Abstract
Background
Recently, there have been a number of studies on the association between MDM2 (Murine Double Minute 2) 309 polymorphism and ovarian cancer risk. However, the results of previous reports remain controversial and ambiguous. Thus, we performed a meta-analysis to explore more precisely the association between MDM2 309 polymorphism and the risk of ovarian cancer.
Methods
A meta-analysis was performed to examine the association between MDM2 309T>G polymorphism and ovarian cancer risk. Odds ratio (OR) and its 95% confidence interval (CI) were used for statistical analysis.
Results
Our publication search identified a total of 6 studies with 1534 cases and 2211 controls. No significant association was found between MDM2 309T>G polymorphism and ovarian cancer risk in total population analysis. In the subgroup meta-analysis by ethnicity, a negative association was shown in Asian subgroup (G vs. T OR = 0.774, 95% CI = 0.628–0.955, P = 0.017, P het = 0.327; GG vs. TT: OR = 0.601, 95% CI = 0.395–0.914, P = 0.017, P het = 0.417; dominant model TG+GG vs. TT: OR = 0.661, 95% CI = 0.468–0.934, P = 0.019, P het = 0.880), and no significant association in any genetic models among Caucasians was observed.
Conclusions
This meta-analysis provides evidence for the association between MDM2 309 polymorphism and ovarian cancer risk, supporting the hypothesis that MDM2 SNP309 G allele acts as an important ovarian cancer protective factor in Asians but not in Caucasians.
Introduction
Epithelial ovarian cancer (OC) is the leading cause of death from gynecologic malignancies. OC is mostly asymptomatic at early-stage, and most of the cases are diagnosed when the tumor has established regional or distant metastases [1].
Therefore, it is important to clarify the molecular mechanism of OC development which can help to detect OC at an early stage, and studies on gene polymorphism that affects the pathways known to influence the neoplastic process may be particularly relevant.
P53 is a tumor suppressor gene, which is involved in multiple pathways including apoptosis, cellular transcriptional control, and cell cycle regulation [2], [3]. MDM2 (mouse double minute 2 homolog) is a crucial negative regulator of the tumor suppressor p53. P53 and MDM2 act in a feedback loop where p53 activates MDM2 at the transcriptional level while MDM2 binds to the N -terminus of p53 protein, inhibits its activity and mediates its location and degradation through E3 ligase activity [4], [5], [6]. The expression level of MDM2 can be affected by several factors, one of which is single nucleotide polymorphism.
In 2004, Bond et al. reported that a polymorphism (SNP309T>G; rs2279744) in the MDM2 intronic promoter P2 affects MDM2 expression levels [7]. SNP309 (rs2279744) enhances the DNA-binding affinity of the transcriptional activator Sp1. This results in elevated MDM2 levels and consequently an attenuation of the p53 pathway associated with susceptibility to certain types of cancer [7], [8], [9], [10], [11], [12]. Following the discovery of the 309 polymorphism, conflicting evidence has linked the G-allele to enhanced cancer risk as well as early cancer diagnosis across different tumor types and ethnic groups [13], [14].
Other meta-analyses suggest that the G/G genotype is associated with an increased risk for lung, endometrial, and hepatocellular carcinomas, but not for breast or colorectal carcinomas [15], [16], [17]. Studies have also found that SNP309G is associated with early diagnosis of several malignancies in females but not in males [7], [18], [19]. Over the last two decades, a number of case–control studies were conducted to investigate the association between MDM2 polymorphism and ovarian cancer risk in humans. However,no quantitative summary of evidence has ever been developed so far since these studies reported conflicting results. The purpose of this meta-analysis is to provide a quantitative summary of evidence.
Materials and Methods
Publication Search
Computer searches were performed independently by two authors, covering all papers published in PubMed, Embase, Medline and Google Scholar before May 2012. The keywords were as follows: ovarian cancer/ovarian carcinoma, polymorphism/variant/genotype/SNP, and murine double minute 2/MDM2. The reference lists of the retrieved articles were hand-searched to obtain other relevant publications. All associated publications were evaluated to identify the most eligible literature. The results were limited to papers published in English.
