Abstract
Background
Vesicular stomatitis virus (VSV) is a novel, anti-cancer therapy that selectively targets cancer cells with defective antiviral responses; however, not all malignant cells are sensitive to the oncolytic effects of VSV. Herein, we explore the mechanistic determinants of mutant M protein VSV (M51R-VSV) susceptibility in malignant melanoma cells.
Methods
Cell viability after VSV infection was measured by the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) viability assay in a panel of melanoma cell lines. VSV infectability, viral protein synthesis and viral progeny production were quantified by flow cytometry, 35S-methionine electrophoresis, and viral plaque assays, respectively. Interferon (IFN) responsiveness was determined using MTS assay after β-IFN pre-treatment. Xenografts were established in athymic nude mice and treated with intratumoral M51R-VSV.
Results
Cell viability after M51R-VSV infection at a multiplicity of infection (MOI) of 10 pfu/mL, 48 hours post-infection) ranged between 0±1 and 59±9% (mean ± standard deviation). Sensitive cell lines supported VSV infection, viral protein synthesis, and viral progeny production. In addition, when pre-treated with β-IFN, sensitive cells became resistant to M51R-VSV, suggesting that IFN-mediated antiviral signaling is defective in these cells. In contrast, resistant melanoma cells do not support VSV infection, viral protein synthesis, or viral replication, indicating that anti-viral defenses remain intact. In a murine xenograft model, intratumoral M51R-VSV treatment decreased tumor growth relative to controls after 26 days in SK-Mel 5 (−21±19% vs. 2100±770%, p<0.0001) and SK-Mel 3 (2000±810% vs 7000±3000%, p=0.008) established tumors.
Conclusions
M51R-VSV is a viable, anti-cancer therapy, but susceptibility varies among melanomas. Future work will exploit specific mechanisms of resistance to expand the therapeutic efficacy of M51R-VSV.
Introduction
Over 68,000 people are diagnosed and almost 9,000 people die from malignant melanoma each year in the United States.1 The current therapeutic options for metastatic melanoma are limited and associated with significant side effects.2–5 These findings suggest that novel approaches to the treatment of metastatic melanoma are warrented.
Vesicular stomatitis virus (VSV) is one of several oncolytic viruses currently being developed as an anticancer therapy. VSV, the prototypical member of the family Rhabdoviridae, is a negative-stranded RNA virus whose genome encodes the nucleocapsid (N), polymerase proteins (L and P), surface glycoprotein (G) and matrix protein (M). Our group has focused on the M51R-VSV virus, which contains a single arginine for methionine amino acid substitution at position 51 in the M protein, as a potential oncolytic virus. The basis for our work lies in the fact that normal cells mount antiviral responses upon infection with mutant M protein VSV strains while cancer cells remain susceptible to mutant M protein VSV infection and cell death due to inherent defects in antiviral signaling. 6–9
In the work presented herein, we analyze the oncolytic effects of recombinant wild-type VSV (rwt-VSV) and M51R-VSV in a panel of malignant melanoma cell lines that demonstrate varying degrees of susceptibility to VSV. Our data not only establish oncolytic M51R-VSV as a viable therapeutic option for malignant melanomas that are susceptible to viral infection, but they also indicate that there is likely considerable variation in the susceptibility to VSV oncolysis among human melanomas. These studies provide a strong framework for future research to determine mechanisms of susceptibility versus resistance and developing methods of predicting response to oncolytic virus treatment.
Materials and Methods
Cells and Viruses
SK-Mel 2, SK-Mel 3, SK-Mel 5, SK-Mel 24, SK-Mel 28, and RPMI 7951 cell lines were obtained from the American Type Culture Collection and were grown in DMEM or McCoy’s 5A medium (both containing 10% FBS, penicillin/streptomycin, and L-glutamine) according to American Type Culture Collection specifications. The recombinant VSV viruses, rwt-VSV and M51R-VSV, were isolated from infectious VSV cDNA clones, and virus stocks were prepared using BHK cells as described previously.10 Cells were grown in monolayers to about 70 to 90% confluence and infected in small volumes at multiplicities of infection (MOIs) as specified in each experiment.
Cell Viability Assays
Melanoma cells were plated in 96-well plates containing 3,000 cells per well. Cells were infected with rwt and M51R viruses at an MOI of 0.1, 1, 5, or 10 plaque-forming units per cell (pfu/cell). At 24 and 48 h post-infection, live cells were measured by the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (CellTiter 96 Aqueous One Solution Cell Proliferation Assay; Promega, Madison, Wisconsin) according to manufacturer’s instructions.
Viral Infectability
The ability of M51R virus to infect melanoma cells was determined by green fluorescent protein (GFP) expression using flow cytometry analysis of infected cells. SK-Mel 3, SK-Mel 5, and SK-Mel 24 cells were plated in 6-well plates and infected with GFP-labeled M51R virus (rGFP-M51R) at MOIs of 0.1, 1, and 10 pfu/mL and incubated for 8h. The cells were then washed and fixed in 2% paraformaldehyde. GFP expression was quantified using a Becton Dickinson FACS Caliber flow cytometer.
Host Cell and Viral Protein Synthesis
SK-Mel 3, SK-Mel 5, and SK-Mel 24 cells were infected with rwt-VSV and M51R-VSV at an MOI of 5 pfu/cell to analyze the effect of host cell protein synthesis to VSV infection and the ability of rwt and M51R viruses to produce viral proteins in melanoma cells. At 4, 8, and 12 h post-infection, cells were methionine starved and then labeled with a 15 min pulse of 35S-methionine (200µCi/mL) in a small volume of methionine-free medium. Cells were washed with PBS and harvested in radioimmunoprecipitation assay (RIPA) buffer. Cell extracts were normalized for protein levels by protein assay (DC Protein Assay Kit; Bio-Rad Laboratories, Hercules, California) and analyzed by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by phosphorescence imaging. Radioactivity of viral N protein bands and background host proteins (two sections from each lane excluding viral protein bands) were quantified with ImageQuant software (Molecular Dynamics, Inc., Sunnyvale, California).
