Abstract
Objectives
This study examines cognitive outcomes for alcohol drinking status over time, across cognitive ability and age groups.
Methods
Data (1998-2005) from N=571 Seattle Longitudinal Study participants age 45+years (middle-aged: 45-64, young-old: 65-75, old-old: 75+) were analyzed to examine the alcohol drinking status effect (e.g. abstinent, moderate (≤7 drinks/week), at-risk (≥8 drinks/week)) on cognitive ability (e.g., Memory, Reasoning, Spatial, Verbal Number, Speed abilities).
Results
Findings indicated that alcohol drinking status was associated with change in verbal ability, spatial ability, and perceptual speed. Decline in verbal ability was seen among alcohol abstainers and moderate alcohol consumers, but at-risk drinkers displayed relative stability. At-risk old-old adults and middle-aged adults (regardless of drinking status), displayed relative stability in spatial ability. Decline in spatial ability was however present among young-old adults across drinking status, and among abstaining and moderate drinking old-old adults. At-risk drinkers showed the most positive spatial ability trajectory. A gender effect in perceptual speed was detected, with women who abstained from drinking displaying the most decline in perceptual speed compared with women that regularly consumed alcohol, and men displaying decline in perceptual speed across drinking status.
Discussion
In this study, consuming alcohol is indicative of cognitive stability. This conclusion should be considered cautiously, due to study bias created from survivor effects, analyzing two time points, health/medication change status, and overrepresentation of higher socioeconomic status and white populations in this study. Future research needs to design studies that can make concrete recommendations about the relationship between drinking status and cognition.
Keywords: alcohol, cognition, aging
INTRODUCTION
Adults are recommended to reduce alcohol consumption as they age. This recommendation stems from a decrease in endogenous water levels in which to dilute alcohol, leading to a higher blood alcohol concentration in older adults compared to younger adults after consuming the same amount of alcohol (Dufour, Archer, & Gordis, 1992). For both men and women it has been suggested that individuals aged 65 and over should, on average, consume no more than one drink a day (Blow, 2004; National Institute on Aging, 2012; National Institute on Alcohol Abuse and Alcoholism, 1992). In addition, compared to younger adults, older adults can unknowingly become more impaired when consuming similar doses of alcohol (Gilbertson, Ceballos, Prather, & Nixon, 2009).
Interestingly, there is evidence for alcohol having a positive effect on multiple health outcomes (Sun et al., 2011). Specifically, multiple studies have shown that moderate alcohol consumption (approximately 1 drink/day) protects cognition in aging adults, compared to abstinent or excessive alcohol usage (Ganguli, Vanderbilt, Saxton, Shen, & Dodge, 2005;Rodgers, et al, 2005). Moderate alcohol use also appears to reduce the risk of late life dementia and cognitive impairment (Chan, Chiu, & Chu, 2010; Solfrizzi et al., 2007; Weyerer et al., 2011).
Alcohol consumption has been speculated to affect cognition through its association with higher levels of high-density lipoprotein (HDL), increased sensitivity to insulin over time (Kiechl et al., 1998), increased cerebral blood flow (CBF; Sano et al., 1993), and evidence for an anti-inflammatory function (Albert, Glynn, & Ridker, 2003; Imhof et al., 2004). Collectively, these factors affect the risk of atherosclerosis, which is linked to progressive neurodegeneration (Yi et al., 2009). Additionally, the anti-inflammatory function of alcohol can decrease chronic cerebral inflammation (McGeer & McGeer, 1999). Together, increased cerebral blood flow and decreased inflammation reduces cognitive decline through reducing the risk of vascular pathology and neurodegeneration (Panza et al., 2008).
Contrary to findings indicating beneficial effects of moderate alcohol consumption in older adults, excessive drinking can be very dangerous. Consuming too much alcohol can lead to accelerated neurodegeneration (Cairney, Clough, Jaragba, & Maruff, 2007; Chan et al., 2010). Excessive alcohol consumption can also have negative interactions with medications commonly used by older adults and is a major risk factor for prescription drug misuse (Culberson & Ziska, 2008; McCabe, Cranford, & Boyd, 2006; Onder et al, 2002; Simoni-Wastila & Strickler, 2004). Older adults are actually hospitalized for alcohol-related complications as often as for myocardial infarctions and medication overdoses in older adults are commonly associated with alcohol (Dukes, Robinson, Thomson, & Robinson, 1992; Finkle, McCloskey, Kiplinger, & Bennett, 1976; Ødegård & Rossow, 2004). Excessive alcohol and prescription drugs can: a) undermine the treatment of existing disorders, b) lead to unintentional injuries and consequences (i.e. falls, cognitive impairment, medical complexities), c) lead to fatal alcohol-drug interactions; and d) and increase morbidity and mortality, and health costs in older adults (Barry, Gallagher, & Ryan, 2008; Finlayson, 1995; Larson, Kukull, Buchner, & Reifler, 1987; Philips, Barker, & Eguchi, 2008).
Another wrinkle that needs to be considered is that there is an association between gender, cognition, and alcohol consumption indicating that consuming moderate amounts of alcohol is potentially beneficial for women (Dufouil, Ducimetiere, & Alperovitch, 1997; Elias et al., 1999; Forette et al., 1998; Onland-Moret et al., 2005; Wise et al., 2001). While not fully understood, gender differences in alcohol associations may be attributed to differences in hormone levels. Women who consume alcohol have elevated levels of estrogen (Gavaler & Love, 1992; Onland-Moret, Peeters, van der Schouw, Grobbee, & van Gils, 2005); estrogen has been controversially associated with cognition in women (Wise, Dubal, Wilson, Rau, & Liu, 2001), but more recent hormone replacement trials have indicated an increased risk for dementia (Coker, et al., 2010). Accordingly there is a need to better understand how alcohol consumption differently affects cognition across gender.
Unfortunately, clear-cut alcohol consumption recommendations pertaining to cognitive outcomes cannot be made due to research being limited by several factors, such as cross-sectional designs (Cooper et al., 2009), inclusion of men only (Gross et al., 2011), inclusion of women only (Espeland et al., 2006; Stampfer et al., 2005), gender variance (findings not consistent across gender) (McGuire, Ajani, & Ford, 2007; Stott et al., 2008), global cognitive measurements (Bond et al., 2005; Galanis et al., 2000), and mixed or unsupportive findings (Panza et al, 2009; Townsend, Devore, Kang, & Grodstein, 2009). Clear evidence for alcohol and cognition guidelines should ideally be consistent across gender, across cognitive measurement, and be longitudinally based. Despite the evidence supporting moderate alcohol consumption as a means to prevent cognitive decline and dementia, relatively little is known about the effects of alcohol on specific cognitive domains (Gross et al., 2011). Given the benefits of moderate alcohol consumption and the risks of excessive drinking, the interplay among alcohol use, cognition, and aging requires further study to formulate a more crystallized understanding of the effect alcohol consumption has on cognitive health outcomes (Chiu, 2008; Panza et al, 2009; Peters, Peters, Warner, Beckett, & Bulpitt, 2008).
This study will advance current knowledge by considering the developmental aging perspective and examining the effects of alcohol consumption on cognition over time (longitudinally) and across age groups (Anstey, 2008; Schaie, 2009). Cognitive level differences that exist between middle age (45-65 years of age) and late life need to be better understood as indicated by age-based associations with cognition (Cherbuin et al., 2009; Jarvenpaa, Rinne, Koskenvuo, Raiha, & Kaprio, 2005; Lang, Wallace, Hupper, & Melzer, 2007;). Furthermore, this study will examine effects across cognitive abilities, considering that distinct cognitive domains do not change unilaterally with aging (Schaie, 2005). For example, perceptual speed is one of the first cognitive abilities to decline, while verbal ability remains steadfast until much later in life, at which very modest declines occur. Finally, alcohol and cognition associations with gender will also be considered due to speculation about a gender-based relationship between alcohol and cognition (Dufouil et al, 1997; Elias et al., 1999; Forette et al., 1998; Onland-Moret et al., 2005; Wise et al., 2001). Accordingly, the objective of this study is to evaluate the relationship between cognitive change and alcohol consumption across abilities and age: middle-aged adults (46-64 years old), young-old adults (65-74 years old), and old-old adults (75+ years of age) and gender, using a longitudinal design. Differences in cognitive change will be examined among a) alcohol abstainers, b) moderate alcohol consumers (≤7 drinks/week), and c) at-risk alcohol consumers (≥8 drinks/week), considering the effects of age and gender.
