Abstract
Purpose
Intraductal papillary mucinous neoplasms are mucin-producing cystic neoplasms of the pancreas. One-third are associated with invasive carcinoma. We examined the benefit of adjuvant chemoradiotherapy (CRT) for this cohort.
Methods and Materials
Patients who had undergone pancreatic resection at Johns Hopkins Hospital between 1999 and 2004 were reviewed. Of these patients, 83 with a resected pancreatic mass were found to have an intraductal papillary mucinous neoplasm with invasive carcinoma, 70 of whom met inclusion criteria for the present analysis.
Results
The median age at surgery was 68 years. The median tumor size was 3.3 cm, and invasive carcinoma was present at the margin in 16% of the patients. Of the 70 patients, 50% had metastases to the lymph nodes and 64% had Stage II disease. The median survival was 28.0 months, and 2- and 5-year survival rate was 57% and 45%, respectively. Of the 70 patients, 40 had undergone adjuvant CRT. Those receiving CRT were more likely to have lymph node metastases, perineural invasion, and Stage II–III disease. The 2-year survival rate after surgery with vs. without CRT was 55.8% vs. 59.3%, respectively (p = NS). Patients with lymph node metastases or positive surgical margins benefited significantly from CRT (p = .047 and p = .042, respectively). On multivariate analysis, adjuvant CRT was associated with improved survival, with a relative risk of 0.43 (95% confidence interval, 0.19–0.95; p = .044) after adjusting for major confounders.
Conclusion
Adjuvant CRT conferred a 57% decrease in the relative risk of mortality after pancreaticoduodenectomy for intraductal papillary mucinous neoplasms with an associated invasive component after adjusting for major confounders. Patients with lymph node metastases or positive margins appeared to particularly benefit from CRT after definitive surgery.
Keywords: Pancreatic adenocarcinoma, intraductal papillary mucinous neoplasm, adjuvant therapy, radiotherapy, invasive carcinoma
INTRODUCTION
Intraductal papillary mucinous neoplasms (IPMNs) are mucin-producing cystic neoplasms of the pancreas that are thought to be a precursor to invasive adenocarcinoma (1). Approximately 30% of resected IPMNs are associated with invasive adenocarcinoma (2). The progression of IPMN to invasive carcinoma shares some characteristics of the pancreatic intraepithelial neoplasia to carcinoma sequence of the more common infiltrating ductal carcinoma of the pancreas; however, clear distinctions exist (3). IPMNs have a lower frequency of mutations in KRAS, TP53, and SMAD4 than pancreatic intraepithelial neoplasia. Many IPMNs express MUC2, and pancreatic intraepithelial neoplasia usually express MUC1 (4–7). Invasive cancer associated with IPMNs can be of tubular/ductal, colloid, undifferentiated (anaplastic), or mixed histologic types.
Adjuvant chemoradiotherapy (CRT) is generally offered to patients with invasive ductal carcinoma and has been shown to improve survival in some prospective and retrospective studies (8–11). The role of adjuvant therapy for resected IPMN with an invasive component, however, remains undefined (5, 12, 13). The purpose of the present study was to examine the use and potential benefits of adjuvant CRT in a large cohort of patients with IPMN-associated invasive carcinoma treated at a single, high-volume institution. We also examined the prognostic factors for survival in these patients. To our knowledge, this is the only study to date to evaluate the role of CRT in the treatment of patients with invasive adenocarcinoma arising in resected pancreatic IPMN.
METHODS AND MATERIALS
Patients
A retrospective review of a prospectively collected institutional review board-approved database of all patients who had undergone pancreatic resection at the Johns Hopkins Hospital between 1999 and 2004 was performed. More than 1,383 pancreatic resections had been performed during this period. Of these, 83 specimens (6%) were found to contain invasive carcinoma arising in association with IPMN (69 were head of pancreas and 14 distal pancreas). Patients with either incomplete information on the status of adjuvant treatment (n = 11) or <30 days of postoperative follow-up (n = 2) were excluded. Thus, 70 patients met the inclusion criteria for the present analysis (59, head of pancreas and 11, distal pancreas). Patient follow-up information was obtained from the paper and electronic hospital charts. Survival was determined and cross-checked by a review of the clinical follow-up information, cancer center abstracting services, and the Social Security Death Index.
