Abstract
Attenuation in gap junctional coupling has consistently been associated with induction of rapid or synchronous cell division in normal and pathological conditions. In the case of the v-src oncogene, gating of Cx43 gap junction channels has been linked to both direct phosphorylation of tyrosines (Y247 and 265) and phosphorylation of serine target of Erk1/2 (S255, 279, and 282) on the cytoplasmic C-terminal domain of Cx43. However, only the latter has been associated with acute rather than chronic gating of the channels immediately after v-src expression, a process that is mediated through a “ball and chain” type mechanism. In this study we show that while ERK1/2 is necessary for acute closure of gap junction channels, it is not sufficient. Rather, multiple pathways converge to regulate Cx43 coupling in response to expression of v-src, including parallel signaling through PKC and MEK1/2, with additional positive and negative regulatory effects mediated by PI3kinase, distinguished by the involvement of Akt.
Keywords: Cx43, Gap Junctions, v-Src, Erk1/2, signal transduction
Introduction
Mammalian gap junctions, composed of membrane proteins called connexins, represent arrays of transmembrane channels that allow low molecular weight molecules and ions to move directly between the cytoplasm of opposed cells (Goldberg et al. 1998, Kumar and Gilula 1996). Reduced communication through gap junction channels has been frequently correlated with increases in cell proliferation of both normal and transformed cells (Berthoud et al. 1993, Cronier et al. 2009). An early response to the stimulation of many cells by growth factors in culture is a decrease in coupling just prior to initiation of mitosis (Lau et al. 1992). Similarly, several oncogenes have been shown to reduce coupling of cells, most notably pp60 v-src, which Ross Johnson first observed 31 years ago to acutely induce closure of gap junction channels (Atkinson et al., 1981). It has been proposed that this uncoupling could serve to either isolate cells from the inhibitory signals of neighboring cells and/or enable the accumulation of positive stimuli within the cells that generate them (Loewenstein 1990). In the current work, we return to a more detailed analysis of the signals that link v-src expression to the closure of Cx43 gap junction channels, to compare the similarities and differences of the oncogenic process to that induced by normal growth factor signaling.
Several growth factors [EGF (Lau et al, 1992), PDGF (Hossain et al., 1998, 1999b), ILGF (Homma et al., 1998)] have been shown to cause acute closure of Cx43 gap junction channels, primarily associated with activation of ERK, and its direct phosphorylation of Cx43(Kanemitsu and Lau, 1993, Hossain et al., 1998). However, other kinase pathways, such as PKC (Hossain et al., 1998, 1999a, 1999b), have also been implicated. Several phosphorylation sites for these, and other kinases, have been mapped on the C-terminal domain of Cx43 (Berthoud et al. 1993; Kanemitsu et al. 1998; Warn-Cramer et al. 1996: reviewed in Lampe and Lau 2004). Consistent with this, removal of the C-terminal domain was shown to ablate gating by Insulin like growth factor, which could then be rescued by addition of the C-terminal domain as a separate peptide (Homma et al., 1998). This led to the model that this phosphorylation driven gating in response to growth factors occurs by a “ball and chain” mechanism, similar to what had been shown for pH gating of Cx43 (Ek-Vitorin et al. 1996; Morley et al. 1996), and much earlier for inactivation of K+ channels (Hoshi et al., 1990). However, while these studies had focused on acute gating of the channels, there is also evidence that growth factors can more chronically induce internalization of gap junction structures over the longer time. Hence, the interaction of the cytoplasmic domain of Cx43 with various signaling [e.g. src, via SH2 and 3 domains (Warn-Cramer et al. 1996)], adapter [e.g. ZO-1via PDZ 1(Giepmans and Moolenaar 1998; Toyofuku et al. 1998); 14-3-3 protein (Park et al., 2007)], and cytoskeletal elements [e.g. tubulin (Giepmans et al. 2001)] may also be relevant to longer term regulation of coupling by growth factors.
While the regulation of gap junctions by v-src was first studied 10 years before that by growth factors, the precise mechanism of how v-src induces loss of coupling either acutely, or chronically, has remained somewhat controversial. By analogy with closure of Cx43 channels by growth factors described above, Zhou et al., 1999, demonstrated that v-src closure of Cx43 channels depended not on the the direct src targets on Cx43 (Y265 and 247 – Swenson et al., 1990, Solan and Lampe, 2008), but on the presence of three ERK1/2 phosphorylation sites (S255, 279 and 282) mapped in the C-terminal domain by Warn-Cramer et al. (1996). The dependence on ERK activity was also demonstrated pharmacologically in normal rat kidney (NRK) cells expressing a temperature sensitive (ts) pp60v-src. Consistent with this,Ito et al. (2006) implicated the Ras-Raf pathway, which directly activates ERK, in the gating of Cx43 gap junction channels by v-src. Others have also implicated Cas as being essential for src gating, although the mechanism of this effect remains unclear (Shen et al. 2007). Finally, truncations of the C-terminal domain led to a loss of response of Cx43 to v-src, which was restored by co-expression of the C-terminal domain as an independent polypeptide, consistent with the “ball and chain” mechanism implicated in growth factor gating of Cx43.
However, contrary results have been published by Swenson, et al. (1990) and Lin et al. (2001), who showed that Y265 and Y247 are required for v-src induced disruption of coupling in both Xenopus oocytes (Swenson et al. 1990) and in a mouse Cx43 knockout cell line transfected with different Cx43 mutants (Lin et al. 2001). In the latter study, the ERK1/2 phosphorylation sites (S255, 279, and 282) were found not to be required for closure. The differences between these seemingly contradictory findings do not correlate with the expression system, as both results have been reported in oocytes and mammalian cells. However, one consistent distinction is that ERK-dependence was reported when src’s effect was exerted on pre-formed Cx43 channels, and was likely associated with initial gating immediately following v-src expression. This is likely to be similar to the transient closing of Cx43 channels in response to cytokines [PDGF (Hossain et al. 1998) and EGF (Kanemitsu and Lau 1993)]. By contrast, tyrosines were implicated when v-src was expressed prior to, or concurrent with, Cx43, and could represent a more chronic mechanism for closure of Cx43 channels that diverges from known growth factor pathways. Using phosphorylation state specific antibodies, Solan and Lampe (2008) show that, in LA-25 cells, tyrosine phosphorylation predominantly occurred in gap junction plaques when v-src was activated. They also show increased phosphorylation of ERK and PKC sites in Cx43 upon v-Src activation, suggesting the role of multiple signaling pathways in gap junction down-regulation during src transformation. However they were unable to address the issue of whether these phosphorylation sites were functionally required for gap junction closure, nor could they assess the timeline of these phosphorylation events following src expression. In most cell lines this poses a problem, unless src expression can be acutely activated, such as in temperature sensitive mutants. Unfortunately, the better characterized ts v-src constructs have often proven to be unstable.
An alternative model system is the Xenopus oocyte, where src can be acutely activated by injection of its encoding RNA, allowing the time course of the response to be followed. Most mitogenic signaling cascades are present and well characterized in Xenopus oocytes, and they have been used extensively in the electrophysiological characterization of gap junction channels. In this study, we have employed this expression system to conduct a comprehensive analysis of the regulatory pathways that mediate the initial gating of Cx43 channels by v-src to explore the degree to which they may use similar or distinct pathways from those implicated in growth factor mediated Cx43 gating.
Materials and Methods
cDNA constructs
Rat Cx43 cDNA (provided by Dr Eric Beyer, University of Chicago, IL) was subcloned into the PGEM-7Zf(+) vector (Promega Corp.) at the EcoRI site. All mutants were provided by Drs Steve Taffet and Mario Delmar (State University of New York Health Science Center at Syracuse, NY). The cDNA for pp60v-src was provided by Dr Marilyn Resh (Memorial Sloan-Kettering Cancer Center, NY). The cDNA for constitutively active MEK1 (human CA-MEK1), a kind gift from Dr Natalie Ahn (University of Colorado, CO) was subcloned into the oocyte expression vector pBluescript MXT at the EcoRV and XbaI restriction sites. Constitutively active and dominant negative PKC (PKCα/ ε) constructs (murine) were provided by Dr Elissavet Kardami (University of Manitoba, Winnipeg, Canada) in the pSVK3 vector.
Preparation of cRNAs
All cRNAs were linearized and transcribed in vitro using Ampliscribe Transcription kits (Epicenter) according to the manufacturer's recommendations. The resultant cRNAs were quantitated after DNase-1 (Sigma Aldrich) treatment by absorbance at 260 nm.
Xenopus oocyte expression system and measurement of junctional conductance
Oocytes were unilaterally extracted from female Xenopus laevis toads and treated with 1mg/ml collagenase (Sigma Aldrich) to digest most of the follicular layer. Oocytes were preinjected with 40nl of 0.2ug/ul of an oligonucleotide complementary to Xenopus Cx38: 5′-75 GCTTTAGTAATTCCCATCCTGCCATGTTTC 45-3′ prior to injection with Cx43 cRNA (2 ng/oocyte) as described byZhou et al. (1999). After final manual stripping of the vitelline membrane the oocytes were paired for ~16 hours prior to measuring junctional currents (Ij) by dual cell voltage clamp as described in Zhou et al. (1900). All experiments were carried out in oocytes batches from at least 5 different females to account for differences in batch specific physiological behavior.
