Abstract
A library of more than 200 novel uncharged oxime reactivators was used to select and refine lead reactivators of human acetylcholinesterase (hAChE) covalently conjugated with sarin, cyclosarin, VX, paraoxon and tabun. N-substituted 2-hydroxyiminoacetamido alkylamines were identified as best reactivators and reactivation kinetics of the lead oximes, RS41A and RS194B, were analyzed in detail. Compared to reference pyridinium reactivators, 2PAM and MMB4, molecular recognition of RS41A reflected in its Kox constant was compromised by an order of magnitude on average for different OP-hAChE conjugates, without significant differences in the first order maximal phosphorylation rate constant k2. Systematic structural modifications of the RS41A lead resulted in several-fold improvement with reactivator, RS194B. Kinetic analysis indicated Kox reduction for RS194B as the main kinetic constant leading to efficient reactivation. Subtle structural modifications of RS194B were used to identify essential determinants for efficient reactivation. Computational molecular modeling of RS41A and RS194B interactions with VX inhibited hAChE, bound reversibly in Michaelis type complex and covalently in the pentacoordinate reaction intermediate suggests that the faster reactivation reaction is a consequence of a tighter RS194B interactions with hAChE peripheral site (PAS) residues, in particular with D74, resulting in lower interaction energies for formation of both the binding and reactivation states. Desirable in vitro reactivation properties of RS194B, when coupled with its in vivo pharmacokinetics and disposition in the body, reveal the potential of this oxime design as promising centrally and peripherally active antidotes for OP toxicity.
Keywords: Oxime reactivation, organophosphate intoxication, CNS AChE reactivation, molecular modeling, peripheral site
1. Introduction
Research on new class of oximes capable of reactivating organophosphate (OP) inhibited acetylcholinesterase (AChE) in the central nervous system (CNS) of nerve agent or pesticide OP exposed individuals has intensified during past years emphasizing the importance of maintaining a balance of cholinergic activity in the CNS (1 – 7). Our efforts to identify efficient uncharged oxime reactivators based on seeking compounds amenable to protonation using medium sized libraries of diverse chemical scaffolds have recently led to identification of N-substituted 2-hydroxyiminoacetamido alkylamines as very promising reactivators of VX, sarin, cyclosarin, paraoxon and tabun inhibited hAChE (6,7). We systematically modified the structure of our initially identified lead, oxime RS41A (6) to result in enhanced in vitro reactivation potency of several new oximes culminating with our current lead RS194B (7). Both in vitro and in vivo reactivation properties of RS194B were recently analyzed in detail (7) indicating to be a most promising centrally active reactivator antidotes of those under in vitro and in vivo investigation. In this study we prepared several close congeners of RS194B with an aim of deconstructing critical structural elements contributing to its in vitro reactivation activity. We furthermore analyzed in detail computational molecular models of RS41A and RS194B bound to the VX-hAChE conjugate in a reversible, Michaelis-Menten type complex and as covalent pentacoordinate reactivation intermediate seeking to identify key enzyme residues involved in this reactivation reaction.
2. Material and Methods
2.1. Enzyme
Highly purified monomeric hAChE was prepared as described earlier (c.f. 6,7).
2.2. Chemicals
Organophosphates
Low toxicity, nonvolatile fluorescent methylphosphonates (Flu-MPs) (8) were used in in vitro experiments as analogues of nerve agents sarin, cyclosarin and VX as described earlier (6,7). Paraoxon was from Sigma-Aldrich (St. Louis MO, USA). Nerve agent OPs tabun, VX, sarin and soman used in in vivo experiments were from NC Laboratory (Spiez, Switzerland).
Oximes
Synthesis and initial characterization of novel oxime reactivators were recently reported (6,7). 2PAM (2-Pyridinealdoxime methiodide), MINA (monoisonitrosoacetone), and DAM (2,3-butanedione monoxime), acetylthiocholine iodide (ATCh) and 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) were from Sigma-Aldrich. Oxime MMB4 (dimethylsulfonate) was kindly donated by Dr. Tony Shih, USAMRICD, Edgewood, USA.
2.3. In vitro oxime reactivation assays and in vivo experiments
All enzyme activity, in vitro oxime reactivation assays and in vivo experiments were performed as reported earlier (6,7).
