Abstract
Optimal management of complex autoimmune diseases requires a multidisciplinary medical team including dentists to care for lesions of the oral cavity. In this review, we discuss the presentation, prevalence, diagnosis and treatment of oral manifestations in chronic Graft-versus-Host Disease (cGVHD) which is a major late complication in patients treated by allogeneic hematopoietic stem cell transplantation. We assess current general knowledge of systemic and oral cGVHD, and present general treatment recommendations based on literature review and our clinical experience. Additionally, we review areas where the understanding of oral cGVHD could be improved by further research, and address tools with which to accomplish the long-term goal of providing better health and quality-of-life to patients with cGVHD.
Introduction
An estimated 25,000 hematopoietic stem cell transplant (HSCT, see Box 1 for definition of abbreviations) procedures occur each year, and this number is on the rise (Gyurkocza et al., 2010). HSCT is performed primarily for the treatment of hematological malignancies, but has also been used to treat autoimmune or other non-malignant diseases such as immunodeficiency or hemoglobinopathies (Li & Sykes, 2012). The hematologic stem cells come from a related or unrelated volunteer donor matched to the recipient (allogeneic transplant). As many as half of allogeneic transplant recipients will experience a post-transplant complication that manifests as an autoimmune-like disease, graft-versus-host disease (GVHD), which, in its chronic form (cGVHD), affects multiple sites including the oral cavity. There has been little progress in preventing or treating GVHD, and the incidence of cGVHD is increasing due to a number of factors, including the increased use of peripheral blood mobilized stem cells, unrelated donor HSCTs, older age of patients, and increasing number of long-term transplant survivors. Total numbers of transplants, especially from unrelated donors, are also on the rise.
Box 1.
| Abbreviation | Term |
|---|---|
| aGVHD | acute graft-versus-host disease |
| BAFF | B Cell Activating Factor of the TNF Family |
| CD | cluster of differentiation |
| cDC | conventional dendritic cell |
| cGVHD | chronic graft-versus-host disease |
| DC | dendritic cell |
| GI | gastrointestinal |
| GVHD | graft-versus-host disease |
| GVT | graft-versus-tumor |
| HLA | human leukocyte antigen |
| HSCT | hematopoietic stem cell transplant |
| HSV | herpes simplex virus |
| IFN | interferon |
| IgA | immunoglobulin A |
| IgG | immunoglobulin G |
| IL | interleukin |
| MHC | major histocompatibility complex |
| MSG | minor salivary gland |
| NIH | National Institutes of Health |
| OMRS | Schubert Oral Mucositis Rating Scale |
| OSCC | oral squamous cell carcinomas |
| PCR | polymerase chain reaction |
| pDC | plasmacytoid dendritic cell |
| PUVA | psoralen and ultraviolet A |
| SCC | Squamous cell carcinomas |
| TBI | total body irradiation |
| TGF | transforming growth factor |
| Th | T helper |
| TNF | tumor necrosis factor |
| Treg | regulatory T cell |
| UV | ultraviolet |
| VH | verrucous hyperplasic hyperplasia |
With the increased burden of cGVHD, incidence of oral cGVHD will also become increasingly common, and the oral health community, both researchers and clinicians, should be prepared to aid in the understanding and treatment of this significant disease. It is important for oral health practitioners and scientists to be aware of the general characteristics, immunological implications, and treatment options for systemic and oral GVHD. The present review was written as an update on the general features of systemic and oral cGVHD, and highlights factors critical for progress in clinical management and the field of oral cGVHD research.
Allogeneic Hematopoietic Stem Cell Transplantation
Allogeneic HSCT is a potentially curative option for many hematological malignancies. The transplantation into the patient of donor hematopoietic stem cells – collected from bone marrow or umbilical cord blood, or mobilized for collection from peripheral blood -- is the basis of this therapy. Preparative regimens before HSCT, myeloablative or reduced-intensity, are used to reduce tumor burden and host resistance to the graft. Malignant stem cells survive chemotherapies used to treat cancers, and can survive the preparative regimens of transplants, but can often be eradicated by the immunologically active donor cells, through graft-versus-tumor (GVT) effect. Transplant grafts act immunologically based on histocompatibility, with the severity of the reaction based on the degree of compatibility in HLA tissue type between the donor and transplant patient. Before transplant, matching is performed on the basis of variability of the HLA gene, and a perfect match at tested loci is ideal. Transplant donors can be related to the patient, in which case a close match may be found, or can be an unrelated donor with a close degree of matching, often found through the National Marrow Donor Program. Immune disparities remain, however, and immunological reactions based on donor T cells interacting with transplant recipient tissue causes GVT effects as well as GVHD, the leading cause of morbidity and mortality after HSCT (Blazar et al., 2012, Copelan, 2006).
The improving success and long-term survival of patients after HSCT, as well as the increasing indications for its use, will continue the steep increase seen in the numbers of HSCT performed each year (Paczesny et al., 2010). In the US, almost 6000 patients underwent HSCT in 2009, an increase of 30% since 2005 (Majhail NS, 2011). With this dramatic increase in HSCT, the incidence and prevalence of GVHD will also continue to increase.
Acute Graft-versus-Host Disease
Graft-versus-host disease can be classified as either acute or chronic in nature. Historically, this determination was based on the time of onset, with aGVHD defined as occurring within 100 days of transplantation (Goker et al., 2001). Most recently, there has been a shift towards defining GVHD status based on clinical features. Acute presents mainly as erythema and maculopapular cutaneous lesions, liver dysfunction, oral mucositis, and upper and lower gastrointestinal involvement and can be characterized as classic, persistent, recurrent or late-onset aGVHD. Classic aGVHD occurs within 100 days after allogeneic HSCT, while persistent, recurrent or late-onset aGVHD occur after 100 days post-allogeneic HSCT (Filipovich et al., 2005).
Chronic Graft-versus-Host Disease
CGVHD is the most common complication following allogeneic HSCT. Nearly 50% of patients who survive longer than one year after transplantation develop the disease. Symptoms of cGVHD generally present within the first 3 years after HSCT, and may affect only one organ or may be widespread, affecting many areas of the body (Filipovich et al., 2005). Patients with cGVHD may suffer from severe morbidity, usually targeting the skin, eyes, mouth, GI tract, liver, lungs, joints, and genitourinary tract, resulting in pain, impaired functional ability, and poor quality-of-life. It is also the leading cause of fatality in long-term survivors of transplant, with a 5-year mortality rate for patients with cGVHD being about 70% (Arora et al., 2011). This high morbidity and mortality burden is most often due to immune dysregulation and suppression, leading to recurrent and opportunistic infections (Filipovich et al., 2005). The syndrome of cGVHD resembles, both clinically and histologically, many autoimmune disorders and other immunologic diseases, such as scleroderma, Sjögren’s syndrome, primary biliary cirrhosis, bronchiolitis obliterans, immune cytopenias, and chronic immunodeficiency (Filipovich et al., 2005). CGVHD is, therefore, defined as a multisystem alloimmune and autoimmune-like disorder characterized by immune dysregulation, immune deficiency, impaired end-organ function, and decreased survival.
Risk Factors for cGVHD
The known risk factors for developing cGVHD include older recipient age, female donor to male recipient, major allelic mismatch for HLA between donor and recipient, TBI conditioning, unrelated donors, peripheral blood stem cell source, donor lymphocyte infusion, and prior acute GVHD (Flowers et al., 2011, Martires et al., 2011). Grafts that include more donor T cells are typically more effective at eradicating malignancy; however, this also leads to a higher incidence of severe cGVHD (Apperley et al., 1988).
Immunopathogenesis of cGVHD
The essential pathogenesis of cGVHD entails alloreactive donor T cells that recognize and attack host tissues in immunocompromised recipients (Barnes et al., 1962, Billingham, 1966). The current understanding of cGVHD involves nuanced interplay among multiple immune cell types from the donor and host. This relationship is complicated by factors including inflammation induced by the conditioning regimen and post-transplant infectious insults. CGVHD has many autoimmune and fibrotic features. Clinically and histologically, cGVHD bears similarity to classic autoimmune diseases including scleroderma, systemic lupus erythematosus, primary biliary cirrhosis, Sjögren’s Syndrome and lichen planus (Filipovich et al., 2005, Baird & Pavletic, 2006). The process has a strong pro-inflammatory T cell component, with additional involvement of B cells and regulatory T cells (Blazar et al., 2012, Imanguli et al., 2009).
The greatest challenge in understanding the immunopathogenesis of cGVHD is the lack of a preclinical model that replicates the major clinical and chronic facets of human cGVHD. Mouse and other animal models typically mismatch major MHC factors, and focus on mortality and weight loss as the major outcomes. Though some minor-MHC mismatch models are in use, none adequately reproduces the temporal and multi-organ nature of human cGVHD (Hakim et al., 2001, Chu & Gress, 2008, Schroeder & DiPersio, 2011). Prospective longitudinal studies of cGVHD in humans require following patients for years after transplant, and often samples are limited to peripheral blood, clinical laboratory values and clinically-indicated tissue biopsies - the variable nature of which makes it difficult to draw conclusions about cGVHD development and pathogenesis. The following discussion highlights what has been learned from both animal models and studies of human cGVHD with an emphasis on human data, and details the contribution of multiple factors and cell types to the complex picture of cGVHD pathogenesis.
Role of T cells
CD4+T Cells
CD4+ T cells differentiate into functionally different subsets Th1, Th2, Th17 and Treg, depending on the cytokine milieu to which they are exposed and the respective developmental pathway that is activated. In the presence of IL-12, CD4+ T cells differentiate into IFN-γ or TNF-producing Th1 cells, whereas in the presence of IL-4, CD4+ T cells differentiate into IL-4-, IL-5-, and IL-13-producing Th2 cells (Yi et al., 2009). Th17 cells are considered to be pro-inflammatory, and produce cytokines IL-17A, IL-17F, and IL-22. Th17 differentiation requires TGF-β and IL-6, furthermore, factors IL-23 and IL-21 are critical for Th17 cell expansion and survival.
The role of Th1, Th2, and Th17 cells in GVHD pathogenesis is not clearly defined. In murine models, mediation of acute and chronic GVHD has been proposed by the action, or lack thereof, of different CD4 subsets in different tissues. Traditionally, aGVHD has been thought of as a Th1-mediated disease with most apoptotic damage to target tissues (skin, gut and liver) being mediated by inflammatory cytokines IFN-γ and TNF. CGVHD does not fit cleanly into either the Th1 or Th2 paradigm (Serody & Hill, 2012, Blazar et al., 2012, Imanguli et al., 2009).
In many autoimmune diseases, Th17 cells are considered to be potent inflammatory mediators (Lock et al., 2002, Koenders & van den Berg, 2010). Th17 cells have been associated with skin and lung GVHD (Serody & Hill, 2012) and were also shown to augment GVHD in some circumstances (Yi et al., 2009). Work is ongoing to understand the role of Th17 cells in GVHD and to develop the Th17 developmental pathway as a therapeutic target for the control of alloimmune disease (Serody & Hill, 2012).
Regulatory T cells
Tregs are a subset of CD4+T cells that express high levels of the IL-2 receptor α-chain CD25 and the transcription factor forkhead box P3 (FOXP3). A similar subset of CD8+T cells also exists, however this discussion focuses on the role of CD4+Treg cells. In the past decade, Tregs have emerged as a major factor in regulation of the immune response, particularly in autoimmune disease. Tregs function to suppress autoreactive lymphocytes thereby controlling innate and adaptive immune responses. Loss or impairment of Treg cell populations is implicated in many human autoimmune diseases, including emerging evidence for a role in cGVHD. In healthy humans, Treg cells comprise 5–10% of the entire CD4+T cell population and are thought to maintain self-tolerance in sites of primary and peripheral immune activation (Koreth et al., 2011). Murine models of GVHD have demonstrated attenuation of GVHD with the adoptive transfer of Treg cells, however, murine Tregs do not exactly mimic human Tregs in form and function, and this work has been problematic to implement clinically (Brusko et al., 2008). Expansion and survival of Treg cells is driven by IL-2, which, at low doses, can be used in humans to drive expansion of Treg cell and natural killer cell populations without inducing cGVHD. A recent human clinical trial demonstrated the efficacy of low-dose subcutaneous IL-2 therapy for the treatment of steroid-refractory cGVHD. In this trial, Koreth et.al demonstrated sustained Treg expansion in a majority of patients that was closely associated with amelioration of clinical manifestations of cGVHD (Koreth et al., 2011). This line of inquiry will continue to expand as more is understood about the relationship between Treg cells and cGVHD.
CD8+T Cells
Donor and recipient CD8+T cells have crucial roles in the development and pathogenesis of cGVHD, and, critically, mediate the therapeutic GVT effect of transplant. Infiltration of CD8+T cells in the skin, intestine and oral mucosa is associated with cGVHD (Wenzel et al., 2008, Imanguli et al., 2009, Panoskaltsis-Mortari et al., 2007). This underscores some of the tissue-specific differences in cGVHD pathogenesis that have been observed in clinical studies, in which all affected organs do not fall into a strict pattern of pathogenesis with regard to infiltrating lymphocyte profile, expression of cytokines and other regulatory factors. In patients with cGVHD, CD8+T cells demonstrate an increased level of proliferation and activation, and contain effector cells that are only selectively sensitive to immunosuppressive treatments (Grogan et al., 2011). Expression of CD134 (OX40) on the surface of CD8+ and CD4+T cells is associated with cGVHD onset, and marks early T cell activation that is often associated with induction by inflammatory cytokines (Miura et al., 2005, Ge et al., 2008, Briones et al., 2011). The details of CD8+T cell involvement and implications for prevention and therapeutic management of cGVHD are evolving areas in cGVHD research.
