Background: The metabolic fate of glycerol in the heart is controversial.
Results: [U-13C3]Glycerol is metabolized to glycogen, but the label is only found in carbon positions 4–6 of each glucose moiety.
Conclusion: This isotopomer pattern can only be ascribed to transaldolase activity.
Significance: This is the first detection of transaldolase activity of an isolated organ using a stable isotope.
Keywords: Glycerol, Heart Metabolism, Isotopic Tracers, NMR, Pentose Pathway, Transaldolase
Abstract
Studies of glycerol metabolism in the heart have largely emphasized its role in triglyceride synthesis. However, glycerol may also be oxidized in the citric acid cycle, and glycogen synthesis from glycerol has been reported in the nonmammalian myocardium. The intent of this study was to test the hypothesis that glycerol may be metabolized to glycogen in mammalian heart. Isolated rat hearts were supplied with a mixture of substrates including glucose, lactate, pyruvate, octanoate, [U-13C3]glycerol, and 2H2O to probe various metabolic pathways including glycerol oxidation, glycolysis, the pentose phosphate pathway, and carbon sources of stored glycogen. NMR analysis confirmed that glycogen production from the level of the citric acid cycle did not occur and that the glycerol contribution to oxidation in the citric acid cycle was negligible in the presence of alternative substrates. Quite unexpectedly, 13C from [U-13C3]glycerol appeared in glycogen in carbon positions 4–6 of glucosyl units but none in positions 1–3. The extent of [4,5,6-13C3]glucosyl unit enrichment in glycogen was enhanced by insulin but decreased by H2O2. Given that triose phosphate isomerase is generally assumed to fully equilibrate carbon tracers in the triose pool, the marked 13C asymmetry in glycogen can only be attributed to conversion of [U-13C3]glycerol to [U-13C3]dihydroxyacetone phosphate and [U-13C3]glyceraldehyde 3-phosphate followed by rearrangements in the nonoxidative branch of the pentose phosphate pathway involving transaldolase that places this 13C-enriched 3-carbon unit only in the bottom half of hexose phosphate molecules contributing to glycogen.
Introduction
Free fatty acids, lactate and glucose are primary substrates for cardiac energy production, but the heart has the capacity to oxidize a wide variety of alternative compounds. In mammalian heart tissue, 14C-enriched glycerol was readily oxidized to 14CO2 even in the presence of glucose or long chain fatty acids (1, 2). Glycerol is phosphorylated by glycerol kinase to form glycerol 3-phosphate, and this intermediate is then converted to dihydroxyacetone phosphate (DHAP)2 which is rearranged to d-glyceraldehyde 3-phosphate (GA3P) via triose phosphate isomerase (TPI; EC 5.3.1.1). GA3P may be further metabolized to pyruvate and enter the citric acid cycle via pyruvate dehydrogenase, or pyruvate may supply carbon to the cycle via pyruvate carboxylase without net oxidation (3). Another metabolic fate of glycerol after phosphorylation is that it is readily utilized in the synthesis of triglycerides and phospholipids in the mammalian heart (2). Although the heart is not a glyconeogenic organ, the enzymes necessary to convert glycerol to glucose 1-phosphate (G1P) and glycogen are all present, and conversion of 14C-enriched glycerol to glycogen in isolated heart tissue of nonmammalian species has been reported (4). The quantitative significance of this conversion in mammalian tissue has not been examined.
The physiological range of glycerol in plasma is 0.04-0.4 mm (5–7), but it may be increased severalfold under the condition of starvation (7), exercise (8), exposure to cold (9), or diabetes (10). The combination of fasting and cold exposure increased plasma glycerol up to ∼1 mm in rodents (9). Furthermore, athletes who drink beverages containing extra glycerol for improving exercise performance and hydration status may reach up to ∼10 mm glycerol in plasma with the dose of 1 g/kg body weight (11, 12). In this study, [U-13C3]glycerol was supplied to isolated rat hearts with the intent of quantifying the contribution of glycerol to assess exchange of glycerol into the glucosyl carbons of glycogen. With full exchange at the level of TPI, labeling in the top and bottom of glucosyl units of glycogen should be equal whereas incomplete exchange in the triose pool should result in preferential enrichment of carbons 1–3 of glucosyl units in glycogen. Surprisingly, we found that [U-13C3]glycerol is transferred exclusively to carbons 4–6 of glucosyl units with no enrichment in carbons 1–3. This observation can only be explained by carbon rearrangements occurring at the level of transaldolase in the nonoxidative portion of the pentose phosphate pathway (PPP).
EXPERIMENTAL PROCEDURES
Materials
[U-13C3]Glycerol (99%), [1,2-13C2]glucose (99%), and 2H2O (99%) were obtained from Cambridge Isotope Laboratories, Inc. (Andover, MA). dl-Isoproterenol hydrochloride, cation-exchange (Dowex 50WX8-200) and anion-exchange (Amberlite IRA-67) resin, and other common chemicals were obtained from Sigma.
Isolated Heart Preparation
The protocol was approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center. Under general anesthesia, hearts (1.5 ± 0.2 g) from overnight-fasted male Sprague-Dawley rats (300–350 g) were rapidly excised and perfused using standard Langendorff methods at 37 °C and 100-cm H2O with a Krebs-Henseleit bicarbonate (KHB) buffer (3). The KHB buffer, bubbled continuously with a 95/5 mixture of O2/CO2, contained 25 mm NaHCO3, 118 mm NaCl, 4.7 mm KCl, 1.2 mm MgSO4, 1.2 mm KH2PO4, 0.5 mm EDTA (sodium salt), 2.5 mm CaCl2, 1.2 mm lactate, and 0.12 mm pyruvate. The heart rate was monitored through an open-ended cannula in the left ventricle. In one set of studies, the heart was preperfused with 8 nm isoproterenol solution containing 0.5 mm octanoate, 1.2 mm lactate, and 0.12 mm pyruvate for 20 min to reduce glycogen content, followed by a 5-min perfusion with a mixture of the same substrates to wash out isoproterenol in the medium. In other studies, the heart was preperfused with a substrate-free KHB buffer without isoproterenol for 20 min to reduce glycogen. In all experiments the heart was perfused subsequently for 60 min with a medium containing 0.5 mm octanoate, 1.2 mm lactate, 0.12 mm pyruvate, 10 mm glucose, 2 mm glycerol, and 0.5% BSA to assure identical substrate conditions for all hearts. Depending on the experiment, deuterated water (2H2O), insulin, or hydrogen peroxide (H2O2) was added, or [U-13C3]glycerol or [1,2-13C2]glucose was substituted for the unlabeled compound, as follows: (i) 2 mm [U-13C3]glycerol and no insulin; (ii) 2 mm [U-13C3]glycerol and 50 microunits/ml insulin; (iii) 2 mm [U-13C3]glycerol, 50 microunits/ml insulin, and 0.1 mm H2O2; or (iv) 2 mm unlabeled glycerol, 10 mm [1,2-13C2]glucose, 50 microunits/ml insulin, and 5% 2H2O. After the perfusion, each heart was freeze clamped.
