Abstract
Colorectal cancer is the leading cause of cancer-related mortality in the western world. It is also the third most common cancer diagnosed in both men and women in the United States with a recent estimate for new cases of colorectal cancer in the year 2012 being around 103,170. Various risk factors for colorectal cancer include life-style, diet, age, personal and family history, and racial and ethnic background. While a few cancers are certainly preventable but this does not hold true for colon cancer as it is often detected in its advanced stage and generally not diagnosed until symptoms become apparent. Despite the fact that several options are available for treating this cancer through surgery, chemotherapy, radiation therapy, immunotherapy, and nutritional-supplement therapy, but the success rates are not very encouraging when used alone where secondary complications appear in almost all these therapies. To maximize the therapeutic-effects in patients, combinatorial approaches are essential. In this review we have discussed the therapies previously and currently available to patients diagnosed with colorectal-cancer, focus on some recent developments in basic research that has shaded lights on new therapeutic-concepts utilizing macrophages/dendritic cells, natural killer cells, gene delivery, siRNA-, and microRNA-technology, and specific-targeting of tyrosine kinases that are either mutated or over-expressed in the cancerous cell to treat these cancer. Potential strategies are discussed where these concepts could be applied to the existing therapies under a comprehensive approach to enhance the therapeutic effects.
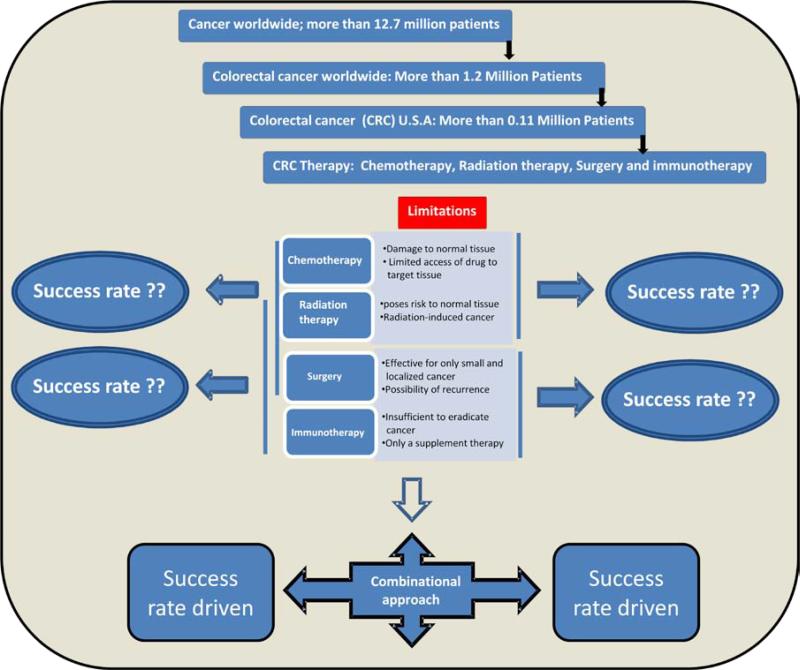
1. Introduction
Colorectal cancer is among the leading cause of cancer related death in the western world which is also the third most common cancer diagnosed in both men and women in the United States [2]. The American Cancer Society's most recent estimate indicates a total of 103,170 new cases of colorectal cancer in the United States for 2012 (http://www.cancer.org/Cancer/ColonandRectumCancer/DetailedGuide/colorectal-cancer-key) [3]. The risk factors for developing colon cancer include age, personal history, family history, racial and ethnic background. Life style and diet related factors also contribute to the development of colon cancer as well [4]. Mostly colorectal cancers occur due to lifestyle and increasing age with only a minority of cases associated with underlying genetic disorders. It typically starts in the lining of the bowel and if left untreated, can grow into the muscle layers underneath, and then through the bowel wall. There are environmental (chemicals, infectious agents, radiation) and genetic (mutations, immune system and hormone dysfunction) factors that can interact in a variety of ways to potentiate carcinogenesis [5, 6]. Activated oncogenes can cause hyperplasia and protect the cancerous cell against apoptosis while tumor suppressor genes can become inactivated in cancer cells, resulting in dysfunctional cellular processes (DNA replication, cell cycle processes, dysplasia, and immune cell interaction) [5, 6]. The goal of cancer therapy is to completely eradicate the cancer without damaging the rest part of the body, and the choice of therapy depends on the state of the patient, location, and stage of the cancer.
There are many scientific and alternative methods of colon cancer treatment. Treatment trials show their higher or lower effectiveness based on at what stage the cancer was detected in the patients and the age of the patients where outcomes for the elderly have been worse compared to younger patients. Nonetheless, standard-of-care treatment for the elderly patients result in equivalent long-term outcomes to those observed in the younger population; and available data support the use of aggressive surgery and adjuvant therapies in well-selected patients [7]. In patients all the methods should not be tried at once because it may be ineffective and sometimes even harmful. Usually cancer specialists offer time-proven standard methods of cancer treatment and in the case of favorable outcome the patient needs only symptom control during the whole life to prevent metastases or return of the disease. The choice of method of colon cancer treatment is very important because each tumor responds to different methods differentially. It is selected according to many factors including tumor type, stage of the disease, patient's age, patient's level of health, and his attitude towards life. Today alternative medicine offers many methods that help a number of people. Scientists also make their researches and present more and more new methods that have all chances to become a panacea. However, specific methods for colon cancer treatment are very limited. In addition, the pathogenesis of colon cancer which involves a combination of many risk factors is poorly understood, and research efforts in these areas are ongoing.
2. Causes, stages, and conventional treatment approaches for colon cancer
2.1 Colon cancer: causes and etiology
The majorities of colorectal cancer cases are sporadic with no obvious heritable tendency or family history. Somatic mutation of the adenomatous polyposis coli (APC) is a distinctive marker of approximately 80-85% of the patients with nonhereditary sporadic adenomatous polyposis (SAP). Mutation of the APC gene is thought to be an early step in the development of colorectal cancer [8]. Causes of the APC mutation are not known but may involve diets, smoking, environmental hazards, viruses, and life styles of different individuals [9]. The accumulation of defective end product of APC gene contributes to subsequent activation of Wnt/β-catenin signaling pathway leading to the transcriptional activation of certain target genes such as c-myc and cyclin D. Following initial genetic change, the process of carcinogenesis involves a series of genetic mutations, phenotypic and pathologic changes such as the loss of p53 tumor suppressor gene toward invasive colorectal carcinoma [10]. A minor group of colon cancer patients (8 to 15% of all cases) is associated with heritable tendency. About 1-2% of these cases develop familial adenomatous polyposis (FAP). Genetic evidence shows that patients with FAP inherit a germ line mutation of the APC gene and the lifetime incidence of colorectal cancer is almost 100%. They are the most high risk group and if untreated, most of the subjects will eventually develop colorectal cancer in their lifetime [11]. The progress from adenoma to carcinoma pathway in FAP is very similar to that of nonhereditary sporadic colon cancer patients. A second group of heritable colorectal cancer patients consists of those who are diagnosed with hereditary nonpolyposis colorectal cancer (HNPCC) (also known as Lynch syndrome). The genetic defects in HNPCC patients are less specific but are known to be associated with the mutations of a number of DNA mismatch repair genes including MLH1, MSH2, MSH6, PMS1 and PMS2 genes [8]. Although HNPCC cases are larger in size (5% of all hereditary cases), the penetrance of HNPCC is significantly much less than that of FAP [12] and patients with HNPCC usually have a higher 5-year survival rate. A much larger third group of “heritable” colorectal cancer is those with a family history of colorectal cancer but is distinct genetically from either FAP or HNPCC cases. The genetic mutations associated with this group of patients appear to be unique involving different sets of genes. The significant insight obtained from these groups of patients with inherited colorectal cancer has become very important in our understanding of sporadic disease. Specifically, the same genetic alterations leading to hereditary colorectal cancer have also been implicated in the pathogenesis of sporadic non-hereditary colon cancer, and these include APC (Wnt/β-catenin pathway), TGF-β, Notch and hedgehog (Hh) signaling pathways [8, 13]. In addition, other signaling pathways such as epidermal growth factor receptor (EGFR)[14], the ras/raf/MAPK cascade [15], and activation of Akt kinase and STAT3 transcription factors have been implicated in the oncogenesis of colorectal cancer [16-18].
2.2 Different stages of colon cancer and the percentage of patient survival
Colorectal cancer is classified into four distinct stages along with a fifth stage called “recurring”. According to American Joint Committee on Cancer, each stage has different treatment options with a five-year survival rates (AJCC 5th edition; http://www.cancerstaging.org). Stage 0; it is the very early stage of colon cancer where polyps are formed in the mucosal lining of the colon. During colonoscopy, the polyps are eradicated fully by polypectomy. This prevents the advanced stages of colon cancer to occur. Stage I; at this stage polyp develops into a tumor and invades the inner-lining of the mucosa. Usually surgery is the main option for treating the colon cancer at this stage where the cancerous portion of the tissues is separated from the non-cancerous portion. Survival rate is around 95% if colon cancer is detected at this stage. Stage II; it is characterized by whether the cancer has spread beyond colon but not to the lymph nodes through metastasis. This stage is subcategorized into Stage IIA, Stage IIB, and Stage IIC depending on the spreading of cancer to the muscular layer, or outermost layer of the colon or beyond colon. Resection surgery is the only option to threat this stage of colon cancer and the survivalist of the patients at this stage is 85%. Stage III; this stage of colon cancer is diagnosed with cancer has already spread all the wall of the colon and also to the surrounding lymph nodes and the survival rate is around 30-60%. This stage of cancer is subcategorized into stage III a, b and c depending on the spreading of the cancer to the inner , middle and outer layer of colon and the surrounding lymph nodes. Along with the surgery, chemotherapy and the other medical therapy is required to treat this cancer. Stage IV; at this stage the cancer has speeded to the other part / organ of the body like liver, ovary, testis, intestines. Survival rate is only 3%. Surgical resection, chemotherapy, radiation therapy and surgical removal of the portion of the other body parts with cancer are opted to treat at this stage of colon cancer. Colonoscopy is recommended for all 50 years or older in their routine checks [19].
2.3 Conventional methods of treating colon cancer
There are several conventional methods for colon cancer treatments that include (a) Polypectomy & Surgery, (b) Radiation therapy, (c) Chemotherapy, and (d) Targeted therapy.
Polypectomy & Surgery: The main surgical methods include radiation therapy and medication treatment. Surgical removal of the pre-cancerous/cancerous tumor has the potential for full recovery of the patient and can be an effective option for small, localized cancerous growths. Surgical removal of the polyps during colonoscopy is called as polypectomy. While recurrence is possible, surgery is often the only way to treat solid tumors that are resistant to radiation and chemotherapy or is inoperable at the time of diagnosis (e.g. pancreatic carcinoma). In addition, for older patients, comprehensive geriatric assessment (CGA) can identify frail patients with significantly increased risk of severe post-surgical complications [20].
Radiation therapy: Radiotherapy is the use of ionizing radiation to control the proliferation of malignant cells, and can be used to treat most common cancer types. Because radiation poses a risk to normal tissue, including the formation of radiation-induced cancers, shaped radiation beams are aimed from several angles of exposure to intersect at the tumor, providing a much larger absorbed dose at the cancer site than in the normal tissue. Apart from surgery, other techniques have also been used particularly for oligometastasis. These include selective internal radiotherapy (SIRT), trans-arterial chemoembolisation (TACE), and radiofrequency ablation (RA). RA has played an important role in managing localized disease but data from reports have documented fairly high local recurrence-rate. Newer techniques developed may likely improve the local recurrence rate and could ultimately shift the balance away from surgical resection [21].
Chemotherapy: This is also the main method that is implemented in review of cancer treatment. It includes medication-intake such as an alkylating agents, antimetabolites, plant alkaloids, antitumor antibiotics, enzymes, hormones and modifiers of biological response that destroy malignant cells, suppress tumor growth or its cells division. Unfortunately, there is no medication that can destroy only the malignant cells without damaging the normal tissues and sometimes their side effects are dangerous. The treatment of metastatic cancer mainly relies on chemotherapy, the method or process of administering a pharmaceutical compound to kill tumor cells by direct cytotoxicity leading to tumor regression. These drugs interfere with cell division pathways including DNA replication and chromosomal separation, and are not specific to cancer cells. Targeting all rapidly dividing cells in the body, only a small portion of the drug reaches the target tissue. This poses a risk of harming healthy tissue, especially those tissues that have a high replacement rate (e.g., intestinal lining and immune cells), though these cells usually repair themselves after discontinuation of drug therapy.
Targeted therapy: Targeted drug delivery systems seek to concentrate anti-cancer agents at the cancer tissue, while reducing the relative concentration of the medication in the proximal tissue. Polymeric micelles increase the accumulation of drugs in tumor tissues utilizing the enhanced permeability and retention effect (EPR-effect) [22]. A variety of drugs can be incorporated into the inner core by chemical conjugation or physical entrapment, and the diameter of the micelles can be manipulated to ensure that the micelles do not pass through normal vessel walls.
