Abstract
Objective
Vasomotor symptoms (VMS) recur after discontinuation of hormonal therapy. Selective serotonin reuptake-inhibitors (SSRI) are used increasingly to treat VMS, but whether VMS recur after cessation of SSRI is unknown. We hypothesized that relapse of VMS back to baseline levels after SSRI cessation would be common and predicted by menopausal and psychological characteristics.
Methods
Recurrence of VMS (frequency, severity, and bother) was measured using daily diaries for 3 weeks after cessation of escitalopram, which was administered in an 8-week randomized placebo-controlled trial in peri/postmenopausal women with hot flashes and night sweats. Blinding of staff and participants was maintained throughout. Relapse was defined as mean daily VMS frequency, severity, or bother ≤20% lower than pre-treatment levels.
Results
Of 76, 57, and 51 women included in the analysis for VMS frequency, severity, and bother, respectively, 34.2%, 38.6%, and 37.3% had relapse of VMS frequency, severity, and bother. In adjusted models, VMS frequency relapse was predicted by higher levels of pre-treatment insomnia symptoms (p=0.02) and a weaker response to escitalopram (p=0.03).
Conclusions
Of women whose VMS improved on escitalopram, approximately one-third relapsed swiftly after discontinuation of the medication. Those with pre-treatment insomnia and those with a weaker response to escitalopram may be at greatest risk for VMS relapse after treatment discontinuation. Women should be educated about the likelihood of VMS symptom relapse when they discontinue SSRI’s after receiving benefit from short-term treatment.
Keywords: hot flash, vasomotor symptoms, SSRI, escitalopram, randomized trial, recurrence, relapse
Background
Selective serotonin reuptake-inhibitors (SSRI) are effective and increasingly used treatments for vasomotor symptoms (VMS). Randomized placebo-controlled trials demonstrating efficacy of SSRI for treatment of VMS have shown benefit within 4 weeks of treatment.1 However, the required duration of treatment to maintain control of VMS is unknown and little data are available to guide women about the likelihood that VMS will recur after cessation of SSRI.
In an 8-week double-blind randomized placebo-controlled trial, we recently showed that escitalopram 10–20-mg/day is more effective than placebo at reducing the number, severity, and bothersomeness of hot flashes and night sweats, while modest effects of placebo were also observed.2 However, by 3 weeks after treatment cessation, the number of VMS per day had increased significantly in the escitalopram-treated group such that the frequency of VMS in those who had discontinued escitalopram approximated that of the group which had stopped placebo.2 Consistent with recurrence of VMS frequency VMS severity and bothersomeness also worsened, suggesting that improvements in the frequency, severity and bothersomeness of VMS were lost rapidly when the SSRI was discontinued.2
Several studies have shown that VMS recur after discontinuation of hormone therapy (HT).3–6 In observational studies of women who were receiving HT for VMS, 37–87% reported that they experienced VMS again when assessed several months to years after they had stopped HT.3, 4 Return of VMS occurs rapidly, within the first few weeks after HT cessation.4 In a follow-up survey conducted 8–12 months after HT discontinuation, VMS had recurred in more than half of women ages 55–59 who had VMS prior to combined estrogen-progestogen therapy (EPT) and whose VMS were suppressed on EPT.5 Taken together, these data suggest that HT is not a curative therapy for VMS but, rather, that VMS are suppressed for the duration of the treatment period.
While the occurrence of VMS after cessation of HT and the SSRI escitalopram appears to be common, it is not known whether VMS are experienced at the same frequency or intensity after treatment discontinuation as was experienced before treatment was started. That is, whether symptoms are in general less frequent or less bothersome, or whether women tend to experience a “relapse” of their symptoms, with VMS recurring at a similar level as prior to initiation of VMS treatment. Consequently, we examined whether women who received escitalopram for treatment of VMS experienced a relapse of their symptoms within a 3-week period after escitalopram was discontinued in our randomized clinical trial. We hypothesized that VMS relapse would occur commonly within the short period after treatment cessation and that baseline menopausal and psychological characteristics would predict the likelihood of VMS recurrence.
Methods
Participants in this study were women enrolled in the clinical trial who improved 20% or more from their baseline symptom level in the frequency, severity and bother of VMS after 8 weeks of escitalopram treatment. This subgroup of women was selected because they responded at least partially to the treatment and they represent the relevant clinical population for VMS treatment and its discontinuation.
The parent study was an 8-week multisite, randomized, placebo-controlled, double-blind trial of escitalopram 10–20-mg/day conducted within the Menopause Strategies: Finding Lasting Answers for Symptoms and Health (MsFLASH) Research Network. Details describing the study population and study procedures are available elsewhere.2 Briefly, participants were 204 perimenopausal, postmenopausal, or hysterectomized women ages 40–62 years who had a minimum of 28 bothersome or severe hot flashes or night sweats per week during a 3-week screening period. Participants were healthy women without clinical depression who were not taking any hormonal treatments, SSRI/SNRI, tamoxifen, aromatase inhibitors, or VMS treatments.
