Background: CART potentiates GSIS in pancreatic beta cells and confers neuroprotection.
Results: CART reduced beta cell apoptosis, induced phosphorylation of IRS, PKB, p44/42 MAPK, and CREB, and increased proliferation.
Conclusion: CART protects beta cells from glucotoxicity and activates kinases important for cell survival and proliferation.
Significance: CART is a novel islet peptide that improves glycemia and beta cell viability.
Keywords: Apoptosis, Beta Cell, Gene Expression, Glucose, Proliferation, Signal Transduction, Beta Cell Viability, CART peptide, Cocaine- and Amphetamine-regulated Transcript, Glucotoxicity
Abstract
Cocaine- and amphetamine-regulated transcript (CART) is an islet peptide that promotes glucose-stimulated insulin secretion in beta cells via cAMP/PKA-dependent pathways. In addition, CART is a regulator of neuronal survival. In this study, we examined the effect of exogenous CART 55–102 on beta cell viability and dissected its signaling mechanisms. Evaluation of DNA fragmentation and chromatin condensation revealed that CART 55–102 reduced glucotoxicity-induced apoptosis in both INS-1 (832/13) cells and isolated rat islets. Glucotoxicity in INS-1 (832/13) cells also caused a 50% reduction of endogenous CART protein. We show that CART increased proliferation in INS-1 (832/13) cells, an effect that was blocked by PKA, PKB, and MEK1 inhibitors. In addition, CART induced phosphorylation of CREB, IRS, PKB, FoxO1, p44/42 MAPK, and p90RSK in INS-1 (832/13) cells and isolated rat islets, all key mediators of cell survival and proliferation. Thus, we demonstrate that CART 55-102 protects beta cells against glucotoxicity and promotes proliferation. Taken together our data point to the potential use of CART in therapeutic interventions targeted at enhancing functional beta cell mass and long-term insulin secretion in T2D.
Introduction
The pancreatic beta cells are key regulators of glucose homeostasis, and type 2 diabetes (T2D)2 evolves due to the inability of the islets to adapt the beta cell mass to the increased insulin demand. Beta cell mass is governed by a fine balance between apoptosis and limited regenerative capacity (1–4). Regeneration is likely due to the replication of the existing beta cells as well as neogenesis from progenitors (5, 6). Dysregulation of any of these physiological processes is likely to cause a decrease in beta cell mass. Therefore, molecular therapies to restore functional beta cell mass are critical.
Recent studies have shown the anorexigenic regulatory peptide cocaine- and amphetamine-regulated transcript (CART) to be neuroprotective (7, 8) and to activate extracellular signal-regulated kinase (ERK) signaling in both primary cortical neurons and neuronal cell lines (7, 9, 10). CART is highly expressed in several islet cell types during development (11), and CART−/− mice exhibit impaired beta cell function (12). Further, CART regulates islet hormone secretion and glucose homeostasis and potentiates the effect of glucagon-like peptide-1 (GLP-1) on glucose-stimulated insulin secretion in INS-1 (832/13) cells and isolated islets from both normal and diabetic GK rats in a 3′-5′-cyclic adenosine monophosphate/protein kinase A (cAMP/PKA)-dependent manner (12, 13). Together these findings suggest that CART is important for normal beta cell function. Although studies to identify the CART receptor are ongoing (9, 10, 14), evidence from nucleus accumbens neurons and differentiated PC12 cells suggests CART to act via a G protein-coupled receptor (10, 15) that mediates inhibition of adenylate cyclase (Gi/o). However, our previous work in the INS-1 (832/13) cells shows that CART stimulates the production of cAMP (indicative of a Gsα-coupled receptor) in the presence of IBMX (13). In addition to its role in islet hormone secretion, CART has been implicated in the regulation of beta cell growth. This is based on our observations of increased CART in the beta cells of several rodent models of T2D (13) and in endocrine cells under different growth situations (11, 13, 16, 17). However, the role of CART in beta cell survival and the signaling mechanisms involved remain unknown.
In this study, we report that CART expression in INS-1 (832/13) beta cells is regulated by glucose. Importantly, exogenous CART protects beta cells from glucotoxicity via increased proliferation and reduced apoptosis. Furthermore, we show that CART, in addition to increasing cAMP production, induces phosphorylation of cAMP-response element-binding protein (CREB), insulin receptor substrate protein (IRS), protein kinase B (PKB)/forkhead box protein O1 (FoxO1), and p44/42-mitogen-activated protein kinase (p44/42 MAPK)/p90 ribosomal S6 kinase (p90RSK) in the INS-1 (832/13) cells and rat islets.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Rat CART (55–102) peptide (18, 19) was a kind gift from Dr. Lars Thim and Dr. Birgitte S. Wulff (Novo Nordisk A/S, Målöv, Denmark). RPMI 1640, cell culture supplements, High Capacity cDNA reverse transcription kit, precast NOVEX SDS polyacrylamide 4–12% Bis-Tris gels, lauryl dodecyl sulfate sample buffer, Click-iT® TUNEL Alexa Fluor® 488 kit, Hoechst 33342, ProLong® Gold antifade, Opti-MEM, and fetal bovine serum (FBS) were purchased from Invitrogen. The following were purchased as indicated: H89 (BioMol), MicroBCA protein assay and SuperSignal® West Pico (Pierce), NucleoSpin (Machery-Nagel), real-time PCR Primers (DNA Technologies A/S), Brilliant® II SYBR Green master mix (Agilent Technologies),Complete protease inhibitors, XTT reagent, and FuGENE HD (Roche Applied Science), Glosensor (Promega), and cAMP enzyme immunoassay (EIA) kit (Cayman Chemical Co. Most of the other reagents were obtained from Sigma unless otherwise mentioned. The following antibodies were used: HRP-conjugated anti-rabbit secondary antibody for Western blots; anti-phospho-PKB (Ser-473) and anti-phospho-IRS-1 (Tyr-612) (BIOSOURCE/Invitrogen); anti-IRS-2 (Upstate Biotech); anti-PKB, anti-phospho-p44/42 MAPK (Thr-202/Tyr-204), anti-p44/42 MAPK, anti-phospho-CREB (Ser-133), anti-CREB, anti-phospho-p90RSK (Ser-380), anti-RSK1/RSK2/RSK3, anti-phospho-FoxO1 (Ser-256), and anti-β-tubulin (Cell Signaling Technologies); anti-CART antibody (20) (a kind gift from Prof. Michael J. Kuhar (Emory University, Atlanta, GA)); guinea pig-anti-proinsulin antibody (Euro-Diagnostica AB); and Texas red anti-guinea pig secondary antibody (Jackson ImmunoResearch Laboratories).
INS-1 (832/13) Clonal Beta Cell Culture
INS-1 832/13 cells (passages 60–70) were cultured in monolayers as described previously (21). Briefly, cells were cultured at 37 °C and 5% CO2 in RPMI 1640 with GlutaMAXTM medium containing 2 g/liter (11.1 mm) d-glucose and supplemented with 10% FBS, 10 mm HEPES, 1 mm sodium pyruvate, 1% penicillin/streptomycin, and 50 μm β-mercaptoethanol.
Isolation of Rat Islets
Wild type female Sprague-Dawley rats (8–10 weeks old; 180–200 g) were purchased from Taconic (Ry, Denmark) and housed under standardized conditions. The study was approved by the Animal Ethics Committees in Lund and Malmö. Islets were isolated by collagenase digestion in Hanks' buffer and handpicked under a microscope. Isolated rat islets were maintained for 24 h in Complete RPMI 1640 medium with 10% FBS. Thereafter, islets were cultured for the respective experiments as specified below.
Hoechst and Terminal Deoxynucleotidyl Transferase-mediated Biotinylated UTP Nick End Labeling Staining (TUNEL)
INS-1 (832/13) cells were seeded on poly-d-lysine-coated 4-well chamber slides and allowed to adhere for 24 h. To examine the effect of glucose and serum on cell toxicity (Hoechst), cells were grown in different glucose concentrations with 10 or 1% FBS for 48 h. To test the effect of exogenous CART on glucotoxicity, cells were exposed to 5 and 25 mm glucose at 1% serum concentration for 48 h followed by exposure to CART 55–102 for an additional 48 h. Prior to processing for Hoechst and TUNEL, the cells were rinsed with PBS followed by fixation in ice-cold 4% buffered paraformaldehyde and permeabilization with 0.25% Triton X-100 in PBS for 20 min at room temperature.