2.4. In silico molecular modeling
Molecular modeling was performed as described (7).
3. Results and Discussion
We present here a multifaceted, collaborative analysis on the interaction of our lead uncharged reactivator RS194B with covalent OP-hAChE conjugates, probed by targeted in vitro structure/activity analysis, in silico molecular modeling and in vivo testing using a mouse model.
In vitro reactivation
Reactivation kinetics of covalent OP-hAChE conjugates obtained from paraoxon, sarin, cyclosarin, VX and tabun inhibition of hAChE was studied by eight congeneric RS194B analogues (Table 1). Modifications of three elements of RS194B structure were considered: the heterocyclic (azepine) ring, the acetamide in the immediate vicinity of the nucleophilic oxime and an intervening aliphatic linker. None of modifications in the eight analogues led to improved reactivation potency over RS194B. Fluorination of the ring in position 4 or substitution of sulfur for carbon in the same position to achieve a modest pKa reduction of the cyclic amine (from the pKa 8.8 (7) to the expected pKa of ~ 8.4) compromised reactivation rates significantly. This reveals a restricted fit of the heterocycle within narrow confines of the OP-hAChE active center gorge. Similarly, quaternization of the cyclic amine reduced average reactivation potency for the RS2-162B oxime by about one order of magnitude, compared to RS194B. Both, k2 and Kox constants were compromised (Table 2; Figure 1) suggesting looser fit in both, reversible complex formation (increase in Kox) and in the pentacoordinate intermediate stabilization (decrease in k2). This is consistent with either loss of hydrogen bonding of the quaternary ammonium or enhanced steric hindrance when compared to protonated tertiary cyclic amine. One of the main stabilizing interactions of RS194B with OP-hAChE conjugates thus could be a hydrogen bond formation, possibly with Asp 74 of the hAChE peripheral site. Methylation of the intervening linker resulted in small reduction of an average reactivation rate (compound RS2-103B, Table 1), but when it was combined with keto group introduction vicinal to the ring nitrogen (in the RS2-99B compound) the loss of reactivation potency was complete. This is consistent with earlier observations that protonation of heterocyclic N resulted in enhanced OP-hAChE reactivation (7). Any structural modification in the immediate vicinity of the oxime nucleophile virtually incapacitated the reactivation properties of the compound (RS283A, RS292A, RS275D) revealing steric restrictions in the approach of the anionic oximate oxygen to the active Ser203 conjugated P atom.
Table 1.
Structures and reactivation rates, kobs (for reactivation of VX-hAChE, sarin-hAChE, cyclosarin-hAChE and paraoxon-hAChE conjugates) by close structural analogues of RS194B at 0.67 mM concentration. Reactivation rates for each oxime are listed as an average of four kobs values determined individually for each of the four OP-hAChE conjugates. Values are shown relative to 2PAM kobs values. All experiments were performed at 37 °C in 0.1M phosphate buffer pH 7.4.
| Oxime | kobs | Oxime | kobs | ||
|---|---|---|---|---|---|
| RS41A |
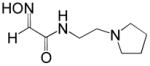
|
0.88 | RS2-140B |
|
0.080 |
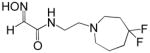
| RS194B |
|
2.5 | RS2-148B |
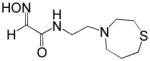
|
0.32 |
| RS2-99B |
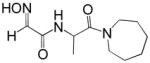
|
0.0 | RS283A |
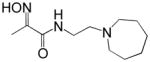
|
0.027 |
| RS2-103B |
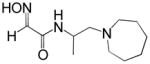
|
1.2 | RS292A |
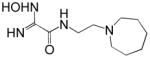
|
0.0060 |
| RS2-162B |
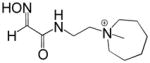
|
0.15 | RS275D |
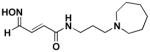
|
0.16 |
Table 2.