Surgery and pathologic analysis
All patients underwent pancreaticoduodenectomy or distal pancreatectomy. The standard surgical approach at our institution has been standard pancreaticoduodenal resection without extended retroperitoneal lymph node dissection with partial pancreatectomy, leaving the body and tail of the pancreas intact. A pylorus-preserving approach is standard, with distal gastric resection added for cancer involving the distal stomach or first portion of the duodenum. Pancreatic-enteric reconstruction was accomplished by pancreaticojejunostomy or pancreaticogastrostomy (14). Standard distal pancreatectomy was used to remove the body and/or tail of the pancreas, leaving the head of the pancreas intact, and typically included splenectomy and lymphadenectomy.
All pathology specimens were reviewed to determine the primary pathologic diagnosis and disease extent. The lymph nodes were considered positive if any lymph node in the resected specimen contained carcinoma (by either direct extension or more distant spread). Resection margins were considered positive if invasive carcinoma was present at the final pancreatic neck, uncinate process, bile duct, or duodenal or retroperitoneal soft-tissue margin. To provide pathologic confirmation of the histologic types of invasive carcinoma, hematoxylin-eosin–stained slides from all available cases were reviewed again by 2 of us (R.H.H., M.G.) and classified as ductal, colloid, or other histologic type.
Adjuvant therapy
The medical records were reviewed to determine which patients had received adjuvant CRT. Adjuvant therapy consisted of concurrent 5-fluorouracil (5-FU)–based chemotherapy with radiotherapy followed by 5-FU based chemotherapy alone. Most patients received continuous infusion 5-FU given at 225 mg/m2. All patients underwent external beam radiotherapy using a computed tomography-based treatment plan. The median radiation dose was 50.4 Gy (range, 45–54).
Statistical analysis
Statistical analyses were performed using STATA, version 9 (StataCorp, College Station, TX). Tests of the differences in patient characteristics, stratified by histologic type and adjuvant treatment, were performed using t tests and chi-square tests. The primary analysis for the present study was the interval to death stratified by the use of adjuvant treatment. For the patients who died, the follow-up time was assessed from the date of surgery to the date of death. For the patients who survived, the follow-up time was assessed from the date of surgery to the date of the last record query, December 18, 2006. The survival curves were estimated using the Kaplan-Meier method, and differences in survival and median survival stratified by adjuvant treatment use were compared using the log–rank test. For 2- and 5-year survival, comparisons were done with the follow-up time censored at the corresponding interval. To further determine whether adjuvant treatment affected survival differently when adjusted for tumor characteristics, the Kaplan-Meier curves were also stratified by nodal status, margin positivity, tumor grade, tumor location, and histologic type. Proportional hazards models were constructed to examine adjuvant treatment use and other patient characteristics as predictors of mortality. Univariate analyses examined nodal positivity, margin positivity, histologic type (ductal, colloid, or other), tumor grade, tumor location, and adjuvant treatment as predictors of mortality. To examine adjuvant treatment use as an independent predictor of mortality, multivariate proportional hazards analysis was performed using these characteristics as covariates.
RESULTS
The median follow-up for patients with IPMN-associated adenocarcinoma was 24.8 months. The characteristics of the 70 patients included in the present study are listed in Table 1. The median age at surgery was 68.0 years (range, 35–85); 47% were men, and 89% were white. Of the 70 patients, 84% had undergone a Whipple procedure and 16% had undergone distal pancreatectomy with or without splenectomy. The median size of the tumor with an invasive component was 3.3 cm (range, 0.2–10.0). Invasive carcinoma at the margin was present in 11 patients (15.7%), and 50% had lymph node metastases. Perineural and perivascular invasion was present in 47% and 27% of the carcinoma specimens, respectively. One patient had Stage III disease, and most had Stage II (64%). The median survival for all patients in the present analysis was 28 months. The 2- and 5-year survival rate was 57% and 45%, respectively.
Table 1.