DNA fragmentation was assayed with Click-iT® TUNEL Alexa Fluor® 488-based apoptosis detection system according to the manufacturer's instructions. Briefly, the fixed and permeabilized cells were incubated with terminal deoxynucleotidyl transferase reaction mixture for 1 h at 37 °C in a humidified chamber. No enzyme and DNase I-treated controls were included. Following the enzymatic reaction, samples were incubated in the dark with the Click-iT® reaction mixture for 30 min at room temperature. INS-1 (832/13) cells were stained with Hoechst and mounted using ProLong® Gold antifade.
Isolated rat islets (60–80) for each condition were cultured in RPMI 1640 medium containing 1% FBS with or without the addition of 100 nm CART 55–102 at 5 or 25 mm glucose for 48 h. The islets were then gently dispersed with 0.25% trypsin/EDTA into a single cell suspension and were centrifuged with a CytoSpin machine onto slides. Finally, cells were fixed and permeabilized as mentioned above. After TUNEL staining, to identify beta cells, rat islets were stained with a guinea pig-anti-pro-insulin antibody at a dilution of 1:2000 overnight at 4 °C. After secondary antibody staining, the samples were stained in the dark for 15 min with 10 μg/ml Hoechst 33342 at room temperature. They were rinsed in 1× PBS, and the slides were mounted using polyvinyl alcohol mounting media with 1,4-diazabicyclo[2.2.2]octane. Fluorescent cell nuclei were visualized in an Olympus BX60 (Tokyo, Japan) epifluorescence microscope at 200× (islets) and 400× (INS-1 cells) magnification, and images were acquired using a digital camera (Nikon DS-2Mv, Nikon, Tokyo, Japan). A total of 1500–2000 cells for each condition were counted to determine the percentage of abnormal nuclei (Hoechst) and the percentage of TUNEL-positive cells. For Hoechst staining, we quantified cells with condensed chromatin, crescent-shaped condensation around the periphery of the nucleus, or the entire nucleus appearing as one or a group of featureless, bright spherical beads as morphological abnormalities, i.e. dying cells. Thereafter, the total number of cells was assessed, and the ratio was calculated.
RNA Extraction and RT-Quantitative PCR
Cells were seeded in 6-well plates and grown at different glucose concentrations. RNA was extracted with TRIzol and purified using a NucleoSpin kit, and 1 μg of RNA was reverse-transcribed using High Capacity cDNA kit as per the manufacturer's instructions. Real-time quantitative PCR was performed using SYBR Green chemistry on Stratagene Mx3005P using the following primers: CART (forward, 5′-TGGATGATGCGTCCCATG, and reverse, 5′-TACTTCTTCTCATAGATCGGAATG), PPIA (forward,, 5′-AATGCTGGACCAAACACAAATG and reverse, 5′-CAATGCTCATGCCTTCTTTCAC), and HPRT-1 (forward, 5′-GTTGGATATGCCCTTGACTATAATG, and reverse, 5′-AGATTCAACTTGCCGCTGTC). CART gene expression was normalized to the reference genes, peptidylprolyl isomerase A (PPIA) and HPRT-1.
Immunoblotting
For immunoblotting, INS-1 (832/13) cells were seeded in 6-well plates and stimulated when they reached 80% confluency as indicated in the figure legends. Isolated rat islets (150–200) for each condition from a total of 12 rats were cultured in RPMI 1640 medium containing 1% FBS at 25 mm glucose for 48 h and stimulated as indicated in the figure legends. At the respective time points, they were rinsed with ice-cold Dulbecco's PBS, and protein was extracted in ice-cold Nonidet P-40 lysis buffer (50 mm Tris-HCl (pH 7.5), 1 mm EGTA, 1 mm EDTA, 1 mm sodium orthovanadate, 10 mm sodium-β-glycerophosphate, 50 mm sodium fluoride, 5 mm sodium pyrophosphate, 1 mm dithiothreitol (DTT), 1% (w/v) Nonidet P-40, and 0.27 m sucrose) with Complete protease inhibitor mixture. Lysates were centrifuged at 14,000 × g for 15 min at 4 °C, and the protein concentration was determined using MicroBCA.
The total cell lysates were heated at 95 °C for 2 min in lauryl dodecyl sulfate sample buffer. Total protein (10–30 μg) was resolved on precast NOVEX 4–12% Bis-Tris gels and transferred onto nitrocellulose membranes. The membranes were blocked for 30 min at room temperature in 50 mm Tris-HCl (pH 7.6), 137 mm NaCl, and 0.2% (w/v) Tween 20 (TBS-T) containing 10% (w/v) nonfat dried milk followed by overnight incubation at 4 °C with the indicated antibodies (1:1000) in TBS-T containing 5% (w/v) protease-free bovine serum albumin. For CART immunoblots, total cell lysates were resolved on precast mini-PROTEAN 16.5% Tris-Tricine gels, transferred onto PVDF membranes, and blocked with 1% (w/v) nonfat dry milk/Tris-buffered saline with Tween 20 (TBS-T). The blots were incubated overnight at 4 °C with C4 anti-CART antibody (1:1000 in 1% (w/v) nonfat dry milk/TBS-T). The bands were visualized by enhanced chemiluminescence using horseradish peroxidase-conjugated secondary antibody, and images were acquired with a Fuji LAS 1000 charge-coupled device camera. The band intensities were quantified using ImageJ software (National Institutes of Health). Although the CART 55–102 (∼5-kDa band) was normalized to β-tubulin, the phosphorylated protein bands were normalized to the respective total protein levels. Changes in protein levels are depicted as relative to the control condition and specified further in the respective figure legends.
XTT Assay
INS-1 (832/13) cells cultured in 96-well plates were exposed to medium containing 1% FBS and 25 mm glucose for 48 h. Cells were thereafter exposed to different kinase inhibitors (1 h; 5 μm H89, PKA inhibitor; 1 μm Akti1/2 and 5 μm MK-2206, PKB inhibitors; and 100 nm PD0325901, MEK1 inhibitor) with or without the addition of 100 nm CART 55–102 for an additional 48 h. Following this, 50 μl of XTT working reagent was added to each well and incubated for 4 h at 37 °C and 5% CO2. Absorbance at 450 and 630 nm was measured using a plate reader.
Cyclic AMP Enzyme Immunoassay (cAMP EIA)
INS-1 (832/13) cells were cultured in 24-well plates and stimulated with compounds when they were 80% confluent. Briefly, the cells were exposed to 25 μm IBMX for 20 min followed by the addition of 100 nm CART 55–102 and 10 μm forskolin for 30 min. At the end of the incubation, 200 μl of 0.1 m hydrochloric acid was added per well and incubated at room temperature for 20 min. Cell lysates were centrifuged at 14,000 × g for 20 min at 4 °C, and supernatants were collected and assayed using the cAMP EIA kit as per the manufacturer's instructions.
GloSensorTM cAMP Assay
INS-1 (832/13) cells were cultured in 35-mm dishes as mentioned above. At 50% confluency, cells were transiently transfected with pGlosensorTM_22F cAMP plasmid using FuGENE® HD in regular growth medium without antibiotics for 48 h. After transfection, the cells were equilibrated in 2% v/v dilution of the GlosensorTM cAMP reagent stock solution in serum-free RPMI 1640 with 10 mm HEPES for 20 min followed by a 10-min incubation with 25 μm IBMX at room temperature. Using the GloMax® 20/20 luminometer, a 5-min preread kinetic measurement was performed to monitor the base line. Subsequently, 100 nm CART or 100 nm GLP-1 was added to the cells, and kinetic traces were acquired over 35 min and then plotted on a linear scale.
Statistical Analysis
Comparisons between any two groups of data were performed using a Student's two-sample t test assuming unequal variance. A p value of ≤ 0.05 was considered statistically significant. Numerical data were expressed as mean ± S.D. with n signifying the number of experiments.