Preparation of Samples for NMR Analysis
Glycogen was extracted from frozen heart tissue using standard methods (13). Isolated glycogen was dissolved in 5 ml of 10 mm sodium acetate solution (pH 4.8) and incubated with amyloglucosidase (50 mg of glycogen/20 units of amyloglucosidase) for 4 h at 50 °C. After neutralizing, the hydrolyzed glycogen solution was applied to an ion exchange column containing 15 ml of the cation-exchange (Dowex 50WX8-200) and 15 ml of the anion-exchange (Amberlite IRA-67) resin. Hydrolyzed glycogen was purified by passage through the column using deionized water as eluent, collected, and lyophilized.
Glucosyl units from tissue glycogen were converted to monoacetone glucose (MAG) as follows. Dried glucose was suspended in 3.0 ml of acetone containing 120 μl of concentrated sulfuric acid. The mixture was stirred for 4 h at room temperature to yield diacetone glucose. After adding 3 ml of water, the pH was adjusted to 2.0 by dropwise addition of 1.5 m Na2CO3. The mixture was stirred for 24 h at room temperature to convert diacetone glucose into MAG. The pH was then further increased to ∼8.0 by addition of Na2CO3. The acetone was evaporated under vacuum, and the sample was freeze-dried. MAG was extracted into 3 ml of hot ethyl acetate (5×), the solutions were combined, and ethyl acetate was removed by vacuum evaporation. The resulting MAG was further purified by passage through a 3-ml DSC-18 cartridge, using 5% acetonitrile as eluent. The effluent was freeze-dried and stored dry before NMR analysis.
A standard perchloric acid extraction procedure was used to extract water-soluble components from some hearts perfused with [U-13C3]glycerol for NMR analysis of the citric acid cycle intermediates and exchanging pools. In addition, effluent from the heart perfused with [1,2-13C2]glucose was treated with perchloric acid for NMR analysis of 13C-enriched lactate.
NMR Spectroscopy
All NMR spectra were collected using a Varian Inova 14.1T spectrometer (Agilent, Santa Clara, CA) equipped using a 3-mm broadband probe with coil tunable to 1H (600 MHz), 2H (92 MHz), or 13C (150 MHz). Hydrolyzed glycogen or perchloric acid extracts were dissolved in 2H2O (160 μl) for 1H and 13C NMR acquisition. 1H NMR was acquired at 25 °C, using a 90° observe pulse, a 2-s acquisition time, and 1-s delay between pulses. The solvent water signal was presaturated using a frequency-selective pulse. Typically, 128 scans were summed requiring ∼6 min. Proton-decoupled 13C NMR spectra were acquired at 25 °C using a 45° pulse (5.0 μs), 34,965-Hz sweep width, 104,986 data points, and a 1.5-s interpulse delay at 25 °C. The samples were signal-averaged over 7,000–30,000 scans requiring 6–25 h. Proton decoupling was performed using a standard WALTZ-16 pulse sequence.
For 2H NMR acquisition, MAG was dissolved in a mixture of 160 μl of acetonitrile and 10 μl of water. Proton-decoupled 2H NMR spectra were acquired using a 90° pulse (12.5 μs), 920-Hz sweep width, 1,836 data points, and a 1-s acquisition time with no further delay at 50 °C. Typically ∼70,000 scans were averaged, requiring ∼21 h. After 2H NMR acquisition, MAG was lyophilized and resuspended in 160 μl of deuterated acetonitrile (99.8%) and 10 μl of water for 13C NMR acquisition. 13C NMR spectra of MAG were collected using 52° pulse (6.06 μs), 20,330-Hz sweep width, 60,992 data points, and a 1.5-s acquisition time with 1.5-s interpulse delay at 25 °C. Typically ∼25,000 scans were averaged, requiring ∼25 h. All NMR spectra were analyzed using ACD/Labs PC-based NMR spectral analysis program (Advanced Chemistry Development, Inc., Toronto, ON, Canada).
Statistics
Data are expressed as means ± S.E. Comparisons between two groups were made using Student's two-tailed t test, where p < 0.05 was considered significant.
Pathway Considerations: 13C Labeling of Glycogen in Hearts Supplied with [U-13C3]Glycerol
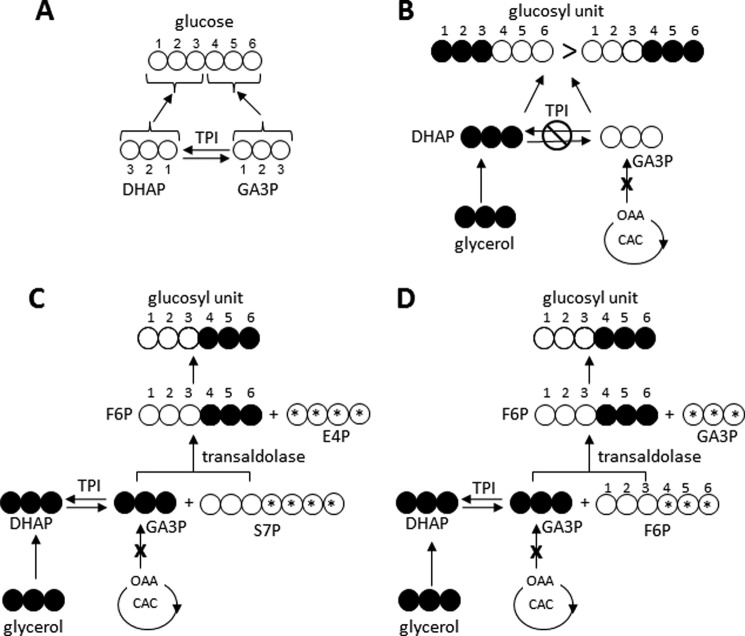
Given that the heart does not generate glycogen from the citric acid cycle, the only possible pathway for [U-13C3]glycerol to contribute to glucosyl units in glycogen must involve exchanges occurring at the level of TPI. In the “standard” gluconeogenesis in general, carbons 1–3 of glucose (the “top half”) are derived from DHAP whereas carbons 4–6 (the “bottom half”) are derived from GA3P (Fig. 1A) and, because phosphorylation of [U-13C3]glycerol would form [U-13C3]DHAP in two steps if complete equilibration at the level of TPI does not occur, this should produce more [1,2,3-13C3]glucosyl units (the DHAP end) than [4,5,6-13C3]glucosyl units (the GA3P end; Fig. 1B). If the trioses fully equilibrate at the level of TPI and this is the only pathway leading to glucosyl units, the labeling of the top and bottom ends of glucosyl units must be identical. On the other hand, if other exchanges occur rapidly in the nonoxidative portion of the PPP, then the labeling pattern could be quite different. For example, transaldolase (EC 2.2.1.2) catalyzes removal of a 3-carbon dihydroxyacetone unit from the nonphosphorylated end of sedoheptulose 7-phosphate (S7P), and this 3-carbon unit can then condense with any [U-13C3]GA3P that might be present to yield [4,5,6-13C3]fructose 6-phosphate (F6P) and erythrose 4-phosphate (Fig. 1C). This [4,5,6-13C3]F6P would be readily converted to [4,5,6-13C3]glucose 6-phosphate (G6P) and subsequently [4,5,6-13C3]G1P and glycogen with glucosyl units labeled only in the 4,5,6 positions.
