Abstract
Mutations in mitochondrial DNA (mtDNA) are associated with serious human diseases and inherited from mother's eggs. Here we investigated the feasibility of mtDNA replacement in human oocytes by spindle transfer (ST). Of 106 human oocytes donated for research, 65 were subjected to reciprocal ST and 33 served as controls. Fertilization rate in ST oocytes (73%) was similar to controls (75%). However, a significant portion of ST zygotes (52%) displayed abnormal fertilization as determined by irregular number of pronuclei. Among normally fertilized ST zygotes, blastocyst development (62%) and embryonic stem cell (ESC) isolation (38%) rates were comparable to controls. All ESC lines derived from ST zygotes displayed normal euploid karyotypes and contained exclusively donor mtDNA. The mtDNA can be efficiently replaced in human oocytes. Although some ST oocytes displayed abnormal fertilization, remaining embryos were capable of developing to blastocysts and producing ESCs similar to controls.
MtDNA is localized in the cell's cytoplasm as opposed to the chromosomal genes confined to the nucleus. Each cell may have thousands of mtDNA copies and all copies could be mutated (homoplasmy) or exist as a mixture (heteroplasmy). The clinical manifestations of mtDNA diseases vary, but often affect organs and tissues with the highest energy requirements including the brain, heart, muscle, pancreas and kidney1. The expression and severity of disease symptoms depends on the specific mutation and heteroplasmy levels1.
An estimated prevalence of inherited mtDNA diseases is 1 in every 5,000-10,000 live births, suggesting that, in USA alone, between 1,000 to 4,000 children are born every year with mtDNA diseases2,3. Based on other estimates, the frequency of pathogenic mtDNA mutations is even higher - 1 in 200 children inherit mutations4. However, not all these children develop the disease at the birth, because mtDNA mutations are present at low heteroplasmy levels.
At present, there are no cures for mitochondrial disorders and available treatments only alleviate symptoms and delay disease progression. Therefore, several strategies for preventing transmission of mtDNA mutations from mothers to their children have been actively pursued.
One approach is to completely replace the mutated mtDNA of a patient's oocyte with the healthy mitochondrial genome from an oocyte donated by another women using ST5. The technique isolates and transplants the chromosomes (nuclear genetic material) from a patient's unfertilized oocyte into the cytoplasm of another enucleated egg, containing healthy mtDNA as well as other organelles, RNA and proteins. A child born after ST procedure will be the genetic child of the patient but carry healthy mitochondrial genes from the donor. Our prior studies in a monkey model demonstrated not only the feasibility, but also that ST is highly effective and compatible with normal fertilization and birth of healthy offspring6. This strategy has been considered clinically to be a highly important future gene therapy to avoid transmission of serious mitochondrial diseases (http://www.hfea.gov.uk/6372.html).