RESULTS
CART Is Expressed in INS-1 (832/13) Clonal Beta Cells and Is Regulated by Glucose
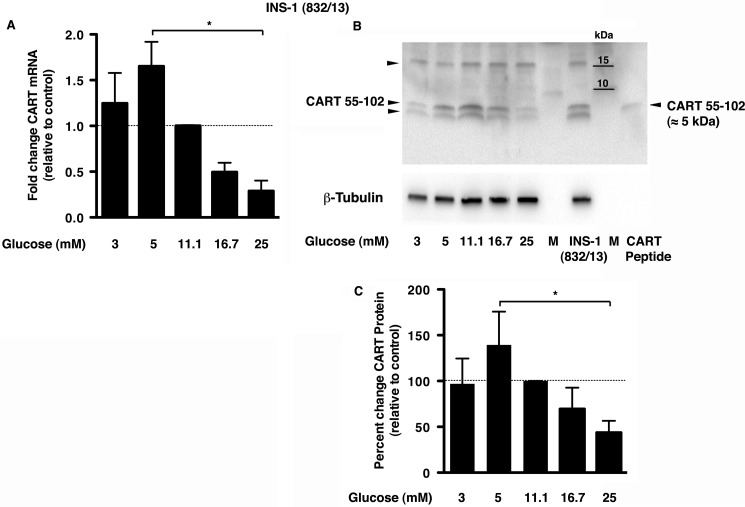
INS-1 (832/13) cells are rat insulinoma cells stably transfected with a plasmid coding for human proinsulin (21, 22). To determine endogenous CART expression in INS-1 (832/13) cells and its regulation by glucose, the cells were grown in medium supplemented with different glucose concentrations at 1% serum. Cells grown at the standard culture concentration of glucose (11.1 mm) were used as control. We found both CART transcript (65 ± 26%) and protein (39 ± 36%) levels to be maximal at 5 mm glucose and decreased at higher glucose concentrations (Fig. 1, A–C). At 25 mm glucose, CART mRNA and protein expression was significantly decreased when compared with the control (71 ± 11%; p = 0.008 and 56 ± 12%; p = 0.0001) and with 5 mm glucose (82 ± 11%; p = 0.01 and 68 ± 12%; p = 0.003). Immunoblotting with the anti-CART antibody in the INS-1 (832/13) cells revealed three bands (Fig. 1B, arrowheads), similar to observations by Dey et al. (23) in βTC3 cells. The ∼15-kDa band corresponds to proCART, the ∼5-kDa band corresponds to CART 55–102 (upper band of the doublet that is concurrent with the CART peptide used as positive control), and a lower band at ∼4.5 kDa corresponds to CART 62–102 peptide.
FIGURE 1.
CART is expressed in INS-1 (832/13) cells with CART mRNA and protein decreased at 25 mm glucose. A, CART mRNA expression determined by RT-quantitative PCR is shown relative to the control (INS-1 (832/13) cells grown at 11.1 mm glucose concentration). B and C, representative immunoblot (B) and quantitation (C) of CART protein in the INS-1 (832/13) cells at different glucose concentrations relative to control (11.1 mm glucose) and normalized to β-tubulin. Purified CART 55–102 peptide (10 ng) was used as a positive control (last lane), which corresponded to the upper band of the observed doublet. INS-1 (832/13), cells grown in regular medium (10% FBS, 11.1.mm glucose); M, molecular mass markers with kilodaltons (kDa) depicted. The arrowheads correspond to 15-kDa proCART, 5-kDa CART 55–102, and ∼4.5-kDa CART 62–102. The data in panels A and C are presented as mean ± S.D. (n = 3–4 experiments done in replicates of 2–3). *, p ≤ 0.05 between 5 mm and 25 mm.
CART Protects Beta Cells from Glucotoxicity
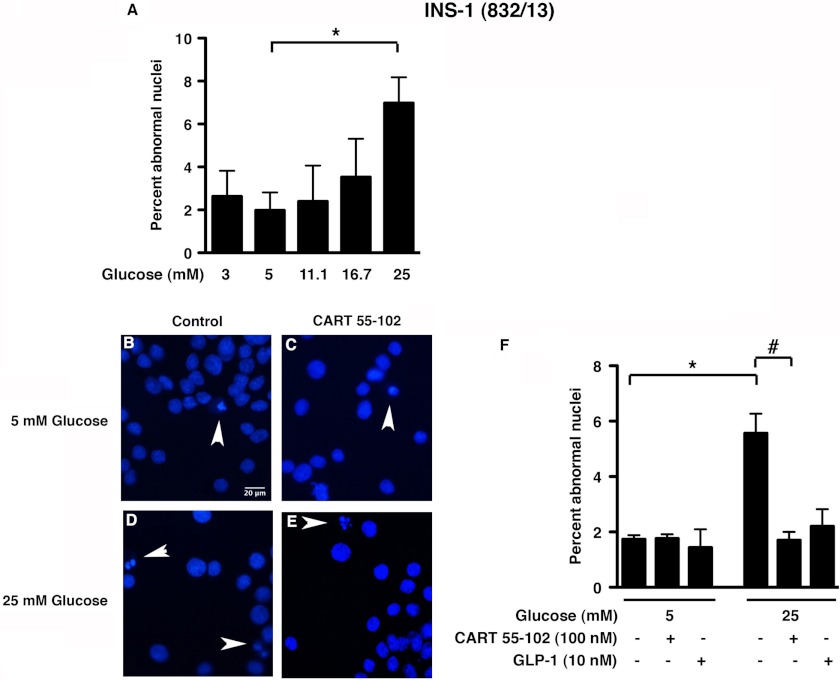
Given the altered beta cell morphology in the CART−/− mice (12), and having observed reduced expression of endogenous CART in the INS-1 (832/13) cells at 25 mm glucose, we wanted to investigate whether exogenous CART has a protective effect at this potentially cytotoxic glucose concentration. To confirm that 25 mm glucose was toxic to the INS-1 (832/13) cells, we exposed cells to varying glucose concentrations at 1% serum for 24 h. As expected, Hoechst staining revealed a higher percentage of abnormal nuclei at 25 mm when compared with 5 mm glucose (Fig. 2A; p = 0.0001). Taking these data together with the CART expression data (Fig. 1), we subsequently chose to use glucose concentrations of 5 and 25 mm at 1% serum and 100 nm CART (55–102) peptide. We chose this concentration of CART based on previous studies on the effect of CART on glucose-stimulated insulin secretion (13). Exposure of INS-1 (832/13) cells to CART decreased cell toxicity by 69% (p = 0.013) at 25 mm glucose, with no significant change observed at 5 mm glucose (Fig. 2, B–F).
FIGURE 2.