FIGURE 1.
Possible metabolic routes for unequal 13C enrichment of carbons 1–3 versus 4–6 of glucosyl units of glycogen in heart supplied with [U-13C3]glycerol. A, during glucose production through standard gluconeogenic pathways, carbons 1–3 of glucose originate from DHAP whereas carbons 4–6 originate from GA3P. B, in hearts exposed to [U-13C3]glycerol, one would anticipate that incomplete equilibration at the level of TPI would yield more [1,2,3-13C3]glucosyl units than [4,5,6-13C3]glucosyl units because [U-13C3]glycerol is converted first to [U-13C3]DHAP before [U-13C3]GA3P is produced at the level of TPI. C, in comparison, rapid equilibration of [U-13C3]GA3P with S7P at the level of transaldolase would yield only [4,5,6-13C3]F6P and E4P. D, transaldolase exchange between carbons 4–6 of F6P and [U-13C3]GA3P would also yield only [4,5,6-13C3]F6P. OAA, oxaloacetate; E4P, erythrose 4-phosphate; CAC, citric acid cycle; open circles, 12C; filled circles, 13C. The x indicates that flow of 13C from the citric acid cycle into glycogen production is not considered in these examples. The asterisks in C and D indicate the carbons that are replaced by [U-13C3]GA3P through transaldolase activity to form [4,5,6-13C3]F6P.
Transaldolase Exchange
Ljungdahl et al. (14) demonstrated that isolated transaldolase can exchange carbons 4–6 of F6P with GA3P even in the absence of other enzymes or intermediates of the PPP. If this exchange between F6P and [U-13C3]GA3P should occur in heart, this would also result in exclusive labeling of the 4–6 carbons of glucosyl units of glycogen (Fig. 1D).
RESULTS
Hearts Supplied with [U-13C3]Glycerol
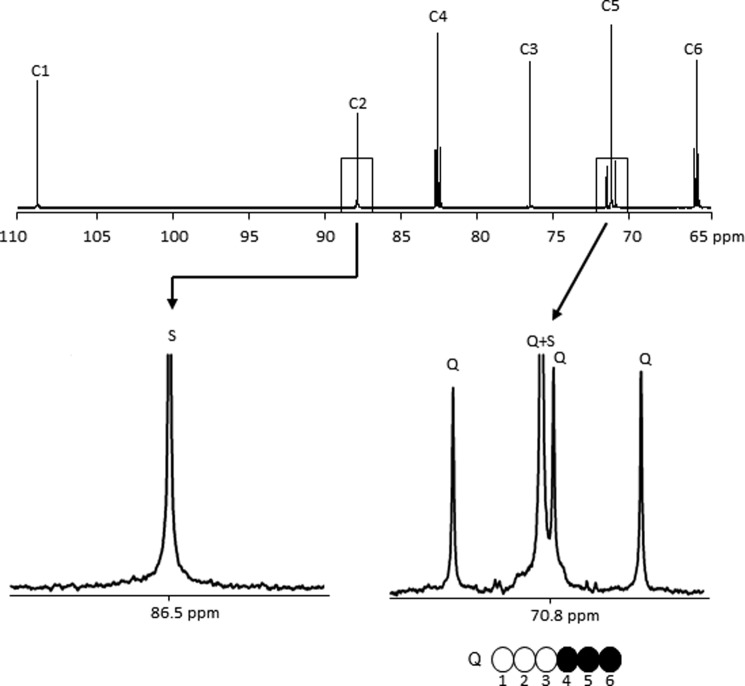
A 13C NMR spectrum of MAG derived from hydrolyzed glycogen is shown in Fig. 2. Clearly, excess 13C was evident only in carbons 4, 5, and 6 of glucosyl units of glycogen in hearts supplied with [U-13C3]glycerol and other natural abundance substrates. Preferential labeling of the bottom half could in principle arise from metabolism of [U-13C3]glycerol in the citric acid cycle followed by conversion of 13C-enriched citric acid cycle intermediates to phosphoenolpyruvate and ultimately GA3P, but this is highly unlikely because only intact, three-carbon 13C-enriched units were found in glycogen as [4,5,6-13C3]glucose moiety.
FIGURE 2.
13C NMR spectrum of MAG derived from hydrolyzed glycogen of a heart supplied with [U-13C3]glycerol. The multiplets seen in the C4, C5, and C6 resonances but not in the C1, C2, and C3 resonances indicate that carbons originating in [U-13C3]glycerol find their way only into the bottom half of glucosyl units of glycogen. The singlets detected in each glucosyl unit resonance reflect largely natural abundance levels of 13C. Q, doublet of doublets, or quartet, arising from coupling of C5 with both C4 and C6; S, singlet.
