometry, CD45 expression can be used to exclude haematopoietic cells from the analysis. Theoretically, CD146 is expressed in endothelial cells but not platelet, and CD133 expression can be used to differentiate platelet and endothelial microparticles from CEP. However, lack of DNA staining is at a risk since a nuclear-staining molecule can be useful to exclude aggregated platelets and/or endothelial microparticles (which share some surface markers with CECs and CEPs) from the CEC count [42].
Longitudinal follow-up found that the levels of CEC and V-CEC increased from the baseline before chemotherapy to the first day of the first cycle of chemotherapy, but this did not occur with the CEPs (Figure 2). Current thought is that CECs are mature endothelial cells that have detached from their basement membrane in response to a blood vessel injury [24,42]. The increase in CEC and V-CEC levels we observed may be due to vessel damage caused by implanting port-A catheters or by tumor resection surgery, since all of the patients were treated with one of these two procedures before the first day of the first cycle of chemotherapy. The CEPs are progenitor cells that are recruited from bone marrow rather than detached from the vessel wall, and their level did not change in a similar manner.
Since several studies have indicated that levels of CEC and CEP correlate with tumor size and grade, [20,40,43-46] we assumed that tumor-bearing patients would have higher CEC and CEP levels than patients without tumors, but our analysis found no significant differences. Perhaps CEC and CEP levels correlate with not just the presence of a tumor, but with many factors, including tumor size, tumor grade, vascular invasion, and lymphatic invasion. Although patients who received adjuvant chemotherapy mostly had no gross residual tumor, but vascular or lymphatic invasion, even cancer related cytokines was possible and may explain for the CEC and CEP kinetics. Since our analysis of CEC and CEP levels in tumor–bearing and non–tumor bearing patients included a mix of many variables, we cannot make a conclusion about which variables affect their levels. Here we provided some representative figures of the CEC and CEP kinetics after administration of chemotherapy in tumor-bearing and non-tumor bearing patients (Additional file 6: Figure S6). The CEC and CEP kinetics consistently showed similar wave pattern and had no obvious differences between patients with and without tumor bearing. This explanation is also consistent with a study that found that CEC and CEP levels did not significantly change before and after surgery in 15 breast cancer patients, although vascular invasion and tumor size were independently associated with the CEC levels [43].
Administering GCSF to mice has been found to elevate CEP and accelerate tumor growth [47-49]. A human study analyzing CEC levels in four patients (one with seminoma, two with nonseminoma testis cancer, and one with small-cell lung cancer) receiving chemotherapy with the support of GCSF found that three out of four patients had CEC amounts increased 8- to 9-fold 3–8 days after GCSF administration [50]. In the same study, the CEC level was almost undetectable in leukopenic patient. This implies that the GCSF effect on CEC levels may be markedly related to bone marrow recovery after chemotherapy. When we compared the seven patients who received GCSF during chemotherapy courses to the eight who did not, we found no differences in the dynamic trends in the CEC and CEP levels between these groups. Since our patients received different chemotherapy regimens and may have different grades of bone marrow suppression which lead to different timing of bone marrow recovery. We suggest that in our study, the effects of chemotherapy on CEC and CEP may mask the GCSF effect.
We wanted to understand whether CEC and CEP levels are affected differently by different chemotherapy agents. Certain chemotherapy drugs, such as taxane and fluorouracil, have been demonstrated to rapidly induce CEP mobilization and subsequent tumor homing, while others, such as gemcitabine, cisplatin, and doxorubicin, do not [37]. Our analysis of 29 cycles of taxane-based chemotherapy and 26 cycles of non-taxane-based chemotherapy found no significant differences in the dynamic trends of these two groups. However, this is not conclusive, since all of the chemotherapy regimens in this study used multiple chemotherapy agents and it is difficult to evaluate the effect of single chemotherapy agent on CEC/CEP kinetics. We provided some representative figures of the CEC and CEP kinetics after administration of different types of chemotherapy regimens in five different patients (Additional file 7: Figure S7). Their CEC and CEP kinetics consistently show similar wave pattern. It suggests that dynamic changes of CEC and CEP induced by chemotherapy may have more significant effect than using different drugs. It supports our conclusion that timing of measurement of CEC and CEP after chemotherapy should be seriously considered and unified in the future studies using CEC and CEP as endpoint.