Kinetic constants for reactivation of paraoxon-, sarin-, cyclosarin-, VX- and tabun-hAChE conjugates by RS194B, RS41A, quaternary, charged RS194B analogue RS2-162B and reference oximes 2PAM, MMB4, MINA and DAM. Maximal reactivation rate constant (k2, min−1), apparent dissociation constant of [oxime * OP-hAChE conjugate] reversible complex (Kox, mM) and overall second order reactivation rate constant (kr, M−1min−1) were determined as described before (7) at 37 °C in 0.1M phosphate buffer pH 7.4. Standard errors of determined kinetic constants were typically less than 30% of the mean.
| Oxime | Paraoxon | Sarin | Cyclosarin | VX | Tabun | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k2 | Kox | kr | k2 | Kox | kr | k2 | Kox | kr | k2 | Kox | kr | k2 | Kox | kr | |
| RS194B* | 0.38 | 7.4 | 51 | 2.5 | 1.9 | 1300 | 0.88 | 3.9 | 230 | 3.1 | 2.1 | 1600 | 0.0018 | 1.8 | 1.0 |
| RS2-162B | nd | nd | nd | 0.38 | 3.5 | 110 | 0.50 | 18 | 28 | 0.40 | 4.2 | 95 | nd | nd | nd |
| RS41A* | 0.15 | 4.3 | 35 | 3.7 | 11 | 340 | 1.9 | 21 | 90 | 3.1 | 4.6 | 670 | 0.00082 | 3.6 | 0.22 |
| MINA* | > 0.20 | >20 | 8.4 | 1.6 | 14 | 120 | 1.2 | 16 | 75 | >0.7 | >6.0 | 110 | - | - | 0.039 |
| DAM* | 0.027 | 46 | 0.58 | 0.13 | 88 | 1.5 | >0.30 | >100 | 2.5 | 0.20 | >100 | 1.7 | - | - | 0.0038 |
| 2PAM* | 0.27 | 1.8 | 150 | 1.1 | 0.34 | 3200 | 0.73 | 6.6 | 110 | 0.65 | 0.25 | 2600 | 0.0058 | 1.5 | 3.8 |
| MMB4 | 0.70 | 1.5 | 470 | 4.0 | 0.82 | 4900 | 8.2 | 2.5 | 3500 | 3.4 | 1.3 | 2600 | nd | nd | nd |
Data from (7).
nd – not determined.
Figure 1.
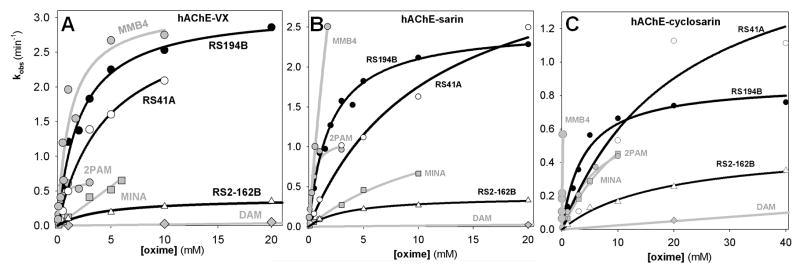
Concentration dependence of oxime reactivation of A) VX, B) sarin, and C) cyclosarin inhibited (conjugated) hAChE. Dependence for oximes RS194B (●) and RS41A (○) compared to RS2-162B, the charged analogue of RS194B and reference uncharged (DAM;
 and MINA;
and MINA;
 ) and cationic (2PAM;
) and cationic (2PAM;
 , MMB4;
, MMB4;
 ) oximes (measured at 37 °C in 0.10M phosphate buffer pH 7.4).
) oximes (measured at 37 °C in 0.10M phosphate buffer pH 7.4).