3. Recent advances in treatment methods for colon cancer
In combination with the conventional methods of treatment many a time current treatments also include targeted therapies predominantly for advance stages of colorectal cancer. In addition to the above strategies, some additional approaches are listed;
3.1 Immunotherapy
Immunotherapy is based on the fact that immune system can help fight against cancer. People with weakened immune systems are more likely to get certain cancers. In other cases, people with normal immune systems still develop cancer where immune system fail to recognize the cancer cells as foreign or the cancer cells do not express their antigens which are different enough from those of normal cells. Sometimes the immune system does recognize the cancer cells, but the responses are not strong enough to induce apoptosis in these cells. Cancer cells themselves may also secret immunosuppressant that may keep the immune system in check. To overcome these, researchers have designed ways to help the immune system recognize cancer cells and strengthen its response so that it can destroy the cancer cells. Treatments using immunotherapy are based on these premises. Immunotherapy (also called biological therapy or biotherapy) not only strengthens body's own immune system to detect and kill cancer cells but also in many a cases reduces treatment-related side effects. It is used to halt or suppress the processes that allow cancer growth, help the immune system identify cancer cells, and promote the body's natural ability to repair or replace cells that have been damaged by cancer treatments. Immunotherapy has become an integral part of modern treatment options in oncology, as it is not associated with many of the drawbacks of conventional therapies. Cancer immunotherapy attempts to stimulate host immune system thereby increases its capability to reject and destroy tumors, and the complexity of the regulation of the immune system gives rise to many different treatment approaches. Both chemotherapy and the tumor itself are known to potentially inhibit immune response. Cancer cells create an immunosuppressive microenvironment within the tumor that allows for escape from immune surveillance. Immunosuppressive tumor-associated macrophages, myeloid-derived suppressor cells, and regulatory T cells reside in tumors, and their products along with tumor derived products, create a microenvironment that counters immune activation and attack [23]. Utilizing a combination of anti-cancer therapies is often necessary, despite the potency of cytotoxic anticancer agents and specificity of immunotherapy, because neither by itself is often sufficient to eradicate the disease. T-cell enhancement, one of the immunotherapy, refers to the induction of T-cell responses against tumor-associated antigens is particularly important in tumor vaccination strategies. These strategies to stimulate the dormant immune system against tumors are varied and warrant further investigation for their potential to cancer therapy in the future [24]. Adoptive T-cell transfer (ACT) using autologous tumor-infiltrating lymphocytes has emerged as an effective treatment for patients with metastatic melanoma [25]. Immunotherapy is widely considered as the fourth treatment modality for patients with cancer, and uses constantly increasing knowledge in molecular biology, cell biology and immunology. Some biotherapies use naturally occurring biological molecules (e.g., cytokines and antibodies) to manipulate normal biological mechanisms to control or inhibit tumor growth. Among important achievements in anticancer drug development are immunotherapeutic strategies recently approved by the US FDA which are supported by clinical data from cancer patients under clinical trials [26]. These utilize dendritic cells harvested from a patient to activate a cytotoxic response towards patient-specific cancer-antigens. These cells are either activated with an antigen or transfected with a viral vector followed by placing back the activated dendritic cells into the patient. Once placed in patient, these cells then present the antigens to effecter lymphocytes which initiate a cytotoxic response to the cancerous cells bearing these antigens. T-cells with a naturally occurring reactivity to a patient's cancer can be found infiltrated in the patient's own tumors. The tumor is harvested, and these tumor-infiltrating lymphocytes (TIL) are expanded in vitro using high concentrations of interluekin-2 (IL-2), anti-CD3 and allo-reactive feeders. These T cells are then transferred back into the patient along with administration of exogenous IL-2 and GM-CSF. Our group has shown that a tight regulation of IL-2 concentration is necessary for maintaining a balance between apoptosis and proliferation in epithelial cells [27]. These increase immune cells availability in the tumor vicinity, and thus improve both antigen presentation and T-cell activation and proliferation. Cytotoxic T-lymphocyte antigen 4 (CTLA-4)-blocking monoclonal antibodies enhance immune activity by prolonging T-cell activation. Despite the evidence that immune effectors can play a significant role in controlling tumor growth under natural conditions or in response to therapeutic manipulation, it is still unclear how malignant cells evade immune surveillance in most cases [28].
So far as classification is concerned, the field of immunotherapy is broadly composed of (a) Alternative Medicines-Chinese herbs, dietary supplements and homeopathic medicines, (b) Biological-Pharmaceutical grade products developed by biotechnology/drug companies that are clinically tested and require government approval/clearance for marketing. Within the field of biological therapy there are three main categories of immunotherapy:
Passive immunotherapy: these refer to antibodies or other immune-system components that are made outside of the body (i.e. in the laboratory) and administered to patients to provide immunity against cancer, or sometimes to help them fight off an infection. However it does not stimulate a patient's immune system to “actively” respond to a disease in the way a vaccine does. Examples of passive immunotherapies include different antibodies created outside patient body using different molecular and cell biological techniques to destroy the cancer cells.
Active immunotherapy: these immunotherapies stimulate the body's own immune system to fight the tumor. It includes cancer vaccines, cellular therapies, and adjuvants. Cancer vaccines as well as the cellular therapies are discussed in detail in a separate section below. In adjuvant-immunotherapy an adjuvant is injected together with an antigenic protein or other bio-molecule like monoclonal antibodies (anti-idiotypic vaccine) or cancer-antigen that increases or boosts the immune response to that particular antigenic part of the vaccine or the cancer antigen. Examples include: BCG, KLH, IFA, QS21, Detox, DNP, GM-CSF. However the limitations to adjuvant-immunotherapies include adjuvant associated toxicities. Moreover many adjuvants can only be administered once or twice to patients, and can only be administered sub-cutaneously, and cannot be infused.
Combination immunotherapy: this immunotherapy constitutes drugs that possess both active and passive immunotherapy activity. As with other drugs this immunotherapy may also cause side effects. Compared to the side effects of chemotherapeutic drugs, the side effects of naked monoclonal antibodies (mAbs) which are generally administered intravenously are usually fairly mild and are often more like an allergic reaction. In most cases it occurs during very first administration to patients. The possible side effects may include fever, chills, weakness, headache, nausea, vomiting, diarrhea, low blood pressure, rashes etc. These side effects can interfere with one's ability to participate in daily-activities. Therefore, to be effective immunotherapy is often provided in conjunction with other treatment modalities, such as surgery, radiation therapy and chemotherapy.
Limitations of immunotherapies
Some of the limitations of current immunotherapeutic drugs include; Provenge an autologous cancer vaccine that works by stimulating the patient's own immune system to target prostate cancer cells. Though it represents an important clinical success and has shown very positive results, however it is very hard to produce in large quantities. It is an autologous vaccine, meaning one patient-one vaccine (prepared from the patient's own cancer cells). The targets can differ among different patients, and the targets can change when cancer cells mutate. The second major limitation of current immunotherapies includes drug administration to patients at a late-stage in the cancer therapy cycle, at which the patient's immune system is already weakened. In order to achieve a meaningful immunotherapeutic effect when treating cancer, immunotherapy should be used as early treatment in the disease process. It should be used before any potential effect on the immune system that might be caused by radiation, chemotherapy and surgery, and before the cancer has possibly become “tolerated” by the affected individual's immune system. Future advances in cancer therapy will require an integrative immunological approach to inform on the finer details of the immune signaling networks that will be directly applicable for designing novel anticancer strategies. Inflammation plays a dominant role at all stages of tumor development viz. initiation, progression, and metastasis [29]. Tumor-associated inflammation causes a decline in immune function and overrides tumor immunosurveillance and immunotherapy [30]. Understanding the immune regulatory mechanisms of inflammation and balancing them in favor of tumor immunity will help improve cancer immunotherapy approaches. The success of an immune effecter response depends on a fine productive balance between the innate and adaptive components of immunity. Besides providing an effecter response, cognate adaptive immune cells are also necessary to mediate tissue specificity in the chemokine promoted recruitment of innate immune cells to the site of cancer or other lesions following a pathological insult and to generate their effecter responses in a controlled fashion. Identification of the underlying signaling mechanisms responsible for the cross-talk between innate cells and the various populations of resting, effector, and regulatory T cells, as well as B cells, will help decipher new networks of immune regulation. This will reveal new intervention targets applicable for cancer therapy and prevention [31-33]. Immunotherapy represents a new and powerful tool for cancer treatment. For that reason immunotherapies involving cytokines and antibodies have now become part of standard cancer treatment these days. Besides these there are other examples of immunotherapy that are still experimental. Although many clinical trials of new forms of immunotherapy are in progress, an enormous amount of research remains to be done before the findings can be widely applied.
3.2 Cancer vaccines
The idea behind cancer vaccine is generally meant to boost the immune system to fight against the cancer just like vaccine to infection. Developing an effective vaccine against cancer is both fascinating and promising. Though most of the cancer vaccines are in clinical trials, research suggests promise in the therapeutic potential of a prototypic melanoma vaccine based on recombinant adenovirus expressing tumor-antigen. In the presence of a tumor however, the magnitude of T-cell immunity evoked by the vaccine was significantly reduced. Success of any cancer vaccine would depend on the induction of an effective tumor-specific immune response to break tolerance and to elicit long lasting anti-tumor immunity. Though preventative vaccines, like those that protect against viruses or the flu are given before a person becomes sick, in recent years scientists have attempted to develop therapeutic vaccines with the first successful prostate cancer vaccine called Provenge approved in 2010 by the US FDA [34]. In contrast to preventive vaccines the therapeutic cancer vaccines are given to a person who already has the disease. Cancer vaccines are active immunotherapies because they trigger the patient's immune system to respond. These are also targeted because they not only boost the immune system in general but also cause the immune system to attack the cancer cells through honing in on one or more specific tumor antigens. Cancer vaccines typically consist of a source of cancer-associated material (antigen), along with other components, to further stimulate the immune response against the antigen. The challenge has been to find better antigens as well as to package the antigen in such a way as to enhance the patient's immune system to fight cancer cells that have those antigens. Few examples of Cancer vaccines include: Tumor cell vaccines, Antigen vaccines, Dendritic cell vaccines (Provenge), Anti-idiotype vaccines, DNA vaccines, and vector-based vaccines. Thus a cancer vaccine may contain cancer cells, parts of cells, or purified tumor-specific antigens with overall goal to increase the targeted immune response against cancer cells already present in the patient. Cancer vaccines are also conjugated to carrier called adjuvants that help boost the immune response even further. Antigen presenting cells (APC) are key players in the initiation of an effective immune response [32]. Dendritic cells (DC), which reside in peripheral tissues, are professional APC. Appropriate activation of dendritic cells (DC) is essential for successful vaccination and induction of cell-mediated immunity [35]. Although high systemic levels of chemotherapeutic agents are invariably lethal to immune effector cells, they can actually activate DC when applied locally and might thus act as an adjuvant in vaccination settings [36]. Studies showed that combined effect of DC vaccination with paclitaxel treatment, resulted in increased anti-tumor responses [37]. Cancer vaccines are either cell-based vaccine, where cells from the patient's own cancer cells are presented to the patient's own immune system cells albeit in an in-vitro condition in laboratory. These in-vitro activated immune cells are then injected back to the same patient with other proteins (e.g., IL-2) to further facilitate immune activation of these tumor antigens primed immune cells. In other instances of vector-based cancer vaccines, engineered viruses or other vectors are used to introduce cancer specific proteins and other molecules to the patient in order to stimulate the patient's immune system to recognize the tumor and mount immune response against cancer cells. Both approaches are designed to stimulate the patient's own immune system to attack tumors.
Limitations of cancer vaccines
Since cancer vaccines are targets, the limitations of such vaccines are very similar to the limitations of other targeted therapies like mAbs. Tumor cells mutate as a result of chemotherapy or radiation treatment, and therefore the target antigens on the tumor cells at which the therapies are aimed also change. Thus if the targets change the vaccines which are target-specific become ineffective. Other vaccine limitations include autologous nature of the vaccine customized for the same patient which presents scale up challenges for commercialization as it may not be effective in other patients. These make autologous therapy is very costly. Moreover many cancer vaccines are poorly immunogenic and require the use of adjuvants to elicit an effective immune response. Though addition of adjuvants increases immunogenicity of the vaccine but also cause increased toxicity. The increased antigenicity of the patient's own cellular derived materials used to produce autologous cancer vaccines in many instances also cause auto-reactivity and subsequent development of an autoimmune diseases. Patients treated with genetically engineered vaccines may also produce neutralizing antibodies which could cause subsequent therapies with the same product ineffective. Antigen selection and the identification of new target antigens are of high importance in the field of vaccination strategies. Also active vaccination with Tumor Associated Antigen (TAA) peptides or passive vaccination with specific lymphocytes against these TAAs did not however demonstrate encouraging results in clinical trials. Attempts at cytokine therapy were also met with challenges of high systemic toxicity and a lack of specific lymphocyte activation [38].