B cells in cGVHD
Though cGVHD therapies are classically focused on T cell dysregulation, the role of B cell involvement in the disorder has begun to emerge (Ratanatharathorn et al., 2009). This is driven in part by advances in understanding of basic B cell biology and also by the development and success of several biological therapies targeting B cells in human disease, including rituximab, a biologic therapy that targets and depletes host B cells though the CD20 receptor. Unlike many classical autoimmune diseases, in cGVHD, a consistent pattern of autoantibodies, i.e. those that could be used as cGVHD biomarkers or therapeutic targets, has not been detected in patient cohorts. However, in subsets of patients, autoantibodies against a few select groups of host antigens have been correlated with cGVHD activity. Specifically, antibodies targeting male HY antigen have correlated with cGVHD activity in female to male donor to host pairs, and anti-double-stranded DNA and organ-specific autoantibodies have been associated in select patients with the onset and severity of cGVHD (Fujii et al., 2008, Svegliati et al., 2007, Miklos et al., 2005). Clinical trials have established the efficacy of rituximab in the prevention, delay or attenuation of cGVHD manifestations (Cutler et al., 2006, Ratanatharathorn et al., 2009, Kim et al., 2010), which supports an important role for B cell dysregulation in cGVHD pathogenesis and clinical management (Blazar et al., 2012).
Emerging evidence from translational studies indicates that a regulatory cytokine for B cell activity and survival, B Cell Activating Factor of the TNF Family (BAFF) is elevated in cGVHD patients (Sarantopoulos et al., 2007, Fujii et al., 2008, Kuzmina et al., 2011, Sarantopoulos et al., 2009). BAFF is constitutively produced as a homeostatic cytokine for B cells, and BAFF levels are thought to be modulated by B cell consumption (Schiemann et al., 2001). Circulating BAFF concentration has functional significance, as elevated levels of BAFF have been correlated with increased numbers of transitional B cells, increased B cell sensitivity to inflammatory pattern receptor signaling, and increased production of memory B cells (Kuzmina et al., 2011, Sarantopoulos et al., 2009, She et al., 2007). Many transplant regimens, by design, result in long-term deficits of B cells. Elevated levels of BAFF have been detected after B cell depletion by transplant conditioning regimens or rituximab therapy in patients who developed cGVHD, but declined in transplant patients who did not develop cGVHD and also declined in patients who responded to rituximab therapy (Sarantopoulos et al., 2009, Sarantopoulos et al., 2011). In future studies, BAFF levels may serve as a biomarker for cGVHD activity and provide clues for new therapeutic targets.
Role of Antigen presenting cells
Dendritic cells (DCs) are specialized immune cells that process and present antigens to other immune cells, particularly T cells. In addition to the well-known capacity of DCs to stimulate innate and adaptive immune responses, DCs may also induce and maintain immune tolerance (Matta et al., 2010). DCs arise from specific progenitor subsets of hematopoietic bone marrow stem cells. DCs are strong contributors to the beneficial GVT effect of HSCT but also have a crucial role in the development and pathogenesis of GVHD. Both donor and recipient DCs contribute to these effects. Two types of DCs have been implicated in the induction of acute and chronic GVHD (Stenger et al., 2012, Young et al., 2007, Koyama et al., 2009). Broadly, conventional DCs (cDCs) are classic DCs with morphologic dendrites and function effective to uptake, process and present antigens to lymphocytes in a highly effective manner. These include many specific DC subsets that are defined both by location in the body and specific surface markers, such as skin epidermal Langerhans cells and resident DCs present in lymphoid organs. Upon activation, cDCs produce IL-12 and promote Th1 cell differentiation and CD8+ cytotoxic T cell responses (Young et al., 2007, Koyama et al., 2009). Although most cDCs are immunostimulatory, some subsets, including epidermal Langerhans cells, have been shown to be tolerogenic or immunostimulatory, depending on maturation state, antigen and cytokine milieu at activation. The second broad category of human DC is the precursor DC. These include plasmacytoid DCs (pDCs), which have an immature phenotype and plasma cell morphology during steady-state. Once activated, pDCs acquire a classic DC morphology, like cDCs, however, their efficacy of antigen processing and loading is lower than that of cDCs, which leads to altered immunologic outcomes, often inducing T cell tolerance secondary to less effective T cell stimulation when compared with cDCs. After stimulation, pDCs secrete large amounts of type I interferons which activate CD4+ and CD8+ T cells, however because pDCs display lower surface levels of MHC and costimulatory molecules, full activation of T cells is often prevented. Furthermore, paces have intrinsic tolerogenic properties in a healthy human that vary by tissue location. In the human immune system, thymic pDCs induce Tregs, liver pDCs induce oral tolerance, and airway pDCs regulate mucosal tolerance (Stenger et al., 2012).
The role of DCs in the induction of human GVHD is based largely on information from mouse models of GVHD, which contain significant phenotypic and functional differences in DC subsets, and from ex vivo work with human DCs. Clinical studies show an association between decreased numbers of total DC at time of engraftment and three negative outcomes: decreased patient survival, increased relapse of malignancy and increased acute GVHD (Stenger et al., 2012). The role of DCs and of specific DC subsets in cGVHD is an area of intense investigation. Therapeutic strategies under investigation include generation and infusion of tolerogenic DCs for GVHD patients, in vivo stimulation of pDC and other tolerogenic subsets using pharmacological strategies, and direct depletion of activated DCs using monoclonal antibodies against their specific surface markers. An additional promising clinical strategy includes the use of mesenchymal stem cells, a subset of rare, nonhematopoietic human pluripotent cells, to induce immune tolerance via DCs in GVHD patients (Aldinucci et al., 2010, Li et al., 2008, Baron & Storb, 2012, Le Blanc et al., 2004, Gyurkocza et al., 2010).
Biomarkers in cGVHD
Biology-based markers, or biomarkers, are defined as a metric that is objectively measured and evaluated as an indicator of a normal biologic or pathogenic process, a pharmacologic response to a therapeutic intervention, or a surrogate end point intended to substitute for a clinically important end point (Biomarkers Definitions, 2001). Biomarkers in cGVHD are an important area for research and clinical trials and management; the reasons for this were highlighted in the cGVHD Biomarker Working Group 2006 NIH Consensus paper and include: 1) predicting response to therapy, 2) measuring disease activity and distinguishing irreversible damage from continued disease activity, 3) predicting the risk of developing cGVHD, 4) diagnosing cGVHD, 5) predicting the prognosis of cGVHD, 6) evaluating the balance between cGVHD and graft-versus-transplant effects, and 7) serving as surrogate end points for therapeutic response, particularly for clinical trial research. Both hypothesis and discovery-based biomarker studies are encouraged to further this field of research (Schultz et al., 2006). There are no currently validated biomarkers for cGVHD, and the research steps needed to fill this need (discovery, validation, and clinical testing) are complicated by the complex and incompletely understood pathophysiology of cGVHD (Rozmus & Schultz, 2011, Levine et al., 2012).
Process-specific classifications of cGVHD biomarkers have been suggested (Schultz et al., 2006). Allogeneic disparity between non-HLA polymorphisms, regulatory T cell populations, Th1/Th2 balances, and B cell-related biomarkers are all areas of intense current research (Rozmus & Schultz, 2011, Levine et al., 2012). Blood-based biomarkers are routinely used in the management of HSCT patients, and a study by Grkovic, et. al. has described the non-immunologic biomarker profile of cGVHD patients. Lower albumin, higher C-reactive protein, and higher platelets, as measured by routine laboratory analysis, were associated with active disease defined as the clinician’s intention to intensify or alter systemic therapy due to a lack of response (Grkovic et al., 2012). Fassil, et. al. recently reported that lower albumin and higher total complement were further associated with clinically significant oral cGVHD (Fassil et al., 2012).
Chronic GVHD is not only a systemic disorder, but also affects specific end-organ systems, including the skin, liver, and mouth. In the case of oral cGVHD, its manifestation can be confused or worsened by other causes, such as infection or drug-induced mucositis (Imanguli et al., 2008a). Biomarkers in this case which are cGVHD and mouth specific may improve the diagnosis, prognosis, and treatment of patients after HSCT (Levine et al., 2012). The environment of the biomarker medium may be very different between organs in a patient with cGVHD, and the use of solely blood-based biomarkers may not reflect what is happening in a specific organ (Schultz et al., 2006). In oral cGVHD, this argues for the discovery of oral-specific biomarkers taken from specimens at the site of disease, either from oral tissue biopsy or saliva.
Salivary biomarker research holds great promise due to the non-invasive nature of saliva procurement as a specimen and its ease of collection (Baum et al., 2011). This is especially true in the field of oral cGVHD research, and several studies have now focused on cGVHD salivary biomarker discovery. Chronic GVHD causes increased inflammation of the salivary glands and increased oral epithelial permeability, increasing salivary concentrations of albumin, IgG, electrolytes such as Na+ and Cl−, and altering lactoferrin levels (Rozmus & Schultz, 2011, Imanguli et al., 2010).
Patients who have undergone HSCT appear to have a significantly altered salivary proteome, though the specific protein changes are still emerging. Imanguli, et. al. described the elevation of salivary lactoferrin and secretory leukocyte protease inhibitor in saliva collected serially from 41 patients undergoing HSCT; this change persisted at least for 6 months after transplant (Imanguli et al., 2007). Recently, the quantitative salivary proteome was analyzed between two patient groups, both of which had cGVHD but were divided by having oral cGVHD. Of 180 proteins identified, 102 changed in abundance at least 2 fold, including 12 proteins identified only in the group with oral cGVHD. The reduction of salivary lactoperoxidase, lactotransferrin, and several cysteine proteinase inhibitors suggests impaired oral antimicrobial host immunity in cGVHD patients, and may explain some oral GVHD manifestations (Bassim et al., 2012).
Diagnosis and Scoring of cGVHD
In 2005, the National Institute of Health (NIH) Consensus Working Group for Diagnosis and Staging of cGVHD (Filipovich et al., 2005) recommended a new definition of cGVHD, based on characteristic clinical and pathologic features. The diagnosis of cGVHD requires the following: 1) distinction from aGVHD, 2) presence of at least 1 diagnostic clinical sign of cGVHD or presence of at least 1 distinctive manifestation confirmed by pertinent biopsy or other relevant tests, and 3) the exclusion of other possible diagnoses. There is no time restriction placed on when cGVHD can be diagnosed, and can occur at any time after transplant. Further, a clinical scoring system of 0–3 was proposed to evaluate the involvement of individual organs and sites (focusing on the skin, mouth, eyes, female genitalia, GI tract, lungs, and connective tissues), as well as a global assessment of severity (mild, moderate, or severe). Systemic therapy is recommended for patients who meet the criteria for moderate or severe cGVHD.
Oral Chronic Graft-versus-Host Disease
Clinical Presentation of Oral cGVHD
Although cGVHD can affect many various organs, the oral cavity is the second most commonly involved organ system, behind skin involvement, with a historically reported 45–83% prevalence among cGVHD patients (Schubert & Correa, 2008). Oral cGVHD can present as mucosal lesions, salivary gland dysfunction, and restricted mouth opening (Woo et al., 1997, Filipovich et al., 2005, Imanguli et al., 2008a, Schubert & Correa, 2008). The spectrum of clinical presentation of cGVHD is diverse in the type and severity of tissue changes and can involve any site in the oral cavity: the lips, labial and buccal mucosa, tongue, hard and soft palate, floor of mouth, and gingiva should be evaluated, as well as salivary function and mouth movement. Patient-reported oral pain and sensitivity, subjective oral dryness, and inquiries into oral movement dysfunction or restriction can add important elements to the clinical exam and guide the practitioner to more site specific evaluations.
Oral Mucosal Lesions
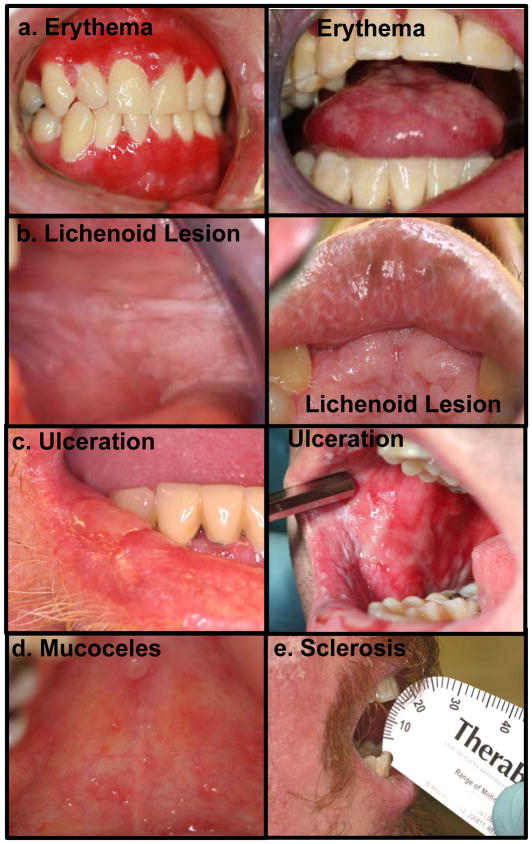
When evaluating oral mucosal lesions, the surface area of intraoral mucosa affected as well as the severity of the lesion can be variable and both should be assessed (Schubert & Correa, 2008). Typically, cGVHD oral mucosal lesions are characterized as erythema, lichenoid, ulcerative, or as mucoceles (Figure 1) (Filipovich et al., 2005).
Figure 1.
The spectrum of the clinical presentation of oral cGVHD includes (a) erythema of the oral mucosa and tongue, (b) lichenoid lesions that occur on the buccal mucosa, lips and other areas of the oral cavity, (c) oral ulcerations, (d) mucoceles on the hard palate and occasionally lower labial mucosa, and (e) peri-oral sclerosis that limits mouth-opening.