Inclusion and Exclusion Criteria
The following criteria were used to select studies for further meta-analysis: (1) the studies were case-control study; (2) the studies were about MDM2 309T>G polymorphism and risk of ovarian cancer; (3) the studies contained at least two comparison groups (cancer group vs. control group); (4) the studies included detailed genotyping data.
Data Extraction
The extraction of data from all eligible publications was performed by two investigators independently, according to the inclusion and exclusion criteria listed above. For each study, information extracted were author’s last name, year of publication, country of origin, ethnicity, cancer type, sources of control and case groups, specimen of cases, genotyping methods for MDM2 SNP309T/G, total number of cases and controls as well as number of cases and controls with T/T, T/G and G/G genotypes. All the case and control groups were well controlled. The non-cancer controls had no history of gynecologic disease, and there was no present evidence of gynecologic cancer, any malignant disease or genetic disease. All case patients and control subjects were unrelated. There was no statistically significant difference in terms of age distribution, smoking habits or menstrual status between case and control groups.
Statistical Analysis
Hardy-Weinberg equilibrium (HWE) for the control group of each study was assessed using goodness-of-fit test (χ2 of Fisher’s exact test). Based on both fixed effects and random-effects models, a pooled OR with 95% CI was used to assess the strength of association between MDM2 SNP309T/G polymorphism and ovarian cancer risk, depending on the heterogeneity of the analysis. In the overall and the subgroup meta-analysis, pooled ORs and 95% CIs for GG vs. TT, TG vs. TT, dominant model (TG+GG vs. TT), and recessive model (GG vs. TG+TT) were all calculated. Heterogeneity was assessed using Q-test and I2 score. If the result of heterogeneity test was P>0.1, ORs were pooled according to the fixed-effects model (Mantel-Haenszel model). Otherwise, ORs were pooled according to the random-effects model (DerSimonian and Laird model). I2 was used to qualify variation in OR attributable to heterogeneity.
Publication bias was assessed using Egger test and Begg test. All statistical tests were performed using the software STATA v.10.0 (Stata Corporation, College Station, TX, USA). The results were considered statistically significant if P<0.05.
Results
Study Selection
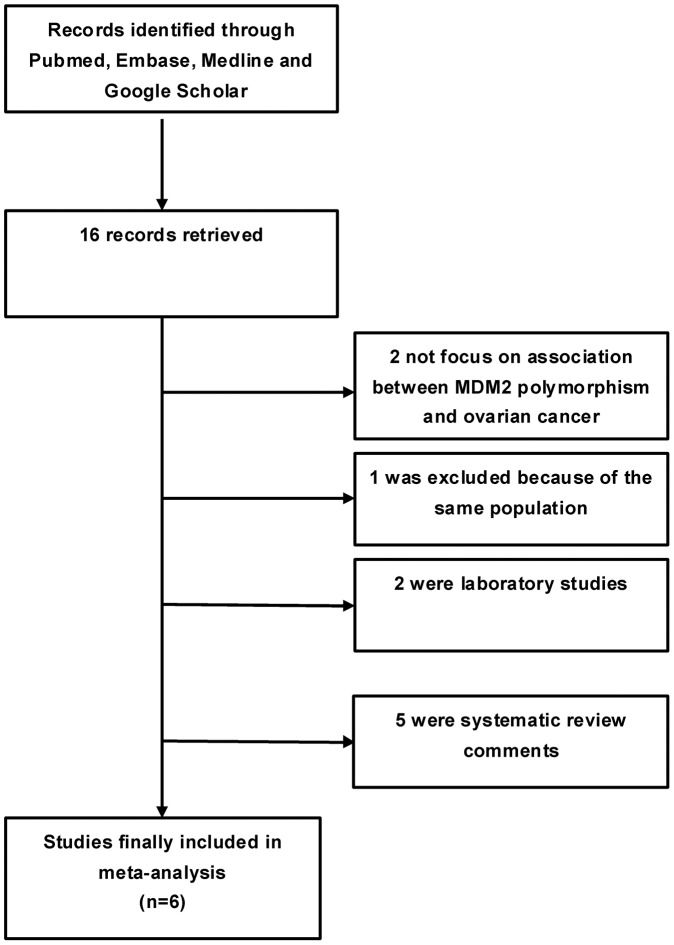
A total of 16 records that fulfilled our search criteria were preliminarily identified for further detailed evaluation, which excluded ten studies (Figure 1). Two studies [20], [21] were not focused on MDM2 SNP309T/G polymorphism and ovarian cancer risk. One study (a conference abstract) [22] was excluded because it used the same population as an included study [23]. Two others [24], [25] were laboratory studies, and the rest of the 5 studies [17], [26], [27], [28], [29] were systematic review comments. At last, six studies [23], [30], [31], [32], [33], [34] on MDM2 SNP309 genotypes and ovarian cancer risk were identified, including a total of 1534 ovarian cancer cases and 2211 controls.