Here we present a comprehensive study demonstrating the feasibility and outcomes of ST with human oocytes donated by healthy volunteers. To measure success, we fertilized reconstructed oocytes in vitro and assessed the normalcy of fertilization and embryo development to blastocysts. In addition, we derived ESCs and carried out detailed genetic analyses to assess efficacy of gene replacement and possible chromosomal abnormalities associated with ST. We also conducted additional studies in a rhesus macaque model to investigate the feasibility of using cryopreserved oocytes for ST and postnatal development of ST offspring.
Mitochondrial DNA replacement in human oocytes
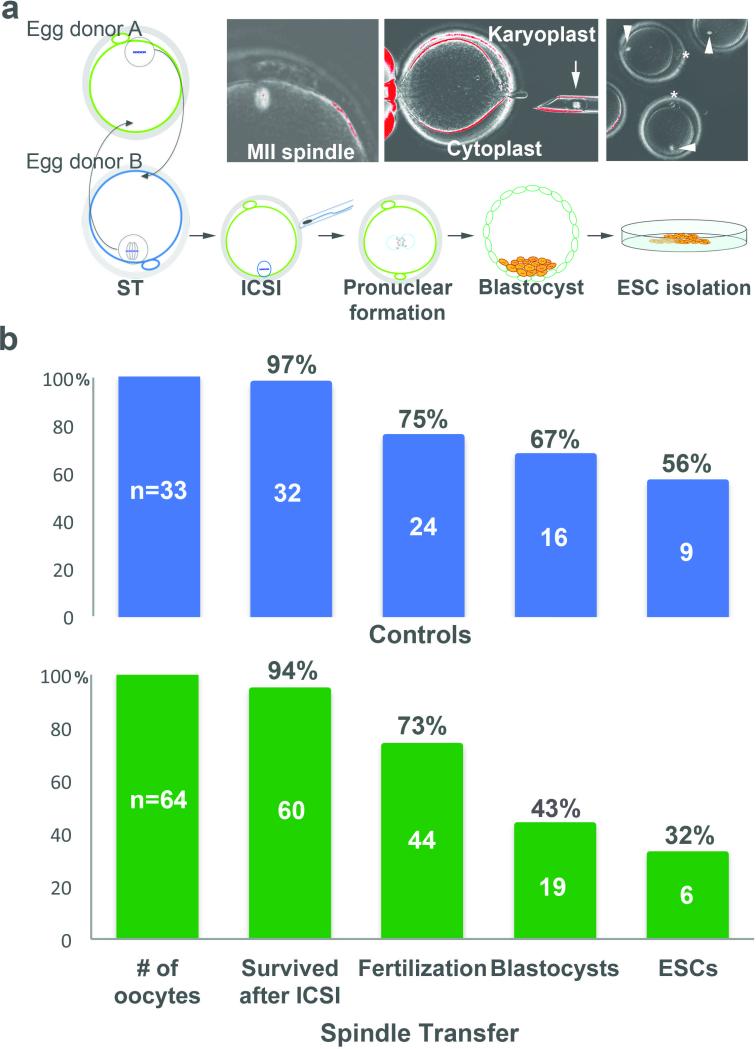
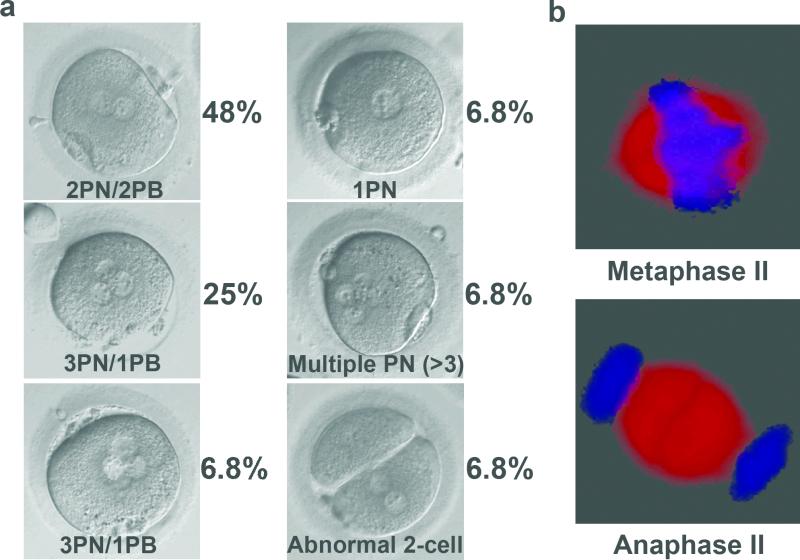
Seven volunteers (age 21-32 years) underwent ovarian stimulation and a total of 106 mature MII oocytes were retrieved (range of 7–28, or a mean of 15 oocytes per donor cycle). Participants were synchronized in 3 separate experiments, so that at least two fresh oocyte cohorts were available on the same day for reciprocal ST. We selected a total of 65 MII oocytes for the ST procedure and 33 served as non-manipulated controls (Fig. 1a). We successfully transferred the spindle surrounded by a membrane and small amount of cytoplasm (karyoplast), between 64 oocytes (98%; Fig. 1a, b). Sixty oocytes survived fertilization by intracytoplasmic sperm injection (ICSI; 94%) and 44 formed visible pronuclei (73%). These outcomes were similar to controls, where 32 oocytes survived ICSI (97%) and 24 (75%) formed pronuclei (Fig. 1b). Microscopic evaluations determined that almost half of ST zygotes (21/44, 48%) contained normal 2 pronuclei and 2 polar bodies (2PN/2PB) (Fig. 2a). However, the remaining ST zygotes displayed an irregular number of pronuclei and/or polar bodies (Fig. 2a). Abnormal fertilization was also observed in the intact control group, albeit at a lower incidence (3/24, 13%).
Figure 1. Experimental design and main outcomes after ST with human oocytes.
a, Oocytes were retrieved from two unrelated donors and spindle-chromosomal complexes were reciprocally exchanged. Reconstructed oocytes were fertilized by ICSI and monitored for in vitro development to blastocysts and ESCs. Images in boxes depict a human mature MII oocyte with the spindle visualized under polarized microscope (left), isolated cytoplast and karyoplast (middle) and intact spindles inside recipient cytoplasts after transfers (right). Original magnifications: left and middle, x200; right, x100. b, Experimental outcomes after ST in human oocytes. The top and bottom graphs represent fertilization, blastocyst and ESC isolation rates for intact control and ST embryos, respectively. No statistical differences were found between ST and controls in survival after ICSI, fertilization, blastocyst development and ESC derivation rates (P>0.05).
