INTRODUCTION
The Drosophila visual system is composed of the retina and the optic lobes, which are the ganglia where photoreceptors project and initial processing of visual inputs occur. This protocol outlines procedures for dissecting the optic lobes from Drosophila larvae, pupae, and adults. It also describes methods for visualizing the anatomy of brain neural circuits by staining with fluorescent secondary antibodies and primary antibodies specific for various neuronal populations and architectural features.
MATERIALS
RECIPES: Please see the end of this article for recipes indicated by <R>.
It is essential that you consult the appropriate Material Safety Data Sheets and your institution’s Environmental Health and Safety Office for proper handling of equipment and hazardous materials used in this protocol.
Reagents
CO2 (for anesthetizing adult fiies)
Drosophila at stage of interest
Glycerol (50%)
-
Normal goat serum (Lampire Biological Laboratories) (5%, v/v in PBST)
Aliquot in very small volumes and store at −20°C. Paraformaldehyde (16%) (Electron Microscopy Sciences)
PBST (PBS containing 0.3% Triton X-100)
<R>Phosphate-buffered saline (PBS) (1×, pH 7.4)
Primary antibodies (see Table 1)
Secondary antibodies (see Table 2)
Vectashield mounting medium (for preserving fluorescence) (Vector Laboratories)
Table 1.
Primary antibodies used to label Drosophila brains
| Primary antibody | Source | Dilution |
|---|---|---|
| To detect expression of green fluorescent protein(GFP)- or lacZ-based constructs: | ||
| Rabbit anti-GFP | Molecular probes; A11122 | 1:1000 |
| Sheep anti-GFP | Serotec; 4745-1051 or similar | 1:1000 |
| Rabbit anti-β-Gal | ICN/Cappel or similar | 1:20,000 |
| Mouse anti-β-Gal | Promega; Z3781 | 1:500 |
| To label relevant architectural features: | ||
| Mouse anti-nc82 (to label general larval, pupal, and adult neuropil) | Developmental studies Hybridoma bank | 1:50 |
| Rat anti-DE-cadherin (DCAD2) (to label the larval neuroepithelium and neuropil in larvae and early pupae) | Developmental studies Hybridoma bank | 1:50 |
| Rat anti-DN-cadherin (DN-EX 8) (to label general neuropil in larvae, pupae, and adult) | Developmental studies Hybridoma bank | 1:50 |
| To label neuronal populations in larvae: | ||
| Mouse anti-24B10 (to label photoreceptors and their projections) | Developmental studies Hybridoma bank | 1:50 |
| Mouse anti-Dachshund (mAbdac 2-3) (to label lamina precursors and lobula cells) | Developmental studies Hybridoma bank | 1:100 |
| Mouse anti-Elav (9F8A9) (to label neurons) | Developmental studies Hybridoma bank | 1:25 |
| Rat anti-Elav (7E8A10) (to label photoreceptors and their projections) | Developmental studies Hybridoma bank | 1:25 |
Table 2.
Secondary antibodies used to label Drosophila brains
| Secondary antibody | Source | Dilution |
|---|---|---|
| Donkey anti-sheep Alexa488 | Molecular probes; A-11015 | 1:1000 |
| Donkey anti-mouse Alexa488 | Molecular probes; A-21202 | 1:1000 |
| Goat anti-rabbit Alexa488 | Molecular probes; A-11034 | 1:1000 |
| Donkey anti-mouse Alexa555 | Molecular probes; A-31570 | 1:1000 |
| Donkey anti-rabbit Alexa555 | Molecular probes; A-31572 | 1:1000 |
| Goat anti-guinea pig Alexa555 | Molecular probes; A-21435 | 1:500 |
| Goat anti-rat Alexa555 | Molecular probes; A-21434 | 1:500 |
| Donkey anti-mouse Alexa647 | Molecular probes; A-31571 | 1:500 |
| Donkey anti-rabbit Alexa647 | Molecular probes; A-31573 | 1:500 |
| Donkey anti-rat Cy5 | Jackson ImmunoResearch Europe; 712-175-153 | 1:400 |
| Goat anti-guinea pig Alexa647 | Molecular probes; A-21450 | 1:500 |
Equipment
Cover glasses (18 × 18-mm and 24 × 50-mm) (Fisher Scientific)
Dissecting microscope
Dissection dishes (three-well, glass) (Fisher Scientific)
Forceps (two pairs; Dumont #55; Fine Science Tools)
Micropipettor and tips
Microscope slides (Fisher Scientific)
Minutien pins (0.1-mm diameter) (Fine Science Tools 26002-10)
Mounted pins (fine)
Nail polish (clear)
Orbital shaker (Bellco Biotechnology)
Paintbrush (small)
Petri dishes
Pin holders (12-cm) (Fine Science Tools 26016-12)
Slide holder (dark)
Sylgard 184 Silicone Elastomer Kit (Dow Corning) (Stern 1999)
Tubes, 1.5-mL
METHOD
Dissection
The dissection procedure should take ~10–25 min per experiment. It is critical to minimize dissection time to avoid tissue degradation.