Participants were randomly assigned to receive escitalopram 10-mg/day or matching placebo for the first 4 weeks of the study. Treatment assignment was stratified on race (White, African-American, or other). After 4 weeks of treatment, the dose was increased to escitalopram 20-mg/day or matching placebo (2 pills per day in each treatment group) for the remaining 4 weeks of the trial if a participant had not experienced at least a 50% reduction in VMS frequency or VMS severity on a daily VMS diary. Those whose symptoms had responded by 50% or more after 4 weeks of treatment continued on escitalopram 10-mg/day or matching placebo for the remaining 4 weeks of the trial. All participants provided written informed consent and the study was approved by the Institutional Review Board at each participating MsFLASH site.
Measurement of VMS Frequency, Severity, and Bother
A hot flash diary was completed daily through the entire trial and post-treatment period. The frequency, severity, and bother of hot flashes and night sweats were recorded at the end of the day to describe daytime symptoms and upon awakening for nighttime symptoms. Our outcomes were 7-day means of hot flash frequency, severity (0–3; none, mild, moderate, or severe), and bother (0–3; none, a little, moderate, or a lot).
Post-treatment Phase
The double-blind was not broken during the post-treatment phase, and participants and staff remained unaware of which treatment had been received. For women who received escitalopram 10-mg/day, treatment was stopped at the conclusion of the 8-week trial. Those on escitalopram 20-mg/day took a total of 3 additional pills of escitalopram 10-mg/day every other day during the first post-treatment week. For the remainder of the 3-week period, participants took no study medication.
At the end of the 3-week period, post-treatment VMS diaries were collected together with abbreviated Discontinuation-Emergent Signs and Symptoms Scale, used to assess 17 common SSRI withdrawal symptoms (anxious/nervous, irritable, crying or tearfulness, agitation, difficulty concentrating or paying attention, mood swings, insomnia, unusual and/or vivid dreams, excessive sweating, muscle tension, muscle aches, fatigue or tiredness, headaches, dizziness or lightheadedness, chills, nausea, or diarrhea).7 Participants indicate whether each symptom is new, old but more severe, old but at the same intensity level, or old but less severe, or not present. For this analysis, a symptom was considered a withdrawal symptom if it was reported as new or old but more severe.
Predictors of VMS Relapse
A priori characteristics of interest examined as potential predictors of hot flash relapse were age, race (White vs. African-American), baseline levels of hot flash frequency, severity, and bother, menopause status (postmenopausal vs. perimenopausal), smoking status, body-mass index (BMI), number of years since the final menstrual period (FMP, categorized as <1, 1 to <3, and 3 or more years), duration of hot flashes (categorized as 0–2, 3–5, 6 or more years), prior HT use, final dose of escitalopram (20-mg/day vs. 10-mg/day), and level of response to escitalopram therapy (≥50% vs. 20–49.9% reduction in VMS frequency, severity, or bother). Other menopause-related and psychological characteristics examined were baseline levels of insomnia symptoms on the Insomnia Severity Index (ISI),8 depressive symptoms using the 9-item Patient Health Questionnaire (PHQ-9),9 and anxiety symptoms using the 7-item Generalized Anxiety Disorder (GAD-7).10
Statistical Methods
The primary outcome was relapse in hot flash frequency. This was defined as a difference of 20% or less in the frequency of hot flashes at 3 weeks after treatment cessation (week 11) compared to the pretreatment baseline. For example, a woman with 10 VMS at baseline who had 8.1 or more VMS 3 weeks after treatment cessation was considered to have had VMS frequency relapse. This definition of relapse was selected to identify those who no longer showed any benefit from the SSRI. All participants randomized to escitalopram who completed week 11 hot flash diaries were included in the analysis unless their symptoms did not improve by at least 20% during the 8-week trial. Analyses of VMS severity and bother relapse were conducted using the same approach, with relapse defined similarly (i.e., a difference of 20% or less in the reports at week 11 relative to the pretreatment baseline).
Logistic regression models were used to estimate the relationships between VMS relapse and covariates of interest. Variables with univariate p-values <0.05 were included in a multivariable analysis, along with baseline hot flash frequency. This logistic modeling process was then repeated for the VMS severity and bother recurrence outcomes adjusting for the corresponding baseline VMS measure. Associations between VMS relapse and pre-treatment insomnia symptoms (ISI score >7 vs. ≤7 to indicate the presence of at least mild insomnia)8 were further explored using Fisher’s exact tests for small cells. The statistical significance of associations between VMS relapse and SSRI withdrawal symptoms were examined using chi-squared tests.
Analyses were conducted using SAS Version 9.2 (SAS Institute, Cary, NC) with 2-sided p-values <0.05 considered statistically significant. Secondary analyses are considered exploratory and should be interpreted with caution.
Results
Of 205 women who entered the parent trial, 104 were randomly assigned to escitalopram. Of those, 97 (93.3%) completed the 8-week trial and 93 (89.4%) completed the post-treatment phase of the trial. Among those completing the post-treatment phase of the trial, 76 (81.7%) were improved with escitalopram (defined as a 20% or larger reduction in VMS frequency from baseline to the end of the 8-week treatment period) and included in this analysis. Analyses of relapse in VMS severity and bother were conducted on 57 (61.3%) and 51 (54.8%) women, respectively, who had improved by at least 20% on these dimensions in the 8-week treatment trial.