Viral Growth Curves
The ability of rwt-VSV and M51R-VSV to replicate in melanoma cells was quantified by viral plaque assays. SK-Mel 3, SK-Mel 5, and SK-Mel 24 cells were infected with rwt and M51R viruses at MOIs of 0.1 and 10 pfu/cell. One hour post-infection, the media was aspirated, the cells were washed with phosphate buffer solution to remove all free virions, and fresh media was replaced on the cells. At specified times postinfection, a small aliquot of media was collected and stored at −80° C. The viral yield was determined b y plaque assays on BHK cells as described previously.11
Interferon Responsiveness
Melanoma SK-Mel 3, SK-Mel 5 and SK-Mel 24 cells were plated onto 96-well dishes and pretreated with varying amounts (0 to 25,000 IU/ml/100,000 cells) of human β-IFN (PBL InterferonSource, Piscataway, New Jersey) for 8 h. Cells were then inoculated with rwt-VSV and M51R-VSV at an MOI of 5 pfu/cell. After 48 or 72 h, the percentage of live cells was measured by MTS assay according to manufacturer’s instructions. Controls included mock-treated cells infected with virus and β-IFN treated cells not challenged with VSV.
Xenograft Treatment
SK-Mel 3 and SK-Mel 5 cells were checked for animal pathogens (service provided by RADIL, Columbia Missouri) and harvested from semiconfluent cultures. Cells were suspended at 2×106 cells in 0.2mL of their respective culture medium and injected subcutaneously in the right flank of athymic C57BL/6-nu/nu mice. Animals were monitored for tumor development three times a week by visual inspection and palpation of the injection site. Palpable tumors were measured with calipers, and the tumor volume was calculated using the formula: volume = width2×length/2. Once tumors reached a threshold volume of 5mm3, tumor-bearing mice were assigned randomly to receive intratumoral injection of 7×107 pfu of M51R-VSV or intratumoral mock injection with culture medium as negative controls. Tumor volume and animal mass were measured three times a week with calipers as described above. Two mice were selected from each group to be sacrificed on post-treatment day two, and the tumors were harvested for immunohistochemical analysis.
Immunohistochemistry
Harvested tumors were fixed in 10% formalin, embedded in paraffin, and sectioned at 5µm. Sections were stained with hematoxylin and eosin for histologic examination. For immunohistochemical staining, cells were fixed in descending series of ethanol washes, quenched with 0.3% peroxide in PBS, and blocked in 5% goat serum. Serial sections were incubated overnight with antibodies against VSV envelope glycoprotein (rabbit anti-G, Research Diagnostics, Inc., Flanders, New Jersey). Secondary antibody (biotinylated anti-rabbit; Biogenex Supersensitive Kit, San Ramon, California) was incubated on sections at room temperature for 30 min. Primary antibody detection was accomplished using a streptavidin alkaline phosphatase detection kit (Supersensitive Detection Kit; Biogenex, San Ramon, California). Vector Red Substrate Kit #1 for alkaline phosphatase (Vector Laboratories, Inc., Burlingame, California) was used to visualize the antibody-antigen complex. Nuclei were counterstained using Mayer’s hematoxylin. Negative controls consisted of histologic sections processed with the addition of primary antibody, but incubated instead with 1% goat serum or mouse IgG (Reagent Grade, 0.33 mg/mL; Sigma Chemical, Saint Louis, Missouri).
Statistical analysis
A longitudinal, mixed models approach was used to compare experimental groups on mean percent changes in tumor volume over time for the murine xenograft experiments taking into account the repeated measures of each animal. In this model, the mouse was considered a random effect, and time and group were considered as fixed effects. In these models, the time by group interaction was of primary interest, because if this interaction was important, it would suggest that the mean percent changes in tumor volumes in the two groups differed over time. In addition, when the time by group interaction was found to be significant, we examined contrasts comparing groups at different time points to determine at what time point the groups became significantly different from each other. All analyses were performed using SAS version 9.2. Values were considered statistically significant if p-values were <0.05. The values are summarized as X ± S.D.
Results
Sensitivity of malignant melanoma cells to VSV
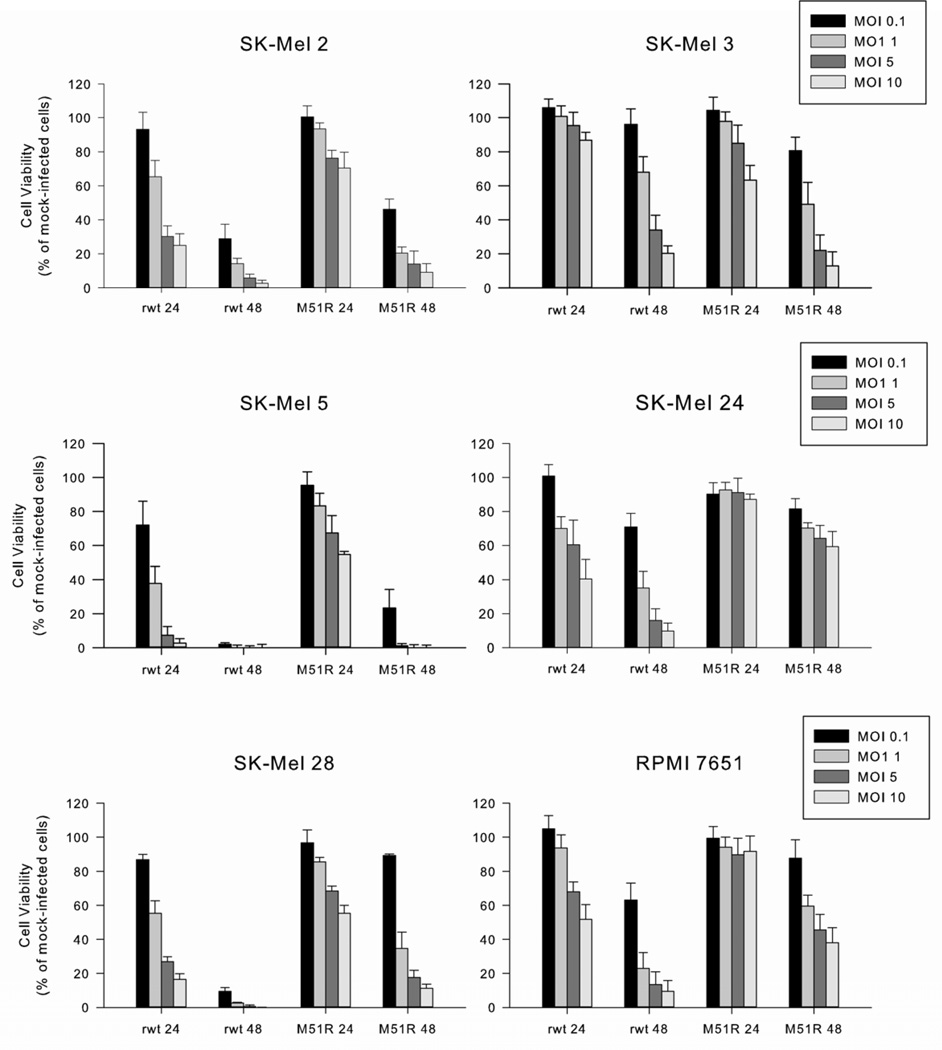
The six melanoma cell lines displayed varying oncolytic effects to VSV (figure 1). SK-Mel 2 and SK-Mel 5 cells were very sensitive to rwt-VSV and M51R-VSV under both multi-cycle and single-cycle infection conditions. SK-Mel 28 cells were sensitive to rwt-VSV; however, these cells were much less susceptible to the oncolytic effects of M51R-VSV 48 hours post-infection under multi-cycle infection conditions (89 ± 8% viability, MOI 0.1 pfu/cell) but were sensitive to single-cycle infections (11 ± 2% viability, MOI 10 pfu/cell). This pattern of relative resistance to multi-cycle infection was also seen in SK-Mel 3 cells; however, these cells were equally resistant to both rwt-VSV and M51R-VSV (96 ± 9% and 81 ± 8% viability respectively, MOI 0.1 pfu/cell) at 48 hours post-infection. This resistance in SK-Mel 3 cells was also overcome by single-cycle infections (20 ± 4% and 13 ± 8% viability respectively, MOI 10 pfu/cell). In contrast, SK-Mel 24 and RPMI 7651 cells remain relatively resistant to the oncolytic effects of M51R-VSV 48 hours after infection even when infected at 10 MOI pfu/cell (59 ± 9% and 38 ± 9%, respectively).
