Abstract
Background
The emergence of multidrug-resistant strains of Mycobacterium tuberculosis (Mtb) has intensified efforts to discover novel drugs for tuberculosis (TB) treatment. Targeting the persistent state of Mtb, a condition in which Mtb is resistant to conventional drug therapies, is of particular interest.
Methods
This study is focused on propargyl acetate derivatives. Eight molecules were designed based on propargyl alcohols and different acid anhydrides.
Results
All the synthesized compounds and commercially available ones were evaluated for anti-tuberculosis activity.
Conclusions
Inhibitors against Mtb have been identified and characterized for further development into potential novel anti-tubercular drugs.
Keywords: Mycobacterium tuberculosis, BCG, Propargyl alcohol, Acetylenic compounds (propargyl acetate derivatives)
Background
Tuberculosis (TB) is a deadly infectious disease and is the leading cause of death worldwide, killing around 2 million people annually, primarily in developing countries. The World Health Organization (WHO) estimates that over one third of the world’s population is infected with TB with approximately 8 million new cases of infection diagnosed every year [1-3]. After human immunodeficiency virus (HIV)/AIDS, TB is the second most common cause of death due to an infectious disease, and current trends suggest that TB will still be among the 10 leading causes of global disease burden in the year 2020 [4]. TB incidence is also on the rise because of the correspondingly high HIV infection rates. These two diseases progress at faster rates in co-infected individuals. The immune systems have been compromised by HIV/AIDS, so individuals fall victims to TB which takes opportunity of their weakened immune systems. There is a great interest in the scientific field to come up with a new drug(s) to combat TB. Tuberculosis is an airborne infectious disease caused by mycobacteria, mainly Mycobacterium tuberculosis (Mtb) [5]. The success of mycobacteria in producing disease relies entirely on its ability to utilize macrophages for its replication and more importantly, the maintenance of viability of host macrophages that sustain mycobacteria.
In the present scenario, due to the emergence of multi-drug resistant tuberculosis (MDR-TB) and association between HIV and TB, Directly Observed Treatment Short course (DOTS) is becoming rapidly ineffective in controlling tuberculosis. Recent reports indicate that, areas where there is a high incidence of MDR-TB, DOTS is failing to control the disease [6]. In such circumstances, the second line drugs are prescribed in combination with DOTS. However, this combination of drugs is very expensive, are less effective and thus has to be administered for a longer duration (e.g. p-amino salicylic acid), has significant side effects (e.g., cycloserine) and some are unavailable in many developing countries (e.g. fluoroquinolones).
Hence, it is clear that there is an urgent need to develop novel anti-TB drugs with improved properties such as enhanced activity against multidrug-resistance, reduced toxicity, shortened duration of therapy, rapid mycobactericidial mechanism of action, ability to penetrate host cells and exert anti-mycobacterial effect in the intracellular environment.
Alkynes or acetylenic compounds play an important role as building blocks in many synthetic transformations, and in new materials. In addition acetylenic group is common structural motifs found in various natural products and also great interest in medicinal chemistry and the pharmaceutical industry [7]. It moreover functions as a key pharmacophoric unit in acetylenic antibiotics [8] and its presence in anticancer [9] and anti-tubercular [10] agents is noteworthy.
Due to above-mentioned reasons and as a part of our ongoing research program on the synthetic methods in organic compounds [11,12], and also our drug discovery program [13,14], here a series of new acetylenic compounds (propargyl acetate derivatives ) were synthesized and evaluated for anti-tubercular activity.
Methods
General
Infrared spectra were determined with a Perkin-Elmer 843 spectrometer (USA). Proton nuclear magnetic resonance (1H NMR) spectra and carbon nuclear magnetic resonance (13C NMR) spectra were determined on a Bruker Avance DRX 500 MHz spectrometer (Germany) and chemical shifts are reported as δ (ppm) in CDCl3 solution (0.05% v/v TMS). GC/MS spectra were obtained using Agilent 7000 Triple Quad (USA). (EI at 70 eV). The chemicals used in this work were purchased from Merck (Germany), Fluka (Germany) and Sigma-Aldrich (USA) Chemical Companies.