METHODS
The study sample (N = 571) contained adults age 45+ years participating in the 1998 and 2005 waves of the Seattle Longitudinal Study (SLS). The 1998 and 2005 waves were selected because alcohol consumption patterns and cognitive data were collected simultaneously. The SLS database consists of psychological assessments conducted during seven major testing cycles (1956, 1963, 1970, 1977, 1984, 1991, 1998, and 2005). Written consent was acquired during each wave with the understanding that all research data would remain unidentified. Approximately 6000 people have now participated at some time in this study. Of the original participants, 26 people remain who have now been in the study for 50 years. Current participants range in age from 22 to 101 years. Throughout its course, SLS has lost participants to attrition, resulting in the overrepresentation of people with better cognitive performance and good health. No statistical methodology was implemented to account for attrition/missing data. All participants were members of the Group Health Cooperative health maintenance organization of Puget Sound in Washington State at the time they entered into the study. At each interval, all persons who had previously participated were asked to participate in subsequent waves. Potential participant were randomly selected using a sampling-with-replacement methodology within the 420,000 member organization. The research interviews took place in an in-person group setting for cognitive assessments (using a classroom testing approach), with a mail-in homework component for supplement surveys. A more detailed description of the SLS is available in a previous publication (Schaie, 2005).
Measurement Variables
Participants assessed in 1998 (Time 1) and 2005 (Time 2) were given an extensive assessment test battery from which alcohol consumption, cognitive ability, and demographic measurements were extracted.
Alcohol Consumption
Alcohol consumption was calculated from the sum of three open-ended questions assessed in the SLS Health Behavior Questionnaire: 1) How many GLASSES OF WINE did you drink last week?; 2) How many BOTTLES OR CANS OF BEER did you drink last week?; and 3) How many drinks containing HARD LIQUOR did you drink last week? Having three individual alcohol questions can reduce the common practice of underreporting alcohol consumption (Ekholm, Strandberg-Larsen, & Grønbæk, 2011). Furthermore, a one-week recall period is a valid and reliable time frame for alcohol recall (Dawson, 2003). Accordingly, the number of total drinks was categorized into drinking status categories: 1) Alcohol abstainer: no alcohol consumed, 2) Moderate alcohol consumer: no more than 7 drinks/week, and 3) At-risk alcohol consumers: more than 7 drinks/week (Blow, 2004; National Institute on Aging, 2012; National Institute on Alcohol Abuse and Alcoholism, 1992). Alcohol consumption classifications were equivalent across gender and age.
Cognitive Ability
Twenty-nine cognitive ability scores were transformed into six standardized cognitive domain scores; mean score = 50, standard deviation = 10. The cognitive domains were based on Thurstone Primary Mental Abilities (Thurstone, 1962; Thurstone & Thurstone, 1949). The cognitive battery was then expanded and transformed using structural equational modeling to represent latent variables for memory, reasoning, spatial, verbal, numeric, and perceptual speed abilities using well-validated instruments (Schaie, 2005). Transitioning from an observed to a latent variable was done to create more stabilized cognitive constructs. Details on the structural analysis and individual instruments are available in Schaie, 2005. Briefly described, the targeted cognitive domains are:
Memory Ability - memorization and recall of meaningful language units. (Measured by: Immediate Recall (Zelinski, Gilewski, & Schaie, 1993), Delayed Recall (Zelinski, Gilewski, & Schaie, 1993), and Primary Mental Abilities (PMA) Word Fluency (Thurstone & Thurstone, 1949))
Reasoning Ability - recognize and understand novel concepts or relationships; solve logical problems, and foresee and plan. (Measured by: PMA Reasoning (Thurstone & Thurstone, 1949), Adult Development and Enrichment Project (ADEPT) Letter Series (Blieszner, Willis, & Baltes, 1981), Word Series (Schaie, 1985), and Number Series (Thurstone, 1962))
Spatial Ability - visualize and mentally manipulate spatial configurations in two or three dimensions, maintain orientation with respect to spatial objects, and perceive relationships among objects in space. (Measured by: PMA Space (Thurstone & Thurstone, 1949), Object Rotation (Quayhagen, 1979; Schaie, 1985), Alphanumeric Rotation (Willis & Schaie, 1983), and Cube Comparison (Ekstrom et al., 1976))
Verbal ability - understand ideas expressed in words. (Measured by: PMA Verbal Meaning (Thurstone & Thurstone, 1949), Educational Testing Service (ETS) Vocabulary V-2 (Ekstrom et al., 1976), and ETS Vocabulary V-4 (Ekstrom et al., 1976))
Numeric ability - understand numerical relationships, work with figures, and solve simple quantitative problems rapidly and accurately. (Measured by: PMA Number (Thurstone & Thurstone, 1949), Addition (Ekstrom et al., 1976), and Subtraction & Multiplication (Ekstrom et al., 1976))
Perceptual speed - find figures, make comparisons, and carry out simple tasks involving visual perception with speed and accuracy. (Measured by: Identical Pictures (Ekstrom et al., 1976), Findings As (Ekstrom et al., 1976), and Number Comparison (Ekstrom et al., 1976))
Demographics
Age, gender, education, and income were obtained from the 1998 self-report SLS: Life Complexity Inventory Questionnaire, and smoking status from the SLS: Health Behavior Questionnaire.
Data analysis
Twelve linear mixed models were analyzed in SAS 9.1 (Time × Drinking Status × Age and Time × Drinking Status × Gender) for each cognitive domain: memory, reasoning, spatial, verbal, numeric, and speed). The models included Time (2 levels: 1998, 2005), Drinking Status (3 levels: abstinent, moderate, at-risk), and Age-group (3 levels: middle-aged, young-old, old-old) or Gender (2 levels: male, female) as independent variables. Age was treated as a categorical variable to make distinctions and detect patterns across age group instead of creating implications for 50 age categories (Nagin, 1999), as indicated by the age range of study participants. Existing literature has determined education, income and smoking have significant effects on drinking status (Dufouil et al, 1997; Gavaler et al., 1992; Hellwig, 2011; Kawas et al., 1997; Onland-Moret et al., 2005; Schmidt et al., 1996; Wise et al., 2001). Therefore, analyses controlled for these factors in addition to gender and age group and baseline drinking levels (beer, wine, and liquor) to account for within drinking category variation in alcohol consumed. To assess the gender effect for drinking status, linear mixed models were conducted with time, drinking status, and gender serving as independent variables (while still controlling for age-group, education, income, and drinking levels), and smoking status. In all analyses, the cognitive domains served as the dependent variables.
RESULTS
Sample characteristics
The 1998 sample (N=839) consisted of 371 males (44%), with a mean age of 67.44 years (range 45-94). Forty-two percent were middle-aged adults (46-64 years of age), 25% young-old adults (65-74 years of age), and 33% old-old adults (75+ years of age). Between 1998 and 2005, 68% (N=571) of the 1998 sample returned for the 2005 follow-up. Sample differences indicated that the returning sample was less likely to never drink (41% vs. 53%; p=.0055) and reported higher consumption of wine (1.1 vs. 1.8 glasses of wine/week; p=.0023) and proportion of wine consumed, compared to total alcohol consumption (0.21 vs. 0.31; p=.0008) in 1998. The returning sample was also younger (73.8 vs. 64.7 years of age; p=.0001), with more education (15 vs. 16 years; p=.0001) and income ($39,199 vs. $48,749; p=.0001), and higher cognitive abilities across all domains (p<.0001).
