Background: ΔNp63 exhibits an oncogenic potential and is often overexpressed in squamous cell carcinomas.
Results: Arsenic degrades ΔNp63 protein at least in part via Pirh2-mediated proteolysis, and inhibition of ΔNp63 expression facilitates tumor cells to arsenic-induced death.
Conclusion: Arsenic trioxide induces Pirh2 to target ΔNp63 for degradation.
Significance: Targeting ΔNp63 may be explored to manage tumors overexpressing ΔNp63.
Keywords: Cell Death, E3 Ubiquitin Ligase, p63, Protein Degradation, Protein Stability, ΔNp63, Pirh2, Arsenic, Cell Survival, p53 Family
Abstract
Transcription factor p63, a member of the p53 family, shares a high degree of sequence similarity with p53. Because of transcription from two distinct promoters, the p63 gene encodes two isoforms, TAp63 and ΔNp63. Although TAp63 acts as a tumor suppressor, ΔNp63 functions as an oncogene and is often overexpressed in squamous cell carcinomas. Thus, therapeutic agents targeting ΔNp63 might be used to manage tumors that overexpress ΔNp63. Here we found that arsenic trioxide, a frontline agent for acute promyelocytic leukemia, inhibits ΔNp63 but not TAp63 expression in time- and dose-dependent manners. In addition, we found that arsenic trioxide decreases the stability of ΔNp63 protein via a proteasome-dependent pathway but has little effect on the level of ΔNp63 transcript. Furthermore, we found that arsenic trioxide activates the Pirh2 promoter and consequently induces Pirh2 expression. Consistent with this, we found that knockdown of Pirh2 inhibits, whereas ectopic expression of Pirh2 enhances, arsenic-induced degradation of ΔNp63 protein. Importantly, we found that knockdown of ΔNp63 sensitizes, whereas ectopic expression of ΔNp63 inhibits, growth suppression induced by arsenic. Together, these data suggest that arsenic degrades ΔNp63 protein at least in part via Pirh2-dependent proteolysis and that inhibition of ΔNp63 expression facilitates tumor cells to arsenic-induced death.
Introduction
The transcription factor p63, a member of the p53 family, shares a high degree of sequence similarity with p53, particularly in the central DNA-binding domain (1). Because of transcription from two distinct promoters, the p63 gene yields two types of transcripts, TAp63 and ΔNp63 (2, 3). Both TAp63 and ΔNp63 transcripts consist of at least five variants because of alternative splicing at the 3′ terminus (4). TAp63 contains an activation domain similar to the first activation domain in p53 and, thus, has a strong transcriptional activity. Like p53, overexpression of TAp63 is capable of inducing cell cycle arrest and apoptosis (5, 6). In contrast, ΔNp63 loses such an activation domain but gains 14 unique residues at the N terminus. These 14 residues, together with the adjacent proline-rich region, constitute an activation domain for ΔNp63 (7, 8). Thus, ΔNp63 also possesses a transcriptional activity.
Although p53 functions as a classical tumor suppressor, the role for p63 in tumorigenesis is still uncertain. A study showed that p63+/− mice are predisposed to develop spontaneous tumors (9), suggesting that the p63 gene acts as a tumor suppressor. Consistently, TAp63 is found to induce senescence and suppress tumorigenesis in TAp63 conditional knockout mice (10). On the other hand, many studies have highlighted the oncogenic properties of ΔNp63. ΔNp63 is frequently found to be amplified and overexpressed in squamous cell carcinomas (11, 12). ΔNp63α overexpression promotes cell proliferation in vitro and tumor growth in vivo (13, 14). In addition, ΔNp63α represses apoptosis-related genes and, thereby, contributes to chemoresistance of hepatocellular carcinoma (15). In line with this, knockdown of ΔNp63α induces apoptosis and sensitizes cells to DNA damage (16). Clinically, high levels of ΔNp63 expression in tumors are associated with an aggressive phenotype and chemoresistance (17, 18).
The role of ΔNp63 in tumorigenesis might be partially due to its transcriptional activity. We found previously that GPX2 and BMP7, two direct targets of ΔNp63, inhibit oxidative stress-induced apoptosis in a p53-dependent manner and are required for survival of tumor cells (19, 20). Other studies also found that ΔNp63 regulates genes involved in cell cycle progression and cell survival (2, 21). Interestingly, ΔNp63 was found to regulate the splicing pattern of CD44, which may affect the adhesion and metastasis of cancer cells (14). The oncogenic potential of ΔNp63 might be also due to its dominant-negative activities to suppress p53- or TAp63-mediated transactivation (2, 7, 15, 23). In addition, ΔNp63α is found to exhibit a survival function in squamous epithelial malignancy by repressing TAp73-dependent pro-apoptotic activity (24). However, the unique transcriptional and dominant-negative abilities in ΔNp63 may be explored for a new therapeutic approach modulating ΔNp63 expression to manage tumors that overexpress ΔNp63 but harbor TAp63, TAp73, and/or wild-type p53.
Arsenic is a metalloid with a substantial efficacy in treating patients with acute promyelocytic leukemia, myeloma, and myelodysplastic syndromes (25). Evidences showed that arsenic functions as an anticancer agent at least in part via targeting proteins for degradation (26–31). Recently, we found that arsenic targets mutant p53 for degradation, which mediates arsenic-induced growth suppression in solid tumor cells (32). The structural and functional similarity between ΔNp63 and mutant p53 prompts us to examine whether arsenic has an effect on ΔNp63 expression. Indeed, we found that arsenic induces ΔNp63 degradation via the proteasome-dependent pathway. Our finding suggests that targeting ΔNp63 with arsenic may be explored further to manage tumors that are carrying a high level of ΔNp63.
EXPERIMENTAL PROCEDURES
Cell Culture
Human keratinocyte cell line HaCaT, human cervical carcinoma cell line ME-180, and human pancreatic cancer cell line MIA PaCa-2 were cultured in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (Hyclone). The human mammary epithelial cell line MCF10A was cultured in DMEM/F12 supplemented with 5% donor horse serum, 20 ng/ml of EGF, 10 μg/ml of insulin, 0.5 μg/ml of hydrocortisone, and 100 ng/ml of cholera toxin.
Antibodies
Mouse anti-p63(4A4) was purchased from Santa Cruz Biotechnology, Inc. Rabbit anti-Pirh2 was purchased from Bethyl Laboratories, Inc. Rabbit anti-actin and mouse anti-FLAG were purchased from Sigma.