The characteristics of the study participants in this analysis (Table 1) were similar in each of the 3 outcome groups (frequency, severity and bother). The mean age was 53.7 ± 4.3 years; two-thirds were postmenopausal and the remaining were perimenopausal or had a hysterectomy. At baseline, the mean number of VMS was 10.0 ± 6.5 per day and approximately two-thirds rated their average daily VMS severity and average daily VMS bother as predominately severe. Prior to treatment randomization, 40% of participants had mild insomnia symptoms and 28% had moderate-to-severe insomnia symptoms. The dose of escitalopram was increased to 20-mg/day in 51.3% of the VMS frequency analysis sample and 64.5% had at least a 50% reduction in VMS frequency during the 8-week escitalopram trial.
Table 1.
Demographic and clinical characteristics among escitalopram-treated participants who had >20% reduction in hot flash frequency, severity, or bother during the 8-week trial.
| Participant Characteristics | Frequency (n=76) | Severity (n=57) | Bother (n=51) | |||
|---|---|---|---|---|---|---|
| Age at screening, mean (SD) | 53.74 (4.30) | 52.96 (3.95) | 52.65 (4.04) | |||
| N | % | N | % | N | % | |
| Race | ||||||
| White | 46 | 60.5 | 31 | 54.4 | 26 | 51.0 |
| African American | 27 | 35.5 | 23 | 40.4 | 22 | 43.1 |
| Other | 3 | 3.9 | 3 | 5.3 | 3 | 5.9 |
| Marital status | ||||||
| Never married | 12 | 15.8 | 11 | 19.3 | 10 | 19.6 |
| Divorced / separated | 10 | 13.2 | 5 | 8.8 | 4 | 7.8 |
| Widowed | 3 | 3.9 | 3 | 5.3 | 3 | 5.9 |
| Married / marriage-like relationship | 51 | 67.1 | 38 | 66.7 | 34 | 66.7 |
| Education | ||||||
| ≤ High school diploma or GED | 13 | 17.1 | 9 | 15.8 | 8 | 15.7 |
| School / training after high school | 29 | 38.2 | 19 | 33.3 | 17 | 33.3 |
| College graduate | 34 | 44.7 | 29 | 50.9 | 26 | 51.0 |
| Body-mass index (kg/m2) | ||||||
| <25 | 26 | 34.2 | 20 | 35.1 | 19 | 37.3 |
| 25 – <30 | 23 | 30.3 | 17 | 29.8 | 14 | 27.5 |
| ≥ 30 | 27 | 35.5 | 20 | 35.1 | 18 | 35.3 |
| Smoking | ||||||
| Never | 41 | 53.9 | 29 | 50.9 | 27 | 52.9 |
| Past | 22 | 28.9 | 17 | 29.8 | 15 | 29.4 |
| Current | 13 | 17.1 | 11 | 19.3 | 9 | 17.6 |
| Menopause status | ||||||
| Post-menopause | 51 | 67.1 | 38 | 66.7 | 31 | 60.8 |
| Peri-menopause | 17 | 22.4 | 13 | 22.8 | 14 | 27.5 |
| Hysterectomy / unknown | 8 | 10.5 | 6 | 10.5 | 6 | 11.8 |
| Years since final menstrual period | ||||||
| Unknown 1 | 18 | 23.7 | 14 | 24.6 | 14 | 27.5 |
| < 1 | 19 | 25.0 | 14 | 24.6 | 15 | 29.4 |
| 1 < 3 | 19 | 25.0 | 16 | 28.1 | 12 | 23.5 |
| 3+ | 19 | 25.0 | 12 | 21.1 | 9 | 17.6 |
| Years of hot flashes | ||||||
| 0 – 2 | 23 | 30.3 | 22 | 38.6 | 21 | 41.2 |
| 3 – 5 | 25 | 32.9 | 16 | 28.1 | 15 | 29.4 |
| 6+ | 27 | 35.5 | 18 | 31.6 | 14 | 27.5 |
| Hormone therapy use (ever) | ||||||
| No | 52 | 68.4 | 42 | 73.7 | 37 | 72.5 |
| Yes | 24 | 31.6 | 15 | 26.3 | 14 | 27.5 |
| Insomnia Severity Index (ISI) score | ||||||
| ≤ 7 | 24 | 31.6 | 21 | 36.8 | 18 | 35.3 |
| 8 – 14 | 30 | 39.5 | 18 | 31.6 | 18 | 35.3 |
| > 14 | 21 | 27.6 | 17 | 29.8 | 14 | 27.5 |
| Patient Health Questionnaire (PHQ-9) depression score | ||||||