Figure 2. Abnormal pronuclear formation and spindle morphology in human ST zygotes.
a, Proportion of normally fertilized zygotes with 2 pronuclei and 2 polar bodies (2PN/2PB) vs. abnormal zygotes (3PN/1PB; 3PN/2PB; 1PN; multiple PN and 2-cell) after ST. b, Integrity of meiotic spindles in human ST oocytes depicting normal metaphase II (top), and premature progression to the anaphase II (bottom).
Blastocyst formation rate in the normally fertilized ST group (13/21, 62%) was statistically similar to controls (16/21, 76%). However, the majority of abnormal ST zygotes arrested with only 26% (6/23) reaching blastocysts (Suppl. Table 1). Interestingly, blastocyst development of 3PN/1PB zygotes was noticeably higher (4/11, 36%) compared to other abnormally fertilized ST groups (Suppl. Table 1).
Derivation and genetic analysis of human ESCs
To provide additional insights into the developmental competence of ST-produced human embryos and to obtain sufficient material for molecular and cytogenetic analyses, we derived ESCs from blastocysts. Nine ESC lines (hESO lines) were established from 16 control blastocysts (56%; Fig. 1b). This ESC derivation rate is significantly higher than currently reported for embryos donated by IVF patients7. Similarly, 13 ST blastocysts developed from normally fertilized zygotes produced 5 ESC lines (38%; hESO-ST lines). We also plated 4 ST blastocysts that originated from 3PN/1PB zygotes and derived one ESC line (25%; Suppl. Table 1).
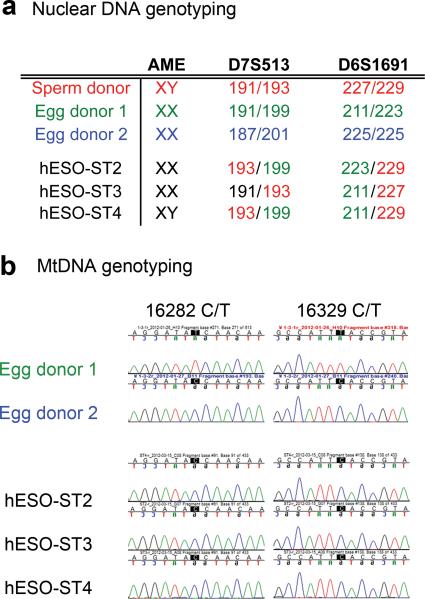
The ESCs derived from the ST embryos displayed normal morphology and were indistinguishable from controls (Suppl. Fig. 1a, b). All ESCs expressed standard pluripotency markers, including OCT-4, SOX2, SSEA-4, TRA-1-81 and TRA-1-60 (Suppl. Fig. 1a, b). Following injection into immunodeficient mice, experimental ESCs formed teratoma tumors consisting of cells and tissues representing all three germ layers (Suppl. Fig. 1c). Detailed analysis of nuclear DNA employing microsatellite markers confirmed that all hESO-ST lines inherited their chromosomes from the spindle donor oocytes (Fig. 3a, Table 1, Suppl. Fig. 2a, d). Analysis of mtDNA confirmed that all hESO-ST cell lines derived their mtDNA from the cytoplast donors (Fig. 3b, Table 1, Suppl. Fig. 2b,e, and 3)6,8.
Figure 3. Genetic analysis of ESCs derived from human ST embryos.
a, Nuclear DNA origin of hESO-ST2, -ST3 and -ST4 determined by microsatellite parentage analysis. The microsatellite markers for D7S513 and D6S1691 loci demonstrate that the nuclear DNA in these ESC lines was from the egg donor 1 (the spindle donor). b, mtDNA genotyping by direct sequencing show that the mtDNA in hESO-ST2, -ST3 and -ST4 is originated from the egg donor 2.
Table 1.
Genetic analysis of human ESCs derived from ST blastocysts
| Cell line | hESO-ST-2 | hESO-ST-3 | hESO-ST-4 | hESO-ST-5 | hESO-ST-6 | hESO-ST-7 |
|---|---|---|---|---|---|---|
| Nuclear donor | Donor 1 | Donor 1 | Donor 1 | Donor 4 | Donor 3 | Donor 6 |
| Cytoplast donor | Donor 2 | Donor 2 | Donor 2 | Donor 3 | Donor 4 | Donor 7 |
| Fertilization | 2PN/2PB | 2PN/2PB | 2PN/2PB | 2PN/2PB | 3PN/1PB | 2PN/2PB |
| Karyotype (passage no.) | 46 XX, P4 | 46 XX, P7 | 46 XY, P7 | 46 XX, P4 | 69 XXX, P4 | 46 XY, P3 |
| Nuclear DNA origin (by STR) | Donor 1 | Donor 1 | Donor 1 | Donor 4 | Donor 3 | Donor 6 |
| mtDNA origin | Donor 2 | Donor 2 | Donor 2 | Donor 3 | Donor 4 | Donor 7 |
| mtDNA carryover (RFLP) | Undetectable | Undetectable | Undetectable | Undetectable | Undetectable | NT |
| mtDNA carryover (ARMS-qPCR) | 0.20% | 0.01% | 1.70% | NT | NT | NT |
NT, not tested.