3.3 Cellular therapies
Cellular therapies are usually single cell type agents derived from the cancer patient which are modified in the laboratory to become more adept at recognizing and killing the patient's own tumor. This type of active immunotherapy is designed to boost specific parts of the immune system to cause tumor cell death. Vaccines, in contrast, attempt to get the body's immune system to react to specific antigens. Examples of cellular therapies include: Lymphocyte activated killer cells therapy, tumor infiltrating lymphocyte with IL-2, and suppressor regulatory T cell. Limitations: Not all tumor infiltrating lymphocytes grow well enough in cultured conditions to generate enough quantity of cells that would be required to produce a useful anti-tumor effect when they are infused back into the patient. Even when they are able to grow outside, infusing back into a cancer patient of billions of cells that have been grown or modified genetically “in vitro” is not completely without risk and sometimes may be associated with immediate and delayed hypersensitivity reactions that can be life threatening. Autologous therapy is cumbersome, very costly, and does not easily lend itself to the commercial scale mass production techniques necessary to reach the multitude of cancer patients world-wide.
3.4 Gene therapy
Gene therapy is the transfer of gene/s to dysfunctional cells to correct a deficiency in the DNA or genome of a patient. Gene therapy provides a still poorly explored opportunity to treat cancer by “active” immunotherapy as it enables the transfer of genes encoding antibodies directed against specific oncogenic proteins. Gene therapy can be applied to genetic disorders as well as diseases acquired over the lifetime of an individual, such as cancer or infection, to confer a specific property to the cell allowing it to combat the disease. Gene therapy is mostly done through delivery of genes using viral and non-viral mediated methods which involves introducing foreign DNA into host cells. Current efforts in the area of gene delivery include the development of targeted delivery in which the gene is active only in the target area of the body. Telomerase is the enzyme responsible for immortalizing the cell and is specific to cancer cells, making it an excellent potential to target cell sets. Telomerase activation is a critical step for human carcinogenesis through the maintenance of telomeres, but the activation mechanism during carcinogenesis remains unclear. If a drug can deactivate telomerase, telomere degradation will resume and cancer cells will age and die like normal cells. Telomerase inhibition in many types of cancer cells grown in culture has led to the massive death of the cell population. Current development of vaccine methods attempt to teach the human immune system to attack cancer cells expressing telomerase. Three examples of gene delivery targets are; angiogenesis-associated targeting, targeting to uncontrolled cell proliferation markers, and tumor cell targeting [39]. There are several physical methods of gene delivery which include microinjection, gene gun, and electroporation. Sonication represents a means of increasing membrane permeability via ultrasound, and holds potential to be advantageous as a gene delivery mechanism [40]. Apart from genes other bio-molecules such as peptide and protein, antibody, vaccine and gene based drugs, might be susceptible to enzymatic degradation or cannot be absorbed into the systemic circulation efficiently due to molecular size and charge issues and must be delivered by injection. A potential way to overcome these is ultrasound, which can be used to increase the efficacy of genes for improving cancer treatment by affecting tissue permeability thereby enhancing the delivery of conventional agents [41].
Virus-mediated gene delivery
these utilize the ability of a virus to infect and integrate its genetic material into host cell genome. A gene of interest that is intended for delivery is packaged into a viral particle and allowed to transfect cells. Viral vectors are tailored to their specific applications but generally share a few key properties. A viral vector should have low toxicity and minimal effect on the physiology of the host cell that it infects. Viruses that are genetically unstable and can rapidly rearrange their genomes are not desirable gene therapy candidates. In fact these may be detrimental because of their unpredictability and non-reproducibility. Cell type specificity is also important because most viral vectors are engineered to infect a wide range of cell types. In cancer treatment, it may be desirable to have a viral vector that is specific for cancer cells. In order to accomplish this, the viral receptor can be modified to target the virus to these types of cells. A drawback may be the inability to easily track or monitor virus activity during treatment. Oncolytic viruses can be further engineered to delete immunosuppressive viral components and to insert transgenes that enhance antitumor immunity. Transcriptional regulation of the human telomerase reverse transcriptase (hTERT) gene is the major mechanism for cancer-specific activation of telomerase, and a number of factors have been identified to directly or indirectly regulate the hTERT promoter. These have been used as tools for cancer diagnostics and therapeutics by utilizing the hTERT promoter [42]. The utilization of rAdv-shRNA-hTERT promoter binding system could efficiently deactivate the RNA template of telomerase, therefore not granting the replicative immortality to cancer cells. Combining new immunomodulating agents (cyclophosphamide) or cell therapy approaches will likely further augment specific antitumor immunity using viral vector based therapy [43]. Oncolytic virotherapy has demonstrated multimodal antitumor mechanisms like simultaneous cytoreduction and conferring personalized anticancer immunity, without the need for personalized manufacture. Although viral vectors are occasionally created from pathogenic viruses, they are modified in such a way as to minimize the risk of handling them. This usually involves the deletion of a part of the viral genome critical for viral replication. Lentiviruses are retroviral RNA virus vectors that are used in the packaging and transduction of genetic components into cells. The viral genome in the form of RNA is reverse-transcribed when the virus enters the cell to produce DNA, which is then inserted into the genome at a random position by the viral integrase enzyme. The vector remains in the genome and is passed on to the progeny of the cell when it divides. For safety reasons lentiviral vectors never carry the genes required for their replication. An advantage lentivirus has over other RNA viruses are its ability to integrate into the genome of non-dividing cells. The site of integration is unpredictable, which can pose a problem, as the provirus can disturb the function of cellular genes and lead to activation of oncogenes promoting the development of cancer, which raises concerns for possible applications of lentivirus in gene therapy. By a bidirectional lentiviral vector, the monoclonal antibody gene transferred into live animals by systemic administration or by local intratumoral delivery resulted in substantial inhibition of tumor growth. These data provide proof of concept both for targeting the Met receptor and for a gene transfer-based immunotherapy strategy [44]. Adenoviruses are another class of DNA viruses that do not integrate into the genome and is not replicated during cell division. Their primary applications are in gene therapy and vaccination. Transduction with recombinant, replication-defective adenoviral vectors (rAdv) encoding a transgene is an efficient method for gene transfer into human dendritic cells [45]. Research has shown that adenovirus showed strong tumor-cell selectivity in vitro and antitumor efficacy in mouse models, suggesting potential clinical applications for the treatment of solid tumors [46]. Reports suggest intralesional administration of the adenovirus into subcutaneous tumor xenograft significantly suppressed tumor growth and provided a survival benefit. The results demonstrated that a human telomerase reverse transcriptase (hTERT) specific oncolytic adenovirus expressing an hTERT specific transgene is applicable for cancer therapy [47]. Conditionally replicative adenovirus (CRAD) represents another approach for rAdv cancer therapy, with high specificity to cancer cells and low cytotoxicity to normal cells [48]. Telomelysin, a telomerase specific replication-competent adenovirus with hTERT promoter, showed a strong anticancer effect by inducing cell lysis of human non-small cell lung cancer and colorectal cancer cells [49]. Because adenoviruses commonly come into contact with humans, causing respiratory, gastrointestinal and eye infections, they trigger a rapid immune response with potentially dangerous consequences. This problem must be overcome before treating cancer effectively in a large population, by way of finding adenoviruses to which humans do not have immunity. Recombinant Adeno-associated virus (rAAV) is a small virus which infects humans and some other primate species. AAV is not currently known to cause disease and consequently the virus causes a very mild immune response. AAV can infect both dividing and non-dividing cells and may incorporate its genome into that of the host cell. However, recombinant AAV has low in vivo transduction efficiency contrasted with the undesirably strong immunogenicity of adenovirus which has limited their clinical utilization in cancer gene therapy. A combinatorial application of rAAV - hTERT and a therapeutic dose of rAdv-hTERT is potentially a promising treatment for some types of cancer [50]. Another class of viruses known as Sendai Virus (HVJ) is an enveloped viral vector which combines the advantages of viral and non-viral vector systems as a safe and easy ‘‘non-viral” transfection reagent that has the ability to deliver of various molecules including plasmid DNA, siRNA, protein, antisense oligonucleotides, and wide usability from in vitro to in vivo [51]. Non-viral vehicles has received great attention due to their several favorable properties, including low toxicity and immunogenicity, resistance to nucleases, and their high affinity for DNA targets [52]. The two main modes of delivery of nucleic acids are chitosan-based systems and non-chitosan-based systems. These delivery systems can traverse the gut and gain entry into the bloodstream in sufficient quantities for efficacy in diseased tissues at distant sites [53]. Moreover, viral mediated –shRNA delivery system may hold promise as an effective cancer therapy agent [54].
3.5 Targeted therapy
Chemotherapy not only eradicates the cancer cells but also upsets the normal cells. Treating the cancer with the monoclonal antibodies drugs that specifically target the cancerous cells comes under targeted therapies (table 1). These and other drugs have been developed over the last five years that target on signaling pathways that control cell division and tumor angiogenesis mainly for non small cell lung cancer (NSCLC). Angiogenesis inhibitors, especially monoclonal antibody to VEGF, evacizumab, have also been developed in the last few years. Bevacizumab associated with classical cytotoxic chemotherapy led, in selected patients to an increase of median survival to more than 12 months with tolerable toxicity. Panitumumab is a fully human monoclonal antibody directed against the epidermal growth factor receptor (EGFR) and has been used in association with best supportive care (BSC) for metastatic colorectal cancer treatment [55]. Combination of some of these targeted therapy with chemotherapy have shown to achieve a median survival of over 2 years [56]. Other drugs that have both anti-EGFR activity and anti-angiogenic properties will soon be developed, since future bioactive anti-cancer drugs will have to be multi-targeted drugs [57]. These drugs are used as a combinational therapy along with chemotherapy or by themselves when chemo is no longer working. Though they have side effects but are less severe. A positive feedback loop has been indentified between tumor cells and macrophages that propagates the growth and promotes the survival of colon cancer cells. Tumor cells stimulate macrophages to secrete IL-1β, which in turn, promotes Wnt signaling and stabilizes Snail in tumor cells, conferring resistance to TRAIL. Vitamin D3 halts this amplifying loop by interfering with the release of IL-1β from macrophages. Accordingly, vitamin D3 sensitizes tumor cells to TRAIL-induced apoptosis, suggesting that the therapeutic efficacy of TRAIL could be augmented by this readily available chemo preventive agent (http://www.cancer.gov/cancertopics/factsheet/Therapy/targeted). Targeted therapy using antibodies is generally done on patients with advance cancer. New class of drugs available to patients under targeted therapy includes; Monoclonal antibody (mAb): Several monoclonal antibodies are now used to treat different types of cancer including colorectal cancer. Clinical trials are also using vaccines and many other immunotherapies as adjuvant to surgery, with and without chemotherapy. Success of mAbs as cancer therapeutics relies substantially on their ability to engage the immune system having significant efficacy in cancer therapy without the side effects of conventional chemotherapy or radiotherapy. Since the approval of rituximab by the FDA in 1997, several other mAbs have been validated for the treatment of cancer, both for solid tumors and hematologic malignancies [58]. More targeted agents have been developed in recent years to specifically stimulate immune cells. Targeted immunotherapy is usually directed to a single target on cancer cells such as an antigen or a receptor sites on cancer cells. Many a times these are specific enzymes or proteins on cancer cells. Although some of these immunostimulatory agents have yet to be tested in clinical trials, preclinical data evaluating their combination with antitumor mAbs appear promising where targeting immune effector cells might enhance the efficacy of antitumor mAbs. Typical immune cell candidates include NK cells (through anti-killer immunoglobulin-like receptor; KIR and anti-CD137 mAb), macrophages (through anti-CD47 mAb), gd T-cells (through gd T cell agonists), and DCs (through toll-like receptors; TLRs agonists) [59]. Monoclonal antibodies bind only to cancer cell-surface specific antigens. When an antibody recognizes the antigen against which it is directed, they fit together like two pieces of a puzzle setting off a cascade of events leading to tumor cell death. Examples of monoclonal antibodies include: Avastin, Erbitux, Rituxan, Herceptin, Mylotarg, Campath, Zevalin, Bexxar, Vectibix. Tumors that develop drug resistance would still be a suitable target for immunotherapy [60]. Immune evasion is one of the major problems in the development of cancer immunotherapy. The STAT3 signal in immune cells appears to be one of the therapeutic targets to overcome some of the limitations of cancer immunotherapy. The negative role of STAT3 for DC activation in both mice and humans is an attractive target for the development of effective cancer immunotherapies that do not induce harmful inflammatory responses [61]. Dendritic cells (DCs) play a pivotal role in the induction of Ag-specific T-cell immune responses [62]. Immunotherapies using DCs have been attempted for various diseases. For cancer patients, a number of DC immunotherapies have been developed and evaluated in preclinical and clinical settings [63-65]. However, the effectiveness of DC vaccines has been limited [66]. Therefore, improvements in DC therapy are essential for successful cancer immunotherapy. Limitations; Despite being the most widely used form of cancer immunotherapy, monoclonal antibodies have not been as successful as expected. This is because of several reasons a few of which are discussed here. The mAb products were not always as pure or specific as they initially were thought to be and were generally more toxic when given systemically, especially when given at high doses. Many mAbs are not administered as first-line therapy and are usually administered as a second, third, or last resort cancer treatment when the immune system is already weakened by chemotherapy, surgery, and radiations. These also limit their effectiveness. In general, response rates of these “targeted therapies” appear to be around 20 to 30%. To optimize this type of therapy, it would be necessary to identify each subgroup of patients with a specific cancer and develop therapies targeted to, or directed specifically at, their individual cancers. Tumor cells mutate as a result of chemotherapy and radiation treatment, and therefore the target antigen on the tumor cell at which the therapy is aimed is also changed. As the target changes, the mAbs which target those specific antigens become ineffective. In addition the toxicity associated with some of the targeted therapies can be significant.