Figure 1.
Malignant melanoma cell viability after rwt-VSV or M51R-VSV infection. Six melanoma cell lines were infected at indicated MOIs. After 24 and 48 h post-infection, live cells were quantified by MTS assay. Data are expressed as a percentage of mock-infected cell viability and represent the mean ± standard deviation of at least three independent experiments.
Taken as a whole, these results suggest that anti-viral defenses vary among melanoma cell lines in response to M51R-VSV infection; this observation suggests that some melanoma lines are very sensitive (SK-Mel 2 and SK-Mel 5), others are relatively resistant (SK-Mel 24 and RPMI 7651) and others are intermediate in their sensitivity (SK-Mel 28 and SK-Mel 3). In general, host antiviral responses played a large role in the relative resistance of cell lines, as shown by cells in which rwt-VSV was more potent than M51R-VSV and by the relative resistance to low versus high MOI infection.
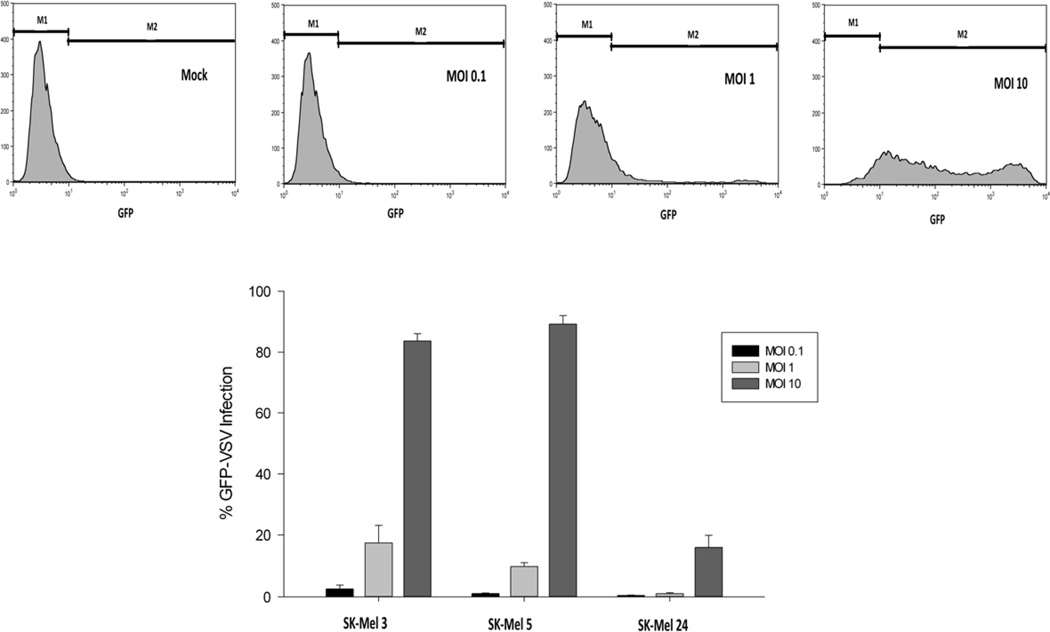
VSV infectability in malignant melanoma cells
The therapeutic effect of oncolytic VSV depends in part on its ability to replicate and spread throughout a tumor. Therefore, we tested the ability of VSV to infect and replicate using the most sensitive cell line (SK-Mel 5), an intermediately sensitive cell line (SK-Mel 3), and the most resistant cell line (SK-Mel 24). The experiments summarized in figure 2 determined the ability of the inoculating virus to establish infection in malignant melanoma cells. SK-Mel 3 and SK-Mel 5 cells were similar in their VSV infectability with less than 3% of cells infected at an MOI of 0.1 pfu/cell and increased to greater than 83% infected at an MOI of 10 pfu/cell. In contrast, SK-Mel 24 cells were much more resistant to VSV infection with 16 ± 4% GFP-positive cells at an MOI of 10 pfu/cell.
Figure 2.
Malignant melanoma cell infectability by GFP-labeled M51R-VSV. Cells were infected with rGFP-M51R virus at indicated MOIs. After 8h, cells were analyzed for GFP expression by flow cytometry. (a) Representative histograms from SK-Mel 3 cells show different proportions of cells expressing low and high levels of GFP at varying MOIs. (b) The percentage of melanoma cells expressing GFP under each condition, expressed as the mean ± standard deviation from three independent experiments.