Table 1 depicts 1998 demographic characteristics across drinking status for the study sample. There were significant differences (p < .05) for all variables across drinking status, with the exception of smoking status. Abstainers were older and reported less income and education, and there were fewer female at-risk alcohol consumers. As expected, amount of alcohol consumed was significantly greater for the at-risk group across drinking variables. For the moderate drinking group (N=252), the median level of drinking was 3 drinks, the mode was 1 drink, with 25% quartile reporting 1 drink and 75% quartile reporting 5 drinks. For the at-risk drinking group (N=82), the median level of drinking was 12.5 drinks, the mode was 8 drinks, with 25% quartile reporting 9 drinks and 75% quartile reporting 17 drinks. Cognition levels were significantly (p ≤ .05) different across drinking categories, with the exception of Time 2 memory ability, with abstainers generally reporting lower cognitive performance (Table 2).
Table 1.
Baseline (1998) Sample Demographics (N=571)
| Drinking Status | |||
|---|---|---|---|
|
| |||
| Abstainers (n=237, 42%) |
Moderate (n=252, 44%) |
At-risk (n=82, 14%) |
|
| Age (mean(SD) range) | 65.8 (11.1) | 63.6 (11.7) | 62.6 (11.0) |
| Range=45-93 | Range=45-90 | Range=45-84 | |
| Gender (% female) | 63% | 57% | 29% |
| Income (median range) | $40,000-$34,999 | $50,000-$54,999 | $55,000-59,999 |
| Education years (mean (SD)) | 15.0 (2.6) | 16.2 (2.5) | 16.1 (2.9) |
| Range=8-20 | Range=10-20 | Range=7-20 | |
| Smoking (% smokers) | 3% | 5% | 7% |
| Mean Total Alcohol Drinks/ Week (mean (SD)) | 0 | 3.3 (2.1) | 14.1 (6.1) |
| Range = 1-7 | Range = 8-33 | ||
| Mean Beer Drinks/Week (mean (SD)) | 0 | 0.7 (1.2) | 3.5(6.1) |
| Range = 0-7 | Range = 0-30 | ||
| Beer Proportion/ Total Alcohol Drinks | 0 | .24 | .23 |
| Mean Wine Drinks/Week (mean (SD)) | 0 | 1.8 (1.9) | 6.8 (5.0) |
| Range = 0-7 | Range = 0-20 | ||
| Wine Proportion/ Total Alcohol Drinks | 0 | .54 | .52 |
| Mean Liquor Drinks/Week (mean (SD)) | 0 | 0.8 (1.5) | 3.8 (6.0) |
| Range = 0-7 | Range = 0-30 | ||
| Liquor Proportion/ Total Alcohol Drinks | 0 | .24 | .25 |
Note. Significant differences (p≤.05) across drinking status for all variables, with the exception of smoking status.
Table 2.
Cognitive Levels Across Time (N=571)
| Drinking Status | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Abstainers (n=237, 42%) |
Moderate (n=252, 44%) |
At-risk (n=82, 14%) |
||||
|
| ||||||
| Time1 | Time2 | Time 1 | Time 2 | Time 1 | Time 2 | |
| Memory (mean (SD)) | 48.6 (9.5) | 46.9 (10.3) | 50.8 (9.0) | 48.8 (10.6) | 51.1 (9.2) | 49.3 (9.4) |
| Reasoning (mean (SD)) | 50.1 (8.0) | 47.8 (8.9) | 52.4 (7.9) | 50.1 (9.4) | 52.7 (7.1) | 50.8 (8.4) |
| Spatial (mean (SD)) | 49.2 (8.5) | 47.3 (9.6) | 51.3 (8.2) | 49.6 (9.3) | 52.1 (8.0) | 50.6 (8.3) |
| Verbal (mean (SD)) | 50.4 (8.4) | 49.2 (8.6) | 53.8 (6.5) | 53.1 (7.1) | 52.8 (6.6) | 52.8 (6.6) |
| Number (mean (SD)) | 48.8 (8.8) | 45.2 (9.4) | 50.4 (8.3) | 46.9 (9.1) | 51.8 (7.9) | 49.2 (8.3) |
| Speed (mean (SD)) | 49.3 (7.1) | 46.4 (8.8) | 50.8 (6.6) | 48.3 (8.9) | 50.9 (5.6) | 48.8 (7.5) |
Note. Significant differences (p ≤ .05) across drinking status for all variables, with the exception of Time 2 memory ability.
Cognitive Effects
The linear mixed models (Table 3) indicated that there is a significant Time × Drinking status effect for verbal ability (F(2, within groups df) = 3.10; p = .0459), Time × Age group × Drinking status effect for spatial ability (F(4, within groups df) = 2.92; p = .0208), and Time × Gender × Drinking status effect on perceptual speed (F(2, within groups df) = 4.84; p = .0083). No other significant effects were identified.
Table 3.
Drinking Effects on Cognitive Change (1998-2005) by Age Group (f-values)
| Memory | Reasoning | Spatial | Verbal | Number | Speed | |
|---|---|---|---|---|---|---|
|
| ||||||
| Age Group Models | ||||||
| Time | 35.22*** | 210.93*** | 63.12*** | 31.61*** | 363.44*** | 187.96*** |
| Time × Age Group | 6.14* | 35.26*** | 16.01*** | 18.56*** | 42.52*** | 32.84*** |
| Time × Drinking Status | 1.14 | 1.66 | 0.10 | 3.10* | 2.08 | 0.53 |
| Time × Age × Drinking Status | 2.05 | 0.90 | 2.92* | 1.26 | 1.22 | 1.42 |
| Gender Models | ||||||
| Time | 21.75 *** | 113.85*** | 29.38*** | 10.69** | 207.28*** | 90.34*** |
| Time × Gender | 0.76 | 0.01 | 1.14 | 0.01 | 0.58 | 1.04 |
| Time × Drinking Status | 0.72 | 0.51 | 0.10 | 2.98 | 1.49 | 0.92 |
| Time × Gender × Drinking Status | 1.51 | 0.74 | 1.64 | 0.89 | 0.92 | 4.84** |
Note. Analyses conducted in SAS 9.1 using Proc Mixed, controlling for Income, Education, Drinking Levels for Beer, Wine, Liquor, Smoking Status, and Gender in Age Group models and Age Group in Gender models.
Time indicated two points: Time 1 = 1998 and Time 2 = 2005.
Bolding indicates significant (p <.05) differences;
=p ≤ .05;
=p ≤ .01;
=p ≤ .001.
Time × Drinking status interaction indicated the greatest degree of decline in verbal ability was seen among alcohol abstainers (Differences of Least Squares Means estimate (est.) = 1.5421; SE = 0.2299; df= 539; p < .0001), followed by moderate alcohol consumers ((est.= 1.1830; SE = 0.2407; df= 539; p < .0001) see Figure 1). At-risk drinkers showed relative stability in verbal ability (est. = 0.3350; SE = 0.4306; df= 539; p = .4370). Ad-hoc analyses identified significant differences in change over time between alcohol abstainers and at-risk drinkers (F(1, within groups df) = 6.16; p = .0136), with abstainers showing more decline.
Figure 1.