Plasmids
Myc-tagged ΔNp63α and 2 × FLAG-tagged Pirh2 cDNAs in pcDNA3 expression vector were described previously (8, 33). To generate the luciferase reporter under the control of the Pirh2 promoter, a 2043-bp DNA fragment containing the Pirh2 promoter (from nucleotides −2000 to +43) was amplified using human genomic DNA with forward primer 5′-CTCGAGCCTATCTGAAATGATATCCAGA-3′ and reverse primer 5′-AAGCTTCCACTAGCGACAATATGGCT-3′. The PCR product, Pirh2–2000, was cloned into the pGEM-T-Easy vector and confirmed by DNA sequencing. After digesting with XhoI and HindIII, Pirh2–2000 was cloned into the pGL2-Basic vector, and the resulting luciferase reporter was designated pGL2-Pirh2–2000. Using pGL2-Pirh2–2000 as a template, several deletion constructs were generated by PCR using the above reverse primer and one of the following forward primers: Pirh2-1000, 5′-CTCGAGAAAGAAAATTAGAATGTTAAGAG-3′); Pirh2-500, (5′-CTCGAGGTCATGGTGG AGTGTG-3′); or Pirh2-250, (5′-CTCGAGGGAGGACCCGTCACAG-3′).
siRNA
A siRNA against Pirh2 (5′-CAU GCC CAA CAG ACU UGU G dTdT-3′), a siRNA against p63 (5′-CGA CAG UCU UGU ACA AUU U dTdT-3′), and a scrambled siRNA (5′-GCA GUG UCU CCA CGU ACU A dTdT-3′), were purchased from Dharmacon RNA Technologies (Chicago, IL).
Reverse Transcription PCR Assay
Total RNA was isolated from cells using TRIzol reagent (Invitrogen). cDNA was synthesized using an IscriptTM cDNA synthesis kit (Bio-Rad). To measure ΔNp63 mRNA, RT-PCR was done with forward primer 5′-TGGCAAAATCCTGGAGCCAG-3′and reverse primer 5′-GTCTGTGTTATAGGGACTGG-3′. To measure Pirh2 mRNA, RT-PCR was done with forward primer 5′-CTGCGAGCACTATGACAGAG-3′ and reverse primer 5′-TTCATAGCTAGGCATAAGTTAC-3′. Actin was amplified with forward primer 5′-TCCATCATGAAGTGTGACGT-3′ and reverse primer 5′-TGATCCACATCTGCTGGAAG-3′.
Protein Half-life Assay
ME-180 cells were left untreated or pretreated with 10 μm arsenic trioxide for 1 h and then incubated with 50 μg/ml of cycloheximide to inhibit de novo protein synthesis for various times. The relative level of ΔNp63α protein was quantified by densitometry and normalized by the level of actin protein, which was then plotted versus time (h) to calculate the half-life of ΔNp63α.
Proteasome Inhibition Assay
Cells were seeded for 24 h, left untreated or pretreated with proteasome inhibitor MG132 (5 μm) for 4 h, and then treated with arsenic trioxide for various times.
Luciferase Assay
The dual luciferase assay was done in triplicate according to the instructions of the manufacturer (Promega). Briefly, 0.5 μg of a luciferase reporter and 3 ng of pRL-SV40-Renilla luciferase reporter (Promega) were cotransfected into HaCaT cells by using the Expressfect transfection reagent (Denville). The fold increase in relative luciferase activity is a product of the luciferase activity induced by arsenic treatment divided by that induced by mock treatment.
Cell Survival Assay
To determine whether ectopically expressed ΔNp63α makes tumor cells resistant to arsenic trioxide treatment, ME-180 cells were transiently transfected with pcDNA3 or pcDNA3-myc-ΔNp63α for 1 day and then left untreated or treated with 7.5 μm arsenic trioxide for 2 days. The survival cells were collected and counted.
To determine whether knockdown of ΔNp63 sensitizes tumor cells to arsenic trioxide treatment, ME-180 cells were transfected with scrambled siRNA or siRNA against p63 for 1 day and then left untreated or treated with 7.5 μm arsenic trioxide for 2 days. The survival cells were collected and counted.
Statistics
All experiments were performed in triplicates. Two-group comparisons were analyzed by two-sided Student's t test. p values were calculated, and p < 0.05 was considered significant.
RESULTS
Arsenic Trioxide Inhibits ΔNp63 Expression in Time- and Dose-dependent Manners
Arsenic trioxide is a therapeutic agent for acute promyelocytic leukemia, which potentially targets proteins for degradation (26–31). We found previously that arsenic-induced degradation of mutant p53 leads to growth suppression (32). ΔNp63, a member of p53 family, shares the high degree of sequence similarity to mutant p53 (1) and confers a proliferative and chemoresistant advantage to tumor cells (16, 18). Thus, we rationalized that targeting ΔNp63 with arsenic may be explored as a therapeutic strategy to tumors that carry a high level of ΔNp63.
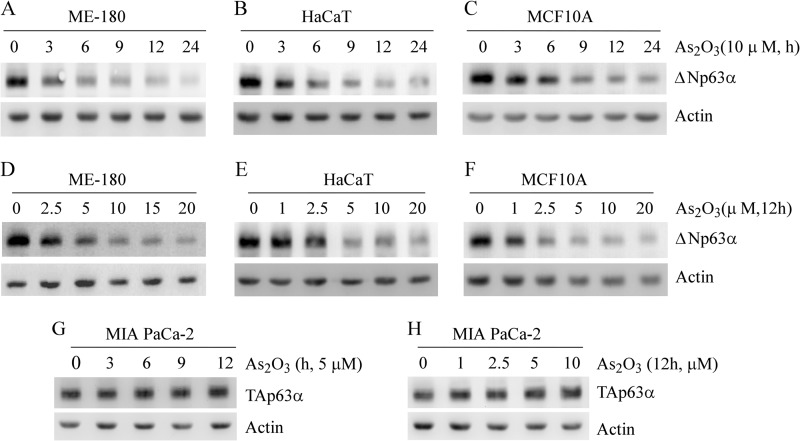
Studies showed that although TAp63 is expressed at a very low level, ΔNp63, especially ΔNp63α, is highly expressed in MCF10A mammary epithelial cells, HaCaT keratinocyte cells, and ME-180 cervical carcinoma cells (34–36). Thus, the effect of arsenic on ΔNp63 expression was examined in these cells. We found that upon treatment with 10 μm arsenic trioxide, the level of ΔNp63α protein was promptly decreased in ME-180 cells, reaching maximum reduction within 12–24 h (Fig. 1A, top panel). The level of actin protein was used as a loading control (Fig. 1A, bottom panel). Similar results were found in HaCaT cells (Fig. 1B) and MCF10A cells (C).