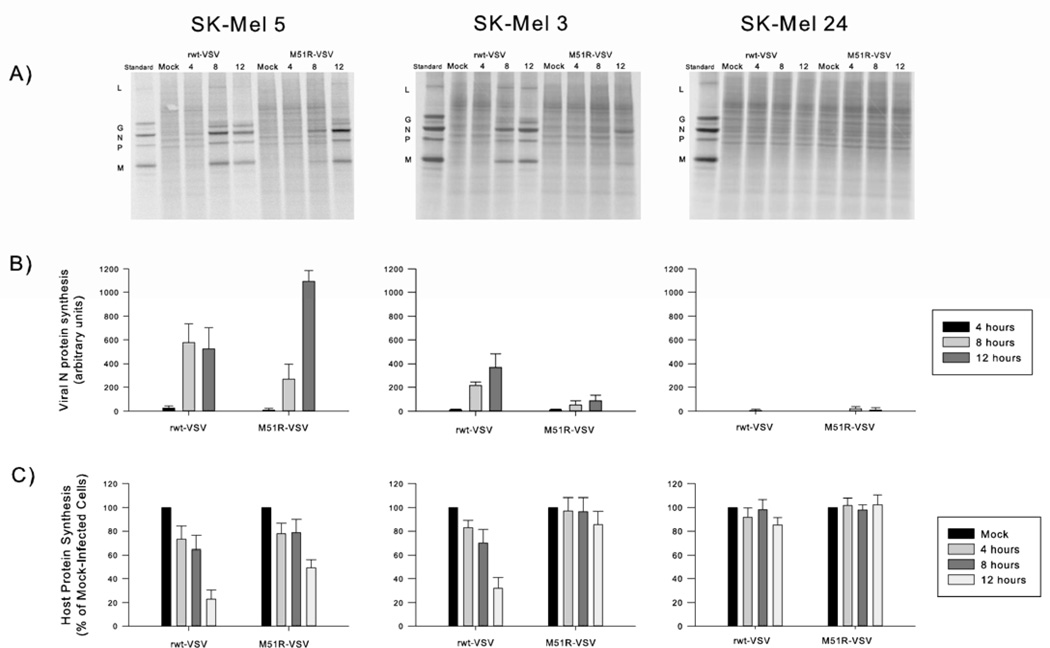
VSV protein synthesis in malignant melanoma cells
The time course of viral protein synthesis was determined in SK-Mel 3, SK-Mel 5, and SK-Mel 24 cells at 4, 8, and 12 hours after infection with rwt-VSV or M51R-VSV using 35S-methionine-based phosphorescence imaging techniques. Representative phosphorescence images from each cell line are shown in figure 3A. Within 8 to 12 hours, SK-Mel 3 and SK-Mel 5 cells supported viral protein synthesis as shown by the intense signals corresponding to viral protein bands greater than the background signal of host proteins. In contrast, there was minimal if any viral protein synthesis in SK-Mel 24 cells. The capacity of viral protein synthesis in each cell line was measured by quantifying the intensity of the viral N protein (the most abundant viral protein) greater than above background and was expressed as the percentage of intensity measured from a comparable region in mock-infected cells (see figure 3B). In sensitive SK-Mel 5 cells, high levels of viral protein synthesis were observed after infection by both viruses. The decrease in overall protein synthesis in the SK-Mel 5 cells at 12 hours after rwt-VSV infection is typical of translation control in VSV-infected cells.12 In comparison, the intermediately sensitive SK-Mel 3 cells supported less viral protein synthesis overall; however, significant levels of viral protein synthesis were observed following rwt-VSV, but only low levels were observed after M51R-VSV infection. There was no detectable viral protein production in SK-Mel 24 cells over time in response to infection by rwt-VSV or M51R-VSV.
Figure 3.
Viral and host cell protein synthesis in response to rwt-VSV and M51R-VSV infections. Cells were infected at a multiplicity of infection of 5 pfu/cell and were labeled with [35S]methionine at the indicated times. Proteins were analyzed by SDS-PAGE and phosphorescence imaging. (A) Representative phosphorescence images. Viral proteins (L, G, N, P, and M) are shown in the first lane in each image as reference. (B) Viral protein synthesis. In order to compare cell types, the radioactivity of the N protein in each lane was quantified and normalized by dividing the intensity of the N protein band by the intensity of a comparable region in mock-infected cells. (C) Host cell protein synthesis. The radioactivity of two sections in each lane between viral protein bands was quantified and is expressed as a percentage of mock-infected cells. Data are expressed as the mean of each experimental result ± standard deviation of three independent experiments.
The corresponding inhibition of host cell protein synthesis as a result of VSV infection was determined from the same phosphorescence images by analyzing the radioactive signal greater than background in two areas in each lane devoid of viral protein bands. The results are expressed as the percentage of host protein synthesis in mock-infected cells (figures 3C). As expected, host cell protein synthesis was inhibited in SK-Mel 5 cells in response to rwt-VSV to a greater extent than M51R-VSV infection (23 ± 7 and 49 ± 7% of mock-infected protein synthesis at 12 hours, respectively). Host protein synthesis was inhibited in SK-Mel 3 cells in response to rwt viral infection (32 ± 9% of mock-infected protein synthesis at 12 hours); however, there was no significant inhibition of host cell protein synthesis in SK-Mel 3 cells 12 hours after M51R-VSV treatment. In contrast to the other cell lines, SK-Mel 24 protein synthesis was not inhibited by either rwt-VSV or M51R-VSV infection.
