Abstract
The National Research Council has consistently recommended housing densities for animals used in science and agriculture. For mice, the recommended density is 77.4 cm2 (12 in2) for a 15–25 gm mouse. The Council noted that its recommendations were based on “best professional judgment” and encouraged alternatives that were data driven. As part of a continual effort of The Jackson Laboratory to ensure the health and well-being of production and research mice while promoting cost-effective, state-of-the-art research, several density-driven studies have been conducted by lab researchers. The objectives of this study were to determine the effect of housing density on parameters related to mouse physiology and air quality in the cages and to assess the value of specific measured parameters in such studies. The study discussed in this report monitored C57BL/6J mice in individually ventilated cages from weaning until 9 months of age. Housing densities were equivalent to 66.4 and 36.8 cm2/mouse (10.3 and 5.7 in2), representing increases in density of 17% and 110%, respectively, over the National Research Council recommendation. Clinical physiological parameters representing general health and well-being were measured. Hematological traits, plasma lipids and glucose, growth, bone mineral density and percent body fat did not differ between densities. In the more densely housed mice, however, adrenal glands were significantly smaller, heart rates were significantly lower, and food consumption was less. Cage air microenvironment was evaluated for ammonia, carbon dioxide, temperature and humidity in cages changed weekly or every 2 weeks. The cage microenvironment remained within acceptable limits at the higher density of mice at both cage-changing frequencies. The results suggest that mice housed in individually ventilated cages for up to 9 months at up to twice the density currently recommended by the National Research Council show no measurable adverse effects. Continued re-evaluation of the recommendation by measuring additional relevant parameters of health and general well-being and studying additional strains is warranted.
Keywords: adrenal weight, animal husbandry, cage air microenvironment, heart rate, mouse housing density, animal well-being
INTRODUCTION
To ensure humane treatment of research animals, in 1963 the National Research Council set standards for housing density based on the best professional judgment at the time. Forty-nine years later, in the Eighth Edition of the Guide for the Care and Use of Laboratory Animals (Guide; National Research Council, 2011), the standards remain unchanged. For adult mice weighing 15–25 g the Guide recommends housing in at least 77.4 cm2 (12 in2) of cage space. But much has changed in animal husbandry since 1963. Mouse rooms are cleaner; many are specific pathogen free. Many institutions use positive individually ventilated (PIV) caging systems, reducing exposure to infectious agents, more efficiently removing waste gases such as ammonia and carbon dioxide (CO2), and inhibiting growth of ammonia-producing bacteria.
At The Jackson Laboratory, a critical component of our effort to ensure the welfare of mice is ongoing evaluation of new technology and data relevant to housing density and mouse health. The current study was designed to test the hypothesis that mice housed in PIV cages at the density recommended by the Guide would show optimal health and that mice housed at higher densities would reveal measurable adverse physiological effects. Mice used in this study were part of an ongoing phenotype-driven study aimed at identifying physiologically relevant mutations in mutagenized C57BL/6J (B6) mice. These mice were viable subjects for our study: they were already being monitored for a battery of physiological tests, including hematology, plasma lipids, body composition, and heart function. For our study, mice were housed at densities of 66.4 cm2 (10.3 in2) or 36.8 cm2 (5.7 in2) per mouse. Additional parameters to those measured in the mutagenesis program, namely growth, food and water consumption, cage air quality, and adrenal weight, were measured to enhance the quantification of physiological well-being. Results did not support the hypothesis.
MATERIALS AND METHODS
Mice and Animal Husbandry
Consistent with the principal of reducing the use of animals whenever possible, this study of housing density used mice from an ongoing study being carried out by The Jackson Laboratory Heart, Lung, and Blood Mutagenesis Program (Svenson et al., 2003). Male C57BL/6J (B6) mice had been mutagenized with N-ethyl-N-nitrosourea (ENU) and bred to B6 females to produce third generation offspring of the original males carrying new mutations in the heterozygous or homozygous state. The Mutagenesis Program phenotyped these mice in a battery of weekly, high throughput, non-invasive screens between the ages of 7 and 15 weeks. In the Mutagenesis Program, if a mouse presented a deviant phenotype, it was removed from the pipeline for heritability testing of the deviant trait. Our density study used cohorts of these mice, housed at weaning at the 2 densities for this study, allowing them to follow the Mutagenesis Program pipeline. Of the 280 mice selected for our study, none was found to be pheno-deviant for any measured trait. Between 15 and 39 weeks of age, growth, food and water consumption, cage air microenvironment, observations of aggression or fighting, and adrenal weight at necropsy were recorded. All tests were selected as performance indices to assess animal well-being under conditions of increased density.
Two housing densities were set up at weaning age in duplex cages (Thoren Caging Systems, Inc, Hazelton, PA) comprising 2 pens, each with 333 cm2 (51.7 in2) of floor space. Mice were housed at either 5 (D5) or 9 (D9) mice per pen, for housing densities of either 66.4 or 36.8 cm2 (10.3 or 5.7 in2) per mouse, respectively. Each pen was individually pressurized and set to operate at 60 air changes/hour. Bedding was autoclaved pine shavings (Crobb Box, Ellsworth, ME). Mice were provided ad libitum access to acidified water (pH 2.8 to 3.1) and pelleted autoclaved 5K52 diet, modified from the NIH 31M open formula with 6% fat by weight (LabDiet® 5K52 6% Fat, St. Louis, MO). At the end of the mutagenesis protocol, when mice were 15 weeks of age, the number of cages was reduced randomly to 6 per sex and density group, for a total of 12 cages (24 pens; 168 mice) and maintained until mice were 39 weeks of age.
