Abstract
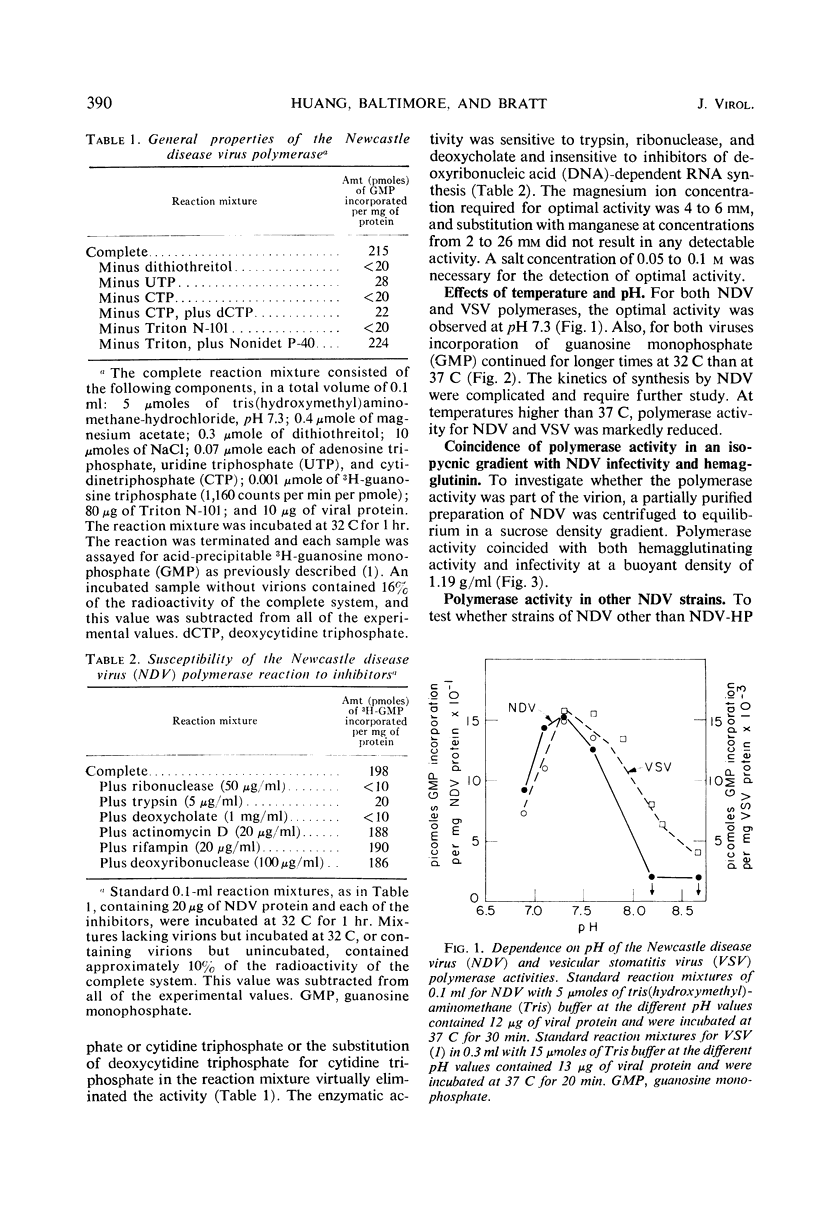
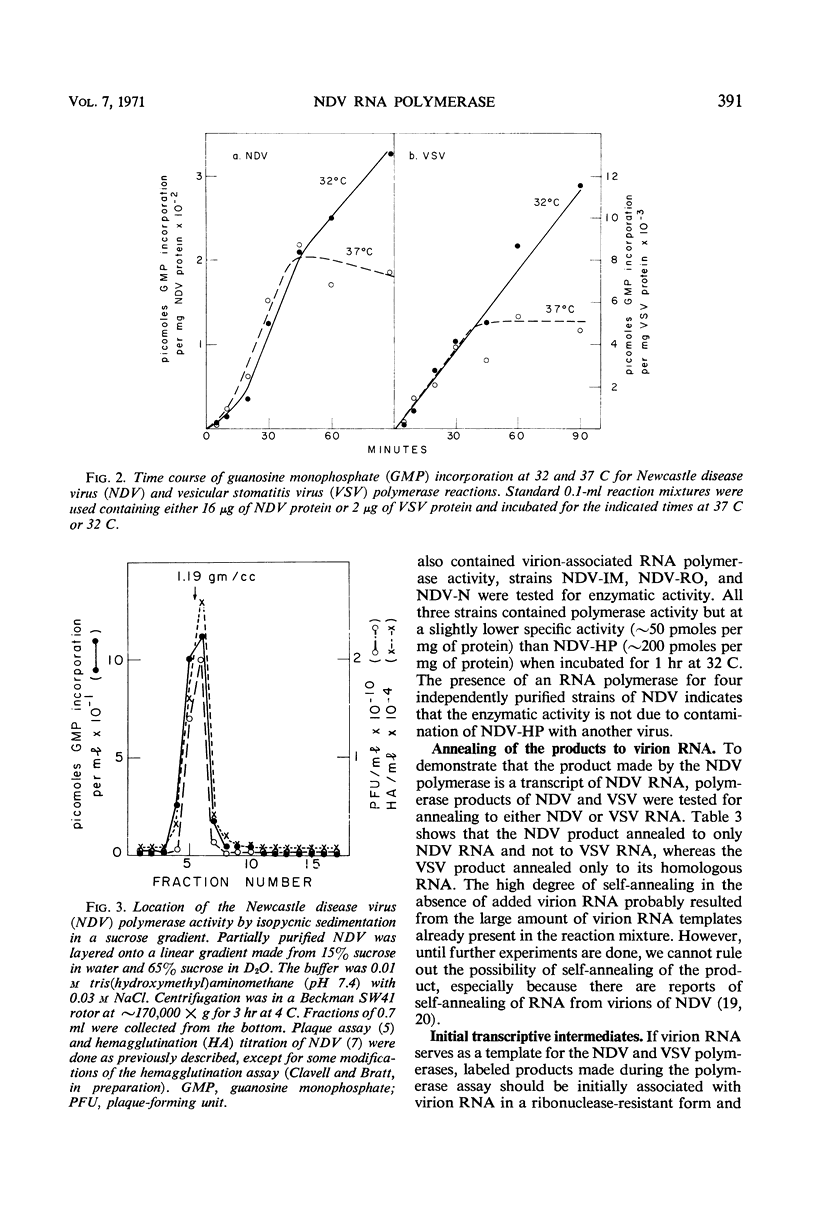
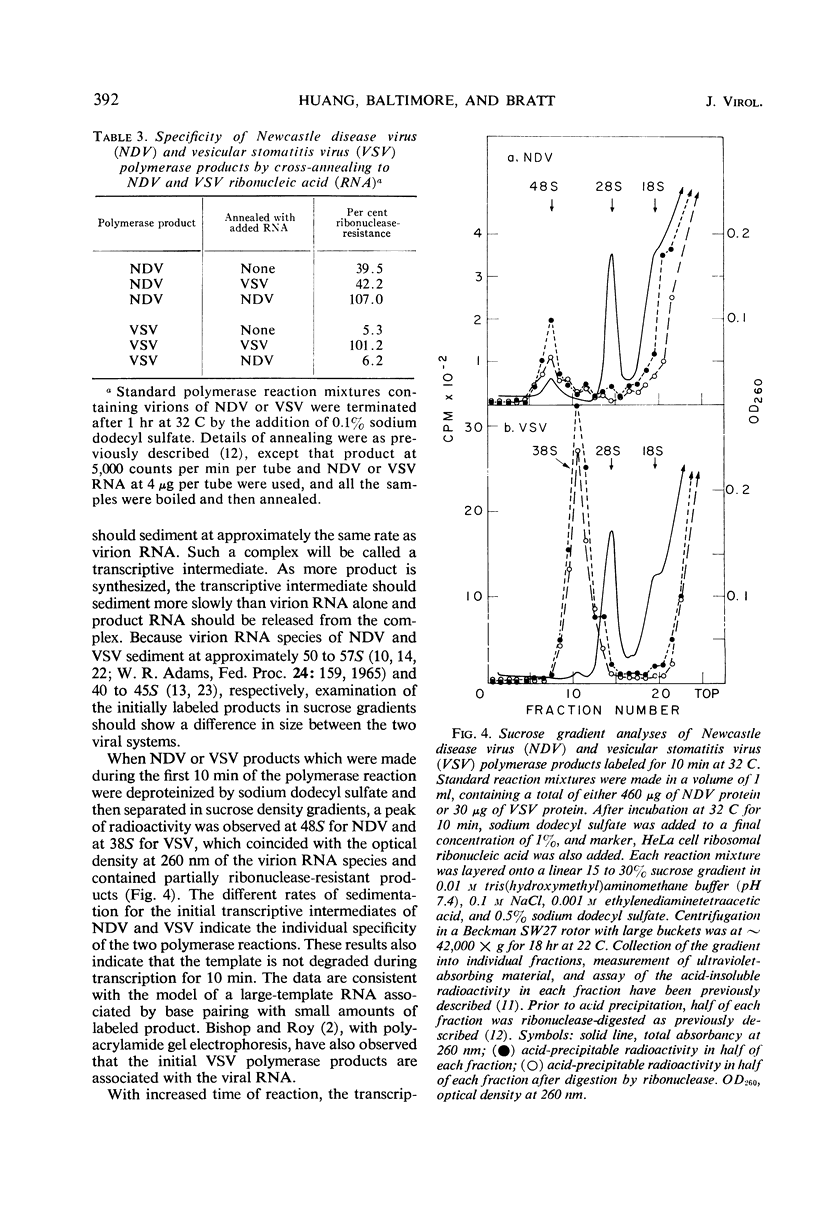
The virions of Newcastle disease virus (NDV) contained an enzyme that catalyzed the incorporation of ribonucleotides into ribonucleic acid (RNA). Optimal conditions for this polymerase activity were identical to the conditions for the vesicular stomatitis virus (VSV) polymerase, and both enzymes were active for longer times at 32 C than at 37 C. However, the specific activity of the NDV polymerase was less than 3% that of the VSV polymerase. Product RNA species from the NDV and VSV polymerase reactions annealed specifically to the homologous virion RNA species. Transcriptive intermediates containing product RNA attached to the respective virion RNA could be identified in both systems.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C. D., Robinson W. S. Replication of Sendai virus. I. Comparison of the viral RNA and virus-specific RNA synthesis with Newcastle disease virus. Virology. 1968 Aug;35(4):537–549. doi: 10.1016/0042-6822(68)90284-5. [DOI] [PubMed] [Google Scholar]
- Blair C. D., Robinson W. S. Replication of Sendai virus. II. Steps in virus assembly. J Virol. 1970 May;5(5):639–650. doi: 10.1128/jvi.5.5.639-650.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratt M. A., Gallaher W. R. Preliminary analysis of the requirements for fusion from within and fusion from without by Newcastle disease virus. Proc Natl Acad Sci U S A. 1969 Oct;64(2):536–543. doi: 10.1073/pnas.64.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choppin P. W., Compans R. W. Phenotypic mixing of envelope proteins of the parainfluenza virus SV5 and vesicular stomatitis virus. J Virol. 1970 May;5(5):609–616. doi: 10.1128/jvi.5.5.609-616.