FIGURE 1.
Arsenic trioxide inhibits ΔNp63 but not TAp63 expression in time- and dose-dependent manners. A–C, Western blot analyses were prepared with extracts from ME-180 (A), HaCaT (B), and MCF10A (C) cells left untreated or treated with 10 μm arsenic trioxide for 3–24 h and then probed with antibodies against p63 and actin, respectively. D–F, Western blot analyses were prepared with extracts from ME-180 (D), HaCaT (E), and MCF10A (F) cells left untreated or treated with 1.0–20 μm arsenic trioxide for 12 h and then probed with antibodies against p63 and actin, respectively. G, Western blot analyses were prepared with extracts from MIA PaCa-2 cells, which were left untreated or treated with 5 μm arsenic trioxide for 3 to 12 h and then probed with antibodies against p63 and actin, respectively. H, the experiments were performed as in G except that MIA PaCa-2 cells were left untreated or treated with 1–10 μm arsenic trioxide for 12 h.
To determine the dose-response relationship between arsenic and ΔNp63α expression, ME-180 cells were treated with various doses of arsenic trioxide for 12 h. We found that the level of ΔNp63α protein in ME-180 cells was decreased markedly by arsenic trioxide at a concentration of as low as 2.5 μm (Fig. 1D). This concentration is close to that for degradation of mutant p53 in cultured cells (32) and the plasma peak values of 1.5–3.4 μm in acute promyelocytic leukemia patients treated with arsenic trioxide (37). As arsenic concentration was increased to 5 μm, the level of ΔNp63α was decreased further in ME-180 cells (Fig. 1D). However, the arsenic-induced decrease of ΔNp63α was not further enhanced at concentrations of more than 5 μm (Fig. 1D). A similar result was seen in HaCaT cells (Fig. 1E) and MCF10A cells (F).
Because TAp63 isoforms function as tumor suppressors by inducing senescence (10) and preventing invasiveness and metastasis (38), it is vitally important to determine how arsenic alters TAp63 expression in tumor cells. For this purpose, MIA PaCa-2 cells, which carry a high level of TAp63α, were left untreated or treated with arsenic trioxide as indicated (Fig. 1, G and H). Interestingly, we found that arsenic trioxide had little, if any, effect on the level of TAp63α protein in MIA PaCa-2 cells (Fig. 1, G and H).
Arsenic Trioxide Decreases the Stability of ΔNp63 Protein but Has Little Effect on the Level of ΔNp63 Transcript
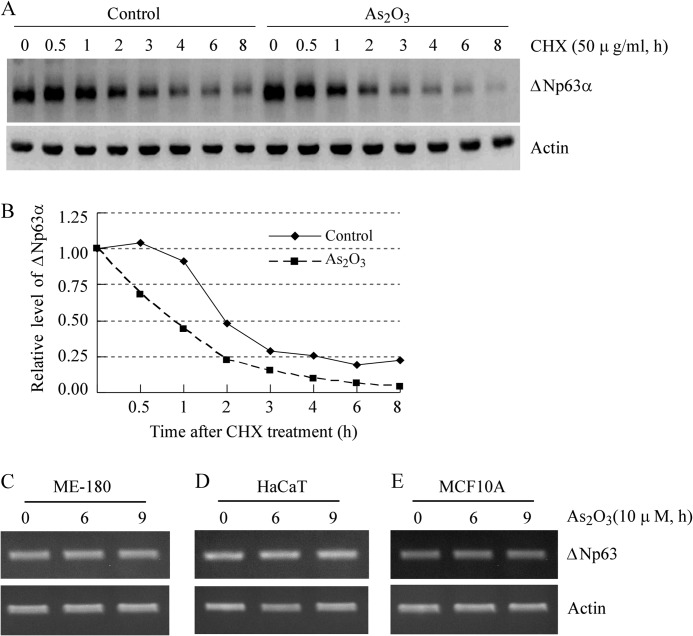
To examine whether arsenic-induced reduction of ΔNp63 is through a posttranslational mechanism, ME-180 cells were treated with 50 μg/ml of cycloheximide for various times in the absence or presence of arsenic trioxide. The relative level of ΔNp63α protein was quantified by densitometry and normalized by level of actin protein, which was then plotted versus time (h) to calculate the half-life of ΔNp63α. We found that the half-life for ΔNp63α protein was decreased from about 2 h in the control cells to about 1 h in cells treated with arsenic trioxide (Fig. 2, A and B).
FIGURE 2.
Arsenic trioxide decreases the stability of ΔNp63α protein but has little effect on the level of ΔNp63 transcript. A, the half-life of ΔNp63α protein was shortened by arsenic trioxide in ME-180 cells. Western blot analyses were prepared with extracts from ME-180 cells that were treated with cycloheximide (50 μg/ml) (CHX) in the absence or presence of 10 μm arsenic trioxide for 0–8 h and then probed with antibodies against ΔNp63 and actin, respectively. B, the relative levels of ΔNp63 protein measured in A were normalized by levels of actin protein and then plotted versus time. C–E, the level of ΔNp63 transcript is not obviously altered by arsenic trioxide. RT-PCR analysis was performed with total RNAs isolated from ME-180 (C), HaCaT (D), and MCF10A (E) cells untreated or treated with 10 μm arsenic trioxide for 6 or 9 h. Actin mRNA was amplified as a loading control.
Next, to test whether ΔNp63 transcription is suppressed by arsenic trioxide, RT-PCR analysis was performed to measure the level of ΔNp63 transcript in ME-180, HaCaT, and MCF10A cells, which were mock-treated or treated with 10 μm arsenic trioxide as indicated. The actin transcript was measured as a control. We found that the level of ΔNp63 mRNA was not obviously altered by arsenic trioxide in ME-180 (Fig. 2C), HaCaT (D), and MCF10A (E) cells. These results suggest that arsenic trioxide does not inhibit ΔNp63 transcription but rather decreases the stability of ΔNp63 protein.