Figure 1. Flow chart of study study selection based on the inclusion and exclusion criteria.
Study Characteristics
Characteristics of the studies included in this meta-analysis are presented in Table I. All studies are case-control studies. Of these 6 studies, 2 used allele specific PCR, 2 used PCR-RFLP and 2 used pyrosequencing. The studies were carried out in Japan, UK, China, Norway and Czech Republic. Two studies were on Asians and four studies were on Caucasians. The studies carried out in China and Japan were used in Asian subgroup, and others were used in Caucasian subgroup. The distribution of genotypes in the controls was consistent with Hardy-Weinberg equilibrium (P>0.05) in all studies except for one study of Ueda et al. (P = 0.021) [34].
Table 1. MDM2 SNP309T>G Genotype Distribution and Allele Frequency in Cases and Controls.
| Author-Year | Country | Genotype (N) | Allele frequency (N, %) | P HWE Controls | ||||||||||
| Case | Control | Case | Control | |||||||||||
| total | TT | TG | GG | total | TT | TG | GG | T | G | T | G | |||
| Kang et al. (2006) | China | 257 | 77 | 120 | 60 | 257 | 56 | 121 | 80 | 274(53.3%) | 240(46.7%) | 233(45.3%) | 281(54.7%) | 0.422 |
| Knappskog et al. (2011) | Norway | 832 | 296 | 437 | 99 | 1337 | 561 | 617 | 159 | 1029(61.8%) | 635(38.2%) | 1739(65.0%) | 935(34.9%) | 0.591 |
| Ueda et al. (2009) | Japan | 85 | 21 | 45 | 19 | 108 | 20 | 66 | 22 | 87(51.2%) | 83(48.8%) | 106(49.1%) | 110(50.9%) | 0.021 |
| Campbell et al. (2006) | UK | 302 | 117 | 133 | 52 | 258 | 105 | 111 | 42 | 367(60.8%) | 237(39.2%) | 321(62.2%) | 195(37.8%) | 0.172 |
| Copson et al. (2006) | UK | 14 | 6 | 4 | 4 | 102 | 48 | 38 | 16 | 16(57.1%) | 12(42.9%) | 134(65.7%) | 70(34.3%) | 0.079 |
| Krekac et al. (2008) | Czech Republic | 44 | 24 | 18 | 2 | 149 | 61 | 71 | 17 | 66(75.0%) | 22(25.0%) | 193(64.8%) | 105(35.2%) | 0.591 |
Quantitative Data Synthesis
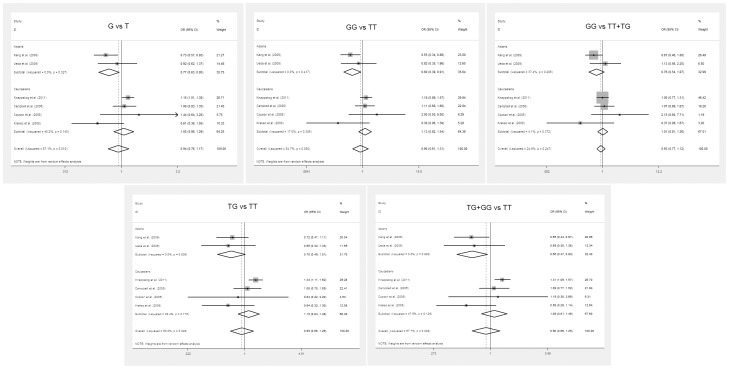
The results on the associations between MDM2 SNP309 polymorphism and ovarian cancer risk, and of the heterogeneity test are shown in Table 2. The combined results based on all studies showed that variant genotypes are not associated with increased ovarian cancer risk in different genetic models (OR = 0.942, 95% CI = 0.760–1.167 for G vs. T, P = 0.583; OR = 0.895, 95% CI = 0.611–1.313 for GG vs. TT, P = 0.571; OR = 0.929, 95% CI = 0.684–1.261 for TG vs. TT, P = 0.635; OR = 0.905, 95% CI = 0.657–1.246 for the dominant model TG+GG