Measure of kinase effects
About 16 hr after pairing, oocytes were recorded for approximately 20 min to ensure stable conductance levels before secondary injection of cRNAs for the kinase of interest [i.e. v-src (8ng) or CA-PKC (8ng) or CA-MEK (8ng) The effects of secondary injection on gap junctional conductance were assessed after 6h, and expressed as fractional decrements of the conductance recorded from the same oocytes pair before introduction of the kinase cRNA. Thus, effects of kinases were normalized within the same oocyte. In cases where dominant negative constructs were used, the cRNA encoding DN-PKAα (8ng) and DNPKCε (8ng) were injected at the same time as v-src cRNA injection. Expression of each construct was tested by western blot analysis in each batch of oocytes as described ahead.
Pharmacological inhibitors
Bis(indolyl-1)Maleamide (BIM) (Calbiochem, La Jolla, CA) was added to 0.1 µm final concentration, U0126 and LY294002 (Cell Signaling technology, MA) were added to 50 µm final concentration, Wortmannin (Sigma Aldrich, MO) was added to 0.05 µm final concentration and Akt inhibitor VIII (Akti-1/2, Calbiochem, La Jolla, CA) was added to 10 µm final concentration. All inhibitors were added to L-15 Xenopus oocyte incubation media (Sigma Aldrich, MO) just before injection of the cRNA for pp60v-src, CA-MEK1/2 or CA-PKCε. The media with the relevant inhibitor was changed every hour to ensure its full effect over the entire duration of the experiment.
Immunoprecipitation and western blot
Oocyte lysates were prepared from approximately 15–20 oocytes, injected with the same schedule as used in the functional assays. The lysates were prepared in modified RIPA buffer (1% NP-40, 0.1% SDS, 50ml Tris pH7.4, 100mM NaCl, 2mM EDTA, 50mM NaF, 40mM β-glycerophosphate, 1mM Na2VO4, and protease inhibitors). The supernatant was collected from clarified lysates and immunoprecipitated with Anti-Cx43 polyclonal antibody (1:200; Santa Cruz Biotechnology, CA) or Anti-Erk1/2 polyclonal Antibody (1:500; Upstate Biotechnology, NY) overnight at 4°C. For Western blot analysis of oocyte lysates, oocytes were lysed in 200ul of RIPA buffer and clarified at 14K RPM for 30 mins. Lysates were resolved on a 10% SDS-polyacrylamide gel before transfer onto PVDF membrane (Millipore Corp. MA. Since, Xenopus oocytes express only Erk2 (Ferrell 1999), therefore a single band is observed when probed for Erk1/2 or phospho-Erk1/2. For Cx43 detection, anti-Cx43 monoclonal antibody (Chemicon, CA) targeted to the C-terminal tail of Cx43 was used as the primary antibody. To test for expression of kinase constructs, anti-avian Src antibody (clone EC10; 1:1000; Upstate Biotechnology, NY), anti-PKCε (Cell Signaling Technologies, Beverly, MA), anti-PKCε mouse monoclonal antibody (1:1000; Santa Cruz Biotechnology) were used.
Radiolabelled assay for Cx43 phosphorylation
To assay for MEK induced Cx43 phosphorylation, ATP-γ32 (2–10 µCi/oocyte; 250 µCi/µl, Amersham Biosciences) was injected along with CA-MEK1 mRNA into Cx43 coupled oocytes. After 6 hours oocytes were lysed as described inZhou et al. (1999). Briefly for each experiment, approximately six labeled oocytes were homogenized in 200 µl/oocyte of modified RIPA buffer. The homogenate was brought to 2% Triton X-100 after boiling for 5 min and cleared in a microcentrifuge at 13,000 rpm for 5 min. 1 µl primary antibody/oocyte (crude rabbit antisera against Cx43 residue 302–319) was added to the supernatant and pulled down by preswollen protein A-Sepharose CL-4B beads (Sigma Aldrich) The beads were washed three times in the same RIPA buffer used for oocyte lysis, before solubilization of the immunoprecipitated material by boiling for 10 min in 2× SDS sample buffer and subsequent separation by SDS-PAGE on a 10% gel.. The dried gel was analyzed by exposure to a PhosphoImaging cassette (model 425E using ImageQuant v.4.2 software; Molecular Dynamics Inc.) for several hours and then bands were quantitated after reading on a PhosphoImager.
Results
ERK1/2 is necessary in v-src induced acute closure of Cx43 gap junctions
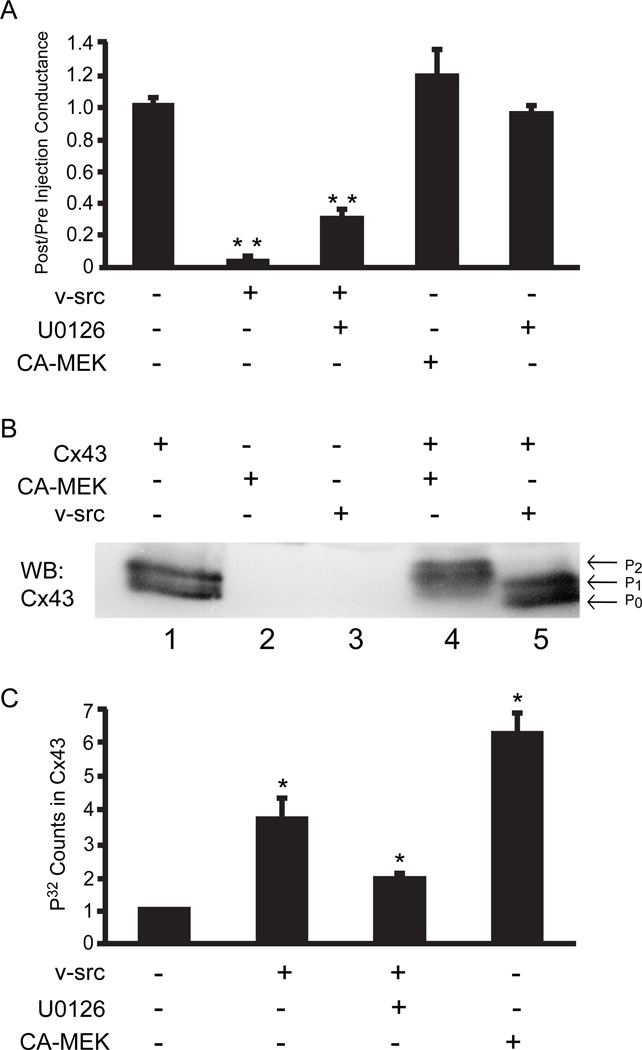
Oocytes have proven to be an effective system for analysis of acute gating of Cx43 channels by src as the v-src protein can be expressed after Cx43 gap junction channels are stably established. This is in contrast to most mammalian cell studies where src is expressed concurrently with Cx43, and can affect many processes including assembly and degradation. We had previously shown a requirement for ERK phosphorylation of Cx43 for acute gating by src in oocytes. In mammalian cells, inhibition of both ERK (Zhou et al. 1999) and Ras (Ito et al. 2006) largely prevents src gating of Cx43, a result we now tested in oocytes. In the presence of the highly specific inhibitor of MEK1/2 activation, U0126 (Davies et al., 2000), we observed only a 2.5 fold drop in conductance of Cx43 coupled oocytes (Fig. 1A). This was slightly greater than the non-specific effect of v-src on Cx43 with the ERK targets deleted, or Cx32 which has no endogenous src or ERK kinase targets, and was dramatically less than the150-fold drop induced by v-src alone. This indicated the ERK was required for src gating of Cx43, consistent with the previous observation that the consensus ERK phosphorylation sites on Cx43 are required for src gating (Zhou et al., 1999).
Fig. 1.
a. ERK1/2 is necessary, but not sufficient, for v-src gating of Cx43: Cx43 paired oocytes were injected with water, v-src or CA-MEK1, and conductance 6 hours post-injection measured as a ratio of the pre-injection conductance. v-src induced almost complete inhibition of coupling, which could be partially prevented by inhibition of MEK1/2 with U0126. However, CA-MEK1 injection alone had no significant effect on Cx43 coupling. U0126 alone had no effect on coupling. The data represent means of six separate experiments each experiment having a minimum of 8 coupled oocyte pairs. Statistical analysis was performed by use of the Student’s t-test comparing each group to the Cx43 expressing oocytes (* *= P<0.001). In this and all subsequent graphs, bars represent mean values with standard errors indicated.
b. CA-MEK induces phosphorylation of Cx43: Cx43 coupled oocytes, subjected to various treatments as indicated, were lysed and then immunoprecipitated with anti-Cx43 polyclonal antibody, followed by analysis via Western blot probed with a Cx43 monoclonal antibody. No bands were evident in the absence of Cx43 injection (lanes 2 and 3). A doublet corresponding to P0 and P1, a phosphorylated form, is found in cells injected with only Cx43 (lane 1). CA-MEK induces retardation in electrophoretic mobility of Cx43, producing a more highly phosphorylated P2 form (lane 4). In contrast, v-src (lane 5) failed to induce a significant shift in mobility.
c. Src phosphorylation of Cx43 occurs partially through ERK, but with low efficiency: Cx43 coupled oocytes were co-injected with ATP- 32 and either v-src or CA-MEK1 cRNA. After 6 hrs of incubation oocytes pairs were lysed and immunoprecipitated with anti- Cx43 antibody and separated by SDS-PAGE. Incorporated 32P was quantitated by phosphoimage analysis. Src induces phosphorylation, which is about 50% inhibited by the MEK inhibitor U0126. CA-MEK induces significantly higher phosphorylation levels than does src. Data shown represent the mean ± S.E. from three separate experiments with equal numbers of oocytes pairs in each set. Data are normalized, and compared statistically to, oocytes expressing only Cx43; statistical significance was determined by Student t-test (*P<0.05).