Patient characteristics
| Characteristic | Value |
|---|---|
| Patient demographics | |
| Patients (n) | 70 |
| Age at surgery (y) | |
| Mean ± SD | 67.8 ± 10.3 |
| Median | 68.0 |
| Range | 35–85 |
| Male (n) | 33 (47.1) |
| Race (n) | |
| White | 62 (88.6) |
| Black | 5 (7.1) |
| Other | 3 (4.3) |
| Tumor characteristcs | |
| Location (n) | |
| Head of pancreas | 59 (84.3) |
| Distal pancreas | 11 (15.7) |
| Tumor diameter (cm) | |
| Mean ± SD | 3.5 ± 2.2 |
| Median | 3.3 |
| Range | 0.2–10.0 |
| Stage (n) | |
| I | 24 (34.3) |
| II | 45 (64.3) |
| III | 1 (1.4) |
| Nodal status (n) | |
| N0 | 35 (50.0) |
| N1 | 35 (50.0) |
| Margin status (n) | |
| Negative | 59 (84.3) |
| Positive | 11 (15.7) |
| Perineural invasion (n) | |
| No | 26 (37.1) |
| Yes | 33 (47.2) |
| Unknown | 11 (15.7) |
| Vascular invasion (n) | |
| No | 42 (60.0) |
| Yes | 19 (27.1) |
| Unknown | 9 (12.9) |
| Histologic type (n) | |
| Ductal | 34 (48.6) |
| Colloid | 23 (32.9) |
| Other | 13 (18.6) |
| Histologic grade (n) | |
| Well/moderate | 44 (62.9) |
| Poor/anaplastic/undifferentiated | 17 (24.3) |
| Unknown | 9 (12.9) |
| Treatment/survival | |
| Adjuvant CRT (n) | 40 (57.1) |
| Median follow-up time (mo) | 24.8 |
| Median survival (mo) | 28.0 |
| 2-y Survival rate (%) | 57.4 |
| 5-y Survival rate (%) | 45.3 |
Abbreviations: SD = standard deviation; CRT = chemoradiotherapy. Data in parentheses are percentages.
The evaluation of the histologic features of the invasive carcinoma associated with IPMN revealed that 49% of the patients had tubular adenocarcinoma and 33% had colloid carcinoma; 24% of patients had other histologic types, including undifferentiated (anaplastic) and mixed.
Of the 70 patients with an IPMN-associated invasive component, 40 underwent adjuvant therapy and 30 did not (Table 2). The patients who had not received adjuvant therapy were more likely to be ≥65 years old; however, the difference was not statistically significant (p = .095). The median size of the invasive component was 3.1 cm for those receiving CRT and 3.5 cm for those who did not (p = .859). The proportion of patients receiving CRT who had positive margins was 20%. In contrast, only 10% of patients who did not receive CRT had positive margins (p = .255). Significantly more patients who received CRT were more likely to have positive lymph nodes (65% vs. 30%), Stage II–III disease (80% vs. 47%), and perineural invasion (63% vs. 27%) than patients who did not receive CRT (all p <.007). Patients with ductal histologic tumor were more likely than those with colloid histologic tumor to have Stage II–III disease, positive lymph nodes, vascular invasion, and perineural invasion. Patients with a ductal histologic type were significantly more likely to undergo adjuvant CRT than those with a colloid histologic type (p = .005).
Table 2.