vs. TT, P = 0.540; OR = 0.927, 95% CI = 0.770–1.116 for the recessive model GG vs. TT+TG, P = 0.424) (Figure 2). In the subgroup analysis by ethnicity, in Asian population, the results revealed significant associations between the MDM2 SNP309 polymorphism and ovarian cancer in three genotype distributions (G vs. T: OR = 0.774, 95% CI = 0.628–0.955, P = 0.017, P het = 0.327; GG vs. TT: OR = 0.601, 95% CI = 0.395–0.914, P = 0.017, P het = 0.417; dominant model TG+GG vs. TT: OR = 0.661, 95% CI = 0.468–0.934, P = 0.019, P het = 0.880), but not in the other two genotype distributions (TG vs. TT: OR = 0.702, 95%CI = 0.486–1.013, P = 0.059, P het = 0.806; GG vs. TT+TG: OR = 0.763, 95%CI = 0.543–1.072, P = 0.119, P het = 0.206). In contrast, no significant association in any genetic models was observed in Caucasians (G vs. T: OR = 1.053, 95% CI = 0.856–1.294, P = 0.626, P het = 0.140; GG vs. TT: OR = 1.125, 95% CI = 0.823–1.538, P = 0.459, P het = 0.306; dominant model TG+GG vs. TT: OR = 1.091, 95% CI = 0.814–1.462, P = 0.560, P het = 0.126; TG vs. TT: OR = 1.115, 95%CI = 0.840–1.480, P = 0.450, P het = 0.176; GG vs. TT+TG: OR = 1.008, 95%CI = 0.807–1.258, P = 0.946, P het = 0.372).
Table 2. Meta-analysis of the association between MDM2 SNP309 polymorphism and ovarian cancer risk.
| Comparisons | Odds ratio | 95%Confidence Interval | P value | Heterogeneity | Effects model | |
| I2 | P value | |||||
| G vs T | 0.942 | 0.760–1.167 | 0.583 | 67.1% | 0.010 | Random |
| Asians | 0.774 | 0.628–0.955 | 0.017 | 0.0% | 0.327 | |
| Caucasians | 1.053 | 0.856–1.294 | 0.626 | 45.2% | 0.140 | |
| GG vs TT | 0.895 | 0.611–1.313 | 0.571 | 54.7% | 0.050 | Random |
| Asians | 0.601 | 0.395–0.914 | 0.017 | 0.0% | 0.417 | |
| Caucasians | 1.125 | 0.823–1.538 | 0.459 | 17.0% | 0.306 | |
| TG vs TT | 0.929 | 0.684–1.261 | 0.635 | 60.0% | 0.028 | Random |
| Asians | 0.702 | 0.486–1.013 | 0.059 | 0.0% | 0.806 | |
| Caucasians | 1.115 | 0.840–1.480 | 0.450 | 39.4% | 0.176 | |
| TG+GG vs TT | 0.905 | 0.657–1.246 | 0.540 | 67.7% | 0.008 | Random |
| Asians | 0.661 | 0.468–0.934 | 0.019 | 0.0% | 0.880 | |
| Caucasians | 1.091 | 0.814–1.462 | 0.560 | 47.5% | 0.126 | |
| GG vs TT+TG | 0.927 | 0.770–1.116 | 0.424 | 24.9% | 0.247 | Fixed |
| Asians | 0.763 | 0.543–1.072 | 0.119 | 37.4% | 0.206 | |
| Caucasians | 1.008 | 0.807–1.258 | 0.946 | 4.1% | 0.372 | |
Figure 2. Forest plots of MDM2 309T/G polymorphism in ovarian cancer vs. normal control and subgroup analyses.