The levels of CEC and V-CEC significantly decreased as the number of chemotherapy courses progressed (P = 0.02), but the CEP levels significantly increased during the first three courses of chemotherapy (P = 0.02) and then declined during later treatment courses. Although this study was restricted by the small number of patients and chemotherapy cycles, the trends we observed were consistent with the analysis of Furstenberger et al. [39]. They analyzed the levels of CEC and CEP before and after neoadjuvant chemotherapy in breast cancer patients and found that, after two cycles of chemotherapy, the CEC levels decreased and the CEP levels increased. This phenomenon may be explained by the CEPs behaving as progenitor cells that could be mobilized from the bone marrow by a regular dose of chemotherapy. Although the CEPs would gradually differentiate into CECs, the next course of chemotherapy might destroy some cells that were in transition before they could fully differentiate into CECs. This may explain why CEC levels decreased and CEP levels increased after cycles of chemotherapy.
Many preclinical studies have indicated that CEPs contribute to tumor growth [23,40,43], which warrants further concern that chemotherapy may remobilize CEPs and trigger additional tumor growth. We demonstrated that the CEC and CEP levels decreased in the first week of chemotherapy, CEC increased after one week with each cycle of chemotherapy, and CEP rebounded even more slowly than CEC. This suggests to us that the rate of tumor control achieved by chemotherapy may theoretically be helped by adding metronomic chemotherapy or an antiangiogenic agent one week after chemotherapy to suppress the rebound of CEC and CEP. This hypothesis warrants further prospective study.
Our observation that CEC rebounds earlier than CEP has two possible explanations: 1) CEP is not the only source of CEC, which has three main sources: tumor vasculature, normal vasculature, and CEP. The three sources could explain why CEC is not fully synchronized with CEP. 2) The CEPs are a kind of progenitor cell where one CEP may differentiate into a population of CECs, which would explain the earlier rise in the slope of the CECs as compared to the CEPs.
Conclusions
The levels of CEC and CEP change dynamically during and after each course of chemotherapy. Careful selection of the timing of sample collection in each chemotherapy cycle is needed when using CEC and CEP as surrogate markers of angiogenesis.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Yu-Hsuan Kuo, Ching-Hung Lin, Wen-Yee Shau, Te-Jung Chen, Shih-Hung Yang, Shu-Min Huang, Chun Hsu, Yen-Shen Lu, Ann-Lii Cheng. AL Cheng, YS Lu and Chin-Hung Line participated in the design of the study. TJ Chen and SM Huang carried out the six-color flow cytometry. WY Shau performed the statistical analysis. SH Yang and C Hsu contribute to acquisition of data. YH Kuo integrate the data and help to draft the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
CEC and CEP test were preformed within 24 hours of collection of blood samples. The gating strategy is described below. Exclude debris and red blood cells first. CEC and CEPs are within CD45dim population (P2). CD31/CD146 double positive population (Q2) were defined as CECs (S-Figure 1 and 2). Both CD31/CD133 (Q2-1) and CD146/CD133 (Q2-2) double positive were CEPs (S-Figure 1 and 2). CEPs number presented here were the average of Q2-1 dot number and Q2-2 dot number. For gating viable-CEC, excluding debris and red blood cells first and CEC and CEPs are within CD45dim population (P2). CD146 was CEC maker and 7AAD staining was used to identify the cell viability. Cells in Q2 are apoptotic CECs and in Q4 are viable CECs. (S-Figure 3 and 4) Unstain sample was used as a negative control (S-Figure 1 and 3). CEC/CEP and viable CEP gating was follow the unstain one. (S-Figure 2 and 4).
CEC and CEP test were preformed within 24 hours of collection of blood samples. The gating strategy is described below. Exclude debris and red blood cells first. CEC and CEPs are within CD45dim population (P2). CD31/CD146 double positive population (Q2) were defined as CECs (S-Figure 1 and 2). Both CD31/CD133 (Q2-1) and CD146/CD133 (Q2-2) double positive were CEPs (S-Figure 1 and 2). CEPs number presented here were the average of Q2-1 dot number and Q2-2 dot number. For gating viable-CEC, excluding debris and red blood cells first and CEC and CEPs are within CD45dim population (P2). CD146 was CEC maker and 7AAD staining was used to identify the cell viability. Cells in Q2 are apoptotic CECs and in Q4 are viable CECs. (S-Figure 3 and 4) Unstain sample was used as a negative control (S-Figure 1 and 3). CEC/CEP and viable CEP gating was follow the unstain one. (S-Figure 2 and 4).