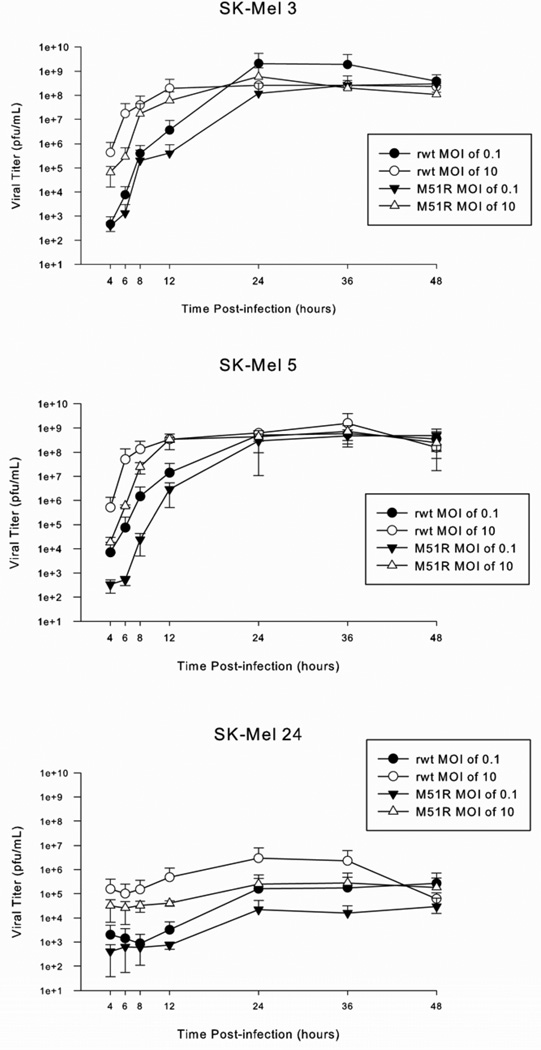
Production of progeny virus by malignant melanoma cells
The production of progeny virus was quantified in malignant melanoma cells after infection with rwt-VSV or M51R-VSV under multi-cycle (MOI 0.1 pfu/cell) and single-cycle (MOI 10 pfu/cell) conditions using viral plaque assays on BHK cells (figure 4). As expected, the sensitive SK-Mel 5 cells yielded high levels of viral progeny that peaked between 1×108 and 1×109 pfu/mL by 12 hours under single-cycle infection conditions and by 24 hours under multi-cycle infection conditions. While rwt-VSV produced slightly more progeny than M51R virus early post-infection, by 12 to 24 hours, the amount of viral progeny produced by both viruses was equivalent. Despite demonstrating intermediate sensitivity to VSV infection, the viral progeny growth curves for SK-Mel 3 cells were essentially the same as the highly sensitive SK-Mel 5 cells.
Figure 4.
Viral progeny production in malignant melanoma cells. Cells were treated with rwt-VSV and M51R-VSV under multiple-cycle (MOI 0.1 pfu/mL) or single-cycle (MOI 10 pfu/mL) infection conditions. At various times post-infection, small aliquots of supernatant were removed and the amount of viral progeny was determined by plaque assay. Data are expressed as the mean ± standard deviation from three independent experiments.
In contrast, SK-Mel 24 cells did not support significant viral replication. At 48 hours post-infection, only between 1×104 and 1×105 pfu/mL progeny were produced, and while single-cycle infection produced slightly greater levels of viral progeny, there was relatively little increase in production over time under all conditions tested. Collectively, these results support earlier studies that show that the M51R M protein mutation has little effect on the viral assembly function of the M protein.7, 13 In addition, they also indicate that the resistance of SK-Mel 24 cells to the oncolytic effects of VSV is related to the inability of these cells to support viral replication.
IFN responsiveness in malignant melanoma cells
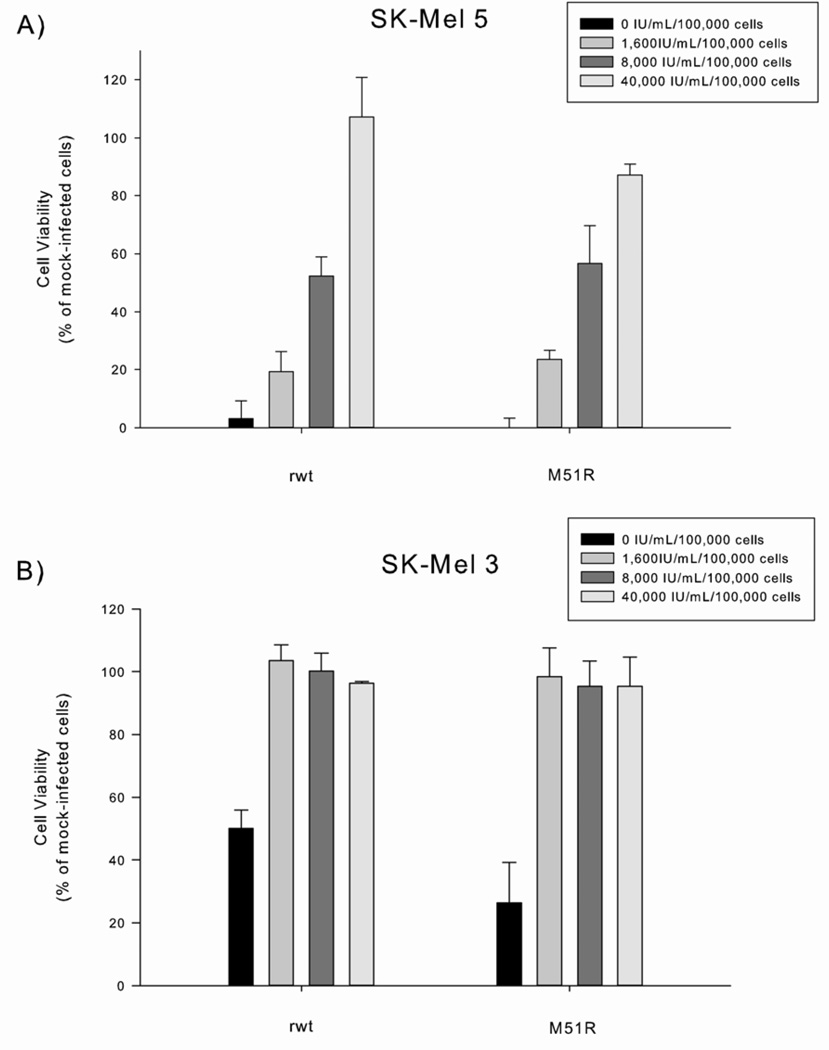
The hypothesis that VSV can target tumor cells selectively over normal tissues is attributed to the observation that tumor cells acquire defects in antiviral mechanisms, such as type I IFN responses, as they lose the ability to regulate normal cell growth. To evaluate whether IFN signaling was defective in VSV-sensitive melanoma cells, we performed interferon responsiveness assays to determine how malignant melanoma cells would respond to extrinsic β-IFN. SK-Mel 3 and SK-Mel 5 were pre-treated with increasing concentrations of β-IFN prior to VSV infection. Both SK-Mel 3 and SK-Mel 5 cells became resistant to the oncolytic effects of VSV after treatment with high dose β-IFN (see figure 5). The ability of high-dose β-IFN to protect these cells from VSV suggests that these cells are not defective completely in IFN-mediated, anti-viral responses. Interestingly, less β-IFN was required to rescue SK-Mel 3 cells, indicating again that the anti-viral defects in SK-Mel 3 cells are less potent.
Figure 5.