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Choppin P. W. Isolation and properties of the helical nucleocapsid of the parainfluenza virus SV5. Proc Natl Acad Sci U S A. 1967 Apr;57(4):949–956. doi: 10.1073/pnas.57.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Robinson W. S. Isolation of the nucleic acid of Newcastle disease virus (NDV). Proc Natl Acad Sci U S A. 1965 Sep;54(3):794–800. doi: 10.1073/pnas.54.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Balitmore D. Initiation of polyribosome formation in poliovirus-infected HeLa cells. J Mol Biol. 1970 Feb 14;47(3):275–291. doi: 10.1016/0022-2836(70)90302-5. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus. 3. Multiple complementary messenger RNA molecules. Virology. 1970 Dec;42(4):946–957. doi: 10.1016/0042-6822(70)90343-0. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Comparative sedimentation coefficients of RNA extracted from plaque-forming and defective particles of vesicular stomatitis virus. J Mol Biol. 1966 Dec 28;22(2):381–384. doi: 10.1016/0022-2836(66)90143-4. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W., Darlington R. W. Isolation and properties of Newcastle disease virus nucleocapsid. J Virol. 1968 Mar;2(3):248–255. doi: 10.1128/jvi.2.3.248-255.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. W. Newcastle disease virus RNA. I. Isolation and preliminary characterization of RNA from virus particles. J Mol Biol. 1966 Jun;18(1):195–203. doi: 10.1016/s0022-2836(66)80085-2. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W. Newcastle disease virus RNA. II. Preferential synthesis of RNA complementary to parental viral RNA by chick embryo cells. J Mol Biol. 1966 Jun;18(1):204–214. doi: 10.1016/s0022-2836(66)80086-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahy B. W., Hutchinson J. E., Barry R. D. Ribonucleic acid polymerase induced in cells infected with Sendai virus. J Virol. 