Abstract
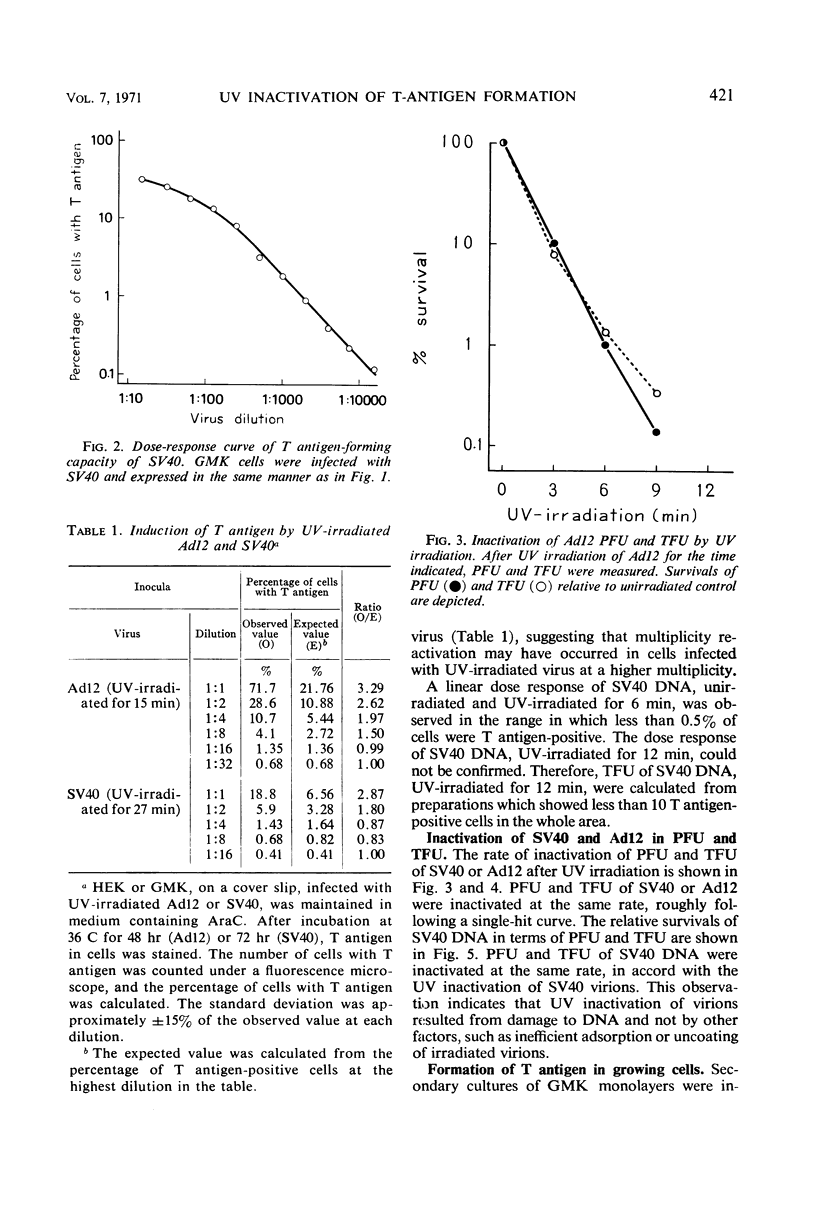
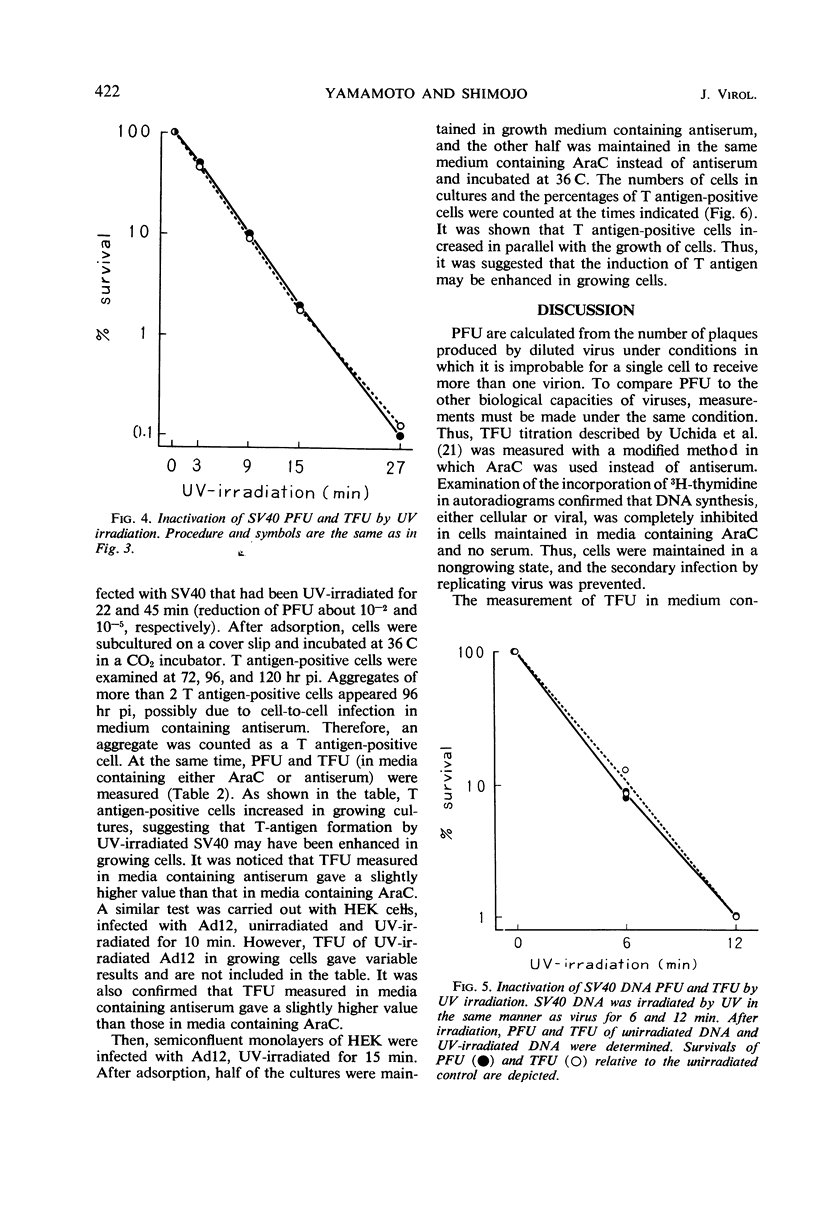
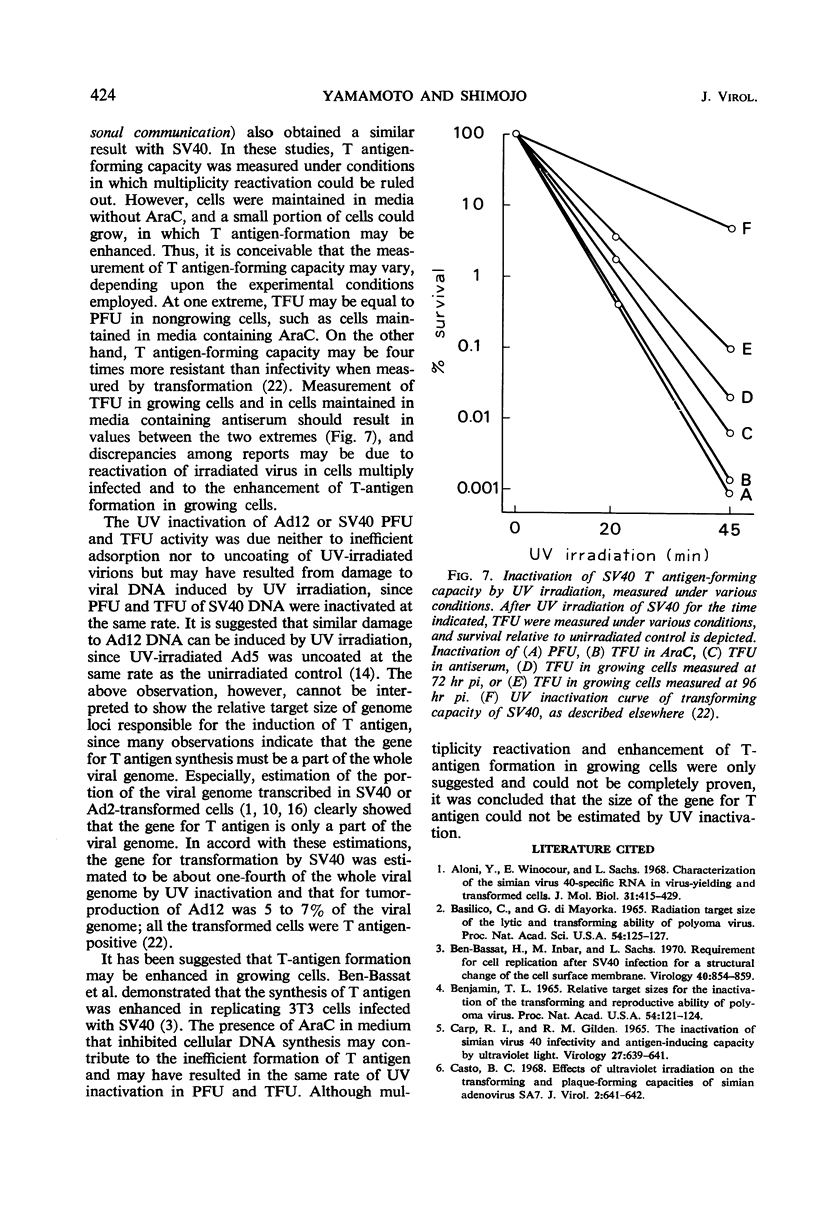
Methods to measure T antigen-forming capacities of simian virus 40 (SV40) and adenovirus 12 (Ad12) were investigated, and a method to measure the capacity in terms of T antigen-forming units was employed by the use of cytosine arabinoside. Plaque-forming units and T antigen-forming units of SV40, SV40 deoxyribonucleic acid, or Ad12 were inactivated by ultraviolet (UV) irradiation at the same rate, roughly following a single-hit curve. T-antigen formation by UV-irradiated SV40 and Ad12 was enhanced in cells multiply infected and in cells in a growing state. These observations showed that it was difficult or impossible to estimate the size of the gene for T antigen by UV inactivation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Winocour E., Sachs L. Characterization of the simian virus 40-specific RNA in virus-yielding and transformed cells. J Mol Biol. 1968 Feb 14;31(3):415–429. doi: 10.1016/0022-2836(68)90418-x. [DOI] [PubMed] [Google Scholar]
- Basilico C., Di Mayorca G. Radiation target size of the lytic and the transforming ability of polyoma virus. Proc Natl Acad Sci U S A. 1965 Jul;54(1):125–127. doi: 10.1073/pnas.54.