The responsiveness of SK-Mel 5 and SK-Mel 3 melanoma cells to β-interferon (β-IFN). Cells were incubated with varying concentrations of β-IFN (0 to 40,000 IU/mL/100,000 cells) for 8 h and then challenged with rwt-VSV or M51R-VSV (MOI of 5 pfu/cell). Cell viability was measured by MTS assay 48 h after VSV infection. Data are expressed as the percentage of β-IFN treated, mock-infected cells and presented as the mean ± standard deviation of three independent experiments.
In vivo oncolytic effects of M51R-VSV in a mouse xenograft model
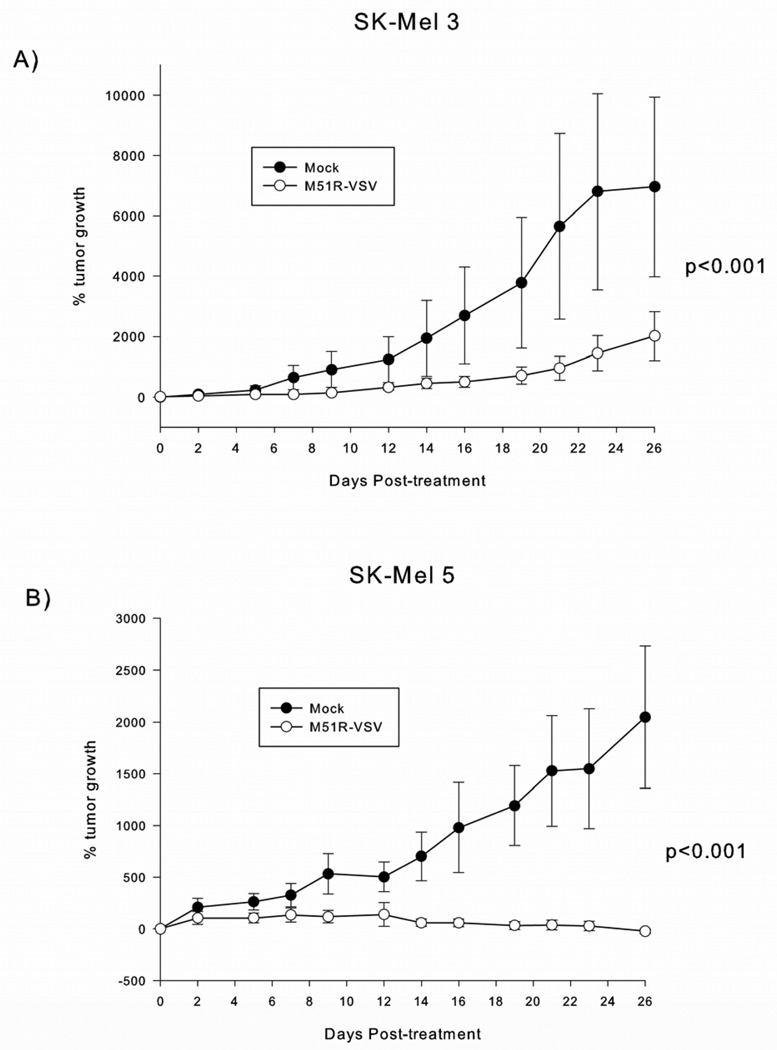
In order to test the in vivo oncolytic effects of M51R-VSV in sensitive malignant melanoma cells, SK-Mel 3 or SK-Mel 5 cells were injected subcutaneously into the flanks of athymic nude mice and treated with mock injections or M51R-VSV. Mock-treated xenografts from both cell lines continued to grow exponentially. SK-Mel 3 xenografts treated with M51R-VSV exhibited slowed but continued tumor growth compared to controls (figure 6A). This rate of growth was different from mock-treated tumors as demonstrated by a time by group interaction (p<0.001). When we examined the separate days within a longitudinal mixed model, we found that the two groups first separated at 21 days (950 ± 400% vs 5700 ± 3100%, p=0.011) and were consistently different thereafter (p=0.0039 and p=0.0082 at 23 and 26 days, respectively). In contrast, xenografts established from sensitive SK-Mel 5 did not grow significantly after M51R-VSV treatment (figure 6B). The difference in tumor growth compared to the mock-infected tumors was highly significant (p<0.001). In addition, we found that the two groups were different by day 16 (57 ± 38% vs 980 ± 440%, p=0.032), which became more pronounced over time (p=0.0073, p=0.0006 and p=0.0005, on days 19, 21 and 23 respectively) and by day 26, the difference of tumor growth between M51R-VSV treated and mock-treated tumors was −21 ± 19% and 2100 ± 770% respectively (p<0.0001). In three of ten mice with SK-Mel 5 tumors, treatment with M51R-VSV resulted in the complete disappearance of tumor and viable tumor cells were not seen on histologic examination (images not shown).
Figure 6.
Intratumoral M51R-VSV treatment of melanoma xenografts derived from SK-Mel 3 and SK-Mel 5 cells. Subcutaneous xenografts were established in the right flank of athymic nude mice. Once tumors formed, the mice were assigned randomly to a single intratumoral injection of M51R-VSV (7×107 pfu) or culture medium as negative controls. Tumor volume was measured with calipers. SK-Mel 3 (n=8 per group) and SK-Mel 5 (n=10 per group) tumor growth is presented as the percentage of tumor size on day 0 post-infection and is expressed as the mean percent tumor growth ± standard deviation. P-values shown represent the difference in tumor growth rate between M51R-VSV treated and mock-treated xenografts calculated using the time by group interaction in a longitudinal mixed model.