Erythema, defined as redness of the oral mucosa without obvious tissue breakdown, is often a sign of infection or inflammation, and can be associated with atrophy and/or edema of the mucosa (Figure 1a). Patients often complain of generalized oral sensitivity with mucosal erythema.
Lichenoid oral cGVHD is characterized by white or milky reticular streaks or lacey lines on oral mucosa, resembling the Wickham’s striae observed in oral lichen planus (Figure 1b). These can be associated with hyperkeratotic leukoplakias: white plaques or thickened, hyperplastic mucosa. These oral mucosal lesions are considered diagnostic for oral cGVHD in the context of post-HSCT oral evaluation, and are often not painful (Filipovich et al., 2005).
Ulcerations represent a breakdown in oral mucosa and can be associated with pseudomembranes as poor wound-healing evolves over time (Figure 1c). Ulcers can be very painful, to the point of limiting eating and the ability to maintain oral hygiene (Imanguli et al., 2008a). Ulcers also represent the most obvious route for infection to enter the bloodstream of a patient from the oral cavity, as the integrity of the oral mucosal barrier is completely broached.
Mucoceles are superficial subepithelial extravasations of saliva from minor salivary glands into the epithelial-connective tissue interface resulting from fibrotic occlusions of the glandular duct openings (Imanguli et al., 2008a). They present clinically as fluid-filled domed lesions, covered and surrounded by normal appearing oral mucosa (Figure 1d). They are seen only on the palate and the lips, and are generally asymptomatic. Salivary gland inflammation may block salivary ducts, which is worsened by decreased secretion and increased saliva viscosity, leading to mucocele formation (Filipovich et al., 2005).
Limited Oral Opening
Sclerotic fibrosis of the perioral tissue as a consequence of chronic inflammation can result in restricted oral range-of-motion in patients with oral cGVHD; this resembles tissue changes observed in patients with scleroderma (Filipovich et al., 2005, Schubert & Correa, 2008). Scleroderma-like cGVHD can involve any of the orofacial tissues, resulting in fibrosis and limited mouth opening, often presenting as a “purse-string” mouth (Figure 1e) (Schubert & Correa, 2008, Woo et al., 1997). Continued inflammation can cause scarring of the oral cavity, further restricting mouth movement. Limitations of mouth opening could lead to problems with oral hygiene and eating, potentially contributing to infection and malnutrition (Imanguli et al., 2008a, Schubert & Correa, 2008). Other morbidities related to cGVHD, including muscle wasting, muscle cramping, and joint range-of-motion problems may affect the oral cavity.
Salivary Dysfunction
Inflammatory damage to salivary gland tissue may result in diminished salivary flow and subsequent dry mouth characteristically seen in oral cGVHD. Salivary dysfunction of cGVHD can be characterized by Sjögren syndrome-like manifestations: hyposalivation (objective saliva flow reduction) and xerostomia (subjective dry mouth) (Woo et al., 1997, Imanguli et al., 2010). A dry mouth can cause problems with speaking, chewing, and swallowing, and is strongly associated with increased dental and oral mucosal disease, including caries and oral Candida infections (Figure 2a-c) (Mathews et al., 2008).
Figure 2.
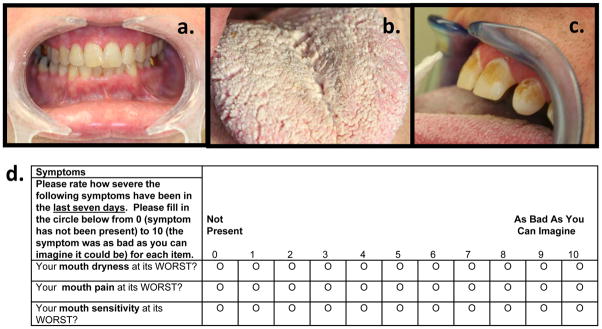
Salivary gland dysfunction results in extreme dry mouth (a). This contributes to increased susceptibility to opportunistic infections and fungal overgrowth (b), and reduced remineralization of tooth enamel, resulting in cervical carious lesions (c). Patient-reported oral dryness, pain, and sensitivity scales, used for oral cGVHD symptom measurement (d).
In a recent study, Imanguli, et. al carefully described salivary gland involvement in GVHD (Imanguli et al., 2010). In this cohort, xerostomia was complained of by 77% of patients with cGVHD, most often associated with dry eye complaints. Salivary flow rates were low (≤ 0.2mL/min) in 43% of patients with cGVHD, and histopathological changes of mononuclear infiltration and/or fibrosis/atrophy were present in all patients with hyposalivation. Further, patients with salivary gland cGVHD had decreased oral specific quality-of-life and a lower body mass index. Salivary dysfunction in oral cGVHD appears to be a distinct entity from the mucosal manifestations seen, and little correlation exists between these two manifestations. Imanguli concludes that formal salivary function testing is needed in the evaluation of cGVHD, and should be considered as an important sign and symptom in the diagnosis, treatment, and staging of the disease.
Diagnosis of Oral cGVHD
The 2005 NIH Consensus Working Group for Diagnosis and Staging of cGVHD (Filipovich et al., 2005) standardized the criteria for the diagnosis of oral cGVHD. Diagnostic signs and symptoms of oral cGVHD are defined as manifestations that establish the presence of cGVHD without the need for further testing. These include lichen planus-like changes (intraoral white lines and lacy-appearing lesions) and hyperkeratotic leukoplakias. Distinctive features of oral cGVHD, which may require further testing to confirm a diagnosis, include xerostomia, the presence of mucoceles, mucosal atrophy, pseudomembranes, and ulcerations. Manifestations that are common to both acute and chronic GVHD include gingivitis, mucositis, erythema, and pain.
Diagnosis of oral cGVHD requires that other causes of oral symptoms be excluded, as oral infections often complicate the differential diagnosis of cGVHD (Filipovich et al., 2005). For oral cGVHD, this means that oral infections, including herpes simplex and candidiasis, must be considered when diagnosing oral mucosal lesions in this patient group. Samples should be taken for any suspected oral infection and analyzed by culturing for yeast or PCR analysis of viral involvement, as appropriate for individual cases. Drug reactions and recurrent or new malignant lesions must be excluded prior to a definitive diagnosis of oral cGVHD. Further testing in support of an oral cGVHD diagnosis includes biopsy of oral GVHD lesions (non-ulcerated tissue) or adjacent tissue, including labial minor salivary glands (MSGs) in xerostomic patients. Biopsies should be formalin fixed, processed for routine pathology (H&E stained) and read by an experienced pathologist as “consistent with,” or “unequivocal” cGVHD, (Pavletic et al., 2006a, Meier et al., 2011).
Oral Histopathology
Histopathological samples from cGVHD-involved sites afford investigators a glimpse into the tissue architecture, cell populations, and pathological mechanisms at the sites of active cGVHD. The oral cavity, like the skin, is a site that allows for straightforward biopsy collection under local anesthesia. This affords a unique opportunity to use biopsies of the oral buccal mucosa and MSGs to ask directed questions about the character and immunopathogenic mechanisms of oral cGVHD, in addition to diagnosis of oral cGVHD. Knowledge of the histological features of oral cGVHD is not well-crystallized, as present reports of cGVHD oral histopathology have examined small cohorts of patients with this rare disease; thus, the field would greatly benefit from comparative analysis of oral cGVHD in a larger population.
All soft-tissue areas of the oral cavity may be impacted by cGVHD. Most frequently affected are the oral mucosa and salivary glands. Labial MSGs are more often involved than is oral mucosa, according to several small cohort studies of buccal mucosa and minor salivary gland involvement (Soares et al., 2005, Nakamura et al., 1996). In addition to being part of the highly vascular oral mucosa, which provides ready access for circulating auto-reactive pathogenic lymphocytes, the salivary glands also express high levels of the histocompatibility antigen HLA-DR (Hiroki et al., 1996, Soares et al., 2005). When the MHC antigens, major or minor, expressed by the salivary glands are mis-matched with those of the donor lymphocytes, this marks the MSGs as a target for auto-immune assault. Although a subset of cGVHD patients exhibit Sjögren’s Syndrome-like clinical symptoms including xerostomia and xeropthalmia, MSG histology is not identical in the two diseases (Nakamura et al., 1996). CGVHD patients most often have diffuse lymphocytic infiltration with expression of adhesion molecules including ICAM-1 and E-selectin on both the ductal epithelial and endothelial cells only in areas with infiltrating lymphocytes (Hiroki et al., 1996). In contrast, MSG sections from Sjögren’s Syndrome patients classically exhibit periductal and focal lymphocytic infiltrates with adhesion molecule expression on ductal and endothelial cells in the presence and absence of lymphocytic infiltrates (Hiroki et al., 1996). In most reports, the infiltrating lymphocytes in cGVHD MSG tissue consist primarily of CD3+T cells with a predominance of CD8+T cells over CD4+T cells, with occasional identification of B cells, and increased prevalence of macrophages. One study reports parotid gland biopsies from 3 cGVHD patients that were paired with MSG and buccal mucosa biopsies (Hiroki et al., 1994). Similar pathology in the parotid gland to that of the MSGs was reported for 2 cases, and, for the third, the parotid gland was the only tissue sampled from the patient with evidence of cGVHD, though hepatic and ocular involvement manifested 2 months later (Hiroki et al., 1994). This suggests that the salivary glands may be one of the earliest tissues involved in cGVHD, and that MSG biopsy provides a representative sample to assess salivary gland involvement.
Buccal mucosa biopsies are more frequently obtained than are MSG biopsies due to clinical ease and patient acceptance of the procedure. However, based on the above detailed reports, histopathological diagnosis of cGVHD in buccal mucosa is likely less sensitive than in MSG tissue, though this has not been rigorously examined. In a recent study of buccal tissue from cGVHD patients, Imanguli, et al found that clinical severity of oral cGVHD was correlated with apoptotic epithelial cells, and that the apoptotic cells were often found adjacent to infiltrating effector-memory T cells expressing markers of type I cytokine polarization and cytotoxicity (Imanguli et al., 2009). The data from this study strongly support the development of oral cGVHD as a result of type I IFN–driven immigration, proliferation, and differentiation of effector T cells. In support of this hypothesis, a separate study found significantly higher numbers of Langerhans cells, which work to stimulate T cells and are recruited by inflammatory chemokines, in the buccal mucosal tissue of oral cGVHD patients than in non-GVHD post-transplant patients and healthy controls (Orti-Raduan et al., 2009). It is not known if these findings are generalizable to all oral cGVHD, as data from MSG tissue has thus far focused on cellular phenotype rather than cell function or pathway activation.
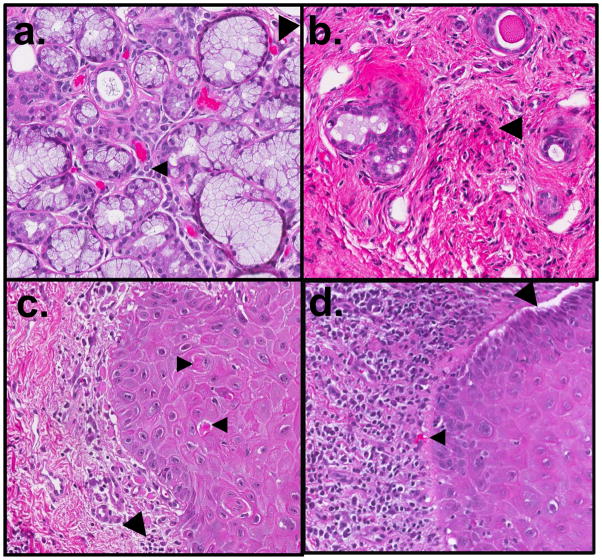
Key histological features of cGVHD in MSG tissue are diffuse lymphocytic infiltration and damage of the intralobular ducts, inflammation with atrophy and destruction of acinar tissues, and fibroplasia in periductal stroma (Figure 3) (Shulman et al., 2006). The standard for minimal histologic criteria for diagnosis of oral cGVHD in any oral tissue, according to the 2006 cGVHD Pathology Working Group report, are localized or generalized epithelial changes (lichenoid surface inflammation, exocytosis and apoptosis) similar to those seen in cutaneous cGVHD, or the presence of intralobular, periductal lymphocytes with or without plasma cells and lymphocyte exocytosis (without neutrophils) into intralobular ducts and acini (Shulman et al., 2006). In 1989, Nakhleh refined these minimal criteria for GVHD as greater than 3 mucosal apoptotic bodies and for salivary gland changes, a greater-than 10% loss of acinar tissue or ductal epithelial cell necrosis (Pavletic et al., 2006b, Nakhleh et al., 1989). The specific guidelines, as suggested by the 2006 Working Group report, may change when more is known about correlations among histopathological features and clinical data (Shulman et al., 2006). There are several histologic grading criteria sometimes used for GVHD that were originally developed for other diseases including Sjogren’s Syndrome. However, GVHD-specific grading criteria most accurately reflect the disease stage and pathogenesis. The scale developed by Horn et al. for salivary gland cGVHD is based on the degree of lymphocytic infiltration and destruction of glandular acini (Horn et al., 1995).
Figure 3.
Oral histopathologic changes in cGVHD include alterations in the minor salivary glands (MSG) and buccal mucosa. In the MSG, (a) sialadentis is often present, and fibrosis and atrophy of the gland are frequently observed. Lymphocytic infiltration (small arrow) and apoptotic cells (large arrow) may be observed in active stages of disease. (b) In late cGVHD, MSGs may have marked atrophy with only mild residual inflammation and few to no observable apoptotic cells. Destroyed glandular acini are often replaced by loose fibrotic stroma with associated lymphocytes (large arrow). In the buccal mucosal tissue, (c) generalized or band-like lymphocytic infiltration (large arrow) may be observed in the submucosa, near the junction of nonkeratinized squamous mucosa. Apoptotic cells (small arrows), often associated with lymphocytes, and may be observed in active stages of disease. (d) In severe cases, separation or clefting of the basal epithelial layer may be observed (large arrow), often in conjunction with heavy lymphocytic infiltrate and apoptotic bodies (small arrow). Original magnification 20×.