To test if it was also sufficient, a CA-MEK1, which directly activates Erk1/2, was injected into Cx43 coupled oocytes with no significant effect on gap junction coupling (Fig 1A). This was not due to failure of expression or activity of CA-MEK1 in the oocyte system, as CA-MEK1 induced both a significantly greater mobility shift of Cx43 in SDS polyacrylamide electrophoresis [this has been correlated with serine phosphorylation (Musil et al. 1990; Lampe et al. 2000) (Fig 1B)] and 32P incorporation into Cx43.(Fig. 1C) than was caused by v-src. Nonetheless, about 50% of src-induced phosphorylation of Cx43 could be attributed to ERK, as it could be blocked by U0126. However, the efficiency of the signal is less than that achieved by directly activating ERK through MEK. Thus, while Erk1/2 appears to be necessary for acute closure of gap junctions by v-src, it is not sufficient. We have also shown previously that direct gating of Cx43 channels by v-src is not dependent on the tyrosine targets of v-src on Cx43 (Zhou et al. 1999). Thus, it seems reasonable to deduce that other signaling pathways are likely to be involved in acute closure of Cx43 gap junctions.
PKC is also required for v-src induced closure of Cx43 gap junctions
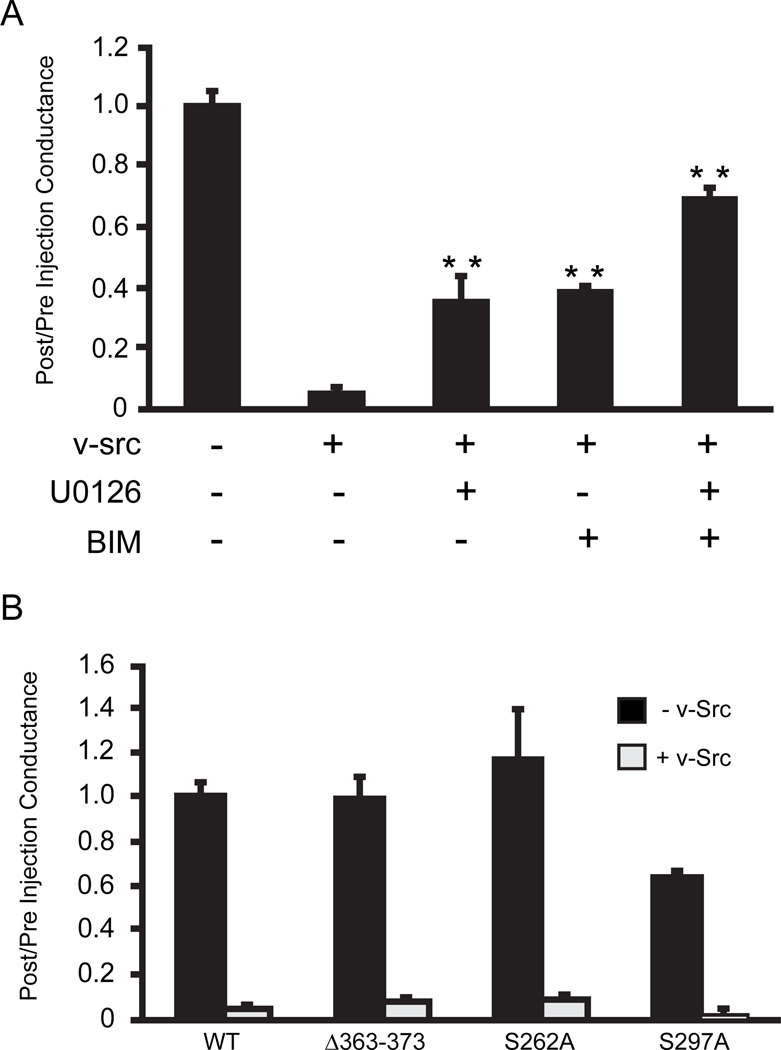
Given previous documentation of direct effects of PKC on Cx43 coupling (Moreno et al. 1994) and its implication in uncoupling Cx43 expressing cells in response to PDGF (Hossain et al. 1998) or v-src (Solan and Lampe 2008), we tested the effects of the broad spectrum PKC inhibitor bis(indolyl1)Maleamide (BIM). This significantly reduced the ability of v-src to close Cx43 gap junctions, although, as in the case of the MEK1/2 inhibitor U0126, this was not complete (Fig 2A). When added together, MEK1/2 (U0126) and PKC inhibitors (BIM) appeared to act in an additive fashion. In fact, the rescue of coupling to 2/3rds of the level before src injection is comparable to that seen in src injections of Cx32 cells (a connexin that lacks src or ERK targets) and has been interpreted as reflecting non-gap junction specific effects of src, possibly on cell adhesion. To assess if the role of PKC in v-src gating required direct phosphorylation of Cx43, the PKC targets on Cx43 were deleted. Cx43Δ363–373 removes PKC phosphorylation sites that have been definitively mapped (S368, S372 (Lampe et al. 2000)), while Cx43S262A and Cx43S297A delete serines within consensus PKCε phosphorylation sites (Doble et al. 2001). Neither the deletion, nor site specific mutants, showed any significant effect on v-src gating of Cx43 (Figure 2B), demonstrating that PKC must affect signaling pathways upstream of the channel itself. S297 appears to be required for optimal function of Cx43, as Cx43S297A expressing oocytes showed consistently lower coupling, despite injection of similar cRNA levels. However, the src gating response was not impacted (Fig. 2B).
Fig. 2.
a. Both MEK1/2 and PKC play a role in v-src induced Cx43 gating: Cx43 coupled oocytes were incubated in medium alone, or in the presence of PKC inhibitor, BIM (0.1 m),or MEK inhibitor, U0126 (50 m), or both. Individually, both U0126 and BIM decreased pp60v-src induced Cx43 closure. Combined treatment with both inhibitors showed an additive effect, indicating that MEK and PKC may be acting through parallel pathways. The data represent means of six separate experiments, each experiment having a minimum of 8 coupled oocyte pairs. Statistical analysis was performed by use of the Student’s t-test comparing each group to the Cx43+src injected oocytes (** = P<0.001).
b. PKC phosphorylation of Cx43 is not required for gating: Ablation of all documented PKC phosphorylation targets (Cx43 363–373), as well as consensus PKC phosphorylation sites on Cx43 (S362A and S297A), failed to significantly affect v-src gating of the channels. The reduced coupling in Cx43S297A cells may reflect the independent role of this site in efficient Cx43 expression. Grey bars represent pre-/post-conductance ratios in the absence of Src, and black bars represent conductance ratios pre- and post- v-src cRNA injection.
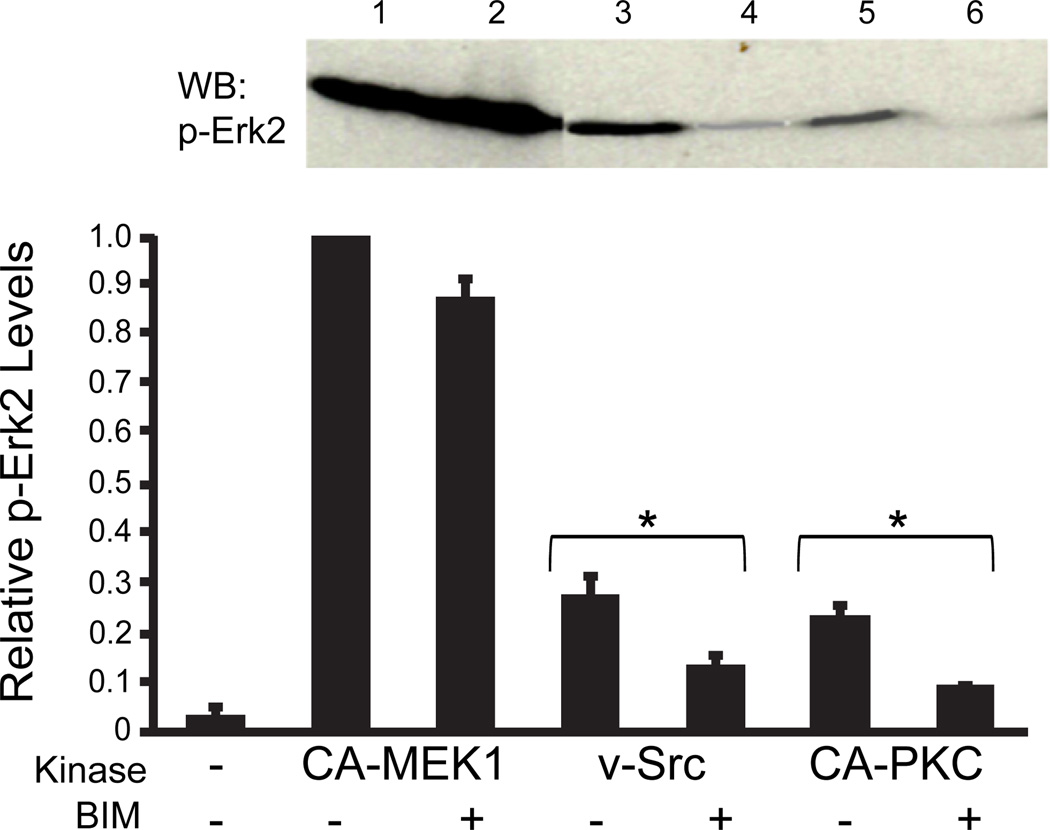
PKC has been demonstrated in other systems to be required for optimal ERK1/2 activity in response to upstream signals (Schonwasser et al. 1998). To test this in the oocyte system, we first showed that direct activation of ERK1/2 by CA-MEK1, which caused a large increase in the phospho form, was unaffected by BIM (Fig 3, lanes 1 and 2). However, ERK2 activation by the upstream effector v-src (Fig. 3, lane 3), is substantially inhibited by BIM (Fig. 3, lane 4). We can also show that ERK1/2 can be directly activated by CA-PKC (Fig 3, lane 5), which, as might be expected was almost completed clocked by BIM (Fig 3, lane 6).