| No depression symptoms (0 – 4) | 58 | 76.3 | 42 | 73.7 | 38 | 74.5 |
| Depression symptoms (5 – 13) | 18 | 23.7 | 15 | 26.3 | 13 | 25.5 |
| Generalized Anxiety Disorder (GAD-7) score | ||||||
| No anxiety symptoms (0 – 4) | 62 | 81.6 | 44 | 77.2 | 39 | 76.5 |
| Anxiety symptoms (5 – 9) | 14 | 18.4 | 13 | 22.8 | 12 | 23.5 |
| Average daily hot flash frequency at screening, mean (SD) | 9.30 (4.39) | 9.00 (4.38) | 9.18 (4.51) | |||
| < 6 | 17 | 22.4 | 13 | 22.8 | 10 | 19.6 |
| 6 < 9 | 26 | 34.2 | 20 | 35.1 | 20 | 39.2 |
| 9 < 12 | 17 | 22.4 | 15 | 26.3 | 12 | 23.5 |
| ≥ 12 | 16 | 21.1 | 9 | 15.8 | 9 | 17.6 |
| Average hot flash severity at screening2 | ||||||
| Predominately mild/moderate | 28 | 36.8 | 21 | 36.8 | 18 | 35.3 |
| Predominately severe | 48 | 63.2 | 36 | 63.2 | 33 | 64.7 |
| Average hot flash bother at screening3 | ||||||
| Predominately a little/moderate | 30 | 39.5 | 21 | 36.8 | 18 | 35.3 |
| Predominately a lot of bother | 46 | 60.5 | 36 | 63.2 | 33 | 64.7 |
| Escitalopram dose escalated at week 4 | ||||||
| Yes | 39 | 51.3 | 27 | 47.4 | 24 | 47.1 |
| No | 34 | 44.7 | 28 | 49.1 | 26 | 51.0 |
| Hot flash reduction at week 8 > 50% | ||||||
| Yes | 49 | 64.5 | 45 | 78.9 | 27 | 52.9 |
| No | 27 | 35.5 | 12 | 21.1 | 24 | 47.1 |
Hysterectomy and/or oöphorectomy
Predominately mild/moderate defined as an average weekly severity score ≤2. Predominately severe defined as average weekly severity score > 2.
Predominately a little/moderate defined as an average weekly bother score ≤3. Predominately a lot defined as average weekly severity score > 3.
VMS Frequency, Severity, and Bother Relapse
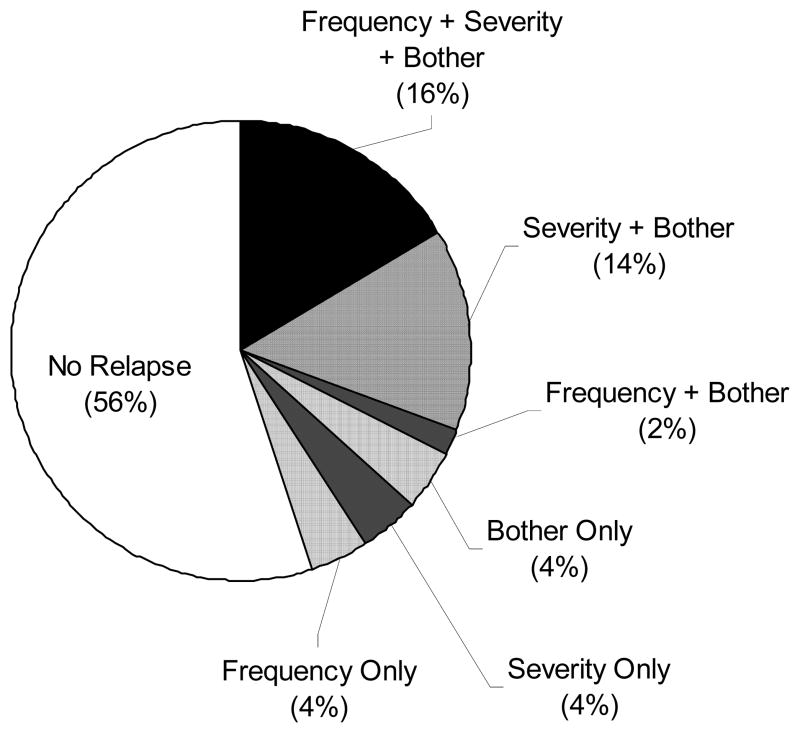
Three weeks after cessation of escitalopram, 26 (34.2%) women met relapse criteria in VMS frequency, while 22 (38.6%) and 19 (37.3%) met relapse criteria for VMS severity and bother, respectively. Among the subgroup that had >20% reduction on all three VMS relapse parameters (frequency, severity, and bother) after 8 weeks of treatment (n=49), 16.3% of participants met relapse criteria for all three VMS endpoints (frequency, severity, and bother), while 56.0% did not relapse for any VMS endpoints (Figure 1). For the group that relapsed, the mean daily VMS frequency changed from 8.9 ± 4.2 at baseline to 9.6 ± 4.5–3 weeks after escitalopram cessation, and from 9.5 ± 4.5 to 4.4 ± 3.0 for those who did not relapse. This translates to a 15.0% mean increase (95% confidence interval [CI] ranging from 4.0% reduction to 34.1% increase) in VMS frequency at the end of the post-treatment phase relative to their pre-treatment baseline for the group that relapsed. Similarly, the group that had VMS severity relapse had a mean increase in VMS severity of 10.7% (95% CI 4.8% reduction to 26.3% increase) and those with VMS bother relapse had a mean reduction in VMS bother of 1.5% (95% CI 10.6% reduction to 7.6% increase). Further analysis showed that VMS frequency, severity, and bother increased after treatment cessation by at least 50% of the benefit gained from baseline to week 8 in 61.8%, 47.4%, and 54.9% of women who had experienced at least a 20% improvement during the intervention, respectively.