Arsenic Trioxide Degrades ΔNp63 Protein via the Proteasome-dependent Pathway
Because the stability of ΔNp63 protein was decreased by arsenic, we further explored whether ΔNp63 is degraded via the proteasome-dependent pathway. To test this, ME-180 cells were left untreated or treated with 4 μm MG132, an inhibitor of 26 S proteasome, in the absence or presence of arsenic trioxide. We found that arsenic-induced ΔNp63 degradation was almost abolished by MG132 (Fig. 3A). Similarly, we found that arsenic-induced degradation of ΔNp63 protein was inhibited by MG132 in HaCaT cells (Fig. 3B). Taken together, these results suggest that arsenic-induced ΔNp63 degradation is at least in part via the proteasome-dependent pathway.
FIGURE 3.
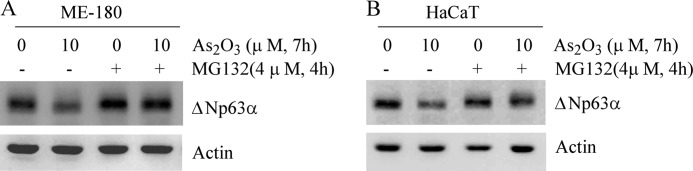
Arsenic-induced degradation of ΔNp63 protein is inhibited by MG132, an inhibitor of 26 S proteasome. A and B, Western blot analyses were prepared with extracts from ME-180 (A) and HaCaT (B) cells that were left untreated or pretreated with 4 μm MG132 for 4 h and then left untreated or treated with arsenic trioxide for 7 h.
Arsenic Trioxide Induces Expression of Pirh2 E3 Ligase
Next, we sought to identify the proteins that may mediate arsenic-induced ΔNp63 degradation. Multiple E3 ligases were reported to degrade p63 protein. For example, MDM2 and Fbw7 cooperate to induce p63 protein degradation following DNA damage and cell differentiation (39). WW domain-containing E3 ubiquitin protein ligase 1 targets p63 protein for ubiquitin-mediated proteasomal degradation (40). Hect-containing Nedd4-like ubiquitin protein ligase Itch promotes the degradation of p63 via ubiquitylation (41, 42). We also found recently that Pirh2 (p53-induced RING-H2) E3 ubiquitin ligase physically interacts with p63 and targets p63 for proteasome-dependent degradation (43).
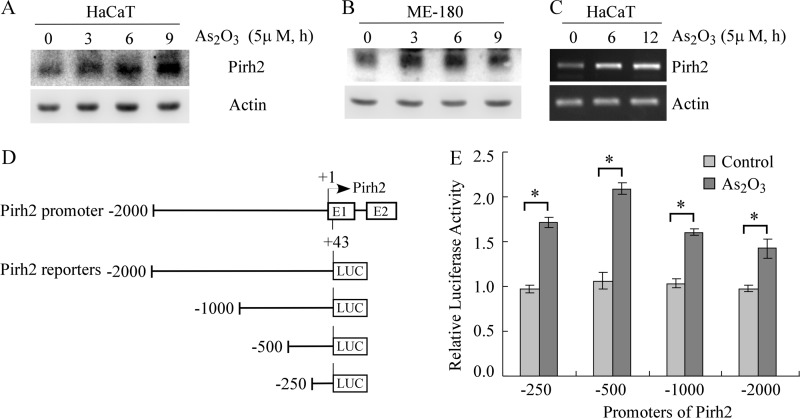
Here, multiple E3 ligases, known to degrade p63 or other p53 family members, were screened in arsenic-treated cells with a Western blot assay. We found that upon treatment with arsenic trioxide, the level of Pirh2 protein was promptly increased in HaCaT cells (Fig. 4A) and ME-180 cells (B). To further examine whether arsenic increased the level of Pirh2 mRNA, RT-PCR analysis was performed with HaCaT cells, which were left untreated or treated with 5 μm arsenic trioxide for 6 or 12 h. We found that the level of Pirh2 mRNA in HaCaT cells was obviously increased by arsenic (Fig. 4C).
FIGURE 4.
Arsenic trioxide induces expression of Pirh2 E3 ligase. A and B, Western blot analyses were prepared with extracts from HaCaT (A) and ME-180 (B) cells left untreated or treated with 5 μm arsenic trioxide for 3–9 h and then probed with antibodies against Pirh2 and actin, respectively. C, the level of Pirh2 transcript is increased by arsenic trioxide. RT-PCR analysis was performed with total RNAs isolated from HaCaT cells left untreated or treated with 5 mm arsenic trioxide for 6 or 12 h. Actin mRNA was amplified as a loading control. D, schematic presentation of the Pirh2 promoter luciferase reporters. E, arsenic treatment transactivates the Pirh2 promoter. The dual luciferase assay was performed with HaCaT cells that were cotransfected with 0.5 μg of a luciferase reporter (LUC) and 3 ng of pRL-SV40-Renilla vector for 24 h and then left untreated or treated with 5 μm arsenic trioxide for 8 h. *, p < 0.05.
Next, to explore how arsenic trioxide transactivates the Pirh2 gene, several luciferase reporters containing various regions of the Pirh2 promoter were generated (Fig. 4D). Each of these reporters was cotransfected into HaCaT cells with pRL-SV40-Renilla. We found that arsenic was able to increase the luciferase activities of all the Pirh2 promoter reporters (Fig. 4E). Interestingly, the effect of arsenic trioxide on the luciferase activity reached the maximum in Pirh2–500 promoter reporter and then slightly decreased in longer promoter reporters. Thus, it is most likely that the response element to arsenic treatment is located in the proximal region of the Pirh2 promoter. Together, these results suggest that arsenic trioxide can transactivate the expression of Pirh2 gene.
