Abstract
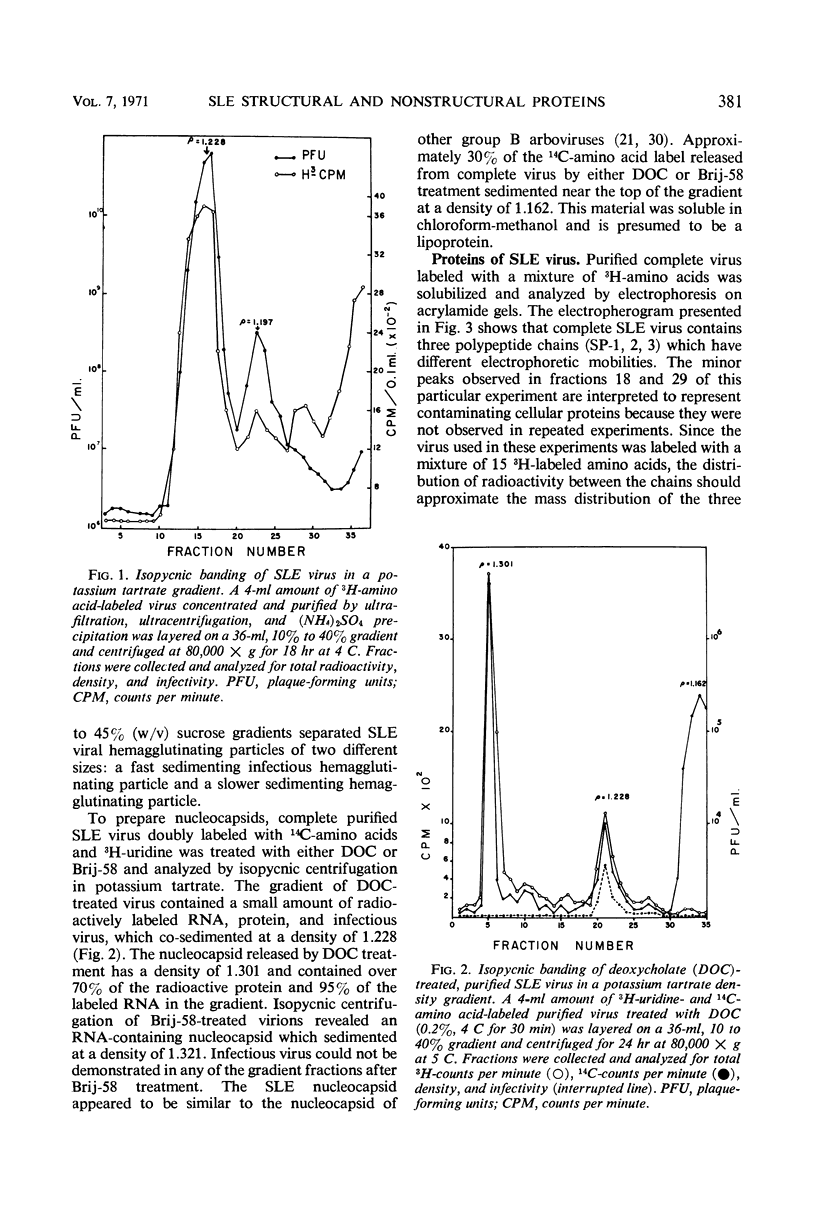
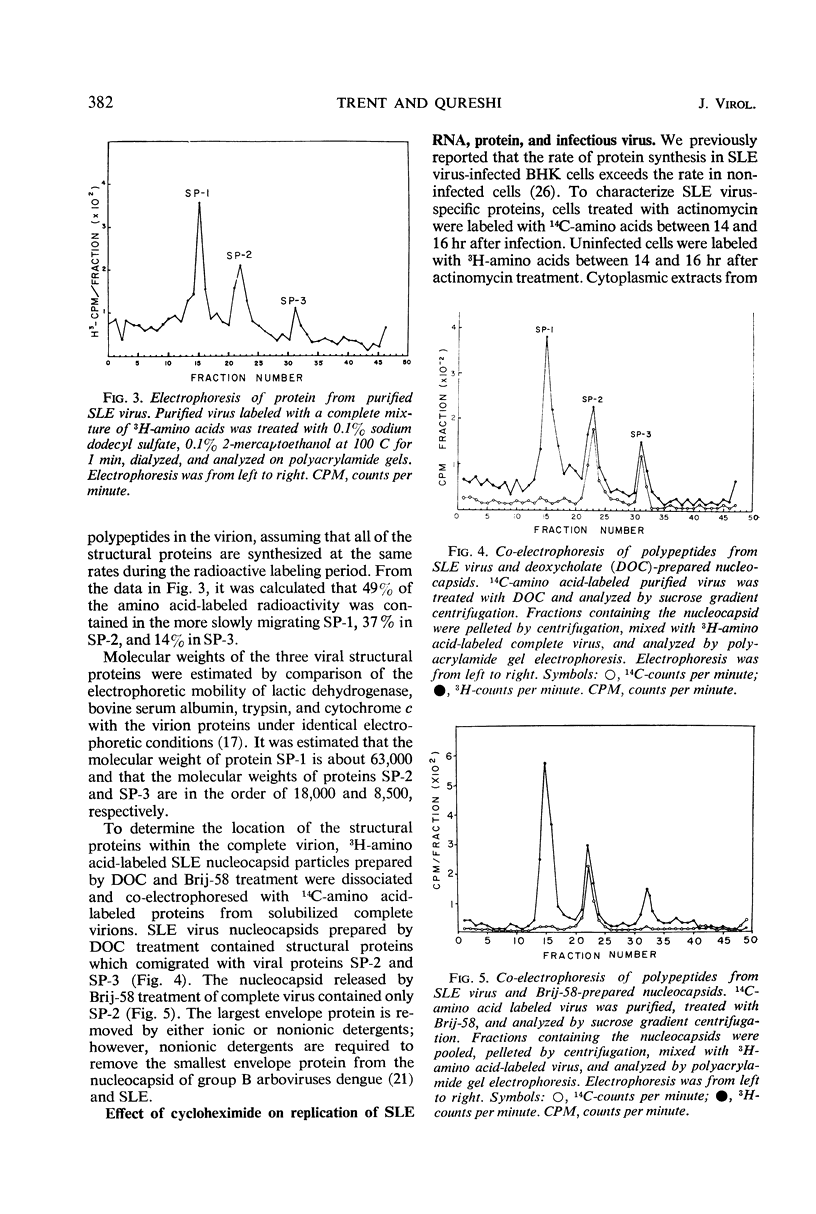
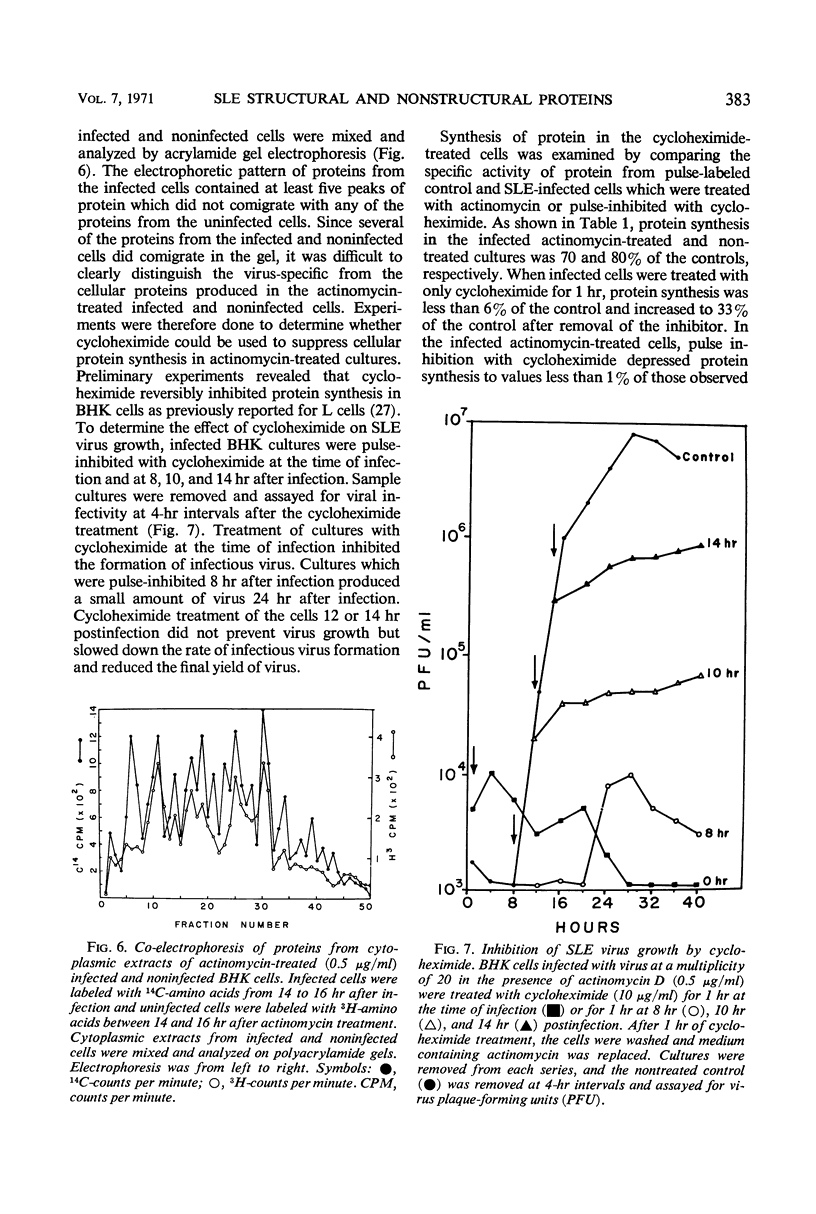
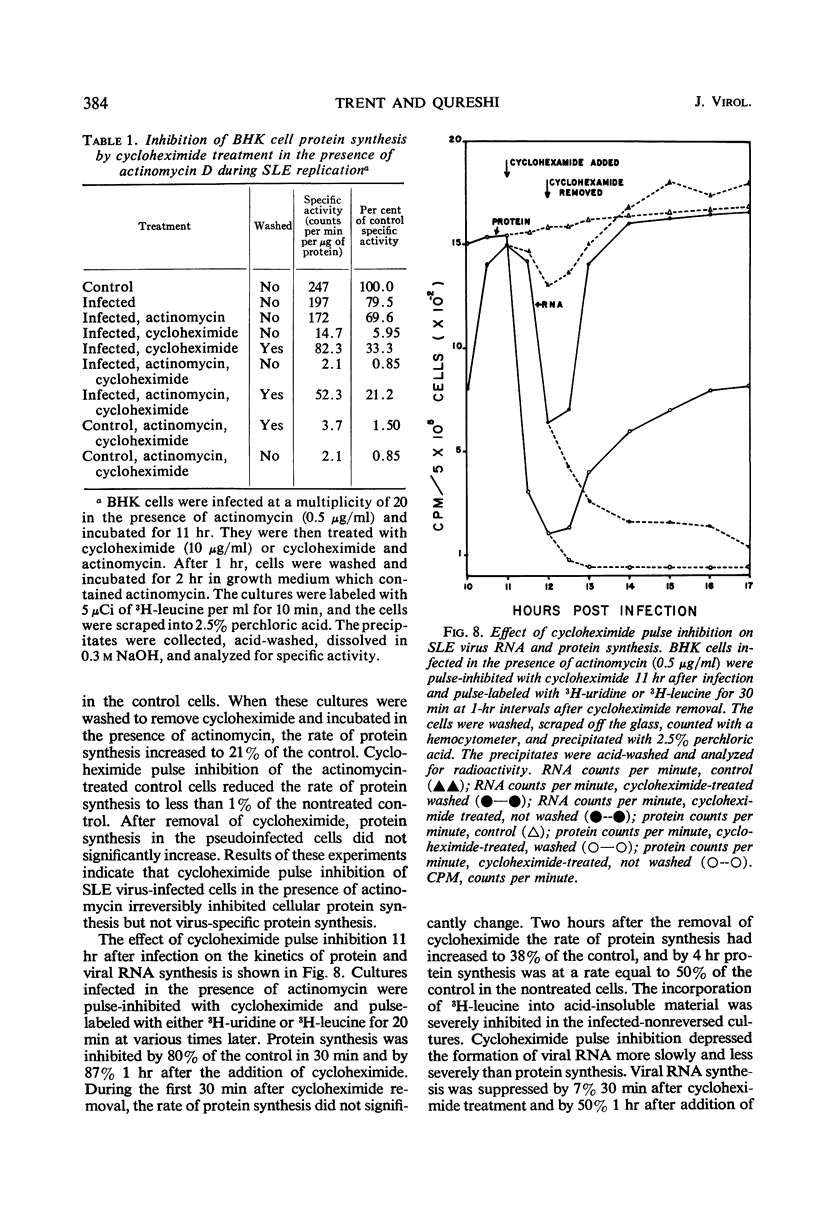
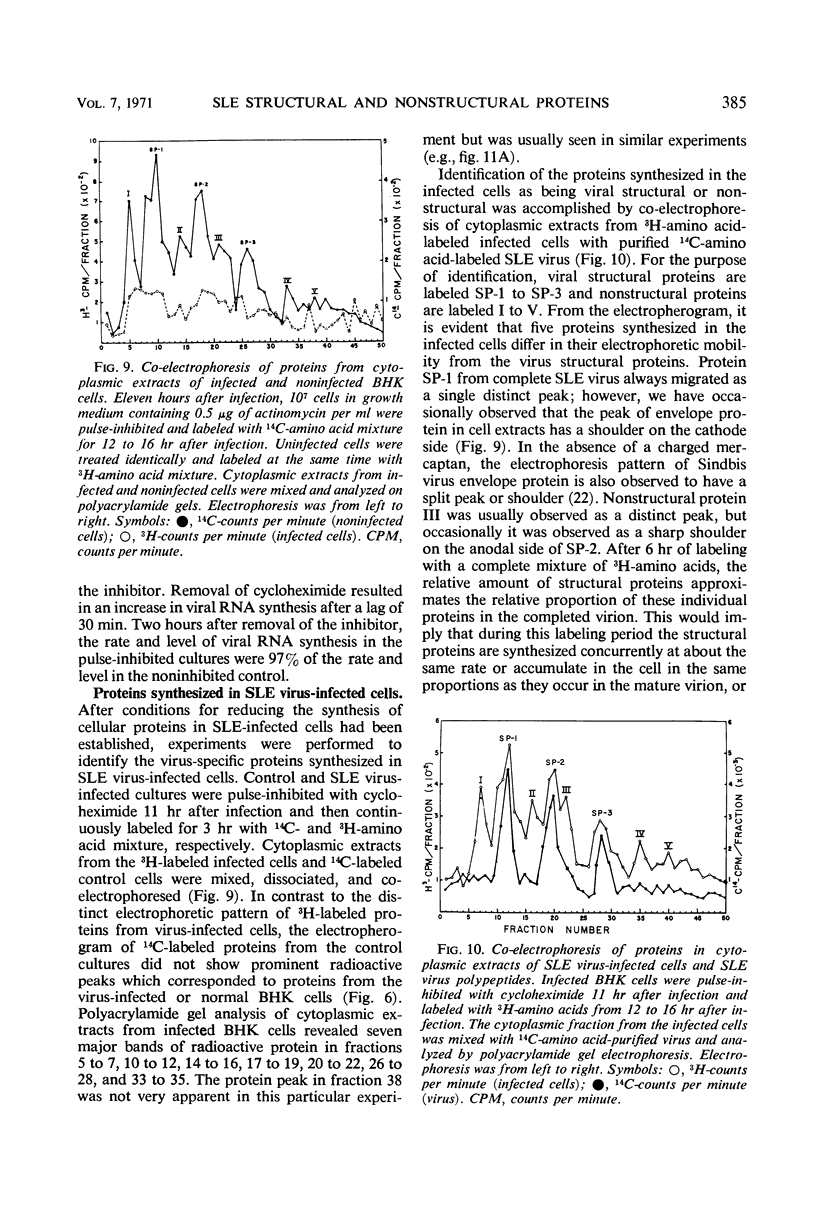
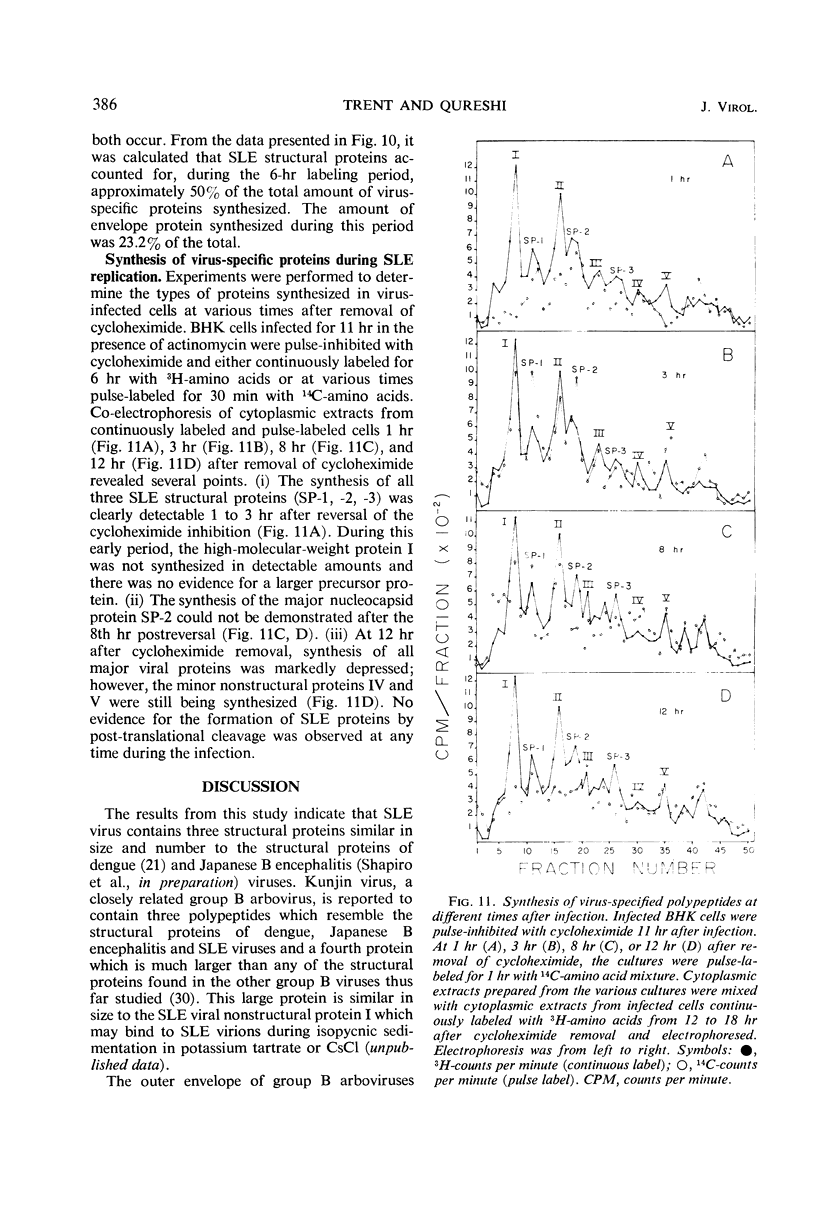
Analysis of purified Saint Louis encephalitis (SLE) virus by acrylamide gel electrophoresis revealed that the virions contained three structural proteins designated SP-1, SP-2, and SP-3 which had molecular weights of 63,000, 18,000, and 8,500, respectively. The envelope contained proteins SP-1 and SP-3 which were removed from the nucleocapsid by nonionic detergent treatment. Nucleocapsids prepared by deoxycholate treatment of complete virions had a density of 1.301 in potassium tartrate and contained SP-2 and SP-3. Brij-58-prepared SLE nucleocapsids had a density of 1.321 and contained only SP-2. Cycloheximide treatment for 1 hr in the presence of actinomycin irreversibly inhibited BHK cellular protein synthesis and reversibly inhibited the synthesis of SLE viral