In silico modeling
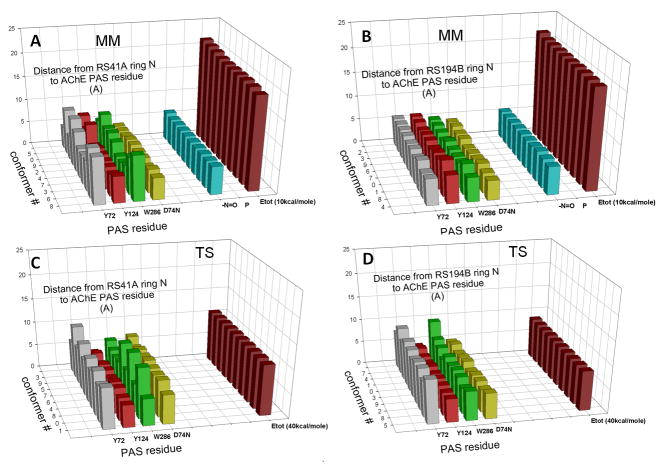
Computational analysis of interactions of RS41A and RS194B oximes with O-ethyl, methylphosphonylated human AChE at the active Ser203 resulting from covalent inhibition by VX shows extensive interactions of heterocyclic oxime rings with peripheral site (PAS) residues of hAChE in all calculated conformers (Figure 2). This was observed in models of both reversible Michaelis type complexes (MM state) and pentacoordinate reaction intermediates (TS state). Visual comparison of geometries of two reaction states for each of the two oximes reveals higher similarity for the in vitro and in vivo more reactive RS194B reactivator. A detailed inventory of distances between ring nitrogen atoms of an oxime and individual PAS residues of the VX-hAChE conjugate reveals generally tighter binding of RS194B oxime models in both reaction states (Figure 3; Table 3). The heterocyclic oxime nitrogen of RS41A was on average 5.9 Å and 6.9 Å removed from four residues of the PAS (Y72, Y124, W286 and D74) in the reversible (Michaelis-Menten) complex and the pentacordinate reaction intermediate, respectively. Corresponding average distances in models of RS194B conformers were 5.3 Å and 6.3 Å, respectively. Out of all PAS residues the ring nitrogen was closest to D74 in the MM state for both oximes (4.3 ± 0.3 Å vs. 4.5 ± 0.3 Å for RS194B vs. RS41A). The rank order of distances to other PAS residues was different. While the MM complex of RS194B was primarily stabilized by W286 and to a lesser extent by Y72 and Y124, all within 0.9 Å distance range (5.2 Å–6.1 Å), the RS41A complex interfaced closer with Y124, than W286 and Y72, but at 2.5 Å range (5.2 Å–7.7 Å). In the TS step RS194B was equally close to Y124 and D74 (4.8 Å and 5.1 Å, respectively) while RS41A was closest to Y124 (4.7 Å) and all other residues were more than 7.1 Å distant. This distance inventory uncovers subtle differences in stabilization of the two oximes within the PAS with RS194B assuming a tighter fit and better overall stabilization in both states. The average oximate–phosphorus distance for both oximes was the same in both MM step (5.9 ± 0.1 Å) and the TS step (covalent bond). The overall conclusion is thus that a tighter fit of RS194B within the PAS is reflected in lower Kox and higher k2 values for this oxime (Table 2; Figure 1) and enhanced overall reactivation efficiency, in vitro.
Figure 2.
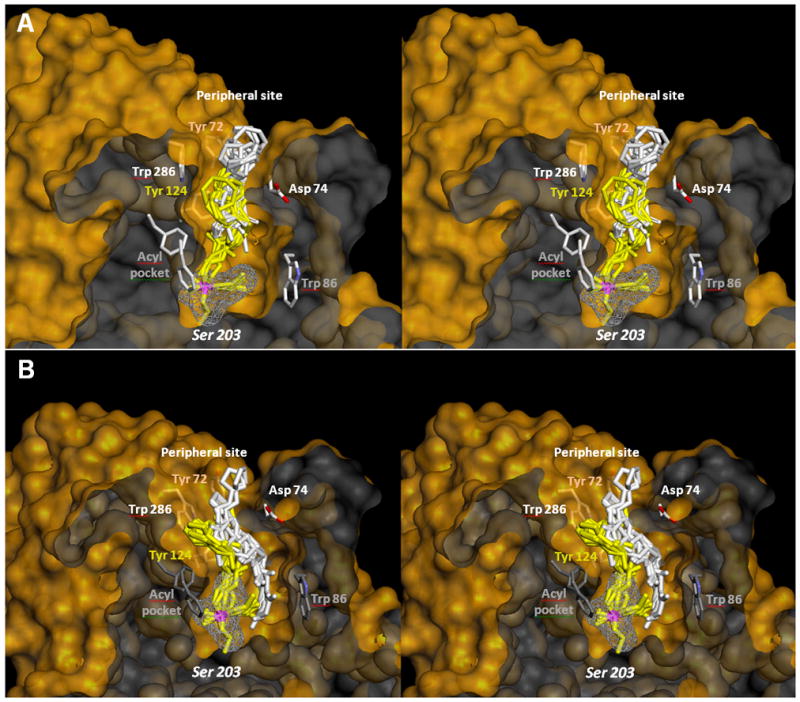
Stereo representation of in silico molecular modeling of VX inhibited AChE showing the reversible Michaelis type complex (white sticks) and covalent pentacoordinate trigonal bipyramidal intermediate (yellow sticks) for interaction of A) oxime RS194B and B) the oxime RS41A. Ten conformers of each oxime are shown in each of two interaction states. Phosphorus atom is colored purple. Solvent accessible part of the hAChE Connolly surface is represented in yellow and solvent inaccessible part of the hAChE molecule in dark grey. Overlapping similarity in global geometries of the reversible complex and trigonal bipyramidal intermediate was observed for RS194B oxime, but not with RS41A oxime.