As reported previously6, small amounts of mtDNA is usually co-transferred with the karyoplast during the ST causing a low carryover heteroplasmy. In clinical situations this may result in the transmission of mutant mtDNA to ST embryos and children. Therefore we conducted both qualitative and quantitative mtDNA assays to determine the degree of mtDNA carryover in ST embryos and ESC lines. We identified mtDNA sequence differences between oocyte donors and unique restriction enzyme recognition sites for RFLP assay 6. For example, mtDNA of the egg donor 1 (spindle contributor for hESO-ST2, -ST3 and -ST4) possessed a unique EcoRV digestion sequence GATATC (Suppl. Fig. 4a). In contrast, egg donor 2 (mtDNA contributor) carried a single nucleotide polymorphism GATACC precluding enzyme recognition. The results confirmed that mtDNA in ST cell lines was exclusively derived from the cytoplast donors with no detectable mtDNA carryover (Table 1, Suppl. Fig. 2c and 4a). We also utilized more sensitive ARMS-qPCR that allows measuring heteroplasmy below 1%9,10. The mean mtDNA carryover in ST oocytes and embryos was 0.5% (SD: ± 0.4; range 0-0.9%) and in hESO-ST cell lines was 0.6% (SD: ±0.9; range 0-1.7%) (Suppl. Fig. 4b, Table 2). These results are consistent with our previous data from a nonhuman primate6 and suggest negligible mtDNA carryover in ST offspring.
Table 2.
Fertilization and embryo development of frozen rhesus oocytes
| Exp | Group | N | Survived after ST (%) | Survived after ICSI (%) | Fertilized (%) | Blastocysts (%) |
|---|---|---|---|---|---|---|
| # 1 | Fresh oocytes | 32 | NA | 30 (94) | 29 (97) | 15(52)† |
| Vitrified oocytes | 26 | NA | 25 (96) | 18 (72) | 1(6)‡ | |
| # 2 | Control Fresh oocytes | 34 | NA | 33 (97) | 30 (91)† | 17 (57)† |
| Fresh Cytoplasts Vit spindles | 36 | 34 (94) | 32 (94) | 28 (88)† | 19 (68)† | |
| Vit Cytoplasts Fresh spindles | 35 | 35 (100) | 34 (97) | 17 (50)‡ | 0‡ | |
Different symbols within columns (dagger and double dagger) indicate significant differences (P<0.05). Data were analyzed using ANOVA.
NA: not applicable
Cytogenetic analyses, using G-banding, indicated that all 5 hESO-ST lines derived from normal ST embryos contained diploid male or female karyotypes, with no evidence of detectable numerical or structural chromosomal abnormalities (Table 1 and Suppl. Fig. 5). However, 2 of 9 lines derived from control embryos displayed numerical aberrations. Notably, hESO-6 carried a 47XYY karyotype, while hESO-9 was 45XO (Suppl. Table 3 and Suppl. Fig. 6). G-banding revealed that hESO-ST6, the cell line derived from abnormally fertilized ST zygote, contained abnormal triploid female chromosome complement (Suppl. Fig. 5). In addition, detailed microsatellite analysis of nuclear DNA in this cell line confirmed the presence of three alleles for most STR loci (Suppl. Table 4). Based on the allele inheritance, we concluded that the triploid karyotype was caused by retention of the genetic material of the 2nd polar body. This was consistent with observation of the extra pronucleus but lack of the second polar body in the zygote.
Origin of abnormal fertilization in human zygotes produced by ST
Abnormal fertilization observed in some human ST oocytes was unexpected since this was not observed in monkey studies 6. Therefore, additional experiments were conducted to investigate possible underlying mechanisms. Genetic analysis of the hESO-ST6 cell line hinted that some ST oocytes and embryos retain extra chromosomes that are normally extruded into the 2nd polar body. This was likely caused by suboptimal conditions during the ST procedure that disturbed spindle integrity. Initially, we focused on the effect of cytochalasin B (CB), a microfilament inhibitor known to inhibit cytokinesis and to block the 2nd PB extrusion in oocytes11,12. CB is used acutely during the ST procedure and oocytes are thoroughly rinsed before fertilization, but residual CB could interrupt the 2nd PB extrusion. Therefore, we extended incubation time between ST and ICSI, or decreased CB concentration. However, abnormal fertilization persisted even in the absence of CB (Suppl. Tables 5, 6) suggesting that abnormal meiotic segregation is not likely caused by CB exposure.