protein and ribonucleic acid. Three structural proteins and five virus-specific nonstructural proteins were detectable in SLE virus-infected BHK cells treated with actinomycin and pulse-inhibited with cycloheximide. Formation of each individual viral structural protein was detectable within 30 min after cycloheximide removal and continued with only minor changes from 12 to 18 hr after infection. Late in the infection cycle, synthesis of the nucleocapsid structural protein SP-2 and SP-3, the small envelope protein, was no longer detectable.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967 May;32(1):128–143. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- Acheson N. H., Tamm I. Structural proteins of Semliki Forest virus and its nucleocapsid. Virology. 1970 Jun;41(2):321–329. doi: 10.1016/0042-6822(70)90084-x. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Functional defects of temperature-sensitive mutants of Sindbis virus. J Mol Biol. 1968 Jul 14;35(1):193–205. doi: 10.1016/s0022-2836(68)80047-6. [DOI] [PubMed] [Google Scholar]
- Burrell C. J., Martin E. M., Cooper P. D. Posttranslational cleavage of virus polypeptides in arbovirus-infected cells. J Gen Virol. 1970 Feb;6(2):319–323. doi: 10.1099/0022-1317-6-2-319. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Grimley P. M. Inhibition of arbovirus assembly by cycloheximide. J Virol. 1969 Sep;4(3):292–299. doi: 10.1128/jvi.4.3.292-299.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M. Primary gene products of an arbovirus. Biochem Biophys Res Commun. 1969 Oct 8;37(2):369–373. doi: 10.1016/0006-291x(69)90744-x. [DOI] [PubMed] [Google Scholar]
- Friedman R. M. Structural and nonstructural proteins of an arbovirus. J Virol. 1968 Oct;2(10):1076–1080. doi: 10.1128/jvi.2.10.1076-1080.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimley P. M., Berezesky I. K., Friedman R. M. Cytoplasmic structures associated with an arbovirus infection: loci of viral ribonucleic acid synthesis. J Virol. 1968 Nov;2(11):1326–1338. doi: 10.1128/jvi.2.11.1326-1338.