In vitro evaluation of anti-mycobacterial activity
In vitro anti-mycobacterial activity evaluations of the compounds were done by the broth microtiter dilution method) against BCG (1173P2) and ethambutol were used as standard controls.
The test compounds were initially dissolved in DMSO to give a concentration of 1 or 2 mg/L. All wells of micro plates received 100 μL of freshly prepared Middle broke 7H9 medium (Himedia, India), except first column. 200 μL of distilled water was added to the first column of 96 well plates to minimize evaporation of the medium in the test wells during incubation. Then 100 μL of test compounds with desired concentrations (1000 or 2000 μL) were added to the wells of the first row (each concentration was assayed in duplicate) and serial dilution was made from the first row to the last. Microbial suspension of BCG (1173P2) (100 μL), which had been prepared with standard concentration of 0.5 Mcfarland and diluted with 1:10 proportion by the distilled water, was added to all test wells. Plates were then sealed and incubated for 4 days at 37°C. After that 12 μl Tween 80 10% and 20 μl Alamar blue 0.01% (Himedia, India) were added to each test well. The results were assessed after 24 and 48 hours. A blue color was interpreted as no bacterial growth, and color change to pink was scored as bacterial growth. Wells with a well-defined pink color were scored as positive for growth. The MIC (minimum inhibitory concentration) was defined as the lowest drug concentration, which prevented a color change from blue to pink. Ethambutol (Irandaru, Tehran) were used as positive control and DMSO as negative control [15].
General procedure for the preparation of propargyl acetate derivatives 3a-h
To a magnetically stirred solution of anhydride (1.2 mmol) was added 3 drops of concentrated sulfuric acid and mix thoroughly holding the large test tube containing the acetic anhydride in cold water. Then, the alcohol (1 mmol) was added to it in several increments and the reaction mixture stirred at room temperature. After, the reaction mixture was placed in hot water at about 70°C to complete the reaction; the progress of the reaction was monitored by TLC (ethyl acetate/n-hexane, 2:1). The purification of product was done according to the literature [16].
Prop-2-ynyl acetate (3a): Yellow liquid (yield 65%). 1H NMR (500 MHz, CDCl3): δH (ppm) 2.07 (3H, s, CH3), 2.46 (1H, t, 4JHH = 2.5 Hz, ≡CH), 4.64 (1H,d, 4JHH = 2.5 Hz, OCH2). 13C NMR (125 MHz, CDCl3): δC (ppm) 30.7 (CH3), 51.9 (OCH2), 74.8 and 77.6 (two acetylenic carbons), 170.1 (C = O). IR (KBr) (νmax,cm-1): 3468, 3296, 2948, 2125, 1733. GC EI-MS, m/z: 99 (M+· + H+, 3%).
But-3-yn-2-yl acetate (3b): Yellow liquid (yield 70%). 1H NMR (500 MHz, CDCl3): δH (ppm) 1.49 (3H, d, CH3), 2.06 (3H, s, CH3), 2.44 (1H, s, ≡CH), 5.39-5.43 (1H, m, OCH). 13C NMR (125 MHz, CDCl3): δC (ppm) 21.00 (CH3), 21.2 (CH3), 60.0 and 82.1 (two acetylenic carbons), 72.8 (OCH), 169.8 (C = O). IR (KBr) (νmax,cm-1): 3480, 3308, 2999, 2131, 1739. GC EI-MS, m/z: 113 (M+· + H+, 10%).
3-Methylpent-1-yn-3-yl acetate (3c): Yellow liquid (yield 63%). 1H NMR (500 MHz, CDCl3): δH (ppm) 1.02 (3H, t, 3JHH = 7.4 Hz, CH3), 1.06 (3H, s, CH3), 1.84 (2H, q, 3JHH = 7.4 Hz, CH2), 2.03 (3H, s, CH3), 2.54 (1H, s, ≡CH). IR (KBr) (νmax,cm-1): 3306, 2967, 2867, 2135, 1716. GC EI-MS, m/z: 140 (M+·, 10%).