The squares and horizontal lines correspond to the study specific OR and 95% CI. The area of the squares reflects the weight (inverse of the variance). The diamond represents the summary OR and 95% CI. OR: odds ratio.
Tests of Heterogeneity
Statistically significant heterogeneity was observed between trials of the following analyses using Q statistic (G vs. T: P = 0.010, I2 = 67.1%; GG vs. TT: P = 0.050, I2 = 54.7%; TG vs. TT: P = 0.028, I2 = 60.0%; dominant model TG+GG vs. TT: P = 0.008, I2 = 67.7%) (Table 2), and the random-effects model was employed in these studies. We did not find the significant heterogeneity for the recessive model GG vs. TT+TG (P = 0.247, I2 = 24.9%), and a fixed-effects model was performed.
Publication Bias
Begg’s test and Egger’s test and were performed to assess publication bias. Egger weighted regression method did not indicate evidence for publication bias for four of the five genetic models (G vs. T: P = 0.354; GG vs. TT: P = 0.679; dominant model TG+GG vs. TT: P = 0.063; recessive model GG vs. TT+TG: P = 0.656). This result was confirmed by Begg rank correlation method (G vs. T: P = 0.707; GG vs. TT: P = 0.707; TG vs. TT: P = 0.707; dominant model TG+GG vs. TT: P = 0.707; recessive model GG vs. TT+TG: P = 0.707) (Table 3).
Table 3. Publication bias test for MDM2 SNP309 polymorphism.
| Comparisons | Egger test | Begg testP value | ||
| Coefficient | P value | 95% CI | ||
| G vs T | −1.427 | 0.354 | −5.209–2.356 | 0.707 |
| GG vs TT | −0.644 | 0.679 | –4.659–3.370 | 0.707 |
| TG vs TT | −2.764 | 0.014 | −4.587–0.941 | 0.707 |
| TG+GG vs TT | −2.574 | 0.063 | −5.367–0.219 | 0.707 |
| GG vs TT+TG | 0.641 | 0.656 | −3.063–4.345 | 0.707 |
Discussion
It has been discovered that a variant of SNP309G affects Sp1 binding to the MDM2 P2 promoter, which results in increased MDM2 transcript and protein [7]. After this discovery, studies described that the 309G status is associated with an early diagnosis and tumor formation in Li-Fraumeni syndrome and several malignancies, including breast cancer, soft tissue sarcomas, large cell lymphomas and colorectal cancer [7], [18], [19]. Interestingly, these associations were observed among females only, showing that SNP309G status could be due to the effects of gender-specific hormones. Another study further supported the fact that SNP309G’s impact on age of cancer onset is the largest among women below the average age of menopause [18], [19]. As ovarian cancer is largely hormone related, it is important to study the impact of MDM2 309 polymorphism on women with ovarian cancer.
Previous investigations found that the frequency distribution of SNP309G allele is significantly varied among different ethnicities, which led to conflicting evidence on the association between MDM2 309 T/G polymorphism and risk of cancer, particularly in Caucasian populations [13], [14]. It was also found that the G/G genotype is associated with an increased risk for lung, endometrial, and hepatocellular carcinomas, but not for breast or colorectal carcinomas [15], [16], [17]. Another study also showed that MDM2 SNP309 G allele probably acts as an important head and neck squamous cell carcinoma (HNSCC) protective factor in Caucasians, but not in Asians [35]. As conflicting results between studies or ethnic groups have been reported, it is necessary to make a quantitative summary to evaluate MDM2 309 T/G polymorphism and risk of cancer.
A commonly occurring T-to-G polymorphism at nucleotide 309 (T309G) of MDM2 has been the focus of many case-control association studies of ovarian cancer in different ethnic populations. However, these studies indicated different or even conflicting results. Kang et al. found that MDM2 SNP309G allele significantly reduced the risk of ovarian cancer and might be a potential protective factor for ovarian cancer development in Chinese women [32]. But knappskog et al. found that MDM2 SNP309G allele significantly increased the risk of ovarian cancer [23]. So it is worthy to make a meta-analysis to evaluate relationship between MDM2 SNP309 polymorphism and ovarian cancer.