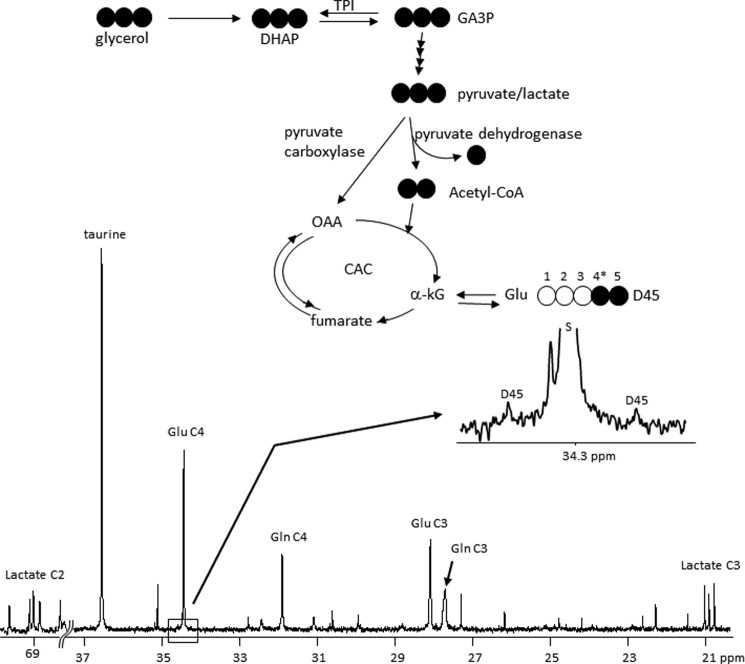
Although glycogen synthesis from the citric acid cycle is not considered active in the heart, we reexamined the possibility that [U-13C3]glycerol under these conditions could be metabolized to pyruvate followed by oxidation to acetyl-CoA or carboxylation to oxaloacetate. The presence of [U-13C3]lactate resonances in the 13C NMR spectra of heart tissue extracts demonstrated that the glycerol was indeed metabolized to pyruvate (Fig. 3). However, the 13C signals in glutamate were almost exclusively at natural abundance levels except the very small amount of [4,5-13C2]glutamate (0.022 ± 0.006%, n = 5). This labeling at C4–C5 of glutamate demonstrated that [U-13C3]glycerol did indeed enter the citric acid cycle as acetyl-CoA, but the fraction of acetyl-CoA derived from glycerol was <1%. Pyruvate carboxylation to oxaloacetate was not detected as evidenced by the absence of excess 13C enrichment in glutamate C1–C3. Clearly, small amount of [4,5-13C2]glutamate formed in the cycle could not have contributed significantly to the [4,5,6-13C3]glucose moiety in glycogen.
FIGURE 3.
13C NMR spectrum of a heart tissue extract after perfusion with [U-13C3]glycerol. Only natural-abundance singlets were detected in glutamate, glutamine, and taurine, with the exception that [4,5-13C2]glutamate was observed at a very low level (0.022 ± 0.006%, n = 5). Resonance of glutamate C4 is expanded. The multiplets in lactate resonances are from [U-13C3]lactate, which is in exchange with [U-13C3]pyruvate in tissues. Because glutamate is in rapid exchange with α-ketoglutarate (α-kG), the presence of the small doublets due to 13C-13C spin-spin coupling between C4 and C5 (D45) indicates that a small portion of [U-13C3]pyruvate entered the citric acid cycle through acetyl-CoA. D45, doublet arising from coupling of C4 with C5; S, singlet.
The labeling pattern in glycogen cannot be explained by simple condensation of GA3P and DHAP at the level of TPI. Incomplete equilibration of 3-carbon units at TPI would result in excess enrichment in the top half of glucosyl units, i.e. [1,2,3-13C3]glucose (Fig. 1B). The appearance of [4,5,6-13C3]glucosyl units alone, however, must reflect some combination of transaldolase activity between [U-13C3]GA3P and S7P (Fig. 1C), and transaldolase exchange between the bottom half carbons of F6P and [U-13C3]GA3P (Fig. 1D). The 13C enrichment in cardiac glycogen was measured using 13C NMR analysis of α- and β-glucose C6 resonances after the hydrolysis of glycogen, assuming that the singlet peaks seen in the C6 resonances represent natural abundance 13C. The areas of the multiplets in the C6 resonances indicated that [U-13C3]glycerol contributed to ∼0.51% and ∼1.21% of the total glucose derived from tissue glycogen in the absence and presence of insulin, respectively (Fig. 4, A and B). Thus, insulin enhanced 13C enrichment in glycogen approximately 2-fold whereas it did not affect glycogen content (Fig. 4C).
FIGURE 4.
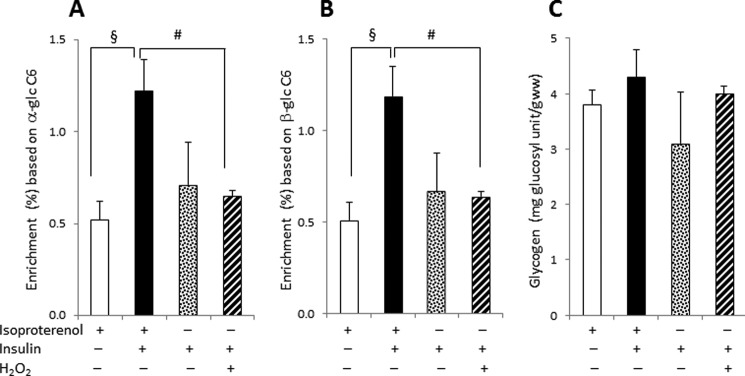
Effect of isoproterenol pretreatment, insulin, or H2O2 on 13C enrichment and total glycogen content in hearts supplied with [U-13C3]glycerol. A and B, insulin increased the enrichment in the [4,5,6-13C3]glucosyl units of glycogen based on the 13C NMR analysis of hydrolyzed α-glucose C6 (A) and β-glucose C6 (B), but H2O2 decreased the enrichment. C, glycogen content remained the same by the presence of insulin or H2O2. Glycogen content was not different in hearts preperfused with isoproterenol compared with preperfusion with a substrate-free buffer without isoproterenol. glc C6, carbon 6 of glucosyl unit; #, p < 0.05; §, p < 0.01.
The unexpected labeling pattern in glycogen remained the same whether hearts were preperfused with isoproterenol or with a substrate-free KHB buffer, confirming that the asymmetry was not related to a residual effect of isoproterenol (Fig. 4). The enrichment of [4,5,6-13C3]glucosyl units from the hearts preperfused with a buffer was not different statistically from the hearts pretreated with isoproterenol (Fig. 4, A and B). In contrast, adding H2O2 in the hearts preperfused with a buffer decreased the enrichment compared with the hearts pretreated with isoproterenol. However, glycogen content was not affected by insulin, H2O2, or pretreatment with isoproterenol (Fig. 4C). All of the hearts under the various conditions were functioning well as evidenced by heart beating rates and oxygen consumption (Table 1).
TABLE 1.
Heart rates and O2 consumption
Hearts were preperfused with isoproterenol solution for 20 min or with a substrate-free buffer without isoproterenol for 20 min to reduce glycogen content. A 60-min perfusion followed with media containing octanoate, lactate, pyruvate, glucose, and [U-13C3]glycerol. Insulin and/or H2O2 were added in some cases, which are specified below.
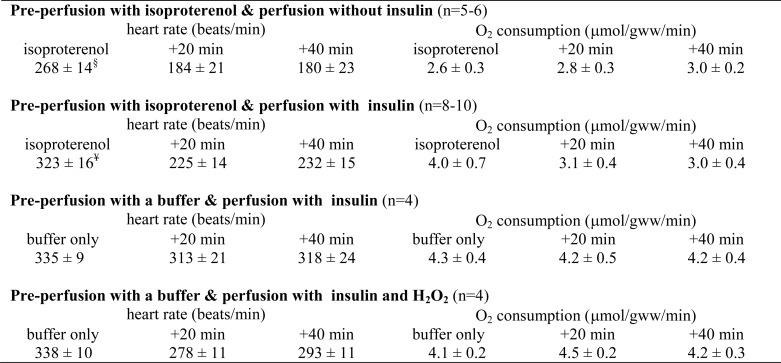
§, p ≤ 0.01; ¥, p < 0.001 compared with the time point at either +20 min or +40 min.