Oct-1-yn-3-yl acetate (3d): Yellow liquid (yield 60%). 1H NMR (500 MHz, CDCl3): δH (ppm) 0.88 (3H, t, 3JHH = 7.0 Hz, CH3), 1.30 (2H, m, CH2), 1.43 (2H, m, CH2), 1.75 (2H, m, CH2), 2.07 (3H, s, CH3), 2.44 (1H, d, 4JHH = 2.5 Hz, ≡CH), 5.32 (H, t of d, 3JHH = 5.0 Hz, 4JHH = 2.5 Hz, OCH). 13C NMR (125 MHz, CDCl3): δC (ppm) 13.9 (CH3), 20.9 (CH3), 22.2 (CH2), 24.4 (CH2), 31.0 (CH2), 34.4 (CH2), 63.7 and 77.2 (two acetylenic carbons), 82.0 (OCH2), 169.9 (C = O). IR (KBr) (νmax,cm-1): 3300, 2964, 2874, 2132, 1740. GC EI-MS, m/z: 168 (M+·, 10%).
But-3-ynyl acetate (3e): Yellow liquid (yield 80%). 1H NMR (500 MHz, CDCl3): δH (ppm) 2.01 (1H, t, 4JHH = 3.0 Hz, ≡CH), 2.10 (3H, s, CH3), 2.54 (2H, t of d, 3JHH = 6.5 Hz, 4JHH = 3.0 Hz, CH2), 4.19 (2H, t, 3JHH = 7.0 Hz, OCH2).13C NMR (125 MHz, CDCl3): δC (ppm) 18.8 (CH3), 20.7 (CH2), 61.7 and 70.1 (two acetylenic carbons), 80.0 (OCH2), 170.8 (C = O). IR (KBr) (νmax,cm-1): 3315, 3028, 2900, 2290, 1738. GC EI-MS, m/z: 113 (M+· + H+, 10%).
Prop-2-ynyl heptanoate (3f): Yellow liquid (yield 78%). 1H NMR (500 MHz, CDCl3): δH (ppm) 1.31 (3H, t, 3JHH = 7.5 Hz, CH3), 1.63 (8H, m, 4CH2), 2.33 (2H, t, 3JHH = 8.0 Hz, CH2), 4.45 (1H, t, 4JHH = 2.0 Hz, ≡CH), 4.65 (2H, d, 4JHH = 2.0 Hz, OCH2). 13C NMR (125 MHz, CDCl3): δC (ppm) 13.8 (CH3), 22.2, 24.4, 31.1, 33.8, (4CH2), 51.6 and 74.6, (two acetylenic carbons), 77.7 (OCH2), 172.9 (C = O). IR (KBr) (νmax,cm-1): 3291, 2932, 2865, 2128, 1738. GC EI-MS, m/z: 168 (M+·, 10%).
Oct-1-yn-3-yl heptanoate (3g): liquid (yield 55%). 1H NMR (500 MHz, CDCl3): δH (ppm) 0.89 (6H, m, 2CH3), 1.31 (4H, m), 1.43 (4H, m), 1.63 (4H, m), 1.75 (4H, m), 2.37 (2H, m), 2.42 (1H, d, 4JHH = 2.5 Hz, ≡CH), 5.34 (1H, t of d, 3JHH = 6.5 Hz, 4JHH = 2.0 Hz, OCH). IR (KBr) (νmax,cm-1): 3310, 2963, 2872, 2127, 1736. GC EI-MS, m/z: 239 (M+·, 10%).
But-3-ynyl heptanoate (3h): Yellow liquid (yield 70%). 1H NMR (500 MHz, CDCl3): δH (ppm) 0.87 (3H, t, 3JHH = 7.0 Hz, CH3), 1.30 (4H, m, CH2), 1.61 (2H, m, CH2), 1.98 (1H, t, 4JHH = 2.5 Hz, ≡CH), 2.32 (2H, m, CH2), 2.50 (2H, t of d, 3JHH = 7.0 Hz, 4JHH = 3.0 Hz, CH2), 4.16 (2H, t, 3JHH = 7.0 Hz, OCH2 ). 13C NMR (125 MHz, CDCl3): δC (ppm) 13.9 (CH3), 19.0, 22.3, 24.4, 31.2, 34.14 (5CH2), 61.9 and 69.9 (two acetylenic carbons), 80.1(OCH2), 173.6 (C = O). IR (KBr) (νmax,cm-1): 3304, 2961, 2868, 2126, 1735. GC EI-MS, m/z: 182 (M+·, 5%).