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Bassat H., Inbar M., Sachs L. Requirement for cell replication after SV40 infection for a structural change of the cell surface membrane. Virology. 1970 Apr;40(4):854–859. doi: 10.1016/0042-6822(70)90131-5. [DOI] [PubMed] [Google Scholar]
- Benjamin T. L. Relative target sizes for the inactivation of the transforming and reproductive abilities of polyoma virus. Proc Natl Acad Sci U S A. 1965 Jul;54(1):121–124. doi: 10.1073/pnas.54.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp R. I., Gilden R. V. The inactivation of simian virus 40 infectivity and antigen-inducing capacity by ultraviolet light. Virology. 1965 Dec;27(4):639–641. doi: 10.1016/0042-6822(65)90194-7. [DOI] [PubMed] [Google Scholar]
- Casto B. C. Effects of ultraviolet irradiation on the transforming and plaque-forming capacities of simian adenovirus SA7. J Virol. 1968 Jun;2(6):641–642. doi: 10.1128/jvi.2.6.641-642.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppey J., Wicker R. Inactivation par les radiations U.V., X et gamma de deux propriétés du virus SV-40: infectivité et induction de l'antigène nucléaire précoce. Ann Inst Pasteur (Paris) 1968 Sep;115(3):478–485. [PubMed] [Google Scholar]