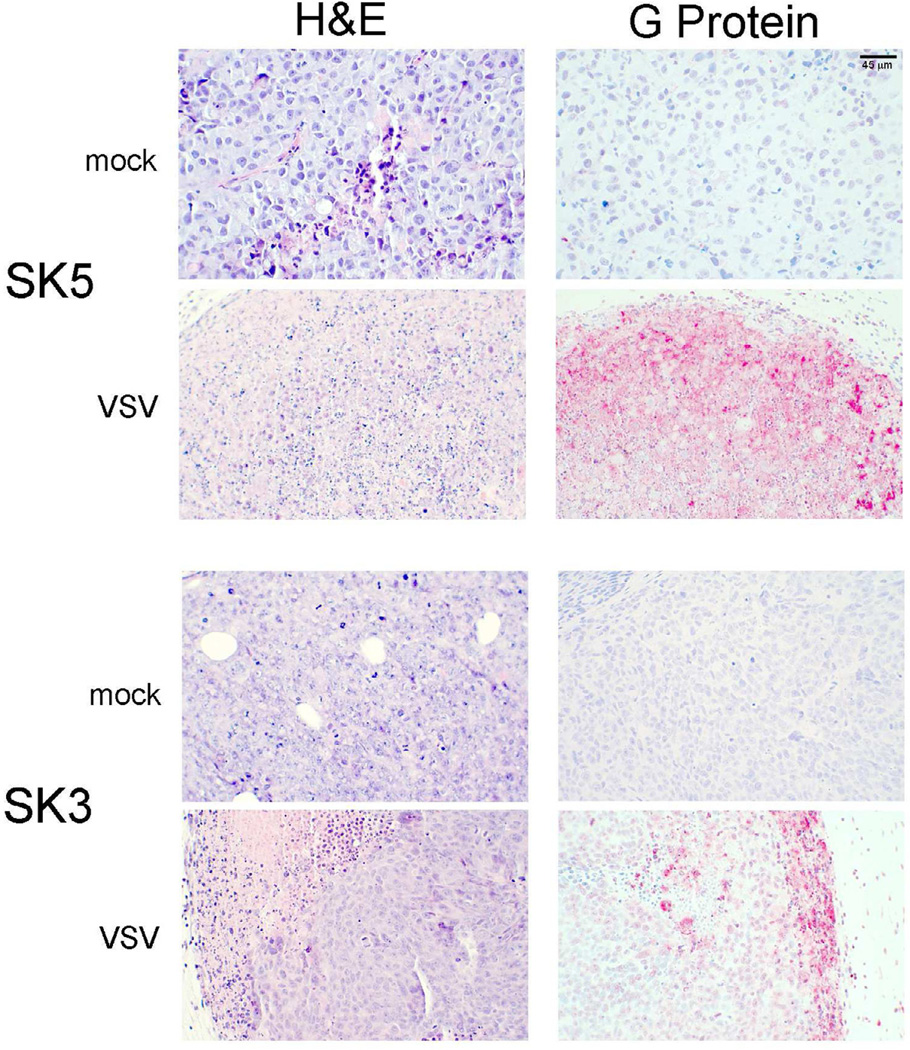
Histologic and immunohistochemical examination of tumors is shown in figure 7. Mock-treated SK-Mel 3 and SK-Mel 5 tumors showed uniform cells with well-defined borders and nuclei. Minimal necrosis was seen in the mock-treated tumors. In contrast, SK-Mel 5 tumors treated with M51R-VSV showed necrosis throughout the tumor, characterized by loss of nuclear staining, increased cytoplasmic eosinophilia, and loss of cellular detail and borders. To a lesser degree, M51R-VSV treated SK-Mel 3 tumors also showed areas of necrosis interspersed between viable tumor, but these treated tumors displayed more necrosis than mock treated tumors.
Figure 7.
Histological and immunohistochemical analysis of melanoma xenografts treated with M51R-VSV. Mock-infected tumors with injected with culture medium. M51R-VSV treated tumors received a single intratumoral injection (7×107 pfu). Tumors were harvested at post-infection day two. Representative sections stained with hematoxylin and eosin (H&E) and immunohistochemical staining for VSV surface glycoprotein (G-protein) are shown from mock-infected and M51R-VSV infected xenografts from each cell line as indicated.
These tumors were also stained with antibodies against VSV surface glycoprotein (G-protein). As expected, viral G-protein was observed throughout SK-Mel 5 tumors treated with M51R-VSV. SK-Mel 3 tumors, in contrast, showed less G-protein staining concentrated at the periphery of the tumor. The areas of G-protein staining in SK-Mel 3 tumors correlate to areas of necrosis seen on H&E staining. Taken together, these data indicate that the in vivo sensitivity of established melanoma xenografts to local M51R-VSV treatment correlate to the sensitivity of malignant melanoma cells to M51R-VSV in vitro. Sensitive SK-Mel 5 tumors promote viral replication and spread, become necrotic, and decrease in size or resolve completely after M51R-VSV treatment. M51R-VSV is also able to replication and spread in SK-Mel 3 tumors but to a much lesser extent consistent with their in vitro intermediate sensitivity to M51R-VSV.
Discussion
Our results reveal varying degrees of sensitivity to M51R-VSV in malignant melanoma cells. By demonstrating the efficacy of VSV in several melanoma cell lines, future translational and clinical studies can be designed specifically for malignant melanoma. Before VSV can be used in clinical trials however, several important issues must be established. First, one of the greatest challenges with oncolytic viral therapy is the ability of the virus to target cancer cells while sparing normal tissues. Prior VSV studies using malignant melanoma cells have used rwt-VSV as a backbone 14, 15 which inhibits global host protein production, thereby decreasing the ability of normal cells to mount anti-viral responses. In contrast, mutant M protein variants of VSV such as M51R-VSV are more selective for cancer cells by allowing normal cells to mount anti-viral responses, while tumor cells with defects in these responses remain susceptible to viral infection and killing. Previous studies by our group and others have shown that normal fibroblasts and hepatocytes are resistant to M51R-VSV,9 while other studies have demonstrated significant oncolysis in normal prostate epithelial cells13 and benign mammary cells16 after M51R-VSV infection. Nevertheless, multiple studies including the present study have shown effective oncolysis after M51R-VSV treatment in in vivo models without apparent adverse effects to surrounding normal tissue. 9, 11, 16 Further research will better establish the selectively of M51R-VSV to cancer cells in vivo using syngeneic models and use viral vectors and/or concomitant therapies to enhance VSV selectivity for cancer cells.
A second issue that must be addressed prior to clinical trials with VSV is to understand the mechanisms that produce the variation in susceptibility seen among tumor subtypes. A basic tenant of oncolytic viral therapy describes the development of defects in anti-viral responses during tumorogenesis that make tumor cells relatively susceptible to viral infection while normal cells retain the ability to prevent viral infection and killing. Our results suggest that not all melanoma cells possess these defects. Specifically, SK-Mel 24 cells were resistant to VSV infection and did not promote VSV replication or viral protein production, indicating that anti-viral defenses remain intact in these tumor cells.