In this meta-analysis, after a critical review of the 6 studies on MDM2 SNP309 polymorphism (a total of 1534 cases and 2211 controls), a comprehensive assessment was performed to investigate whether polymorphisms in MDM2 SNP309 was significantly associated with risk of ovarian cancer. Although no associations between the MDM2 SNP309 polymorphism and ovarian cancer were observed based on total population, significant associations were found in Asian population in subgroup analysis by ethnicity.
The prevalence of homozygous SNP309 variant genotype in Caucasian patients with ovarian cancer was 7.8–17.2% [24], [25], [30], while in healthy Caucasians, the prevalence was 12% [7]. No observable link was established between MDM2 SNP309 and ovarian cancer susceptibility of Caucasian women in two case-control studies [30], [34]. In contrast, the prevalence of the G/G genotype was 31% in healthy Chinese women, and the presence of at least one G-allele significantly decreased the risk for ovarian cancer in Chinese women [32]. In our meta-analysis, the frequency of variant allele MDM2 309G was 46.7%–48.8% among Asian population, and 25.0%–48.8% among Caucasians. This might lead to MDM2 SNP309 polymorphism genotype distribution disequilibrium when all ethnic populations were pooled together. As ethnicity was significantly associated with risk of ovarian cancer, it was essential to conduct a subgroup analysis based on ethnicities.
In the subgroup meta-analysis based on ethnicity, compared with T allele, a significantly reduced risk of ovarian cancer is associated with G allele; compared with TT genotype, a significantly reduced risk of ovarian cancer is associated with GG genotype, TG genotype and the combined TG/GG genotypes in Asian subgroup. Further investigations on large scale on Asian populations may confirm this result. In Caucasian subgroup, no significant association was found in different genetic models. Our results indicate that ethnicity may be a critical factor on the effects of the polymorphic alleles.
Although the case and control groups of the included studies were well controlled by age distribution, smoking habits and menstrual status, there are still a number of limitations in this meta-analysis. First, the analysis did not consider gene-gene and gene-environment interactions because of the lack of sufficient data; second, specific environmental and lifestyle factors may influence the results of this analysis; third, while no publication bias was identified, there is still a possibility that our meta-analysis was biased toward a positive result since negative results were likely to be unreported. In order to provide a more precise estimation by adjustment for confounders, future studies must be taken in larger samples and take potential confounders such as P53 and BRCA1/2 into account.
In summary, positive results have been shown on the search for polymorphic variants influencing the risk of ovarian cancer. This meta-analysis provides evidence of the association between MDM2 309 polymorphism and ovarian cancer risk, supporting the hypothesis that MDM2 SNP309 G allele probably acts as an important ovarian cancer protective factor in Asians, but not in Caucasians. Since the results of our meta-analysis are preliminary and may be biased by the relatively small number of subjects, it still needs to be validated by well-designed studies using larger samples in the future.