CEC and CEP test were preformed within 24 hours of collection of blood samples. The gating strategy is described below. Exclude debris and red blood cells first. CEC and CEPs are within CD45dim population (P2). CD31/CD146 double positive population (Q2) were defined as CECs (S-Figure 1 and 2). Both CD31/CD133 (Q2-1) and CD146/CD133 (Q2-2) double positive were CEPs (S-Figure 1 and 2). CEPs number presented here were the average of Q2-1 dot number and Q2-2 dot number. For gating viable-CEC, excluding debris and red blood cells first and CEC and CEPs are within CD45dim population (P2). CD146 was CEC maker and 7AAD staining was used to identify the cell viability. Cells in Q2 are apoptotic CECs and in Q4 are viable CECs. (S-Figure 3 and 4) Unstain sample was used as a negative control (S-Figure 1 and 3). CEC/CEP and viable CEP gating was follow the unstain one. (S-Figure 2 and 4).
CEC and CEP test were preformed within 24 hours of collection of blood samples. The gating strategy is described below. Exclude debris and red blood cells first. CEC and CEPs are within CD45dim population (P2). CD31/CD146 double positive population (Q2) were defined as CECs (S-Figure 1 and 2). Both CD31/CD133 (Q2-1) and CD146/CD133 (Q2-2) double positive were CEPs (S-Figure 1 and 2). CEPs number presented here were the average of Q2-1 dot number and Q2-2 dot number. For gating viable-CEC, excluding debris and red blood cells first and CEC and CEPs are within CD45dim population (P2). CD146 was CEC maker and 7AAD staining was used to identify the cell viability. Cells in Q2 are apoptotic CECs and in Q4 are viable CECs. (S-Figure 3 and 4) Unstain sample was used as a negative control (S-Figure 1 and 3). CEC/CEP and viable CEP gating was follow the unstain one. (S-Figure 2 and 4).
Representative flow cytometry dot plot for defining viable CECs and apoptotic CECs, (A) Exclude debris and red blood cells. (B) CEC and CEPs are within CD45dim population (P2). (C)CD146 was CEC maker and 7AAD staining was used to identify the cell viability. Cells in Q2 are apoptotic CECs and in Q4 are viable CECsRepresentative data of dynamic change of CEC, CEP (S-Figure 5a, 5c, 5e and 5 g) and viable-CEC (S-Figure 5b, 5d, 5f and 5 h) levels during second cycle of chemotherapy from one patient. CEC, CEP (S-Figure 5a) and viable-CEC (S-Figure 5b) at the day before chemotherapy (for this patient, taxotere /epirubicin /cyclophosphamide) were shown in S-Figure 5a and 5b. Patient’s CEC, CEP and viable-CEC levels were dropping at day 4 and day 7 (S-Figure 5c, 5d, 5e and 5f) after chemotherapy. Three weeks after chemotherapy, CEC and CEP levels were increased again(S-Figure 5e) and most of the CECs were viable (S-Figure 5f).
Standardized trend of CEC, V-CEC, CEP, as a function of chemotherapy the day before tumor resection (A-D), or after tumor resection.(E-G). The CEC and CEP kinetics consistently showed similar wave pattern and had no obvious differences between patients with and without tumor bearing.
Standardized trend of CEC, V-CEC, CEP, as a function of different chemotherapy regimens. (A) Data from a patient who received adjuvant docetaxel, cisplatin, and herceptin. (B) Data from a patient who received adjuvant cyclophosphamide, epirubicin, and Fluorouracil. (C) Data from a patient who received adjuvant docetaxel, epirubicin, and cyclophophamide. (D) Data from a patient who received neoadjuvant docetaxel, Epirubicin, and cyclophophamide. (E) Data from a patient who received neoadjuvant vinorelbine and infusion fluorouracil. Their CEC and CEP kinetics consistently show similar wave pattern. It suggests that dynamic changes of CEC and CEP induced by chemotherapy may have more significant effect than using different drugs.
Contributor Information
Yu-Hsuan Kuo, Email: beethovan@gmail.com.
Ching-Hung Lin, Email: chinghlin@ntu.edu.tw.