Table 1.
| Target Molecule | Mechanism | Drug (reference) | |
|---|---|---|---|
| EGFR | Inhibits activating by growth signals | Cetuximab (Erbitux) | http://www.cancer.gov/cancertopics/factsheet/Therapy/targeted |
| EGFR | Prevents sending growth signals | Panitumumab (Vectibix) | |
| VEGF | Prevents it from interacting with receptors on endothelial cells | Bevacizumab (Avastin) | |
| Macrophages | * | Vit D3 | [1] |
3.5 Nutritional supplement therapy
The highest rates of these therapies were found in Australia, New Zealand, Europe, and North America. On the other hand the lowest rates were found in Africa and South Central Asia [67]. Diet or food is also one of the factors that make a human susceptible to cancer. For example the amounts of red meat (beef, pork), refined sugars and alcohol consumption are more compared to plant derived foods in the countries with high incidence cancer compared to the countries with the low incidence. It was reported that a few ingredients derived from plants could help in fighting cancer (http://www.cancer.gov/cancertopics/factsheet/Therapy/targeted). Discussing them all is beyond the scope of this review; however a few of them are described here. Most of the drugs used to treat cancers are a plant-derived product that is the reason they are also called as to have privileged structures. Fermented wheat germ extract (FWGE); Mueller et al. reported that FWGE in combination with anti-neoplastic chemotherapy drug dacarbazine showed a significant benefit for patients in terms of progression free survival (anti-metastatic) and overall survival. FWGE showed significant anti-proliferative effects and induce apoptosis in cancer cells via caspase and poly [ADP Ribose] polymerase dependent pathways. It also regulated the immune system by down regulation of MHC-I complex expression and induction of TNF-α and other interleukins [68]. Curcumin: Recurrence of tumor after treatment with chemotherapy is a major problem for the colorectal cancer patients and it was partly attributed to cancer stem cells (CSCs) that had the ability to relapse and metastasize by giving rise to new tumors. Studies showed that curcumin with dasatinib (a Src kinase inhibitor) has the ability to inhibit the growth and other properties of carcinogenesis of chemo-resistant colon cancer cells that are enriched in CSCs sub-population. The combinatorial approach showed substantial decrease (80-90%) in the expression of CSC markers ALDH, CD44, CD133, CD166 and inhibition of cellular growth, invasion, and colonsphere [69]. Since curcumin is also implicated in amelioration of inflammation in uncreative colitis, hence may play a role in decreasing the risk of inflammation induced colorectal cancer. STAT3 phosphorylation is commonly detected in various cancers including colorectal cancer. It was reported that an analogue of curcumin GO-Y030 and curcumin itself inhibits STAT3 phosphorylation, cell viability, and tumorsphere formation in colon cancer stem cells. It was also found that GO-Y030 was more potent than curcumin in inhibiting cancer growth. GO-Y030 reduced the downstream target gene expression and induced apoptosis in colon cancer stem cells and suppressed tumor growth in SW480 and HCT116 cancer cell lines in mouse model. Curcumin also has the ability to elicit cell cycle arrest followed by the suppression of cell proliferation in HCT116 cells [70]. Quercetin: Knock down of p53 (a tumor suppressor) has been linked to abrogation of apoptosis and induction of colorectal cancer (CRC). CRC with microsatellite instability showed remarkable resistance to chemotherapy with 5-fluorouracil (5-FU, a pharmacological drug for CRC treatment). Studies showed that a significant induction of apoptosis when CRC cells (CO115 and HCT115 cells) were incubated with quercetin and 5-FU through increased p-53 expression and activating apoptotic via mitochondrial pathways [71]. Besides, when APC (Min/+) mice were treated with 0.02% of quercetin in diet for 4-20 weeks it was found that the total intestinal polyps were decreased by 67%. Quercetin also decreased the macrophage number and spleen weight an indicator of infection and inflammation [72]. Aberrant crypt foci (ACF) are the abnormal tube like glands seen before colorectal polyps in the lining of colon and rectum that many a time leads to cancer. Quecertin decreased the number of ACF's by 4 fold in an azoxymethane (AOM) induced rat colon cancer model. It was reported that quecertin induced apoptosis by mitochondria-induced apoptotic pathways through caspase-9 [73]. Garlic: Diallyl disulfide (DADS) is an organosulfur compound and is one of the principal components of the distilled oil of garlic. It is believed that this dietary constituent prevents the development of colorectal cancer. Though the detailed mechanism is unclear, it was demonstrated that DADS selectively induced redox stress in cancerous cells that led to apoptosis. Treatment of HT-29 cells (a colon adenocarcinoma cells) with DADS initiates a decrease in cellular proliferation, translocation of phosphatidylserine to the plasma-membrane outer layer, activation of caspase-3, and -9, genomic DNA fragmentation, and G(2)/M phase cell-cycle arrest. DADS when combined with butyrate enhanced the apoptotic effect on HT29 cells [74]. The studies showed that DADS induced anti-proliferative effects on HT-29 cells could be through DHDG and DHUG genes [75]. In addition some other foods that have anticancer activity include pomegranate, wine, fish, milk, and tomatoes.
4. Emergence of new concepts from the basic research
4.1 micro RNAs (miRNAs)
In recent years miRNAs, a new class of naturally occurring small (19 to 25-nucleotides) non-coding single-stranded RNA molecules, have gained attention as a family of molecules involved in cancer development. Emerging evidence suggests that miRNAs function as key regulators in tumor oncogenesis, progression, invasion, metastasis, and angiogenesis [76]. Highly specific alterations in miRNA expression profiles have been reported for various cancer entities that are likely to have diagnostic and prognostic value. In addition, an increasing number of miRNAs has been functionally investigated and have shown to possess tumor suppressor or oncogenic activity [77, 78]. Recent evidence suggests that miRNAs cause posttranscriptional gene silencing by inducing target mRNA degradation via the RNA induced silencing complex (RISC) or by repressing the translation process upon binding to the 3’-untranslated region of their target mRNAs [79-82]. It was reported that miR-21 induces invasion/intravasation/metastasis in colorectal cancer cells. Findings also suggest the involvement of let-7 miRNA in the growth of colon cancer cells; therefore inhibitory strategies against miR-21 or let-7 will have a strong rationale for future therapeutic against colon cancer [83, 84]. Besides, miR-34b/c and BTG4 are recently discovered tumor suppressors in colorectal cancer and that the miR-34b/c CpG island, which bi-directionally regulates miR-34b/c and BTG4, is a frequent target of epigenetic silencing in colorectal cancer [85].
4.2 Short interfering nucleic acid (siRNA)
Interfering nucleic acids include interfering RNA (RNAi), small-interfering RNA (siRNA), and short-hairpin RNA (shRNA). Cancer is one of the diseases for which RNA interference is a potential therapeutic approach. Genes involved in the promotion or maintenance of tumor growth is obvious targets for RNAi. RNA interference is a posttranscriptional gene-silencing event in which short double-stranded RNA degrades target mRNA. These interference molecules can be used to induce an effective “gene knockout” by binding genetic moieties and preventing their subsequent translation into undesirable gene products. For instance, the promoter of hTeRT is highly active in cancer cells, with approximately 90% of all tumors expressing telomerase reverse transcriptase. In cancer cells this enzyme prevents cellular senescence by preventing telomeric degradation. RNAi-mediated specific gene-silencing has been shown to significantly suppress tumor growth via intratumoral delivery. SiRNA has shown a high specificity for their molecular target mRNAs as they can selectively inhibit cancer-promoting genes that differ from normal cells by a point mutation. Research also suggests that siRNA can be used for genotype-specific inhibition of tumor growth targeting in vivo [86-88]. It has been demonstrated that conventional anti-leukemic drugs have small or no differential effects under cell-culture conditions, whereas both imatinib and specific RNAi significantly inhibit proliferation of BCR-ABL-expressing TonB cells compared to normal tissue [89]. Therapeutic applications of small interfering RNA however require efficient vehicles for stable complexation, protection, and extra- and intra-cellular delivery. The delivery of RNAi effecter to the target cells is one of the key factors determining therapeutic efficacy, because gene silencing is limited to cells reached by RNAi effectors. SiRNA formulated in stable nucleic acid lipid particles (SNALP) displayed potent antitumor efficacy in both hepatic and subcutaneous tumor models. This was correlated with target gene silencing following a single intravenous administration that was sufficient to cause extensive mitotic disruption and tumor cell apoptosis. SiRNA formulations induced no measurable immune response, minimizing the potential for nonspecific effects [90]. To overcome cancer, long-term suppression of target transcripts in all cells without toxic effects may be required. The design of DNA sequences including shRNA contained in the vector and virus-host interactions during infection needs to be carefully considered [91, 92]. Little is known about shRNA in-vivo processing, accumulation, functional kinetics, and side effects related to shRNA saturation of the cellular gene silencing machinery. Findings suggest that a minimal amount of processed shRNA is required for efficient silencing in vivo, and that adenoviral vectors can deliver sufficient shRNA to mediate inhibition of gene expression without saturating the silencing machinery [93]. Quantitative analyses of the gene-silencing effect revealed that short-hairpin RNA expressing plasmid DNA (pshRNA) has more durable effects than siRNA [94]. The combination of adenovirus and shRNA to form an anticancer expression system against C5aR and IL-8 (a potent pro-angiogenic factor) has gained some ground as a potential therapeutic agent [95, 96]. The yield of infectious adenoviral vector particles was increased about 10-fold in a novel packaging cell with stable production of an shRNA that can silence the transgene, as compared to the yield in standard packaging line, and the consumption of nutrient in the novel packaging cell line is decreased due to silence of adenoviral transgene expression. An oncolytic adenovirus containing a promoter to express short hairpin RNA directed against a membrane-associated inhibitor of apoptosis protein that protects cells against apoptosis that was up-regulated in certain tumor cells [97]. Constitutive Wnt signaling is required to complement downstream mutations in the evolution of colorectal cancer, thus a blockade of the Wnt signal using siRNA may have a therapeutic role in the treatment of colorectal cancer [98].
4.3 Nanoparticles
Nanoparticles hold great promise as non-viral gene delivery systems for therapeutics in cancer. These particles range in size from 1-100 nm, have size-dependent properties, stability in solvents, precise size for delivery within the body, and tunable surface chemistry that makes them ideal for targeted delivery within the body [99]. Early clinical results suggest that nanoparticle therapeutics can show enhanced efficacy, while simultaneously reducing side effects, owing to properties such as more targeted localization in tumors, and active cellular uptake [100]. Non-chitosan based systems like sustained release liposomes, represent a type of nanoparticle that can interact with cells. Tumor growth and metastasis is facilitated by interactions between tumor cells and supporting cells, which consist of adaptive and innate immune cells. Liposomal systems have been designed to target supporting cells in tumor tissue [101]. Research showed that transferrin (Tf) associated liposomes for siRNA complexation and gene silencing holds promise. Complexation of siRNA with the cationic liposomes associated with Tf form stable siRNA lipoplex with reduced toxicity and enhanced specific gene knockdown activity compared to conventional polymeric gene carriers (polyplex) [102]. Thus polyplex could also hold promise as delivery agents. Drug polymer conjugates like the polyelectrolyte complex (PEC), a micelle-based siRNA delivery system have been developed for anti-angiogenic gene therapy. The interaction between poly ethylene glycol (PEG)-conjugated vascular endothelial growth factor siRNA (VEGF siRNA-PEG) and polyethylenimine (PEI) led to the spontaneous formation of nanoscale polyelectrolyte complex micelles (VEGF siRNA-PEG/PEI PEC micelles) having a characteristic siRNA/PEI PEC inner core with a surrounding PEG shell layer [103]. The cationic polymer PEI has been widely used for nucleic acid delivery due to its versatility and efficiency. In particular, the last generation of linear PEI (L-PEI) is being more efficient in vivo than the first generation of branched PEI. No major production of pro-inflammatory cytokines or hepatic enzymes was observed after injection of DNA or oligonucleotides active for RNA interference complexed with L-PEI [104]. Efficient intracellular processes including cytosolic release and unpacking of siRNA from the carrier in the cytoplasm are some of the efficiency determining steps involved in achieving successful gene silencing. Acid-degradable ketalized linear polyethylenimine (KL-PEI) was synthesized for efficient, intracellular target-specific, and biocompatible siRNA delivery. The siRNA/KL-PEI polyplex resulted in much higher RNA interference efficiency than unmodified L-PEI via selective cytoplasmic localization of the polyplex and efficient disassembly of siRNA from the polyplex [105]. Chitosan, a natural cationic linear polymer, has also emerged as an alternative non-viral gene delivery system. These drug delivery systems have been developed in attempts to deliver therapeutics specifically to the target lesion site. Correlations have been established between the structure and the properties of chitosan-pDNA polyplex in-vitro [106]. These findings suggest a high distribution with a high level of transgenic expression combined with efficiency comparable to that of commonly used cationic lipids. The use of chitosan as a gene delivery system is a nontoxic alternative to other cationic polymers. With DNA enzyme and siRNA encapsulation into chitosan nanoparticles, the therapeutics can be targeted to tumors [107].