Supporting Information
(DOC)
(DOC)
Funding Statement
This work was supported by the National Natural Science Foundation of China (No.81071991), Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents and Medicine and Project of Science and Technology of Zhejiang Province (No. 2010C 33018). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Leitao MM Jr, Hummer A, Dizon DS, Aghajanian C, Hensley M, et al. (2003) Platinum retreatment of platinum-resistant ovarian cancer after nonplatinum therapy. Gynecol Oncol 91: 123–129. [DOI] [PubMed] [Google Scholar]
- 2. Dulic V, Kaufmann WK, Wilson SJ, Tlsty TD, Lees E, et al. (1994) p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell 76: 1013–1023. [DOI] [PubMed] [Google Scholar]
- 3. Woods DB, Vousden KH (2001) Regulation of p53 function. Exp Cell Res 264: 56–66. [DOI] [PubMed] [Google Scholar]
- 4. Haupt Y, Maya R, Kazaz A, Oren M (1997) Mdm2 promotes the rapid degradation of p53. Nature 387: 296–299. [DOI] [PubMed] [Google Scholar]
- 5. Honda R, Tanaka H, Yasuda H (1997) Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett 420: 25–27. [DOI] [PubMed] [Google Scholar]
- 6. Kubbutat MH, Jones SN, Vousden KH (1997) Regulation of p53 stability by Mdm2. Nature 387: 299–303. [DOI] [PubMed] [Google Scholar]
- 7. Bond GL, Hu W, Bond EE, Robins H, Lutzker SG, et al. (2004) A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell 119: 591–602. [DOI] [PubMed] [Google Scholar]
- 8. Bond GL, Hu W, Levine A (2005) A single nucleotide polymorphism in the MDM2 gene: from a molecular and cellular explanation to clinical effect. Cancer Res 65: 5481–5484. [DOI] [PubMed] [Google Scholar]
- 9. Bond GL, Levine AJ (2007) A single nucleotide polymorphism in the p53 pathway interacts with gender, environmental stresses and tumor genetics to influence cancer in humans. Oncogene 26: 1317–1323. [DOI] [PubMed] [Google Scholar]
- 10. Hong Y, Miao X, Zhang X, Ding F, Luo A, et al. (2005) The role of P53 and MDM2 polymorphisms in the risk of esophageal squamous cell carcinoma. Cancer Res 65: 9582–9587. [DOI] [PubMed] [Google Scholar]
- 11. Zhang X, Miao X, Guo Y, Tan W, Zhou Y, et al. (2006) Genetic polymorphisms in cell cycle regulatory genes MDM2 and TP53 are associated with susceptibility to lung cancer. Hum Mutat 27: 110–117. [DOI] [PubMed] [Google Scholar]
- 12. Zhou G, Zhai Y, Cui Y, Zhang X, Dong X, et al. (2007) MDM2 promoter SNP309 is associated with risk of occurrence and advanced lymph node metastasis of nasopharyngeal carcinoma in Chinese population. Clin Cancer Res 13: 2627–2633. [DOI] [PubMed] [Google Scholar]
- 13. Economopoulos KP, Sergentanis TN (2010) Differential effects of MDM2 SNP309 polymorphism on breast cancer risk along with race: a meta-analysis. Breast Cancer Res Treat 120: 211–216. [DOI] [PubMed] [Google Scholar]
- 14. Hu Z, Jin G, Wang L, Chen F, Wang X, et al. (2007) MDM2 promoter polymorphism SNP309 contributes to tumor susceptibility: evidence from 21 case-control studies. Cancer Epidemiol Biomarkers Prev 16: 2717–2723. [DOI] [PubMed] [Google Scholar]
- 15. Li Y, Zhao H, Sun L, Huang L, Yang Q, et al. (2011) MDM2 SNP309 is associated with endometrial cancer susceptibility: a meta-analysis. Hum Cell 24: 57–64. [DOI] [PubMed] [Google Scholar]
- 16. Liu GY, Jiang DK, Shen SQ, Yu L (2011) MDM2 SNP309T>G polymorphism with hepatocellular carcinoma risk: a meta-analysis. Arch Med Res 42: 149–155. [DOI] [PubMed] [Google Scholar]
- 17. Wilkening S, Bermejo JL, Hemminki K (2007) MDM2 SNP309 and cancer risk: a combined analysis. Carcinogenesis 28: 2262–2267. [DOI] [PubMed] [Google Scholar]
- 18. Bond GL, Hirshfield KM, Kirchhoff T, Alexe G, Bond EE, et al. (2006) MDM2 SNP309 accelerates tumor formation in a gender-specific and hormone-dependent manner. Cancer Res 66: 5104–5110. [DOI] [PubMed] [Google Scholar]
- 19. Bond GL, Menin C, Bertorelle R, Alhopuro P, Aaltonen LA, et al. (2006) MDM2 SNP309 accelerates colorectal tumour formation in women. J Med Genet 43: 950–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knappskog S, Trovik J, Marcickiewicz J, Tingulstad S, Staff AC, et al. (2012) SNP285C modulates oestrogen receptor/Sp1 binding to the MDM2 promoter and reduces the risk of endometrial but not prostatic cancer. Eur J Cancer 48: 1988–1996. [DOI] [PubMed] [Google Scholar]
- 21. Yarden RI, Friedman E, Metsuyanim S, Olender T, Ben-Asher E, et al. (2008) MDM2 SNP309 accelerates breast and ovarian carcinogenesis in BRCA1 and BRCA2 carriers of Jewish-Ashkenazi descent. Breast Cancer Res Treat 111: 497–504. [DOI] [PubMed] [Google Scholar]
- 22.Bjornslett M, Knappskog S, Lonning PE, Dorum A (2011) Associations between MDM2 SNP309 and SNP285C haplotypes and ovarian cancer risk in BRCA1 mutation carriers. J Clin Oncol 29: (suppl; abstr 5030).