Finally, the 2006 Working Group developed a set of worksheets to facilitate transfer of clinical information to the pathologist and to aid in clincopathologic studies. These are available from the American Society for Blood and Marrow Transplantation at http://www.asbmt.org, under “Guidelines, Policy Statements and Reviews.” Although these tools appear well-designed to accurately reflect the critical histological features of cGVHD, they have not been validated in a large-scale cGVHD cohort for research or clinical diagnostic purposes.
The decision to diagnose and treat oral cGVHD is not based on a histologic gold-standard positive biopsy. CGVHD is a complex and dynamic biologic process, and a biopsy represents tissue in a specific area at a specific time. Furthermore, a number of technical issues may preclude the identification of positive tissue. However, a biopsy can be used to rule out a drug-reaction or certain infections that may mimic cGVHD, which can be instrumental in determining treatment course. Biopsy of both minor salivary glands and buccal mucosa or other suspected sites of oral cGVHD can give information about disease in the oral cavity that is frequently non-synchronous. Taken together, the information obtained from oral biopsies should be used in conjunction with clinical exam and other data to inform treatment decisions about an individual patient.
Predictive Factors of Oral cGVHD
Prior acute GVHD and the use of peripheral blood stem cell source have been reported to be risk factors for oral cGVHD (Hull et al., 2012). Other research suggests that salivary gland involvement in oral cGVHD may be the result of TBI during pre-transplant conditioning (Garming-Legert et al., 2011, Panoskaltsis-Mortari et al., 2007). Most recently, an extensive multivariate logistical regression analysis revealed that oral cGVHD was significantly associated with patient-reported mouth pain as well as several laboratory markers of inflammation, including lower albumin levels and higher total complement levels (Fassil et al., 2012).
Prognostic Factors of Oral cGVHD
The consequences of oral cGVHD can impact on many aspects of health, including increased oral infections, decreased oral epithelial integrity and ability for repair, oral pain, increased caries risk, and negative influences on nutrition, speech, eating, and quality-of-life (Meier et al., 2011, Fassil et al., 2012). Although oral cGVHD is not associated with poor long-term survival, it has a tremendous deleterious impact on the oral health, functional capacity, symptoms and quality-of-life of affected patients. Oral cGVHD is significantly associated with the severity of patient reported oral pain (Fassil et al., 2012). Patients with oral cGVHD are at a very high risk for developing extensive cervical decay within two years of transplantation (Castellarin et al., 2012). Current research also shows that patients with oral cGVHD have reported experiencing taste alteration and increased levels of oral related pain and dryness as compared to patients without oral cGVHD (Fall-Dickson et al., 2010). Oral cGVHD also increases the risk of having diminished oral cavity specific quality-of-life and lower body mass index scores (Imanguli et al., 2010). Patients with salivary gland atrophy or dysfunction often have difficulty swallowing, an increased risk for developing dental carious lesions due to impaired remineralization and frequent co-infections possibly due to diminished salivary defenses including secretary IgA (Meier et al., 2011).
Oral cGVHD Characteristics from the NIH cGVHD Patient Cohort
In 2004, a research study was initiated at the National Institute of Health (NIH) Clinical Center to assess patients with cGVHD (clinicaltrials.gov #NCT00331968, Prospective Assessment of Clinical and Biological Factors Determining Outcomes in Patients With cGVHD). Patients are referred to the study with a diagnosis of cGVHD and are enrolled if they have cGVHD according to the definition of the NIH Consensus Group criteria, as described above (Filipovich et al., 2005). Subjects undergo a four-day, one-time visit for evaluation by a multi-disciplinary team of clinical experts in dermatology, ophthalmology, dentistry, rehabilitation medicine, gynecology, pain and palliative care, and HSCT care. Clinical assessments, patient-reported forms and questionnaires, and laboratory data are recorded at the time of the subject visit using pre-defined data collection instruments. The assessments were undertaken with the understanding and written consent of each subject and according to ethical principles, including the World Medical Association Declaration of Helsinki (2002 version). This cohort provides a detailed description of the demographic, transplant, and GVHD characteristics of a large group of patients with cGVHD, which has been described in a recent paper from our group (Table 1) (Fassil et al., 2012). The majority of patients had undergone myeloblative conditioning and received transplants from HLA-matched related donors and received peripheral blood stem cell grafts. Patients developed cGVHD at a median of 7 months (6–67 months) after allogeneic HSCT and were enrolled at a median of 36 months (6–223 months) after HSCT. Most patients had moderate (30%) or severe (68%) cGVHD, received moderate or high intensity of systemic immunosuppression at the time of enrollment (75%), and had failed multiple lines, median of 3 lines (0–9 lines), of prior systemic therapies for cGVHD. This describes a group of patients with cGVHD who are generally more severely affected than those seen at single transplant centers, but which allows for a detailed and comprehensive analysis of oral cGVHD characteristics from this study.
Table 1.
Patient’s with cGVHD characteristics at the time of enrollment
| Patient characteristics | n (%) or (range) |
|---|---|
| Total number of patients | 187 |
| Age (median, range) | 46 (4–70) |
| Gender | |
| Male | 103 (55 %) |
| Female | 85 (45 %) |
| Disease | |
| ALL/AML/MDS | 79 (46 %) |
| Lymphoma/CML/MM | 71 (41 %) |
| CLL | 12 (7 %) |
| Aplastic Anemia/PNH | 6 (4 %) |
| Other non-malignant | 3 (2 %) |
| Conditioning regimen | |
| Myeloblative | 106 (57 %) |
| Total Body Irradiation (TBI) | 72 (39 %) |
| Donor relationship | |
| Unrelated | 72 (39%) |
| Related | 113 (61 %) |
| Cell source | |
| Bone Marrow | 35 (19 %) |
| Peripheral Blood | 146 (79 %) |
| Cord Blood | 4 (2 %) |
| HLA match | |
| Yes | 148 (82 %) |
| No | 32 (18 %) |
| cGVHD onset type | |
| Progressive | 70 (38 %) |
| Quiescent | 52 (29 %) |
| De Novo | 60 (33 %) |
| Activity by therapeutic intent a | |
| Active | 79 (53 %) |
| Not Active | 69 (47 %) |
| Unknown (other) | 50 (25 %) |
| Intensity of immunosuppression b | |
| None/mild | 46 (25 %) |
| Moderate | 62 (34 %) |
| Severe | 75 (41 %) |
| NIH average total number of organs involved | 5 |
| Mouth | 135 (68%) |
| Skin | 145 (79%) |
| Eyes | 148 (80 %) |
| Lung | 141 (77%) |
| Liver | 96 (52%) |
| Joints or Fascia | 115 (63%) |
| Genitourinary Tract | 42(50%) |
| Gastrointestinal Tract | 84 (46%) |
| NIH Average Score | 1.0(0–2.33) |
| NIH Global Score | |
| Mild | 3 (2%) |
| Moderate | 59 (30%) |
| Severe | 134 (68%) |
| Median number of months from transplant to enrollment | 51 (4–258) |
For all values in above table, continuous variables are shown as median values with ranges and categorical variables are shown as frequencies with percentages.
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; MDS, myelodysplastic syndrome; CML, chronic myeloid leukemia; CLL, chronic lymphocytic leukemia; MM, Multiple Myeloma PNH, paroxysmal nocturnal hemoglobinuria; M, male; F, female; HLA, human leukocyte antigen.
active: 1) increase systemic therapy because cGVHD is worse; 2) substitute systemic therapy due to lack of response; and 3) withdraw systemic therapy due to lack of response. Non-active: 1) decrease systemic therapy because cGVHD is better; 2) not change current systemic therapy because cGVHD is stable;
Intensity of Immunosupression: Mild, single agent prednisone < 0.5; Moderate, prednisone ≥ 0.5mg/kg/day and/or any singe agent/modality; High, 2 or more agents/modalities ± prednisone ≥ 0.5mg/kg/day.
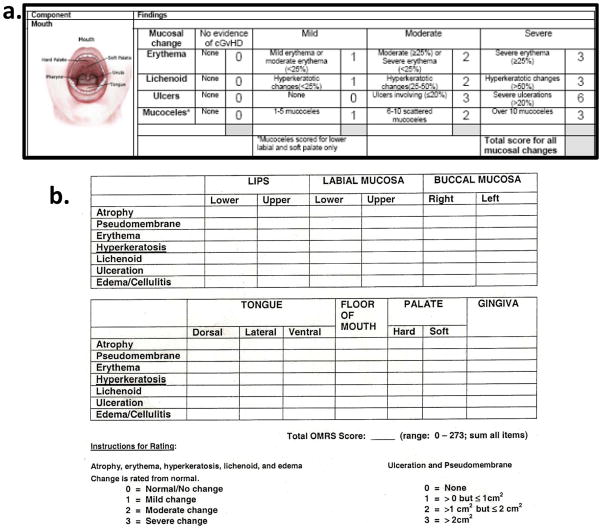
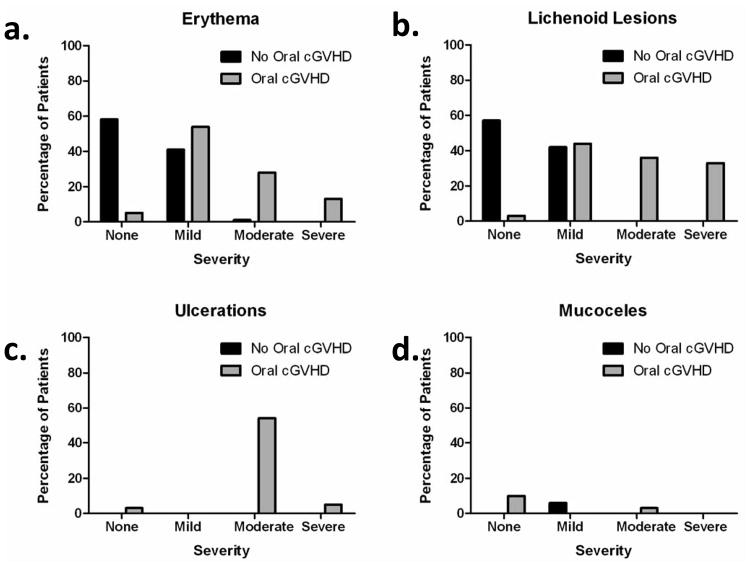
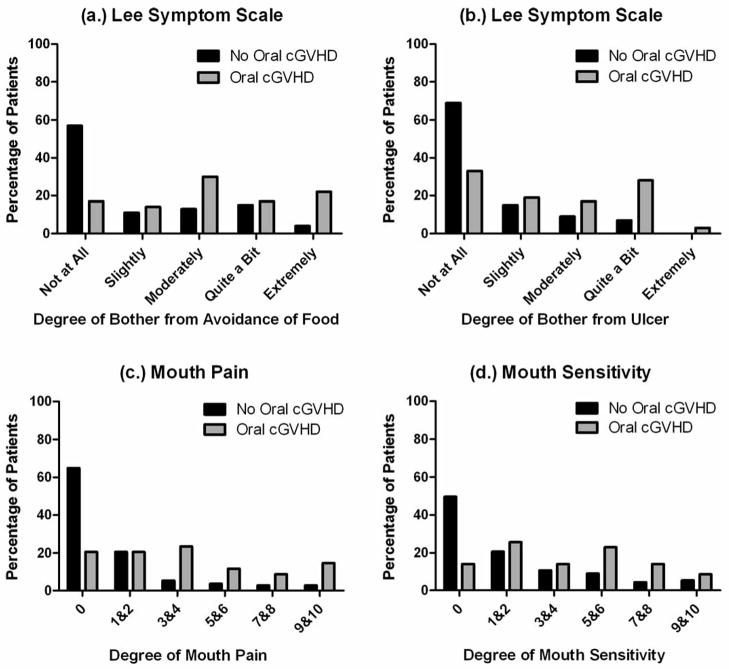
Within this group, 44 (24%) of 187 patients total were reported to have clinically important oral cGVHD when using the NIH Mouth cGVHD Activity Assessment Scale (Figure 4a) to score the severity of oral cGVHD activity based on four major manifestations of oral cGVHD: erythema, lichenoid, ulcers and mucoceles. This is a lower prevalence of oral cGVHD than is normally reported and can be attributed to the conservative and strict definition of oral cGVHD used in the analysis (NIH Activity Assessment Score of above 2, scale 0–15). The majority of patients with oral cGVHD presented with erythema (54%) and lichenoid changes (54%) of the oral mucosa while only a few presented with ulcerations (4%) and mucoceles (7%). Figure 5 shows the range of oral cGVHD seen based on the NIH Oral Score by the type of mucosal change observed. Most patients with cGVHD reported subjective oral dryness (67%), though there was not an association between having oral cGVHD and reporting a dry mouth. This suggests that the NIH Activity Assessment Score does not capture dry mouth issues that may be important in assessing oral cGVHD. Finally, many patients with cGVHD reported oral pain (46%), and sensitivity (59%), and all patient self-reported symptom measures analyzed (Lee symptoms scores for degree of bother from avoidance of foods and from ulcerations, mouth pain, and mouth sensitivity) were significantly associated with oral cGVHD status (Figure 6) (Fassil et al., 2012).
Figure 4.