The animal room was supplied with HEPA-filtered air at 19 air changes/hour and maintained at a temperature of 22 ± 2°C, a relative humidity of 35 ± 4%, and a 12:12 hour light:dark cycle beginning at 0600 h. The animal room was specific pathogen free with controlled personnel access. The colony was routinely monitored for, and found to be free of, 15 viruses (mouse hepatitis virus, reovirus, 2 mouse parvoviruses, Theiler’s mouse encephalomyelitis virus, ectromelia virus, mouse rotavirus, thymic virus, pneumonia virus of mice, Sendaivirus, murine cytomegalovirus, lactic dehydrogenase-elevating virus, K virus, mouse adenovirus, and polyoma virus), 17 bacterial species (including Helicobacter spp.), 2 Mycoplasma spp., external and internal parasites, and Encephalitozoon cuniculi.
All procedures were approved by the Institutional Animal Care and Use Committee at The Jackson Laboratory and are consistent with the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals. The mice were euthanized with CO2 gas at the end of the experiments.
Protocols
Initially, the study comprised 10 pens (5 duplex cages) of each sex at both densities for a total of 40 pens (20 cages) and 280 mice. These mice first went through the Mutagenesis Program screening protocol, including a blood sample at 7 weeks of age for complete hematological analysis; a blood sample at 8 weeks for total and high density lipoprotein (HDL) cholesterol, glucose, and triglycerides; an electrocardiogram at 11 weeks; and a body composition scan at 12 weeks. Detailed methods are published (Svenson et al., 2003, 2007) and online (http://pga.jax.org/protocols.html), but are briefly summarized here. Plasma was obtained after food was removed from mice for 4 hours in the morning (0700–1100 h), and lipids and glucose were analyzed using a Beckman Coulter Synchron CX5 Delta Chemistry Analyzer according to the manufacturer’s instructions (Beckman Coulter, Fullerton, CA). Body composition (percent fat and bone mineral content) was determined in mice anesthetized with 0.02 mL/gm body weight of tribromoethanol using dual-energy X-ray absorptiometry (Lunar PIXImus DXA, Luan, Madison, WI). EDTA-anticoagulated whole blood was obtained from the retro-orbital sinus, and complete blood counts were determined using an Advia 120 Multispecies Hematology Analyzer (Bayer Diagnostics, Tarrytown, NY) as described previously (Peters et al., 2002). Electrocardiograms were obtained from unanesthetized mice using the ECGenie system (Mouse Specifics, Quincy MA). In this system mice were placed on a platform standing 18 inches from the laboratory bench and fitted with 3 pediatric conductance leads. Mice acclimated on the platform for 5 to 10 minutes, and readings were initiated and recorded in the next 3 to 5 minutes whenever 3 of the mouse’s feet simultaneously contacted the leads.
When mice were between 15 and 39 weeks of age, the following observations were recorded daily: evidence of aggressive behavior (e.g., fighting, wounds, bites), barbering or whisker plucking, hair loss, and morbidity or mortality. Starting when mice were 23 weeks of age, body weight and food and water consumption were recorded weekly over a 16-week period. Adrenal weights were recorded at necropsy.
Cage Air Quality
Air quality within pens was monitored for CO2, ammonia, temperature, and humidity using a multi-point gas analyzer (Innova AirTech Instruments A/S, Ballerup, Denmark) as previously described (Reeb et al., 1998). Each pen used for monitoring had 2 small ports drilled into the front wall 5 cm above the floor, one for a temperature probe and one for a gas sampling line. Probes were inserted several hours before measurements were taken, to allow mice to adjust to the novelty of them. Ports were sealed when not in use. Bedding was changed every 2 weeks. In one experiment, however, 6 pens/group (3 cages/group) that had bedding changed weekly were compared to 6 pens/group that had bedding changed every 2 weeks. Air quality was measured 8 times per pen (192 total measurements). Measurements occurred between 1300 and 1700 h, just before the bedding change. Occasionally, very high ammonia readings can be caused by a mouse that buries the sampling probe (Reeb et al., 1998). Eight aberrantly high readings attributable to this phenomenon were observed in 4 out of the 24 pens and more than once in 3 of these 4 pens. These high values were removed from the analysis.
Statistical Analysis
To compare the effects of density for each phenotype, the values for all mice in each pen were averaged as technical replicates and the pen means were used as biological replicates. The statistical package SAS v9.3 was used. Model terms were deemed significant at P-value < 0.05. Details of the analyses used for each of the phenotypes are described below.
Hematology, Plasma Lipids, Body Composition and Electrocardiogram
Linear regression models were fit to phenotypes using PROC MIXED. Each phenotype was investigated for a significant effect of sex, density, and their interaction. Additionally, body weight was incorporated as a covariate in the predictive model for fat percentage. When necessary, values were transformed to meet the assumptions of the analysis.
Food and Water Consumption and Body Weight
Repeated measures linear regression models were fit to food and water consumption (normalized to a 25-gram mouse) and body weight using PROC MIXED. Each phenotype was investigated for a significant effect of density, sex, time (in weeks), and their 2-way interactions. A compound symmetry covariance structure was used for each model. A Tukey’s multiple testing correction was applied to P-values resulting from multiple comparisons performed on the levels of the significant week-by-density factor within the food consumption model. The assumptions of repeated measures linear regression were met by the data.
