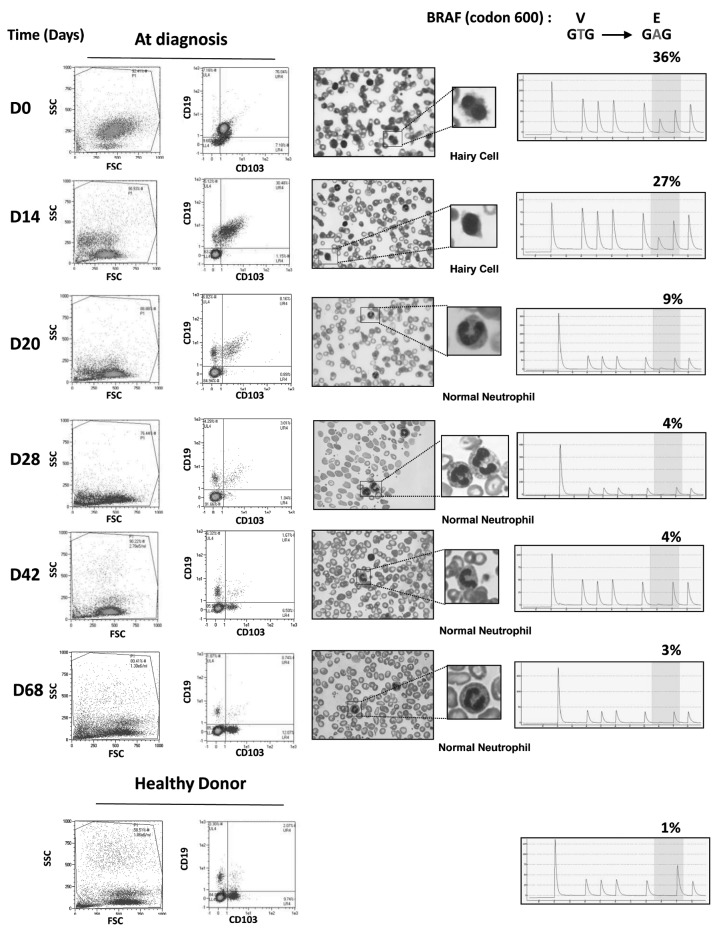
Hairy-cell leukemia (HCL) is a lymphoproliferative disorder characterized by the presence of CD103-positive circulating B cells, pancytopenia and splenomegaly.1 HCL cases were recently shown to harbor a mutation at codon 600 of BRAF (V600E), suggesting that this genetic event represents a key driver of the disease.2 Recently, Dietrich et al. reported a case of refractory HCL treated with escalating doses of vemurafenib. Vemurafenib exhibited remarkable activity, with rapidly decreased splenomegaly, increased platelets, and normalization of hemoglobin and granulocyte counts.3 In consideration of these data, we used vemurafenib to treat a 72-year old patient who presented with a relapse of HCL and the BRAF V600E mutation. In 1989, our then 49-year old male patient was diagnosed with HCL, treated with a splenectomy and follow-up interferon, and demonstrated a complete hematologic response. However, in 2003 and 2009, the patient presented with relapses and was treated with cladribine. Tolerance was very poor with grade IV infectious toxicities after both treatments. In June 2012, the patient presented with a new progression, as revealed by the presence of dyspnea and bicytopenia (platelet count 24×109/L, hemoglobin 6.9 g/dl), a normal neutrophil count, and a circulating HCL level of 4×109/L (morphological examination). A bone marrow aspiration was performed but was not conclusive. The V600E mutation was detected in blood leukemic cells. Considering the patient's medical history, and after obtaining informed consent, we initiated vemurafenib at a dose of 240 mg twice daily (Day 0). The kinetics of the hemogram and hairy-cell leukemia are detailed in (Table 1). Our patient experienced grade III anemia and grade II neutropenia without infection or other complications. At Day 19, the CD19+/CD103+ cell fraction had decreased from 86% to 5%, and the patient remained cytopenic (Figure 1) and required one final red blood cell transfusion. Due to the satisfactory treatment efficiency and the persistent cytopenia, we maintained the treatment with vemurafenib at a constant dose (240 mg x 2), which is in contrast to the management of previously published case of HCL.3 The percentage of cells harboring the V600E mutation was monitored using a pyrosequencing assay, and this method has recently been described as rapid, sensitive and quantitative.4 At Day 56, the immunophenotyping and percentage of mutated cells were comparable to those of a healthy volunteer donor (Figure 2). The blood cell counts at Day 56 had improved, with a hemoglobin level of 9.2 g/dL without transfusion, leukocytes 3.200×109/L and platelets 253×109/L. A bone marrow aspiration showed a CD19+/CD103+ cell frequency of 5%. At this point, the vemurafenib was stopped. One month after completion of the treatment (Day 90), the patient showed complete remission, as defined by Grever (hematologic blood count was normal and blood morphological examination showed no evidence of HCL cells).5 However, close follow up was maintained. Our observations suggest that the inhibition of mutated BRAF by vemurafenib may represent an important therapeutic advancement for the treatment of HCL. Vemurafenib is used as a targeted therapy against mutated BRAF in HCL, as imatinib is used against BCR-ABL tyrosine kinase mutations in chronic myelogenous leukemia. According to these data, the detection of the BRAF V600E mutation is intriguing in cases of HCL. In contrast with the previously reported case of HCL, our data suggest that 240 mg of vemurafenib twice a day is sufficient to obtain hematologic, cytological and molecular responses. However, we did not use a loading dose or escalation dose, which could improve tolerance in frail patients. As in the previously reported study by Dietrich et al., the response to treatment was very rapid and reached a maximum within 21 days. This indicates that a shorter duration of treatment could be sufficient to obtain a response, although the patient should be closely followed up to evaluate if this early complete response is followed by a prolonged disease-free period. If minimal biological disease reappears, evaluation should be made using a sequential pyrosequencing assay. Because very few data are available concerning the use of vemurafenib in HCL, additional cases are needed to confirm this demonstrated efficiency. A phase II study would be of considerable interest to determine the dosage, the vemurafenib rate and the duration of response in HCL cases. In addition, a phase II study would help to define the complete molecular response in HCL cases. Despite the very promising results of the present study, this step is likely mandatory for the clinical development of vemurafenib treatment for HCL.