Clinical and research focused rating tools used for assessment of oral GVHD. (a) The NIH cGVHD Activity Scale scores the oral cavity for percent of surface area involved with erythema, lichenoid lesions and hyperkeratosis, ulceration and mucoceles (Pavletic et al., 2006b). (b) The Oral Mucositis Rating Scale (OMRS) rates 13 locations and 7 types of lesions (Schubert et al., 1992).
Figure 5.
NIH oral cGVHD scale breakdown by type of mucosal change. The frequency and severity of mucosal changes in (a) erythema, (b) lichenoid lesions, (c) ulceration and (d) mucoceles were assessed in patients with and without oral cGVHD. Reprinted with permission (Fassil et al., 2012).
Figure 6.
Patient-Reported Symptoms of oral cGVHD. The distribution of self-reported symptoms is compared here in patients with and without oral cGVHD (n=187 total). The oral cGVHD status is compared across the distribution of the Lee Symptom Scale (a) on the degree of bother from the avoidance of food and (b) degree of bother from ulceration. (c) Mouth pain and (d) mouth sensitivity are grouped by the patient-reported 0–10 score to show the percentage of each group affected at that level. Reprinted with permission (Fassil et al., 2012).
Oral cGVHD Scoring for Clinical Use
The NIH Consensus report proposed a clinical scoring system that assesses the severity and extent of oral cGVHD on scale of 0–3 based on clinical presentation and functional impact (Filipovich et al., 2005). This score is designed for use at clinical visit evaluations of oral cGVHD or for baseline or calendar-driven documentation in clinical trials, and is designed for the transplant practitioner’s ease and practicality of use. The scoring of oral cGVHD in this context is as a specific organ evaluation for a broader measure of global cGVHD extent and severity, contributing to a total cGVHD NIH severity score. Scoring does not distinguish between active disease and the consequence of previous disease activity, and is based on an evaluation of current status and not history of disease.
This is a 4-point scale (0–3), with 0 representing no mouth involvement and 3 reflecting severe symptoms, disease signs on examination, and major limitation of oral intake (Filipovich et al., 2005). These NIH Consensus recommendations were based solely on expert opinion, and evidence-based standardized criteria for the staging of oral cGVHD have yet to be defined.
The scoring of oral mucosal biopsy specimens to judge the severity of oral cGVHD is not currently possible, due to lack of necessary research in this area. To date, the pathology results of ‘consistent with’ oral cGVHD provide information to support the diagnosis of oral cGVHD, but not its staging or severity (Pavletic et al., 2006a).
Oral cGVHD Scoring for Research Use and Response Criteria
Quantitative measurements of oral cGVHD manifestations have been proposed to serve as more specific scoring in the context of treatment response and in clinical trials. This led to the development of the NIH Mouth cGVHD Activity Assessment Scale, an oral mucosal score of 0–15 (Figure 4a), which is a simplification and modification of the (Figure 4b) Schubert Oral Mucositis Rating Scale (OMRS, Schubert et al., 1992). There are 4 oral cGVHD manifestations assessed with the NIH Mouth cGVHD Activity Assessment Scale: 1) erythema (0–3, based on color intensity), 2) lichenoid-type hyperkeratosis (0–3, based on oral surface area affected), 3) ulcerations (0–6, based on oral surface area affected), and 4) presence of mucoceles (0–3, total number). Clinically meaningful oral cGVHD has been proposed to be a Score of 2 and above with a minimally detectable change of 2 points within the scale being clinically relevant (Mitchell et al., 2011, Fassil et al., 2012). Therefore, 0 represents no oral mucosal cGVHD activity and 15 shows very severe activity. This scale is meant for use primarily by transplant practitioners in clinical trials.
For even more specific or targeted oral cGVHD activity scoring, the OMRS may still be utilized, which scores 13 intraoral locations for erythema, lichenoid, hyperkeratosis, pseudomembrane, ulceration, atrophy, and edema on a 0–3 scale, making a scale from 0–273 (Figure 4b) (Schubert et al., 1992). This scale is recommended exclusively for the research setting and for exclusive use by dental or oral medicine specialists.
Patient reported symptoms of oral cGVHD are important pieces of information to be captured in the evaluation of a patient with cGVHD and are recommended to be documented separately (Pavletic et al., 2006b). For the mouth, three 0–10 scales, rated to measure the peak severity of the symptom within the last week, were proposed to interrogate: 1) dry mouth (subjective decrease in oral moistness), 2) mouth pain (mouth symptoms without stimulation), and 3) mouth sensitivity (mouth symptoms in the presence of normal stimulation, such as irritation when eating spicy foods or hot liquids) (Figure 2d). Here, 0 indicates no symptoms and 10 represents very severe symptoms. The NIH Mouth cGVHD Activity Scale and patient reported mouth symptom scales are to be measured at minimum every 3 months or more frequently whenever a major change is made in treatment regimen or response anticipated, to judge treatment response or longitudinal change.
Another instrument that complements scoring of cGVHD symptoms in general and mouth related symptoms in particular is the Lee Symptom Scale (Lee et al., 2002). This validated questionnaire reports on the degree of bother that a patient with cGVHD experienced within the last 4 weeks due to symptoms in 7 areas (skin, eyes and mouth, breathing, eating and digestion, muscles and joints, energy, and emotional distress). Mouth specific symptoms include questions on the need to avoid foods due to mouth pain, mouth ulcers, and difficulty swallowing food or liquids.
For the manifestations of cGVHD other than mucosal findings and patient-reported symptoms, further instruments targeted to the finding can be used. For limited mouth opening secondary to oral sclerosis, maximum mouth opening could be recorded. For xerostomia, a 5-minute saliva-flow test (either stimulated or unstimulated), can provide some quantitative measure of salivary dysfunction (Imanguli et al., 2010). These measures have not been validated for use in cGVHD care or research, and would be ancillary data for these cGVHD studies.
Finally, biopsy of oral tissue is a necessary step in oral cGVHD research, and collection of saliva may be of interest. The accessibility of oral tissue and oral fluids, without the need for significantly invasive procedures, allows for the mouth to be a unique environment to study end-organ cGVHD involvement (Imanguli et al., 2010, Imanguli et al., 2007). A mucosal biopsy of oral soft-tissue lesions, MSG biopsy in the context of salivary gland dysfunction, and oral fluid collection could be the constituents for much needed research on the diagnosis, pathogenesis, and therapeutics involved in cGVHD.
Squamous Cell Carcinomas
A major late complication of HSCT is the dramatically increased risk of secondary malignancies, with 2–6% of post-HSCT patients having developed a secondary solid tumor at 10 years (Mawardi et al., 2011). Squamous cell carcinomas (SSCs) of the skin and mouth account for about one-third of these secondary solid tumors, with oral SSCs making up about half of the SSC cases seen (Montebugnoli et al., 2011). Oral cGVHD is a significant risk factor for the development of oral SCC, with an analysis of the International Bone Marrow Transplant Registry in 1997 showing that a relative risk of 6.0 can be associated with oral cGVHD in the development of oral SCC after HSCT (Mawardi et al., 2011). Mechanisms proposed to explain this dramatically increased risk include radiation mutagenesis, cGVHD-related inflammation, prolonged immunosuppression from cGVHD therapy, immunologic dysfunction, and carcinogenic and cytotoxic medication effects (Demarosi et al., 2005, Curtis et al., 2005, Mawardi et al., 2011).
Mawardi et. al. recently described a group of post-HSCT patients who had developed either oral epithelial dysplasia, including verrucous hyperplasic hyperplasia (VH), or malignant oral lesions, including oral SCC and verrucous carcinoma (Mawardi et al., 2011). Of 26 patients identified, 3(12%) developed VH, 5(19%) developed dysplasia, and 19(69%) developed invasive carcinoma. Twenty-three (89%) of patients with these oral lesions had clinically significant oral cGVHD. VH was found on the gingiva, the hard palate, and the buccal mucosa, and always appeared as a white plaque (100%, leukoplakia or proliferative verrucous leukoplakia) clinically. Dysplasia was found on the lower lip predominately, as well as the tongue, and presented as white or red/white (leukoplakia or erythroleukoplakia) plaques (40%), ulcerations (40%), crusting (40%), or papillary lesions (40%). These oral epithelial dysplasias were mostly non-painful and asymptomatic. Invasive carcinomas were seen throughout the anatomical locations of the mouth with a varying clinical appearance (red, white, or red/white plaques (50%), exophytic (39%), ulceration (28%), erythema (17%), papillary (11%), or crusting (6%)). These carcinomas presented with pain (61%) and sometimes with paresthesia (11%) (Mawardi et al., 2011). CGVHD patients should be regularly screened for oral cancer, and any suspicious lesions should be biopsied to rule out dysplasia or malignancy.
Clinical Management
CGVHD patients have specialized oral health needs that can be addressed in a general dental practice setting by a well-educated clinician, and it is important that oral care is available for cGVHD patients as their numbers continue to increase. The NIH Consensus working group on ancillary therapy and supportive care for cGVHD has provided recommendations for the management of symptoms and guidelines for the prevention of infections and other common complications of treatment related to cGVHD (Couriel et al., 2006). This section will provide a brief overview of systemic therapy for cGVHD, but will focus on the nuances of treatment of oral cGVHD and its associated symptoms.
Goals of therapy for oral cGVHD patients include (1) management of mucosal disease (2) palliation of oral pain and (3) management of symptoms that impact quality of life including dry mouth. Early detection and diagnosis, appropriate therapeutic management and regular follow-up are essential to ensure optimal outcomes and improved quality of life of patients with oral cGVHD. Although oral cGVHD symptoms reduce quality of life and, in severe cases, lead to malnutrition, they are generally not life-threatening (Jacobsohn et al., 2002). Despite the direct and indirect impact of oral cGVHD on the well-being of those affected, a standard of care for the management of oral cGVHD has not been defined. Treatment, particularly any systemic therapy, should be coordinated in conjunction with the medical team.
CGVHD patients should be regularly screened for oral cancer, as oral squamous cell carcinoma has been reported in this population. Suspicious lesions should be biopsied. Patients on systemic or oral topical corticosteroids are prone to overgrowth of oral Candida, and it is important to differentiate cGVHD-related hyperkeratosis and erythema from an ongoing oral fungal infection before starting an aggressive course of treatment. Although cGVHD patients are regularly screened for serum antibodies to viruses, virus-related oral ulcers (such as HSV) may still occur in serum-negative or therapy-refractory patients, and PCR-based screening from an oral swab or biopsy is recommended when clinically indicated. Additional non-cGVHD causes of oral ulcers include neutropenia or chemotherapy associated ulcers that are closely associated with the early post-transplant period, and sirolimus-induced ulcers, which may occur whenever systemic sirolimus therapy is increased. CGVHD induces changes in both the quality and quantity of saliva, which leaves patients susceptible to dental decay (Castellarin et al., 2012). Excellent professional and home oral hygiene, use of supplemental fluoride and mild dentifrice are strongly indicated in cGVHD patients. Patients should be counseled on use of sugar-free beverages, sialagogues, and saliva substitute products.
Systemic Therapy
Therapy for cGVHD is a challenging area, as progress is slow and there is no good prevention. Systemic therapy is usually initiated to manage more than mild cGVHD (more than two organs involved or any organ score of 2 or more) and typically includes prednisone with or without a calcineurin inhibitor (cyclosporine or tacrolimus) (Koc et al., 2002, Sullivan et al., 1988, Couriel et al., 2006). About 50% of patients fail this front line therapy. There is no standard second line treatment, and numerous agents are used, most commonly mycophenolate, sirolimus, extracorporeal photopheresis or rituximab (Martin & Pavletic, 2009). The average length of immunosuppressive treatment for those afflicted by cGVHD is 2–3 years, therefore increasing the risk of avascular bone necrosis, steroid myopathy and other complications (Enright et al., 1990, Lee et al., 2006, Lee et al., 2004, Socie et al., 1997, Wingard et al., 2002).
Therapy for oral cGVHD
Management of oral cGVHD includes titration of effective systemic therapy, and may also require topical immunosuppressive treatment and ancillary supportive care. Oral cGVHD flares may occur during taper of systemic immunosuppression, when a patient goes off of systemic immunosuppression, or when drug levels are adjusted. For example, increase in blood levels of sirolimus are known to induce oral ulceration. Patients should be carefully monitored for oral lesions when these changes occur. Most therapy currently recommended for the management of mucosal manifestations of oral cGVHD is directed at the use of topical high and ultra-high potency corticosteroids (Table 2), calcineurin inhibitors and analgesics (Wolff et al., 2010, Couriel et al., 2006).
Table 2.
Oral Topical Therapy Recommendations for cGvHD
| Agent | Indications | |||||
|---|---|---|---|---|---|---|
| Oral Rinse | Corticosteroid | Oral Ulcers | Soft Tissue Sensitivity | Caries | Hyperkeratosis/Lichenoid | Candidiasis |
| Budesoinde | Yes | Yes | Yes | Yes | ||
| Chlorhexidine | Yes | |||||
| Clobetasol | Yes | Yes | Yes | Yes | ||
| Cyclosporine | Yes | Yes | Yes | |||
| Dexamethasone | Yes | Yes | Yes | Yes | ||
| Dyclonine | Yes | Yes | Yes | |||
| Lidobenalox (magic mouth wash) | only when formulated with dexamethasone | Yes | Yes | Yes | ||
| Nystatin | Yes | |||||
| Sodium Fluoride rinse | Yes | |||||
| Tacrolimus | Yes | Yes | Yes | |||
| Other Topicals | ||||||
| Azathioprine Ointment | Yes | |||||
| Clobetasol Ointment | Yes | Yes | ||||
| Clotrimazole Troches | Yes | |||||
| Cyclosporine Ointment | Yes | |||||
| Fluoride Gel | Yes | |||||
| Thalidomide Ointment | Yes | Yes | ||||
These topical treatments are not always effective, and also carry the risk of systemic absorption due to a breakdown in mucosal integrity in oral cGVHD patients. Efficacy may be improved by compounding some of the topical agents to an oral rinse or oral adhesive formulation; however, clinical trials are lacking to support specific agents and dosing schedules.