Fighting, Barbering and Hair Loss
Logistic regression models were fit to the events of fighting, barbering, and hair loss using PROC GENMOD. Each type of event was investigated for a significant effect of sex and density (individually). The sample size used in the logistic regression analyses was relatively small (24 samples total), although it did meet the recommended sample size of 10 cases for each predictor/independent variable (Agresti, 2007) when evaluating either the effect of sex or density alone. There were no instances of fighting recorded; hence statistical analysis of the data was omitted. The assumptions of logistic regression were met by the data analyzed.
Air Quality
Repeated measures linear regression models were fit to air quality metric using PROC MIXED. Each metric (ammonia, carbon dioxide, humidity, and temperature) was investigated for a significant effect of cage changing interval, density, sex, time (in weeks), and their 2-way interactions. A compound symmetry covariance structure was used for each model. When necessary, air quality metrics were transformed to meet the assumptions of the analysis.
Adrenal Weight
A linear regression model was fit to adrenal weight using PROC MIXED. Significant effects of density, sex, and their interaction were investigated. Body weight was also investigated as a potential covariate. The assumptions of linear regression were met by the data.
RESULTS
Hematology, Plasma Lipids, and Glucose
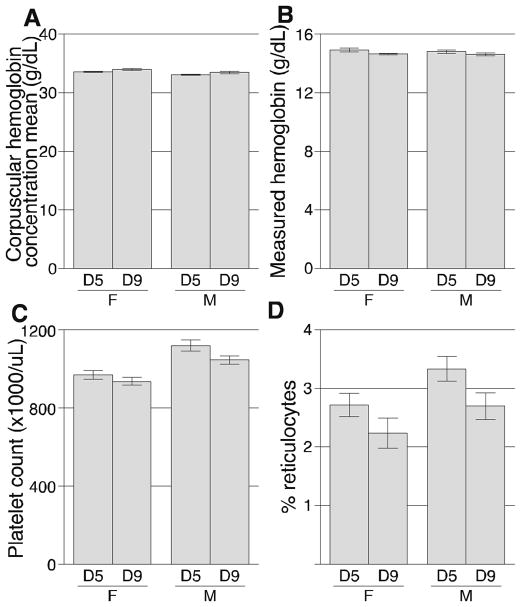
Complete blood counts, plasma lipids, and plasma glucose were analyzed. Of the 17 hematological parameters analyzed, no significant differences were found between mice housed at the 2 densities for the following 13 parameters: red blood cells, white blood cells, percent lymphocytes, percent monocytes, percent neutrophils, percent eosinophils, mean corpuscular volume, red cell distribution width, mean corpuscular hemoglobin, mean cellular hemoglobin concentration, hemoglobin distribution width, hematocrit, and mean platelet volume. Differences between density group means for 4 parameters had P-values < 0.05 (Figure 1). One parameter, corpuscular hemoglobin concentration mean, was greater in mice housed at the higher density (P = 0.002; Figure 1A). Three parameters were lower in mice housed at the higher density: measured hemoglobin (P = 0.033; Figure 1B), platelets (P = 0.028; Figure 1C), and reticulocytes (P = 0.014; Figure 1D). Although these 4 parameters were statistically different between density groups, all values were within normal physiological ranges. Although some whole blood parameter values varied by sex, the statistical analyses revealed no interaction between sex and density; thus, sex differences did not account for the density differences (see Methods). All hematological data are presented in Supplemental Table 1.
Figure 1.
Hematological measurements that varied between mice housed at 5 (D5) or 9 (D9) mice/pen. Parameters that differed were (A) corpuscular hemoglobin concentration mean; (B) measured hemoglobin; (C) platelets; and (D) reticulocytes. Values for all hematological parameters are presented in Supplemental Table 1. F, female; M, male.
For plasma lipids and glucose, no significant differences were found between densities for any of the measurements: total cholesterol, HDL-cholesterol, triglycerides, and glucose. All 4 measurements differed by sex. All plasma lipid and glucose data are presented in Supplemental Table 2.
Electrocardiogram
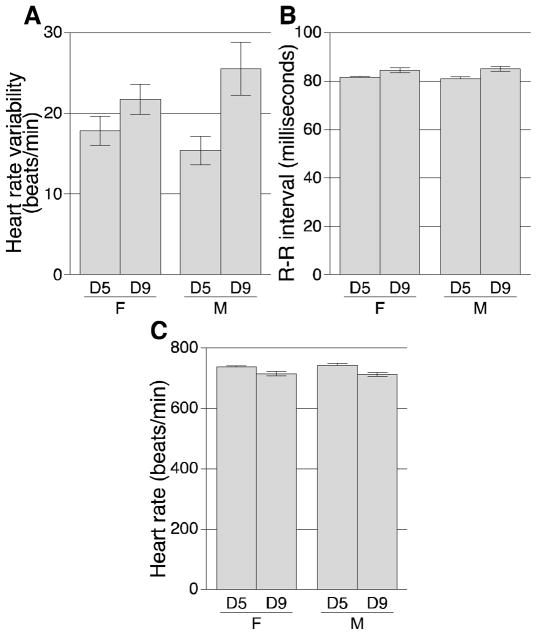
Of the 7 electrocardiogram parameters analyzed, no differences were found between density groups for 4 of them: PR interval, QRS duration, PQ interval, and QTc. The QTc is the QT interval that has been corrected for heart rate. Differences between density group means for 3 parameters had P-values < 0.05 (Figure 2). Two parameters were greater at the higher density: heart rate variability (HRV; P = 0.003; Figure 2A) and R–R interval (P = 0.0001; Figure 2B). Heart rate (HR) was lower at the higher density (P = 0.0002; Figure 2C). In an electrocardiogram, heart rate is inversely correlated to the R–R interval, so the lower HR observed at the higher density is consistent with the increased R–R interval. All electrocardiogram data are presented in Supplemental Table 3.