Figure 3.
Distances between heterocyclic nitrogen atoms of individual conformers of oximes RS41A and RS194B and PAS residues determined in models of their Michaelis-Menten type reversible complexes (MM) and pentacoordinate reaction intermediates (TS) with a model of VX-hAChE conjugate. Distances between the oxime oxygen (-N=O) as a anionic oximate and the electrophilic phosphorus atom (P) are also shown, along with calculated energies of interaction (Etot). Amino acid atoms used in measurements were Cz for tyrosine, CE2 for tryptophan and OE for aspartate.
Table 3.
Distances between heterocyclic nitrogen atoms of oximes RS41A and RS194B and PAS residues determined in models of their Michaelis-Menten type reversible complexes (MM) and pentacoordinate reaction intermediates (TS) with a model of VX-hAChE conjugate. Distances of ten individual conformers, shown in Figure 3 are averaged. Amino acid atoms used in measurements were Cz for tyrosine, CE2 for tryptophan and OE for aspartate. Determined distances for the lowest energy conformer (LEC) are also given.
| oxime | Reaction step | Distance from ring N (A) ± SD (n=10) | ||||
|---|---|---|---|---|---|---|
| Y72 | Y124 | W286 | D74 | average | ||
| RS41A | MM | 7.7 ± 2.2 | 5.2 ± 1.1 | 6.2 ± 1.9 | 4.5 ± 0.3 | 5.9 ± 1.9 |
| LEC | 9.9 | 5.6 | 9.6 | 4.6 | 7.5 | |
| TS | 8.5 ± 1.3 | 4.7 ± 0.5 | 7.1 ± 2.5 | 7.1 ± 0.9 | 6.9 ± 2.0 | |
| LEC | 8.6 | 4.6 | 5.6 | 6.1 | 6.2 | |
| RS194B | MM | 5.8 ± 0.4 | 6.1 ± 0.5 | 5.2 ± 0.5 | 4.3 ± 0.3 | 5.3 ± 0.8 |
| LEC | 5.4 | 5.8 | 5.0 | 4.0 | 4.5 | |
| TS | 8.7 ± 0.8 | 4.8 ± 0.2 | 6.6 ± 1.3 | 5.1 ± 0.3 | 6.3 ± 1.7 | |
| LEC | 9.3 | 4.9 | 6.2 | 5.5 | 6.5 | |
In vivo efficacy
Exceptionally low toxicity of RS194B determined for i.m. administration in mice (LD50 ≥ 500mg/kg), favorable pharmacokinetic properties, including rapid brain penetration in mice (7), and good in vitro reactivation efficacy all represent essential elements for an oxime to become an efficacious antidote in treatment of OP intoxication. Indeed both therapeutic and a combination of therapeutic and prophylactic efficacies of RS194B in treatment of nerve agent and pesticide OP exposed mice yielded surprisingly high protective indices upon i.m. oxime administration (Table 4; 7).
Table 4.
Treatment of OP exposed mice with oxime RS194B. In therapy mice were administered i.m. with 125 mg/kg RS194B (+ atropine) 1 min after s.c. administration of OP. In the combined treatment mice were pretreated with 125 mg/kg RS194B i.m. 15 min before OP and then administered with another 125 mg/kg RS194B (+ atropine) 1 min after s.c. administration of OP. Protective index is the ratio of LD50 values for OP exposed animals with and without oxime treatment. Modified from (7).