Knockdown of Pirh2 Inhibits whereas Ectopic Expression of Pirh2 Promotes Arsenic-induced Degradation of ΔNp63 Protein
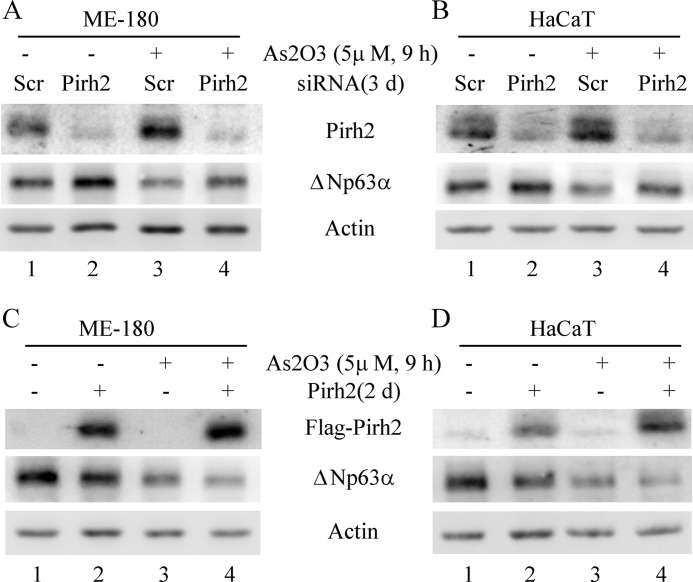
To further examine whether Pirh2 mediates arsenic-induced degradation of ΔNp63 protein, ME-180 and HaCaT cells were transiently transfected with scrambled siRNA or siRNA against Pirh2 for 3 days and then mock-treated or treated with arsenic. We showed that the level of Pirh2 was significantly decreased by Pirh2 but not scrambled siRNA (Fig. 5, A and B). Importantly, we found that Pirh2 knockdown obviously increased ΔNp63 expression regardless of arsenic treatment (Fig. 5, A and B, compare lanes 1 and 3 with lanes 2 and 4, respectively). We also noted that arsenic treatment increased Pirh2 expression concomitantly with a decreased expression of ΔNp63 (Fig. 5, A and B, compare lane 1 with lane 3).
FIGURE 5.
Knockdown of Pirh2 inhibits whereas ectopic expression of Pirh2 promotes arsenic-induced degradation of ΔNp63 protein. A and B, Western blot analyses were prepared with extracts from ME-180 (A) and HaCaT (B) cells that were transfected with scrambled siRNA or siRNA against Pirh2 for 3 days and then treated with 5 μm arsenic trioxide for 9 h. The blots were then probed with antibodies against Pirh2, ΔNp63, and actin, respectively. C and D. Western blot analyses were prepared with extracts from ME-180 (C) and HaCaT (D) cells transfected with pcDNA3 or pcDNA3–2×FLAG-Pirh2 for 2 days and then treated with 5 μm arsenic trioxide for 9 h. The blots were then probed with antibodies against the FLAG tag, ΔNp63, and actin, respectively.
Next, we examined whether ectopic expression of Pirh2 enhances arsenic-induced degradation of ΔNp63 protein. Consistently, we found that upon ectopic expression of Pirh2 in ME-180 and HaCaT cells, the level of ΔNp63 protein was obviously decreased under normal and arsenic-treated conditions (Fig. 5, C and D, compare lanes 1 and 3 with lanes 2 and 4, respectively). Together, these data suggest that arsenic-induced degradation of ΔNp63 protein is at least in part mediated by Pirh2 E3 ligase.
Knockdown of ΔNp63 Sensitizes whereas Ectopic Expression of ΔNp63 Desensitizes Tumor Cells to Arsenic Treatment
Because ΔNp63 is implicated in promoting cell survival and conferring tumor cells resistant to DNA damage (16), we tested whether ΔNp63 knockdown affects cell survival in arsenic-treated cells. To this end, ME-180 cells were transfected with scrambled siRNA or siRNA against p63. We found that the level of ΔNp63α was significantly decreased by p63 but not scrambled siRNA (Fig. 6A, compare lane 1 with lane 2). We also found that when combined with arsenic treatment, the level of ΔNp63α was further decreased in ME-180 cells with p63 knockdown (Fig. 6A, compare lane 4 with lanes 1–3). In addition, we found that short-term knockdown of ΔNp63 alone had little effect on cell survival in ME-180 cells (Fig. 6B). However, we found that upon arsenic treatment, ΔNp63 knockdown significantly reduced the number of survival cells by 42.2% as compared with that in control cells (Fig. 6B).
FIGURE 6.

Knockdown of ΔNp63 sensitizes tumor cells to arsenic treatment. A, Western blot analysis was performed with extracts from ME-180 cells that were transfected with scrambled siRNA or siRNA against p63 for 1 day and then untreated or treated with 7.5 μm arsenic trioxide for 2 days. The blots were then probed with antibodies against p63 and actin, respectively. B, ME-180 cells were treated as in A. The survival cells were collected and counted. *, p < 0.05.
Next, we tested whether ectopic expression of ΔNp63 is capable of making tumor cells resistant to arsenic treatment. For this purpose, ME-180 cells were transiently transfected with an empty vector or a vector expressing ΔNp63α for 24 h and then treated with or without arsenic trioxide for 48 h. We found that the level of ΔNp63α was increased significantly in ME-180 cells transfected with a vector expressing ΔNp63α, regardless of arsenic treatment (Fig. 7A, compare lanes 1 and 3 with lanes 2 and 4, respectively). As expected, we found that arsenic also decreased the ectopically expressed ΔNp63α (Fig. 7A, compare lane 2 with lane 4). Furthermore, we found that upon arsenic treatment, ectopic expression of ΔNp63 significantly increased the number of survival cells by 1.6-fold of that in cells transfected with an empty vector. We would like to mention that short-term overexpression of ΔNp63 alone had little effect on cell survival in ME-180 cells (Fig. 7B). Together, these results suggest that ΔNp63 plays a role in arsenic-induced inhibition on cell survival and that knockdown of ΔNp63 sensitizes tumor cells to arsenic trioxide.
FIGURE 7.
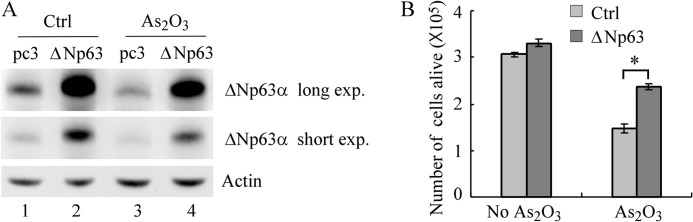
Ectopic expression of ΔNp63 makes cells resistant to arsenic treatment. A, Western blot analysis was performed with extracts from ME-180 cells that were transfected with pcDNA3 or pcDNA3-Myc-ΔNp63 for 1 day and then left untreated or treated with 7.5 μm arsenic trioxide for 2 days. The blots were then probed with antibodies against p63 and actin, respectively. B, ME-180 cells were treated as in A. The survival cells were collected and counted. *, p < 0.05.