We next addressed if oocyte polarity during displacement of spindles leads to abnormal meiosis. Typically, spindles in MII oocytes are adjacent to the 1st polar bodies. However, during the ST procedure, karyoplasts are reintroduced on the opposite side (referred to as 180 degree)5,6. We reintroduced spindles next to or at the 90 degree from the 1st PB (Suppl. Fig. 7). However, ST zygotes exhibited similar pronuclear abnormalities (Suppl. Fig. 7).
Lastly, we reasoned that human meiotic spindles may undergo premature activation during the ST manipulations leading to incomplete resumption of meiosis after fertilization. Spindle morphology and meiotic stage was analysed in intact and ST human oocytes following immunolabeling with α- and β-tubulins. Analysis demonstrated that in some ST oocytes spindles already progressed to the late anaphase II, whereas all control oocytes maintained uniform metaphase II (Fig. 2b).
Oocyte cryopreservation prior to ST
Current ST protocols use fresh oocytes and require that both patient and healthy mtDNA egg donors undergo synchronous retrievals. However, it is difficult to manage the same-day egg retrievals due to differences in the ovarian cycle and responses to gonadotropins. In addition, an equal number of patient and donor eggs retrieved would be ideal to avoid oocyte wastage. Therefore, oocyte freezing, storage and thawing will be critical for clinical applications of the ST. Recent advances in oocyte vitrification procedures suggest that cryopreserved human MII oocytes can be used in clinical IVF practice with the same efficiency as fresh eggs13,14. To evaluate the feasibility of using cryopreserved oocytes for ST, we turned to the nonhuman primate model. We tested a commercially available vitrification kit (CRYOTOP) and determined that survival and recovery of rhesus macaque MII oocytes post-thaw is high. After ISCI, 72% formed pronuclei, but only 6% developed to blastocysts (P<0.05) (Table 2). Thus, this cryopreservation method compromises blastocyst development, since blastocyst formation of fresh oocytes from the same cohort was 52% (Table 2)6,9.
We next conducted reciprocal ST between fresh and frozen-thawed monkey oocytes and examined fertilization and embryo development (Suppl. Fig. 8). When, fresh spindles were transplanted into vitrified cytoplasts, fertilization after ICSI was impaired (50%) compared to controls (91%) (Table 2). Moreover, all embryos in this ST group arrested before reaching blastocysts, while 57% controls progressed to blastocysts. These ST results were similar to that seen with frozen-thawed intact controls (Table 2). However, when spindles from vitrified oocytes were transferred into fresh cytoplasts, fertilization (88%) and blastocyst formation (68%) rates were similar to fresh controls (Table 2). These results suggest that vitrification causes damage primarily within the cytoplasm rather than to the spindle apparatus.
To further evaluate developmental potential, we plated 6 ST blastocysts derived from vitrified spindles onto feeder cells and established two ESC lines (33%). We transplanted 4 ST blastocysts from vitrified spindles into a recipient that resulted in the timely birth of a healthy infant (Suppl. Fig. 8).
Postnatal development of monkey ST offspring
While the technical feasibility of mtDNA replacement is documented for human embryos and ESCs, questions remain whether a “mismatch” between mtDNA and nuclear DNA haplotypes may cause mitochondrial dysfunctions in ST children15. To address these concerns, we conducted a 3-year follow up studies on monkey ST offspring born in 20096.
The growth and development of four healthy infants following ST procedure was evaluated during the postnatal period (Suppl. Fig. 9a). Their overall health, including routine blood and body weight measurements monitored from birth to 3 years were comparable to age-matched controls. The values for haemoglobin, red blood and white blood cell counts, mean corpuscular volume, and haemoglobin concentrations were all within normal ranges (Suppl. Table 7). We also measured blood chemistry and arterial blood gas parameters and demonstrated that metabolic status of ST offspring is comparable to controls (Suppl. Table 6). In addition, the body weight gains for the ST juvenile monkeys were similar to that of age-matched controls (Suppl. Fig. 9b). We also confirmed that ATP levels and mitochondrial membrane potential (ΔΨm) in skin fibroblasts were similar to controls (Suppl. Fig. 10). Finally, there were no significant changes in mtDNA carryover and heteroplasmy in blood and skin samples with age (Suppl. Fig. 9c).