Beta cell death (detected as abnormal nuclei in Hoechst staining) is increased under glucotoxic conditions, and the addition of CART 55–102 rescues the INS-1 (832/13) cells. A, quantification of abnormal nuclei of cells grown in different glucose concentrations in the presence of 1% serum. B–E, representative images of cells exposed to 5 mm (B and C) or 25 mm glucose (D and E) with or without the addition of 100 nm CART 55–102. F, quantification of cells with abnormal nuclei in Hoechst staining. Random fields totaling 1500–2000 cells were counted for each condition. 10 nm GLP-1 was used as a positive control. Data are represented as mean ± S.D. of n = 3 independent experiments. *, p ≤ 0.05, comparing 5 and 25 mm glucose, and #, p ≤ 0.05, comparing 25 mm glucose with and without CART. Scale bar = 20 μm
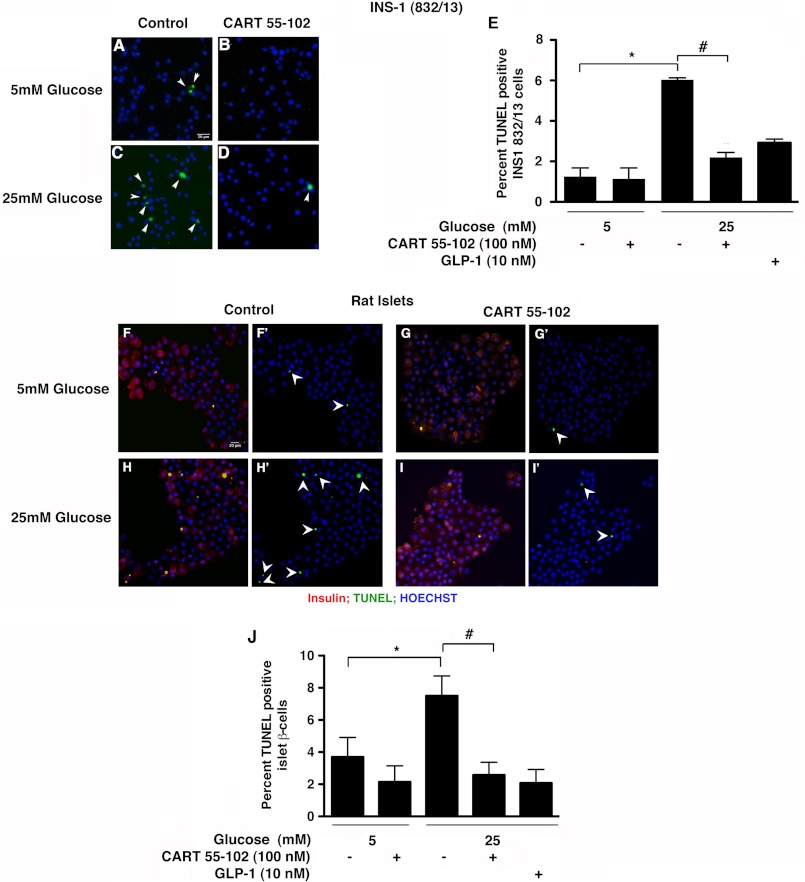
We next evaluated DNA fragmentation as a measure of apoptosis using TUNEL assay. We used GLP-1 as a positive control because previous studies have shown GLP-1 to protect against glucotoxicity in beta cells (24). The addition of CART decreased glucotoxicity-induced apoptosis by 63% (p = 0.03) in the INS-1 (832/13) cells (Fig. 3, A–E) in accordance with Hoechst staining. This effect of CART was comparable with that of GLP-1 (Fig. 3E). We next examined the effect of CART in isolated rat islets using TUNEL. CART prevented beta cell apoptosis in the rat islets by 66% (p = 0.001) at 25 mm glucose (Fig. 3, F–J), thus corroborating the effects observed in INS-1 (832/13) cells.
FIGURE 3.
CART 55–102 prevents beta cell apoptosis in both INS-1 (832/13) cells and isolated rat islets. Representative images of INS-1 (832/13) cells (A–D) and rat islets (F–I′) cultured in 5 mm (A, B, F, F′, G, and G′) or 25 mm glucose (C, D, H, H′, I, and I′) with or without the addition of CART 55–102. (F, G, H, and I) show the merged image of insulin (red), TUNEL (green), and Hoechst for nuclei (blue). F′, G′, H′, and I′ show the same images with TUNEL and Hoechst staining. E and J), quantification of TUNEL-positive INS-1 (832/13) cells (E) and rat beta cells (J). 10 nm GLP-1 was used as a positive control. Rat islet beta cells were identified using anti-proinsulin antibody. Random fields totaling 1500–2000 cells were counted. The data are presented as mean ± S.D. of n = 2–4 independent experiments. *, p ≤ 0.05, 5 versus 25 mm glucose, and #, p ≤ 0.05, comparing 25 mm glucose with and without CART. Scale bar = 20 μm
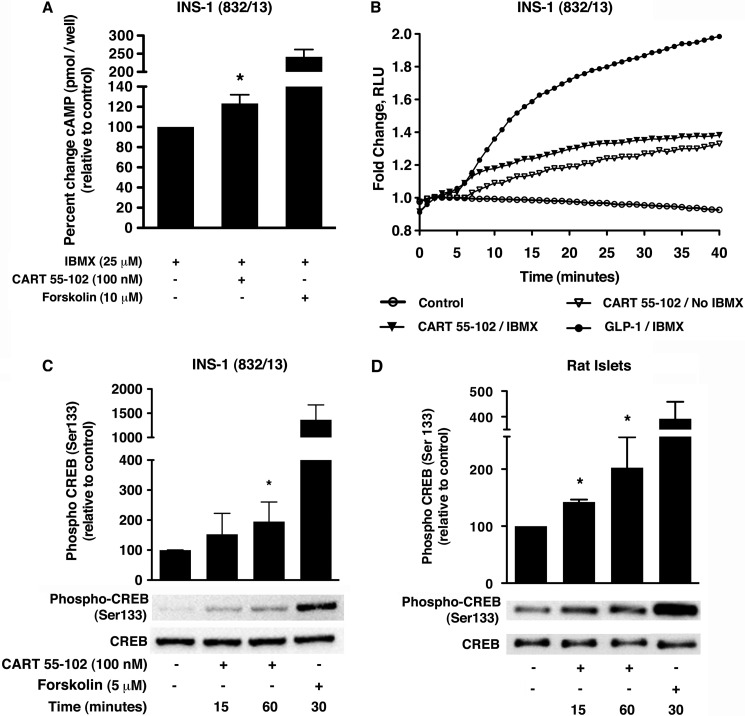
CART Elevates cAMP and Increases CREB Phosphorylation in Beta Cells
We have previously shown exogenous CART to raise intracellular cAMP and to potentiate glucose-stimulated insulin secretion in a PKA-dependent manner in INS-1 (832/13) cells in the presence of 100 μm IBMX (13). In this study, we aimed at providing further evidence for the stimulatory effect of CART on cAMP levels using two independent assays. cAMP levels were monitored at steady state by cAMP EIA (Fig. 4A) and in real time by the GloSensorTM cAMP assay (Fig. 4B). Forskolin and GLP-1 were used as positive controls. We observed an increase in cAMP production by CART in the presence of 25 μm IBMX in both assays, reflecting the activation of adenylate cyclase. Importantly, CART also increased cAMP in the absence of IBMX. Based on these observations and our previous studies (13), we hypothesized that CART, via enhanced cAMP production, regulates downstream effectors including PKA and MAPK. The cAMP-PKA-CREB signaling pathway is known to be important in the physiological function of beta cells including efficient glucose sensing, insulin signaling, and survival (25, 26). We therefore examined whether CART activates CREB by inducing its phosphorylation at Ser-133 in the beta cells. In the INS-1 (832/13) cells, chronic exposure (48 h) to 25 mm glucose followed by acute stimulation with CART did not affect CREB phosphorylation (data not shown), but in the rat islets, CREB phosphorylation increased by 42 ± 2% (p = 0.003) and 102 ± 31% (p = 0.03) at 15 and 60 min, respectively (Fig. 4D). However, when the INS-1 (832/13) cells were glucose-starved for 2 h in Krebs-Ringer buffer with HEPES (KRB-H with 2.8 mm glucose) following 48 h of culture in 25 mm glucose and then stimulated with CART for 15 and 60 min, we observed that CREB phosphorylation was elevated by 95 ± 65% (p = 0.03) at the latter time point (Fig. 4C).
FIGURE 4.
CART 55–102 increases intracellular cAMP and induces phosphorylation of CREB at Ser-133 in INS-1 (832/13) cells and rat islets. A, the intracellular cAMP levels were assessed by EIA using 10 μm forskolin as positive control. The data are presented as mean ± S.D. of n = 3–4 independent experiments each performed in replicates of 3–4 wells per condition. The addition of 100 nm CART 55–102 elevated cAMP in the presence of IBMX, *, p ≤ 0.05 when compared with no CART control. B, kinetic response of the pGloSensorTM-22F biosensor variant after transient expression. 100 nm CART 55–102 elevated cAMP both in the presence and in the absence of IBMX. The data are shown as a representative trace from two experiments each performed in quadruplicates for each compound. RLU, relative light units. C and D, CART 55–102-mediated phosphorylation of CREB at Ser-133 in INS-1 (832/13) cells (C) exposed to glucotoxic conditions followed by 2 h of starvation in KRB-H buffer and in isolated rat islets (D). A representative immunoblot is shown with 5 μm forskolin as a positive control. The bar graph represents quantification of the relative levels of phospho-CREB to total CREB. The data are represented as mean ± S.D. (n = 3–6 experiments) with each condition performed in replicates (2–3) for INS-1 (832/13) and isolated rat islets. *, p ≤ 0.05, when compared with no CART control.