DISCUSSION
ΔNp63 is often highly expressed in squamous cell carcinomas (11, 12, 44). In addition to transactivation of pro-survival genes, ΔNp63 protein is dominant-negative over TAp63, TAp73, and wild-type p53 (2, 7, 15, 23, 24). Thus, the imbalance of ΔNp63 with TA isoforms of the p53 family members and wild-type p53 may lead to tumorigenesis via altered expression of genes related to cell cycle arrest, apoptosis, and inhibition of metastasis. Here we showed that arsenic trioxide, a frontline therapeutic agent for acute promyelocytic leukemia, inhibits ΔNp63 but not TAp63 expression in time- and dose-dependent manners. In addition, we found that arsenic trioxide destabilizes ΔNp63 protein via the proteasome-dependent pathway. Importantly, we found that knockdown of ΔNp63 sensitizes whereas ectopic expression of ΔNp63 inhibits growth suppression induced by arsenic trioxide. Thus, identification of arsenic trioxide as an effective agent for ΔNp63 but not TAp63 degradation provides a promising approach to promptly decrease the level of ΔNp63 in tumor cells. This finding is of vital importance and potentially developed as a therapeutic strategy to tumors highly expressing ΔNp63, given that high ΔNp63 expression confers proliferative and chemoresistant advantage to tumor cells (16–18).
In an effort to probe into the mechanisms by which arsenic promotes ΔNp63 degradation, we found that arsenic potently up-regulates the level of Pirh2 E3 ligase. Knockdown of Pirh2 inhibits whereas ectopic expression of Pirh2 promotes arsenic-induced degradation of ΔNp63 protein. Pirh2, a p53-induced RING finger E3 ubiquitin ligase, is involved in the negative regulation of both TAp63 and ΔNp63 expression through physical interaction and ubiquitin-mediated and proteasome-dependent proteolysis (43). Interestingly, we found that the protein level of ΔNp63 but not TAp63 is decreased by arsenic. This result is similar to the selective decrease of mutant p53 protein, but not wild-type p53 protein, in arsenic-treated cells (32). This may be due to arsenic-activated signal pathways related to reactive oxygen species (45, 46). Consistent with the postulation, reactive oxygen species generated from hypoxia and reoxygenation increase the expression of p63 protein in human lymphocytes (47), a group of cells in which TAp63 is dominantly expressed (48).
Previously, Pirh2 was regarded as an oncogene because of overexpression in tumor tissues (49, 50) and targeting several tumor suppressors for proteasome-dependent degradation, such as p53 (51), TAp73 (52), and p27 (53). However, Pirh2 has an opposing function by suppressing tumorigenesis. A recent study showed that Pirh2 expression is reduced in various human cancers and that lower levels of Pirh2 expression correlate with decreased survival of patients with lung, breast, or ovarian cancer (22). Furthermore, Pirh2 negatively regulates the expression of c-Myc oncoprotein in vivo (22). Here, we showed that arsenic treatment induces Pirh2, which then targets ΔNp63 for proteasome-dependent degradation. Thus, Pirh2 may function as a tumor suppressor. Although the Pirh2 promoter was found to be activated in response to treatment of arsenic trioxide, we are still challenged with unresolved questions, including the identity of the transcriptional factor recruited by arsenic to induce Pirh2 expression. Thus, future studies are warranted to solve this issue.
Footnotes
This work was supported, in whole or in part, by National Institutes of Health Grant R01 CA102188.
REFERENCES
- 1. Harms K., Nozell S., Chen X. (2004) The common and distinct target genes of the p53 family transcription factors. Cell Mol. Life Sci. 61, 822–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Westfall M. D., Mays D. J., Sniezek J. C., Pietenpol J. A. (2003) The Δ Np63 α phosphoprotein binds the p21 and 14-3-3 σ promoters in vivo and has transcriptional repressor activity that is reduced by Hay-Wells syndrome-derived mutations. Mol. Cell. Biol. 23, 2264–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. King K. E., Weinberg W. C. (2007) p63:defining roles in morphogenesis, homeostasis, and neoplasia of the epidermis. Mol. Carcinog. 46, 716–724 [DOI] [PubMed] [Google Scholar]
- 4. Vanbokhoven H., Melino G., Candi E., Declercq W. (2011) p63, a story of mice and men. J. Invest. Dermatol. 131, 1196–1207 [DOI] [PubMed] [Google Scholar]
- 5. Osada M., Ohba M., Kawahara C., Ishioka C., Kanamaru R., Katoh I., Ikawa Y., Nimura Y., Nakagawara A., Obinata M., Ikawa S. (1998) Cloning and functional analysis of human p51, which structurally and functionally resembles p53. Nat. Med. 4, 839–843 [DOI] [PubMed] [Google Scholar]
- 6. Trink B., Okami K., Wu L., Sriuranpong V., Jen J., Sidransky D. (1998) A new human p53 homologue. Nat. Med. 4, 747–748 [DOI] [PubMed] [Google Scholar]
- 7. Yang A., Kaghad M., Wang Y., Gillett E., Fleming M. D., Dötsch V., Andrews N. C., Caput D., McKeon F. (1998) p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell 2, 305–316 [DOI] [PubMed] [Google Scholar]
- 8. Dohn M., Zhang S., Chen X. (2001) p63α and ΔNp63α can induce cell cycle arrest and apoptosis and differentially regulate p53 target genes. Oncogene 20, 3193–3205 [DOI] [PubMed] [Google Scholar]
- 9. Flores E. R., Sengupta S., Miller J. B., Newman J. J., Bronson R., Crowley D., Yang A., McKeon F., Jacks T. (2005) Tumor predisposition in mice mutant for p63 and p73. Evidence for broader tumor suppressor functions for the p53 family. Cancer Cell 7, 363–373 [DOI] [PubMed] [Google Scholar]