Fig. 3.
ERK1/2 phosphorylation is regulated by PKC: Cx43 coupled oocytes were incubated in L-15 medium, with or without BIM (0.1 m), and subsequently injected with cRNA for pp60v-src, CA-MEK1 or CA-PKC, and immuno-blotted with anti-phospho-ERK1/2 antibody (inset). ERK1/2 activation was evident by all three kinases (lanes 1, 3, and 5), although direct activation by MEK1/2 was by far the most effective. BIM did not affect ERK1/2 activation by CA-MEK1 (lane 2), but significantly inhibited its activation by pp60v-src and CA-PKC (lanes 4 and 6, respectively). Data shown is from three separate experiments, each experiment having equal numbers of coupled oocyte pairs for each set (n=10). Statistical significance of each treatment +/− src or +/− CA-PKC was assessed by the Student t-test , *P<0.05).
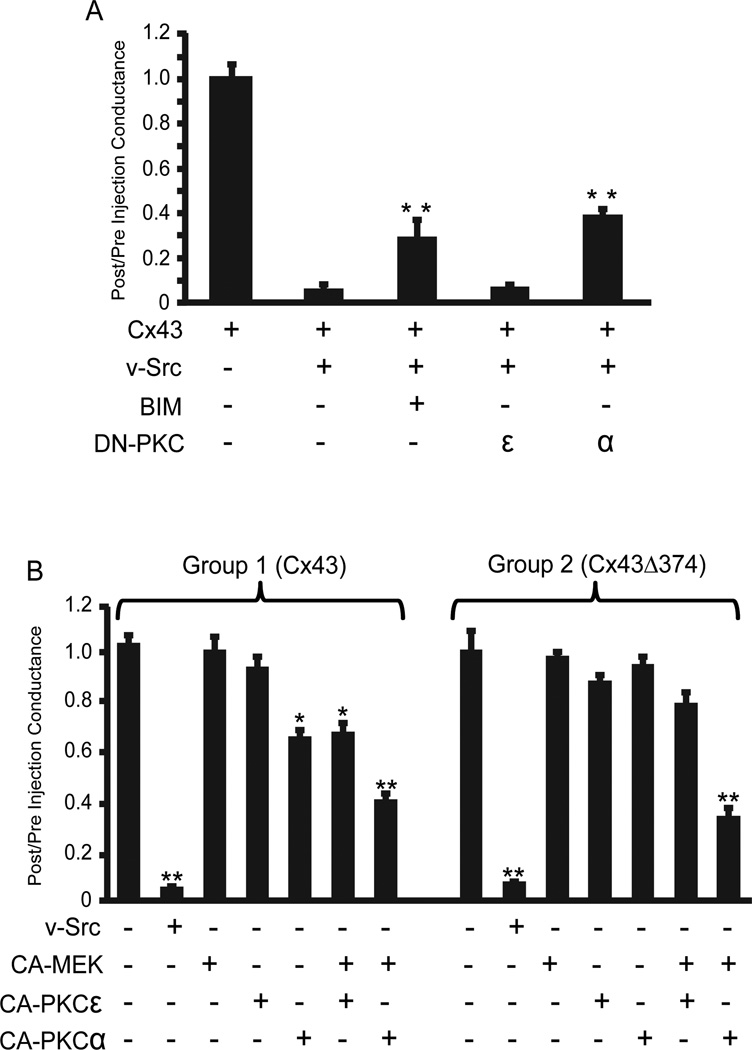
While BIM is an effective inhibitor of many PKC isoforms, it also can have off-target effects against other kinases like MAP kinase activated kinase, S6 kinase, GSK3 and PI dependent protein kinase (Davies et al., 2000). Dominant-negative (DN) PKC constructs represent more specific reagents, but these are isotype specific. The two likely candidates in this case were PKCε and PKCα, since their direct phosphorylation sites on Cx43 have been identified (31, 32) and their activation by v-src is well documented (Zang et al. 1995). When DN constructs specific for each isoform were injected into oocytes, only DN-PKCα was effective, producing a similar level of inhibition of v-src gating of Cx43 as seen with BIM (Fig. 4A). Consistent with the isoform specificity of the dominant negative constructs, only the α-isoform of CA-PKC induced partial closure of Cx43 channels (Fig. 4B, Group 1). In conjunction with CA-MEK1, both isoforms had some effect on partially closing Cx43 channels, but this effect was larger for PKCα. However, the maximum reduction in coupling (~60%) was still far less than that induced by v-src (>99%) (Fig. 4B, Group 1), indicating that additional pathways still need to be considered beyond PKC.
Fig. 4.
a. The PKCα isoform mediates v-src gating of Cx43 in oocytes: The effects of co-injection of dominant negative (DN) isoforms of PKCα and PKCε on v-src induced Cx43 gating were compared to those of the generic PKC inhibitor (BIM). DN-PKCα showed similar effects to BIM, while DN-PKCε had no effect. Data represent means of six separate experiments, each experiment having a minimum of 8 coupled oocyte pairs. Statistical analysis was performed by use of the Student’s t-test, comparing each treatment to the Cx43+src group (** = P<0.001).
b. PKC , in conjunction with MEK, selectively inhibits Cx43 coupling, independent of the C-terminal binding domain for ZO-1. Group 1 – CA-PKC , but not CA-PKC , inhibits Cx43 coupling when injected into oocyte pairs. Uncoupling was enhanced by co-injection of CA-MEK, but this did not approach the degree of uncoupling generated by v-src. Group 2 – Truncation of Cx43 at residue 374, removing the PDZ domain which mediates ZO-1 binding, eliminated the effect of CA-PKC but did not change the combined MEK/PKC effect on coupling. The data represent means of six separate experiments, each experiment having a minimum of 8 coupled oocyte pairs. Statistical analysis was performed by use of the Student’s t-test, comparing each group to oocytes expressing Cx43 only (**P<0.001, *P<0.05).
ZO-1 binding site on Cx43 does not appear to play a role in v-src induced closure of Cx43 gap junctions
Since v-src gating of Cx 43 has been characterized as operating through a “ball and chain” type mechanism, that would presumably require a free C-terminus. Thus, we also investigated the potential role of ZO-1 binding to the C-terminus, as this would “tether” the tail to the cytoskeleton. This interaction, which occurs through a PDZ binding site at the very C-terminus of Cx43 (Giepmans and Moolenaar 1998; Toyofuku et al. 1998), has been shown byToyofuku et al. (2001) andSorgen et al. (2004) to be disrupted by constitutively active c-src, invoking a possible direct role for src in gating other than through tyrosine phosphorylation. However, deletion of the ZO-1 binding site by truncation of the nine C-terminal residues (Cx43Δ374, a gift from Dr M. Delmar) had no effect on the ability of v-src to close the channels, and did not enhance the ability of MEK1, or CA-PKCα (separately or coordinately) to close the channel (Fig. 4B, Group 2). The modest inhibition of wt Cx43 coupling observed with CA-PKCα alone (Fig. 4B, Group 1), was not seen with this C-terminal truncation mutant (Fig. 4B, Group 2), despite the fact that none of the consensus PKCα sites were directly eliminated by this truncation.