4.4 Janus kinases (Jaks,a family of tyrosine kinases) based therapeutics
Jak-1 is involved in interferon responses. Jak-1 has been shown to be essential for signal transduction processes mediated by the receptors for IFN-alpha, IFN-gamma, G-CSF, and prolactin. Cells lacking Jak1 showed complete defect in interferon responses. Jak-2 is associated with the receptors for growth hormone (Epo) and is involved in signal transduction processes mediated by these factors. It facilitates the coupling of Epo binding to its receptor to tyrosine phosphorylation and mitogenesis. Jak-2 is also activated by IL-3, GM-CSF, G-CSF, Prolactin, and IFN-gamma. Jak-2 tends to associate with the receptor subunit that is shared with the receptors for GM-CSF, IL3, and IL5. Signaling molecules cooperating with Jak-2 have been identified as STAM-1 and STAM-2. Tyrosine kinase-2 (Tyk-2) is associated with interferon gamma. Jak-3 is closely related to Jak-2. Like other Jak-kinases, Jak-3 is a cytoplasmic non-receptor tyrosine kinase known to be expressed in hematopoietic cells. It associates with the common gamma chain of theof the receptors for IL-2, IL-4, IL-7, IL-9, and IL-15. Studies of whole organ homogenates showed that Jak-3 is also expressed in the intestines of both human and mice. However, neither its expression nor its function was defined in intestinal epithelial enterocytes. We have demonstrated for the first time that functional Jak-3 is expressed in both human intestinal enterocytes including colon cancer cells. In enterocytes Jak-3 played an essential role in the epithelial cell migration in response to IL-2 [108]. Under neoplastic condition this could be correlated to metastatic potential of colon cancer cells. IL-2-stimulated redistribution of Jak-3 was inhibited by the Jak-3 specific inhibitors. Besides, Jak-3 also induced redistribution of the actin cytoskeleton in migrating cells through tyrosine phosphorylation of cytoskeletal protein villin. In these cells Jak-3 interacted with the cytoskeletal protein villin in an IL-2 dependent manner. Inhibition of Jak-3 activation resulted in loss of tyrosine phosphorylation of villin and a significant decrease in cell migration of the intestinal epithelial cells [108]. Besides we have also determined the molecular mechanism and the structural determinants of Jak-3 that regulates its interactions with villin (Mishra et. al. 2012 manuscript under review). Moreover, increased levels of autoantibody to villin were identified in the serum of colon cancer patients. Anti-villin antibody was most prevalent in patients with colon cancer at significantly higher (P < 0.005) levels than normal controls[109]. It appears that villin expression was deregulated in these cancerous cells resulting in its leakage to the serum. On the other hand, deregulated Jak-3 expression has been linked to different types of cancer including colon cancer [108]. Cytoplasmic Janus protein tyrosine kinases (JAKs) are crucial components of diverse signal transduction pathways that govern cellular survival, proliferation, differentiation and apoptosis. Activated JAKs create binding sites for the signal transducers and activators of transcription (STAT) via phosphorylation (rate-limiting event) of specific tyrosine residues on cytokine receptor subunits. STAT transcription factors translocate from receptor docking sites at the membrane, dimerize, and translocate to the nucleus where they initiate the transcription of genes containing appropriate regulatory sequences in their promoter regions. While it is likely that activation of STAT proteins may be an important function attributed to the JAK, it is certainly not the only function performed by this key family of cytoplasmic tyrosine kinases. Emerging evidence indicates that phosphorylation of cytokine and growth factor receptors may be the primary functional attribute of JAK. Aberrations in JAK kinase activity, that may lead to derailment of one or more of the above mentioned pathways could disrupt normal cellular responses and result in disease states. Thus, over activation of JAK kinases has implications in tumorigenesis. In contrast, loss of JAK kinase function has been found to result in disease states such as severe combined immunodeficiency. The full potential of Jak kinases based therapeutic is yet to be exploited. [110].
4.5 Pharmacogenetics and pharmacogenomics
Pharmacogenetics is the study of the variability in drug response and (or) drug toxicity and its association with the polymorphisms in certain genes involved in the metabolism of those drugs. On the other hand pharmacogenomics is the study of the influence of genomic variability on drug response. Under phamraccogenomics, the association of groups of different single nucleotide polymorphism (SNPs) distributed throughout the genome are studies as a functions of the variation in drug response. The goal of this field of science is to adapt drugs to a patient's specific genetic background and therefore make them more efficacious and safe. The wider use of pharmacogenetic testing is viewed by many as an outstanding opportunity to improve prescription safety and efficacy. Comparisons of the list of drugs most commonly implicated in adverse drug reactions with the list of metabolizing enzymes with known polymorphisms found that drugs commonly involved in adverse drug reactions were also those that were metabolized by enzymes with known polymorphisms. Variants in genes that influence either the efficacy or toxicity of common drugs used in the treatment of inflammatory bowel diseases (IBD) such as ulcerative colitis (UC), Crohn's disease (CD), and colon cancer include sulfasalazine, mesalazine, azathioprine (AZA), 6-mercaptopurine (6-MP), methotrexate (MTX), glucocorticosteroids (CSs), and infliximab [111]. Although pharmacogenetics is a promising field that already contributed to a better understanding of some of the underlying mechanisms of action of drugs used in IBD, the only discovery translated until now into daily practice is the relation between thiopurine S-methyltransferase (TPMT) gene polymorphisms and hematological toxicity of thiopurine treatment. Clinical use of targeted therapies such as tyrosine kinase and angiogenesis inhibitors via molecular profiling may allow for highly individualized prognostic, predictive and therapeutic treatment plans tailored for each patient based on the molecular diagnostic profile of their tumor. Advances in genetic susceptibility, early detection, and individualized therapy based on each tumor's unique biological properties, all hold promise for the future management of cancer [112]. As a greater number of genes responsible for drug metabolism are discovered, future chemo/bio/genetic therapeutic agents used to treat cancer can be tailored to the patient's genetic polymorphisms. This will allow for the most efficient dosing while minimizing side-effects.
5. Combinatorial approach
5.1 Prevention and combined treatment for colon cancer
Given, the high frequencies of occurrence and recurrence of the disease following treatment, chemoprevention of colorectal cancer has attracted great attention in recent years [113]. Effective chemopreventive strategies may provide an attractive alternative in the current approach to reducing the morbidity and mortality from this disease. Chemoprevention is defined as the use of specific natural or synthetic chemical agents to reverse, suppress or prevent the progress from adenoma to invasive colorectal cancers. Epidemiologic data showed that chronic intake of traditional non-steroidal anti-inflammatory drugs (NSAIDs) could reduce the incidence of colorectal cancer [114, 115]. Recent clinical trial studies showed that celecoxib, a selective COX-2 inhibitor, is effective in reducing colorectal adenomas in animal models and patients with FAP, yet with superior GI safety as compared to traditional NSAIDs [116]. Two COX-2 inhibitors (celecoxib and refocoxib) have been approved by FDA as adjuncts to usual care in FAP patients, and are currently being studied in patients with sporadic adenomas and other types of cancers [113]. In addition to COX-2 inhibitors, a number of pharmacological and nonpharmacological agents have also been examined as potential chemopreventive agents for colorectal cancer [117]. These include difluoromethylornithine, an irreversible inhibitor of ornithine decarboxylase, hepatic hydroxymethylglutaryl coenzyme A reductase inhibitor, ursodexoycholic acid, the 7-β epimer of chenodeoxycholic acid, calcium, folate, vitamins, selenium and its derivatives, and dietary fiber. The mechanism of action of each agent appears to be unique and different in lowering the incidence of colorectal cancer. The exact mechanisms of chemoprevention by these agents are not fully understood. However, at least five potential pathways and mechanisms are likely to be involved by these agents: (a) Increased sensitivity of cancerous cells to apoptosis, (b) Inhibition of angiogenesis, (c) Modulation of inflammation and immune responses, (d) Decreased metastasis and (e) Inhibition and removal of endogenous carcinogen formation in-vivo [115, 118-120]. These agents, either alone or in combination with other chemotherapeutic agents, are being studied in various preclinical settings of colorectal cancer. The success of these studies will have far-reaching impact on our current understanding and approach to treatment and prevention of colorectal cancer. It is also common to combine radiotherapy with surgery, chemotherapy, hormone therapy, called “combination chemotherapy”. Systemic treatment decisions are guided by specific tumor characteristics and individual patient factors. Agents targeting angiogenesis, the epidermal growth factor receptor, and various signal transduction pathways have been combined with chemotherapy and possess excellent biologic activity [121]. However, emerging data focus on another aspect of cancer chemotherapy, the antitumor immunity effect as well. Although cancer chemotherapy was usually considered as immunosuppressive, some chemotherapeutic agents have recently been shown to activate an anticancer immune response, which is involved in the curative effect of these treatments. Cancer development often leads to the occurrence of an immune tolerance that prevents cancer rejection by the immune system and hinders efficacy of immunotherapy. They can restore an efficient immune response that contributes to the therapeutic effects of chemotherapy. Taking into account the immunostimulatory capacity of chemotherapeutic agents, and using them in combined chemo-immunotherapy strategies when tumor-induced tolerance is overcome [122]. Water soluble conjugates of doxorubicin with N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer carrier have emerged as efficient therapeutics because they are able to not only directly destroy cancer cells but also elicit systemic tumor-specific anticancer responses [123]. Anthracyclines and gemcitabine, are effective boosters of the immune response through tumor-specific antigen over-expression followed by apoptotic tumor cell destruction. The treatment of some leukemia's and lymphomas requires the use of high-dose chemotherapy, and a total body irradiation (TBI). This treatment ablates the bone marrow, and hence the body's ability to recover and repopulate the blood. For this reason, bone marrow, or peripheral blood stem cell harvesting is carried out before the ablative part of the therapy, to enable “rescue” after the treatment has been given, which have the potential of harming normal, otherwise healthy, non-neoplastic cells.
5.2 Combination of chemotherapy and immunotherapy
Chemotherapeutic agents have shown immunomodulatory activities that enhance the efficacy of tumor cell vaccines and favor the activity of adoptively transferred tumor-specific T-cells. Synergy between monoclonal antibodies and chemotherapy or peptide vaccination is based upon the induction of endogenous humoral and cellular immune responses [124]. The direct effects of chemotherapy on tumor or host environment, such as induction of tumor cell death, elimination of regulatory T cells, and/or enhancement of tumor cell sensitivity to lysis by CTL may account for enhancement of immunotherapy by chemotherapy. On the other hand, immunotherapy may directly modulate the tumor's sensitivity to chemotherapy. Anti-tumor mAb can increase the sensitivity of tumor cells to chemotherapeutic drugs and patients treated first with immunotherapy followed by chemotherapy showed higher clinical response rates than patients that had received chemotherapy alone. In essence, combination of active specific immunotherapy or adoptive mAb or lymphocyte immunotherapy with chemotherapy has great potential for the treatment of cancer patients which needs to be confirmed in larger controlled and randomized Phase III trials [125].
5.3 Tailoring immunotherapy to radiation therapy
Radiation may act synergistically with immunotherapy to enhance or broaden antitumor immune responses in part, because of radiation-induced phenotypic alterations of tumor cells that render them more susceptible to immune-mediated killing [126]. The interactions between the host and the tumor are complicated as there are many tumor-escape mechanisms. Immunotherapy is an attractive option for patients with high risk neuroblastoma due to their poor long-term survival rates after conventional treatment. Neuroblastoma cells express tumor antigens not widely seen in normal cells, monoclonal antibodies target these tumor associated antigens using tumor vaccines and adoptive transfer of tumor-directed T-cells [127]. Investigation of adjuvant treatment settings and combinations of vaccination and other treatment modalities show promising results in clinical trials. For instance, the combination of vaccination with high-dose cyclophosphamide was able to skew the response toward the target antigen and enhanced both the quantity and quality of antigen-specific CD8+ and CD4+ T-cell responses in tumor-bearing mice, which resulted in the inhibition of tumor growth. When tumor-specific antigens were targeted by the vaccine, the combination therapy actually produced tumor regression, which appeared to result from the high frequency of antigen-specific T-cells. Recombinant adenovirus vaccines are compatible with conventional high-dose chemotherapy and that the combined treatment resulted in improved therapeutic outcomes relative to either agent individually [128]. Although a firm conclusion cannot be drawn on T-cell induction in cancer patients during vaccination therapy, results show that CRC patients retain their antiviral T cells, suggesting a potential susceptibility to immunotherapy [129]. Strategies have improved because of advances in the characterization of tumor-associated antigens, the development of improved vaccine delivery systems, and the combination of vaccines with cytokines and other immunostimulants to enhance immune responses [130]. DNA vaccines, dendritic cell based vaccines, HSP based vaccines, and gene transfer technology are being developed to further refined and overcome their inherent limitations [38]. Anti-apoptotic molecules like survivin that enhance the survival of cancer cells and facilitate their escape from cytotoxic therapies are vaccination candidates. T-cell death upon drug exposure may be immunogenic or non-immunogenic depending on the specific chemotherapeutics. Also, chemotherapy represents one of several options available for clearance of CD4+ Foxp3+ regulatory T cells. Moreover, therapies based on monoclonal antibodies may have synergistic potential in combination with vaccination, both when used for targeting of tumor cells and endothelial cells [131].