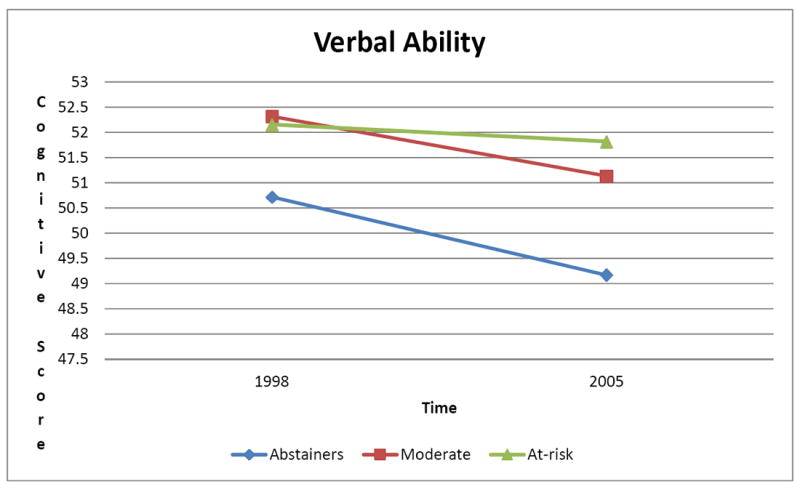
Changes in Verbal Ability
Time × Age group × Drinking status effects on spatial ability (see Figure 2) indicated relative stability in spatial ability in middle-aged adults, across drinking status (abstainers: est. = -0.6239; SE = 0.4847; df= 539; p =.1986; moderate drinkers: est. = 0.5144; SE = 0.4258; df= 539; p = .2275; at-risk drinkers: est. = 0.9236; SE = 0.7402; df= 539; p = .2126). There was spatial ability decline in young-old adults across drinking status (abstainers: est. = 3.4527; SE = 0.6174; df= 539; p <.0001; moderate drinkers: est. = 1.7330; SE = 0.6628; df= 539; p = .0092; at-risk drinkers: est. = 3.4151; SE = 1.1651; df= 539; p = .0035). In old-old adults, there was decline among abstainers (est. = 3.4972; SE = 0.6446; df= 539; p < .0001) and moderate drinkers (est. = 4.5655; SE = 0.7157; df= 539; p <.0001), but no detectable change among at-risk drinkers for spatial ability (est. = 1.6224; SE = 1.3064; df= 539; p = .2148). Ad-hoc analyses identified significant differences in change over time between young-old alcohol abstainers and moderate drinkers (F(1, within groups df) = 3.86; p = .0517), with abstainers showing more decline in spatial ability. In addition, ad-hoc analyses identified significant differences in change over time between old-old moderate and at-risk drinkers (F(1, within groups df) = 4.46; p = .0390), with moderate drinkers showing more decline in spatial ability.
Figure 2.
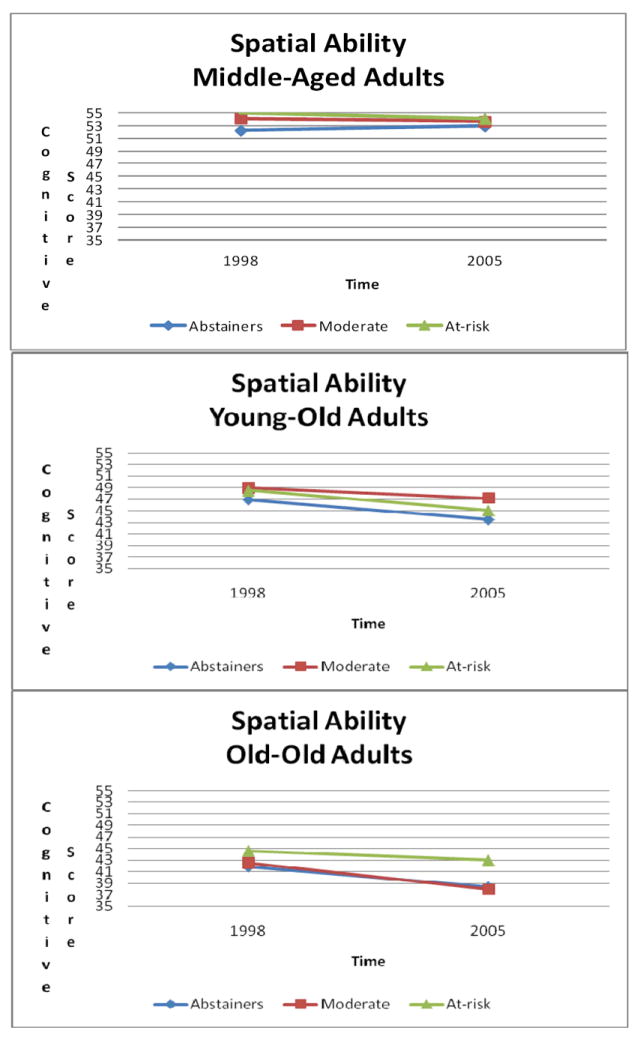
Changes in Spatial Ability
Time × Gender × Drinking status effect on perceptual speed (Figure 3) indicated perceptual speed remained stable for women with at-risk drinking status, but declined for the other drinking categories (abstainers: est. = -3.2427; SE = 0.3744; df= 542; p < .0001; moderate drinkers: est. = 2.1230; SE = 0.3871; df=542; p < .0001; at-risk drinkers: est. = 0.5580; SE = 0.9150; df= 542; p = .5422). On the other hand, men’s cognitive ability declined across drinking status (abstainers: est. = 1.9079; SE = 0.4887; df= 542; p =.0001; moderate drinkers: est. = 2.5570; SE = 0.4409; df= 542; p < .0001; at-risk drinkers: est. = 2.8811; SE = 0.6168; df= 542; p < .0001). Ad-hoc analyses identified significant differences in change over time between female alcohol abstainers showing more decline in perceptual speed compared with both moderate drinkers (F(1, within groups df) = 4.36; p = .0378) and at-risk drinkers (F(1, within groups df) = 9.06; p = .0030).
Figure 3.
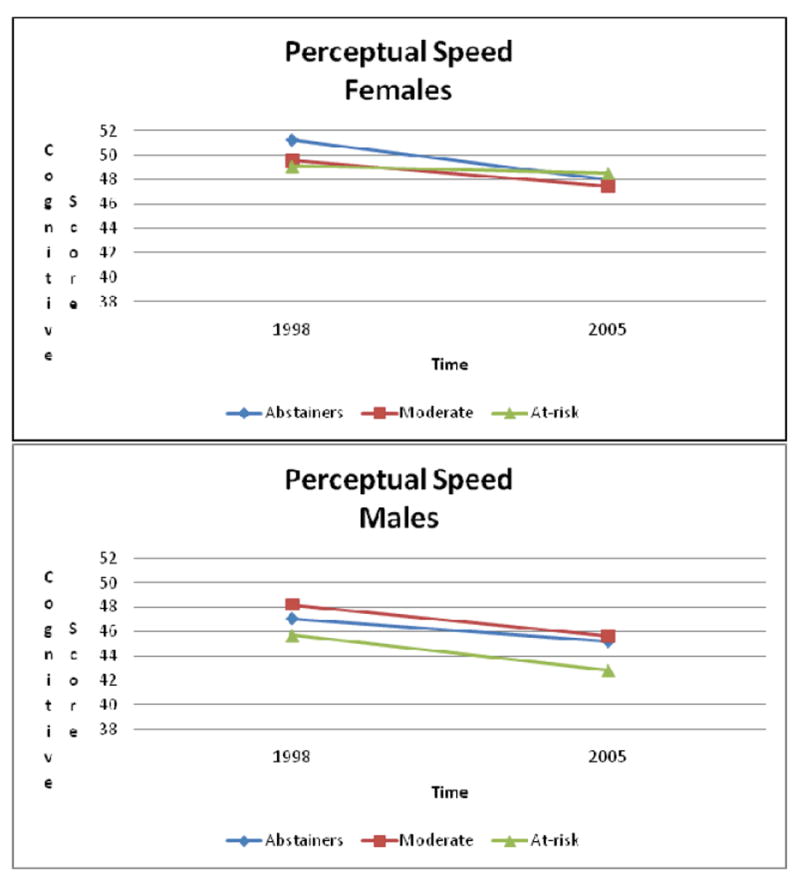
Changes in Perceptual Speed
DISCUSSION
In the current study alcohol consumption was linearly associated with decline in verbal ability over time such that abstainers showed the greatest decline, moderate drinkers showed less decline, and at-risk drinkers showed relative stability. A similar pattern for alcohol consumption was found for spatial ability, with middle-aged adults remaining stable, regardless of drinking status, while young-old adults’ spatial ability declined across drinking status, and old-old adults spatial ability was relatively stable among at-risk drinkers. In addition, while previous studies have reported a greater protective effect of alcohol consumption on cognition among women in cross-sectional (Dufouil et al., 1997; Elias et al., 1999; Forette et al., 1998; Onland-Moret et al., 2005) and longitudinal studies (McGuire et al., 2007; Stott et al, 2008), in the current study there was only evidence that alcohol consumption was connected with less decline in perceptual speed among at-risk drinking women, an effect not previously seen in the literature. Surprisingly, across models there was no significant alcohol effect for memory, reasoning, and number abilities.