Results and discussion
Esterification of alcohols using acid anhydrides in the presence of acid or base catalyst has been known and extensively used by organic chemists for nearly 100 years [17]. Hydroxyl compounds are often converted to acetate derivatives for protection or characterization of the structure. For this purpose, acetic anhydride is commonly employed with an acid or base catalyst, such as zinc chloride, concentrated sulphuric acid, anhydrous sodium [18] acetate or, most often, pyridine [19].
In the present work, propargyl acetate derivatives 3a-h were prepared by acylation of propargyl alcohols 1 with acid anhydrides 2 in H2SO4 (Scheme 1) [20].
Scheme 1.
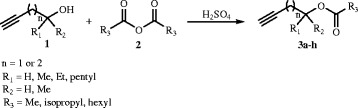
Synthesis of propargyl acetate derivatives.
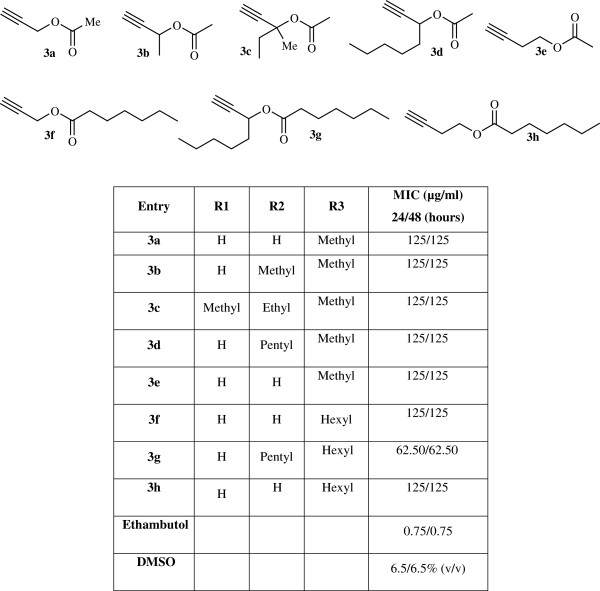
The reactions are straightforward and as indicated in Scheme 1, treatment of various propagyl alcohols 1 with acid anhydrides 2 in H2SO4 led to products 3a-h. To explore the scope and variety of biological activity for products of this reaction, we have examined various primary, secondary and tertiary propagyl alcohols in the presence of aliphatic and aromatic acid anhydrides in H2SO4 at room temperature. As indicated Figure 1, the reaction proceeds efficiently and led to highly functionalized propargyl acetate derivatives 3a–h in good yields.
Figure 1.
Synthesized propargyl acetate derivatives and their MIC.
The structures of compounds 3a–h were deduced from their 1H NMR and 13C NMR spectroscopic data. For example, the 1H NMR spectrum of 3a exhibited a singlet at 2.07 (CH3), a triplet at 2.46 (4JHH = 2.5Hz) for acetylenic hydrogen (≡CH) and a doublet at 4.64 (4JHH = 2.5 Hz, OCH2). The 1H decoupled 13C NMR spectrum of 3a showed 5 distinct resonances, and partial assignment of these resonances is given in experimental section.
All the synthesized compounds were evaluated for anti-tubercular activity and the results are summarized in Figure 1.