CART Activates IRS Proteins, PKB/FoxO1, and p44/42 MAPK/p90RSK
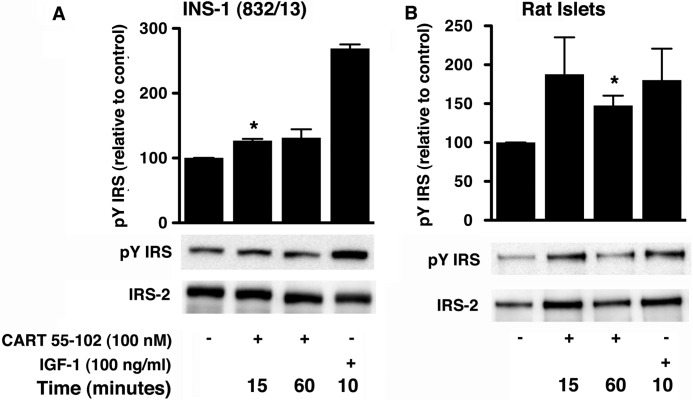
Previous studies have implicated IRS-2 and its tyrosine phosphorylation in the regulation of beta cell mass and survival (27–29). Using the anti-phospho-Tyr-612 IRS-1 antibody, following 48 h of chronic glucotoxicity and an acute exposure to CART in the INS-1 (832/13) cells, there was an increase in tyrosine phosphorylation after 15 min (27 ± 2%; p = 0.04; Fig. 5A). In the rat islets, there was a significant increase at 60 min (35 ± 5%; p = 0.01; Fig. 5B).
FIGURE 5.
CART 55–102 induces tyrosine phosphorylation of IRS in INS-1 (832/13) cells (A) and isolated rat islets (B) exposed to 48 h glucotoxic conditions followed by stimulation with CART 55–102 for indicated times. Representative immunoblots are shown with IGF-1 as positive control. The bar graphs represent quantification of relative levels of phospho-Tyr IRS (pY IRS) normalized to total IRS-2. The data are presented as mean ± S.D. of n = 3 independent experiments with each condition performed in replicates (2–3). *, p ≤ 0.05, when compared with no CART control.
PKB-mediated signaling pathways have been implicated in preventing lipo-, gluco-, and glucolipotoxicity in the parental INS-1 as well as in the INS-1 (832/13) cells (24, 30). Moreover, CART activated MAPKs in rodent pituitary-derived cells (9) and conferred neuroprotection against cerebral ischemia (7). These findings prompted us to investigate whether CART activated these protein kinases in beta cells.
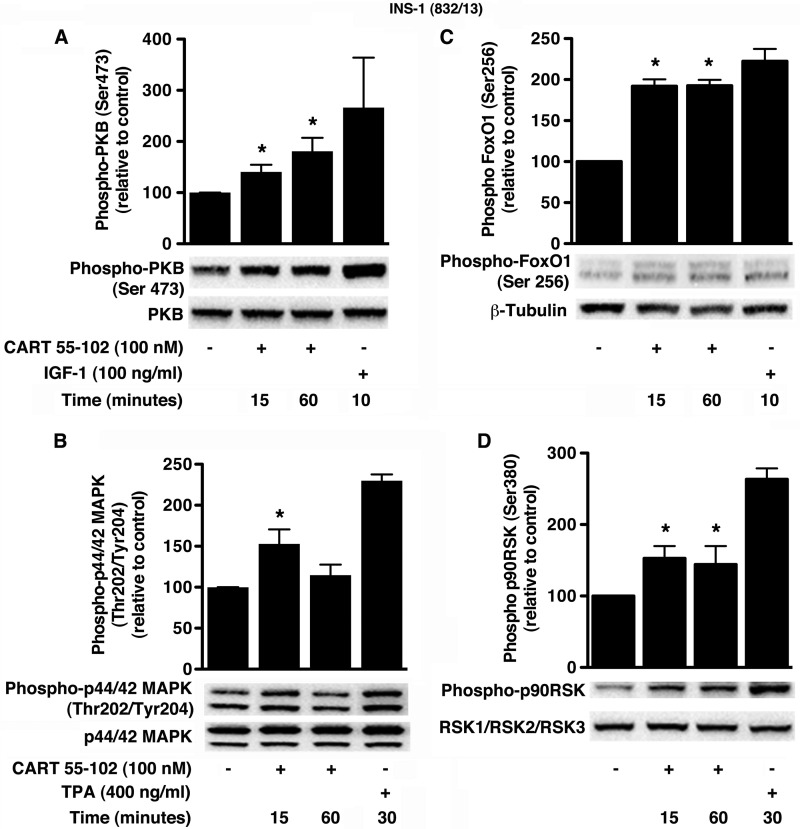
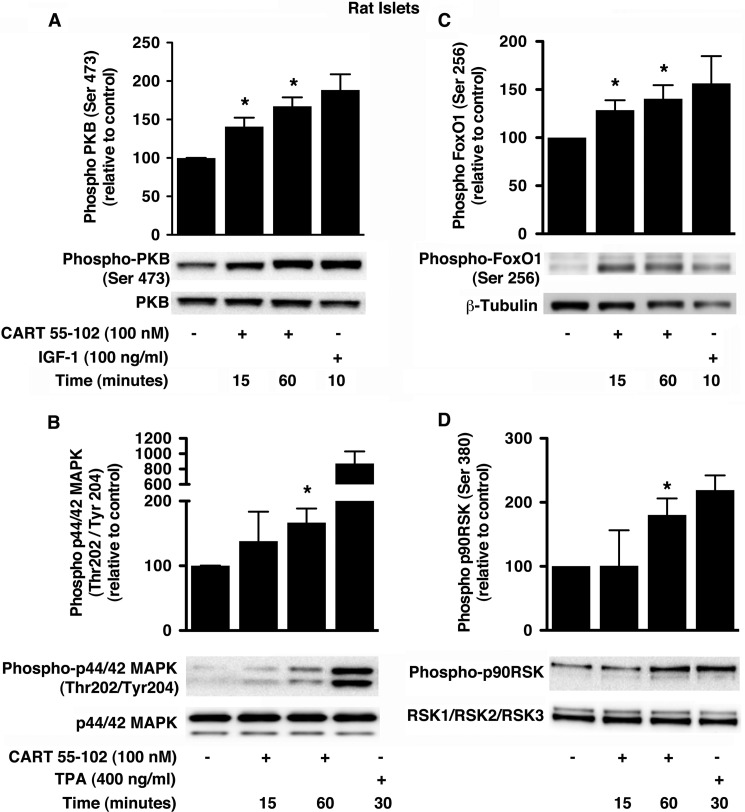
In INS-1 (832/13) cells and isolated rat islets cultured in glucotoxic conditions, the addition of CART resulted in elevated phospho-PKB (Figs. 6A and 7A) and phospho-p44/42 MAPK (Figs. 6B and 7B). As positive controls for PKB and p44/42 MAPK activation, we used 100 ng/ml IGF-1 and 400 ng/ml 12-O-tetradecanoylphorbol-13-acetate, respectively. The phosphorylation of PKB at Ser-473 was significantly increased at 15 min in INS-1 (832/13) cells (40 ± 14%; p = 0.037) and rat islets (40 ± 6%; p = 0.027). This was further elevated at 60 min by 81 ± 26%; p = 0.034 (INS-1 (832/13) cells) and 67 ± 6%; p = 0.01 (rat islets). On the other hand, p44/42 MAPK phosphorylation at Thr-202/Tyr-204 was elevated by 53 ± 18%; p = 0.035 after 15 min in INS-1 (832/13) cells with no significant change at 60 min. However, in rat islets, we observed a 67 ± 13% (p = 0.034) increase in p44/42 MAPK phosphorylation at 60 min but no change at the earlier time point. Several protein substrates downstream of PKB and p44/42 MAPK are known to promote beta cell survival and/or proliferation (31, 32). Activation of PKB and subsequent inactivation of FoxO1 are implicated in glucose-dependent insulinotropic polypeptide-mediated beta cell survival (33). In addition, in beta cells, p44/42 MAPK is known to activate p90RSK in response to GLP-1, and this signaling is suggested to mediate survival (34). Indeed, further investigation showed that CART induced phosphorylation of both FoxO1 at Ser-256 (Figs. 6C and 7C) and p90RSK at Ser-380 (Figs. 6D and 7D). The phospho-FoxO1 (Ser-256) antibody (Cell Signaling Technology) recognizes a doublet that was quantified. In the INS-1 (832/13) cells, the temporal increase in phosphorylation of FoxO1 (92 ± 8% at 15 and 60 min; p ≤ 0.04) and p90RSK (53 ± 17% at 15 min; p = 0.03) was concomitant to that of PKB and p44/42 MAPK, respectively. Importantly, we observed similar temporal increases in the phosphorylation of FoxO1 (28 ± 6%; p < 0.04 and 40 ± 8%; p = 0.04 at 15 and 60 min, respectively) and p90RSK (80 ± 14%; p = 0.03 at 60 min) in the rat islets.