5.4 Combinatorial immunotherapy
Combination immunotherapy is generally considered to be multi-targeted because it is aimed at multiple targets on the cancer cell and because preliminary data suggests that it has the potential to activate multiple cellular components of the immune system which will then target tumor cells and may cause an anti-tumor immune response. The combination of anthraquinone-based chemotherapy and the administration of DC differentiation-inducing cytokines (e.g., GM-CSF) might lead to the simultaneous rapid maturation of functional DC and the release of tumor associated antigens from dying tumor cells, a seemingly ideal scenario for in-vivo tumor immunization. In line with this, Apetoh et al. previously showed that anthracyclins (i.e., doxorubicin) can induce immunogenic tumor cell death due to the release of the high mobility group box 1 (HMGB1) protein from dying cells, which can induce DC activation through interaction with TLR4 [33]. The development of new treatment modalities, including specific immunotherapy, is of great importance in the field of cancer immunotherapy. Recently, new paradigms have emerged in the field of cancer vaccine research. In particular, the potential use of combination therapies that incorporated immune modulators and standard radio- and chemo-therapy synergized with cancer vaccines. Cytotoxic chemotherapy is generally considered immunosuppressive, because of its toxicity for dividing cells in the bone marrow and peripheral lymphoid tissue. Therefore, the combination of cancer vaccines with chemotherapies has been considered to be inappropriate because the immunosuppressive effects of the chemotherapy would negate the efficacy of cancer vaccines. However, increasing evidences have been mounting to suggest that immunotherapy has the possibility of achieving better success when used in combination with conventional chemotherapy [132, 133]. A standard cytotoxic agent, gemcitabine, not only exerts direct antitumor activity, but also mediates immunological effects relevant for cancer immunotherapy [100–102]. Cross-presentation of TAAs by DCs is essential for induction of augmented CTL responses. Treatment of cancer cells and DCs with gemcitabine resulted in enhanced cross-presentation of TAAs by DCs, CTL expansion, and infiltration of the tumor, all of which are associated with augmented CTL [134].
5.5 Investigational combination immunotherapy
A potential investigational combination immunotherapy in what may become a new class of immunotherapy drugs. Multikine (Leukocyte, Interleukin Injection) is an investigational new drug being studied as a potential combination immunotherapy. This type of immunotherapy is thought to work on multiple fronts against advanced cancer. Multikine investigational therapy is comprised of a mixture of cytokines and is not one cell or one protein. It is a combination of molecules and proteins (interleukins, interferons, chemokines, and colony stimulating factors) derived from the stimulation in culture of normal immune system cells. Multikine investigational therapy is a patented defined mixture of biologically active, natural cytokines, for which preliminary evidence from studies to date suggests the potential to simulate the body's healthy immune response [135].
Conclusion and Future Direction
Cancer in general and colorectal cancer in particular is a very complex disease to treat, not only because of its ability to avoid being recognized by the immune system, but also because of its ability to immortalize and continue to divide endlessly. A number of therapies are available to cancers patients including those having colorectal cancer depending on the stage of the cancer and the degree of complications. All the therapies are having their own success-rate and limitations. Effectiveness of a therapy would depend on the survival-rate of the patients and level of recurrence. In this review we have highlighted and emphasized the combinatorial approach along with the conventional methods of cancer treatment that could be much more effective and would enhance the patient's survival. Success-rate driven combinatorial therapy holds a promise and could be foreseen as treatment of choice and the clinical trials for such an approach should be encouraged in future (Fig 1). Also it would be imperative in future, to find out the success rate for different combinatorial approach to overcome the limitations of treating the colorectal cancer.
Figure 1.
Limitations and future of treating cancer through combinatorial approach
Acknowledgments
Funding
The work was supported by grants from Crohn's & Colitis Foundation of America (CCFA Ref. No. 1351 and 2188) and National Institutes of Health Grants DK-081661 (to N. Kumar)
Biography
Dr. Narendra Kumar completed his PhD from the Indian Institute of Technology and postdoctoral training at the University of Tennessee Health Science Center Memphis. After post-doctoral training, he held a joint appointment as instructor of Physiology and Pediatric Gastroenterology at the University of Tennessee HSC and Le Bonheur Children Medical Center, respectively. Since 2008 he has been Assistant Professor at Texas A&M Health Science Center. Rated among top 25% in the college by the students, he teaches immunology, biochemistry, pharmacogenomics and principles of drug action under the PharmD program. In research he is the recipient of several national awards, such as the Crohn's and Colitis Foundation of America (CCFA) Research Fellowship Award, CCFA Career Development Award, National Institute of Health (NIH) Basic Scientist Career Development Award (K01), American Gastroenterology Association (AGA) Research Scholar Award to name a few. His research is currently funded by NIH and CCFA. He has published more than 20 peer reviewed research articles, 30 abstracts, and a book chapter. He also has three patents to his credit, two of which are already commercialized while the third is under negotiation for licensing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Disclosure: No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Kaler P, Galea V, Augenlicht L, Klampfer L. Tumor associated macrophages protect colon cancer cells from TRAIL-induced apoptosis through IL-1beta-dependent stabilization of Snail in tumor cells. PloS one. 2010;5:e11700. doi: 10.1371/journal.pone.0011700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colorectal Cancer Screening. Rockville (MD): 1998. [Google Scholar]
- 3.Chen MJ, Cheng YM, Lai PH, Wu JF, Hsu YC. In vitro biocompatibility of thermally gelling liquid mucoadhesive loaded curcuminoids in colorectal cancer chemoprevention. International journal of colorectal disease. 2012 doi: 10.1007/s00384-011-1393-3. [DOI] [PubMed] [Google Scholar]
- 4.Slattery ML. Diet, lifestyle, and colon cancer. Seminars in gastrointestinal disease. 2000;11:142–6. [PubMed] [Google Scholar]
- 5.Nakaji S, Umeda T, Shimoyama T, Sugawara K, Tamura K, Fukuda S, et al. Environmental factors affect colon carcinoma and rectal carcinoma in men and women differently. International journal of colorectal disease. 2003;18:481–6. doi: 10.1007/s00384-003-0485-0. [DOI] [PubMed] [Google Scholar]
- 6.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. The Journal of nutrition. 2001;131:3109S–20S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 7.Anaya DA, Becker NS, Abraham NS. Global graying, colorectal cancer and liver metastasis: new implications for surgical management. Crit Rev Oncol Hematol. 2011;77:100–8. doi: 10.1016/j.critrevonc.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Saif MW, Chu E. Biology of colorectal cancer. Cancer J. 2010;16:196–201. doi: 10.1097/PPO.0b013e3181e076af. [DOI] [PubMed] [Google Scholar]
- 9.Niv Y, Goel A, Boland CR. JC virus and colorectal cancer: a possible trigger in the chromosomal instability pathways. Current opinion in gastroenterology. 2005;21:85–9. [PubMed] [Google Scholar]
- 10.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 11.Burn J, Mathers J, Bishop DT. Genetics, Inheritance and Strategies for Prevention in Populations at High Risk of Colorectal Cancer (CRC). Recent Results Cancer Res. 2012;191:157–83. doi: 10.1007/978-3-642-30331-9_9. [DOI] [PubMed] [Google Scholar]
- 12.Emery J, Lucassen A, Murphy M. Common hereditary cancers and implications for primary care. Lancet. 2001;358:56–63. doi: 10.1016/S0140-6736(00)05257-0. [DOI] [PubMed] [Google Scholar]
- 13.Fearon ER. Molecular genetics of colorectal cancer. Annual review of pathology. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 14.Lievre A, Blons H, Laurent-Puig P. Oncogenic mutations as predictive factors in colorectal cancer. Oncogene. 2010;29:3033–43. doi: 10.1038/onc.2010.89. [DOI] [PubMed] [Google Scholar]
- 15.Siddiqui A, Piperdi B. <i>KRAS Mutation in Colon Cancer: A Marker of Resistance to EGFR-I Therapy. Annals of Surgical Oncology. 2010;17:1168–76. doi: 10.1245/s10434-009-0811-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baba Y, Nosho K, Shima K, Hayashi M, Meyerhardt JA, Chan AT, et al. Phosphorylated AKT expression is associated with PIK3CA mutation, low stage, and favorable outcome in 717 colorectal cancers. Cancer. 2010;117:1399–408. doi: 10.1002/cncr.25630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corvinus FM, Orth C, Moriggl R, Tsareva SA, Wagner S, Pfitzner EB, et al. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia. 2005;7:545–55. doi: 10.1593/neo.04571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin L, Deangelis S, Foust E, Fuchs J, Li C, Li PK, et al. A novel small molecule inhibits STAT3 phosphorylation and DNA binding activity and exhibits potent growth suppressive activity in human cancer cells. Mol Cancer. 2010;9:217–27. doi: 10.1186/1476-4598-9-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heidelbaugh JJ, Tortorello M. The adult well male examination. Am Fam Physician. 2012;85:964–71. [PubMed] [Google Scholar]
- 20.Kristjansson SR, Nesbakken A, Jordhoy MS, Skovlund E, Audisio RA, Johannessen HO, et al. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: a prospective observational cohort study. Crit Rev Oncol Hematol. 2010;76:208–17. doi: 10.1016/j.critrevonc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Marshall JL. Managing potentially resectable metastatic colon cancer. Gastrointest Cancer Res. 2008;2:S23–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Matsumura Y. Polymeric micellar delivery systems in oncology. Japanese journal of clinical oncology. 2008;38:793–802. doi: 10.1093/jjco/hyn116. [DOI] [PubMed] [Google Scholar]
- 23.Morse MA, Hall JR, Plate JM. Countering tumor-induced immunosuppression during immunotherapy for pancreatic cancer. Expert opinion on biological therapy. 2009;9:331–9. doi: 10.1517/14712590802715756. [DOI] [PubMed] [Google Scholar]
- 24.de la Cruz-Merino L, Grande-Pulido E, Albero-Tamarit A, Codes-Manuel de Villena ME. Cancer and immune response: old and new evidence for future challenges. The oncologist. 2008;13:1246–54. doi: 10.1634/theoncologist.2008-0166. [DOI] [PubMed] [Google Scholar]
- 25.Halama N, Zoernig I, Jager D. [Immunotherapy for cancer--modern immunologic strategies in oncology]. Dtsch Med Wochenschr. 2008;133:2105–8. doi: 10.1055/s-0028-1091251. [DOI] [PubMed] [Google Scholar]
- 26.Kotlan B, Umansky V, Malyguine AM, Marincola FM, Shurin MR. Conference Scene: Immunotherapy reaches new milestones in cancer eradication. Immunotherapy. 2011;3:1131–7. doi: 10.2217/imt.11.114. [DOI] [PubMed] [Google Scholar]
- 27.Mishra J, Waters CM, Kumar N. Molecular Mechanism of Interleukin-2-induced Mucosal Homeostasis. Am J Physiol Cell Physiol. 2011 doi: 10.1152/ajpcell.00316.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mocellin S, Nitti D. Therapeutics targeting tumor immune escape: towards the development of new generation anticancer vaccines. Medicinal research reviews. 2008;28:413–44. doi: 10.1002/med.20110. [DOI] [PubMed] [Google Scholar]
- 29.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soudja SM, Wehbe M, Mas A, Chasson L, de Tenbossche CP, Huijbers I, et al. Tumor-initiated inflammation overrides protective adaptive immunity in an induced melanoma model in mice. Cancer research. 2010;70:3515–25. doi: 10.1158/0008-5472.CAN-09-4354. [DOI] [PubMed] [Google Scholar]