Based on the results from the current study, the consumption of over 7 alcohol drinks/week is positively related to verbal and spatial ability performance in older adults. Also alcohol consumption appears to be positively related to perceptual speed in women. Findings indicate a stronger relationship between alcohol consumption and cognition exists in older adults, compared to their younger counterparts. These findings cannot be extended to memory, reasoning, and number abilities. The mix in effects on various cognitive domains may be due to the structural and functional heterogeneity (Tisserand et al., 2002) of the frontal lobe. Verbal ability (Frey et al., 2008), spatial ability (Ganis et al., 2004), perception (Roca et al., 2009), reasoning (Greene and Haidt, 2002), and numeric ability (Pesenti, Thioux, Seron & De Volder, 2000) are each performed in highly localized regions of the frontal lobe (Duncan & Owen, 2000). This localization may explain why certain functions are affected by alcohol while others remain unaffected (Moselhy et al., 2001). The current study also did not find evidence that alcohol consumption is related to memory. This finding may be due to the Time 2 cohort being significantly younger than the Time 1 cohort (73.8 vs 64.7 years of age; p=0.0001), considering that significant changes in memory do not typically manifest until 70 years of age or older (Aartsen et al.,2002).
These results must be interpreted with some of the caveats that accompany studies examining alcohol consumption effects on cognitive functioning over time among older adults. For example, preserved spatial ability for at-risk drinkers, compared to moderate drinkers or abstainers in old-old adults seen in the current study may indicate a survivor effect. Individuals with poorer spatial ability who engaged in at-risk drinking behaviors may have dropped out of the study due to complications associated with their greater alcohol consumption (De Labry et al., 1992). Such differential dropout would leave only the healthiest (both physically and cognitively) old-old at-risk drinkers, because the drinkers with poor cognitive trajectories may be deceased or too disabled to participate. Another limitation to this study that needs to be considered is that only two data collection points (1998 and 2005) assessed alcohol consumption patterns and cognition simultaneously. It is difficult to make definitive conclusions based on the analysis of two time points (Singer & Wilett, 2003). Also any relationship between alcohol and cognitive benefits is curvilinear, with cognition worse among chronic alcoholics over time (Cairney, et al., 2007; Chan et al., 2010).
Along with the challenge of differentiating a survivor effect from a true consequence of alcohol consumption, other explanations should be considered. One such explanation for the alcohol-cognition effect can be a positive neuro-physiological response on cognition due to alcohol consumption reducing vascular risk factors (Albert et al, 2003;Imhof et al., 2004; Kiechl et al., 1998; McGeer et al, 1999; Panza et al., 2008;Sano et al., 1993; Yi et al., 2009). Another explanation to consider for the alcohol-cognition effect is the positive correlation between alcohol consumption and social activity (Menon et al., 2010). Older adults with higher cognitive function are likely to remain engaged in social activities (Murphy et al., 2007) which may involve alcohol consumption, leading such individuals to drink more than those with declining cognition that withdraw from social activities (James et al., 2011). Evidence has indicated that social drinkers (i.e., persons that consume alcohol while in the presence of others; Spijkerman et al., 2010) are at a decreased risk for cognitive decline (Leroi et al., 2002) and many types of dementias (for a review, see Neafsey & Collins, 2011). Alternatively, the positive cognitive effect could be an artifact of older adults with better cognition simply having a tendency to consume more alcohol than older adults with poorer cognition (Cooper et al., 2009). These plausible explanations require future controlled studies in both laboratory and community settings to determine the exact mechanism for the alcohol-cognition relationship.
Despite the contribution this study makes to the science of alcohol on cognitive function, it is limited by several factors. The majority of participants were White and had high levels of income and education, representing the upper 75th tier of income in the US (Schaie, 2005). Accordingly, it is unclear how the current study’s findings would translate to sample populations with lower income, less education, and/or different races. Additionally, because the average annual income of participants was well above the poverty line (Federal Register, 1998), it is likely that results were attenuated by the shielding effect of affluence on mental and physical health (Kitagawa & Hauser, 1973). Future research should assess the interplay among aging, alcohol drinking status, and cognition among populations with greater educational, economic, and racial diversity.
Finally it is important to consider the reliability of the self-reporting of alcohol. While most research points to the fact the self-reported alcohol consumption is a reliable method for detecting drinking patterns (Chu, et al., 2010; Dawson, 2003; Ekholm, et al., 2011), research also indicates that past-week alcohol recall as measured in this study is not as reliable for sporadic drinkers (Gmel & Daeppen, 2007). For example, asking about drinks a week does not differentiate between drinkers that consume 1 drink a day compared to individuals that consume the same quantity over 1-2 days or even those that did not have a drink during the last week because they drink certain weeks of a month (e.g. pay weeks) or the year (e.g. weddings). Future studies should incorporate methodologies for tracking sporadic, irregular, or binge alcohol consumers to elucidate the alcohol and cognition relationship and associated health outcomes. It is also important to consider the limitations of the snapshot of alcohol measurement approach utilized in the current study does not account for change in lifetime drinking patters caused by life events such as health incidents and medications (Molander, Yonker, & Krahn, 2010). Research indicates that for the most part drinking patterns remain consistent, with heavy drinkers more likely to decline their drinking levels, with abstinent and moderate drinkers remaining abstinent and moderate respectively throughout their lifespan (Molander, Yonker, & Krahn, 2010). In the current study, lifetime drinking patterns and health/medication was not considered. Accordingly future studies should incorporate the measurement of lifetime drinking patters, including accounting for changes in health status and medications to achieve a more accurate understanding of individual drinking level status.
Furthermore, the at-risk criteria (consuming more than 7 drinks/week) used in this study is quite conservative and can classify a range of individuals (Moore, et al., 2011). For example, the at-risk criteria in this study places individuals that report drinking 8 drinks in the last week and those that report consuming 33 drinks in the last week in the same category. Typically someone that consumes 8 drinks a week compared to someone that consumes 33 drinks can be quite different, but not so much difference between someone that reports consuming 7 drinks compared to 8 drinks in the last week. However in the current study, drinking levels were significantly different (p<.05) across groups with mean drinking levels for moderate drinkers being 3 drinks in the last week (s.d. = 2; range 1-7) and for at-risk drinkers being 14 drinks in the last week (s.d. = 6; range 8-33). Further, analysis in this study controlled for within drinking category ranges, by accounting for number of drinks. Future studies should examine variations in at-risk drinking levels on cognitive outcomes; however a sufficient sample size of at-risk drinkers will need to be recruited to examine the relationship.
Even with acknowledging the inherent challenges, this study contributes significantly to a growing body of research examining alcohol and cognition. In this study, there was evidence of cognitive stability in certain domains, among older adults that consumed alcohol. This study is one of very few longitudinal studies assessing the effects of alcohol consumption in several cognitive ability domains across age groups and gender. Longitudinal studies such as this study can enlighten findings that may be missed or wrongly identified in cross-sectional research. For instance, cross-sectional studies can only provide evidence for group differences, whereas longitudinal work provides insights about actual change, and the factors that can affect change across groups. The strength of the current study rests in the large sample of community-dwelling, middle aged and older adults who have been followed over time to assess changes in behavioral patterns and cognitive performance. Given the expenses and complications associated with longitudinal research (Ruspini, 2002; Schaie, 2005), such well-characterized samples are limited. Also, breaking down and investigating cognition by domain was extremely important. In this study interestingly there was evidence that alcohol consumption is related to verbal, spatial, and perceptual speed, but not memory, reasoning, and number abilities.