PI3K plays a complex regulatory role in v-src closure of Cx43 gap junctions
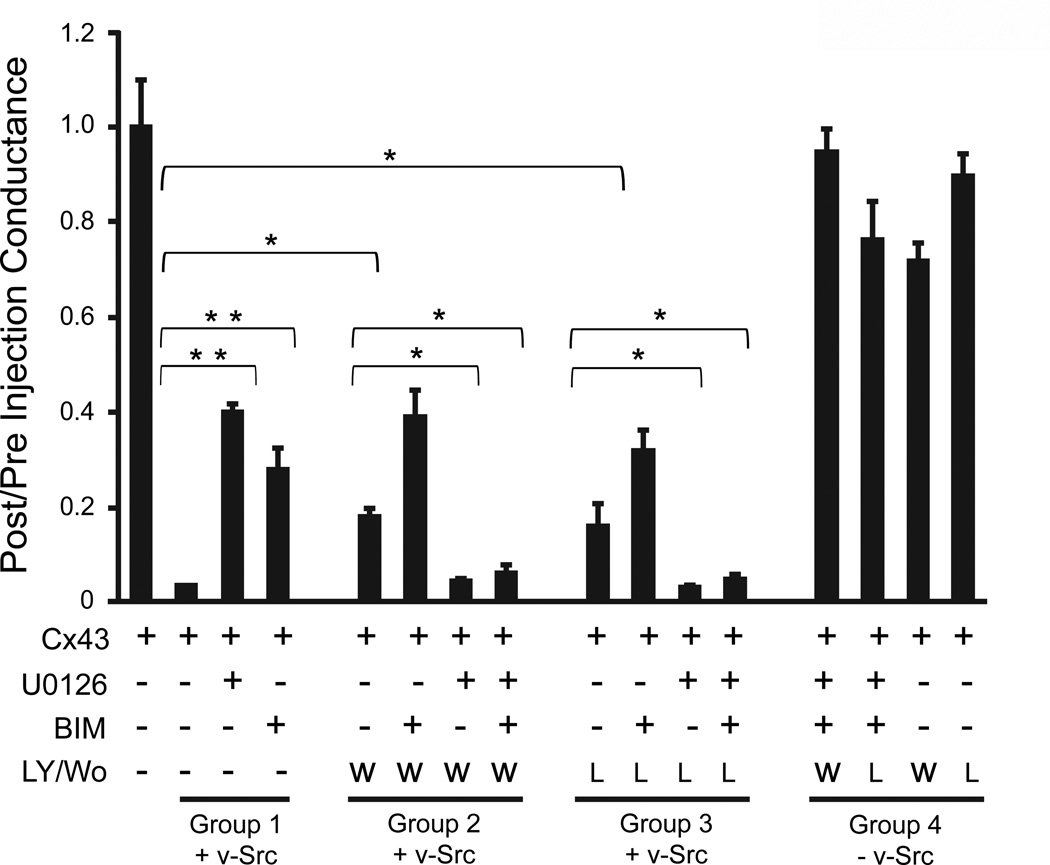
Another potential pathway that has been implicated in src signaling is phosphotidylinositol-3-kinase (PI3K). To probe the role of this pathway in src gating of Cx43, we used two different PI3K inhibitors, reversible LY294002 and irreversible Wortmannin. The former has a lower affinity, and shows some cross-reactivity with casein kinase II and GSK3β, while the latter has a higher affinity and different cross-reactivity with smooth muscle myosin light chain kinase (Davies et al., 2000), likely not relevant in the current study. Despite the different properties of these two inhibitors, both caused similar inhibition (P<0.05) of v-src gating (Fig. 5, Groups 2 and 3, respectively), albeit less efficiently than we had observed with either MEK1/2 (U0126) or PKC inhibitors (BIM) (Figure 5, Group 1, P<0.001). No significant increase in inhibition was seen when either Wortmannin or LY294002 were used in combination with BIM, compared to BIM alone. However, the application of U0126 in concert with Wortmannin or LY294002 caused an unexpected restoration of v-src gating of Cx43, reversing the inhibition seen with either inhibitor alone. The application of all three inhibitors caused a similar “annulment” of their individual effects, and the synergistic effects of PKC and ERK pathways, and restored full closure of Cx43 channels by v-src. None of the inhibitors, alone or in combination, affected Cx43 coupling in the absence of v-src (Fig. 5, Group 4), indicating no deleterious effects on oocytes from the combination of inhibitors used.
Fig. 5.
PI3K plays a complex role in v-src induced gating: Inhibition of PI3K by Wortmannin or LY294002 partially blocked the ability of v-src to close Cx43 channels (compare first bars in Groups 2 or 3, respectively, with second bar in Group 1). When added together with BIM, both Wortmannin and LY294002 slightly enhanced the inhibition of v-src gating (second bars, Groups 2 and 3), although this was only significant in the case of Wortmannin. However, when Wortmannin or LY294002 were added in conjunction with U0126, with or without BIM (third and fourth bars, Groups 2 & 3), the effects of both inhibitors appeared to be annulled, restoring full closure of Cx43 by v-src. None of the inhibitors, alone or in combination, had a significant effect on Cx43 coupling in the absence of v-src (Group 4). Data shown represent the mean ± S.E. from three separate experiments with equal number of oocytes pairs in each set. Inverted brackets indicate specific comparisons subjected to Student’s t-test statistical analysis yielding differences at the **P<0.001 or the *P<0.05 level.
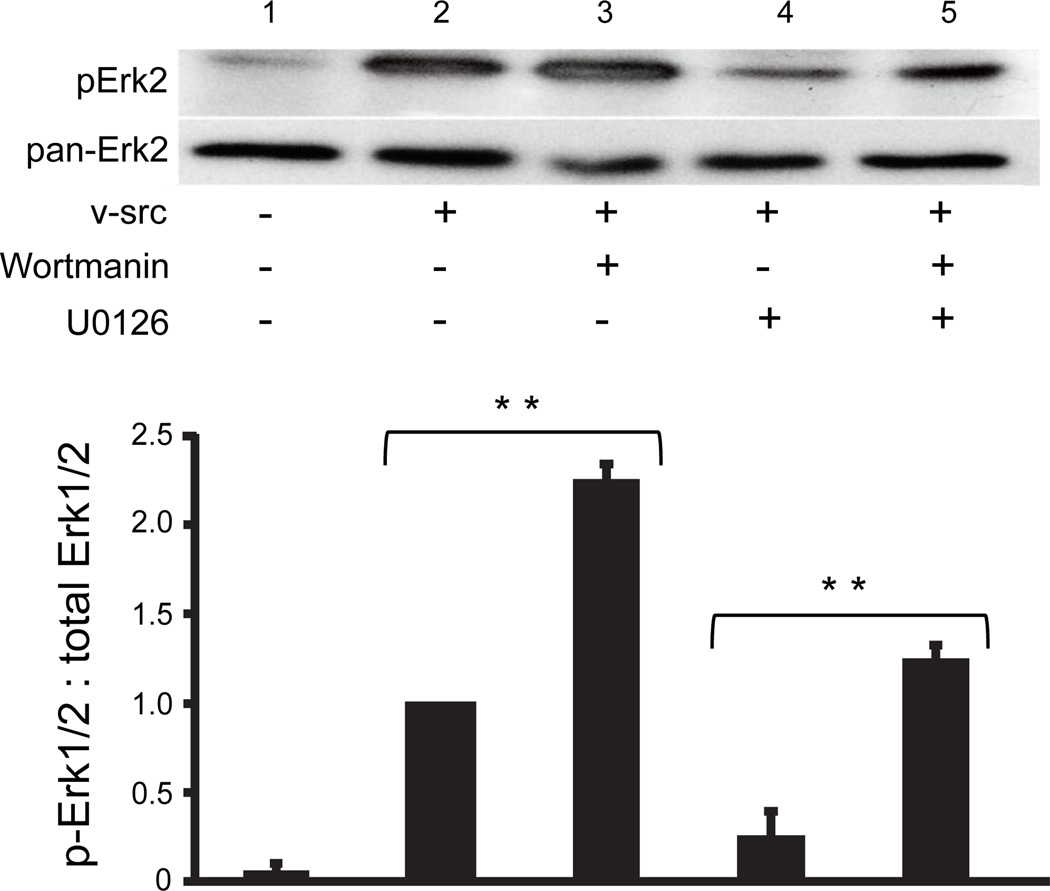
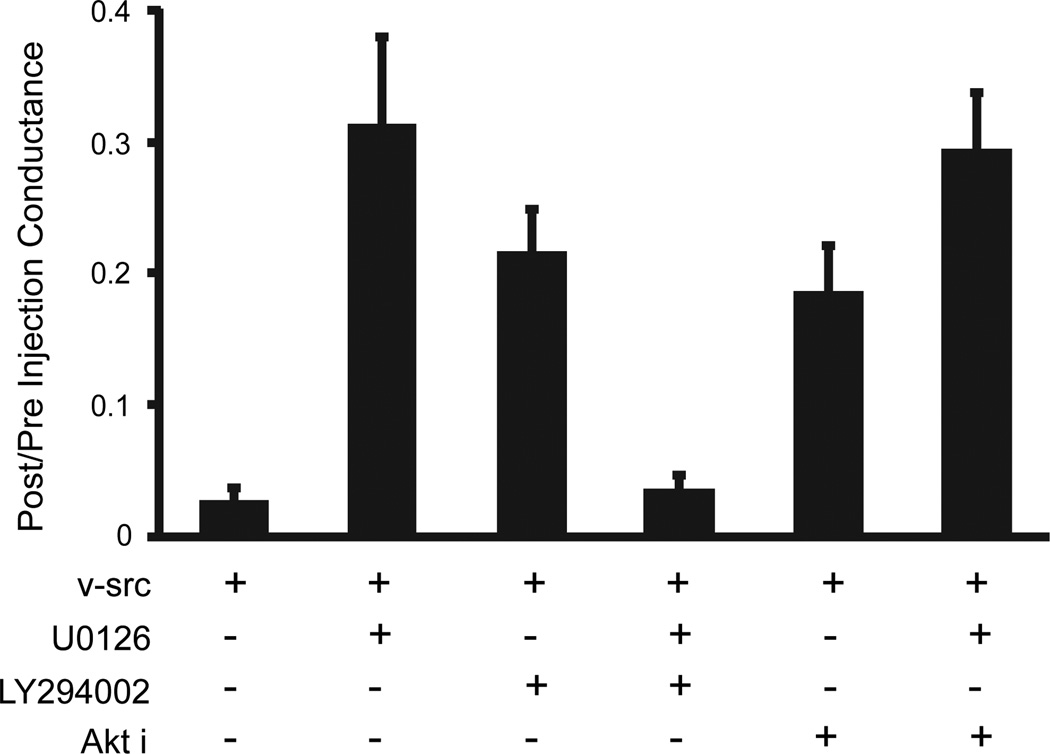
Further insights into apparently antagonistic effects of Wortmannin and U0126, were obtained by directly examining levels of activated ERK under the different treatments. Not only did Wortmannin treatment enhance the activation of ERK1/2 following v-src treatment (Fig. 6, lanes 2 and 3; P<0.001), it also reversed the reduction of src induced ERK phosphorylation caused by U0126 (Fig. 6, lanes 4 and 5; P<0.05). Thus, PI3K may play dual roles in the src gating of Cx43, facilitating the process, possibly through PKC, while also exerting inhibitory influence through effects on ERK activation. In an effort to distinguish which aspects of the diverse PI3K pathways might mediate these apparently opposing effects on v-src gating, we tested the role of the major PI3K effector, Akt. Akti, a highly specific inhibitor of both Akt isoforms 1 and 2 (Barrett et al, 2005), caused a similar level of inhibition of v- src gating as had the PI3Kinase inhibitor LY294002 (Fig. 7). However, unlike the PI3 kinase inhibitors, application of Akti in conjunction with the ERK inhibitor, U0126, caused no restoration of v-src gating (Fig 7). Thus, it appears that PI3K’s role in contributing to v-src induced closure of Cx43 channels is mediated by Akt, while its positive effects on Cx43 coupling through antagonism of the ERK pathway do not involve Akt.