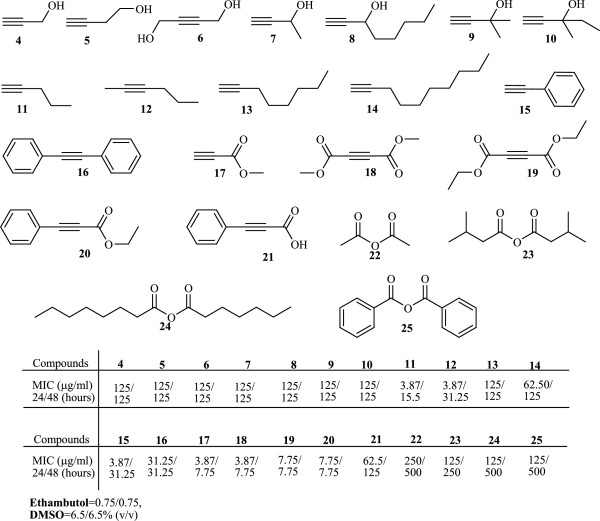
Compounds 3a-h have almost the same activity except 3 g that is slightly stronger; it shows that the changing of R1, R2 and R3 is not important in these compounds. Therefore, we did not synthesis more derivatives from these compounds. In continuation of the work, we investigated different groups of organic compounds that all of these compounds containing acetylenic group in difference situation, such as alcohols (4–10), acetylenic compounds (11–16), esters (17–20), acids (21), and anhydrides (22–25) (without acetylenic group). In these compounds (4–25), the nature and location of mentioned functional group can affect the anti-bacterial activity of acetylenic compounds. As can be seen from Figure 2, among these acetylenic compounds (11,12,15, 16) and esters (17–20), have almost similar effect and more bioactivity than others. It seems that replacement of alkyne hydrogen with an alkyl or aryl group in case of compounds 12 and 16 and a balanced hydrophobicity playing with special features, in case of compounds 11 and 15 have beneficial effect on the bioactivity. However, it is necessary to examine the possible mechanism of action in detail to clear the reason for the variations of activity.
Figure 2.
Commercially available substrates and their MIC (minimal inhibition concentration).
It is interesting to note that comparison of compounds 3a-h in Figure 1 and 17–20 in Figure 2 which are ester shows that 17–20 more active than 3a-h (3.87 vs 125). This can be because of difference in structure of two classes of esters. The main structural difference between 3a-h and 17–20 is that in 17–20 the ester group is terminal whilst in 3a-h is internal. The conjugation of double bond with the triple bond and the resulting dipole moment is a feature that can explain this difference. The same effect can play a role for the better activity of compounds 15 and 16.
Conclusions
In conclusion, the synthesized new class of propargyl acetate derivatives and commercially available compounds were evaluated for anti-tuberculosis activity. Among them, 11, 12, 15, 17 and 18 exhibited promising activity against of M. bovis.
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
First person: synthesis of the molecules, bioassay and writing some parts of the draft, second person: preparation of the draft, third person: design of the molecules and PI, fourth person: interpretation of MS and IR spectra, fifth person: obtaining MS spectra, sixth person: obtaining IR spectra. All authors read and approved the final manuscript.
Contributor Information
Parisa Azerang, Email: p.azerang@gmail.com.
Ali Hossein Rezayan, Email: ahrezayan@ut.ac.ir.
Soroush Sardari, Email: ssardari@hotmail.com.
Farzad Kobarfard, Email: farzadkf@yahoo.com.
Mitra Bayat, Email: bayat.mitra@gmail.com.
Kimia Tabib, Email: Kimia.tabib@gmail.com.
Acknowledgements
We gratefully acknowledge the Pasteur Institute of Iran and Ministry of Health and Medical Education in Iran for partial funding of this project as part of grant number 456.