Discussion
This report summarizes our effort to test an mtDNA replacement in unfertilized human oocytes, initially developed and optimized in a monkey model. The results demonstrate that the ST procedure can be performed with high efficiency in human oocytes. Manipulated oocytes also supported high fertilization rates similar to that of controls. However, approximately half of the human ST zygotes exhibited abnormal fertilization primarily, as a result of excessive pronuclear numbers. This was an unexpected outcome that was not observed with monkey oocytes. Our follow up studies indicated that this is caused by the failure to complete meiosis and segregate chromosomes into the 2nd PB, likely due to premature activation. A set of haploid genetic material is normally discarded during asymmetrical cell division into the 2nd PB, while the other half forms the female pronucleus. By genetic analysis of ESCs derived from abnormally fertilized zygote, we confirmed the triploid nature and presence of two sets of female chromosomes.
The spindle-chromosomal apparatus in MII oocytes is an extremely sensitive structure that can be easily perturbed by physical or chemical manipulations. Our initial attempts to isolate and transplant monkey MII spindles were unsuccessful due to similar problems with spontaneous resumption of meiosis6. Procedures were optimized to avoid this negative outcome and current ST protocols allow maintenance of an intact MII spindle and normal fertilization. It appears that human MII oocytes are more sensitive to spindle manipulations and further improvements and optimizations will be required for future clinical applications. Maintenance of meiotic spindles in MII oocytes is dependent on the activity of M-phase-specific kinases including maturation-promoting factor (MPF) and MAP kinase16. Under normal conditions, sperm entry triggers degradation of kinase activities and chromosome segregation mediated by oscillations of intracellular Ca2+ concentrations17. However, an influx of calcium induced by mechanical or chemical manipulations can induce parthenogenetic activation of oocytes and resumption of meiosis18. Thus, ST manipulations in a medium without Ca2+ or supplementations with MG132 could potentially avoid problems with spontaneous activation19,20.
Morphological evaluation of fertilization and early detection of abnormal pronuclear and/or polar body formation appears to be critical to separate normal and abnormal ST embryos. Blastocyst development and ESC isolation in normally fertilized ST zygotes was similar to controls. We also confirmed that all ESC lines derived from these ST embryos are karyotypically normal.
Two of the 9 ESC lines (22%) derived from non-manipulated oocytes also showed chromosomal abnormalities. Since aberrations were confined to the sex chromosomes (47XYY and 45XO), it is possible that this was induced by sperm carrying either two Y or without Y chromosome.
Despite the risk of abnormal pronuclear formation and aneuploidy in a portion of ST zygotes, embryo development and ESC isolation rates in normal ST zygotes are comparable to intact controls. Based on our estimates of retrieving on average 12 MII oocytes, 35% normal (2PN/2PB) fertilization rates, and 60% blastocyst development, at least 2 ST blastocysts suitable for transfers can be generated during a single cycle for each patient.
The safety of the ST procedure is also dependent on the amount of mutated mtDNA co-transferred with spindles. Importantly, mtDNA carryover in ST embryos and ESC lines is technically undetectable or below 1%. In most patients with mtDNA diseases, a threshold of 60% or higher of mutated mtDNA must be reached for clinical features to appear. Thus, it is unlikely that low mtDNA carryover during ST would cause disease in children. Segregation of mutated mtDNA to specific tissues during development and aging may hypothetically result in a significant accumulation of the mutant load. However, analysis of mtDNA carryover in monkey ST offspring discovered no detectable mtDNA segregation into different tissues9. In addition, there were no changes in heteroplasmy levels during postnatal development of monkeys. Thus, carryover, segregation and tissue-specific accumulation of mutant mtDNA molecules in ST children seems unlikely to be a major concern.
Birth of a healthy monkey infant after oocyte freezing marks an important milestone in applying the ST technology to patients. Transplantation of vitrified spindles into fresh cytoplasms yields the best results, comparable to controls. However, fertilization of vitrified cytoplasts even with fresh spindles was compromised. These remarkable findings suggest that the damage after cryopreservation is confined mainly to the eggs’ cytoplasm, not to the chromosomes and spindles as commonly believed21. Our observations also reveal another unexpected potential clinical application of the ST technique, suggesting that spindles in sub-optimally cryopreserved oocytes can be rescued by transplanting into fresh cytoplasts.
Follow up postnatal studies in four monkeys produced by ST provide convincing evidence that oocyte manipulation and mtDNA replacement procedures are compatible with normal development. These monkeys were derived by combining of nuclear and mtDNA from the two genetically distant subpopulations of rhesus macaques. Mitochondrial and nuclear genetic differences between these monkeys are considered to be as distant as those between some different primate species22, thus imitating haplotype differences between humans. Concerns have been raised that nuclear and mtDNA incompatibilities between mtDNA patients and cytoplast donors may cause a “mismatch” and mitochondrial dysfunctions in ST children even in the absence of mutations15. Based on our long-term observations, it is reasonable to speculate that nuclear-mtDNA interactions are conserved within species.