Tumor characteristics and survival for patients receiving or not receiving adjuvant therapy
| Characteristic | Adjuvant treatment
|
p | |
|---|---|---|---|
| No (n = 30) Yes (n = 40) | |||
| Age ≥65 y (n) | 23 (76.7) | 23 (57.5) | .095 |
| Tumor size (cm) | .859 | ||
| Mean ± SD | 3.4 ± 2.5 | 3.5 ± 2.0 | |
| Median | 3.5 | 3.1 | |
| Positive margins (n) | 3 (10.0) | 8 (20.0) | .255 |
| Positive lymph nodes (n) | 9 (30.0) | 26 (65.0) | .004 |
| Perineural invasion (n) | .007 | ||
| No | 14 (46.6) | 12 (30.0) | |
| Yes | 8 (26.7) | 25 (62.5) | |
| Unknown | 8 (26.7) | 3 (7.5) | |
| Vascular invasion (n) | .212 | ||
| No | 20 (66.6) | 22 (55.0) | |
| Yes | 5 (16.7) | 14 (35.0) | |
| Unknown | 5 (16.7) | 4 (10.0) | |
| Stage II–III disease (n) | 14 (46.7) | 32 (80.0) | .004 |
| Histologic type (n) | .005 | ||
| Ductal | 8 (26.7) | 26 (65.0) | |
| Colloid | 15 (50.0) | 8 (20.0) | |
| Other/unknown | 7 (23.3) | 6 (15.0) | |
| Distal Pancreatectomy (n) | .394 | ||
| No | 24 (80.0) | 35 (87.5) | |
| Yes | 6 (20.0) | 5 (12.5) | |
| Histologic grade (n) | .286 | ||
| Well/moderate | 22 (73.3) | 22 (55.0) | |
| Poor/anaplastic/undifferentiated | 5 (16.7) | 12 (30.0) | |
| Unknown | 3 (10.0) | 6 (15.0) | |
| Median survival rate (%) | 91.9 | 25.8 | .923 |
| 2-y Survival rate (%) | 59.3 | 55.8 | .930 |
| 5-y Survival rate (%) | 50.2 | 41.4 | .744 |
Data in parentheses are percentages.
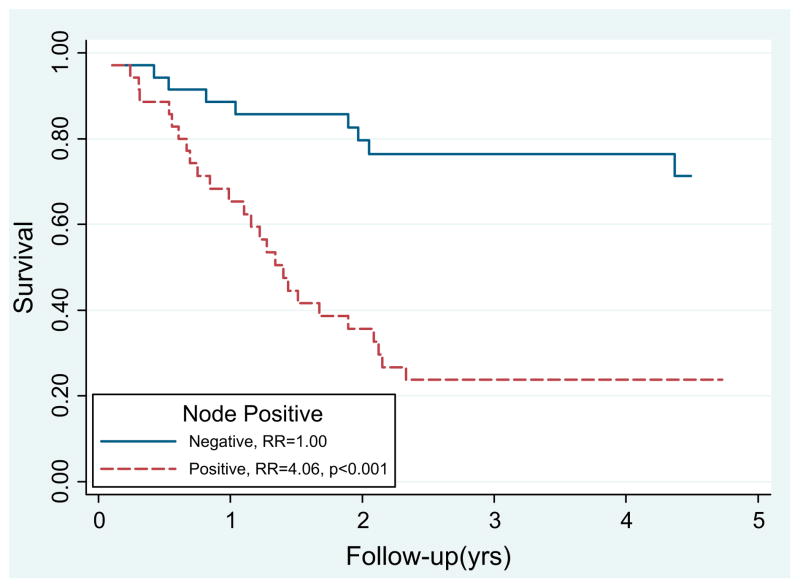
Univariate and multivariate analyses were performed to identify the prognostic factors in patients with IPMN-associated invasive carcinoma (Table 3). Patients with lymph node positive disease had significantly inferior survival on univariate analysis (relative risk [RR] 4.06, p <.001; Fig. 1). Margin-positive resections resulted in inferior survival on univariate analysis (RR 2.42, p = .023). Poor, anaplastic, or undifferentiated tumors resulted in a worse prognosis compared with well or moderate-grade tumors (RR 2.25, p = .026). Patients with colloid histologic features had a nonsignificant improvement in survival compared with those with ductal histologic features (RR 1.94, p = .120).
Table 3.