FIGURE 6.
CART 55–102 activates PKB- and MAPK-dependent pathways in INS-1 (832/13) cells exposed to glucotoxic conditions. A–D, representative immunoblots are shown with respective positive controls, IGF-1 (A and C) and 12-O-tetradecanoylphorbol-13-acetate (TPA) (B and D). The bar graphs represent quantification of relative levels of phospho-PKB (Ser-473) (A), phospho-FoxO1 (Ser-256) (C), phospho-p44/42 MAPK (Thr-202/Tyr-204) (B), and phospho-p90RSK (Ser-380) (D). All phosphorylated protein bands were normalized to that of the corresponding total protein. The data are presented as mean ± S.D. of n = 3–5 independent experiments with each condition performed in replicates (2–3). *, p ≤ 0.05, when compared with no CART control.
FIGURE 7.
CART 55–102 activates PKB- and MAPK-dependent pathways in isolated rat islets exposed to glucotoxic conditions. A–D, representative immunoblots are shown with respective positive controls IGF-1 (A and C) and 12-O-tetradecanoylphorbol-13-acetate (TPA) (B and D). The bar graphs represent quantification of relative levels of phospho-PKB (Ser-473) (A), phospho-FoxO1 (Ser-256) (C), phospho-p44/42 MAPK (Thr-202/Tyr-204) (B), and phospho-p90RSK (Ser-380) (D). All phosphorylated protein bands were normalized to that of the corresponding total protein. The data are presented as mean ± S.D. of n = 3 independent experiments with each condition performed in duplicates. *, p ≤ 0.05, when compared with no CART control.
CART Increases Proliferation in INS-1 (832/13) Cells via PKA-, PKB-, and MAPK-dependent Pathways
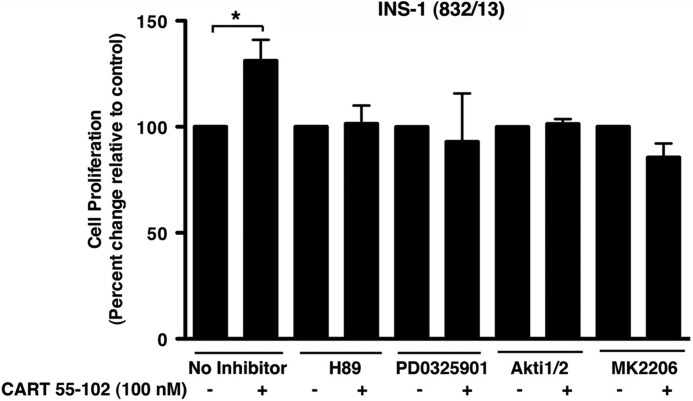
After having established that CART promotes cell viability and reduces apoptosis under glucotoxic conditions, we examined the effect of CART on proliferation. At 25 mm glucose, the addition of CART increased cell proliferation by 31 ± 4% (p = 0.002) in the INS-1 (832/13) cells (Fig. 8). CART had no effect at 5 or 11.1 mm glucose (data not shown). Having established that CART activates PKB, p44/42 MAPK, and CREB in the INS-1 (832/13) cells, we next studied whether these pathways are required for the effect of CART on proliferation. Indeed we observed that CART-mediated proliferation in INS-1 (832/13) was prevented by the addition of pharmacological inhibitors of PKA (H89), PKB (Akti1/2 and MK-2206), and MEK1 (PD-0325901) (Fig. 8).
FIGURE 8.
CART 55–102 increases cell proliferation in INS-1 (832/13) cells via PKA-, MAPK-, and PKB-dependent pathways. The addition of 100 nm CART 55–102 increased proliferation in INS-1 (832/13) cells cultured in 25 mm glucose for 48 h. The addition of pharmacological inhibitors of PKA (H89), MEK1 (PD0325901), and PKB (Akti1/2 and MK2206) abolished the proliferative effect of CART. The data are presented as mean ± S.D. of n = 3–5 independent experiments each performed in replicates of 6–8 wells per condition. *, p ≤ 0.05 when compared with no CART control for each inhibitor.
DISCUSSION
T2D is characterized by hyperglycemia, impaired islet hormone secretion, and reduced beta cell survival (35, 36). Here we show that the novel islet peptide CART protects against glucotoxicity-induced apoptosis and increases cell proliferation in beta cells. Further, we show that CART activates key signaling molecules in cell survival and proliferation including CREB, IRS proteins, PKB, and p44/42 MAPK.
Interestingly, we observed reduced CART mRNA and protein expression at high glucose concentrations. These findings gain support from a previous report on parental INS-1 cells (37). We believe that the reduced endogenous levels of beta cell CART under glucotoxic conditions in the INS-1 (832/13) cells could play a role in the observed reduced viability, thus pointing to the importance of CART in normal beta cell function. Our present findings that CART regulates beta cell viability is in line with previous observations that CART is up-regulated in endocrine cells in different situations of cell growth, for example, during rodent embryonic development (11), in beta cells of rodent models of T2D (13), as well as in endocrine tumors (16, 17). Our data from Hoechst and TUNEL staining show that glucotoxicity-mediated beta cell apoptosis was reduced by CART, thus promoting cell viability.
CART regulates glucose homeostasis and potentiates GLP-1-mediated glucose-stimulated insulin secretion in INS-1 (832/13) cells and isolated islets from both wild type and GK rats in a cAMP/PKA-dependent manner (12, 13). Concurrent with these observations, we report that CART elevated cAMP in the presence of IBMX in the INS-1 (832/13) cells. This indicates that CART, similar to GLP-1, signals through a Gs-coupled G protein-coupled receptor in beta cells. It is noteworthy that the GloSensor cAMP assay used in this study, in comparison with the cAMP EIA, accurately tracks both the magnitude and the kinetics of cAMP alterations. When discussing CART-mediated cAMP signaling, it is important to note that although the CART receptor is still unidentified, it is currently considered to be coupled to pertussis-sensitive cAMP reduction rather than stimulation (9, 14). On the contrary, our results indicate the beta cell CART receptor to be coupled to Gαs rather than Gαi/o, suggesting the presence of more than one CART receptor, which warrants further investigation.
Previous studies have shown glucose and GLP-1 receptor agonists to regulate beta cell mass and survival in an IRS-2-dependent manner (38–41). Transgenic mice overexpressing Irs2 in pancreatic beta cells are protected from development of T2D (27), whereas Irs2 knock-out mice are characterized by reduced beta cell mass and development of T2D (42, 43). Further, the tyrosine phosphorylation of IRS-2 has been implicated in increased beta cell growth and survival (28). In light of this, our observation that CART induces the tyrosine phosphorylation of IRS in beta cells following a glucotoxic insult provides further support for a role of CART in cell viability. The antibody used was raised against an epitope of human IRS-1 containing Tyr-612 (the sequence being conserved in mouse, rat, and chicken). This site, and its surrounding sequence, is, however, conserved in IRS-2, and given the predominance of IRS-2 in pancreatic beta cells (29, 43–47), it is likely that the anti-pY612 IRS-1 antibody also recognizes the IRS-2 isoform. The observation that acute CART exposure resulted in increased levels of total IRS-2 protein (data not shown) is likely related to altered protein stability. IRS proteins are known to be regulated via proteasomal degradation, for example, during prolonged treatment with insulin/IGF-1, and potentially also glucose. It is possible that CART inhibits signaling pathways that trigger this degradation.