Hearts Supplied with [1,2-13C2]Glucose and 2H2O
To determine whether the observed asymmetry required flux of exogenous glucose into the triose phosphate pool, a second group of hearts were supplied with medium containing [1,2-13C2]glucose and 5% 2H2O in addition to unlabeled glycerol. The 2H NMR spectrum of MAG derived from glycogen showed ∼0.5–0.6% 2H enrichment in H1 and H2 positions, ∼0.07–0.08% enrichment in H4 and H5, but no excess enrichment in other proton positions (Fig. 5). 2H enrichment at the H1 and H2 positions was the result of exchange reactions occurring at the level of hexose phosphates, whereas 2H enrichment at H4 and H5 originate from exchange reactions occurring at the level of triose phosphates. The absence of enrichment at H3 informed that DHAP did not contribute to carbons 1–3 of glucosyl units of glycogen through the standard glyconeogenic pathways from the triose pool because 2H incorporation at H3 position also occurs at the level of TPI (DHAP ← GA3P) (15). Otherwise, the enrichment at H3 of glucosyl units would be observed, which originates from H1 position of DHAP. The absence of enrichment at H6 confirmed that the citric acid cycle did not contribute to glycogen because 2H incorporation at H6 occurs at the level of fumarase (16). The dominant 2H enrichment at the H1 and H2 positions of [1,2-13C2]glucose units from glycogen (Fig. 5) informed that newly synthesized glycogen was mainly from exogenous glucose incorporation. The 13C NMR spectra confirmed that the overwhelming majority of glycogen was produced by direct phosphorylation of [1,2-13C2]glucose into glycogen rather than breakdown to the level of trioses followed by synthesis to glycogen (spectra not shown).
FIGURE 5.
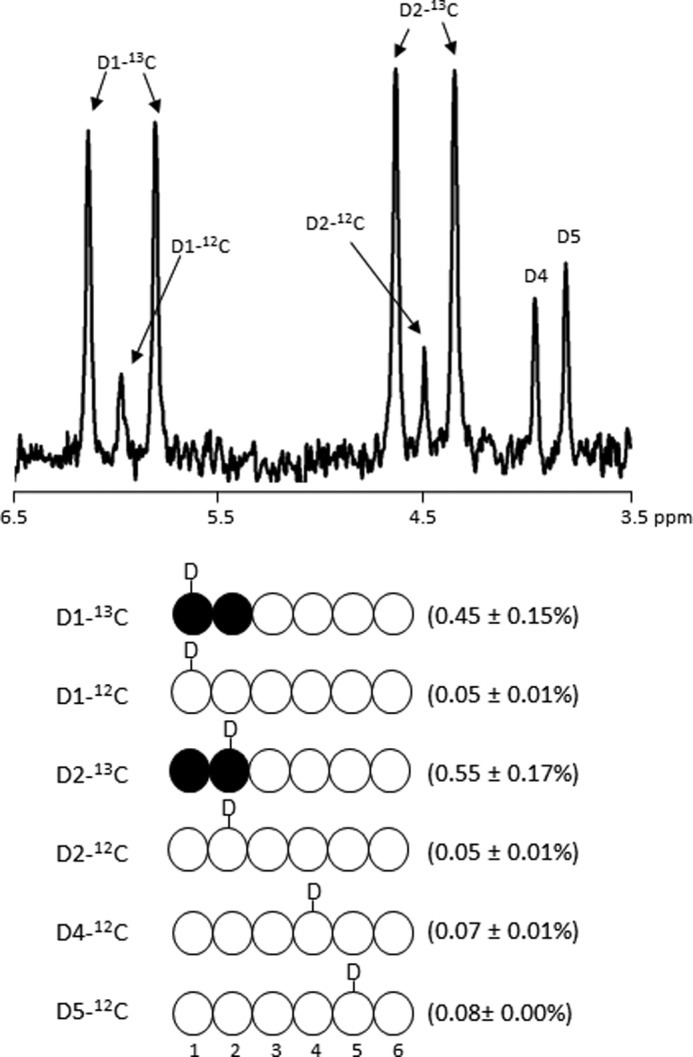
2H NMR spectrum of MAG derived from glycogen isolated from a heart supplied with [1,2-13C2]glucose and 2H2O. In the presence of 2H2O, deuterium (2H or D) enrichment is found in positions H1, H2, H4, and H5, but not in H3, H6R, and H6S. The absence of enrichment at H3 informs that DHAP did not contribute to carbons 1–3 of glucosyl units of glycogen through the standard glyconeogenic pathways (DHAP + GA3P → Fru-1,6-P2 → F6P → G6P → G1P → glycogen). The absence of 2H enrichment at the H6 position demonstrates the absence of glyconeogenesis from the citric acid cycle. The enrichment values shown in parentheses are average ± S.E. (n = 3). D1, D2, etc. indicate deuterium at glucosyl unit C1 position, C2 position, etc.
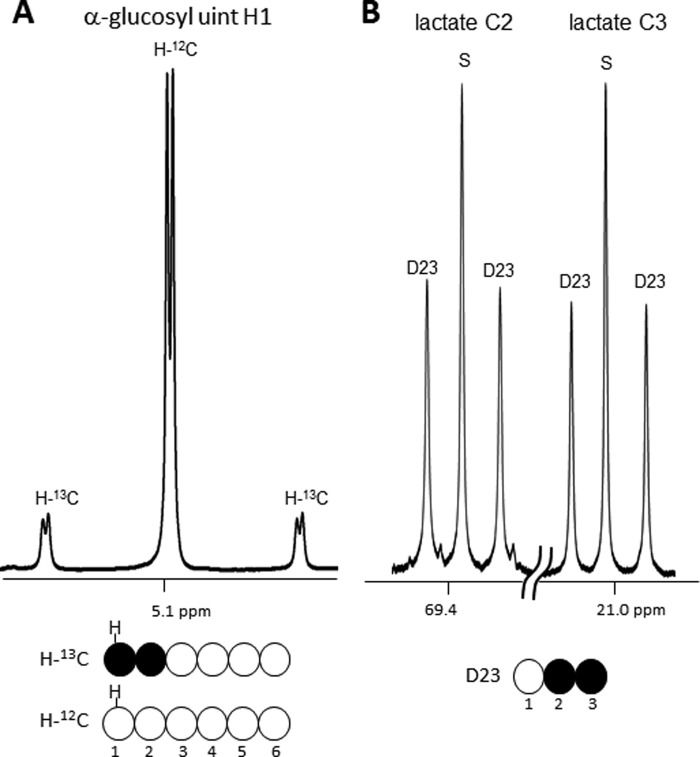
The fraction of newly synthesized glycogen that occurred during the perfusion period was determined by 1H NMR analysis of hydrolyzed glycogen (Fig. 6A). Fig. 6A shows the 1H NMR spectrum of glucose derived from glycogen with the H1 resonance showing the 13C satellite peaks characteristic of enrichment in carbon 1. Because the proton signals in positions 1 and 2 are both split due to JCH scalar coupling, the fraction of glycogen from perfusate [1,2-13C2]glucose is simply the area of the doublet relative to total signal. In this case, 23 ± 1% (n = 6) of total glycogen was derived from [1,2-13C2]glucose. This demonstrates that pretreatment with isoproterenol did not deplete the entire glycogen pool.