Figure 1.
Relapse at week 11 on measures of hot flash frequency, severity, and bother among participants who improved* with 8 weeks of escitalopram treatment (n=49).
*Sample is restricted to the subgroup of women who had at least a 20% reduction on all three VMS measures (frequency, severity, and bother).
The proportion of women who relapsed was similar when more stringent definitions of treatment response were used to define the subgroup in the analysis. For those who had at least a 33% reduction in VMS from baseline to the end of the 8-week treatment period, 28.8%, 37.3% and 34.2% met criteria for VMS frequency, severity, and bother relapse, respectively. Results were consistent when the analysis was restricted to those who had experienced at least a 50% reduction in symptoms on escitalopram.
Predictors of VMS Frequency, Severity and Bother Relapse
In univariate analysis (Table 2), characteristics associated with relapse of VMS frequency were higher levels of insomnia symptoms at baseline (p=0.01) and a weaker response to escitalopram therapy (p=0.02). Both characteristics remained significant predictors of VMS frequency relapse in multivariable analyses. For every one-point difference in the ISI, there was an 11% higher odds of relapse (p=0.02); those whose VMS responded less well to escitalopram at week 8 had over three-fold higher odds of relapse at week 11. Women who had insomnia at baseline (ISI score >7, indicating mild or greater levels of insomnia) were more likely to relapse than those without insomnia (43% vs. 17%, p=0.04).
Table 2.
Univariate and Multivariate Predictors of Relapse (≤20% Reduction) of Hot Flash Frequency at Week 11 Relative to Baseline (n=76)
| Variable | Univariate Analyses | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age (5 year increase) | 1.08 (0.62, 1.88) | 0.78 | ||
| Race | 0.071 | |||
| African-American | 1.00 (ref) | |||
| White | 2.69 (0.92, 7.92) | |||
| BMI (5 kg/m2 increase) | 1.17 (0.81, 1.70) | 0.39 | ||
| Smoking | ||||
| Never | 1.00 (ref) | |||
| Past | 0.90 (0.30, 2.72) | |||
| Current | 1.21 (0.33, 4.38) | |||
| Menopausal status2 | 0.31 | |||
| Postmenopausal | 1.00 (ref) | |||
| Perimenopausal | 0.52 (0.15, 1.82) | |||
| Years since final menstrual period | 0.743 | |||
| < 1 | 1.00 (ref) | |||
| 1 – <3 | 1.58 (0.42, 5.95) | |||
| 3+ | 1.00 (0.26, 3.93) | |||
| Duration of hot flashes (years) | 0.78 | |||
| 0 – 2 | 1.00 (ref) | |||
| 3 – 5 | 0.73 (0.21, 2.48) | |||
| 6+ | 1.10 (0.35, 3.52) | |||
| Hormone therapy use (ever) | 0.35 | |||
| No | 1.00 (ref) | |||
| Yes | 1.61 (0.59, 4.38) | |||
| Baseline hot flash frequency (1 flash increase) | 0.97 (0.86, 1.08) | 0.54 | ||
| Insomnia Severity Index score (1 point increase) | 1.11 (1.02, 1.20) | 0.01 | 1.11 (1.02, 1.20) | 0.02 |
| PHQ-9 depression score (1 point increase) | 1.08 (0.92, 1.26) | 0.35 | ||
| GAD-7 anxiety score (1 point increase) | 1.02 (0.87, 1.19) | 0.84 | ||
| Escitalopram dose escalated at week 4 | 0.28 | |||
| No | 1.00 (ref) | |||
| Yes | 1.74 (0.64, 4.71) | |||
| Response to escitalopram at week 8 | 0.02 | 0.03 | ||
| 20 – <50% reduction | 1.00 (ref) | 1.00 (ref) | ||
| ≥ 50% reduction | 0.30 (0.11, 0.82) | 0.32 (0.11, 0.90) | ||
p-value from a contrast comparing African-American vs. White participants
p-value from a contrast comparing postmenopausal vs. perimenopausal participants
p-value from a contrast testing for any difference between participants with <1, 1-<3, or 3+ years since final menstrual period (comparison does not include participants with a hysterectomy or oöphorectomy at baseline)
Results of univariate analyses to identify predictors of relapse in VMS severity showed that higher pre-treatment levels of insomnia (p=0.02) and depressive symptoms (p=0.04) were associated with a greater likelihood of relapse (Table 3). However, neither remained a significant predictor of relapse in multivariable analyses.
Table 3.