Figure 2.
Electrocardiogram parameters that varied between mice housed at 5 (D5) or 9 (D9) mice/pen. Parameters that differed were (A) heart rate variability; (B) R—R interval; and (C) heart rate. Values for all electrocardiogram parameters are presented in Supplemental Table 3. F, female; M, male.
Body Composition, Growth, Food and Water Consumption, and Adrenal Weight
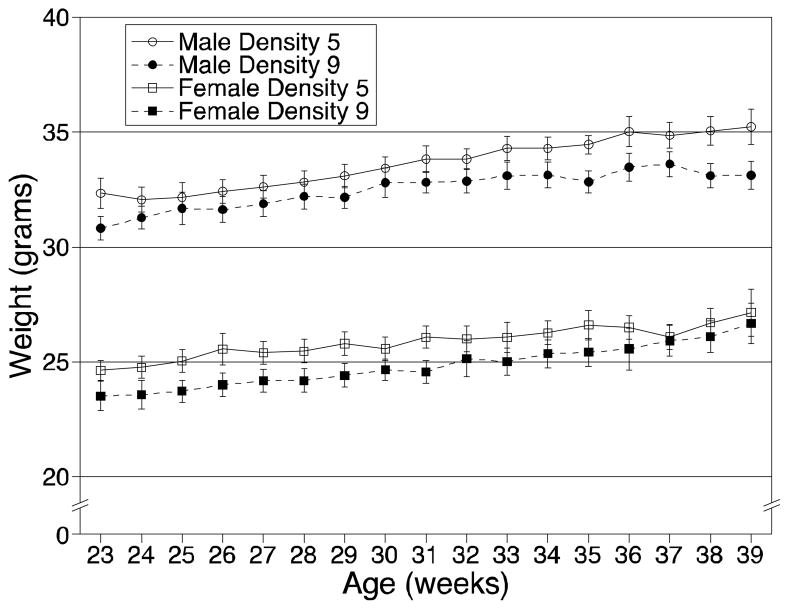
Body composition (bone mineral density, percent body fat) did not differ between the 2 housing densities (Table 1). Growth curves, generated from weekly measurements of body weight from age 23 weeks until the end of the study, did not differ between the 2 housing densities (Figure 3). While growth rate did not differ, mice at the higher density were consistently lighter than mice at the lower density (P = 0.016). Over the 16-week period of growth curve measurements, females at the higher density were an average of 1.04 ± 0.09 g lighter than mice at the lower density, and males at the higher density were an average of 1.14 ± 0.12 g lighter. The group housed more densely had reduced food consumption (P = 0.017) and slightly reduced water consumption (P = 0.053), a difference that nearly met the threshold for significance (Table 1). Adrenal weight, measured after euthanasia, was lower in the more densely housed mice (P = 0.001; Table 1). Sex differences were found for body composition, growth, and adrenal weight (Table 1).
Table 1.
Body composition, food and water consumption, and adrenal weight
| Test | Density (mice/pen) | Density group
|
Sex
|
|||
|---|---|---|---|---|---|---|
| Mean ± SEM | P value | Mean ± SEM
|
P value | |||
| Females | Males | |||||
| Bone mineral density (g/cm2)*1 | 5 | 0.0469 ± 0.0003 | ns | 0.0460 ± 0.0003 | 0.0477 ± 0.0003 | 0.0002 |
| 9 | 0.0476 ± 0.0004 | 0.0466 ± 0.0005 | 0.0484 ± 0.0004 | |||
| % Body fat*1 | 5 | 20.9 ± 1.3 | ns | 23.8 ± 1.6 | 17.9 ± 0.6 | 0.001 |
| 9 | 21.4 ± 0.6 | 22.6 ± 1.0 | 20.4 ± 0.4 | |||
| Food consumption (g/wk)2 | 5 | 18.5 ± 0.2 | 0.02 | 20.2 ± 0.2 | 16.9 ± 0.2 | <0.0001 |
| 9 | 17.5 ± 0.2 | 19.2 ± 0.2 | 15.8 ± 0.1 | |||
| Water consumption (g/wk)2 | 5 | 17.8 ± 0.2 | 0.053 | 19.8 ± 0.2 | 16.0 ± 0.2 | <0.0001 |
| 9 | 17.5 ± 0.2 | 19.6 ± 0.3 | 15.3 ± 0.2 | |||
| Adrenal weight (mg)*3 | 5 | 6.8 ± 0.4 | 0.001 | 7.6 ± 0.4 | 6.0 ± 0.5 | <0.0001 |
| 9 | 5.4 ± 0.4 | 6.4 ± 0.3 | 4.4 ± 0.2 | |||
The difference between sexes reached statistical significance at P < 0.05; however, the interaction between sex and density did not.
Transformations applied:sqrt.
Food and water consumption are normalized to a 25-gram mouse.
For adrenal weight, body weight was not found to be a significant covariate (P < 0.05).
Figure 3.
Growth curves of mice housed at 5 (D5) and 9 (D9) mice/pen. Open symbols and solid lines are mice housed at D5; closed symbols and dotted lines are mice housed at D9. Squares are females and circles are males. Each symbol represents the average of 6 pens/group ± standard error of mean (SEM).