| Treatment | Protective index
|
||||
|---|---|---|---|---|---|
| VX | Sarin | Paraoxon | Soman | Tabun | |
| Therapy | 18 | 10 | 9.4 | 1.8 | 1.5 |
| Pretreatment + Therapy | 45 | 4.5 | 22 | 2.8 | 2.0 |
Low molecular weight compounds that cross the blood-brain barrier by passive diffusion such as RS194B should be expected to be absorbed orally, since both require passage across a lipid membrane. Studies are currently underway to measure bioavailability and rates of oral absorption in the mouse (data not shown). The findings, to date, look sufficiently promising to begin studies of a loading and maintenance dose regimen for antidotes to ascertain: (a) concentrations in blood, skeletal muscle and brain, (b) oral bioavailability from areas under the curve following i.v. and p.o. dosing, (c) volume of distribution and clearance, (d) rates of oral absorption, and (e) suitable loading and maintenance dose regimens to follow organophosphate exposure. Since the individual organophosphates themselves differ in their toxicokinetics and body disposition, pharmacokinetic considerations are likely to be important criteria for optimizing efficacy of the antidotes.
4. Conclusion
Our in vitro and in silico analyses presented in this study consistently indicate that RS194B owes its enhanced reactivation potency to the extensive interaction of its puckered azepine ring with the peripheral site of hAChE, while directing its reactive oximate anion towards the electrophilic phosphorus of the OP-hAChE conjugate. The primary stabilizing interactions are with D74 and W286A residues of the PAS. The possibility of hydrogen bond formation between protonated cyclic amine and D74 should be considered and justifies future mutagenesis studies in this region.
Outstanding in vivo efficacy of RS194B in the treatment of OP exposed mice coupled with very promising preliminary pharmacokinetic results of oral dosing in mice reveals antidotal potential of this oxime becoming widely used in prophylaxis as well as in antidotal therapy of OP exposure and intoxication.
Highlights.
RS194B interaction with OP-hAChE peripheral site enhances reactivation in vitro.
In silico models show RS194B azepine nitrogen in close contact with D74 and W286.
Oral intake of RS194B into OP exposed mice could improve the antidotal treatment.
Acknowledgments
This research was supported by the CounterACT Program, National Institutes of Health Office of the Director (NIH OD), and the National Institute of Neurological Disorders and Stroke (NINDS), Grant Number U01 NS058046.
Footnotes
Conflict of Interest. None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mercey G, Verdelet T, Saint-André G, Gillon E, Wagner A, Baati R, Jean L, Nachon F, Renard PY. Chem Commun. 2011;475:295–5297. doi: 10.1039/c1cc10787a. [DOI] [PubMed] [Google Scholar]
- 2.de Koning MC, van Grol M, Noort D. Toxicol Lett. 2011;206:54–59. doi: 10.1016/j.toxlet.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Kalisiak J, Ralph EC, Zhang J, Cashman JR. J Med Chem. 2011;54:3319–3330. doi: 10.1021/jm200054r. [DOI] [PubMed] [Google Scholar]
- 4.Demar JC, Clarkson ED, Ratcliffe RH, Campbell AJ, Thangavelu SG, Herdman CA, Leader H, Schulz SM, Marek E, Medynets MA, Ku TC, Evans SA, Khan FA, Owens RR, Nambiar MP, Gordon RK. Chem Biol Interact. 2010;187:191–198. doi: 10.1016/j.cbi.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia GE, Campbell AJ, Olson J, Moorad-Doctor D, Morthole VI. Chem Biol Interact. 2010;187:199–206. doi: 10.1016/j.cbi.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 6.Sit RK, Radć Z, Gerardi V, Zhang L, Garcia E, Katalinić M, Amitai G, Kovarik Z, Fokin VV, Sharpless KB, Taylor P. J Biol Chem. 2011;286:19422–19430. doi: 10.1074/jbc.M111.230656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radić Z, Sit RK, Kovarik Z, Berend S, Garcia E, Zhang L, Amitai G, Green C, Radić B, Fokin VV, Sharpless KB, Taylor P. J Biol Chem. 2012;287:11798–11809. doi: 10.1074/jbc.M111.333732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amitai G, Adani R, Yacov G, Yishay S, Teitlboim S, Tveria L, Limanovich O, Kushnir M, Meshulam H. Toxicology. 2007;233:187–198. doi: 10.1016/j.tox.2006.09.020. [DOI] [PubMed] [Google Scholar]