FIGURE 6.
1H NMR and 13C NMR spectra from a heart supplied with [1,2-13C2]glucose and 2H2O. A, 1H NMR spectrum of hydrolyzed glycogen (H1 region of α-glucosyl units) from an isolated heart. The doublet due to scalar carbon-proton coupling in C1 of α-glucosyl units indicates that 23% of the glycogen was synthesized by direct phosphorylation of [1,2-13C2]glucose. B, glycolysis of [1,2-13C2]glucose produces [2,3-13C2]lactate. In contrast, flux through the oxidative arm of the PPP results in loss of carbon 1 with subsequent generation of [3-13C1]lactate. The singlet components in lactate C2 and C3 were essentially identical so must be assigned to natural abundance lactate. This shows that there was no significant flux of [1,2-13C2]glucose through the oxidative portion of the PPP. D23, doublet arising from coupling of C2 with C3; S, singlet due to natural abundance.
Lactate in the effluent was also examined by 13C NMR. Glycolysis of [1,2-13C2]glucose will produce [2,3-13C2]lactate whereas any flux through the oxidative portion of the PPP would yield [3-13C1]lactate. The spectra shown in Fig. 6B provide direct evidence that no [3-13C1]lactate was formed as a result of glucose passing through the PPP. Here, the singlet components seen in the C2 and C3 resonances of lactate have identical intensities so must reflect only natural abundance levels of 13C. This demonstrates that the oxidative portion of the PPP was not active in these hearts.
DISCUSSION
The observation that carbons from [U-13C3]glycerol appear exclusively in the 4,5,6-positions of glucosyl units of newly formed glycogen in hearts perfused with a mixture of substrates confirms earlier observations that glycerol can indeed supply carbons to glycogen in the nonmammalian heart (4). The lack of 13C enrichment in the carbons 1, 2, or 3 demonstrates that conversion of glycerol to glycogen does not occur via the standard glyconeogenic pathways (DHAP + GA3P → Fru-1,6-P2 → F6P → G6P → G1P → glycogen) but rather must involve rearrangements in the nonoxidative portion of the PPP. The rather dramatic 13C asymmetry found in glycogen (Fig. 2) was quite surprising. The data shown here by 13C NMR isotopomer analysis of tissue glutamate showed that very little [U-13C3]glycerol (<1%) contributes to acetyl-CoA in the citric acid cycle even though [U-13C3]lactate is formed. Furthermore, 2H NMR of MAG derived from tissue glycogen in hearts exposed to 2H2O indicates that, within error of the measurement, none of the glucosyl units in glycogen comes from the level of the citric acid cycle (Fig. 5), as expected for this nonglyconeogenic tissue. Thus, the only reasonable explanation for the observations seen here must involve carbon rearrangements in the PPP catalyzed by transaldolase.
The PPP is described as having two components, the oxidative segment that produces NADPH and ribulose 5-phosphate from 6-phosphogluconate and the nonoxidative segment that interconverts sugar phosphates formed in glycolysis with the pentose phosphates. Transaldolase catalyzes exchange of 3-carbon sugar units, with the most widely accepted reaction being interconversion of GA3P and S7P to form erythrose 4-phosphate and F6P. However, it has also been reported that isolated transaldolase can directly exchange the carbons 4,5,6 of F6P with GA3P even in the absence of other enzymes or intermediates of the PPP (14). Transaldolase is active in the heart, although at a lower level compared with the kidney or liver (17, 18). The fact that the fraction of glycogen derived through the transaldolase reaction increased in the presence of insulin suggests that flux through this pathway may be physiologically significant. However, at least in normal myocardium the fraction of glycogen labeled with 13C from [U-13C3]glycerol was small, on the order of 5–6%, compared with the glycogen derived directly from exogenous glucose. Nevertheless, the fact that genetic defects in transaldolase have been associated with cardiomyopathy (19) suggests that transaldolase may play a significant role under some pathophysiological conditions.
Transaldolase was also reported to play a key role in redox homeostasis (20, 21), and oxidative damage is involved in many diseases including heart failure (22–24). The decreased [U-13C3]glycerol incorporation to glycogen via transaldolase in the presence of H2O2 (0.1 mm) supports the idea that it may play a role in regulation of redox. Reduced transaldolase activity was reported to correlate with increased activities of glucose-6-phosphate dehydrogenase (G6PD) and 6-phosphogluconate dehydrogenase (6PGD), and to increased level of reduced glutathione (GSH) in Jurkat human leukemic T cells (20). GSH is the primary antioxidant in most cells involved in reducing H2O2 to water (H2O). The NADPH supply from the oxidative segment of the PPP is essential in maintaining GSH, and it is produced at the levels of G6PD and 6PGD. However, the precise mechanism remains to be investigated as to how transaldolase plays a role in antioxidant defense along with the enzymes of the oxidative segment. We also perfused hearts with a higher concentration of H2O2 (0.225 mm), which reduced the enrichment of [4,5,6-13C3]glucosyl units dramatically by ∼10-fold, but the hearts functioned poorly, and the perfusions were discontinued before the planned 60-min duration (data not shown).
Although the pathophysiological relevance of transaldolase in heart is not well understood, its activity in the intact liver has been examined in some detail in part because it may result in overestimation of gluconeogenic flux using the 2H2O method introduced by Landau and his co-workers (25–28). The distribution of 2H in glucose, after the administration of 2H2O, is commonly used to measure the relative activity of gluconeogenesis and glycogenolysis in hepatic glucose production. In principle, 2H labeling should occur in three-carbon units derived from either glycerol or the citric acid cycle at the level of TPI. The hydrogen destined to become the H5 of glucose exchanges with any 2H in tissue water and thus reports the fraction of glucose derived from the sum of glycerol plus the citric acid cycle contribution, assuming equilibration in the TPI reaction. However, 2H labeling of glucose H5 through transaldolase exchange was reported to result in overestimation of gluconeogenesis (26). The distribution of 13C in urinary glucuronides was also reported to be sensitive to activity of liver transaldolase (28).