Other Observations
No deaths or signs of poor health were observed throughout the study, nor were signs of aggression, such as fighting, wounds or bites (Table 2). Barbering and whisker plucking were observed in several pens, but no differences were found between the density groups (Table 2). B6 mice are prone to barber each other, a behavior that has been shown to increase in frequency when housed at 20.6 cm2/mouse (3.2 in2) (Smith et al., 2004), which is almost twice that of the highest density in our study. Hair loss was observed in several pens, but did not differ between densities.
Table 2.
Aggression, barbering, and hair loss
| Trait | Density (mice/pen) | Number of pens with observed behavior |
|---|---|---|
| Fighting, wounds, bites | 5 | 0 |
| 9 | 0 | |
| Barbering, whisker plucking | 5 | 5 |
| 9 | 7 | |
| Hair loss | 5 | 7 |
| 9 | 7 |
All pens (12 per density; 6 per sex/density) were monitored daily for the occurrence of fighting, barbering and hair loss from 22–36 weeks of age.
Cage Microenvironment
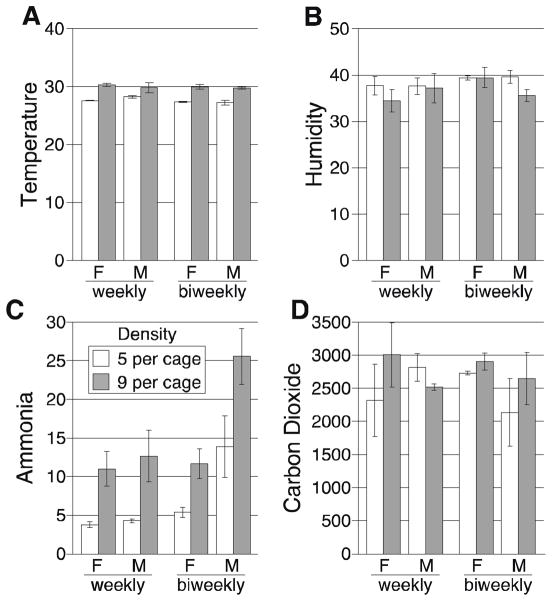
Increased housing density resulted in increased pen temperature of about 3°C (P < 0.0001). At the lower density, temperature ranged from 27–28°C, and in the higher density, it ranged from 30–31°C. Frequency of bedding change did not affect temperature. Increased density also increased ammonia levels (P < 0.0001; D5 = 6.8 ± 1.5 ppm; D9 = 15.2 ± 2.2 ppm). Humidity and CO2 levels did not change with density or cage changing frequency. All cage microenvironment data are presented in Figure 4.
Figure 4.
Air quality within pens as a function of housing density and frequency of cage changing. (A) Temperature; (B) humidity; (C) ammonia; (D) carbon dioxide. For females (F) and males (M), each bar is the mean and SEM of 3 pens/group. Each pen was measured at 8 different times, so each bar represents the mean of 24 measurements. Open bars are 5 mice/pen (D5); closed bars are 9 mice/pen (D9). Bedding was changed weekly or every 2 weeks as shown, and air was measured on the day prior to change (day 6 or 13).
DISCUSSION
The purpose of this study was to compare measures of well-being in mice housed at the density that has been recommended by the NRC for 49 years to those of mice housed at a higher density. We tested the hypothesis that B6 mice housed at twice the recommended density would exhibit measurable adverse effects. The performance indices used to assess well-being included hematology and plasma chemistries, growth and body composition, electrocardiogram, food and water consumption, home cage behavior, cage air quality, and adrenal weight.
Comparison of Physiological Measurements
No differences were observed between the 2 density groups for most measurements, including hematological parameters, plasma lipids and glucose, percent body fat, and bone mineral density. However, heart weight, adrenal weight and body weight were reduced in the more densely housed mice.
Heart rate is often used as a measure of stress (Bernberg et al., 2009; Gilmore et al., 2008; Meijer et al., 2009) and was therefore chosen for evaluation in our study. Our results confirm previous studies that demonstrated reduced heart rate with increased housing density (Nicholson et al., 2009; Van Loo et al., 2007). These previous studies used the invasive technique of surgically implanting telemeters to record resting heart rate over 24-h periods. While the method used in this study differs significantly from the previous studies, the results are similar, and the method has been validated as a reliable technique for measuring heart rate (Svenson et al., 2003). The use of our technique further demonstrates that reduced heart rate in the more densely housed mice is maintained even when they are removed from their home cage and placed in a novel situation.
Increased adrenal weight is also often used as a measure of stress (Naidu et al., 1995; Schmidt et al., 2010; Sterlemann et al., 2008). The rationale is that when animals are chronically stressed, their adrenal glands weigh more because of an increased production of corticosterone. In the previous studies, both adrenal weight and either plasma or urinary corticosterone were measured. In general, adrenal weight was a more robust measure because the variation between replicates was less than in replicate measurements of corticosterone. Our results support findings from a previous housing density study (Peters and Festing, 1990), in which adrenal weight was reduced in the more densely housed mice. In a study in which 3 density groups were compared, plasma corticosterone was slightly reduced as density increased, but the differences were not statistically significant due to the high variation in values between mice (Reeb et al., 1998). Plasma corticosterone may not be a robust indicator of chronic stress in mice because it can rise within a few minutes in response to removing a mouse from the pen to draw blood. Mice may respond differently and sampling time may also vary, resulting in highly variable results among animals. Fecal corticosterone may represent a better way to assess the stress level (Harper et al., 2003; Nicholson et al., 2009; Rakowski-Anderson et al., 2012) because it represents average corticosterone over a longer period of time.