Univariate and multivariate analyses of prognostic variables
| Characteristic | Patients (n) | Univariate RR (95% CI) | p | Multivariate RR (95% CI) | p |
|---|---|---|---|---|---|
| Adjuvant CRT | .923 | .044 | |||
| No | 30 (42.9) | 1.00 | 1.00 | ||
| Yes | 40 (57.1) | 1.03 (0.53–2.01) | 0.43 (0.19–0.98) | ||
| Nodal status | <.001 | <.001 | |||
| Negative | 35 (50.0) | 1.00 | 1.00 | ||
| Positive | 35 (50.0) | 4.06 (1.94–8.50) | 5.31 (1.96–14.39) | ||
| Margin status | .023 | .273 | |||
| Negative | 59 (84.3) | 1.00 | 1.00 | ||
| Positive | 11 (15.7) | 2.42 (1.13–5.17) | 1.65 (0.67–4.05) | ||
| Histologic type | |||||
| Colloid | 23 (32.9) | 1.00 | — | 1.00 | — |
| Ductal | 34 (48.6) | 1.96 (0.85–4.49) | .113 | 0.91 (0.32–2.64) | .865 |
| Other | 13 (18.6) | 2.23 (0.86–5.79) | .101 | 0.86 (0.27–2.68) | .790 |
| Distal Pancreatectomy | .588 | .805 | |||
| No | 59 (84.3) | 1.00 | 1.00 | ||
| Yes | 11 (15.7) | 0.75 (0.26–2.13) | 0.86 (0.26–2.82) | ||
| Histologic grade | |||||
| Well/moderate | 44 (62.9) | 1.00 | — | 1.00 | — |
| Poor/anaplastic/undifferentiated | 17 (24.3) | 2.25 (1.10–4.61) | .026 | 1.45 (0.61–3.43) | .397 |
| Unknown | 9 (12.9) | 0.62 (0.18–2.17) | .452 | 0.87 (0.22–3.53) | .850 |
Abbreviations: RR = relative risk; CI = confidence interval; CRT = chemoradiotherapy.
Fig. 1.
Survival after pancreaticoduodenectomy for intraductal papillary mucinous neoplasm with associated invasive adenocarcinoma was inferior for node-positive patients (relative risk [RR] 4.06, p <.001).
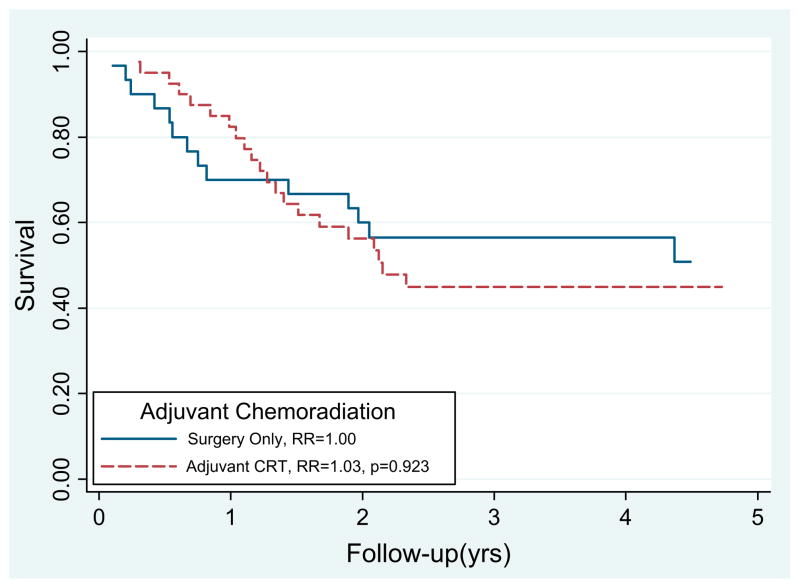
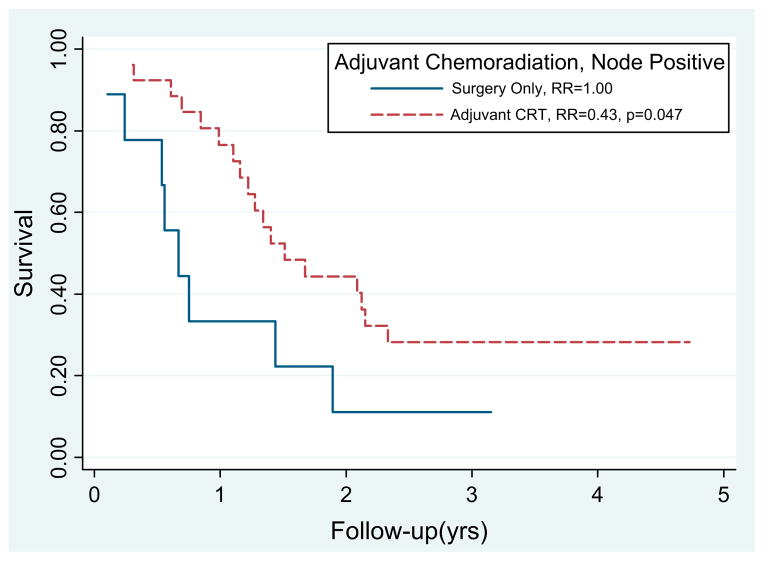
The median survival time and 2-year survival rate after surgery with and without CRT was 25.8 months and 56% and 91.9 months and 59%, respectively (p = .923; Fig. 2). Node-positive patients who received adjuvant CRT had significantly improved survival compared with those who did not receive CRT (RR 0.43, p = .047; Fig. 3). Although the numbers were small (n = 11), the margin-positive patients who received CRT (n = 9) had improved survival compared with those who did not (p = .042). On multivariate analysis, adjuvant CRT was associated with improved survival (RR 0.43; 95% confidence interval, 0.19–0.95; p = .044) after adjusting for nodal disease, margin status, tumor location, tumor grade, and histologic type.
Fig. 2.
Survival after pancreaticoduodenectomy for intraductal papillary mucinous neoplasm with associated invasive adenocarcinoma was not significantly different statistically for those who received adjuvant chemoradiotherapy (CRT) (relative risk [RR] 1.023, p <.923).
Fig. 3.