- 31.Shanker A, Marincola FM. Cooperativity of adaptive and innate immunity: implications for cancer therapy. Cancer immunology, immunotherapy : CII. 2011;60:1061–74. doi: 10.1007/s00262-011-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annual review of immunology. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 33.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature medicine. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 34.Kruger C, Greten TF, Korangy F. Immune based therapies in cancer. Histology and histopathology. 2007;22:687–96. doi: 10.14670/HH-22.687. [DOI] [PubMed] [Google Scholar]
- 35.van de Ven R, Reurs AW, Wijnands PG, van Wetering S, Kruisbeek AM, Hooijberg E, et al. Exposure of CD34+ precursors to cytostatic anthraquinone-derivatives induces rapid dendritic cell differentiation: implications for cancer immunotherapy. Cancer immunology, immunotherapy : CII. 2012;61:181–91. doi: 10.1007/s00262-011-1039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Limpens J, Van Meijer M, Van Santen HM, Germeraad WT, Hoeben-Schornagel K, Breel M, et al. Alterations in dendritic cell phenotype and function associated with immunoenhancing effects of a subcutaneously administered cyclophosphamide derivative. Immunology. 1991;73:255–63. [PMC free article] [PubMed] [Google Scholar]
- 37.Yu B, Kusmartsev S, Cheng F, Paolini M, Nefedova Y, Sotomayor E, et al. Effective combination of chemotherapy and dendritic cell administration for the treatment of advanced-stage experimental breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:285–94. [PubMed] [Google Scholar]
- 38.Chaudhuri D, Suriano R, Mittelman A, Tiwari RK. Targeting the immune system in cancer. Current pharmaceutical biotechnology. 2009;10:166–84. doi: 10.2174/138920109787315114. [DOI] [PubMed] [Google Scholar]
- 39.Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Advanced drug delivery reviews. 2008;60:1615–26. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Li YS, Davidson E, Reid CN, McHale AP. Optimising ultrasound-mediated gene transfer (sonoporation) in vitro and prolonged expression of a transgene in vivo: potential applications for gene therapy of cancer. Cancer letters. 2009;273:62–9. doi: 10.1016/j.canlet.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 41.Frenkel V. Ultrasound mediated delivery of drugs and genes to solid tumors. Advanced drug delivery reviews. 2008;60:1193–208. doi: 10.1016/j.addr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kyo S, Takakura M, Fujiwara T, Inoue M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer science. 2008;99:1528–38. doi: 10.1111/j.1349-7006.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li QX, Liu G, Wong-Staal F. Oncolytic virotherapy as a personalized cancer vaccine. International journal of cancer Journal international du cancer. 2008;123:493–9. doi: 10.1002/ijc.23692. [DOI] [PubMed] [Google Scholar]
- 44.Vigna E, Pacchiana G, Mazzone M, Chiriaco C, Fontani L, Basilico C, et al. “Active” cancer immunotherapy by anti-Met antibody gene transfer. Cancer research. 2008;68:9176–83. doi: 10.1158/0008-5472.CAN-08-1688. [DOI] [PubMed] [Google Scholar]
- 45.Chen L, Tang XD, Yu ST, Ai ZH, Fang DC, Cai YG, et al. Induction of anti-tumour immunity by dendritic cells transduced with hTERT recombinant adenovirus in mice. The Journal of pathology. 2009;217:685–92. doi: 10.1002/path.2493. [DOI] [PubMed] [Google Scholar]
- 46.Lei N, Shen FB, Chang JH, Wang L, Li H, Yang C, et al. An oncolytic adenovirus expressing granulocyte macrophage colony-stimulating factor shows improved specificity and efficacy for treating human solid tumors. Cancer gene therapy. 2009;16:33–43. doi: 10.1038/cgt.2008.46. [DOI] [PubMed] [Google Scholar]
- 47.Davis JJ, Wang L, Dong F, Zhang L, Guo W, Teraishi F, et al. Oncolysis and suppression of tumor growth by a GFP-expressing oncolytic adenovirus controlled by an hTERT and CMV hybrid promoter. Cancer gene therapy. 2006;13:720–3. doi: 10.1038/sj.cgt.7700944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su C, Na M, Chen J, Wang X, Liu Y, Wang W, et al. Gene-viral cancer therapy using dual-regulated oncolytic adenovirus with antiangiogenesis gene for increased efficacy. Molecular cancer research : MCR. 2008;6:568–75. doi: 10.1158/1541-7786.MCR-07-0073. [DOI] [PubMed] [Google Scholar]
- 49.Huang P, Watanabe M, Kaku H, Kashiwakura Y, Chen J, Saika T, et al. Direct and distant antitumor effects of a telomerase-selective oncolytic adenoviral agent, OBP-301, in a mouse prostate cancer model. Cancer gene therapy. 2008;15:315–22. doi: 10.1038/cgt.2008.3. [DOI] [PubMed] [Google Scholar]
- 50.Gao Y, Ng SS, Chau DH, Yao H, Yang C, Man K, et al. Development of recombinant adeno-associated virus and adenovirus cocktail system for efficient hTERTC27 polypeptide-mediated cancer gene therapy. Cancer gene therapy. 2008;15:723–32. doi: 10.1038/cgt.2008.33. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Q, Li Y, Shi Y, Zhang Y. HVJ envelope vector, a versatile delivery system: its development, application, and perspectives. Biochemical and biophysical research communications. 2008;373:345–9. doi: 10.1016/j.bbrc.2008.06.055. [DOI] [PubMed] [Google Scholar]
- 52.Goyal AK, Khatri K, Vyas SP. Patents on non-viral mediated gene delivery. Recent patents on DNA & gene sequences. 2008;2:44–60. doi: 10.2174/187221508783406594. [DOI] [PubMed] [Google Scholar]
- 53.Dass CR, Choong PF. Chitosan-mediated orally delivered nucleic acids: a gutful of gene therapy. Journal of drug targeting. 2008;16:257–61. doi: 10.1080/10611860802088523 . [DOI] [PubMed] [Google Scholar]
- 54.Chu L, Gu J, Sun L, Qian Q, Qian C, Liu X. Oncolytic adenovirus-mediated shRNA against Apollon inhibits tumor cell growth and enhances antitumor effect of 5-fluorouracil. Gene therapy. 2008;15:484–94. doi: 10.1038/gt.2008.6. [DOI] [PubMed] [Google Scholar]
- 55.Gravalos C, Cassinello J, Garcia-Alfonso P, Jimeno A. Integration of panitumumab into the treatment of colorectal cancer. Crit Rev Oncol Hematol. 2010;74:16–26. doi: 10.1016/j.critrevonc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez J, Viudez A, Ponz-Sarvise M, Gil-Aldea I, Chopitea A, Garcia-Foncillas J, et al. Improving disease control in advanced colorectal cancer: Panitumumab and cetuximab. Crit Rev Oncol Hematol. 2010;74:193–202. doi: 10.1016/j.critrevonc.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Zalcman G, Richard N, Bergot E. [New biological treatments for lung cancer]. Revue de pneumologie clinique. 2007;63:20–8. doi: 10.1016/s0761-8417(07)90085-1. [DOI] [PubMed] [Google Scholar]
- 58.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nature reviews Immunology. 2010;10:317–27. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Houot R, Kohrt HE, Marabelle A, Levy R. Targeting immune effector cells to promote antibody-induced cytotoxicity in cancer immunotherapy. Trends in immunology. 2011;32:510–6. doi: 10.1016/j.it.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Weng D, Song B, Durfee J, Sugiyama V, Wu Z, Koido S, et al. Induction of cytotoxic T lymphocytes against ovarian cancer-initiating cells. International journal of cancer Journal international du cancer. 2010 doi: 10.1002/ijc.25851. [DOI] [PubMed] [Google Scholar]
- 61.Iwata-Kajihara T, Sumimoto H, Kawamura N, Ueda R, Takahashi T, Mizuguchi H, et al. Enhanced cancer immunotherapy using STAT3-depleted dendritic cells with high Th1-inducing ability and resistance to cancer cell-derived inhibitory factors. J Immunol. 2011;187:27–36. doi: 10.4049/jimmunol.1002067. [DOI] [PubMed] [Google Scholar]
- 62.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 63.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nature medicine. 2004;10:475–80. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 64.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–83. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 65.Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunological reviews. 2007;220:129–50. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- 66.Kawakami Y, Fujita T, Kudo C, Sakurai T, Udagawa M, Yaguchi T, et al. Dendritic cell based personalized immunotherapy based on cancer antigen research. Frontiers in bioscience : a journal and virtual library. 2008;13:1952–8. doi: 10.2741/2814. [DOI] [PubMed] [Google Scholar]
- 67.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 68.Mueller T, Voigt W. Fermented wheat germ extract--nutritional supplement or anticancer drug? Nutrition journal. 2011;10:89. doi: 10.1186/1475-2891-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nautiyal J, Kanwar SS, Yu Y, Majumdar AP. Combination of dasatinib and curcumin eliminates chemo-resistant colon cancer cells. Journal of molecular signaling. 2011;6:7. doi: 10.1186/1750-2187-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin L, Liu Y, Li H, Li PK, Fuchs J, Shibata H, et al. Targeting colon cancer stem cells using a new curcumin analogue, GO-Y030. British journal of cancer. 2011;105:212–20. doi: 10.1038/bjc.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xavier CP, Lima CF, Rohde M, Pereira-Wilson C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer chemotherapy and pharmacology. 2011;68:1449–57. doi: 10.1007/s00280-011-1641-9. [DOI] [PubMed] [Google Scholar]
- 72.Murphy EA, Davis JM, McClellan JL, Carmichael MD. Quercetin's effects on intestinal polyp multiplicity and macrophage number in the Apc(Min/+) mouse. Nutrition and cancer. 2011;63:421–6. doi: 10.1080/01635581.2011.535954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Volate SR, Davenport DM, Muga SJ, Wargovich MJ. Modulation of aberrant crypt foci and apoptosis by dietary herbal supplements (quercetin, curcumin, silymarin, ginseng and rutin). Carcinogenesis. 2005;26:1450–6. doi: 10.1093/carcin/bgi089. [DOI] [PubMed] [Google Scholar]
- 74.Altonsy MO, Andrews SC. Diallyl disulphide, a beneficial component of garlic oil, causes a redistribution of cell-cycle growth phases, induces apoptosis, and enhances butyrate-induced apoptosis in colorectal adenocarcinoma cells (HT-29). Nutrition and cancer. 2011;63:1104–13. doi: 10.1080/01635581.2011.601846. [DOI] [PubMed] [Google Scholar]
- 75.Chen CY, Huang CF, Tseng YT, Kuo SY. Diallyl disulfide induces Ca2+ mobilization in human colon cancer cell line SW480. Archives of toxicology. 2012;86:231–8. doi: 10.1007/s00204-011-0748-4. [DOI] [PubMed] [Google Scholar]
- 76.Dhayat S, Mardin WA, Mees ST, Haier J. Epigenetic markers for chemosensitivity and chemoresistance in pancreatic cancer--a review. International journal of cancer Journal international du cancer. 2011;129:1031–41. doi: 10.1002/ijc.26078. [DOI] [PubMed] [Google Scholar]
- 77.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–8. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 78.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Du T, Zamore PD. microPrimer: the biogenesis and function of microRNA. Development. 2005;132:4645–52. doi: 10.1242/dev.02070. [DOI] [PubMed] [Google Scholar]
- 80.Schwarz DS, Hutvagner G, Du T, Xu Z, Aronin N, Zamore PD. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115:199–208. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- 81.Hutvagner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002;297:2056–60. doi: 10.1126/science.1073827. [DOI] [PubMed] [Google Scholar]
- 82.Martinez J, Tuschl T. RISC is a 5' phosphomonoester-producing RNA endonuclease. Genes & development. 2004;18:975–80. doi: 10.1101/gad.1187904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–36. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 84.Akao Y, Nakagawa Y, Naoe T. let-7 microRNA functions as a potential growth suppressor in human colon cancer cells. Biological & pharmaceutical bulletin. 2006;29:903–6. doi: 10.1248/bpb.29.903. [DOI] [PubMed] [Google Scholar]
- 85.Toyota M, Suzuki H, Sasaki Y, Maruyama R, Imai K, Shinomura Y, et al. Epigenetic silencing of microRNA-34b/c and B-cell translocation gene 4 is associated with CpG island methylation in colorectal cancer. Cancer research. 2008;68:4123–32. doi: 10.1158/0008-5472.CAN-08-0325. [DOI] [PubMed] [Google Scholar]
- 86.Mook OR, Baas F, de Wissel MB, Fluiter K. Allele-specific cancer cell killing in vitro and in vivo targeting a single-nucleotide polymorphism in POLR2A. Cancer gene therapy. 2009;16:532–8. doi: 10.1038/cgt.2008.104. [DOI] [PubMed] [Google Scholar]