-
1
Dissect Drosophila brains as described below according to the developmental stage desired.
Larval brains
-
i
Collect larvae at the stage of interest either from the walls of the vial using forceps or with a PBS-soaked paintbrush for younger larvae. Alternatively, fill the vial with 50% glycerol and larvae will come up to the surface.
-
ii
Place larvae in a Petri dish layered with Sylgard transparent resin containing drops of PBS.
-
iii
Remove the brain lobes by holding the larval body with one pair of forceps and pulling from the larval mouth hook with a second pair. Discard the larval body. Remove the excess tissue surrounding the brain lobes using fine mounted pins.
-
iv
Place the clean brain lobes in a glass dissection dish well containing 150 μL of PBS. Keep the dish on ice.
A typical experiment requires 10–20 larval brains.
Pupal brains
-
v
Collect pupae at the appropriate developmental stage in a Petri dish layered with Sylgard transparent resin containing drops of PBS.
Pupae can be distinguished from slow-moving third-instar larvae because they stop crawling and remain attached to the wall of the vial. White pupae are considered the earliest stage in pupal development, whereas older pupae are dark brown. To study specific pupal development stages, wait the appropriate number of hours before collecting. -
vi
Submerge pupae in PBS and hold the pupal case through the abdominal part with one pair of forceps. With another pair, open the pupal case and pull the pupae out. Gently open the pupae and push the brain out. Remove excess tissue surrounding the brain lobes using fine mounted pins.
-
vii
Using a pair of sharp forceps held closed, place the clean brain lobes in a glass dissection dish well containing 150 μL of PBS. Keep the dish on ice.
A typical experiment requires six to 10 pupal brains. -
viii
Using fine pins, remove or gently separate the pupal eye to avoid interference with visualization of the pupal optic lobe.
This step is critical for visualizing the medulla neuropil.
Adult brains
-
ix
Anesthetize adult flies of the appropriate genotype with CO2. Decapitate and place the heads in a glass dissection dish well containing PBS. Keep the dish on ice.
-
x
Perform the dissection in a Petri dish layered with Sylgard transparent resin containing drops of PBS, or alternatively, in the dissection dish well containing PBS.
-
xi
Holding the head through the maxillary cavity, submerge it in PBS, and remove the maxillary palps with forceps. Hold both sides of the maxillary cavity and gently pull the two pairs of forceps away from each other to open the head cuticle. Remove the brain from the head cuticle and remove any excess surrounding tissue using fine mounted pins.
-
xii
Using a pair of sharp forceps held closed, place the clean adult brain lobes in a glass dissection dish well containing 150 μL of PBS. Keep the dish on ice.
A typical experiment requires six to 10 adult brains. -
xiii
Remove the retina and lamina to avoid interference with the visualization of the medulla/ lobula complex and poor antibody penetration.
This step is critical.
Fixation
The fixation and staining of brains should take ~1.5 d.
-
2
Add 50 μL of 16% paraformaldehyde to the glass wells containing 150 μL of PBS and the dissected tissue. Fix the larval, pupal, or adult brains by incubating on an orbital shaker with gentle agitation for 20 min at room temperature.