- Finklestein J. Z., McAllister R. M. Ultraviolet inactivation of the cytocidal and transforming activities of human adenovirus type 1. J Virol. 1969 Mar;3(3):353–354. doi: 10.1128/jvi.3.3.353-354.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Green M. Mechanism of viral carcinogenesis by DNA mammalian viruses. VII. Viral genes transcribed in adenovirus type 2 infected and transformed cells. Proc Natl Acad Sci U S A. 1970 Feb;65(2):375–382. doi: 10.1073/pnas.65.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilead Z., Ginsberg H. S. Comparison of the rates of ultraviolet inactivation of the capacity of type 12 adenovirus to infect cells and to induce T antigen formation. J Bacteriol. 1966 Dec;92(6):1853–1854. doi: 10.1128/jb.92.6.1853-1854.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latarjet R., Cramer R., Montagnier L. Inactivation, by UV-, x-, and gamma-radiations, of the infecting and transforming capacities of polyoma virus. Virology. 1967 Sep;33(1):104–111. doi: 10.1016/0042-6822(67)90098-0. [DOI] [PubMed] [Google Scholar]
- Lawrence W. C., Ginsberg H. S. Intracellular uncoating of type 5 adenovirus deoxyribonucleic acid. J Virol. 1967 Oct;1(5):851–867. doi: 10.1128/jvi.1.5.851-867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Oda K., Dulbecco R. Regulation of transcription of the SV40 DNA in productively infected and in transformed cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):525–532. doi: 10.1073/pnas.60.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo H., Yamamoto H., Abe C. Differentiation of adenovirus 12 antigens in cultured cells with immunofluorescent analysis. Virology. 1967 Apr;31(4):748–752. doi: 10.1016/0042-6822(67)90213-9. [DOI] [PubMed] [Google Scholar]