References
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO global surveillance and monitoring project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan M, Mujahid M, Yogeeswari P, Sriram D. Syntheses and biological evaluation of new triazole-spirochromone conjugates as inhibitors of Mycobacterium tuberculosis. Tetrahedron Lett. 2011;52:2387–2389. doi: 10.1016/j.tetlet.2011.02.099. [DOI] [Google Scholar]
- Agrawal YK, Bhatt HG, Raval HG, Oza PM, Vaidya HB, Manna K, Gogoi P. Emerging trends in tuberculosis therapy. J Sci Indus Res. 2007;66:191–208. [Google Scholar]
- Murray CJ, Salomon JA. Modeling the impact of global tuberculosis control strategies. Proc Natl Acad Sci USA. 1998;95:13881–13886. doi: 10.1073/pnas.95.23.13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma V, Abbas AK, Fausto N, Mitchell R. Robbins Basic Patholog. 8th Edition. London: Elsevier Health Sciences; 2007. [Google Scholar]
- Kimerling ME, Kluge H, Vezhnina N, Iacovazzi T, Demeulenaere T, Portaels F. Inadequacy of the current WHO re-treatment regimen in a central Siberian prison: treatment failure and MDR-TB. Int J Tuberc Lung Dis. 1999;3:451–453. [PubMed] [Google Scholar]
- Hans RH, Guantai EM, Lategan C, Smith PJ, Wanc B, Franzblau SG, Gut J, Rosenthal PJ, Chibale K. Synthesis, antimalarial and antitubercular activity of acetylenic chalcones. Bioorg Med Chem Lett. 2010;20:942–944. doi: 10.1016/j.bmcl.2009.12.062. [DOI] [PubMed] [Google Scholar]
- Maretina IA, Trofimov BA. Enediyne antibiotics and their models: new potential of acetylene chemistry. Russ Chem Rev. 2006;75:825–845. doi: 10.1070/RC2006v075n09ABEH002479. [DOI] [Google Scholar]
- Siddiq A, Dembitsky V. Acetylenic anticancer agents. Anticancer Agents Med Chem. 2008;8:132–170. doi: 10.2174/187152008783497073. [DOI] [PubMed] [Google Scholar]
- Deng S, Wang Y, Inui T, Chen SN, Farnsworth NR, Cho S, Franzblau SG, Pauli GF. Anti-TB polyynes from the roots of Angelica sinensis. Phytother Res. 2008;22:878–882. doi: 10.1002/ptr.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaabani A, Rezayan AH, Kishipour S, Sarvary A, Heidary M, Ng SW. Synthesis of highly stable unusual charge-separated pyridinium-, isoquinolinium-, quinolinium-, and N-methylimidazoliumtetronic acid zwitterions. Tetrahedron. 2009;65:6063–6068. doi: 10.1016/j.tet.2009.05.055. [DOI] [Google Scholar]
- Shaabani A, Sarvary A, Ghasemi S, Rezayan AH, Ghadari R, Ng SW. An environmentally benign approach for the synthesis of bifunctional sulfonamide-amide compounds via isocyanide-based multicomponent reactions. Green Chem. 2011;13:582–585. doi: 10.1039/c0gc00442a. [DOI] [Google Scholar]
- Soltani S, Dianat S, Sardari S. Forward modeling of the coumarin antifungals; SPR/SAR based perspective. Avicenna J Med Biotech. 2009;1:95–103. [PMC free article] [PubMed] [Google Scholar]
- Mehravar M, Sardari S. Screening of antimicrobial membrane-active metabolites of soil microfungi by using chromatic phospholipid/polydiacetylene vesicles. J Mycol Med. 2011;21:188–197. doi: 10.1016/j.mycmed.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Camacho-Corona MR, Ramirez-Cabrera MA, Santiago OG, Garza-Gonzalez E, Palacios Idel P, Luna-Herrera J. Activity against drug resistant-tuberculosis strains of plants used in Mexican traditional medicine to treat tuberculosis and other respiratory diseases. Phytother Res. 2008;22:82–85. doi: 10.1002/ptr.2269. [DOI] [PubMed] [Google Scholar]
- Pearson AL, Roush WJ. Handbook of Reagents for Organic Synthesis: Acetylating Agents and Protecting Groups. Chichester: John Wiley; 1999. [Google Scholar]
- Verley A, Bolsing F. Esterbildung und Bestimmung von Alkoholen resp. Phenolen Ber Dtsch Chem Ges. 1901;34:3354. doi: 10.1002/cber.19010340318. [DOI] [Google Scholar]
- Secen H, Kalpar AH. An efficient acetylation of primary and secondary aliphatic alcohols with acetic anhydride in the presence of graphite. Turk J Chem. 1999;23:27–30. [Google Scholar]
- March J. Advanced Organic chemistry: Reactions, mechanisms, and structure. 4th Edition. Wiley, New York: the University of Michigan; 1992. [Google Scholar]
- Krause P, Hilterhaus L, Fieg G, Liese A, Bornscheuer U. Chemically and enzymatically catalyzed synthesis of C6–C10 alkyl benzoates. Eur J Lipid Sci Technol. 2009;111:194–201. doi: 10.1002/ejlt.200800135. [DOI] [Google Scholar]