Patients with resected intraductal papillary mucinous neoplasm with invasive component and node-positive disease benefited from adjuvant chemoradiotherapy (CRT) (relative risk [RR] 0.43, p = .044).
DISCUSSION
The number of surgical resections for IPMN performed at our institution has increased dramatically since the late 1990s as this diagnosis has become increasingly recognized (15). IPMNs are believed to represent precursor neoplasms, some of which follow a predictable progression to invasive carcinoma from noninvasive IPMN with mild dysplasia (adenoma) to noninvasive IPMN with moderate dysplasia (borderline) to noninvasive IPMN with marked dysplasia (carcinoma in situ) to invasive carcinoma. Invasive carcinoma arising in association with IPMNs is believed to be a genetically and clinically distinct entity separate from pancreatic ductal adenocarcinoma not arising in association with IPMN (1). Controversy still exists regarding the prognostic variables and optimal management of invasive carcinoma arising in association with IPMN.
Numerous studies have suggested that patients with resected pancreatic ductal adenocarcinoma should receive adjuvant therapy (8, 9). After adjuvant therapy, the 5-year survival rate for patients with resected ductal adenocarcinoma has been approximately 25%. Patients with nodal metastasis or margin-positive resections generally have an inferior prognosis and are more likely to benefit from adjuvant therapy (10, 16, 17). In the present study, the 5-year survival rate for all IPMN patients with an associated invasive component was 45%, comparable to that of other published studies (range, 36–70%) (13, 15, 18). Salvia et al. (13) postulated that the reported differences in survival might have resulted from the inclusion of both main duct and branch duct IPMNs or from differences in the pathologic interpretation. Another possible explanation for the range in survival is institutional bias toward or against adjuvant therapy for patients with resected IPMN. The patients in the present series were generally offered adjuvant CRT if they had an associated invasive component and lymph node metastases or positive margins. Given this selection bias, no significant benefit was found for adjuvant CRT on univariate analysis. However, in the subgroup analyses, patients with node- or margin-positive resection experienced significantly improved survival with the addition of CRT. Furthermore, on multivariate analysis, after controlling for nodal status, margin status, and histologic type, CRT was associated with improved survival. Drawing conclusions from these comparisons is difficult, however, given the small patient numbers and inherent selection bias associated with them.