Growth curves did not differ between density groups in our study, although mice at higher densities weighed less. The lighter body weight of mice at the higher density is consistent with our observation that they consumed less food. In most previous studies growth was not affected, but in some studies growth was reduced at higher densities (Laber et al., 2008; Ortiz et al., 1984). No mortality was observed at either density in our study. In 2 previous studies, increased density lowered mortality (Fullwood et al., 1998; McGlone et al., 2001).
Comparison of Cage Temperature and Air Quality
At the greater density, pen temperature was about 3°C higher. Mice at the higher density consumed less food, likely because they needed fewer calories to stay warm. The Guide does not discuss temperature inside the cage, but recommends an animal room temperature of 18–26°C. The animal room for this experiment was maintained at 22 ± 2°C. The literature indicates that the core temperature of mice is 37.5°C and that their thermoneutral zone is 30–34°C (Gordon, 2004). When given a choice, mice seek a warmer temperature, preferring 30°C compared to cooler temperatures (Gaskill et al., 2009, 2012; Gordon et al., 2001; Murakami and Kinoshita, 1978). Therefore, in our study, the elevated pen temperature of males to 31°C at the higher density was within their thermoneutral zone. However, our experiments were not designed to test temperature limits. Should further experimental evidence suggest that 31°C is too high for the comfort and well-being of mice, cage temperature could be lowered by increasing the airflow within the cage or by lowering the room temperature slightly.
Our study was carried out in PIV caging with an airflow rate of 60 air changes/h within the pen. Humidity and ammonia levels remained within acceptable limits at both densities, whether cages were changed weekly or every 2 weeks. In particular, ammonia remained below 25 ppm on the average, although some individual values exceeded that. It is often stated that ammonia levels should be less than 25 ppm. However, as summarized by Reeb-Whitaker and colleagues (Reeb-Whitaker et al., 2001), this threshold is largely based on a single study in rats infected with respiratory mycoplasmosis (Broderson et al., 1976). An ammonia level of 25 ppm is the threshold for humans (American Conference of Governmental Industrial Hygienists, 1996). As previously reported (Broderson et al., 1976), the most sensitive indicator of damage from chronic ammonia exposure in rats is histological changes in nasal passages. We have tested levels >100 ppm on young mice and found no effect on the histology of nasal passages (Reeb-Whitaker et al., 2001). In our current study, we report average levels of ammonia. If an institution plans to house mice more densely and wishes to avoid all ammonia levels greater than 25 ppm, it has 2 options: increase the frequency of cage changes or increase the airflow (most ventilated caging can handle 100 air changes/h).
Comparing this Study with Previous Studies
A number of studies have examined the effects of housing animals at different densities, evaluating health and well-being by growth, survival, aggression, plasma corticosterone levels, adrenal weight, immune response, and behavior (Davidson et al., 2007; Fullwood et al., 1998; Laber et al., 2008; McGlone et al., 2001; Nicholson et al., 2009; Ortiz et al., 1984; Ortiz et al., 1985; Peters and Festing, 1990; Smith et al., 2005; Smith et al., 2004; Van Loo et al., 2001).
Many studies report positive health benefits in more densely housed mice, including enhanced immune response (Fullwood et al., 1998; McGlone et al., 2001), reduced anxiety as shown by behavioral tests (Davidson et al., 2007), and increased grooming behavior (McGlone et al., 2001). Our survey of the literature, along with published reviews (Nicholson et al., 2009; Smith and Corrow, 2005), reveals that multiple study designs have come to the same general conclusion that, for most strains, mice can be housed at a density twice that recommended by the Guide. Our current study upholds this observation. However, comparing housing density studies to identify general best practices for husbandry is hindered by the wide variation in experimental design among them. Previous studies usually had a duration of less than 4 months, often only one sex was considered, and the numbers and varieties of measures of health and well-being were limited. Additionally, some studies were underpowered (Davidson et al., 2007; Laber et al., 2008; McGlone et al., 2001; Ortiz et al., 1984, 1985), and some (Laber et al., 2008; Ortiz et al., 1984, 1985) considered each mouse as a biological replicate. These studies have not led to changes in recommendations for housing density, even in the most recent edition of the Guide, which acknowledges the difficulty in comparing studies. Our design addressed some of the limitations of previous studies by using both sexes and larger numbers of mice, using each mouse within the pen as a technical replicate and each pen as a biological replicate, extending the study length to 9 months, and using a broader range of physiological measurements.
Limitations of This Study
No adverse effects of increased housing density were found in our study. This might suggest that our study did not include performance indicators to detect these effects or did not achieve study densities high enough to result in adverse effects. A previous study that found no adverse effect of increased density (Smith et al., 2004) was immediately followed by an experiment with several higher densities, which did show an adverse effect (increased rate of barbering) (Smith et al., 2005) when mice were housed at 20.6 cm2 (3.2 in2), about 4 times the density recommended in the Guide. Thus, future studies should include higher densities.
Our study had adequate power for detecting differences in those measurements that were quantitative, but did not have adequate power for measurements such as aggression or barbering, measured only as present or absent in a pen. In addition, the B6 strain is not particularly aggressive. Inbred strains vary considerably in aggression, and future studies should include multiple strains and quantitative measures of behavior. In light of a previous report suggesting that social contact is more important as mice age (Van Loo et al., 2004), a study that considers the lifespan of mice at various housing densities would be especially informative.