- 87.Liu X, Yang J, Shang F, Hong C, Guo W, Wang B, et al. Silencing GIRK4 expression in human atrial myocytes by adenovirus-delivered small hairpin RNA. Molecular biology reports. 2009;36:1345–52. doi: 10.1007/s11033-008-9318-0. [DOI] [PubMed] [Google Scholar]
- 88.Takahashi Y, Nishikawa M, Takakura Y. Suppression of tumor growth by intratumoral injection of short hairpin RNA-expressing plasmid DNA targeting beta-catenin or hypoxia-inducible factor 1alpha. Journal of controlled release : official journal of the Controlled Release Society. 2006;116:90–5. doi: 10.1016/j.jconrel.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 89.Chaturvedi A, Battmer K, Schaefer D, Ganser A, Eder M, Scherr M. Comparison between molecularly defined and conventional therapeutics in a conditional BCR-ABL cell culture model. Oligonucleotides. 2007;17:22–34. doi: 10.1089/oli.2006.0054. [DOI] [PubMed] [Google Scholar]
- 90.Judge AD, Robbins M, Tavakoli I, Levi J, Hu L, Fronda A, et al. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. The Journal of clinical investigation. 2009;119:661–73. doi: 10.1172/JCI37515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rauschhuber C, Xu H, Salazar FH, Marion PL, Ehrhardt A. Exploring gene-deleted adenoviral vectors for delivery of short hairpin RNAs and reduction of hepatitis B virus infection in mice. The journal of gene medicine. 2008;10:878–89. doi: 10.1002/jgm.1207. [DOI] [PubMed] [Google Scholar]
- 92.Zhu H, Zhu Y, Hu J, Hu W, Liao Y, Zhang J, et al. Adenovirus-mediated small hairpin RNA targeting Bcl-XL as therapy for colon cancer. International journal of cancer Journal international du cancer. 2007;121:1366–72. doi: 10.1002/ijc.22856. [DOI] [PubMed] [Google Scholar]
- 93.Narvaiza I, Aparicio O, Vera M, Razquin N, Bortolanza S, Prieto J, et al. Effect of adenovirus-mediated RNA interference on endogenous microRNAs in a mouse model of multidrug resistance protein 2 gene silencing. Journal of virology. 2006;80:12236–47. doi: 10.1128/JVI.01205-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takahashi Y, Nishikawa M, Takakura Y. [In vivo siRNA delivery to tumor cells and its application to cancer gene therapy]. Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan. 2007;127:1525–31. doi: 10.1248/yakushi.127.1525. [DOI] [PubMed] [Google Scholar]
- 95.Yoo JY, Kim JH, Kim J, Huang JH, Zhang SN, Kang YA, et al. Short hairpin RNA-expressing oncolytic adenovirus-mediated inhibition of IL-8: effects on antiangiogenesis and tumor growth inhibition. Gene therapy. 2008;15:635–51. doi: 10.1038/gt.2008.3. [DOI] [PubMed] [Google Scholar]
- 96.Sun L, Gao H, Sarma VJ, Guo RF, Ward PA. Adenovirus-mediated in vivo silencing of anaphylatoxin receptor C5aR. Journal of biomedicine & biotechnology. 2006;2006:28945. doi: 10.1155/JBB/2006/28945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang L, Qi X, Shen R, Sun Y, Tuveson DA. An shRNA silencing a non-toxic transgene reduces nutrient consumption and increases production of adenoviral vectors in a novel packaging cell. Journal of cellular physiology. 2009;219:365–71. doi: 10.1002/jcp.21679. [DOI] [PubMed] [Google Scholar]
- 98.He B, Reguart N, You L, Mazieres J, Xu Z, Lee AY, et al. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene. 2005;24:3054–8. doi: 10.1038/sj.onc.1208511. [DOI] [PubMed] [Google Scholar]
- 99.Gil PR, Parak WJ. Composite nanoparticles take aim at cancer. ACS nano. 2008;2:2200–5. doi: 10.1021/nn800716j. [DOI] [PubMed] [Google Scholar]
- 100.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature reviews Drug discovery. 2008;7:771–82. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 101.Schiffelers RM, Storm G. Liposomal nanomedicines as anticancer therapeutics: beyond targeting tumor cells. International journal of pharmaceutics. 2008;364:258–64. doi: 10.1016/j.ijpharm.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 102.Cardoso AL, Simoes S, de Almeida LP, Pelisek J, Culmsee C, Wagner E, et al. siRNA delivery by a transferrin-associated lipid-based vector: a non-viral strategy to mediate gene silencing. The journal of gene medicine. 2007;9:170–83. doi: 10.1002/jgm.1006. [DOI] [PubMed] [Google Scholar]
- 103.Kim SH, Jeong JH, Lee SH, Kim SW, Park TG. Local and systemic delivery of VEGF siRNA using polyelectrolyte complex micelles for effective treatment of cancer. Journal of controlled release : official journal of the Controlled Release Society. 2008;129:107–16. doi: 10.1016/j.jconrel.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 104.Bonnet ME, Erbacher P, Bolcato-Bellemin AL. Systemic delivery of DNA or siRNA mediated by linear polyethylenimine (L-PEI) does not induce an inflammatory response. Pharmaceutical research. 2008;25:2972–82. doi: 10.1007/s11095-008-9693-1. [DOI] [PubMed] [Google Scholar]
- 105.Shim MS, Kwon YJ. Acid-responsive linear polyethylenimine for efficient, specific, and biocompatible siRNA delivery. Bioconjugate chemistry. 2009;20:488–99. doi: 10.1021/bc800436v. [DOI] [PubMed] [Google Scholar]
- 106.Koping-Hoggard M, Tubulekas I, Guan H, Edwards K, Nilsson M, Varum KM, et al. Chitosan as a nonviral gene delivery system. Structure-property relationships and characteristics compared with polyethylenimine in vitro and after lung administration in vivo. Gene therapy. 2001;8:1108–21. doi: 10.1038/sj.gt.3301492. [DOI] [PubMed] [Google Scholar]
- 107.Tan ML, Choong PF, Dass CR. Cancer, chitosan nanoparticles and catalytic nucleic acids. The Journal of pharmacy and pharmacology. 2009;61:3–12. doi: 10.1211/jpp/61.01.0002. [DOI] [PubMed] [Google Scholar]
- 108.Kumar N, Mishra J, Narang VS, Waters CM. Janus kinase 3 regulates interleukin 2-induced mucosal wound repair through tyrosine phosphorylation of villin. J Biol Chem. 2007;282:30341–5. doi: 10.1074/jbc.C600319200. [DOI] [PubMed] [Google Scholar]
- 109.Rimm DL, Holland TE, Morrow JS, Anderson JM. Autoantibodies specific for villin found in patients with colon cancer and other colitides. Dig Dis Sci. 1995;40:389–95. doi: 10.1007/BF02065426. [DOI] [PubMed] [Google Scholar]
- 110.Rane SG, Reddy EP. Janus kinases: components of multiple signaling pathways. Oncogene. 2000;19:5662–79. doi: 10.1038/sj.onc.1203925. [DOI] [PubMed] [Google Scholar]
- 111.Smith MA, Marinaki AM, Sanderson JD. Pharmacogenomics in the treatment of inflammatory bowel disease. Pharmacogenomics. 2010;11:421–37. doi: 10.2217/pgs.10.4. [DOI] [PubMed] [Google Scholar]
- 112.Lam WK, Watkins DN. Lung cancer: future directions. Respirology. 2007;12:471–7. doi: 10.1111/j.1440-1843.2007.01105.x. [DOI] [PubMed] [Google Scholar]
- 113.Chu AJ, Chou TH, Chen BD. Prevention of colorectal cancer using COX-2 inhibitors: basic science and clinical applications. Frontiers in bioscience : a journal and virtual library. 2004;9:2697–713. doi: 10.2741/1429. [DOI] [PubMed] [Google Scholar]
- 114.Klampfer L. Cytokines, inflammation and colon cancer. Current cancer drug targets. 2011;11:451–64. doi: 10.2174/156800911795538066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hawk ET, Viner JL, Umar A. Non-steroidal anti-inflammatory and cyclooxygenase-2-selective inhibitors in clinical cancer prevention trials. Prog Exp Tumor Res. 2003;37:210–42. doi: 10.1159/000071375. [DOI] [PubMed] [Google Scholar]
- 116.Bakhle YS. COX-2 and cancer: a new approach to an old problem. Br J Pharmacol. 2001;134:1137–50. doi: 10.1038/sj.bjp.0704365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gwyn K, Sinicrope FA. Chemoprevention of colorectal cancer. Am J Gastroenterol. 2002;97:13–21. doi: 10.1111/j.1572-0241.2002.05435.x. [DOI] [PubMed] [Google Scholar]
- 118.Dempke W, Rie C, Grothey A, Schmoll HJ. Cyclooxygenase-2: a novel target for cancer chemotherapy? J Cancer Res Clin Oncol. 2001;127:411–7. doi: 10.1007/s004320000225. [DOI] [PubMed] [Google Scholar]
- 119.Prescott SM, Fitzpatrick FA. Cyclooxygenase-2 and carcinogenesis. Biochim Biophys Acta. 2000;1470:M69–78. doi: 10.1016/s0304-419x(00)00006-8. [DOI] [PubMed] [Google Scholar]
- 120.Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003;24:96–102. doi: 10.1016/S0165-6147(02)00043-3. [DOI] [PubMed] [Google Scholar]
- 121.Conlin AK, Seidman AD. Beyond cytotoxic chemotherapy for the first-line treatment of HER2-negative, hormone-insensitive metastatic breast cancer: current status and future opportunities. Clinical breast cancer. 2008;8:215–23. doi: 10.3816/CBC.2008.n.024. [DOI] [PubMed] [Google Scholar]
- 122.Menard C, Martin F, Apetoh L, Bouyer F, Ghiringhelli F. Cancer chemotherapy: not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity. Cancer immunology, immunotherapy : CII. 2008;57:1579–87. doi: 10.1007/s00262-008-0505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rihova B, Kovar L, Kovar M, Hovorka O. Cytotoxicity and immunostimulation: double attack on cancer cells with polymeric therapeutics. Trends in biotechnology. 2009;27:11–7. doi: 10.1016/j.tibtech.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 124.Baxevanis CN, Perez SA, Papamichail M. Combinatorial treatments including vaccines, chemotherapy and monoclonal antibodies for cancer therapy. Cancer immunology, immunotherapy : CII. 2009;58:317–24. doi: 10.1007/s00262-008-0576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang T, Herlyn D. Combination of active specific immunotherapy or adoptive antibody or lymphocyte immunotherapy with chemotherapy in the treatment of cancer. Cancer immunology, immunotherapy : CII. 2009;58:475–92. doi: 10.1007/s00262-008-0598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ferrara TA, Hodge JW, Gulley JL. Combining radiation and immunotherapy for synergistic antitumor therapy. Current opinion in molecular therapeutics. 2009;11:37–42. [PMC free article] [PubMed] [Google Scholar]
- 127.Louis CU, Brenner MK. Cellular immunotherapy for neuroblastoma: a review of current vaccine and adoptive T cell therapeutics. Current pharmaceutical design. 2009;15:424–9. doi: 10.2174/138161209787315765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Grinshtein N, Ventresca M, Margl R, Bernard D, Yang TC, Millar JB, et al. High-dose chemotherapy augments the efficacy of recombinant adenovirus vaccines and improves the therapeutic outcome. Cancer gene therapy. 2009;16:338–50. doi: 10.1038/cgt.2008.89. [DOI] [PubMed] [Google Scholar]
- 129.Kiewe P, Wojtke S, Thiel E, Nagorsen D. Antiviral cellular immunity in colorectal cancer patients. Human immunology. 2009;70:85–8. doi: 10.1016/j.humimm.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 130.Mohebtash M, Gulley JL, Madan RA, Ferrara T, Arlen PM. Cancer vaccines: current directions and perspectives in prostate cancer. Current opinion in molecular therapeutics. 2009;11:31–6. [PMC free article] [PubMed] [Google Scholar]
- 131.Andersen MH, Sorensen RB, Schrama D, Svane IM, Becker JC, Thor Straten P. Cancer treatment: the combination of vaccination with other therapies. Cancer immunology, immunotherapy : CII. 2008;57:1735–43. doi: 10.1007/s00262-008-0480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gabrilovich DI. Combination of chemotherapy and immunotherapy for cancer: a paradigm revisited. The lancet oncology. 2007;8:2–3. doi: 10.1016/S1470-2045(06)70985-8. [DOI] [PubMed] [Google Scholar]
- 133.Smith BD, Kasamon YL, Kowalski J, Gocke C, Murphy K, Miller CB, et al. K562/GM-CSF immunotherapy reduces tumor burden in chronic myeloid leukemia patients with residual disease on imatinib mesylate. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:338–47. doi: 10.1158/1078-0432.CCR-09-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Koido S, Homma S, Takahara A, Namiki Y, Tsukinaga S, Mitobe J, et al. Current immunotherapeutic approaches in pancreatic cancer. Clinical & developmental immunology. 2011;2011:267539. doi: 10.1155/2011/267539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Timar J, Forster-Horvath C, Lukits J, Dome B, Ladanyi A, Remenar E, et al. The effect of leukocyte interleukin injection (Multikine) treatment on the peritumoral and intratumoral subpopulation of mononuclear cells and on tumor epithelia: a possible new approach to augmenting sensitivity to radiation therapy and chemotherapy in oral cancer--a multicenter phase I/II clinical Trial. Laryngoscope. 2003;113:2206–17. doi: 10.1097/00005537-200312000-00031. [DOI] [PubMed] [Google Scholar]