Once phosphorylated, IRS proteins are known to interface with SH2 domain-containing proteins and further activate other tyrosine kinases, thus initiating signaling cascades (29, 48, 49). A number of growth factor- and glucose-regulated protein kinase cascades have been implicated in beta cell survival signaling, including PI3K/PKB, Ras/MAPK/RSK, and cAMP/PKA/CREB pathways (24, 32–34, 50, 51). The addition of exogenous CART following exposure of INS-1 (832/13) cells and rat islets to high glucose activated PKB and p44/42 MAPK, two central mediators of cell proliferation and anti-apoptotic action. The observation of CART-mediated phosphorylation of FoxO1 at Ser-256, a substrate for PKB, is suggestive of a PKB/FoxO1 survival cascade in CART-mediated cytoprotection of beta cells. The observed effects of CART on these pathways are reminiscent of the effects of both GLP-1 and glucose-dependent insulinotropic polypeptide (24, 30, 33, 52–55).
We also showed that CART induces phosphorylation of p44/42 MAPK at Thr-202/Tyr-204, a key modulator of both beta cell proliferation and survival. This is in line with prior studies showing CART to activate the MAPK pathway and promoting subsequent neuroprotection (7, 9). Further, we observed the activation of p90RSK at Ser-380, a downstream target of p44/42 MAPK, and a regulator of cell proliferation. In fact, Quoyer et al. (34), have shown GLP-1 to be anti-apoptotic in beta cells through the activation of p44/42 MAPK and p90RSK. In addition, the temporal phosphorylation of FoxO1 and p90RSK is similar to that of PKB and p44/42 MAPK, respectively, indicating the linearity of these signaling molecules downstream of CART. Importantly, we show further evidence for the role of the MAPK and PKB signaling in CART-mediated cell proliferation with the use of pharmacological inhibitors of these kinases.
The cAMP/PKA/CREB pathway has been implicated in cell proliferation with cAMP having an inhibitory role in some cell types, but enhancing proliferation in others (51). In our study, proliferation was blocked by H89, a PKA inhibitor, suggesting that cAMP/PKA signaling might be relevant to cell proliferation in the INS-1 (832/13) cells. Furthermore, our observation that CART activated CREB in the INS-1 (832/13) cells and rat islets is corroborated by findings from prior studies in neuronal models (15, 56, 57). Because glucose by itself is known to trigger these signaling pathways in the beta cells (32, 58, 59), we confirmed that CART indeed activates CREB by starving the cells for an additional 2 h in KRB-H with 2.8 mm glucose after 48 h of glucotoxicity before stimulating with CART. Further, evidence of this pathway being involved in beta cell proliferation comes from in vivo studies showing that beta Gs knock-out mice exhibit reduced beta cell mass due to decreased cell proliferation (60). Studies in mouse beta cells show that IRS-2 is regulated in a CREB-dependent manner promoting cell survival (26, 61, 62). Considering the positive effect of CART on CREB observed in INS-1 832/13 cells and isolated rat islets, it is possible that long-term treatment with CART (such as in our survival assays) indeed leads to a CREB-dependent induction of IRS-2 expression, thus suggesting a link between CREB and IRS-2 in CART action.
Our primary objective in this study was to elucidate the role of CART in regulating beta cell viability and its signaling mechanisms. We demonstrate that CART prevents beta cell apoptosis and increases proliferation under glucotoxic conditions. Further, we show that CART treatment leads to the activation of key pro-survival molecules including cAMP, CREB, IRS proteins, PKB, FoxO1, p44/42 MAPK, and p90RSK in INS-1 (832/13) clonal beta cells and isolated rat islets. These results, together with our previous work (12, 13), suggest a pleiotropic role of CART in beta cells including regulation of insulin secretion, beta cell protection, and proliferation. Our work also indicates the presence of more than one CART receptor subtype. Progressive deterioration and loss of beta cell function have been attributed to hyperglycemia in T2D. Therefore, molecules including CART that reduce beta cell glucotoxicity and promote maintenance of functional beta cell mass may present as novel therapeutics.
Acknowledgments
We thank Ann Helen Thorén-Fischer for technical assistance and Dr. Srikanth Ranganathan for technical assistance, reviewing, and editing of the manuscript.
This work was supported by grants from the Swedish Medical Research Council (Project Number 2008-4216), the Novo Nordisk Foundation, the European Foundation for the Study of Diabetes and Merck Sharpe and Dohme, the Medical Faculty at Lund University, The Crafoord Foundation, The Gyllenstiernska Krapperup Foundation, The Tore Nilsson Foundation, The Åke Wibergs Foundation, The Fredrik and Ingrid Thuring Foundation, The Magnus Bergwall Foundation, and The Albert Påhlsson Foundation.
- T2D
- type 2 diabetes
- CART
- cocaine- and amphetamine-regulated transcript
- GLP-1
- glucagon like peptide-1
- CREB
- cAMP-response element-binding protein
- IRS
- insulin receptor substrate
- p90RSK
- p90 ribosomal S6 kinase
- FoxO1
- forkhead box protein O1
- TUNEL
- terminal deoxynucleotidyl transferase dUTP nick end labeling
- IBMX
- isobutylmethylxanthine
- EIA
- enzyme immunoassay
- Bis-Tris
- 2-(bis(2-hydroxyethyl)amino)-2-(hydroxymethyl)propane-1,3-diol
- KRB-H
- Krebs-Ringer buffer with HEPES
- XTT
- (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide)
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- RSK
- ribosomal s6 kinase.