Housing density was increased in our study by adding more mice to a standard institutional caging system already in place at The Jackson Laboratory. This design confounds the effect of group size with housing density, but it more closely reflects the likelihood that, once an institution has made a major capital investment in caging, it will increase density by adding mice to a cage rather than by switching to different-sized cages. Three previous studies maintained a constant group size and achieved increased density by reducing the size of the cage (Davidson et al., 2007; Fullwood et al., 1998; McGlone et al., 2001); the conclusions in these studies did not differ from ours.
Our study has other limitations. Only one inbred strain was used, so key features must be replicated in other inbred strains with inherently different body size or metabolic rate, and even in hybrid or outbred stocks. Additional measures of well-being, such as fecal corticosterone levels, behavior, and immune function, could also be included. Finally, our study was carried out in PIV caging; it is not clear whether the results can be extended to caging that is not individually ventilated. Although the effects of increased housing density on mice may be independent of cage type, the cage microenvironment is likely to be different in ventilated cages compared to conventional ones, and there would be concerns about air quality in conventional cages.
Conclusions
Because mice are social animals, it makes sense that they are less stressed when in groups, and, as multiple studies have indicated, that their well-being will not be compromised if they are housed more densely than recommended by the Guide. In fact, some evidence indicates that well-being may be improved by increasing housing density. The Guide standards, although based on best professional judgment rather than empirical data, were quite reasonable for the circumstances that existed when they were first set. But now, major refinements in animal room cleanliness and the wide use of ventilated caging have improved air quality and animal health. Additional studies extending our findings to more strains for longer periods of time and including additional indicators of well-being would be useful in reconsidering the current space limitations for mice.
Supplementary Material
Footnotes
The authors wish to thank Ellen Smith, Sara Connolly, Phyllis Magnani, Sarah Mabus, Holly Savage, Linda Jorgenson, Stacey Dannenberg, and Willson Roper III for outstanding technical assistance, Joanne Currer for scientific editing, Jesse Hammer for preparation of figures, and Drs. LeahRae Donahue and James Nelson for their constructive review of the manuscript. This work was supported by grant RR12552 (to BP) from the national Center for Research Resources, National Institutes of Health, by HL66611 (to LLP) from the Program for Genomic Applications, Heart, Lung and Blood Institute, and the National Institutes of Health Cancer Core grant CA34196 to The Jackson Laboratory.
LITERATURE CITED
- Agresti A. Page 138 in An Introduction to Categorical Data Analysis. Wiley; Hoboken, NJ: 2007. Chapter 5: Building and applying logistic regression models. [Google Scholar]
- American Conference of Governmental Industrial Hygienists. 1996 threshold limit values for chemical substances and physical agents and biological exposure indices; 1996. p. 119. [Google Scholar]
- Bernberg E, I, Andersson J, Tidstrand S, Johansson ME, Bergstrom G. Repeated exposure to stressors do not accelerate atherosclerosis in apoe−/− mice. Atherosclerosis. 2009;204:90–95. doi: 10.1016/j.atherosclerosis.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Broderson JR, Lindsey JR, Crawford JE. The role of environmental ammonia in respiratory mycoplasmosis of rats. Am J Pathol. 1976;85:115–130. [PMC free article] [PubMed] [Google Scholar]
- Davidson LP, Chedester AL, Cole MN. Effects of cage density on behavior in young adult mice. Comp Med. 2007;57:355–359. [PubMed] [Google Scholar]
- Fullwood S, Hicks TA, Brown JC, Norman RL, McGlone JJ. Floor space needs for laboratory mice: C56bl/6 males in solid-bottom cages with bedding. ILAR J. 1998;39:29–36. doi: 10.1093/ilar.39.1.29. [DOI] [PubMed] [Google Scholar]
- Gaskill BN, Rohr SA, Pajor EA, Lucas JR, Garner JP. Some like it hot: Mouse temperature preferences in laboratory housing. Applied Anim Behav Sci. 2009;116:279–285. [Google Scholar]
- Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP. Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest. PLoS One. 2012;7(3):e32799. doi: 10.1371/journal.pone.0032799. Epub 2012 Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AJ, Billing RL, Einstein R. The effects on heart rate and temperature of mice and vas deferens responses to noradrenaline when their cage mates are subjected to daily restraint stress. Laboratory animals. 2008;42:140–148. doi: 10.1258/la.2007.06030e. [DOI] [PubMed] [Google Scholar]
- Gordon CJ. Effect of cage bedding on temperature regulation and metabolism of group- housed female mice. Comp Med. 2004;54:63–68. [PubMed] [Google Scholar]
- Gordon CJ, Kimm-Brinson KL, Padnos B, Ramsdell JS. Acute and delayed thermoregulatory response of mice exposed to brevetoxin. Toxicon. 2001;39:1367–1374. doi: 10.1016/s0041-0101(01)00092-7. [DOI] [PubMed] [Google Scholar]