The present analysis was limited by a number of factors. Although the high patient volume at our institution afforded us one of the largest experiences with invasive carcinoma arising in association with IPMNs, our patient numbers were still relatively small. Examining the potential benefits of adjuvant therapy in this cohort was fraught with selection bias, because patients with more aggressive disease were more likely to receive recommendations to undergo adjuvant CRT. Furthermore, the potential benefits of adjuvant CRT might not be realized in this subgroup of patients because our treatment recommendations for invasive carcinoma associated with IPMN have been extrapolated from our experience with pancreatic ductal carcinoma. Nevertheless, the results of the present study suggest that patients with IPMN and an associated invasive component may benefit from adjuvant 5-FU–based CRT if they have lymph node metastasis or margin-positive resections. As with pancreatic adenocarcinoma, prospective clinical trials are necessary to optimize the adjuvant therapy for invasive carcinoma arising in association with IPMNs.
CONCLUSION
Compared with surgery alone, adjuvant CRT conferred a 57% decrease in the RR of mortality after pancreaticoduodenectomy for IPMN with an associated invasive component after adjusting for major confounders. Patients with lymph node metastases or positive margins appeared to particularly benefit from 5-FU–based CRT after definitive surgery.
Footnotes
Presented at the 49th Annual Meeting of the American Society for Therapeutic Radiology and Oncology, Los Angeles, CA 2007.
Conflict of interest: none.
References
- 1.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 2.Azar C, Van de Stadt J, Rickaert F, et al. Intraductal papillary mucinous tumours of the pancreas: Clinical and therapeutic issues in 32 patients. Gut. 1996;39:457–464. doi: 10.1136/gut.39.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brune K, Abe T, Canto M, et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol. 2006;30:1067–1076. [PMC free article] [PubMed] [Google Scholar]
- 4.Hruban RH, Petersen GM, Ha PK, et al. Genetics of pancreatic cancer: From genes to families. Surg Oncol Clin North Am. 1998;7:1–23. [PubMed] [Google Scholar]
- 5.Iacobuzio-Donahue CA, Klimstra DS, Adsay NV, et al. Dpc-4 protein is expressed in virtually all human intraductal papillary mucinous neoplasms of the pancreas: Comparison with conventional ductal adenocarcinomas. Am J Pathol. 2000;157:755–761. doi: 10.1016/S0002-9440(10)64589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adsay NV, Merati K, Andea A, et al. The dichotomy in the pre-invasive neoplasia to invasive carcinoma sequence in the pancreas: Differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol. 2002;15:1087–1095. doi: 10.1097/01.MP.0000028647.98725.8B. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki S, Yamamoto H, Kaneto H, et al. Differential roles of alterations of p53, p16, and SMAD4 expression in the progression of intraductal papillary-mucinous tumors of the pancreas. Oncol Rep. 2003;10:21–25. [PubMed] [Google Scholar]
- 8.Kalser MH, Ellenberg SS. Pancreatic cancer: Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 9.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 10.Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: Results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26:3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: A randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 12.Nakagohri T, Asano T, Kenmochi T, et al. Long-term surgical outcome of noninvasive and minimally invasive intraductal papillary mucinous adenocarcinoma of the pancreas. World J Surg. 2002;26:1166–1169. doi: 10.1007/s00268-002-6254-3. [DOI] [PubMed] [Google Scholar]
- 13.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: Clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239(678):685–687. doi: 10.1097/01.sla.0000124386.54496.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas: 201 patients. Ann Surg. 1995;221(721):731–733. doi: 10.1097/00000658-199506000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: An updated experience. Ann Surg. 2004;239(788):797–799. doi: 10.1097/01.sla.0000128306.90650.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corsini MM, Miller RC, Haddock MG, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: The Mayo Clinic experience (1975–2005) J Clin Oncol. 2008;26:3511–3516. doi: 10.1200/JCO.2007.15.8782. [DOI] [PubMed] [Google Scholar]
- 17.Oettle H, Neuhaus P. Adjuvant therapy in pancreatic cancer: A critical appraisal. Drugs. 2007;67:2293–2310. doi: 10.2165/00003495-200767160-00001. [DOI] [PubMed] [Google Scholar]
- 18.Chari ST, Yadav D, Smyrk TC, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500–1507. doi: 10.1053/gast.2002.36552. [DOI] [PubMed] [Google Scholar]