1970 Jun;5(6):663–671. doi: 10.1128/jvi.5.6.663-671.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A., Kingsbury D. W. Complementary RNAs in paramyxovirions and paramyxovirus-infected cells. Nature. 1970 Dec 19;228(5277):1196–1197. doi: 10.1038/2281196a0. [DOI] [PubMed] [Google Scholar]
- Robinson W. S. Self-annealing of subgroup 2 myxovirus RNAs. Nature. 1970 Mar 7;225(5236):944–945. doi: 10.1038/225944a0. [DOI] [PubMed] [Google Scholar]
- Schaffer F. L., Hackett A. J., Soergel M. E. Vesicular stomatitis virus RNA: complementarity between infected cell RNA and RNA's from infectious and autointerfering viral fractions. Biochem Biophys Res Commun. 1968 Jun 10;31(5):685–692. doi: 10.1016/0006-291x(68)90616-5. [DOI] [PubMed] [Google Scholar]
- Sokol F., Skacianska E., Pivec L. Some properties of Newcastle disease virus ribonucleic acid. Acta Virol. 1966 Jul;10(4):291–300. [PubMed] [Google Scholar]
- Stampfer M., Baltimore D., Huang A. S. Absence of interference during high-multiplicity infection by clonally purified vesicular stomatitis virus. J Virol. 1971 Mar;7(3):409–411. doi: 10.1128/jvi.7.3.409-411.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D., Huang A. S. Ribonucleic acid synthesis of vesicular stomatitis virus. I. Species of ribonucleic acid found in Chinese hamster ovary cells infected with plaque-forming and defective particles. J Virol. 1969 Aug;4(2):154–161. doi: 10.1128/jvi.4.2.154-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. C., Snyder R. M., Schnaitman C. A. Protein composition of the structural components of vesicular stomatitis virus. J Virol. 1969 Jun;3(6):611–618. doi: 10.1128/jvi.3.6.611-618.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