Management of mucosal disease has relied heavily on oral rinses mostly due to the ease and effectiveness of this delivery mode. Several oral rinses are commonly used and may be titrated with systemic medications. Budesonide and dexamethasone elixirs are corticosteroid rinses that can be used to help alleviative symptoms from oral ulcers, soft tissue sensitivity and hyperkeratotic/lichenoid reactions. Corticosteroid topical therapy for the oral cavity may thin the oral mucosal over time, leading to increased oral sensitivity. Additionally, even short-term local corticosteroid therapy may lead to overgrowth of oral yeast species. It is generally recommended that patients be treated prophylactically with an anti-fungal rinse or troche, in addition to any systemic anti-fungal treatment, while on oral topical steroid treatments (Couriel et al., 2006, Wolff et al., 2004). An additional concern with corticosteroid topical therapy is the risk of systemic absorption. Steroids are lipophilic drugs, easily able to transverse the oral mucosa, and, in severe oral cGVHD, there is also breakdown in the integrity of the mucosal barrier. Patients should be monitored for increased adrenal suppression and cushingoid features, and topical treatments should be adjusted as appropriate if such symptoms occur.
Directed topical therapies for the oral cavity have been designed using oral rinseformulations of systemic immunosuppressive medications. Many of these therapies were originally designed and tested for oral lichen planus, which approximates many clinical features of oral cGVHD (Eisen & Ellis, 1990, Feliciani & Tulli, 2002, Byrd et al., 2004). The efficacy of various rinses has been evaluated in limited clinical trials with mixed outcomes for cGVHD patients (Wolff et al., 2004, Elad et al., 2012, Utsman et al., 2008). Topical cyclosporine rinse was evaluated in a limited study (n=11) in patients with oral GVHD refractory to dexamethasone rinse and mucosal disease was reduced in 64% of treated patients (Epstein & Reece, 1994). Byrd et al also reported success with topical tacrolimus used in patients with oral lichen planus (Byrd et al., 2004).
Ointments, another topical delivery mechanism, can be extremely effective in cases of patients with isolated symptoms of oral cGVHD in which ointments may be used as ‘spot treatment.’ Current topical ointments include tacrolimus, azathioprine, cyclosporine, and thalidomide. However, the application of ointment or cream in the moist environment of the oral cavity is problematic. Much of the drug is washed away, despite the best effort of the patient, resulting in reduced contact time of the mucosal surfaces with the therapeutic agent, and increased systemic exposure to intended topical treatment once the drug is swallowed.
Local phototherapy has gained some traction as an adjunct form of oral treatment as it continues to elicit positive results in certain dermatological cGVHD cases. For oral PUVA treatment, the patient is first administered an oral tablet of 8-methoxypsoralen, which sensitizes the oral mucosa to UV exposure. The 8-methoxypsoralen crosslinks cellular DNA with exposure to UV light, then the cell becomes apoptotic (Imanguli et al., 2006, Wolff et al., 2004).
One of the most common complaints from patients who have oral cGVHD is that of oral pain or soft tissue sensitivity. Even though there are many formulations of lidobenalox (“magic mouthwash”), the principal three ingredients remain the same: a local anesthetic (lidocaine), an antihistamine (Benadryl), in an aluminum/magnesium hydroxide (Maalox®) base to coat the oral cavity. Other formulations of this compound rinse that could be beneficial for cGVHD patients include antifungal and corticosteroid ingredients. For cases of severe and intolerable pain, liquid dyclonine (usually 0.5%), a strong topical anesthetic, can be given to patients for palliation of pain, especially when eating. Long-acting or short-acting pre-meal narcotics may also be used for this purpose to allow for adequate nutrition though food intake.
Chlorhexidine oral rinse is a mouth rinse with strong bactericidal properties. The alcohol-free version can be effective in control of oral bacterial flora in patients for whom oral hygiene (brushing and flossing) is painful, or in patients with xerostomia who are especially susceptible to dental decay. Adjunct use of topical fluorides is also recommended in this patient group. Studies have shown that the character of saliva is altered in cGVHD patients, and may not be able to adequately remineralize and clean teeth (Castellarin et al., 2012). Careful attention should be paid to oral hygiene measures and fluoride supplementation in oral cGVHD patients.
Several studies support the use of short-term or prolonged pilocarpine therapy for salivary stimulation in cGVHD patients, however, pilocarpine also increases secretion of gastric fluids, which may be problematic in patients with GI tract GVHD (Nagler & Nagler, 1999, Singhal et al., 1997). Cevimeline, a selective agonist of M1 and M3 cholinergic muscarinic receptors, is also approved for xerostomia therapy in Sjögren’s Syndrome. A small case-series (n=3) reported improvement in cGVHD patient-perceived xerostomia after 10 weeks of cevimeline treatment (Carpenter et al., 2006).
Ongoing Research
In addition to ongoing research to better understand the etiology and pathophysiology of oral cGVHD, there are 4 ongoing clinical trials for the treatment of oral cGVHD registered with clinicaltrials.gov. Each of the trials is investigating different steroidal (budesonide, clobetasol, dexamethasone), or non-steroidal (tacrolimus) oral rinse agents designed for local treatment of the oral cavity. The oral rinse formulation of these agents allows for better coverage of all areas of the oral cavity than does off-label application of the agents in cream or ointment form.
These therapies are focused on treatment of mucosal manifestations of oral cGVHD, and may not affect disease within the salivary glands. Treatment of salivary gland cGVHD specifically has been limited to palliative care measures: use of moisturizing rinses (Biotene) and sialagoges. Although pharmacologic agents including pilocarpine and cevimeline may be helpful to alleviate xerostomia in cGVHD patients, they are understudied in this population. Alternative medicine therapies including acupuncture and acupressure have shown promise in individual cases of cGVHD-related xerostomia, however, controlled studies are absent in this area.
Early detection and diagnosis, appropriate therapeutic management and regular follow-up are essential to insure optimal outcomes and improved quality of life of patients with oral CGHVD. As no evidence-based standards of care exist, patients should be enrolled in clinical trials whenever possible.
Table 3.
Registered Topical Therapy Trials for Oral cGvHD
| Trial | Center | ClinicalTrials.gov Identifier | Agent | Design | Premise | Target Population |
|---|---|---|---|---|---|---|
| Dexamethasone to Prevent Oral cGVHD | National Institutes of Health, NHLBI, Bethesda, MD | NCT00391170 | topical dexamethasone 0.01% oral rinse | Randomized double blind | Prevention of cGvHD | 70–90 d. post transplant, no oral cGVHD |
| Topical Dexamethasone and Tacrolimus for the Treatment of Oral cGVHD | Brigham and Women’s Hospital, Boston, MA | NCT00686855 | tacrolimus oral rinse or dexamethasone oral rinse | Open label, randomized | Efficacy of Treatment for oral GvHD | Post transplant with mild to moderate oral cGvHD sympotoms |
| Efficacy and Safety Study of Budesonide to Treat Oral cGvHD | University of Regensburg, Regensberg, Germany; The Hebrew University, Jerusalem, Israel | NCT00887263 | Budesonide 3 mg Effervescent Tablet | Randomized double blind | Efficacy of Treatment for oral GvHD | Post transplant with mild to severe oral cGvHD sympotoms |
| Clobetasol for Oral GVHD | National Institutes of Health, NCI, Bethesda, MD | NCT01557517 | topical clobetasol 0.05% oral rinse | Randomized double blind | Efficacy of Treatment for oral GvHD | Post transplant with moderate to severe oral cGvHD sympotoms |
Information was extracted from www.clinicaltrials.gov using search terms “oral “ and “GVHD.” Current as of August 27, 2012
Acknowledgments
The authors thank Dr. Francis Hakim for insightful discussion and suggestions regarding this manuscript. We are extraordinarily grateful to the patients and their families for their participation in these studies, and thank the members of the NIH Study Group for continual discussion and collaboration with this patient group. This work was supported by the Intramural Research Program of the National Institutes of Health through the National Institute for Dental and Craniofacial Research and the National Cancer Institute, Center for Cancer Research. H.F. was supported through the Clinical Research Training Program, a public-private partnership supported jointly by the NIH and Pfizer Inc. (via a grant to the Foundation for NIH from Pfizer Inc.).
References
- Aldinucci A, Rizzetto L, Pieri L, Nosi D, Romagnoli P, Biagioli T, Mazzanti B, Saccardi R, Beltrame L, Massacesi L, Cavalieri D, Ballerini C. Inhibition of immune synapse by altered dendritic cell actin distribution: a new pathway of mesenchymal stem cell immune regulation. J Immunol. 2010;185:5102–10. doi: 10.4049/jimmunol.1001332. [DOI] [PubMed] [Google Scholar]
- Apperley JF, Mauro FR, Goldman JM, Gregory W, Arthur CK, Hows J, Arcese W, Papa G, Mandelli F, Wardle D, et al. Bone marrow transplantation for chronic myeloid leukaemia in first chronic phase: importance of a graft-versus-leukaemia effect. Br J Haematol. 1988;69:239–45. doi: 10.1111/j.1365-2141.1988.tb07628.x. [DOI] [PubMed] [Google Scholar]
- Arora M, Klein JP, Weisdorf DJ, Hassebroek A, Flowers ME, Cutler CS, Urbano-Ispizua A, Antin JH, Bolwell BJ, Boyiadzis M, Cahn JY, Cairo MS, Isola L, Jacobsohn DA, Jagasia M, Klumpp TR, Lee SJ, Petersdorf EW, Santarone S, Gale RP, Schouten HC, Spellman S, Wingard JR, Horowitz MM, Pavletic SZ. Chronic GVHD risk score: a Center for International Blood and Marrow Transplant Research analysis. Blood. 2011;117:6714–20. doi: 10.1182/blood-2010-12-323824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird K, Pavletic SZ. Chronic graft versus host disease. Current Opinion in Hematology. 2006;13:426–35. doi: 10.1097/01.moh.0000245689.47333.ff. [DOI] [PubMed] [Google Scholar]
- Barnes DW, Loutit JF, Micklem HS. “Secondary disease” of radiation chimeras: a syndrome due to lymphoid aplasia. Ann N Y Acad Sci. 1962;99:374–85. doi: 10.1111/j.1749-6632.1962.tb45321.x. [DOI] [PubMed] [Google Scholar]
- Baron F, Storb R. Mesenchymal stromal cells: a new tool against graft-versus-host disease? Biol Blood Marrow Transplant. 2012;18:822–40. doi: 10.1016/j.bbmt.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassim C, Ambatipudi K, Mays J, Edwards D, Swatkoski S, Fassil H, Baird K, Gucek M, Melvin J, Pavletic S. Quantitative salivary proteomic differences in oral chronic graft-versus-host disease. J Clin Immunol. 2012 Jul 18;2012 doi: 10.1007/s10875-012-9738-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum BJ, Yates JR, 3rd, Srivastava S, Wong DT, Melvin JE. Scientific frontiers: emerging technologies for salivary diagnostics. Adv Dent Res. 2011;23:360–8. doi: 10.1177/0022034511420433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingham RE. The biology of graft-versus-host reactions. Harvey Lect. 1966;62:21–78. [PubMed] [Google Scholar]
- Biomarkers Definitions WG. Biomarkers definitions working group. Biomarkers and surrogate endpoints: prefered definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12:443–58. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones J, Novelli S, Sierra J. T-cell costimulatory molecules in acute-graft-versus host disease: therapeutic implications. Bone Marrow Res. 2011;2011:976793. doi: 10.1155/2011/976793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–90. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- Byrd JA, Davis MD, Bruce AJ, Drage LA, Rogers RS., 3rd Response of oral lichen planus to topical tacrolimus in 37 patients. Archives of dermatology. 2004;140:1508–12. doi: 10.1001/archderm.140.12.1508. [DOI] [PubMed] [Google Scholar]