- 10. Guo X., Keyes W. M., Papazoglu C., Zuber J., Li W., Lowe S. W., Vogel H., Mills A. A. (2009) TAp63 induces senescence and suppresses tumorigenesis in vivo. Nat. Cell Biol. 11, 1451–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Massion P. P., Taflan P. M., Jamshedur Rahman S. M., Yildiz P., Shyr Y., Edgerton M. E., Westfall M. D., Roberts J. R., Pietenpol J. A., Carbone D. P., Gonzalez A. L. (2003) Significance of p63 amplification and overexpression in lung cancer development and prognosis. Cancer Res. 63, 7113–7121 [PubMed] [Google Scholar]
- 12. Nonaka D. (2012) A study of ΔNp63 expression in lung non-small cell carcinomas. Am. J. Surg. Pathol. 36, 895–899 [DOI] [PubMed] [Google Scholar]
- 13. Hibi K., Trink B., Patturajan M., Westra W. H., Caballero O. L., Hill D. E., Ratovitski E. A., Jen J., Sidransky D. (2000) AIS is an oncogene amplified in squamous cell carcinoma. Proc. Natl. Acad. Sci. U.S.A. 97, 5462–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boldrup L., Coates P. J., Gu X., Nylander K. (2007) ΔNp63 isoforms regulate CD44 and keratins 4, 6, 14 and 19 in squamous cell carcinoma of head and neck. J. Pathol. 213, 384–391 [DOI] [PubMed] [Google Scholar]
- 15. Mundt H. M., Stremmel W., Melino G., Krammer P. H., Schilling T., Müller M. (2010) Dominant negative (ΔN) p63α induces drug resistance in hepatocellular carcinoma by interference with apoptosis signaling pathways. Biochem. Biophys. Res. Commun. 396, 335–341 [DOI] [PubMed] [Google Scholar]
- 16. Li X., Chen J., Yi Y., Li C., Zhang Y. (2012) DNA damage down-regulates ΔNp63α and induces apoptosis independent of wild type p53. Biochem. Biophys. Res. Commun. 423, 338–343 [DOI] [PubMed] [Google Scholar]
- 17. Mitani Y., Li J., Weber R. S., Lippman S. L., Flores E. R., Caulin C., El-Naggar A. K. (2011) Expression and regulation of the ΔN and TAp63 isoforms in salivary gland tumorigenesis clinical and experimental findings. Am. J. Pathol. 179, 391–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zangen R., Ratovitski E., Sidransky D. (2005) ΔNp63α levels correlate with clinical tumor response to cisplatin. Cell Cycle 4, 1313–1315 [DOI] [PubMed] [Google Scholar]
- 19. Yan W., Chen X. (2006) GPX2, a direct target of p63, inhibits oxidative stress-induced apoptosis in a p53-dependent manner. J. Biol. Chem. 281, 7856–7862 [DOI] [PubMed] [Google Scholar]
- 20. Yan W., Chen X. (2007) Targeted repression of bone morphogenetic protein 7, a novel target of the p53 family, triggers proliferative defect in p53-deficient breast cancer cells. Cancer Res. 67, 9117–9124 [DOI] [PubMed] [Google Scholar]
- 21. Barbieri C. E., Perez C. A., Johnson K. N., Ely K. A., Billheimer D., Pietenpol J. A. (2005) IGFBP-3 is a direct target of transcriptional regulation by ΔNp63α in squamous epithelium. Cancer Res. 65, 2314–2320 [DOI] [PubMed] [Google Scholar]
- 22. Hakem A., Bohgaki M., Lemmers B., Tai E., Salmena L., Matysiak-Zablocki E., Jung Y. S., Karaskova J., Kaustov L., Duan S., Madore J., Boutros P., Sheng Y., Chesi M., Bergsagel P. L., Perez-Ordonez B., Mes-Masson A. M., Penn L., Squire J., Chen X., Jurisica I., Arrowsmith C., Sanchez O., Benchimol S., Hakem R. (2011) Role of Pirh2 in mediating the regulation of p53 and c-Myc. PLoS Genet. 7, e1002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crook T., Nicholls J. M., Brooks L., O'Nions J., Allday M. J. (2000) High level expression of ΔN-p63, a mechanism for the inactivation of p53 in undifferentiated nasopharyngeal carcinoma (NPC)? Oncogene 19, 3439–3444 [DOI] [PubMed] [Google Scholar]
- 24. Rocco J. W., Leong C. O., Kuperwasser N., DeYoung M. P., Ellisen L. W. (2006) p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell 9, 45–56 [DOI] [PubMed] [Google Scholar]
- 25. Emadi A., Gore S. D. (2010) Arsenic trioxide, an old drug rediscovered. Blood Rev. 24, 191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang X. W., Yan X. J., Zhou Z. R., Yang F. F., Wu Z. Y., Sun H. B., Liang W. X., Song A. X., Lallemand-Breitenbach V., Jeanne M., Zhang Q. Y., Yang H. Y., Huang Q. H., Zhou G. B., Tong J. H., Zhang Y., Wu J. H., Hu H. Y., de Thé H., Chen S. J., Chen Z. (2010) Arsenic trioxide controls the fate of the PML-RARα oncoprotein by directly binding PML. Science 328, 240–243 [DOI] [PubMed] [Google Scholar]
- 27. Jeanne M., Lallemand-Breitenbach V., Ferhi O., Koken M., Le Bras M., Duffort S., Peres L., Berthier C., Soilihi H., Raught B., de Thé H. (2010) PML/RARA oxidation and arsenic binding initiate the antileukemia response of As2O3. Cancer Cell 18, 88–98 [DOI] [PubMed] [Google Scholar]
- 28. Shackelford D., Kenific C., Blusztajn A., Waxman S., Ren R. (2006) Targeted degradation of the AML1/MDS1/EVI1 oncoprotein by arsenic trioxide. Cancer Res. 66, 11360–11369 [DOI] [PubMed] [Google Scholar]
- 29. Kim J., Lee J. J., Kim J., Gardner D., Beachy P. A. (2010) Arsenic antagonizes the Hedgehog pathway by preventing ciliary accumulation and reducing stability of the Gli2 transcriptional effector. Proc. Natl. Acad. Sci. U.S.A. 107, 13432–13437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mann K. K., Colombo M., Miller W. H., Jr. (2008) Arsenic trioxide decreases AKT protein in a caspase-dependent manner. Mol. Cancer Ther. 7, 1680–1687 [DOI] [PubMed] [Google Scholar]