Table 1.
Monitoring of peripheral blood counts after 3 months of treatment with vemurafenib.
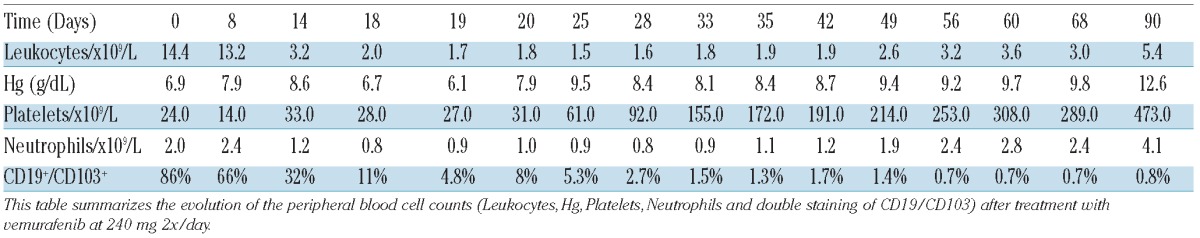
Figure 1.
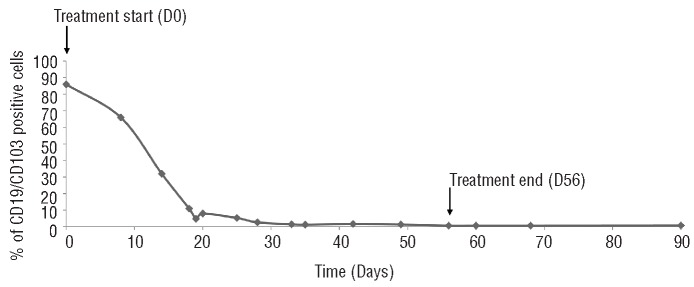
Monitoring of CD19/CD103 positive cells in peripheral blood after three months of treatment with vemurafenib. Determination of CD19+CD103+ cell number in time course of vemurafenib treatment (from Day 0 to Day 56) and one month after the end of treatment (Day 90).
Figure 2.
Treatment with vemurafenib leads to a rapid disappearance of leukemic markers and the mutant form of BRAF in HCL The patient's white blood cells were stained with anti-CD19-VB and anti-CD103-FITC antibodies and analyzed by flow cytometry (left column). Examinations of a peripheral blood smear stained with May-Grünwald-Giemsa were performed at the indicated times (middle column). The pyrosequencing and the identification of the BRAF mutation at position 600 were realized at the indicated times. The results are expressed as the percentage of cells carrying the BRAF V600E mutation versus total peripheral white blood cells (right column).
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Falini B, Tiacci E, Liso A, Basso K, Sabattini E, Pacini R, et al. Simple diagnostic assay for hairy cell leukaemia by immunocytochemical detection of annexin A1 (ANXA1). Lancet. 2004;363(9424):1869-70 [DOI] [PubMed] [Google Scholar]
- 2.Tiacci E, Trifonov V, Schiavoni G, Holmes A, Kern W, Martelli MP, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364(24):2305-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dietrich S, Glimm H, Andrulis M, von Kalle C, Ho AD, Zenz T. BRAF inhibition in refractory hairy-cell leukemia. N Engl J Med. 2012;366(21):2038-40 [DOI] [PubMed] [Google Scholar]
- 4.Verma S, Greaves WO, Ravandi F, Reddy N, Bueso-Ramos CE, O'Brien S, et al. Rapid detection and quantitation of BRAF mutations in hairy cell leukemia using a sensitive pyrosequencing assay. Am J Clin Pathol. 2012;138(1):153-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grever MR. How I treat hairy cell leukemia. Blood. 2010;115(1):21-8 [DOI] [PMC free article] [PubMed] [Google Scholar]