Wen-Yi Shau, Email: wyshau265@cde.org.tw.
Te-Jung Chen, Email: anne_chen@tty.com.tw.
Shih-Hung Yang, Email: b4401064@yahoo.com.tw.
Shu-Min Huang, Email: 496926072@mail.fju.edu.tw.
Chun Hsu, Email: chsu1967@ntu.edu.tw.
Yen-Shen Lu, Email: yslu@ntu.edu.tw.
Ann-Lii Cheng, Email: alcheng@ntu.edu.tw.
Acknowledgements
This study was supported by grants NSC 100-2325-B-002 -067 from the National Science Council and DOH99-TD-C-111-001 from the Department of Health, Taiwan, R.O.C.
References
- Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R. et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL. et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008;26:3523–3529. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR. et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E, Rivera F, Berry S, Kretzschmar A, Michael M, DiBartolomeo M. et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20:1842–1847. doi: 10.1093/annonc/mdp233. [DOI] [PubMed] [Google Scholar]
- Vincenzi B, Santini D, Russo A, Spoto C, Venditti O, Gasparro S. et al. Bevacizumab in association with de Gramont 5-fluorouracil/folinic acid in patients with oxaliplatin-, irinotecan-, and cetuximab-refractory colorectal cancer: a single-center phase 2 trial. Cancer. 2009;115:4849–4856. doi: 10.1002/cncr.24540. [DOI] [PubMed] [Google Scholar]
- Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR. et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–3705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E. et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE) J Clin Oncol. 2008;26:5326–5334. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA. et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- Miles D, Chan A, Romieu G, Dirix LY, Cortes J, Pivot X. et al. Randomised, double-blind, placebo-controlled phase III study of bevacizumab with docetaxel or docetaxel with placebo as first-line therapy for patients with locally recurrent or metastatic breast cnacer: AVADO (abstract) J Clin Oncol. 2008;26:15. [Google Scholar]
- Cobleigh MA, Langmuir VK, Sledge GW, Miller KD, Haney L, Novotny WF. et al. A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol. 2003;30:117–124. doi: 10.1053/j.seminoncol.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Miles DW, Chan A, Dirix LY, Cortes L, Pivot X, Tomczak P. et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V. et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A. et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Fidler IJ. Angiogenesis and lung cancer: potential for therapy. Clin Cancer Res. 2000;6:4604–4606. [PubMed] [Google Scholar]
- Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM. et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Ramalingam SS, Dahlberg SE, Langer CJ, Gray R, Belani CP, Brahmer JR. et al. Outcomes for elderly, advanced-stage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of eastern cooperative oncology group trial 4599. J Clin Oncol. 2008;26:60–65. doi: 10.1200/JCO.2007.13.1144. [DOI] [PubMed] [Google Scholar]
- Sandler AB, Schiller JH, Gray R, Dimery I, Brahmer J, Samant M. et al. Retrospective evaluation of the clinical and radiographic risk factors associated with severe pulmonary hemorrhage in first-line advanced, unresectable non-small-cell lung cancer treated with Carboplatin and Paclitaxel plus bevacizumab. J Clin Oncol. 2009;27:1405–1412. doi: 10.1200/JCO.2008.16.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso P, Burlini A, Pruneri G, Goldhirsch A, Martinelli G, Bertolini F. et al. Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood. 2001;97:3658–3661. doi: 10.1182/blood.v97.11.3658. [DOI] [PubMed] [Google Scholar]
- Dome B, Timar J, Dobos J, Meszaros L, Raso E, Paku S. et al. Identification and clinical significance of circulating endothelial progenitor cells in human non-small cell lung cancer. Cancer Res. 2006;66:7341–7347. doi: 10.1158/0008-5472.CAN-05-4654. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Fellner S, Reiberger T, Ulbrich G, Ferlitsch A, Wacheck V. et al. Differential role of circulating endothelial progenitor cells in cirrhotic patients with or without hepatocellular carcinoma. Dig Liver Dis. 2009;41:902–906. doi: 10.1016/j.dld.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Brunner M, Thurnher D, Heiduschka G, Grasl M, Brostjan C, Erovic BM. et al. Elevated levels of circulating endothelial progenitor cells in head and neck cancer patients. J Surg Oncol. 2008;98:545–550. doi: 10.1002/jso.21139. [DOI] [PubMed] [Google Scholar]