Univariate and Multivariate Predictors of Relapse (≤20% Reduction) of Hot Flash Severity at Week 11 Relative to Baseline (n=57)
| Variable | Univariate Analyses | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age (5 year increase) | 0.84 (0.42, 1.67) | 0.62 | ||
| Race | 0.061 | |||
| African American | 1.00 (ref) | |||
| White | 3.02 (0.94, 9.71) | |||
| BMI (5 kg/m2 increase) | 1.20 (0.81, 1.79) | 0.37 | ||
| Smoking | 0.70 | |||
| Never | 1.00 (ref) | |||
| Past | 0.99 (0.29, 3.35) | |||
| Current | 0.53 (0.12, 2.43) | |||
| Menopausal status2 | 0.95 | |||
| Postmenopausal | 1.00 (ref) | |||
| Perimenopausal | 0.96 (0.26, 3.49) | |||
| Years since final menstrual period | 0.453 | |||
| < 1 | 1.00 (ref) | |||
| 1 – <3 | 1.08 (0.24, 4.79) | |||
| 3+ | 2.52 (0.52, 12.30) | |||
| Duration of hot flashes (years) | 0.99 | |||
| 0 – 2 | 1.00 (ref) | |||
| 3 – 5 | 1.05 (0.28, 3.99) | |||
| 6+ | 1.11 (0.31, 4.03) | |||
| Hormone therapy use (ever) | 0.90 | |||
| No | 1.00 (ref) | |||
| Yes | 1.08 (0.32, 3.62) | |||
| Baseline hot flash severity (1 point increase) | 1.56 (0.46, 5.29) | 0.48 | ||
| Insomnia Severity Index score (1 point increase) | 1.11 (1.02, 1.21) | 0.02 | 1.09 (0.99, 1.20) | 0.07 |
| PHQ-9 depression score (1 point increase) | 1.23 (1.01, 1.49) | 0.04 | 1.15 (0.93, 1.42) | 0.19 |
| GAD-7 anxiety score (1 point increase) | 1.01 (0.86, 1.20) | 0.88 | ||
| Escitalopram dose escalated at week 4 | 0.70 | |||
| No | 1.00 (ref) | |||
| Yes | 1.24 (0.42, 3.68) | |||
| Response to escitalopram at week 8 | 0.12 | |||
| 20 – <50% Reduction | 1.00 (ref) | |||
| ≥ 50% Reduction | 0.36 (0.10, 1.32) | |||
p-value from a contrast comparing African-American vs. White participants
p-value from a contrast comparing postmenopausal vs. perimenopausal participants
p-value from a contrast testing for any difference between participants with <1, 1-<3, or 3+ years since final menstrual period (comparison does not include participants with a hysterectomy or oöphorectomy at baseline)
Univariate analyses of relapse in VMS bother also showed that White women and those with higher pre-treatment levels of insomnia were more likely to relapse (p=0.008 and p=0.009, respectively, Table 4). However, in multivariable analyses, only race was a significant predictor, with White women showing 4.3 times higher odds of relapsing than African-American women.
Table 4.
Univariate and Multivariate Predictors of Relapse (≤20% Reduction) of Hot Flash Bother at Week 11 Relative to Baseline (n=51)
| Variable | Univariate Analyses | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age (5 year increase) | 1.26 (0.62, 2.57) | 0.53 | ||
| Race | 0.0081 | 0.041 | ||
| African-American | 1.00 (ref) | 1.00 (ref) | ||
| White | 6.14 (1.62, 23.29) | 4.31 (1.05, 17.62) | ||
| BMI (5 kg/m2 increase) | 1.15 (0.76, 1.73) | 0.52 | ||
| Smoking | 0.24 | |||
| Never | 1.00 (ref) | |||
| Past | 0.39 (0.01, 1.54) | |||
| Current | 0.31 (0.05, 1.76) | |||
| Menopausal status2 | 0.85 | |||
| Postmenopausal | 1.00 (ref) | |||
| Perimenopausal | 0.88 (0.24, 3.26) | |||
| Years since final menstrual period | 0.573 | |||
| < 1 | 1.00 (ref) | |||
| 1 – <3 | 1.43 (0.30, 6.88) | |||
| 3+ | 2.50 (0.46, 13.65) | |||
| Duration of hot flashes (years) | 0.92 | |||
| 0 – 2 | 1.00 (ref) | |||
| 3 – 5 | 1.33 (0.34, 5.27) | |||
| 6+ | 1.11 (0.27, 4.60) | |||
| Hormone therapy use (ever) | 0.61 | |||
| No | 1.00 (ref) | |||
| Yes | 1.39 (0.40, 4.86) | |||
| Baseline hot flash bother (1 point increase) | 0.55 (0.16, 1.86) | 0.33 | ||
| Insomnia Severity Index score (1 point increase) | 1.15 (1.04, 1.27) | 0.009 | 1.11 (1.00, 1.24) | 0.06 |
| PHQ-9 depression score (1 point increase) | 1.19 (0.98, 1.46) | 0.08 | ||
| GAD-7 anxiety score (1 point increase) | 0.98 (0.82, 1.17) | 0.84 | ||
| Escitalopram dose escalated at week 4 | 0.51 | |||
| No | 1.00 (ref) | |||
| Yes | 0.68 (0.22, 2.16) | |||
| Response to escitalopram at week 8 | 0.54 | |||
| 20 – <50% reduction | 1.00 (ref) | |||
| ≥ 50% reduction | 0.70 (0.22, 2.19) | |||
p-value from a contrast comparing African-American vs. White participants
p-value from a contrast comparing postmenopausal vs. perimenopausal participants
p-value from a contrast testing for any difference between participants with <1, 1-<3, or 3+ years since final menstrual (comparison does not include participants with a hysterectomy or oöphorectomy at baseline)
Withdrawal Symptoms after Cessation of Escitalopram
At the end of the 3-week post-treatment phase, 35 (46.1%) of 76 participants in the VMS frequency relapse analysis reported experiencing at least 2 out of the 17 pre-specified SSRI/SNRI withdrawal symptoms. The most common withdrawal symptoms were excessive sweating, dizziness / lightheadedness, and unusual or vivid dreams, which occurred in 33%, 18%, and 14% of participants. The proportion of women reporting at least two SSRI withdrawal symptoms 3 weeks after treatment cessation did not differ between those who did and did not experience VMS frequency relapse (50% vs. 44%, p=0.62). Similarly, women reporting SSRI/SNRI withdrawal symptoms were not more likely to meet relapse criteria for VMS severity (55% vs. 37%, p=0.20) or VMS bother (58% vs. 41%, p=0.23).