Pioneering work in nonhuman primates is critical for development and safety and efficacy evaluations of new treatments23,24. It is important that scientists and clinicians further optimize ST protocols for human oocytes and assure these procedures are safe. It is also crucial that the FDA initiates careful review of these new developments. Such oversight will be important to establish safety and efficacy requirements and guide clinical trials. Current NIH funding restrictions surrounding these innovative reproductive technologies will also require amendments to support federally-funded clinical trials.
Methods Summary
The study protocols were approved by both the OHSU Embryonic Stem Cell Research Oversight Committee and the Institutional Review Board. Mature oocytes were donated by volunteers and ST procedures were carried out as described5,6. Oocytes were fertilized, cultured to blastocysts and used for ESC isolation. Detailed methods are described in Supplementary information at www.nature.com/nature.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the OHSU Embryonic Stem Cell Research Oversight Committee and the Institutional Review Board for providing oversight and guidance. We thank oocyte and sperm donors and staff at the Women's Health Research Unit at the Center for Women's Health, University Fertility Consultants and Reproductive Endocrinology & Infertility Division at the Department of Obstetrics & Gynecology of Oregon Health & Science University for their support and procurement of human gametes. The Division of Animal Resources, Surgery Team, Assisted Reproductive Technology & Embryonic Stem Cell Core, Endocrine Technology Core, Imaging & Morphology Core, Flow Cytometry Core and Molecular & Cellular Biology Core at the Oregon National Primate Research Center provided expertise and services for the nonhuman primate research. Hamilton Thorne, Inc. donated XYClone laser system for this study. We are grateful to Dr. Warren Sanger and Dianna Zaleski for karyotyping services, Dr. Cecilia Penedo for microsatellite analysis and Dr. Jon Hennebold for consulting on metabolic assays. We are also indebted to Andrea Steele, Rita Cervera Juanes and Erin Wolff for their technical support.
The human oocyte/embryo research was supported by grants from the OHSU Center for Women's Health Circle of Giving and other OHSU institutional funds, as well as the Leducq Foundation. The nonhuman primate study was supported by grants from the National Institutes of Health HD063276, HD057121, HD059946, EY021214, and 8P51OD011092.
Footnotes
Author contribution
MT, PA, JJ, and SM conceived the study, designed experiments and wrote IRB protocols. PA, MS and NMG coordinated recruitment of participants. PA, KM, DB, DL, DW and PP performed ovarian stimulation and oocyte recovery. MT conducted ST micromanipulations. MS, KM and SM performed ICSI. MT, MS, JW, DMS, NMG, RTH and EK conducted ESC derivation and characterization. SG analysed teratoma tumors. MT, HM and DMS performed DNA/RNA isolations, metabolic and mtDNA analyses. CR, MT, MS,H-SL, RS and SM conducted monkey studies. MT, RS, JJ, PP and SM analysed data and wrote the paper.
Supplementary Information accompanies the paper on www.nature.com/nature.
References
- 1.Gropman AL. Diagnosis and treatment of childhood mitochondrial diseases. Current neurology and neuroscience reports. 2001;1:185–194. doi: 10.1007/s11910-001-0015-9. [DOI] [PubMed] [Google Scholar]
- 2.Haas RH, et al. Mitochondrial disease: a practical approach for primary care physicians. Pediatrics. 2007;120:1326–1333. doi: 10.1542/peds.2007-0391. doi:10.1542/peds.2007-0391. [DOI] [PubMed] [Google Scholar]
- 3.Schaefer AM, et al. Prevalence of mitochondrial DNA disease in adults. Annals of neurology. 2008;63:35–39. doi: 10.1002/ana.21217. doi:10.1002/ana.21217. [DOI] [PubMed] [Google Scholar]
- 4.Elliott HR, Samuels DC, Eden JA, Relton CL, Chinnery PF. Pathogenic mitochondrial DNA mutations are common in the general population. American journal of human genetics. 2008;83:254–260. doi: 10.1016/j.ajhg.2008.07.004. doi:10.1016/j.ajhg.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tachibana M, Sparman M, Mitalipov S. Chromosome transfer in mature oocytes. Nature protocols. 2010;5:1138–1147. doi: 10.1038/nprot.2010.75. doi:10.1038/nprot.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tachibana M, et al. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature. 2009;461:367–372. doi: 10.1038/nature08368. doi:nature08368 [pii] 10.1038/nature08368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowan CA, et al. Derivation of embryonic stem-cell lines from human blastocysts. The New England journal of medicine. 2004;350:1353–1356. doi: 10.1056/NEJMsr040330. doi:10.1056/NEJMsr040330. [DOI] [PubMed] [Google Scholar]