-
3
Remove the paraformaldehyde and wash with agitation three times in fresh PBS for 5 min each.
Staining
Day 1
-
4
Add 200 μL of blocking solution (5% normal goat serum in PBST) and incubate the fixed brains on an orbital shaker with gentle agitation for at least 30 min at room temperature in darkness.
-
5
Dilute primary antibodies in PBST in 1.5-mL tubes (you will need 200 μL per well).
-
6
Remove the blocking solution and add primary antibody. Incubate with agitation overnight at room temperature in darkness.
For the majority of commercial antibodies, it is possible to omit the blocking step and incubate the dissected brains directly in primary antibodies overnight.
Day 2
-
7
Remove primary antibody and wash three times in PBS for 20 min each at room temperature.
Primary antibodies can be reused three times or more. -
8
Dilute secondary antibodies in PBST in 1.5-mL tubes (you will need 200 μL per well). Add secondary antibodies to brain preparations. Incubate on an orbital shaker with agitation for 3 h at room temperature in darkness.
Prepare the secondary antibodies fresh and discard after use. -
9
Remove secondary antibodies and wash three times in PBS for 20 min each at room temperature.
Mounting
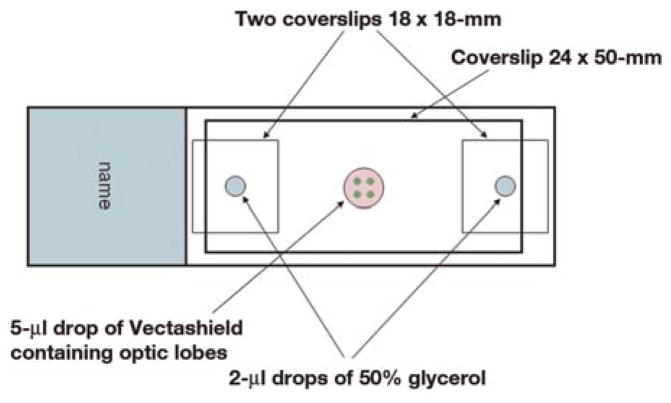
Use the bridge method illustrated in Figure 1 to mount Drosophila brains and preserve their three-dimensional configuration. This procedure should take ~5–10 min per experiment.
FIGURE 1.
Bridge method for mounting Drosophila optic lobes.
-
10
Add two 2-μL drops of 50% glycerol to each end of a microscope slide. Cover these drops with 18 × 18-mm cover glasses.
-
11
Add a 5-μL drop of Vectashield mounting medium in between the two cover glasses.
-
12
Using a pair of sharp forceps held closed, place the brains in the mounting medium. Using fine mounted pins, align all the brains in the same orientation for ease of imaging.
-
13
Place a 24 × 50-mm cover glass on top of the bridge to cover the brains.
-
14
Seal the edges of the cover glass with nail polish and store the samples at 4°C in a dark slide holder.
RECIPES
Phosphate-buffered saline (PBS)
| Reagent | Amount to add (for 1X solution) | Final concentration (1X) | Amount to add (for 10X stock) | Final concentration (10X) |
|---|---|---|---|---|
| NaCl | 8 g | 137 mM | 80 g | 1.37 M |
| KCl | 0.2 g | 2.7 mM | 2 g | 27 mM |
| Na2HPO4 | 1.44 g | 10 mM | 14.4 g | 100 mM |
| KH2PO4 | 0.24 g | 1.8 mM | 2.4 g | 18 mM |
| If necessary, PBS may be supplemented with the following: | ||||
| CaCl2·2H2O | 0.133 g | 1 mM | 1.33 g | 10 mM |
| MgCl2·6H2O | 0.10 g | 0.5 mM | 1.0 g | 5 mM |
PBS can be made as a 1X solution or as a 10X stock. To prepare 1 L of either 1X or 10X PBS, dissolve the reagents listed above in 800 mL of H2O. Adjust the pH to 7.4 (or 7.2, if required) with HCl, and then add H2O to 1 L. Dispense the solution into aliquots and sterilize them by autoclaving for 20 min at 15 psi (1.05 kg/cm2) on liquid cycle or by filter sterilization. Store PBS at room temperature.