Discussion
Among perimenopausal and postmenopausal women who had at least a 20% improvement in VMS in response to escitalopram, approximately one-third experienced relapse of VMS back to pre-treatment symptom levels by 3 weeks after treatment cessation. Symptom relapse occurred regardless of whether relapse was defined by VMS frequency, VMS severity, or VMS bother. The likelihood of VMS relapse was similar when more stringent definitions of treatment response were used to define the analysis sample. Relapse in VMS frequency occurred more commonly in those with higher levels of pre-treatment insomnia symptoms and in those who had a weaker response to escitalopram. Higher pre-treatment levels of insomnia also significantly predicted relapse in VMS severity and bother but only in univariate analyses. Race was associated only with relapse of VMS bother, with African American women less likely to report bother after discontinuation of escitalopram. The likelihood of relapse was not associated with the presence of SSRI withdrawal symptoms.
Our results show that a significant proportion of women discontinuing escitalopram therapy for VMS lose the therapeutic benefit of short-term SSRI treatment soon after stopping treatment. These data suggest that a sizeable number of treatment responders lose benefit rapidly when medication is discontinued, although it is also noteworthy that over half of our participants did not relapse in VMS frequency, severity, or bother during this time period. Our results are consistent with previous studies showing that VMS recur rapidly after short-term treatment of the SNRI venlafaxine,11 although the previous study did not report the proportion of women whose symptoms returned to pre-treatment levels. Because our study was limited to a 3-week post-treatment phase, we do not know whether the likelihood of relapse would continue to increase with time off medication. Conversely, we do not know whether a longer period of initial SSRI treatment would reduce or postpone the return of symptoms after stopping medication.
Our findings are consistent with previous studies that indicate VMS recur after treatment is discontinued in a significant proportion of women receiving HT treatment,3–6 and that VMS occur rapidly after treatment is stopped.4 While the proportion of women that reported VMS recurrence after HT cessation was larger than what we observed in the present study,3–6 our results cannot be compared directly to other studies due to differences in treatments and the duration of time after stopping medication. However, it is noteworthy that our study is the only one to assess the return of symptoms relative to baseline levels rather than a global assessment of symptom recurrence.
These analyses highlight the importance of recognizing insomnia symptoms as a key factor associated with VMS relapse. Identifying women who report both VMS and insomnia prior to initiation of SSRI therapy and counseling them about possible relapse could be helpful. However, it is important to recognize that VMS may not be the only cause of insomnia in this population as stress, sleep apnea, depression, and common medical problems (e.g., GERD, hypertension, obesity) are also frequent causes of insomnia in a peri- and postmenopausal population. These data raise that possibility that women with co-occurring VMS and insomnia symptoms may benefit from a longer duration of VMS therapy or from switching to insomnia treatments as part of an SSRI discontinuation plan.
African-American women are more likely to report VMS during the peri- and postmenopause than are White women,12 and they experience hot flashes for a longer duration.13 It is therefore notable that while African-Americans and Whites did not differ in their VMS response to SSRI therapy,2 African American women were less likely to report bother after cessation of SSRI therapy. Additional studies are needed to better understand racial differences in response to treatments for VMS.
Women who did not respond well to escitalopram were more likely to experience relapse of VMS frequency. Conversely, a more robust response was protective against VMS relapse. The dose increase was not predictive of relapse, nor were withdrawal symptoms. Other characteristics not associated with relapse were age, menopause status, baseline VMS symptom level, duration of VMS, years from final menstrual period, body mass index, or smoking status, all of which we hypothesized would be associated with relapse because they are predictive of VMS occurrence in this population.12
Women who had VMS relapse after SSRI discontinuation were not more likely to report SSRI withdrawal symptoms than those who did not relapse. This observation suggests that withdrawal symptoms did not influence the likelihood of relapse nor were withdrawal symptoms of excessive sweating misinterpreted as VMS recurrence.
This study is the first to describe the likelihood of VMS relapse after discontinuation of non-hormonal treatments for VMS. The analysis is strengthened by the double-blinded discontinuation conditions as participants and staff were unaware of the treatment assignment during the 8-week trial and throughout the post-treatment phase. Limitations of our study include the brief 3-week period of post-treatment assessment after an 8-week treatment period and the modest sample of escitalopram-treated participants, which reduced our ability to assess whether baseline demographic, menopausal, or psychological characteristics (see Tables 2–4) were predictors of VMS relapse. In addition, the categorical definition of relapse may underestimate relapse among those who had a large response to SSRI therapy and a return of symptoms when medication was discontinued if their symptoms did not return to within 20% of baseline levels as defined in this study. We cannot extrapolate our findings to other clinical settings in which SSRI treatment is continued for a longer period of time, nor do we know whether the likelihood of VMS relapse would continue to increase with time off treatment.