- Carpenter PA, Schubert MM, Flowers ME. Cevimeline reduced mouth dryness and increased salivary flow in patients with xerostomia complicating chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2006;12:792–4. doi: 10.1016/j.bbmt.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Castellarin P, Stevenson K, Biasotto M, Yuan A, Woo SB, Treister NS. Extensive Dental Caries in Patients with Oral Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2012 Apr 16;2012 doi: 10.1016/j.bbmt.2012.04.009. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Chu YW, Gress RE. Murine models of chronic graft-versus-host disease: insights and unresolved issues. Biol Blood Marrow Transplant. 2008;14:365–78. doi: 10.1016/j.bbmt.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–26. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- Couriel D, Carpenter PA, Cutler C, Bolanos-Meade J, Treister NS, Gea-Banacloche J, Shaughnessy P, Hymes S, Kim S, Wayne AS, Chien JW, Neumann J, Mitchell S, Syrjala K, Moravec CK, Abramovitz L, Liebermann J, Berger A, Gerber L, Schubert M, Filipovich AH, Weisdorf D, Schubert MM, Shulman H, Schultz K, Mittelman B, Pavletic S, Vogelsang GB, Martin PJ, Lee SJ, Flowers ME. Ancillary therapy and supportive care of chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2006;12:375–96. doi: 10.1016/j.bbmt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Curtis R, Metayer C, Rizzo J, Socié G, Sobocinski K, Flowers M, Travis W, Travis L, Horowitz M, Deeg H. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood. 2005;105:3802–3811. doi: 10.1182/blood-2004-09-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler C, Miklos D, Kim HT, Treister N, Woo SB, Bienfang D, Klickstein LB, Levin J, Miller K, Reynolds C, Macdonell R, Pasek M, Lee SJ, Ho V, Soiffer R, Antin JH, Ritz J, Alyea E. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108:756–62. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarosi F, Lodi G, Carrassi A, Soligo D, Sardella A. Oral malignancies following HSCT: graft versus host disease and other risk factors. Oral Oncol. 2005;41:865–77. doi: 10.1016/j.oraloncology.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Eisen D, Ellis CN. Topical cyclosporine for oral mucosal disorders. Journal of the American Academy of Dermatology. 1990;23:1259–63. discussion 1263–4. [PubMed] [Google Scholar]
- Elad S, Zeevi I, Finke J, Koldehoff M, Schwerdtfeger R, Wolff D, Mohrbacher R, Levitt M, Greinwald R, Shapira MY. Improvement in oral chronic graft-versus-host disease with the administration of effervescent tablets of topical budesonide-an open, randomized, multicenter study. Biol Blood Marrow Transplant. 2012;18:134–40. doi: 10.1016/j.bbmt.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Enright H, Haake R, Weisdorf D. Avascular necrosis of bone: a common serious complication of allogeneic bone marrow transplantation. Am J Med. 1990;89:733–8. doi: 10.1016/0002-9343(90)90214-x. [DOI] [PubMed] [Google Scholar]
- Epstein JB, Reece DE. Topical cyclosporin A for treatment of oral chronic graft-versus-host disease. Bone Marrow Transplantation. 1994;13:81–6. [PubMed] [Google Scholar]
- Fall-Dickson JM, Mitchell SA, Marden S, Ramsay ES, Guadagnini JP, Wu TX, John LS, Pavletic SZ, Hlth NI. Oral Symptom Intensity, Health-Related Quality of Life, and Correlative Salivary Cytokines in Adult Survivors of Hematopoietic Stem Cell Transplantation with Oral Chronic Graft-versus-Host Disease. Biology of Blood and Marrow Transplantation. 2010;16:948–956. doi: 10.1016/j.bbmt.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassil H, Bassim CW, Mays J, Edwards D, Baird K, Steinberg SM, Williams KM, Cowen EW, Mitchell SA, Hakim FT, Taylor T, Avila D, Zhang D, Grkovic L, Datiles M, Gress RE, Pavletic SZ. Oral Chronic Graft-vs.-Host Disease Characterization Using the NIH Scale. Journal of Dental Research. 2012;91:S45–S51. doi: 10.1177/0022034512450881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciani C, Tulli A. Topical cyclosporin in the treatment of dermatologic diseases. Int J Immunopathol Pharmacol. 2002;15:89–93. doi: 10.1177/039463200201500203. [DOI] [PubMed] [Google Scholar]
- Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D, Cowen EW, Dinndorf P, Farrell A, Hartzman R, Henslee-Downey J, Jacobsohn D, McDonald G, Mittleman B, Rizzo JD, Robinson M, Schubert M, Schultz K, Shulman H, Turner M, Vogelsang G, Flowers ME. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Flowers ME, Inamoto Y, Carpenter PA, Lee SJ, Kiem HP, Petersdorf EW, Pereira SE, Nash RA, Mielcarek M, Fero ML, Warren EH, Sanders JE, Storb RF, Appelbaum FR, Storer BE, Martin PJ. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117:3214–9. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Cuvelier G, She K, Aslanian S, Shimizu H, Kariminia A, Krailo M, Chen Z, McMaster R, Bergman A, Goldman F, Grupp SA, Wall DA, Gilman AL, Schultz KR. Biomarkers in newly diagnosed pediatric-extensive chronic graft-versus-host disease: a report from the Children’s Oncology Group. Blood. 2008;111:3276–85. doi: 10.1182/blood-2007-08-106286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garming-Legert K, Remberger M, Ringden O, Hassan M, Dahllof G. Long-term salivary function after conditioning with busulfan, fractionated or single-dose TBI. Oral Dis. 2011;17:670–6. doi: 10.1111/j.1601-0825.2011.01821.x. [DOI] [PubMed] [Google Scholar]
- Ge X, Brown J, Sykes M, Boussiotis VA. CD134-allodepletion allows selective elimination of alloreactive human T cells without loss of virus-specific and leukemia-specific effectors. Biol Blood Marrow Transplant. 2008;14:518–30. doi: 10.1016/j.bbmt.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goker H, Haznedaroglu IC, Chao NJ. Acute graft-vs-host disease: pathobiology and management. Exp Hematol. 2001;29:259–77. doi: 10.1016/s0301-472x(00)00677-9. [DOI] [PubMed] [Google Scholar]
- Grkovic L, Baird K, Steinberg S, Williams K, Pulanic D, Cowen E, Mitchell S, Hakim F, Martires K, Avila D, Aylor T, Salit R, Rowley S, Khang D, Fowler D, Bishop M, Gress R, Pavletic S. Clinical laboratory markers of inflammation as determinants of chronic graft-versus-host disease activity and NIH global severity. Leukemia. 2012;26(4):633–43. doi: 10.1038/leu.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan BM, Tabellini L, Storer B, Bumgarner TE, Astigarraga CC, Flowers ME, Lee SJ, Martin PJ, Warren EH, Hansen JA. Activation and expansion of CD8(+) T effector cells in patients with chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2011;17:1121–32. doi: 10.1016/j.bbmt.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurkocza B, Rezvani A, Storb RF. Allogeneic hematopoietic cell transplantation: the state of the art. Expert Rev Hematol. 2010;3:285–99. doi: 10.1586/ehm.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim F, Fowler DH, Shearer GM, Gress RE. Animal models of acute and chronic graft-versus-host disease. Curr Protoc Immunol. 2001;Chapter 4(Unit 4):3. doi: 10.1002/0471142735.im0403s27. [DOI] [PubMed] [Google Scholar]
- Hiroki A, Nakamura S, Shinohara M, Gondo H, Ohyama Y, Hayashi S, Harada M, Niho Y, Oka M. A comparison of glandular involvement between chronic graft-versus-host disease and Sjögren’s syndrome. Int J Oral Maxillofac Surg. 1996;25:298–307. doi: 10.1016/s0901-5027(06)80062-7. [DOI] [PubMed] [Google Scholar]
- Hiroki A, Nakamura S, Shinohara M, Oka M. Significance of oral examination in chronic graft-versus-host disease. J Oral Pathol Med. 1994;23:209–15. doi: 10.1111/j.1600-0714.1994.tb01115.x. [DOI] [PubMed] [Google Scholar]
- Horn TD, Rest EB, Mirenski Y, Corio RL, Zahurak ML, Vogelsang GB. The significance of oral mucosal and salivary gland pathology after allogeneic bone marrow transplantation. Arch Dermatol. 1995;131:964–5. doi: 10.1001/archderm.131.8.964. [DOI] [PubMed] [Google Scholar]
- Hull KM, Kerridge I, Schifter M. Long-term oral complications of allogeneic haematopoietic SCT. Bone Marrow Transplant. 2012;47(2):265–70. doi: 10.1038/bmt.2011.63. [DOI] [PubMed] [Google Scholar]
- Imanguli MM, Alevizos I, Brown R, Pavletic SZ, Atkinson JC. Oral graft-versus-host disease. Oral Diseases. 2008a;14:396–412. doi: 10.1111/j.1601-0825.2008.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanguli MM, Atkinson JC, Harvey KE, Hoehn GT, Ryu OH, Wu T, Kingman A, Barrett AJ, Bishop MR, Childs RW, Fowler DH, Pavletic SZ, Hart TC. Changes in salivary proteome following allogeneic hematopoietic stem cell transplantation. Exp Hematol. 2007;35:184–92. doi: 10.1016/j.exphem.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanguli MM, Atkinson JC, Mitchell SA, Avila DN, Bishop RJ, Cowen EW, Datiles MB, Hakim FT, Kleiner DE, Krumlauf MC, Pavletic SZ. Salivary gland involvement in chronic graft-versus-host disease: prevalence, clinical significance, and recommendations for evaluation. Biol Blood Marrow Transplant. 2010;16:1362–9. doi: 10.1016/j.bbmt.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanguli MM, Pavletic SZ, Guadagnini JP, Brahim JS, Atkinson JC. Chronic graft versus host disease of oral mucosa: review of available therapies. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:175–83. doi: 10.1016/j.tripleo.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Imanguli MM, Swaim WD, League SC, Gress RE, Pavletic SZ, Hakim FT. Increased T-bet+ cytotoxic effectors and type I interferon-mediated processes in chronic graft-versus-host disease of the oral mucosa. Blood. 2009;113:3620–30. doi: 10.1182/blood-2008-07-168351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsohn D, Margolis J, Doherty J, Anders V, Vogelsang G. Weight loss and malnutrition in patients with chronic graft-versus-host disease. Bone Marrow Transplantation. 2002:231–236. doi: 10.1038/sj.bmt.1703352. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lee JW, Jung CW, Min CK, Cho B, Shin HJ, Chung JS, Kim H, Lee WS, Joo YD, Yang DH, Kook H, Kang HJ, Ahn HS, Yoon SS, Sohn SK, Min YH, Min WS, Park HS, Won JH. Weekly rituximab followed by monthly rituximab treatment for steroid-refractory chronic graft-versus-host disease: results from a prospective, multicenter, phase II study. Haematologica. 2010;95:1935–42. doi: 10.3324/haematol.2010.026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, Sanders JE, Witherspoon RP, Storb R, Appelbaum FR, Martin PJ. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100:48–51. doi: 10.1182/blood.v100.1.48. [DOI] [PubMed] [Google Scholar]
- Koenders MI, van den Berg WB. Translational mini-review series on Th17 cells: are T helper 17 cells really pathogenic in autoimmunity? Clin Exp Immunol. 2010;159:131–6. doi: 10.1111/j.1365-2249.2009.04039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP, 3rd, Armand P, Cutler C, Ho VT, Treister NS, Bienfang DC, Prasad S, Tzachanis D, Joyce RM, Avigan DE, Antin JH, Ritz J, Soiffer RJ. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365:2055–66. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama M, Hashimoto D, Aoyama K, Matsuoka K, Karube K, Niiro H, Harada M, Tanimoto M, Akashi K, Teshima T. Plasmacytoid dendritic cells prime alloreactive T cells to mediate graft-versus-host disease as antigen-presenting cells. Blood. 2009;113:2088–95. doi: 10.1182/blood-2008-07-168609. [DOI] [PubMed] [Google Scholar]
- Kuzmina Z, Greinix HT, Weigl R, Kormoczi U, Rottal A, Frantal S, Eder S, Pickl WF. Significant differences in B-cell subpopulations characterize patients with chronic graft-versus-host disease-associated dysgammaglobulinemia. Blood. 2011;117:2265–74. doi: 10.1182/blood-2010-07-295766. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–41. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Oran B, Saliba RM, Couriel DM, Shin K, Massey P, Neumann J, de Lima M, Champlin R, Giralt S. Steroid myopathy in patients with acute graft-versus-host disease treated with high-dose steroid therapy. Bone Marrow Transplantation. 2006;38:299–303. doi: 10.1038/sj.bmt.1705435. [DOI] [PubMed] [Google Scholar]
- Lee S, Cook EF, Soiffer R, Antin JH. Development and validation of a scale to measure symptoms of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8:444–52. doi: 10.1053/bbmt.2002.v8.pm12234170. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Zahrieh D, Agura E, MacMillan ML, Maziarz RT, McCarthy PL, Jr, Ho VT, Cutler C, Alyea EP, Antin JH, Soiffer RJ. Effect of up-front daclizumab when combined with steroids for the treatment of acute graft-versus-host disease: results of a randomized trial. Blood. 2004;104:1559–64. doi: 10.1182/blood-2004-03-0854. [DOI] [PubMed] [Google Scholar]
- Levine J, Paczesny S, Sarantopoulos S. Clinica applications for biomarkers of actute and chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2012;18:S116–S124. doi: 10.1016/j.bbmt.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HW, Sykes M. Emerging concepts in haematopoietic cell transplantation. Nat Rev Immunol. 2012;12:403–16. doi: 10.1038/nri3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YP, Paczesny S, Lauret E, Poirault S, Bordigoni P, Mekhloufi F, Hequet O, Bertrand Y, Ou-Yang JP, Stoltz JF, Miossec P, Eljaafari A. Human mesenchymal stem cells license adult CD34+ hemopoietic progenitor cells to differentiate into regulatory dendritic cells through activation of the Notch pathway. J Immunol. 2008;180:1598–608. doi: 10.4049/jimmunol.180.3.1598. [DOI] [PubMed] [Google Scholar]
- Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–8. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- Majhail NSME, Omondi NA, Robinett P, Gajewski JL, LeMaistre CF, Confer D, Rizzo JD. Allogeneic transplant physician and center capacity in the United States. Biol Blood Marrow Transplant. 2011;17:956–961. doi: 10.1016/j.bbmt.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PJ, Pavletic SZ. Biology and management of chronic graft-versus-host disease. Cancer Treat Res. 2009;144:277–98. doi: 10.1007/978-0-387-78580-6_12. [DOI] [PubMed] [Google Scholar]