Fig. 6.
PI3K activity inhibits phosphorylation of ERK 1/2: Immunoblot, using anti-phospho ERK1/2 (upper panel) and pan-specific ERK1/2 (lower panel) antibodies, of lysates from Cx43 coupled, v-src injected cells (lanes 2–5) treated with either Wortmannin (lane 3), U0126 (lane 4), or both (lane 5). In either the presence or absence of the MEK1/2 inhibitor, Wortmannin induced an increase in ERK phosphorylation. There was no significant change in the expression of ERK 1/2 in response to the inhibitors. The density of the bands was measured from several experiments, and expressed as a ratio of Phospho- to total ERK1/2 levels and plotted in a histogram below the gel. Inverted brackets indicate pair-wise statistical comparisons of results from three independent experiments yielding differences significant at the **P<0.001 (single asterisk) or the *P<0.05 level; analysis was performed by use of Student’s t-test.
Fig. 7.
The positive, but not the negative regulatory effects of PI3K on src gating are mediated through Akt. As shown above, inhibition of ERK by U0126 or PI3K by LY294002 each partially prevented v-src gating of Cx43. Inhibition of Akt by Akti had the same effect as PI3K inhibition,, indicating that PI3K induced closure of Cx43 is mediated through Akt. By contrast, the “rescue” of src gating by inhibition of both ERK and PI3K was not reproduced by a combination of ERK and Akt inhibition, indicating that the negative influence of PI3K on Cx43 gating by src if mediated through a pathway other than Akt.
Discussion
The mechanisms for inhibition of cell coupling during transformation remain unclear. Chronic uncoupling in response to v-src has been consistently linked to direct phosphorylation of tyrosines in the C-terminal domain of Cx43, mapped to Y265, and a secondary site at Y247. Even after long-term expression of src, the phosphorylation of tyrosine in Cx43 remains in plaques (Solan and Lampe 2008), but it is not clear if the channels are gated or if other processes related to assembly are affected. Serine phosphorylation has also been associated with src expression, but appears not to be required for long-term uncoupling (Lin et al., 2001). In contrast, the initial acute gating of Cx43 gap junction channels by src immediately after its expression has been linked to a “ball and chain” mechanism, triggered, not by tyrosine phosphorylation, but by ERK1/2 phosphorylation of serines in the C-terminal domain of Cx43 (Zhou et al., 1999). These specific sites were the same as those mapped by Warn-Cramer et al. (1996) in cellular responses to growth factors (i.e. serine S255, 279, 282), which suggests potential commonality in these gating mechanisms.
ERK activation alone cannot account for v-src gating
In order to clearly dissect the temporal nature of src regulation of Cx43 and focus on the acute phase, which may be analogous to growth factor induced gating of Cx43, we have employed the Xenopus oocyte expression system. This allows for temporally controlled expression of the v-src kinase in cells that are already stably coupled by Cx43. Both mutation of ERK phosphorylation sites (Zhou et al.,1999), and inhibition of ERK activity (Zhou et al. 1999 and Fig. 1A) prevented v-src gating of Cx43, demonstrating the necessity of ERK for initial gating of Cx43 channels by v-src. However, we show here this it is not sufficient, as activation of ERK by CA-MEK-1 failed to close the Cx43 channels (Fig 1C). This led us to investigate other pathways that could contribute to src gating. Our strategy was to combine the use of various pharmacological blockers of different kinase pathways, which have been implicated in various studies of src action, with more specific knock-outs of their target phosphorylation sites (in the case of ERK), or dominant negative and constitutively active constructs (in the case of PKC). When these were not available, we used multiple inhibitors with different modes of action and specificities (in the case of PI3K). Using this double confirmation approach, we minimized the probability of off-target effects of the pharmacological agents alone (Davies et al., 2000; Anastassiadis et. al., 2011).
Possible roles for PKC in v-src gating
Based on similar inhibitory effects of both BIM and an α-isotype-specific DN-PKC (Fig 4A) on v-src gating of Cx43, we concluded that closure of Cx43 channels by v-src also utilizes the PKC pathway. However, since deletion of all known PKC sites on Cx43 failed to affect gating (Fig 2B), PKC must exert its effects on upstream events. This may be partially through interactions between PKC and ERK, as we show that ERK phosphorylation is enhanced by CA-PKC, and reduced by BIM (Fig 3). However, all of PKC’s effects cannot be mediated through ERK, as CA-MEK1 induced significantly higher levels of ERK phosphorylation than either PKC isotype (Fig 3), but with no effect on gating (Fig 1A). In addition, both α- and ε-isotypes of PKC induce similar enhancement of ERK phosphorylation (data not shown), yet only the former affects gating (Fig 4). The additive effect of U0126 and BIM (MEK and PKC inhibitors, respectively) on preventing v-src block of Cx43 (Fig 2A), and the synergistic action of CA-MEK and CA-PKAα (Fig. 4B) suggests that MEK1/2 and PKC may affect closure of Cx43 channels through independent pathways. The specific PKC pathway remains to be elucidated, but appears not to be mediated through direct phosphorylation of Cx43 on any of the identified consensus sites.
Complexity of PI3K modulation of v-src gating
PI3K, which is also known to be modulated by src activity (Penuel 1999), is also involved in Cx43 gating, as inhibition of PI3K by two independent compounds attenuated v-src block of Cx43 (Fig. 5). This effect of PI3K appears to be mediated by Akt (Fig. 7). This is consistent with previous observations that have associated the PI3K – Akt pathway in both v-src and TNFα closure of Cx43 channels (Ito et al., 2010). In this previous case the effect was linked to Akt1, consistent with the effectiveness of Akti in preventing src gating, as this inhibitor targets Akts 1 and 2 [Barrett et al, 2005]. Also, since inhibition of src gating by PKC was not further enhanced by inhibition of PI3K, these kinases may inhibit Cx43 through a common pathway. However, we also find that PI3K not only plays a role in promoting closure of the Cx43 channels by v-src, but also serves to antagonize this gating. This antagonistic effect appears not to involve Akt action, but is mediated through ERK, as inhibition of PI3K caused an increase in ERK phosphorylation levels (Fig. 6) that may be sufficient to overcome the effects of MEK inhibition on src gating (Fig. 5).