Conclusion
In summary, our findings indicate that approximately one-third of peri- and postmenopausal women who discontinued escitalopram therapy shortly after they improved reported a swift return of VMS. White women and those with higher levels of pre-treatment insomnia symptoms were more likely to experience symptom relapse. VMS relapse was not associated with SSRI withdrawal symptoms or other menopause characteristics in this sample. These data have important clinical implications for women with VMS who respond to SSRI treatment. Counseling women about the likelihood of relapse when medication is discontinued and the risks and benefits of treatment continuation are important considerations. Based on our findings, women should be informed that the therapeutic effect of SSRI’s may be limited to the duration of time that the SSRI is taken, similar to what has been observed with estrogen therapy. In addition, because women who are White, have more insomnia symptoms, or have a less robust response to the treatment appear more likely to relapse after treatment discontinuation, they may benefit from a longer period of treatment. Future studies will examine whether such strategies can reduce the likelihood of VMS relapse when medication is discontinued.
Acknowledgments
Funding source:
The study was supported by a cooperative agreement issued by the National Institute of Aging (NIA), in collaboration with the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD), the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Research and Women’s Health (ORWH), and grants U01 AG032656, U01AG032659, U01AG032669, U01AG032682, U01AG032699, U01AG032700 from the NIA. At the Indiana University site, the project was funded in part with support from the Indiana Clinical and Translational Sciences Institute, funded in part by grant UL1 RR025761 from the National Institutes of Health, National Center for Research Resources, Clinical and Translational Sciences Award. Escitalopram and matching placebo pills were provided by Forest Research Institute.
Footnotes
Summary of disclosures/conflicts of interests:
This study was supported by the National Institutes of Health. Medication for the study was provided by Forest Pharmaceuticals. Dr. Joffe serves on the board of Noven Pharmaceuticals Inc., is an unpaid consultant to Sunovion Pharmaceuticals, and has a research grant from Cephalon Inc. Dr. Cohen consults for Noven Pharmaceutical and PamLab LLC, has received grants from Forest Laboratories Inc., Ortho-McNeil Janssen, Pfizer Inc., Astra-Zeneca Pharmaceuticals, Bristol-Myers and Squib, Cephalon Inc, GlaxoSmithKline, and Sunovion Pharmaceuticals, Inc. Dr. LeCroix is a board member of the Center for Outcomes Research at the University of Massachusetts and the Amgen Scientific Methodology Advisory Committee for Profile. She is also a consultant for the Pfizer Mammographic Breast Density Advisory Meeting. Dr. Freeman has received research grants Forest Laboratories Inc. and Bionovo.
References
- 1.Loprinzi CL, Diekmann B, Novotny PJ, Stearns V, Sloan JA. Newer antidepressants and gabapentin for hot flashes: a discussion of trial duration. Menopause. 2009 doi: 10.1097/gme.0b013e31819c46c7. [DOI] [PubMed] [Google Scholar]
- 2.Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305:267–74. doi: 10.1001/jama.2010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindh-Astrand L, Brynhildsen J, Hoffman M, Hammar M. Vasomotor symptoms usually reappear after cessation of postmenopausal hormone therapy: a Swedish population-based study. Menopause. 2009;16:1213–7. doi: 10.1097/gme.0b013e3181a53221. [DOI] [PubMed] [Google Scholar]
- 4.Grady D, Ettinger B, Tosteson AN, Pressman A, Macer JL. Predictors of difficulty when discontinuing postmenopausal hormone therapy. Obstet Gynecol. 2003;102:1233–9. doi: 10.1016/j.obstetgynecol.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA. 2005;294:183–93. doi: 10.1001/jama.294.2.183. [DOI] [PubMed] [Google Scholar]
- 6.Brunner RL, Aragaki A, Barnabei V, et al. Menopausal symptom experience before and after stopping estrogen therapy in the Women's Health Initiative randomized, placebo-controlled trial. Menopause. 2010;17:946–54. doi: 10.1097/gme.0b013e3181d76953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michelson D, Fava M, Amsterdam J, et al. Interruption of selective serotonin reuptake inhibitor treatment. Double-blind, placebo-controlled trial. Br J Psychiatry. 2000;176:363–8. doi: 10.1192/bjp.176.4.363. [DOI] [PubMed] [Google Scholar]
- 8.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 9.Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 2007;22:1596–602. doi: 10.1007/s11606-007-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–7. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 11.Bordeleau L, Pritchard KI, Loprinzi CL, et al. Multicenter, randomized, cross-over clinical trial of venlafaxine versus gabapentin for the management of hot flashes in breast cancer survivors. J Clin Oncol. 28:5147–52. doi: 10.1200/JCO.2010.29.9230. [DOI] [PubMed] [Google Scholar]
- 12.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: Study of women's health across the nation. Am J Public Health. 2006;96:1226–35. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol. 2011;117:1095–104. doi: 10.1097/AOG.0b013e318214f0de. [DOI] [PMC free article] [PubMed] [Google Scholar]