- Harper JM, Galecki AT, Burke DT, Pinkosky SL, Miller RA. Quantitative trait loci for insulin-like growth factor i, leptin, thyroxine, and corticosterone in genetically heterogeneous mice. Physiological genomics. 2003;15:44–51. doi: 10.1152/physiolgenomics.00063.2003. [DOI] [PubMed] [Google Scholar]
- Laber K, Veatch LM, Lopez MF, Mulligan JK, Lathers DM. Effects of housing density on weight gain, immune function, behavior, and plasma corticosterone concentrations in balb/c and c57bl/6 mice. J Am Assoc Lab Anim Sci. 2008;47:16–23. [PMC free article] [PubMed] [Google Scholar]
- McGlone JJ, Anderson DL, Norman RL. Floor space needs for laboratory mice: Balb/cj males or females in solid-bottom cages with bedding. Contemp Top Lab Anim Sci. 2001;40:21–25. [PubMed] [Google Scholar]
- Meijer MK, van Loo PL, Baumans V. There’s a rat in my room! Now what? Mice show no chronic physiological response to the presence of rats. Journal of applied animal welfare science : JAAWS. 2009;12:293–305. doi: 10.1080/10888700902955849. [DOI] [PubMed] [Google Scholar]
- Murakami H, Kinoshita K. Temperature preference of adolescent mice. Lab Anim Sci. 1978;28:277–281. [PubMed] [Google Scholar]
- Naidu S, Winget CM, Jenner JW, Mele G, Holley DC. Effects of housing density on mouse physiology and behavior in the nasa animal enclosure module simulators. Journal of gravitational physiology : a journal of the International Society for Gravitational Physiology. 1995;2:P140. [PubMed] [Google Scholar]
- Nicholson A, et al. The response of c57bl/6j and balb/cj mice to increased housing density. J Am Assoc Lab Anim Sci. 2009;48:740–753. [PMC free article] [PubMed] [Google Scholar]
- Ortiz R, Armario A, Castellanos JM. Post-weaning differential housing and testosterone secretion in male mice. Experientia. 1984;40:1428–1429. doi: 10.1007/BF01951927. [DOI] [PubMed] [Google Scholar]
- Ortiz R, Armario A, Castellanos JM, Balasch J. Post-weaning crowding induces corticoadrenal hyperreactivity in male mice. Physiol Behav. 1985;34:857–860. doi: 10.1016/0031-9384(85)90003-4. [DOI] [PubMed] [Google Scholar]
- Peters A, Festing M. Population density and growth rate in laboratory mice. Lab Anim. 1990;24:273–279. doi: 10.1258/002367790780866227. [DOI] [PubMed] [Google Scholar]
- Peters LL, et al. Large-scale, high-throughput screening for coagulation and hematologic phenotypes in mice. Physiol Genomics. 2002;11:185–193. doi: 10.1152/physiolgenomics.00077.2002. [DOI] [PubMed] [Google Scholar]
- Rakowski-Anderson T, et al. Fecal corticosterone levels in RCAN1 mutant mice. Comp Med. 2012;62:87–94. [PMC free article] [PubMed] [Google Scholar]
- Reeb CK, et al. Microenvironment in ventilated animal cages with differing ventilation rates, mice populations, and frequency of bedding changes. Contemp Topics in Lab Anim Sci. 1998;37:43–49. [PubMed] [Google Scholar]
- Reeb-Whitaker CK, et al. The impact of reduced frequency of cage changes on the health of mice housed in ventilated cages. Lab Anim. 2001;35:58–73. doi: 10.1258/0023677011911381. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, et al. A novel chronic social stress paradigm in female mice. Hormones and behavior. 2010;57:415–420. doi: 10.1016/j.yhbeh.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Smith AL, Corrow DJ. Modifications to husbandry and housing conditions of laboratory rodents for improved well-being. ILAR J. 2005;46:140–147. doi: 10.1093/ilar.46.2.140. [DOI] [PubMed] [Google Scholar]
- Smith AL, Mabus SL, Muir C, Woo Y. Effects of housing density and cage floor space on three strains of young adult inbred mice. Comp Med. 2005;55:368–376. [PubMed] [Google Scholar]
- Smith AL, Mabus SL, Stockwell JD, Muir C. Effects of housing density and cage floor space on c57bl/6j mice. Comp Med. 2004;54:656–663. [PubMed] [Google Scholar]
- Sterlemann V, et al. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: Implications for stress-related disorders. Hormones and behavior. 2008;53:386–394. doi: 10.1016/j.yhbeh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Svenson KL, Bogue MA, Peters LL. Invited review: Identifying new mouse models of cardiovascular disease: A review of high-throughput screens of mutagenized and inbred strains. J Appl Physiol. 2003;94:1650–1659. doi: 10.1152/japplphysiol.01029.2003. discussion 1673. [DOI] [PubMed] [Google Scholar]
- Svenson KL, et al. Multiple trait measurements in 43 inbred mouse strains capture the phenotypic diversity characteristic of human populations. J Appl Physiol. 2007;102:2369–2378. doi: 10.1152/japplphysiol.01077.2006. [DOI] [PubMed] [Google Scholar]
- Van Loo PL, et al. Impact of ‘living apart together’ on postoperative recovery of mice compared with social and individual housing. Lab Anim. 2007;41:441–455. doi: 10.1258/002367707782314328. [DOI] [PubMed] [Google Scholar]
- Van Loo PL, Mol JA, Koolhaas JM, Van Zutphen BF, Baumans V. Modulation of aggression in male mice: Influence of group size and cage size. Physiol Behav. 2001;72:675–683. doi: 10.1016/s0031-9384(01)00425-5. [DOI] [PubMed] [Google Scholar]
- Van Loo PL, Van de Weerd HA, Van Zutphen BF, Baumans V. Preference for social contact versus environmental enrichment in male laboratory mice. Lab Anim. 2004;38:178–188. doi: 10.1258/002367704322968867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.