Given the inconsistent nature of the evidence for a protective effect of alcohol consumption on older adults’ cognition, firm recommendations for alcohol consumption among older adults cannot be determined with the current evidence. Recommendation cannot be made due to the inherent difficulty in detecting a causal relationship, due to bias created from survivor effects, analyzing two time points, not considering health/medication status, and overrepresentation of higher socioeconomic status and white populations in this study. Based on the results in the current study, having more than 7 alcoholic drinks a week may be connected to decreasing cognitive decline in old-old adults. However, further longitudinal and experimental investigation is required to support this finding prior to making public health recommendations.
Acknowledgments
Funding: This work was supported by grants from the National Institute of Health awarded to K. Warner Schaie (National Institute of Aging R37 AG08055), to Sherry Willis (National Institute of Aging 5R37AG024102), to Tom Curry (National Institute of Drug Abuse 5K12 DA014040), Faika Zanjani (National Institute of Drug Abuse 1K01DA031764), and the Research Trust Challenge Grant awarded to the Graduate Center for Gerontology at the University of Kentucky. Also this research would not have been possible without the support of the participants, Group Health, and research staff.
References
- Adams W, Yuan Z, Barborial JJ, Rimm AA. Alcohol-related hospitalizations of elderly people. Journal of the American Medical Association. 1993;270:1222–1225. [PubMed] [Google Scholar]
- Albert MA, Glynn RJ, Ridker PM. Alcohol consumption and plasma concentration of C-reactive protein. Circulation. 2003;107(3):443–447. doi: 10.1161/01.cir.0000045669.16499.ec. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association. 2010 Alzheimer’s disease facts and figures. Alzheimer’s Dementia. 2010;6(2):158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Anstey KJ. Alcohol exposure and cognitive development: an example of why we need a contextualized, dynamic life course approach to cognitive ageing--a mini-review. Gerontology. 2008;54(5):283–291. doi: 10.1159/000161735. [DOI] [PubMed] [Google Scholar]
- Barry PJ, Gallagher P, Ryan C. Inappropriate prescribing in geriatric patients. Current Psychiatry Reports. 2008;10(1):37–43. doi: 10.1007/s11920-008-0008-3. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurology. 2008;7:184–190. doi: 10.1016/S1474-4422(08)70021-8. [DOI] [PubMed] [Google Scholar]
- Blieszner R, Willis SL, Baltes PB. Training research in aging on the fluid ability of inductive reasoning. Journal of Applied Development Psychology. 1981;2:247–265. [Google Scholar]
- Blow FC. Substance Abuse among older adults: Treatment Improvement Protocol (TIP) series. U S Department of Health and Human Services 2004 [Google Scholar]
- Blow FC, Walton MA, Chermack ST, Mudd SA, Brower KJ. Older adult treatment outcome following elder-specific inpatient alcoholism treatment. Journal of Substance Abuse Treatment. 2000;19:67–75. doi: 10.1016/s0740-5472(99)00101-4. [DOI] [PubMed] [Google Scholar]
- Bond GE, Burr R, McCurry SM, Rice MM, Borenstein AR, Larson EB. Alcohol and cognitive performance: a longitudinal study of older Japanese Americans: The Kame Project. International Psychogeriatrics. 2005;17:653–668. doi: 10.1017/S1041610205001651. [DOI] [PubMed] [Google Scholar]
- Cagney KA, Lauderdale DS. Education, wealth, and cognitive function in later life. Journal of Gerontology B Psychological Science and Social Sciences. 2002;57(2):P163–P172. doi: 10.1093/geronb/57.2.p163. [DOI] [PubMed] [Google Scholar]
- Cairney S, Clough A, Jaragba M, Maruff P. Cognitive impairment in Aboriginal people with heavy episodic patterns of alcohol use. Addiction. 2007;102(6):909–915. doi: 10.1111/j.1360-0443.2007.01840.x. [DOI] [PubMed] [Google Scholar]
- Chan KK, Chiu KC, Chu LW. Association between alcohol consumption and cognitive impairment in Southern Chinese older adults. International Journal of Geriatric Psychiatry. 2010;25(12):1272–1279. doi: 10.1002/gps.2470. [DOI] [PubMed] [Google Scholar]
- Cherbuin N, Reglade-Meslin C, Kumar R, Jacomb P, Easteal S, Christensen H, Anstey KJ, et al. Risk factors of transition from normal cognition to mild cognitive disorder: the PATH through Life Study. Dementia and Geriatric Cognitive Disorders. 2009;28(1):47–55. doi: 10.1159/000229025. [DOI] [PubMed] [Google Scholar]
- Chiu E. Alcohol for the older person--friend or foe? Age and Ageing. 2008;37(5):505–512. doi: 10.1093/ageing/afn157. [DOI] [PubMed] [Google Scholar]
- Chu AY, Meoni LA, Wang NY, Liang KY, Ford DE, Klag MJ. Reliability of alcohol recall after 15 years and 23 years of follow-up in the Johns Hopkins Precursors Study. Journal of Studies in Alcohol and Drugs. 2010;71(1):143–149. doi: 10.15288/jsad.2010.71.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker LH, Espeland MA, Rapp SR, Legault C, Resnick SM, Hogan P, Shumaker SA, et al. Postmenopausal hormone therapy and cognitive outcomes: the Women’s Health Initiative Memory Study (WHIMS) Journal of Steroid Biochemistry and Molecular Biology. 2010;118(4-5):304–310. doi: 10.1016/j.jsbmb.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C, Bebbington P, Meltze H, Jenkins R, Brugha T, Lindesay JE, Livingston G. Alcohol in moderation, premorbid intelligence and cognition in older adults: Results from the Psychiatric Morbidity Survey. Journal of Neurology, Neurosurgery and Psychiatry. 2009;80(11):1236–1239. doi: 10.1136/jnnp.2008.163964. [DOI] [PubMed] [Google Scholar]
- Culberson JW, Ziska M. Prescription drug misuse/abuse in the elderly. Geriatrics. 2008;63(9):22–31. [PubMed] [Google Scholar]
- Dawson DA. Methodological issues in measuring alcohol use. Alcohol Research and Health. 2003;27(1):18–29. [PMC free article] [PubMed] [Google Scholar]
- De Labry LO, Glynn RJ, Levenson MR, Hermos JA, LoCastro JS, Vokonas PS. Alcohol consumption and mortality in an American male population: Recovering the u-shaped curve--Findings from the Normative Aging Study. Journal of Studies on Alcohol and Drugs. 1992;53(1):25–32. doi: 10.15288/jsa.1992.53.25. [DOI] [PubMed] [Google Scholar]
- Dufouil C, Ducimetiere P, Alperovitch A. Sex differences in the association between alcohol consumption and cognitive performance. American Journal of Epidemiology. 1997;146:405–412. doi: 10.1093/oxfordjournals.aje.a009293. [DOI] [PubMed] [Google Scholar]
- Dufour MC, Archer L, Gordis E. Alcohol and the elderly. Clinics in Geriatric Medicine. 1992;8:127–141. [PubMed] [Google Scholar]
- Dukes PD, Robinson GM, Thomson KJ, Robinson BJ. Wellington coroner autopsy cases 1970-89: Acute deaths due to drugs, alcohol and poisons. New Zealand Medical Journal. 1992;105(927):25–27. [PubMed] [Google Scholar]
- Educational Testing Service. Basic Skills Assessment Test—Reading. Princeton, NJ: Educational Testing Service; 1977. [Google Scholar]
- Ekholm O, Strandberg-Larsen K, Grønbæk M. Influence of the recall period on a beverage-specific weekly drinking measure for alcohol intake. European Journal of Clinical Nutrition. 2011;65(4):520–525. doi: 10.1038/ejcn.2011.1. [DOI] [PubMed] [Google Scholar]
- Elias PK, Elias MF, D’Agostino RB, Silbershatz H, Wolf PA. Alcohol consumption and cognitive performance in the Framingham Heart Study. American Journal of Epidemiology. 1999;150(6):580–589. doi: 10.1093/oxfordjournals.aje.a010056. [DOI] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman H, Derman D. Kit of factor-referenced cognitive tests (rev. ed.) Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- Espeland MA, Coker LH, Wallace R, Rapp SR, Resnick SM, Limacher M, Messina CR, et al. Women’s Health Initiative Study of Cognitive Aging. Association between alcohol intake and domain-specific cognitive function in older women. Neuroepidemiology. 2006;27(1):1–12. doi: 10.1159/000093532. [DOI] [PubMed] [Google Scholar]