- Ho JW, Pang RW, Lau C, Sun CK, Yu WC, Fan ST. et al. Significance of circulating endothelial progenitor cells in hepatocellular carcinoma. Hepatology. 2006;44:836–843. doi: 10.1002/hep.21353. [DOI] [PubMed] [Google Scholar]
- Blann AD, Woywodt A, Bertolini F, Bull TM, Buyon JP, Clancy RM. et al. Circulating endothelial cells. Biomarker of vascular disease. Thromb Haemost. 2005;93:228–235. doi: 10.1160/TH04-09-0578. [DOI] [PubMed] [Google Scholar]
- Jubb AM, Harris AL. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol. 2010;11:1172–1183. doi: 10.1016/S1470-2045(10)70232-1. [DOI] [PubMed] [Google Scholar]
- Lin Y, Weisdorf DJ, Solovey Anna, Robert PH. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumov GN, Bender E, Zurakowski D, Kang SY, Sampson D, Flynn E. et al. A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. J Natl Cancer Inst. 2006;98:316–325. doi: 10.1093/jnci/djj068. [DOI] [PubMed] [Google Scholar]
- Peters BA, Diaz LA, Polyak K, Meszler L, Romans K, Guinan EC. et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med. 2005;11:261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- Shaked Y, Voest EE. Bone marrow derived cells in tumor angiogenesis and growth: are they the good, the bad or the evil? Biochim Biophys Acta. 2009;1796:1–4. doi: 10.1016/j.bbcan.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Shaked Y, Ciarrocchi A, Franco M, Lee CR, Man S, Cheung AM. et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–1787. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- Shaked Y, Emmenegger U, Man S, Cervi D, Bertolini F, Ben-David Y. et al. Optimal biologic dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood. 2005;106:3058–3061. doi: 10.1182/blood-2005-04-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaked Y, Emmenegger U, Francia G, Chen L, Lee CR, Man S. et al. Low-dose metronomic combined with intermittent bolus-dose cyclophosphamide is an effective long-term chemotherapy treatment strategy. Cancer Res. 2005;65:7045–7051. doi: 10.1158/0008-5472.CAN-05-0765. [DOI] [PubMed] [Google Scholar]
- Munoz R, Man S, Shaked Y, Lee CR, Wong J, Francia G. et al. Highly efficacious nontoxic preclinical treatment for advanced metastatic breast cancer using combination oral UFT-cyclophosphamide metronomic chemotherapy. Cancer Res. 2006;66:3386–3391. doi: 10.1158/0008-5472.CAN-05-4411. [DOI] [PubMed] [Google Scholar]
- Mancuso P, Colleoni M, Calleri A, Orlando L, Maisonneuve P, Pruneri G. et al. Circulating endothelial-cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood. 2006;108:452–459. doi: 10.1182/blood-2005-11-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellapasqua S, Bertolini F, Bagnardi V, Campagnoli E, Scarano E, Torrisi R. et al. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol. 2008;26:4899–4905. doi: 10.1200/JCO.2008.17.4789. [DOI] [PubMed] [Google Scholar]
- Bertolini F, Paul S, Mancuso P, Monestiroli S, Gobbi A, Shaked Y. et al. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;63:4342–4346. [PubMed] [Google Scholar]
- Shaked Y, Henke E, Roodhart JM, Mancuso P, Langenberg MH, Colleoni M. et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14:263–273. doi: 10.1016/j.ccr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso P, Antoniotti P, Quarna J, Calleri A, Rabascio C, Tacchetti C. et al. Validation of a standardized method for enumerating circulating endothelial cells and progenitors: flow cytometry and molecular and ultrastructural analyses. Clin Cancer Res. 2009;15:267–273. doi: 10.1158/1078-0432.CCR-08-0432. [DOI] [PubMed] [Google Scholar]
- Furstenberger G, von Moos R, Lucas R, Thurlimann B, Senn HJ, Hamacher J. et al. Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy of primary breast cancer. Br J Cancer. 2006;94:524–531. doi: 10.1038/sj.bjc.6602952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik RP, Jin D, Chuang E, Gold EG, Tousimis EA, Moore AL. et al. Circulating endothelial progenitor cells correlate to stage in patients with invasive breast cancer. Breast Cancer Res Treat. 2008;107:133–138. doi: 10.1007/s10549-007-9519-6. [DOI] [PubMed] [Google Scholar]