An integrated model of v-src gatiing of Cx43 – a delicate balance
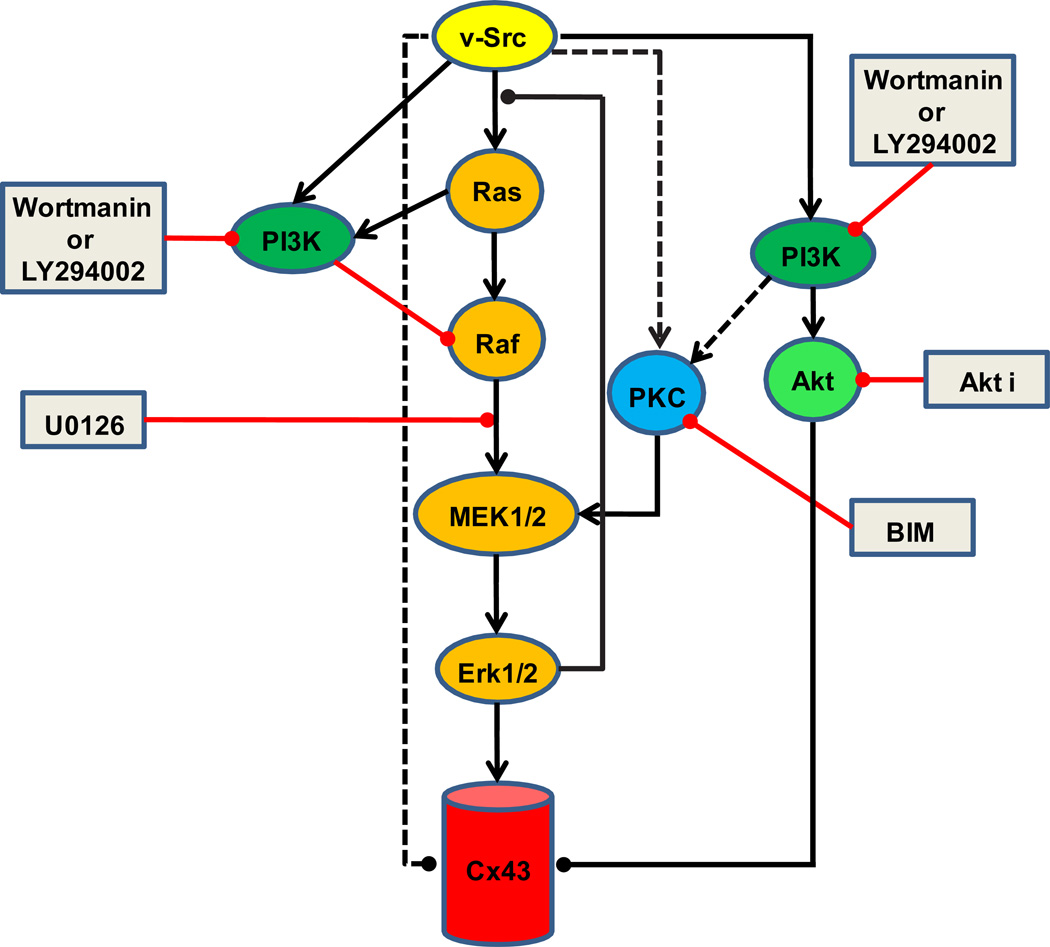
The same three pathways, Ras-Raf-ERK, PKC, and PI3K, which we have identified as mediating initial v-src gating of Cx43, have frequently been implicated in many aspects of growth factor signaling, with growing evidence of cross talk between them at several levels. This is specifically true for the effects of growth factors on Cx43 coupling (Warn-Cramer et al. 1996; Hossain et al. 1999a; Hossain et al. 1999b). Consistent with these known pathways, and the results presented here, one possible model for how v-src may regulate Cx43 coupling is presented in Fig 8. Based on the demonstrated Ras-independent means of activation of ERK1/2 by PKC (Schonwasser et al. 1998; Kolch 2000), PI3K could activate MEK via PKC. This could explain why PI3K inhibitors alone partially blocked closure of Cx43 channels by v-src, and why these inhibitors could not further enhance the effects of inhibition of PKC. Akt1 has also been implicated in the PI3K component of the src gating response of Cx43 (Ito et al. 2010), consistent with our findings that Akti can partially prevent acute src closure of Cx43 channels. The mechanism of Akt action on Cx43 has yet to be resolved, although Akt has been shown to phosphorylate Cx43 at both S369 and 373, leading to association of 14-3-3 with the Cx43 C-terminal domain (Park et al. 2007). This might be expected to affect aspects of Cx43 trafficking, but in the oocyte studies presented here, the gating effects are acute, and could suggest a more direct role of this interaction, or other effects of Akt, on the open state of Cx43 channels.
Fig. 8.
Model for signaling pathways involved in v-src induced initial closure of Cx43: The hypothetical model shown is based on the data presented here, and established (solid lines) or proposed connections (dashed lines) from other studies (see text). We have only noted the simplest model consistent with the data and literature, and more complex connections are possible. Activating effects are indicated by arrowheads (►), and inhibitory effects by lines with solid circles (●). The sites of action of the inhibitors (shown in grey rectangles) used in the current study are also indicated. The central player appears to be ERK1/2 (orange pathway). The PKC pathway (blue) can positively influence the activation of ERK1/2, but may also regulate Cx43 in a manner dependent on its very C-terminal ZO1 binding domain. PI3K (green) can contribute to src gating through PKC (and ERK?), or through Akt (light green), possibly by direct effects on Cx43 that have been reported. PI3K can also antagonize src gating of Cx43, in a yet to be defined pathway that inhibits ERK activation. Together, these pathways may serve to regulate the levels of ERK1/2 activation, perhaps keeping it within a defined range so as not to activate the negative feedback loop of ERK to the activator of Ras. However, since full activation of ERK by CA-MEK fails to induce channel closure, pathways independent of ERK, are likely to present parallel mechanisms for channel closure (dashed lines).
The mechanism by which the PI3K pathway also exerts antagonistic effects on src gating are less clear, although it appears this may be mediated through suppression of ERK activation based on the hyperphosphorylation of ERK that is observed in response to Wortmannin. PI3K has been shown to inhibit the Ras-Raf-MEK1/2-ERK pathway via an antagonistic influence of Akt (Moelling et al. 2002), but this appears not to be the pathway in this study, as Akti had no effect on re-establishing src gating in the presence of MEK inhibitors. These schizophrenic effects of PI3K, as both an activator and inhibitor of the MEK1/2 pathway, are likely to be a critical aspect of the fine tuning of ERK activity that appears to control Cx43 function in response to different mitogenic signals through convergence of several major signaling networks. This balance may be required to avoid activation of the negative feedback loop that has been shown to operate through the serine phosphorylation of SOS by ERK (Langlois et al. 1995). However, artificial elevation of ERK activity through CA-MEK expression, which produces maximal Cx43 phosphorylation, still does not result in gating. Hence, ERK independent pathways must also be required for closure of Cx43 gap junction channels. The nature of these pathways remains to be elucidated (indicated by dotted arrows in Fig 8), although we have eliminated some possibilities, such as direct PKC phosphorylation of Cx43 (Fig. 2A) and binding events, like ZO-1, at the very C-terminal tail of Cx43 (Fig.4B).
Comparison of acute v-src vs growth factor induced closure of Cx43 channels
The data presented here suggest that the initial action of v-src of Cx43 gap junction channels is similar to that of growth factors, with multiple control pathways regulating the process. This is typical of the checks and balances seen in other aspects of the mitogenic response. This initial uncoupling may serve to isolate cells from potential mitogenic inhibitory signals from neighbors, or to allow accumulation of pro-mitogenic activators within the cell. The effects of v-src diverge from that of growth factors in that the suppression of coupling becomes chronic. This effect, which has been linked to tyrosine phosphorylation of Cx43 in several systems, is likely mediated by a mechanism distinct from that of the initial gating response to v-src studied here. Our analyses, including this distinction between acute and chronic gating, provides a basis for reconciliation of diverse reports in the literature. Chronic gating appears to be connected exclusively with tyrosine phosphorylation, while acute gating which has been associated with Ras (Ito et al. 2006) and Cas (Shen et al. 2007) activity, appears to work through synergistic operation of ERK, PKC, and PI3K pathways.
Acknowledgments
We are grateful to Mario Delmar and Steve Taffett for providing various Cx43 mutants, Elissavett Kardami for the PKC constructs, Marilyn Resh for provision of v-src construct and Natalie Ahn for providing the CA-MEK1 construct. We thank Mary Merritt and Eileen Kasperek for technical assistance, Sandra A. Mathis for help in compiling the manuscript, and Edward A. Kalmykov for assistance in preparing the figures for submission. This work was supported by The National Institutes of Health, grant CA048049 to B.J. Nicholson.