Flux through the PPP in heart is controversial in part because of difficulties related to the interpretation of 14CO2 release from [1-14C]glucose compared with 14CO2 release from [6-14C]glucose (29). Pfeiffer et al. concluded that PPP flux does not contribute significantly to NADPH production in the heart (30). However, Burns and Reddy found significant PPP flux in isolated cardiomyocytes (31). The current results provided no information about flux through the oxidative portion of the cycle but did illustrate the high activity of flux through the nonoxidative portion of the cycle.
After exposure of hearts to 2H2O, the absence of excess 2H in glucose H6 demonstrated that none of the trioses involved with glucose production passed through the citric acid cycle. The enrichment of H4 and H5 occurred through 2H incorporation at the levels of triose phosphates. The absence of enrichment at H3 informed that carbons 1–3 of glucosyl units of glycogen were not originated directly from DHAP through the standard glyconeogenic pathways, in which the hydrogen destined to become the H3 of glucosyl units exchanges with 2H in tissue water also occurs at the levels of triose phosphates, precisely originating from the H1 position of DHAP. The highest enrichment in H2 position of glucosyl units was due to 2H incorporation at the level of G6P ↔ F6P exchange. The H1 position was also substantially labeled by 2H, as observed previously in skeletal muscle glycogen (32). 2H labeling at H1 can occur by two processes. First, 2H incorporation at the level of mannose 6-phosphate isomerase (F6P ↔ mannose 6-phosphate) can lead to 2H enrichment of H1 position of glucose (33). The other possibility is intramolecular exchange of 1H in position 1 of F6P with 2H in position 2 of G6P (34).
In contrast to earlier reports (1–2), we found that oxidation of glycerol in the citric acid cycle did not contribute significantly to energy production using a perfusion medium containing physiological amounts of other unlabeled substrates. As noted previously (35, 36), oxidation of any particular substrate is very sensitive to the concentrations of alternative substrates. Consequently, differing results are almost certainly due to differences in the availability of other oxidizable substrates. In the current study, octanoate likely suppressed glycerol oxidation and enabled detection of asymmetric labeling in glycogen.
In summary, 13C labeling in carbons 4,5,6 of glucosyl units of glycogen in the heart after administration of [U-13C3]glycerol reflects flux through the following pathway: [U-13C3]glycerol → → [U-13C3]DHAP → [U-13C3]GA3P → [4,5,6-13C3]F6P → → [4,5,6-13C3]glucosyl units of glycogen. This observation is of interest in part because detection of transaldolase reactions may be important under conditions of oxidative stress.
Acknowledgments
We thank Charles Storey, Angela Milde, and Nicholas Carpenter for excellent technical help in the perfusion experiments.
This study was supported, in whole or in part, by National Institutes of Health Grants K01-DK078933 (to E. S. J.), RR-02584 (to C. R. M.), and HL-34557 (to A. D. S.).
- DHAP
- dihydroxyacetone phosphate
- F6P
- fructose 6-phosphate
- GA3P
- d-glyceraldehyde 3-phosphate
- G1P
- glucose 1-phosphate
- G6P
- glucose 6-phosphate
- G6PD
- glucose 6-phosphate dehydrogenase
- 2H2O
- deuterated water
- KHB
- Krebs-Henseleit bicarbonate
- MAG
- monoacetone glucose
- 6PGD
- 6-phosphogluconate dehydrogenase
- PPP
- pentose phosphate pathway
- S7P
- sedoheptulose 7-phosphate
- TPI
- triose phosphate isomerase.
REFERENCES
- 1. Gambert S., Héliès-Toussaint C., Grynberg A. (2005) Regulation of intermediary metabolism in rat cardiac myocyte by extracellular glycerol. Biochim. Biophys. Acta 1736, 152–162 [DOI] [PubMed] [Google Scholar]
- 2. Gambert S., Héliès-Toussaint C., Grynberg A. (2007) Extracellular glycerol regulates the cardiac energy balance in a working rat heart model. Am. J. Physiol. Heart Circ. Physiol. 292, H1600–1606 [DOI] [PubMed] [Google Scholar]
- 3. Malloy C. R., Sherry A. D., Jeffrey F. M. (1988) Evaluation of carbon flux and substrate selection through alternate pathways involving the citric acid cycle of the heart by 13C NMR spectroscopy. J. Biol. Chem. 263, 6964–6971 [PubMed] [Google Scholar]
- 4. Savina M. V., Wojtczak A. B. (1977) Enzymes of gluconeogenesis and the synthesis of glycogen from glycerol in various organs of the lamprey (Lampetra fluviatilis). Comp. Biochem. Physiol. B 57, 185–190 [DOI] [PubMed] [Google Scholar]
- 5. Lin E. C. (1977) Glycerol utilization and its regulation in mammals. Annu. Rev. Biochem. 46, 765–795 [DOI] [PubMed] [Google Scholar]
- 6. Rémésy C., Demigné C. (1983) Changes in availability of glucogenic and ketogenic substrates and liver metabolism in fed or starved rats. Ann. Nutr. Metab. 27, 57–70 [DOI] [PubMed] [Google Scholar]
- 7. Robinson J., Newsholme E. A. (1969) The effects of dietary conditions and glycerol concentration on glycerol uptake by rat liver and kidney-cortex slices. Biochem. J. 112, 449–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davis S. N., Galassetti P., Wasserman D. H., Tate D. (2000) Effects of gender on neuroendocrine and metabolic counterregulatory responses to exercise in normal man. J. Clin. Endocrinol. Metab. 85, 224–230 [DOI] [PubMed] [Google Scholar]
- 9. Brito N. A., Brito M. N., Bartness T. J. (2008) Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1445–1452 [DOI] [PubMed] [Google Scholar]
- 10. van der Merwe M. T., Schlaphoff G. P., Crowther N. J., Boyd I. H., Gray I. P., Joffe B. I., Lönnroth P. N. (2001) Lactate and glycerol release from adipose tissue in lean, obese, and diabetic women from South Africa. J. Clin. Endocrinol. Metab. 86, 3296–3303 [DOI] [PubMed] [Google Scholar]
- 11. van Rosendal S. P., Osborne M. A., Fassett R. G., Coombes J. S. (2009) Physiological and performance effects of glycerol hyperhydration and rehydration. Nutr. Rev. 67, 690–705 [DOI] [PubMed] [Google Scholar]