- Shaked Y, Bertolini F, Emmenegger U, Lee CR, Kerbel RS. On the origin and nature of elevated levels of circulating endothelial cells after treatment with a vascular disrupting agent. J Clin Oncol. 2006;24:4040–4041. doi: 10.1200/JCO.2006.07.1175. [DOI] [PubMed] [Google Scholar]
- Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- Goon PK, Lip GY, Stonelake PS, Blann AD. Circulating endothelial cells and circulating progenitor cells in breast cancer: relationship to endothelial damage/dysfunction/apoptosis, clinicopathologic factors, and the Nottingham Prognostic Index. Neoplasia. 2009;11:771–779. doi: 10.1593/neo.09490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale D, Phay C, Brown W, Gray-Statchuk L, Furlong P, Lock M. et al. Flow cytometric assessment of monocyte activation markers and circulating endothelial cells in patients with localized or metastatic breast cancer. Cytometry B Clin Cytom. 2009;76:107–117. doi: 10.1002/cyto.b.20449. [DOI] [PubMed] [Google Scholar]
- Kim HK, Song KS, Kim HO, Chung JH, Lee KR, Lee YL. et al. Circulating numbers of endothelial progenitor cells in patients with gastric and breast cancer. Cancer Lett. 2003;198:83–88. doi: 10.1016/s0304-3835(03)00268-4. [DOI] [PubMed] [Google Scholar]
- Richter-Ehrenstein C, Rentzsch J, Runkel S, Schneider A, Schonfelder G. Endothelial progenitor cells in breast cancer patients. Breast Cancer Res Treat. 2007;106:343–349. doi: 10.1007/s10549-007-9505-z. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M. et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Ebihara S, Asada M, Kanda A, Sasaki H, Yamaya M. Granulocyte colony-stimulating factor promotes tumor angiogenesis via increasing circulating endothelial progenitor cells and Gr1+CD11b+ cells in cancer animal models. Int Immunol. 2006;18:1–9. doi: 10.1093/intimm/dxh334. [DOI] [PubMed] [Google Scholar]
- Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M. G-CSF stimulates angiogenesis and promotes tumor growth: potential contribution of bone marrow-derived endothelial progenitor cells. Biochem Biophys Res Commun. 2002;297:1058–1061. doi: 10.1016/s0006-291x(02)02335-5. [DOI] [PubMed] [Google Scholar]
- Beerepoot LV, Mehra N, Vermaat JS, Zonnenberg BA, Gebbink MF, Voest EE. Increased levels of viable circulating endothelial cells are an indicator of progressive disease in cancer patients. Ann Oncol. 2004;15:139–145. doi: 10.1093/annonc/mdh017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CEC and CEP test were preformed within 24 hours of collection of blood samples. The gating strategy is described below. Exclude debris and red blood cells first. CEC and CEPs are within CD45dim population (P2). CD31/CD146 double positive population (Q2) were defined as CECs (S-Figure 1 and 2). Both CD31/CD133 (Q2-1) and CD146/CD133 (Q2-2) double positive were CEPs (S-Figure 1 and 2). CEPs number presented here were the average of Q2-1 dot number and Q2-2 dot number. For gating viable-CEC, excluding debris and red blood cells first and CEC and CEPs are within CD45dim population (P2). CD146 was CEC maker and 7AAD staining was used to identify the cell viability. Cells in Q2 are apoptotic CECs and in Q4 are viable CECs. (S-Figure 3 and 4) Unstain sample was used as a negative control (S-Figure 1 and 3). CEC/CEP and viable CEP gating was follow the unstain one. (S-Figure 2 and 4).
CEC and CEP test were preformed within 24 hours of collection of blood samples. The gating strategy is described below. Exclude debris and red blood cells first. CEC and CEPs are within CD45dim population (P2). CD31/CD146 double positive population (Q2) were defined as CECs (S-Figure 1 and 2). Both CD31/CD133 (Q2-1) and CD146/CD133 (Q2-2) double positive were CEPs (S-Figure 1 and 2). CEPs number presented here were the average of Q2-1 dot number and Q2-2 dot number. For gating viable-CEC, excluding debris and red blood cells first and CEC and CEPs are within CD45dim population (P2). CD146 was CEC maker and 7AAD staining was used to identify the cell viability. Cells in Q2 are apoptotic CECs and in Q4 are viable CECs. (S-Figure 3 and 4) Unstain sample was used as a negative control (S-Figure 1 and 3). CEC/CEP and viable CEP gating was follow the unstain one. (S-Figure 2 and 4).