References
- Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nature Biotech. 2011;29:1039–1045. doi: 10.1038/nbt.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson MM, Menko SS, Johnson RG, Sheppard JR, Sheridan JD. Rapid and reversible reduction of junctional permeability in cells infected with a temperature-sensitive mutant of avian sarcoma virus. J Cell Biol. 1981;91:573–578. doi: 10.1083/jcb.91.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SF, Defeo-Jones D, Fu S, Hancock PJ, Haskell KM, Jones RE, Kahana JA, Kral AM, Leander K, Lee LL, Malinowski J, McAvoy EM, Nahas DD, Robinson RG, Huber HE. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzymespecific Akt inhibitors. Biochem J. 2005;385:399–408. doi: 10.1042/BJ20041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud VM, Rook MB, Traub O, Hertzberg EL, Saez JC. On the mechanisms of cell uncoupling induced by a tumor promoter phorbol ester in clone 9 cells, a rat liver epithelial cell line. Eur J Cell Biol. 1993;62:384–396. [PubMed] [Google Scholar]
- Cronier L, Crespin S, Strale PO, Defamie N, Mesnil M. Gap junctions and cancer: new functions for an old story. Antioxid Redox Signal. 2009;11:323–338. doi: 10.1089/ars.2008.2153. [DOI] [PubMed] [Google Scholar]
- Crow DS, Kurata WE, Lau AF. Phosphorylation of connexin43 in cells containing mutant src oncogenes. Oncogene. 1992;7:999–1003. [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble BW, Ping P, Fandrich RR, Cattini PA, Kardami E. Protein kinase C-epsilon mediates phorbol ester-induced phosphorylation of connexin-43. Cell Commun Adhes. 2001;8:253–256. doi: 10.3109/15419060109080733. [DOI] [PubMed] [Google Scholar]
- Ek-Vitorin JF, Calero G, Morley GE, Coombs W, Taffet SM, Delmar M. PH regulation of connexin43: molecular analysis of the gating particle. Biophys J. 1996;71:1273–1284. doi: 10.1016/S0006-3495(96)79328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell JE. Jr Xenopus oocyte maturation: new lessons from a good egg. Bioessays. 1999;21:833–842. doi: 10.1002/(SICI)1521-1878(199910)21:10<833::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Giepmans BN, Moolenaar WH. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr Biol. 1998;8:931–934. doi: 10.1016/s0960-9822(07)00375-2. [DOI] [PubMed] [Google Scholar]
- Giepmans BN, Verlaan I, Hengeveld T, Janssen H, Calafat J, Falk MM, Moolenaar WH. Gap junction protein connexin-43 interacts directly with microtubules. Curr Biol. 2001;11:1364–1368. doi: 10.1016/s0960-9822(01)00424-9. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Lampe PD, Sheedy D, Stewart CC, Nicholson BJ, Naus CC. Direct isolation and analysis of endogenous transjunctional ADP from Cx43 transfected C6 glioma cells. Exp Cell Res. 1998;239:82–92. doi: 10.1006/excr.1997.3872. [DOI] [PubMed] [Google Scholar]
- Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. Erratum in: Q Rev Biophys 2002 Feb;35(1):109. [DOI] [PubMed] [Google Scholar]
- Homma N, Alvarado JL, Coombs W, Stergiopoulos K, Taffet SM, Lau AF, Delmar M. A particle-receptor model for the insulin-induced closure of connexin43 channels. Circ Res. 1998;83:27–32. doi: 10.1161/01.res.83.1.27. [DOI] [PubMed] [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RW. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990;250:533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Hossain MZ, Ao P, Boynton AL. Platelet-derived growth factor-induced disruption of gap junctional communication and phosphorylation of connexin43 involves protein kinase C and mitogen-activated protein kinase. J Cell Physiol. 1998;176:332–341. doi: 10.1002/(SICI)1097-4652(199808)176:2<332::AID-JCP11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Hossain MZ, Jagdale AB, Ao P, Boynton AL. Mitogen-activated protein kinase and phosphorylation of connexin43 are not sufficient for the disruption of gap junctional communication by platelet-derived growth factor and tetradecanoylphorbol acetate. J Cell Physiol. 1999a;179:87–96. doi: 10.1002/(SICI)1097-4652(199904)179:1<87::AID-JCP11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Hossain MZ, Jagdale AB, Ao P, Kazlauskas A, Boynton AL. Disruption of gap junctional communication by the platelet-derived growth factor is mediated via multiple signaling pathways. J Biol Chem. 1999b;274:10489–10496. doi: 10.1074/jbc.274.15.10489. [DOI] [PubMed] [Google Scholar]
- Ito S, Ito Y, Senga T, Hattori S, Matsuo S, Hamaguchi M. v-Src requires Ras signaling for the suppression of gap junctional intercellular communication. Oncogene. 2006;25:2420–2424. doi: 10.1038/sj.onc.1209263. [DOI] [PubMed] [Google Scholar]
- Ito S, Hyodo T, Hasegawa H, Yuan H, Hamaguchi M, Senga T. PI3K/Akt signaling is involved in the disruption of gap junctional communication caused by v-Src and TNF-α. Biochem Biophys Res Commun. 2010;400:230–235. doi: 10.1016/j.bbrc.2010.08.045. [DOI] [PubMed] [Google Scholar]
- Kanemitsu MY, Lau AF. Epidermal growth factor stimulates the disruption of gap junctional communication and connexin43 phosphorylation independent of 12-0-tetradecanoylphorbol 13-acetate-sensitive protein kinase C: the possible involvement of mitogenactivated protein kinase. Mol Biol Cell. 1993;4:837–848. doi: 10.1091/mbc.4.8.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemitsu MY, Jiang W, Eckhart W. Cdc2-mediated phosphorylation of the gap junction protein, connexin43, during mitosis. Cell Growth Differ. 1998;9:13–21. [PubMed] [Google Scholar]
- Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J 351 Pt. 2000;2:289–305. [PMC free article] [PubMed] [Google Scholar]
- Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol. 2000;149:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois WJ, Sasaoka T, Saltiel AR, Olefsky JM. Negative feedback regulation and desensitization of insulin- and epidermal growth factor-stimulated p21ras activation. J Biol Chem. 1995;270:25320–25323. doi: 10.1074/jbc.270.43.25320. [DOI] [PubMed] [Google Scholar]
- Lau AF, Kanemitsu MY, Kurata WE, Danesh S, Boynton AL. Epidermal growth factor disrupts gap-junctional communication and induces phosphorylation of connexin43 on serine. Mol Biol Cell. 1992;3:865–874. doi: 10.1091/mbc.3.8.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Warn-Cramer BJ, Kurata WE, Lau AF. v-Src phosphorylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication. J Cell Biol. 2001;154:815–827. doi: 10.1083/jcb.200102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein WR. Cell-to-cell communication and the control of growth. Am Rev Respir Dis. 1990;142:S48–S53. doi: 10.1164/ajrccm/142.6_Pt_2.S48. [DOI] [PubMed] [Google Scholar]
- Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M. Regulation of Raf-Akt Cross-talk. J Biol Chem. 2002;277:31099–106. doi: 10.1074/jbc.M111974200. [DOI] [PubMed] [Google Scholar]
- Moreno AP, Saez JC, Fishman GI, Spray DC. Human connexin43 gap junction channels. Regulation of unitary conductances by phosphorylation. Circ Res. 1994;74:1050–1057. doi: 10.1161/01.res.74.6.1050. [DOI] [PubMed] [Google Scholar]
- Morley GE, Taffet SM, Delmar M. Intramolecular interactions mediate pH regulation of connexin43 channels. Biophys J. 1996;70:1294–1302. doi: 10.1016/S0006-3495(96)79686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil LS, Cunningham BA, Edelman GM, Goodenough DA. Differential phosphorylation of the gap junction protein connexin43 in junctional communication-competent and -deficient cell lines. J Cell Biol. 1990;111:2077–2088. doi: 10.1083/jcb.111.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DJ, Wallick CJ, Martyn KD, Lau AF, Jin C, Warn-Cramer BJ. Akt phosphorylates Connexin43 on Ser373, a “mode-1” binding site for 14-3-3. 2007 doi: 10.1080/15419060701755958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penuel E, Martin GS. Transformation by v-Src: Ras-MAPK and PI3K-mTOR mediate parallel pathways. Mol Biol Cell. 1999;10:1693–1703. doi: 10.1091/mbc.10.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonwasser DC, Marais RM, Marshall CJ, Parker PJ. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Khusial PR, Li X, Ichikawa H, Moreno AP, Goldberg GS. SRC utilizes Cas to block gap junctional communication mediated by connexin43. J Biol Chem. 2007;282:18914–18921. doi: 10.1074/jbc.M608980200. [DOI] [PubMed] [Google Scholar]
- Solan JL, Lampe PD. Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim Biophys Acta. 2005;1711:154–163. doi: 10.1016/j.bbamem.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Solan JL, Lampe PD. Connexin 43 in LA-25 cells with active v-src is phosphorylated on Y247, Y265, S262, S279/282, and S368 via multiple signaling pathways. Cell Commun Adhes. 2008;15:75–84. doi: 10.1080/15419060802014016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgen PL, Duffy HS, Sahoo P, Coombs W, Delmar M, Spray DC. Structural changes in the carboxyl terminus of the gap junction protein connexin43 indicates signaling between binding domains for c-Src and zonula occludens-1. J Biol Chem. 2004;279:54695–54701. doi: 10.1074/jbc.M409552200. [DOI] [PubMed] [Google Scholar]
- Swenson KI, Piwnica-Worms H, McNamee H, Paul DL. Tyrosine phosphorylation of the gap junction protein connexin43 is required for the pp60v-src-induced inhibition of communication. Cell Regul. 1990;1:989–1002. doi: 10.1091/mbc.1.13.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem. 1998;273:12725–12731. doi: 10.1074/jbc.273.21.12725. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Akamatsu Y, Zhang H, Kuzuya T, Tada M, Hori M. c-Src regulates the interaction between connexin-43 and ZO-1 in cardiac myocytes. J Biol Chem. 2001;276:1780–1788. doi: 10.1074/jbc.M005826200. [DOI] [PubMed] [Google Scholar]
- Warn-Cramer BJ, Lampe PD, Kurata WE, Kanemitsu MY, Loo LW, Eckhart W, et al. Characterization of the mitogen-activated protein kinase phosphorylation sites on the connexin-43 gap junction protein. J Biol Chem. 1996;271:3779–3786. doi: 10.1074/jbc.271.7.3779. [DOI] [PubMed] [Google Scholar]
- Yao J, Morioka T, Oite T. PDGF regulates gap junction communication and connexin43 phosphorylation by PI 3-kinase in mesangial cells. Kidney Int. 2000;57:1915–1926. doi: 10.1046/j.1523-1755.2000.00041.x. [DOI] [PubMed] [Google Scholar]
- Zang Q, Frankel P, Foster DA. Selective activation of protein kinase C isoforms by v-Src. Cell Growth Differ. 1995;6:1367–1373. [PubMed] [Google Scholar]
- Zhou L, Kasperek EM, Nicholson BJ. Dissection of the molecular basis of pp60(v-src) induced gating of connexin 43 gap junction channels. J Cell Biol. 1999;144:1033–1045. doi: 10.1083/jcb.144.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]