Based on these observations, we can expect that a proportion of melanomas in clinical practice will be innately resistant to VSV as well. In future studies, we will evaluate the mechanism of resistance in SK-Mel 24 cells, specifically the integrity of IFN pathways, and specific components will be exploited in an effort to expand the efficacy of VSV. Several proteins in the IFN pathway are potential candidates for this strategy. STAT1, STAT2, and STAT3 proteins have been shown to modulate early IFN responses.17–20 Viral vectors engineered into M51R-VSV that impede IFN signaling could potentially overcome the VSV-resistance seen in some cells. Other strategies to enhance the oncolytic effects of VSV by introducing suicide genes into the VSV genome have also been described. 15, 21
A final issue that must be addressed before VSV clinical trials is patient safety. While wild-type VSV is thought to cause a non-specific, flu-like illness in humans, it causes encephalitis in mice, resulting in hind limb paralysis and eventually death.22 Our group has developed VSV variants such as M51R-VSV that are less pathogenic than their wild-type counterparts but retain their oncolytic capabilities. In multiple studies, M51R-VSV has been administered, both locally and systemically, at high doses without associated morbidity or mortality in both immunodeficient (nu/nu) and immunocompetent (BALB/c) mice.9, 16 Similarly, none of the mice in our study showed evidence of neurologic sequelae or other derangements after M51R-VSV treatment, yet the oncolytic effects paralleled those seen in vitro. These studies establish the safety of M51R-VSV in pre-clinical trials and promote its consideration in future human testing.
Acknowledgements
This work was supported by Grant no. K08-CA131482 from the National Cancer Institute (JS), 63527 from the Robert Wood Johnson Foundation Harold Amos Faculty Development Award (JS), R01-AI32983 from the National Institute of Allergy and Infectious Diseases (DL), and the Bradshaw Surgical Resident Research Endowment (AB).
We also wish to acknowledge the philanthropic efforts of the Wake Forest Field Hockey Team’s Maria Whitehead Melanoma Research Fund and Jennie’s Walk of Hope in support of this work. We thank Shelby Puckett and Margie McKenzie (Wake Forest School of Medicine, Department of Biochemistry) for their assistance with viral plaque assays and 35S-labeling experiments, Greg Russell (Wake Forest School of Medicine, Division of Public Health Services) for statistical analysis and Hermina Borgerink (Wake Forest School of Medicine, Department of Comparative Medicine) for staining of tissue sections.
Abbreviations
- VSV
Vesicular stomatitis virus
- rwt-VSV
recombinant wild-type vesicular stomatitis virus
- M51R-VSV
M protein mutant vesicular stomatitis virus
- IFN
Interferon
- MOI
Multiplicity of infection
- GFP
green fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no conflicts of interests relating to this manuscript.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Thomas L, Bondarenko I, O'Day S, M DJ, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 4.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 6.Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed M, McKenzie MO, Puckett S, Hojnacki M, Poliquin L, Lyles DS. Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. JVirol. 2003;77:4646–4657. doi: 10.1128/JVI.77.8.4646-4657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebert O, Shinozaki K, Huang TG, Savontaus MJ, Garcia-Sastre A, Woo SL. Oncolytic vesicular stomatitis virus for treatment of orthotopic hepatocellular carcinoma in immune-competent rats. Cancer Res. 2003;63:3605–3611. [PubMed] [Google Scholar]
- 9.Stewart JH, 4th, Ahmed M, Northrup SA, Willingham M, Lyles DS. Vesicular stomatitis virus as a treatment for colorectal cancer. Cancer Gene Ther. 2011;18:837–849. doi: 10.1038/cgt.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopecky SA, Willingham MC, Lyles DS. Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. JVirol. 2001;75:12169–12181. doi: 10.1128/JVI.75.24.12169-12181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed M, Cramer SD, Lyles DS. Sensitivity of prostate tumors to wild type and M protein mutant vesicular stomatitis viruses. Virology. 2004;330:34–49. doi: 10.1016/j.virol.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 12.Connor JH, Lyles DS. Inhibition of host and viral translation during vesicular stomatitis virus infection. eIF2 is responsible for the inhibition of viral but not host translation. J Biol Chem. 2005;280:13512–13519. doi: 10.1074/jbc.M501156200. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed M, Lyles DS. Identification of a consensus mutation in M protein of vesicular stomatitis virus from persistently infected cells that affects inhibition of host-directed gene expression. Virology. 1997;237:378–388. doi: 10.1006/viro.1997.8808. [DOI] [PubMed] [Google Scholar]
- 14.Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez M, Porosnicu M, Markovic D, Barber GN. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J Virol. 2002;76:895–904. doi: 10.1128/JVI.76.2.895-904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed M, Puckett S, Lyles DS. Susceptibility of breast cancer cells to an oncolytic matrix (M) protein mutant of vesicular stomatitis virus. Cancer Gene Ther. 2010;17:883–892. doi: 10.1038/cgt.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong LH, Sim H, Chatterjee-Kishore M, Hatzinisiriou I, Devenish RJ, Stark G, et al. Isolation and characterization of a human STAT1 gene regulatory element. Inducibility by interferon (IFN) types I and II and role of IFN regulatory factor-1. J Biol Chem. 2002;277:19408–19417. doi: 10.1074/jbc.M111302200. [DOI] [PubMed] [Google Scholar]
- 18.Steen HC, Gamero AM. The role of signal transducer and activator of transcription-2 in the interferon response. J Interferon Cytokine Res. 2012;32:103–110. doi: 10.1089/jir.2011.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong LY, Gelbard A, Wei J, Reina-Ortiz C, Wang Y, Yang EC, et al. Inhibition of p-STAT3 enhances IFN-alpha efficacy against metastatic melanoma in a murine model. Clin Cancer Res. 2010;16:2550–2561. doi: 10.1158/1078-0432.CCR-10-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero-Weaver AL, Wang HW, Steen HC, Scarzello AJ, Hall VL, Sheikh F, et al. Resistance to IFN-alpha-induced apoptosis is linked to a loss of STAT2. Mol Cancer Res. 2010;8:80–92. doi: 10.1158/1541-7786.MCR-08-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porosnicu M, Mian A, Barber GN. The oncolytic effect of recombinant vesicular stomatitis virus is enhanced by expression of the fusion cytosine deaminase/uracil phosphoribosyltransferase suicide gene. Cancer Res. 2003;63:8366–8376. [PubMed] [Google Scholar]
- 22.Huneycutt BS, Bi Z, Aoki CJ, Reiss CS. Central neuropathogenesis of vesicular stomatitis virus infection of immunodeficient mice. J Virol. 1993;67:6698–6706. doi: 10.1128/jvi.67.11.6698-6706.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]