CEC and CEP test were preformed within 24 hours of collection of blood samples. The gating strategy is described below. Exclude debris and red blood cells first. CEC and CEPs are within CD45dim population (P2). CD31/CD146 double positive population (Q2) were defined as CECs (S-Figure 1 and 2). Both CD31/CD133 (Q2-1) and CD146/CD133 (Q2-2) double positive were CEPs (S-Figure 1 and 2). CEPs number presented here were the average of Q2-1 dot number and Q2-2 dot number. For gating viable-CEC, excluding debris and red blood cells first and CEC and CEPs are within CD45dim population (P2). CD146 was CEC maker and 7AAD staining was used to identify the cell viability. Cells in Q2 are apoptotic CECs and in Q4 are viable CECs. (S-Figure 3 and 4) Unstain sample was used as a negative control (S-Figure 1 and 3). CEC/CEP and viable CEP gating was follow the unstain one. (S-Figure 2 and 4).
CEC and CEP test were preformed within 24 hours of collection of blood samples. The gating strategy is described below. Exclude debris and red blood cells first. CEC and CEPs are within CD45dim population (P2). CD31/CD146 double positive population (Q2) were defined as CECs (S-Figure 1 and 2). Both CD31/CD133 (Q2-1) and CD146/CD133 (Q2-2) double positive were CEPs (S-Figure 1 and 2). CEPs number presented here were the average of Q2-1 dot number and Q2-2 dot number. For gating viable-CEC, excluding debris and red blood cells first and CEC and CEPs are within CD45dim population (P2). CD146 was CEC maker and 7AAD staining was used to identify the cell viability. Cells in Q2 are apoptotic CECs and in Q4 are viable CECs. (S-Figure 3 and 4) Unstain sample was used as a negative control (S-Figure 1 and 3). CEC/CEP and viable CEP gating was follow the unstain one. (S-Figure 2 and 4).
Representative flow cytometry dot plot for defining viable CECs and apoptotic CECs, (A) Exclude debris and red blood cells. (B) CEC and CEPs are within CD45dim population (P2). (C)CD146 was CEC maker and 7AAD staining was used to identify the cell viability. Cells in Q2 are apoptotic CECs and in Q4 are viable CECsRepresentative data of dynamic change of CEC, CEP (S-Figure 5a, 5c, 5e and 5 g) and viable-CEC (S-Figure 5b, 5d, 5f and 5 h) levels during second cycle of chemotherapy from one patient. CEC, CEP (S-Figure 5a) and viable-CEC (S-Figure 5b) at the day before chemotherapy (for this patient, taxotere /epirubicin /cyclophosphamide) were shown in S-Figure 5a and 5b. Patient’s CEC, CEP and viable-CEC levels were dropping at day 4 and day 7 (S-Figure 5c, 5d, 5e and 5f) after chemotherapy. Three weeks after chemotherapy, CEC and CEP levels were increased again(S-Figure 5e) and most of the CECs were viable (S-Figure 5f).
Standardized trend of CEC, V-CEC, CEP, as a function of chemotherapy the day before tumor resection (A-D), or after tumor resection.(E-G). The CEC and CEP kinetics consistently showed similar wave pattern and had no obvious differences between patients with and without tumor bearing.
Standardized trend of CEC, V-CEC, CEP, as a function of different chemotherapy regimens. (A) Data from a patient who received adjuvant docetaxel, cisplatin, and herceptin. (B) Data from a patient who received adjuvant cyclophosphamide, epirubicin, and Fluorouracil. (C) Data from a patient who received adjuvant docetaxel, epirubicin, and cyclophophamide. (D) Data from a patient who received neoadjuvant docetaxel, Epirubicin, and cyclophophamide. (E) Data from a patient who received neoadjuvant vinorelbine and infusion fluorouracil. Their CEC and CEP kinetics consistently show similar wave pattern. It suggests that dynamic changes of CEC and CEP induced by chemotherapy may have more significant effect than using different drugs.