- 8.Danan C, et al. Evaluation of parental mitochondrial inheritance in neonates born after intracytoplasmic sperm injection. American journal of human genetics. 1999;65:463–473. doi: 10.1086/302484. doi:10.1086/302484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H-S, et al. Rapid Mitochondrial DNA Segregation in Primate Preimplantation Embryos Precedes Somatic and Germline Bottleneck. Cell Reports. 2012;1:10. doi: 10.1016/j.celrep.2012.03.011. doi:10.1016/j.celrep.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgstaller JP, Schinogl P, Dinnyes A, Muller M, Steinborn R. Mitochondrial DNA heteroplasmy in ovine fetuses and sheep cloned by somatic cell nuclear transfer. BMC developmental biology. 2007;7:141. doi: 10.1186/1471-213X-7-141. doi:10.1186/1471-213X-7-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakayama T, Yanagimachi R. The first polar body can be used for the production of normal offspring in mice. Biology of reproduction. 1998;59:100–104. doi: 10.1095/biolreprod59.1.100. [DOI] [PubMed] [Google Scholar]
- 12.Susko-Parrish JL, Leibfried-Rutledge ML, Northey DL, Schutzkus V, First NL. Inhibition of protein kinases after an induced calcium transient causes transition of bovine oocytes to embryonic cycles without meiotic completion. Developmental biology. 1994;166:729–739. doi: 10.1006/dbio.1994.1351. doi:10.1006/dbio.1994.1351. [DOI] [PubMed] [Google Scholar]
- 13.Forman EJ, et al. Oocyte vitrification does not increase the risk of embryonic aneuploidy or diminish the implantation potential of blastocysts created after intracytoplasmic sperm injection: a novel, paired randomized controlled trial using DNA fingerprinting. Fertility and sterility. 2012 doi: 10.1016/j.fertnstert.2012.04.028. doi:10.1016/j.fertnstert.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 14.Rienzi L, et al. Consistent and predictable delivery rates after oocyte vitrification: an observational longitudinal cohort multicentric study. Human reproduction. 2012 doi: 10.1093/humrep/des088. doi:10.1093/humrep/des088. [DOI] [PubMed] [Google Scholar]
- 15.Moreno-Loshuertos R, et al. Differences in reactive oxygen species production explain the phenotypes associated with common mouse mitochondrial DNA variants. Nature genetics. 2006;38:1261–1268. doi: 10.1038/ng1897. doi:10.1038/ng1897. [DOI] [PubMed] [Google Scholar]
- 16.Fisher DL, Brassac T, Galas S, Doree M. Dissociation of MAP kinase activation and MPF activation in hormone-stimulated maturation of Xenopus oocytes. Development. 1999;126:4537–4546. doi: 10.1242/dev.126.20.4537. [DOI] [PubMed] [Google Scholar]
- 17.Runft LL, Jaffe LA, Mehlmann LM. Egg activation at fertilization: where it all begins. Developmental biology. 2002;245:237–254. doi: 10.1006/dbio.2002.0600. doi:10.1006/dbio.2002.0600. [DOI] [PubMed] [Google Scholar]
- 18.Mitalipov SM, Nusser KD, Wolf DP. Parthenogenetic activation of rhesus monkey oocytes and reconstructed embryos. Biology of reproduction. 2001;65:253–259. doi: 10.1095/biolreprod65.1.253. [DOI] [PubMed] [Google Scholar]
- 19.Gao S, Han Z, Kihara M, Adashi E, Latham KE. Protease inhibitor MG132 in cloning: no end to the nightmare. Trends in biotechnology. 2005;23:66–68. doi: 10.1016/j.tibtech.2004.12.007. doi:10.1016/j.tibtech.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Kikuchi K, et al. Maturation/M-phase promoting factor: a regulator of aging in porcine oocytes. Biology of reproduction. 2000;63:715–722. doi: 10.1095/biolreprod63.3.715. [DOI] [PubMed] [Google Scholar]
- 21.Mandelbaum J, et al. Effects of cryopreservation on the meiotic spindle of human oocytes. European journal of obstetrics, gynecology, and reproductive biology. 2004;113(Suppl 1):S17–23. doi: 10.1016/j.ejogrb.2003.11.005. doi:10.1016/j.ejogrb.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Smith DG. Genetic characterization of Indian-origin and Chinese-origin rhesus macaques (Macaca mulatta). Comparative medicine. 2005;55:227–230. [PubMed] [Google Scholar]
- 23.Donnez J, et al. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Annals of medicine. 2011;43:437–450. doi: 10.3109/07853890.2010.546807. doi:10.3109/07853890.2010.546807. [DOI] [PubMed] [Google Scholar]
- 24.Lee DM, et al. Live birth after ovarian tissue transplant. Nature. 2004;428:137–138. doi: 10.1038/428137a. doi:10.1038/428137a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.