- Martires KJ, Baird K, Steinberg SM, Grkovic L, Joe GO, Williams KM, Mitchell SA, Datiles M, Hakim FT, Pavletic SZ, Cowen EW. Sclerotic-type chronic GVHD of the skin: clinical risk factors, laboratory markers, and burden of disease. Blood. 2011;118:4250–7. doi: 10.1182/blood-2011-04-350249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews S, Kurien B, Scofield R. Oral manifestations of Sjogren’s syndrome. J Dent Res. 2008;87:308–18. doi: 10.1177/154405910808700411. [DOI] [PubMed] [Google Scholar]
- Matta BM, Castellaneta A, Thomson AW. Tolerogenic plasmacytoid DC. Eur J Immunol. 2010;40:2667–76. doi: 10.1002/eji.201040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawardi H, Elad S, Correa ME, Stevenson K, Woo SB, Almazrooa S, Haddad R, Antin JH, Soiffer R, Treister N. Oral epithelial dysplasia and squamous cell carcinoma following allogeneic hematopoietic stem cell transplantation: clinical presentation and treatment outcomes. Bone Marrow Transplant. 2011;46:884–91. doi: 10.1038/bmt.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JK, Wolff D, Pavletic S, Greinix H, Gosau M, Bertz H, Lee SJ, Lawitschka A, Elad S. Oral chronic graft-versus-host disease: report from the International Consensus Conference on clinical practice in cGVHD. Clin Oral Investig. 2011;15:127–39. doi: 10.1007/s00784-010-0450-6. [DOI] [PubMed] [Google Scholar]
- Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, Hochberg EP, Wu CJ, Alyea EP, Cutler C, Ho V, Soiffer RJ, Antin JH, Ritz J. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973–8. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SA, Jacobsohn D, Thormann Powers KE, Carpenter PA, Flowers ME, Cowen EW, Schubert M, Turner ML, Lee SJ, Martin P, Bishop MR, Baird K, Bolanos-Meade J, Boyd K, Fall-Dickson JM, Gerber LH, Guadagnini JP, Imanguli M, Krumlauf MC, Lawley L, Li L, Reeve BB, Clayton JA, Vogelsang GB, Pavletic SZ. A Multicenter Pilot Evaluation of the National Institutes of Health Chronic Graft-versus-Host Disease (cGVHD) Therapeutic Response Measures: Feasibility, Interrater Reliability, and Minimum Detectable Change. Biol Blood Marrow Transplant. 2011;17(11):1619–29. doi: 10.1016/j.bbmt.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y, Thoburn CJ, Bright EC, Arai S, Hess AD. Regulation of OX40 gene expression in graft-versus-host disease. Transplant Proc. 2005;37:57–61. doi: 10.1016/j.transproceed.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Montebugnoli L, Gissi DB, Marchetti C, Foschini MP. Multiple squamous cell carcinomas of the oral cavity in a young patient with graft-versus-host disease following allogenic bone marrow transplantation. Int J Oral Maxillofac Surg. 2011;40:556–8. doi: 10.1016/j.ijom.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Nagler RM, Nagler A. Pilocarpine hydrochloride relieves xerostomia in chronic graft-versus-host disease: a sialometrical study. Bone Marrow Transplant. 1999;23:1007–11. doi: 10.1038/sj.bmt.1701752. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Hiroki A, Shinohara M, Gondo H, Ohyama Y, Mouri T, Sasaki M, Shirasuna K, Harada M, Niho Y. Oral involvement in chronic graft-versus-host disease after allogeneic bone marrow transplantation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:556–63. doi: 10.1016/s1079-2104(96)80203-4. [DOI] [PubMed] [Google Scholar]
- Nakhleh RE, Miller W, Snover DC. Significance of mucosal vs salivary gland changes in lip biopsies in the diagnosis of chronic graft-vs-host disease. Arch Pathol Lab Med. 1989;113:932–4. [PubMed] [Google Scholar]
- Orti-Raduan ES, Nunes AJ, Oliveira DT, Lara VS, Taveira LA. Quantitative analysis of Langerhans’ cells in oral chronic graft-vs.-host disease. J Oral Pathol Med. 2009;38:132–7. doi: 10.1111/j.1600-0714.2008.00712.x. [DOI] [PubMed] [Google Scholar]
- Paczesny S, Hanauer D, Sun Y, Reddy P. New perspectives on the biology of acute GVHD. Bone Marrow Transplant. 2010;45:1–11. doi: 10.1038/bmt.2009.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paczesny S, Hanauer D, Sun Y, Reddy P. New perspectives on the biology of acute GVHD. Bone Marrow Transplant. 2010;45:1–11. doi: 10.1038/bmt.2009.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panoskaltsis-Mortari A, Tram KV, Price AP, Wendt CH, Blazar BR. A New Murine Model for Bronchiolitis Obliterans Post–Bone Marrow Transplant. American Journal of Respiratory and Critical Care Medicine. 2007;176:713–723. doi: 10.1164/rccm.200702-335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavletic SZ, Lee SJ, Socie G, Vogelsang G. Chronic graft-versus-host disease: implications of the National Institutes of Health consensus development project on criteria for clinical trials. Bone Marrow Transplant. 2006a;38:645–51. doi: 10.1038/sj.bmt.1705490. [DOI] [PubMed] [Google Scholar]
- Pavletic SZ, Martin P, Lee SJ, Mitchell S, Jacobsohn D, Cowen EW, Turner ML, Akpek G, Gilman A, McDonald G, Schubert M, Berger A, Bross P, Chien JW, Couriel D, Dunn JP, Fall-Dickson J, Farrell A, Flowers ME, Greinix H, Hirschfeld S, Gerber L, Kim S, Knobler R, Lachenbruch PA, Miller FW, Mittleman B, Papadopoulos E, Parsons SK, Przepiorka D, Robinson M, Ward M, Reeve B, Rider LG, Shulman H, Schultz KR, Weisdorf D, Vogelsang GB. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant. 2006b;12:252–66. doi: 10.1016/j.bbmt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Ratanatharathorn V, Pavletic S, Uberti JP. Clinical applications of rituximab in allogeneic stem cell transplantation: anti-tumor and immunomodulatory effects. Cancer Treat Rev. 2009;35:653–61. doi: 10.1016/j.ctrv.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Rozmus J, Schultz K. Biomarkers in chronic graft-versus-host disease. Expert Rev Hematol. 2011;4:329–342. doi: 10.1586/ehm.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantopoulos S, Stevenson KE, Kim HT, Bhuiya NS, Cutler CS, Soiffer RJ, Antin JH, Ritz J. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007;13:6107–14. doi: 10.1158/1078-0432.CCR-07-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantopoulos S, Stevenson KE, Kim HT, Cutler CS, Bhuiya NS, Schowalter M, Ho VT, Alyea EP, Koreth J, Blazar BR, Soiffer RJ, Antin JH, Ritz J. Altered B cell homeostasis and excess BAFF in human chronic graft versus host disease. Blood. 2009;113:3865–74. doi: 10.1182/blood-2008-09-177840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantopoulos S, Stevenson KE, Kim HT, Washel WS, Bhuiya NS, Cutler CS, Alyea EP, Ho VT, Soiffer RJ, Antin JH, Ritz J. Recovery of B-cell homeostasis after rituximab in chronic graft-versus-host disease. Blood. 2011;117:2275–83. doi: 10.1182/blood-2010-10-307819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–4. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- Schroeder MA, DiPersio JF. Mouse models of graft-versus-host disease: advances and limitations. Dis Model Mech. 2011;4:318–33. doi: 10.1242/dmm.006668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert MM, Correa ME. Oral graft-versus-host disease. Dent Clin North Am. 2008;52:79–109. viii–ix. doi: 10.1016/j.cden.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Schubert MM, Williams BE, Lloid ME, Donaldson G, Chapko MK. Clinical assessment scale for the rating of oral mucosal changes associated with bone marrow transplantation. Cancer. 1992;69:2469–2477. doi: 10.1002/1097-0142(19920515)69:10<2469::aid-cncr2820691015>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Schultz KR, Miklos DB, Fowler D, Cooke K, Shizuru J, Zorn E, Holler E, Ferrara J, Shulman H, Lee SJ, Martin P, Filipovich AH, Flowers ME, Weisdorf D, Couriel D, Lachenbruch PA, Mittleman B, Vogelsang GB, Pavletic SZ. Toward biomarkers for chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: III. Biomarker Working Group Report. Biol Blood Marrow Transplant. 2006;12:126–37. doi: 10.1016/j.bbmt.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Serody JS, Hill GR. The IL-17 differentiation pathway and its role in transplant outcome. Biol Blood Marrow Transplant. 2012;18:S56–61. doi: 10.1016/j.bbmt.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She K, Gilman AL, Aslanian S, Shimizu H, Krailo M, Chen Z, Reid GS, Wall D, Goldman F, Schultz KR. Altered Toll-like receptor 9 responses in circulating B cells at the onset of extensive chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13:386–97. doi: 10.1016/j.bbmt.2006.12.441. [DOI] [PubMed] [Google Scholar]
- Shulman HM, Kleiner D, Lee SJ, Morton T, Pavletic SZ, Farmer E, Moresi JM, Greenson J, Janin A, Martin PJ, McDonald G, Flowers ME, Turner M, Atkinson J, Lefkowitch J, Washington MK, Prieto VG, Kim SK, Argenyi Z, Diwan AH, Rashid A, Hiatt K, Couriel D, Schultz K, Hymes S, Vogelsang GB. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant. 2006;12:31–47. doi: 10.1016/j.bbmt.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Singhal S, Powles R, Treleaven J, Rattenbury H, Mehta J. Pilocarpine hydrochloride for symptomatic relief of xerostomia due to chronic graft-versus-host disease or total-body irradiation after bone-marrow transplantation for hematologic malignancies. Leuk Lymphoma. 1997;24:539–43. doi: 10.3109/10428199709055591. [DOI] [PubMed] [Google Scholar]
- Soares AB, Faria PR, Magna LA, Correa ME, de Sousa CA, Almeida OP, Cintra ML. Chronic GVHD in minor salivary glands and oral mucosa: histopathological and immunohistochemical evaluation of 25 patients. J Oral Pathol Med. 2005;34:368–73. doi: 10.1111/j.1600-0714.2005.00322.x. [DOI] [PubMed] [Google Scholar]
- Socie G, Cahn JY, Carmelo J, Vernant JP, Jouet JP, Ifrah N, Milpied N, Michallet M, Lioure B, Pico JL, Witz F, Molina L, Fischer A, Bardou VJ, Gluckman E, Reiffers J. Avascular necrosis of bone after allogeneic bone marrow transplantation: analysis of risk factors for 4388 patients by the Societe Francaise de Greffe de Moelle (SFGM) British journal of haematology. 1997;97:865–70. doi: 10.1046/j.1365-2141.1997.1262940.x. [DOI] [PubMed] [Google Scholar]
- Stenger EO, Turnquist HR, Mapara MY, Thomson AW. Dendritic cells and regulation of graft-versus-host disease and graft-versus-leukemia activity. Blood. 2012;119:5088–103. doi: 10.1182/blood-2011-11-364091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KM, Witherspoon RP, Storb R, Deeg HJ, Dahlberg S, Sanders JE, Appelbaum FR, Doney KC, Weiden P, Anasetti C, et al. Alternating-day cyclosporine and prednisone for treatment of high-risk chronic graft-v-host disease. Blood. 1988;72:555–61. [PubMed] [Google Scholar]
- Svegliati S, Olivieri A, Campelli N, Luchetti M, Poloni A, Trappolini S, Moroncini G, Bacigalupo A, Leoni P, Avvedimento EV, Gabrielli A. Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood. 2007;110:237–41. doi: 10.1182/blood-2007-01-071043. [DOI] [PubMed] [Google Scholar]
- Utsman RA, Epstein JB, Elad S. Budesonide for local therapy of complex oral mucosal immune-mediated inflammatory diseases: case reports. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:e11–7. doi: 10.1016/j.tripleo.2008.02.029. [DOI] [PubMed] [Google Scholar]
- Wenzel J, Lucas S, Zahn S, Mikus S, Metze D, Stander S, von Stebut E, Hillen U, Bieber T, Tuting T. CXCR3 <-> ligand-mediated skin inflammation in cutaneous lichenoid graft-versus-host disease. Journal of the American Academy of Dermatology. 2008;58:437–42. doi: 10.1016/j.jaad.2007.10.647. [DOI] [PubMed] [Google Scholar]
- Wingard JR, Vogelsang GB, Deeg HJ. Stem cell transplantation: supportive care and long-term complications. Hematology Am Soc Hematol Educ Program. 2002:422–44. doi: 10.1182/asheducation-2002.1.422. [DOI] [PubMed] [Google Scholar]
- Wolff D, Anders V, Corio R, Horn T, Morison WL, Farmer E, Vogelsang GB. Oral PUVA and topical steroids for treatment of oral manifestations of chronic graft-vs.-host disease. Photodermatol Photoimmunol Photomed. 2004;20:184–90. doi: 10.1111/j.1600-0781.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- Wolff D, Gerbitz A, Ayuk F, Kiani A, Hildebrandt GC, Vogelsang GB, Elad S, Lawitschka A, Socie G, Pavletic SZ, Holler E, Greinix H. Consensus conference on clinical practice in chronic graft-versus-host disease (GVHD): first-line and topical treatment of chronic GVHD. Biol Blood Marrow Transplant. 2010;16:1611–28. doi: 10.1016/j.bbmt.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Woo SB, Lee SJ, Schubert MM. Graft-vs.-host disease. Crit Rev Oral Biol Med. 1997;8:201–216. doi: 10.1177/10454411970080020701. [DOI] [PubMed] [Google Scholar]
- Yi T, Chen Y, Wang L, Du G, Huang D, Zhao D, Johnston H, Young J, Todorov I, Umetsu DT, Chen L, Iwakura Y, Kandeel F, Forman S, Zeng D. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease. Blood. 2009;114:3101–12. doi: 10.1182/blood-2009-05-219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Merad M, Hart DN. Dendritic cells in transplantation and immune-based therapies. Biol Blood Marrow Transplant. 2007;13:23–32. doi: 10.1016/j.bbmt.2006.10.023. [DOI] [PubMed] [Google Scholar]