- Federal Register. Department of Health and Human Services. 1998;63(36):9235–9238. [Google Scholar]
- Finkle BS, McCloskey KL, Kiplinger GF, Bennett IF. A national assessment of propoxyphene in postmortem medicolegal investigation, 1972-1975. Journal of Forensic Sciences. 1976;21(4):706–741. [PubMed] [Google Scholar]
- Finlayson RE. Misuse of prescription drugs. International Journal of Addiction. 1995;30(13-14):1871–1901. doi: 10.3109/10826089509071059. [DOI] [PubMed] [Google Scholar]
- Forette F, Seux ML, Thijs L, Le Divenah A, Pérol MB, Rigaud AS, Staessen JA, et al. Detection of cerebral aging, an absolute need: Predictive value and cognitive status. European Neurology. 1989;39(suppl 1):2–6. doi: 10.1159/000052063. [DOI] [PubMed] [Google Scholar]
- Galanis DJ, Joseph C, Masaki KH, Petrovich H, Ross GW, White L. A longitudinal study of drinking and cognitive performance in elderly Japanese American men: the Honolulu-Asia aging study. American Journal of Public Health. 2000;90:1254–1259. doi: 10.2105/ajph.90.8.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli M, Vanderbilt J, Saxton JA, Shen C, Dodge HH. Alcohol consumption and cognitive function in late life: a longitudinal community study. Neurology. 2005;65(8):1210–1217. doi: 10.1212/01.wnl.0000180520.35181.24. [DOI] [PubMed] [Google Scholar]
- Gavaler JS, Love K. Detection of the relationship between moderate alcohol beverage consumption and serum levels of estradiol in normal postmenopausal women: Effects of alcohol consumption quantitation methods and sample size adequacy. Journal of Studies on Alcohol and Drugs. 1992;53:389–394. doi: 10.15288/jsa.1992.53.389. [DOI] [PubMed] [Google Scholar]
- Gilbertson R, Ceballos NA, Prather R, Nixon SJ. Effects of acute alcohol consumption in older and younger adults: perceived impairment versus psychomotor performance. Journal of Studies on Alcohol and Drugs. 2009;70(2):242–252. doi: 10.15288/jsad.2009.70.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmel G, Daeppen JB. Recall bias for seven-day recall measurement of alcohol consumption among emergency department patients: implications for case-crossover designs. Journal of Studies on Alcohol Drugs. 2007;68(2):303–310. doi: 10.15288/jsad.2007.68.303. [DOI] [PubMed] [Google Scholar]
- Gross AL, Rebok GW, Ford DE, Chu AY, Gallo JJ, Liang KY, Klag MJ, et al. Alcohol consumption and domain-specific cognitive function in older adults: longitudinal data from the Johns Hopkins Precursors Study. Journals of Gerontology B: Psychological Sciences/Social Sciences. 2011;66(1):39–47. doi: 10.1093/geronb/gbq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwig JP. Smoking and cognitive function: Midlife smoking may double dementia risk. Nursing and Women’s Health. 2011;15(1):19–25. [Google Scholar]
- Imhof A, Woodward M, Doering A, Helbecque N, Loewel H, Amouyel P, Koenig W, et al. Overall alcohol intake, beer, wine, and systemic markers of inflammation in Western Europe: results from three MONICA samples (Augsburg, Glasgow, Lille) European Heart Journal. 2004;25(23):2092–2100. doi: 10.1016/j.ehj.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Järvenpää T, Rinne JO, Koskenvuo M, Räihä I, Kaprio J. Binge drinking in midlife and dementia risk. Epidemiology. 2005;16(6):766–771. doi: 10.1097/01.ede.0000181307.30826.6c. [DOI] [PubMed] [Google Scholar]
- Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Metter E, et al. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: The Baltimore Longitudinal Study of Aging. Neurology. 1997;48(6):1517–1521. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- Kiechl S, Willeit J, Rungger G, Egger G, Oberhollenzer F, Bonora E. Alcohol consumption and atherosclerosis: what is the relation? Prospective results from the Bruneck Study. Stroke. 1998;29(5):900–907. doi: 10.1161/01.str.29.5.900. [DOI] [PubMed] [Google Scholar]
- Kitagawa EM, Hauser PM. Differential Mortality in the United States: A Study of Socioeconomic Epidemiology. Cambridge, MA: Harvard University Press; 1973. [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, Nissinen A, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. British Medical Journal. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang I, Wallace RB, Huppert FA, Melzer D. Moderate alcohol consumption in older adults is associated with better cognition and well-being than abstinence. Age and Ageing. 2007;36:256–261. doi: 10.1093/ageing/afm001. [DOI] [PubMed] [Google Scholar]
- Larson EB, Kukull WA, Buchner D, Reifler BV. Adverse drug reactions associated with global cognitive impairment in elderly persons. Annals of Internal Medicine. 1987;107(2):169–173. doi: 10.7326/0003-4819-107-2-169. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65(4):545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. American Journal of Epidemiology. 2001;154:635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Cranford JA, Boyd CJ. The relationship between past-year drinking behaviors and nonmedical use of prescription drugs: prevalence of co-occurrence in a national sample. Drug Alcohol Dependence. 2006;84(3):281–288. doi: 10.1016/j.drugalcdep.2006.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. Brain inflammation in Alzheimer disease and the therapeutic implications. Current Pharmaceutical Design. 1999;5:821–836. [PubMed] [Google Scholar]
- McGuire LC, Ajani UA, Ford ES. Cognitive functioning in late life: the impact of moderate alcohol consumption. Annals of Epidemiology. 2007;17(2):93–99. doi: 10.1016/j.annepidem.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Rogers RL, Judd BW, Mortel KF, Sims P. Cognition and cerebral blood flow fluctuate together in multi-infarct dementia. Stroke. 1988;19(2):163–169. doi: 10.1161/01.str.19.2.163. [DOI] [PubMed] [Google Scholar]
- Molander RC, Yonker JA, Krahn DD. Age-related changes in drinking patterns from mid- to older age: results from the Wisconsin longitudinal study. Alcohol Clinical and Experimental Research. 2010;34(7):1182–1192. doi: 10.1111/j.1530-0277.2010.01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AA, Blow FC, Hoffing M, Welgreen S, Davis JW, Lin JC, Barry KL, et al. Primary care-based intervention to reduce at-risk drinking in older adults: a randomized controlled trial. Addiction. 2011;106(1):111–120. doi: 10.1111/j.1360-0443.2010.03229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagin DS. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychological Methods. 1999;4(2):139–157. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- National Institute on Aging. AgePage: Alcohol use in older people. National Institutes of Health: NIH…Turning Discovery into Health. U S Department of Health and Human Services 2012 [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. 1992 No. 16 PH 315. [Google Scholar]
- Neafsey EJ, Collins MA. Moderate alcohol consumption and cognitive risk. Neuropsychiatric Disorder Treatment. 2011;7:465–484. doi: 10.2147/NDT.S23159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notkola IL, Sulkava R, Pekkanen J, Erkinjuntti T, Ehnholm C, Kivinen P, Nissinen A, et al. Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer’s disease. Neuroepidemiology. 1998;17(1):14–20. doi: 10.1159/000026149. [DOI] [PubMed] [Google Scholar]
- Ødegård E, Rossow I. Alcohol and non-fatal drug overdoses. European Addiction Research. 2004;10(4):168–172. doi: 10.1159/000079838. [DOI] [PubMed] [Google Scholar]