- Shimojo H., Yamashita T. Induction of DNA synthesis by adenoviruses in contact-inhibited hamster cells. Virology. 1968 Nov;36(3):422–433. doi: 10.1016/0042-6822(68)90167-0. [DOI] [PubMed] [Google Scholar]
- Strohl W. A. The response of BHK21 cells to infection with type 12 adenovirus. 1. Cell killing and T antigen synthesis as correlated viral genome functions. Virology. 1969 Dec;39(4):642–652. doi: 10.1016/0042-6822(69)90003-8. [DOI] [PubMed] [Google Scholar]
- Uchida S., Watanabe S., Kato M. Incomplete growth of simian virus 40 in African green monkey kidney culture induced by serial undiluted passages. Virology. 1966 Jan;28(1):135–141. doi: 10.1016/0042-6822(66)90314-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto H. Inactivation of the transforming capacity of SV40 and the oncogenicity of adenovirus 12 by ultraviolet irradiation. Jpn J Microbiol. 1970 Nov;14(6):487–493. [PubMed] [Google Scholar]
- Zur Hausen H. Induction of specific chromosomal aberrations by adenovirus type 12 in human embryonic kidney cells. J Virol. 1967 Dec;1(6):1174–1185. doi: 10.1128/jvi.1.6.1174-1185.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