- 23. Knappskog S, Bjornslett M, Myklebust LM, Huijts PE, Vreeswijk MP, et al. (2011) The MDM2 promoter SNP285C/309G haplotype diminishes Sp1 transcription factor binding and reduces risk for breast and ovarian cancer in Caucasians. Cancer Cell 19: 273–282. [DOI] [PubMed] [Google Scholar]
- 24. Bartel F, Jung J, Bohnke A, Gradhand E, Zeng K, et al. (2008) Both germ line and somatic genetics of the p53 pathway affect ovarian cancer incidence and survival. Clin Cancer Res 14: 89–96. [DOI] [PubMed] [Google Scholar]
- 25. Galic V, Willner J, Wollan M, Garg R, Garcia R, et al. (2007) Common polymorphisms in TP53 and MDM2 and the relationship to TP53 mutations and clinical outcomes in women with ovarian and peritoneal carcinomas. Genes Chromosomes Cancer 46: 239–247. [DOI] [PubMed] [Google Scholar]
- 26. Atwal GS, Kirchhoff T, Bond EE, Montagna M, Menin C, et al. (2009) Altered tumor formation and evolutionary selection of genetic variants in the human MDM4 oncogene. Proc Natl Acad Sci U S A 106: 10236–10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hirshfield KM, Rebbeck TR, Levine AJ (2010) Germline mutations and polymorphisms in the origins of cancers in women. J Oncol 2010: 297671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Knappskog S, Lonning PE (2011) MDM2 promoter SNP285 and SNP309; phylogeny and impact on cancer risk. Oncotarget 2: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wan Y, Wu W, Yin Z, Guan P, Zhou B (2011) MDM2 SNP309, gene-gene interaction, and tumor susceptibility: an updated meta-analysis. BMC Cancer 11: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Campbell IG, Eccles DM, Choong DY (2006) No association of the MDM2 SNP309 polymorphism with risk of breast or ovarian cancer. Cancer Lett 240: 195–197. [DOI] [PubMed] [Google Scholar]
- 31. Copson ER, White HE, Blaydes JP, Robinson DO, Johnson PW, et al. (2006) Influence of the MDM2 single nucleotide polymorphism SNP309 on tumour development in BRCA1 mutation carriers. BMC Cancer 6: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kang S, Wang DJ, Li WS, Wang N, Zhou RM, et al. (2009) Association of p73 and MDM2 polymorphisms with the risk of epithelial ovarian cancer in Chinese women. Int J Gynecol Cancer 19: 572–577. [DOI] [PubMed] [Google Scholar]
- 33. Krekac D, Brozkova K, Knoflickova D, Hrstka R, Muller P, et al. (2008) MDM2SNP309 does not associate with elevated MDM2 protein expression or breast cancer risk. Oncology 74: 84–87. [DOI] [PubMed] [Google Scholar]
- 34. Ueda M, Yamamoto M, Nunobiki O, Toji E, Sato N, et al. (2009) Murine double-minute 2 homolog single nucleotide polymorphism 309 and the risk of gynecologic cancer. Hum Cell 22: 49–54. [DOI] [PubMed] [Google Scholar]
- 35. Liu J, Zheng Y, Lei D, Liu D, Xu F, et al. (2011) MDM2 309T>G polymorphism and risk of squamous cell carcinomas of head and neck: a meta-analysis. Asian Pac J Cancer Prev 12: 1899–1903. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)