- Onder G, Landi F, Vedova CD, Gambassi G, et al. Moderate alcohol consumption and adverse drug reactions among older adults. Pharmaepidemiology and Drug Safety. 2002;11:385–392. doi: 10.1002/pds.721. [DOI] [PubMed] [Google Scholar]
- Onland-Moret NC, Peeters PH, van der Schouw YV, Grobbee DE, van Gils CH. Alcohol and endogenous sex steroid levels in postmenopausal women: A cross-sectional study. The Journal of Clinical Endocrinology & Metabolism. 2005;90(3):1414–1419. doi: 10.1210/jc.2004-0614. [DOI] [PubMed] [Google Scholar]
- Panza F, Capurso C, D’Introno A, Colacicco AM, Frisardi V, Lorusso M, Solfrizzi V, et al. Alcohol drinking, cognitive functions in older age, predementia, and dementia syndromes. Journal of Alzheimer’s Disease. 2009;17(1):7–31. doi: 10.3233/JAD-2009-1009. [DOI] [PubMed] [Google Scholar]
- Panza F, Capurso C, D’Introno A, Colacicco AM, Frisardi V, Santamato A, Solfrizzi V, et al. Vascular risk factors, alcohol intake, and cognitive decline. Journal of Nutrition Health & Aging. 2008;12(6):376–381. doi: 10.1007/BF02982669. [DOI] [PubMed] [Google Scholar]
- Peters R, Peters J, Warner J, Beckett N, Bulpitt C. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age and Ageing. 2008;37(5):505–512. doi: 10.1093/ageing/afn095. [DOI] [PubMed] [Google Scholar]
- Phillips DP, Barker GE, Eguchi MM. A steep increase in domestic fatal medication errors with use of alcohol and/or street drugs. Archives of Internal Medicine. 2008;168(14):1561–1566. doi: 10.1001/archinte.168.14.1561. [DOI] [PubMed] [Google Scholar]
- Quayhagen M. Training spatial rotation in elderly women. Unpublished docural dissertation. University of California; Los Angeles: 1979. [Google Scholar]
- Reitz C, Tang MX, Luchsinger J, Mayeux R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Archives of Neurology. 2004;61(5):705–714. doi: 10.1001/archneur.61.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Vaughan DE, Stampfer MJ, Glynn RJ, Hennekens CH. Association of moderate alcohol consumption and plasma concentration of endogenous tissue-type plasminogen activator. Journal of the American Medical Association. 1994;272:929–933. doi: 10.1001/jama.1994.03520120039028. [DOI] [PubMed] [Google Scholar]
- Rodgers B, Windsor TD, Anstey KJ, Dear KB, F Jorm A, Christensen H. Non-linear relationships between cognitive function and alcohol consumption in young, middle-aged and older adults: the PATH Through Life Project. Addiction. 2005;100(9):1280–90. doi: 10.1111/j.1360-0443.2005.01158.x. [DOI] [PubMed] [Google Scholar]
- Ruspini E. Introduction to Longitudinal Research. New York, NY: Routledge; 2002. [Google Scholar]
- Sano M, Wendt PE, Wirsén A, Stenberg G, Risberg J, Ingvar DH. Acute effects of alcohol on regional cerebral blood flow in man. Journal of the Study of Alcohol. 1993;54:369–376. doi: 10.15288/jsa.1993.54.369. [DOI] [PubMed] [Google Scholar]
- Schaie KW. Manual for the Schiae-Thurstone Adult Mental Abilities Test (STAMAT) Pal Alto, CA: Consulting Psychological Press; 1985. [Google Scholar]
- Schaie KW. Developmental Influences on Adult Intelligence: The Seattle Longitudinal Study. Oxford: Oxford University Press; 2005. [Google Scholar]
- Schaie KW. “When does age-related cognitive decline begin?” Salthouse again reifies the “cross-sectional fallacy”. Neurobiology of Aging. 2009;30(4):528–529. doi: 10.1016/j.neurobiolaging.2008.12.012. discussion 530-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW, Maitland SB, Willis SL, Intrieri RC. Longitudinal invariance of adult psychometric ability factor structures across seven years. Psychology and Aging. 1998;13:8–20. doi: 10.1037/0882-7974.13.1.8. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Fazekas F, Reinhart B, Kapeller P, Fazekas G, Offenbacher H, Freidi W, et al. Estrogen replacement therapy in older women: a neuropsychological and brain MRI study. Journal of the American Geriatric Society. 1996;44(11):1307–1313. doi: 10.1111/j.1532-5415.1996.tb01400.x. [DOI] [PubMed] [Google Scholar]
- Simoni-Wastila L, Strickler G. Risk factors associated with problem use of prescription drugs. American Journal of Public Health. 2004;94(2):266–268. doi: 10.2105/ajph.94.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Wilett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford, NY: Oxford University Press; 2003. [Google Scholar]
- Solfrizzi V, D’Introno A, Colacicco AM, Capurso C, Del Parigi A, Baldasarre G, Panza F, et al. Italian Longitudinal Study on Aging Working Group. Alcohol consumption, mild cognitive impairment, and progression to dementia. Neurology. 2007;68(21):1790–1799. doi: 10.1212/01.wnl.0000262035.87304.89. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Kang JH, Chen J, Cherry R, Grodstein F. Effects of moderate alcohol consumption on cognitive function in women. New England Journal of Medicine. 2005;352:245–253. doi: 10.1056/NEJMoa041152. [DOI] [PubMed] [Google Scholar]
- Stott DJ, Falconer A, Kerr GD, Murray HM, Trompet S, Westendorp RG, Ford I, et al. Does low to moderate alcohol intake protect against cognitive decline in older people? Journal of the American Geriatric Society. 2008;56(12):2217–2224. doi: 10.1111/j.1532-5415.2008.02007.x. [DOI] [PubMed] [Google Scholar]
- Sun Q, Townsend MK, Okereke OI, Rimm EB, Hu FB, Stampfer MJ, Grodstein F. Alcohol consumption at midlife and successful ageing in women: a prospective cohort analysis in the nurses’ health study. PLoS Medicine. 2011;8(9):e1001090. doi: 10.1371/journal.pmed.1001090. Epub 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen CE, van Boxtel MP, Bosma H, Bosmans E, Delanghe J, De Bruijn C, de Vente J, et al. Inflammation markers in relation to cognition in a healthy aging population. Journal of Neuroimmunology. 2003;134(1-2):142–150. doi: 10.1016/s0165-5728(02)00398-3. [DOI] [PubMed] [Google Scholar]
- Thurstone TG. Primary mental abilities for Grades 9-12. Chicago, IL: Science Research Associates; 1962. [Google Scholar]
- Thurstone LL, Thurstone TG. Examiner manual for the SRA Primary Mental Abilities Test (Form 10-14) Chicago, IL: Science Research Associates; 1949. [Google Scholar]
- Townsend MK, Devore E, Kang JH, Grodstein F. The relation between moderate alcohol consumption and cognitive function in older women with type 2 diabetes. Diabetes Research and Clinical Practice. 2009;85(3):322–327. doi: 10.1016/j.diabres.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyerer S, Schaufele M, Wiese B, Maier W, Tebarth F, van den Bussche H, Pentzek M, Riedel-Heller SG, et al. German AgeCoDe Study group (German Study on Ageing, Cognition and Dementia in Primary Care Patients) Current alcohol consumption and its relationship to incident dementia: results from a 3-year follow-up study among primary care attenders aged 75 years and older. Age and Ageing. 2011;40(4):456–463. doi: 10.1093/ageing/afr007. [DOI] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Wilson ME, Rau SW, Liu Y. Estrogens: Trophic and protective factors in the adult brain. Frontiers in Neuroendocrinology. 2001;22(1):33–66. doi: 10.1006/frne.2000.0207. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Harris T, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61(1):76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- Yi J, Horky LL, Friedlich AL, Shi Y, Rogers JT, Huang X. L-arginine and Alzheimer’s disease. International Journal of Clinical and Experimental Pathology. 2009;2(3):211–238. [PMC free article] [PubMed] [Google Scholar]
- Zelinski EM, Gilewski MJ, Schaie KW. Individual differences in cross-sectional and 3-year longitudinal memory performance across the adult life span. Psychology of Aging. 1993;8(2):176–186. doi: 10.1037//0882-7974.8.2.176. [DOI] [PubMed] [Google Scholar]