- 12. Massicotte D., Scotto A., Péronnet F., M'Kaouar H., Milot M., Lavoie C. (2006) Metabolic fate of a large amount of 13C-glycerol ingested during prolonged exercise. Eur. J. Appl. Physiol. 96, 322–329 [DOI] [PubMed] [Google Scholar]
- 13. Jin E. S., Burgess S. C., Merritt M. E., Sherry A. D., Malloy C. R. (2005) Differing mechanisms of hepatic glucose overproduction in triiodothyronine-treated rats vs. Zucker diabetic fatty rats by NMR analysis of plasma glucose. Am. J. Physiol. Endocrinol. Metab. 288, E654–662 [DOI] [PubMed] [Google Scholar]
- 14. Ljungdahl L., Wood H. G., Racker E., Couri D. (1961) Formation of unequally labeled fructose 6-phosphate by an exchange reaction catalyzed by transaldolase. J. Biol. Chem. 236, 1622–1625 [PubMed] [Google Scholar]
- 15. Jones J. G., Carvalho R. A., Sherry A. D., Malloy C. R. (2000) Quantitation of gluconeogenesis by 2H nuclear magnetic resonance analysis of plasma glucose following ingestion of 2H2O. Anal. Biochem. 277, 121–126 [DOI] [PubMed] [Google Scholar]
- 16. Jones J. G., Solomon M. A., Cole S. M., Sherry A. D., Malloy C. R. (2001) An integrated 2H and 13C NMR study of gluconeogenesis and TCA cycle flux in humans. Am. J. Physiol. Endocrinol. Metab. 281, E848–856 [DOI] [PubMed] [Google Scholar]
- 17. Heinrich P. C., Morris H. P., Weber G. (1976) Behavior of transaldolase (EC 2.2.1.2) and transketolase (EC 2.2.1.1) activities in normal, neoplastic, differentiating, and regenerating liver. Cancer Res. 36, 3189–3197 [PubMed] [Google Scholar]
- 18. James H. M., Williams S. G., Bais R., Rofe A. M., Edwards J. B., Conyers R. A. (1985) The metabolic production of oxalate from xylitol: activities of transketolase, transaldolase, fructokinase and aldolase in liver, kidney, brain, heart, and muscle in the rat, mouse, guinea pig, rabbit and human. Int. J. Vitam. Nutr. Res. Suppl. 28, 29–46 [PubMed] [Google Scholar]
- 19. Verhoeven N. M., Wallot M., Huck J. H., Dirsch O., Ballauf A., Neudorf U., Salomons G. S., van der Knaap M. S., Voit T., Jakobs C. (2005) A newborn with severe liver failure, cardiomyopathy and transaldolase deficiency. J. Inherit. Metab. Dis. 28, 169–179 [DOI] [PubMed] [Google Scholar]
- 20. Banki K., Hutter E., Colombo E., Gonchoroff N. J., Perl A. (1996) Glutathione levels and sensitivity to apoptosis are regulated by changes in transaldolase expression. J. Biol. Chem. 271, 32994–33001 [DOI] [PubMed] [Google Scholar]
- 21. Perl A., Hanczko R., Telarico T., Oaks Z., Landas S. (2011) Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase. Trends. Mol. Med. 17, 395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Belch J. J., Bridges A. B., Scott N., Chopra M. (1991) Oxygen free radicals and congestive heart failure. Br. Heart J. 65, 245–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hill M. F., Singal P. K. (1996) Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am. J. Pathol. 148, 291–300 [PMC free article] [PubMed] [Google Scholar]
- 24. Mallat Z., Philip I., Lebret M., Chatel D., Maclouf J., Tedgui A. (1998) Elevated levels of 8-iso-prostaglandin F2α in pericardial fluid of patients with heart failure: a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation 97, 1536–1539 [DOI] [PubMed] [Google Scholar]
- 25. Landau B. R., Wahren J., Chandramouli V., Schumann W. C., Ekberg K., Kalhan S. C. (1996) Contributions of gluconeogenesis to glucose production in the fasted state. J. Clin. Invest. 98, 378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bock G., Schumann W. C., Basu R., Burgess S. C., Yan Z., Chandramouli V., Rizza R. A., Landau B. R. (2008) Evidence that processes other than gluconeogenesis may influence the ratio of deuterium on the fifth and third carbons of glucose: implications for the use of 2H2O to measure gluconeogenesis in humans. Diabetes 57, 50–55 [DOI] [PubMed] [Google Scholar]
- 27. Basu R., Chandramouli V., Schumann W., Basu A., Landau B. R., Rizza R. A. (2009) Additional evidence that transaldolase exchange, isotope discrimination during the triose-isomerase reaction, or both occur in humans: effects of type 2 diabetes. Diabetes 58, 1539–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones J. G., Garcia P., Barosa C., Delgado T. C., Caldeira M. M., Diogo L. (2008) Quantification of hepatic transaldolase exchange activity and its effects on tracer measurements of indirect pathway flux in humans. Magn. Reson. Med. 59, 423–429 [DOI] [PubMed] [Google Scholar]
- 29. Goodwin G. W., Cohen D. M., Taegtmeyer H. (2001) [5-3H]Glucose overestimates glycolytic flux in isolated working rat heart: role of the pentose phosphate pathway. Am. J. Physiol. Endocrinol. Metab. 280, E502–508 [DOI] [PubMed] [Google Scholar]
- 30. Pfeifer R., Karl G., Scholz R. (1986) Does the pentose cycle play a major role for NADPH supply in the heart? Biol. Chem. Hoppe Seyler 367, 1061–1068 [DOI] [PubMed] [Google Scholar]
- 31. Burns A. H., Reddy W. J. (1977) Hexose monophosphate shunt in isolated cardiac myocytes from normal rats. Am. J. Physiol. 232, E570–573 [DOI] [PubMed] [Google Scholar]
- 32. Jin E. S., Sherry A. D., Malloy C. R. (2009) Evidence for reverse flux through pyruvate kinase in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 296, E748–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chandramouli V., Ekberg K., Schumann W. C., Wahren J., Landau B. R. (1999) Origins of the hydrogen bound to carbon 1 of glucose in fasting: significance in gluconeogenesis quantitation. Am. J. Physiol. Endocrinol. Metab. 277, E717–723 [DOI] [PubMed] [Google Scholar]
- 34. Malaisse W. J., Malaisse-Lagae F., Liemans V., Ottinger R., Willem R. (1990) Phosphoglucoisomerase-catalyzed interconversion of hexose phosphates: isotopic discrimination between hydrogen and deuterium. Mol. Cell. Biochem. 93, 153–165 [DOI] [PubMed] [Google Scholar]
- 35. Taegtmeyer H. (1984) Six blind men explore an elephant: aspects of fuel metabolism and the control of tricarboxylic acid cycle activity in heart muscle. Basic Res. Cardiol. 79, 322–336 [DOI] [PubMed] [Google Scholar]
- 36. Drake A. J., Haines J. R., Noble M. I. (1980) Preferential uptake of lactate by the normal myocardium in dogs. Cardiovasc. Res. 14, 65–72 [DOI] [PubMed] [Google Scholar]