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A. J., Skehel J. J., Burke D. C. Proteins synthesized in chick cells following infection with Semliki Forest virus. J Gen Virol. 1968 Sep;3(2):175–184. doi: 10.1099/0022-1317-3-2-175. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Harrison A. K., Gary G. W., Jr, Whitfield S. G., Forrester F. T. St. Louis encephalitis virus infection in mice. Electron microscopic studies of central nervous system. Lab Invest. 1968 Dec;19(6):652–662. [PubMed] [Google Scholar]
- OTA Z. ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF JAPANESE B ENCEPHALITIS VIRUS IN PORCINE KIDNEY STABLE (PS) CELLS. Virology. 1965 Mar;25:372–378. doi: 10.1016/0042-6822(65)90057-7. [DOI] [PubMed] [Google Scholar]
- SREEVALSAN T., LOCKART R. Z., Jr INHIBITION BY PUROMYCIN OF THE INITIATION OF SYNTHESIS OF INFECTIOUS RNA AND VIRUS BY CHICKEN EMBRYO CELLS INFECTED WITH WESTERN EQUINE ENCEPHALOMYELITIS VIRUS. Virology. 1964 Sep;24:91–96. doi: 10.1016/0042-6822(64)90151-5. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Brandt W. E., Swanson J. L., McCown J. M., Buescher E. L. Physical and biological properties of dengue-2 virus and associated antigens. J Virol. 1970 Apr;5(4):524–532. doi: 10.1128/jvi.5.4.524-532.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T. M., Schlesinger R. W. Studies on the nature of dengue viruses. I. Correlation of particle density, infectivity, and RNA content of type 2 virus. Virology. 1965 Sep;27(1):103–112. doi: 10.1016/0042-6822(65)90147-9. [DOI] [PubMed] [Google Scholar]
- Stollar V. Studies on the nature of dengue viruses. IV. The structural proteins of type 2 dengue virus. Virology. 1969 Nov;39(3):426–438. doi: 10.1016/0042-6822(69)90091-9. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Sindbis virus infection of chick and hamster cells: synthesis of virus-specific proteins. Virology. 1969 Mar;37(3):367–376. doi: 10.1016/0042-6822(69)90220-7. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent D. W., Swensen C. C., Qureshi A. A. Synthesis of Saint Louis encephalitis virus ribonucleic acid in BHK-21-13 cells. J Virol. 1969 Apr;3(4):385–394. doi: 10.1128/jvi.3.4.385-394.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WECKER E., HUMMELER K., GOETZ O. Relationship between viral RNA and viral protein synthesis. Virology. 1962 May;17:110–117. doi: 10.1016/0042-6822(62)90087-9. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Kudo H., Graham A. F. Selective inhibition of reovirus ribonucleic acid synthesis by cycloheximide. J Virol. 1967 Feb;1(1):36–44. doi: 10.1128/jvi.1.1.36-44.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway E. G. Assessment and application of a cell line from pig kidney for plaque assay and neutralization tests with twelve group B arboviruses. Am J Epidemiol. 1966 Nov;84(3):439–456. doi: 10.1093/oxfordjournals.aje.a120657. [DOI] [PubMed] [Google Scholar]
- Westaway E. G., Reedman B. M. Proteins of the group B arbovirus Kunjin. J Virol. 1969 Nov;4(5):688–693. doi: 10.1128/jvi.4.5.688-693.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