- 31. Zhang Q. Y., Mao J. H., Liu P., Huang Q. H., Lu J., Xie Y. Y., Weng L., Zhang Y., Chen Q., Chen S. J., Chen Z. (2009) A systems biology understanding of the synergistic effects of arsenic sulfide and Imatinib in BCR/ABL-associated leukemia. Proc. Natl. Acad. Sci. U.S.A. 106, 3378–3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yan W., Zhang Y., Zhang J., Liu S., Cho S. J., Chen X. (2011) Mutant p53 protein is targeted by arsenic for degradation and plays a role in arsenic-mediated growth suppression. J. Biol. Chem. 286, 17478–17486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jung Y. S., Liu G., Chen X. (2010) Pirh2 E3 ubiquitin ligase targets DNA polymerase η for 20 S proteasomal degradation. Mol. Cell. Biol. 30, 1041–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Debnath J., Muthuswamy S. K., Brugge J. S. (2003) Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30, 256–268 [DOI] [PubMed] [Google Scholar]
- 35. Carroll D. K., Carroll J. S., Leong C. O., Cheng F., Brown M., Mills A. A., Brugge J. S., Ellisen L. W. (2006) p63 regulates an adhesion programme and cell survival in epithelial cells. Nat. Cell Biol. 8, 551–561 [DOI] [PubMed] [Google Scholar]
- 36. Zhang J., Jun Cho S., Chen X. (2010) RNPC1, an RNA-binding protein and a target of the p53 family, regulates p63 expression through mRNA stability. Proc. Natl. Acad. Sci. U.S.A. 107, 9614–9619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shen Y., Shen Z. X., Yan H., Chen J., Zeng X. Y., Li J. M., Li X. S., Wu W., Xiong S. M., Zhao W. L., Tang W., Wu F., Liu Y. F., Niu C., Wang Z. Y., Chen S. J., Chen Z. (2001) Studies on the clinical efficacy and pharmacokinetics of low-dose arsenic trioxide in the treatment of relapsed acute promyelocytic leukemia. A comparison with conventional dosage. Leukemia 15, 735–741 [DOI] [PubMed] [Google Scholar]
- 38. Su X., Chakravarti D., Cho M. S., Liu L., Gi Y. J., Lin Y. L., Leung M. L., El-Naggar A., Creighton C. J., Suraokar M. B., Wistuba I., Flores E. R. (2010) TAp63 suppresses metastasis through coordinate regulation of Dicer and miRNAs. Nature 467, 986–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Galli F., Rossi M., D'Alessandra Y., De Simone M., Lopardo T., Haupt Y., Alsheich-Bartok O., Anzi S., Shaulian E., Calabrò V., La Mantia G., Guerrini L. (2010) MDM2 and Fbw7 cooperate to induce p63 protein degradation following DNA damage and cell differentiation. J. Cell Sci. 123, 2423–2433 [DOI] [PubMed] [Google Scholar]
- 40. Li Y., Zhou Z., Chen C. (2008) WW domain-containing E3 ubiquitin protein ligase 1 targets p63 transcription factor for ubiquitin-mediated proteasomal degradation and regulates apoptosis. Cell Death Differ. 15, 1941–1951 [DOI] [PubMed] [Google Scholar]
- 41. Rossi M., Aqeilan R. I., Neale M., Candi E., Salomoni P., Knight R. A., Croce C. M., Melino G. (2006) The E3 ubiquitin ligase Itch controls the protein stability of p63. Proc. Natl. Acad. Sci. U.S.A. 103, 12753–12758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rossi M., De Simone M., Pollice A., Santoro R., La Mantia G., Guerrini L., Calabrò V. (2006) Itch/AIP4 associates with and promotes p63 protein degradation. Cell Cycle 5, 1816–1822 [DOI] [PubMed] [Google Scholar]
- 43. Jung Y. S., Qian Y., Yan W., Chen X. (2012) Pirh2 E3 ubiquitin ligase modulates keratinocyte differentiation through p63. J. Invest. Dermatol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murata K., Ota S., Niki T., Goto A., Li C. P., Ruriko U. M., Ishikawa S., Aburatani H., Kuriyama T., Fukayama M. (2007) p63. Key molecule in the early phase of epithelial abnormality in idiopathic pulmonary fibrosis. Exp. Mol. Pathol. 83, 367–376 [DOI] [PubMed] [Google Scholar]
- 45. Pelicano H., Feng L., Zhou Y., Carew J. S., Hileman E. O., Plunkett W., Keating M. J., Huang P. (2003) Inhibition of mitochondrial respiration: a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J. Biol. Chem. 278, 37832–37839 [DOI] [PubMed] [Google Scholar]
- 46. Jing Y., Dai J., Chalmers-Redman R. M., Tatton W. G., Waxman S. (1999) Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood 94, 2102–2111 [PubMed] [Google Scholar]
- 47. Choi J. Y., Kim B. M., Kim Y. J., Woo H. D., Chung H. W. (2007) Hypoxia/reoxygenation-induced cytotoxicity in cultured human lymphocytes. Biochem. Biophys. Res. Commun. 352, 366–371 [DOI] [PubMed] [Google Scholar]
- 48. Di Como C. J., Urist M. J., Babayan I., Drobnjak M., Hedvat C. V., Teruya-Feldstein J., Pohar K., Hoos A., Cordon-Cardo C. (2002) p63 expression profiles in human normal and tumor tissues. Clin. Cancer Res. 8, 494–501 [PubMed] [Google Scholar]
- 49. Wang X. M., Yang L. Y., Guo L., Fan C., Wu F. (2009) p53-induced RING-H2 protein, a novel marker for poor survival in hepatocellular carcinoma after hepatic resection. Cancer 115, 4554–4563 [DOI] [PubMed] [Google Scholar]
- 50. Logan I. R., Gaughan L., McCracken S. R., Sapountzi V., Leung H. Y., Robson C. N. (2006) Human PIRH2 enhances androgen receptor signaling through inhibition of histone deacetylase 1 and is overexpressed in prostate cancer. Mol. Cell. Biol. 26, 6502–6510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Leng R. P., Lin Y., Ma W., Wu H., Lemmers B., Chung S., Parant J. M., Lozano G., Hakem R., Benchimol S. (2003) Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell 112, 779–791 [DOI] [PubMed] [Google Scholar]
- 52. Jung Y. S., Qian Y., Chen X. (2011) The p73 tumor suppressor is targeted by Pirh2 RING finger E3 ubiquitin ligase for the proteasome-dependent degradation. J. Biol. Chem. 286, 35388–35395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hattori T., Isobe T., Abe K., Kikuchi H., Kitagawa K., Oda T., Uchida C., Kitagawa M. (2007) Pirh2 promotes ubiquitin-dependent degradation of the cyclin-dependent kinase inhibitor p27Kip1. Cancer Res. 67, 10789–10795 [DOI] [PubMed] [Google Scholar]