REFERENCES
- 1. Bonner-Weir S., Li W. C., Ouziel-Yahalom L., Guo L., Weir G. C., Sharma A. (2010) Beta-cell growth and regeneration: replication is only part of the story. Diabetes 59, 2340–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karaca M., Magnan C., Kargar C. (2009) Functional pancreatic beta-cell mass: involvement in type 2 diabetes and therapeutic intervention. Diabetes Metab. 35, 77–84 [DOI] [PubMed] [Google Scholar]
- 3. Perl S., Kushner J. A., Buchholz B. A., Meeker A. K., Stein G. M., Hsieh M., Kirby M., Pechhold S., Liu E. H., Harlan D. M., Tisdale J. F. (2010) Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J. Clin. Endocrinol. Metab. 95, E234–E239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ritzel R. A. (2009) Therapeutic approaches based on beta-cell mass preservation and/or regeneration. Front. Biosci. 14, 1835–1850 [DOI] [PubMed] [Google Scholar]
- 5. Butler A. E., Janson J., Bonner-Weir S., Ritzel R., Rizza R. A., Butler P. C. (2003) Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52, 102–110 [DOI] [PubMed] [Google Scholar]
- 6. Dor Y., Brown J., Martinez O. I., Melton D. A. (2004) Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41–46 [DOI] [PubMed] [Google Scholar]
- 7. Jia J., Chen X., Zhu W., Luo Y., Hua Z., Xu Y. (2008) CART protects brain from damage through ERK activation in ischemic stroke. Neuropeptides 42, 653–661 [DOI] [PubMed] [Google Scholar]
- 8. Xu Y., Zhang W., Klaus J., Young J., Koerner I., Sheldahl L. C., Hurn P. D., Martínez-Murillo F., Alkayed N. J. (2006) Role of cocaine- and amphetamine-regulated transcript in estradiol-mediated neuroprotection. Proc. Natl. Acad. Sci. U.S.A. 103, 14489–14494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lakatos A., Prinster S., Vicentic A., Hall R. A., Kuhar M. J. (2005) Cocaine- and amphetamine-regulated transcript (CART) peptide activates the extracellular signal-regulated kinase (ERK) pathway in AtT20 cells via putative G-protein coupled receptors. Neurosci. Lett. 384, 198–202 [DOI] [PubMed] [Google Scholar]
- 10. Lin Y., Hall R. A., Kuhar M. J. (2011) CART peptide stimulation of G protein-mediated signaling in differentiated PC12 cells: identification of PACAP 6–38 as a CART receptor antagonist. Neuropeptides 45, 351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wierup N., Kuhar M., Nilsson B. O., Mulder H., Ekblad E., Sundler F. (2004) Cocaine- and amphetamine-regulated transcript (CART) is expressed in several islet cell types during rat development. J. Histochem. Cytochem. 52, 169–177 [DOI] [PubMed] [Google Scholar]
- 12. Wierup N., Richards W. G., Bannon A. W., Kuhar M. J., Ahrén B., Sundler F. (2005) CART knock out mice have impaired insulin secretion and glucose intolerance, altered beta cell morphology, and increased body weight. Regul. Pept. 129, 203–211 [DOI] [PubMed] [Google Scholar]
- 13. Wierup N., Björkqvist M., Kuhar M. J., Mulder H., Sundler F. (2006) CART regulates islet hormone secretion and is expressed in the beta-cells of type 2 diabetic rats. Diabetes 55, 305–311 [DOI] [PubMed] [Google Scholar]
- 14. Jones D. C., Kuhar M. J. (2008) CART receptor binding in primary cell cultures of the rat nucleus accumbens. Synapse 62, 122–127 [DOI] [PubMed] [Google Scholar]
- 15. Jones D. C., Kuhar M. J. (2006) Cocaine-amphetamine-regulated transcript expression in the rat nucleus accumbens is regulated by adenylyl cyclase and the cyclic adenosine 5′-monophosphate/protein kinase a second messenger system. J. Pharmacol. Exp. Ther. 317, 454–461 [DOI] [PubMed] [Google Scholar]
- 16. Landerholm K., Falkmer S. E., Järhult J., Sundler F., Wierup N. (2011) Cocaine- and amphetamine-regulated transcript in neuroendocrine tumors. Neuroendocrinology 94, 228–236 [DOI] [PubMed] [Google Scholar]
- 17. Landerholm K., Shcherbina L., Falkmer S. E., Järhult J., Wierup N. (2012) Expression of cocaine- and amphetamine-regulated transcript is associated with worse survival in small bowel carcinoid tumors. Clin. Cancer Res. 18, 3668–3676 [DOI] [PubMed] [Google Scholar]
- 18. Thim L., Nielsen P. F., Judge M. E., Andersen A. S., Diers I., Egel-Mitani M., Hastrup S. (1998) Purification and characterisation of a new hypothalamic satiety peptide, cocaine and amphetamine regulated transcript (CART), produced in yeast. FEBS Lett. 428, 263–268 [DOI] [PubMed] [Google Scholar]
- 19. Douglass J., McKinzie A. A., Couceyro P. (1995) PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J. Neurosci. 15, 2471–2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuhar M. J., Yoho L. L. (1999) CART peptide analysis by Western blotting. Synapse 33, 163–171 [DOI] [PubMed] [Google Scholar]
- 21. Hohmeier H. E., Mulder H., Chen G., Henkel-Rieger R., Prentki M., Newgard C. B. (2000) Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49, 424–430 [DOI] [PubMed] [Google Scholar]
- 22. Asfari M., Janjic D., Meda P., Li G., Halban P. A., Wollheim C. B. (1992) Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 130, 167–178 [DOI] [PubMed] [Google Scholar]
- 23. Dey A., Xhu X., Carroll R., Turck C. W., Stein J., Steiner D. F. (2003) Biological processing of the cocaine- and amphetamine-regulated transcript precursors by prohormone convertases, PC2 and PC1/3. J. Biol. Chem. 278, 15007–15014 [DOI] [PubMed] [Google Scholar]
- 24. Buteau J., El-Assaad W., Rhodes C. J., Rosenberg L., Joly E., Prentki M. (2004) Glucagon-like peptide-1 prevents beta cell glucolipotoxicity. Diabetologia 47, 806–815 [DOI] [PubMed] [Google Scholar]
- 25. Dalle S., Quoyer J., Varin E., Costes S. (2011) Roles and regulation of the transcription factor CREB in pancreatic β-cells. Curr. Mol. Pharmacol. 4, 187–195 [DOI] [PubMed] [Google Scholar]
- 26. Jhala U. S., Canettieri G., Screaton R. A., Kulkarni R. N., Krajewski S., Reed J., Walker J., Lin X., White M., Montminy M. (2003) cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes Dev. 17, 1575–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hennige A. M., Burks D. J., Ozcan U., Kulkarni R. N., Ye J., Park S., Schubert M., Fisher T. L., Dow M. A., Leshan R., Zakaria M., Mossa-Basha M., White M. F. (2003) Upregulation of insulin receptor substrate-2 in pancreatic beta cells prevents diabetes. J. Clin. Invest. 112, 1521–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lingohr M. K., Dickson L. M., McCuaig J. F., Hugl S. R., Twardzik D. R., Rhodes C. J. (2002) Activation of IRS-2-mediated signal transduction by IGF-1, but not TGF-α or EGF, augments pancreatic beta-cell proliferation. Diabetes 51, 966–976 [DOI] [PubMed] [Google Scholar]
- 29. White M. F. (2002) IRS proteins and the common path to diabetes. Am. J. Physiol. Endocrinol. Metab. 283, E413–E422 [DOI] [PubMed] [Google Scholar]
- 30. Wrede C. E., Dickson L. M., Lingohr M. K., Briaud I., Rhodes C. J. (2002) Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic beta-cells (INS-1). J. Biol. Chem. 277, 49676–49684 [DOI] [PubMed] [Google Scholar]
- 31. Buzzi F., Xu L., Zuellig R. A., Boller S. B., Spinas G. A., Hynx D., Chang Z., Yang Z., Hemmings B. A., Tschopp O., Niessen M. (2010) Differential effects of protein kinase B/Akt isoforms on glucose homeostasis and islet mass. Mol. Cell. Biol. 30, 601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Srinivasan S., Bernal-Mizrachi E., Ohsugi M., Permutt M. A. (2002) Glucose promotes pancreatic islet beta-cell survival through a PI 3-kinase/Akt-signaling pathway. Am. J. Physiol. Endocrinol. Metab. 283, E784–E793 [DOI] [PubMed] [Google Scholar]
- 33. Kim S. J., Winter K., Nian C., Tsuneoka M., Koda Y., McIntosh C. H. (2005) Glucose-dependent insulinotropic polypeptide (GIP) stimulation of pancreatic beta-cell survival is dependent upon phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling, inactivation of the forkhead transcription factor Foxo1, and down-regulation of bax expression. J. Biol. Chem. 280, 22297–22307 [DOI] [PubMed] [Google Scholar]
- 34. Quoyer J., Longuet C., Broca C., Linck N., Costes S., Varin E., Bockaert J., Bertrand G., Dalle S. (2010) GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a beta-arrestin 1-mediated ERK1/2 activation in pancreatic beta-cells. J. Biol. Chem. 285, 1989–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maedler K. (2008) Beta cells in type 2 diabetes – a crucial contribution to pathogenesis. Diabetes Obes. Metab. 10, 408–420 [DOI] [PubMed] [Google Scholar]
- 36. Nolan C. J., Damm P., Prentki M. (2011) Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet 378, 169–181 [DOI] [PubMed] [Google Scholar]
- 37. Arumugam R., Fleenor D., Freemark M. (2007) Lactogenic and somatogenic hormones regulate the expression of neuropeptide Y and cocaine- and amphetamine-regulated transcript in rat insulinoma (INS-1) cells: interactions with glucose and glucocorticoids. Endocrinology 148, 258–267 [DOI] [PubMed] [Google Scholar]
- 38. Jansson D., Ng A. C., Fu A., Depatie C., Al Azzabi M., Screaton R. A. (2008) Glucose controls CREB activity in islet cells via regulated phosphorylation of TORC2. Proc. Natl. Acad. Sci. U.S.A. 105, 10161–10166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lingohr M. K., Briaud I., Dickson L. M., McCuaig J. F., Alárcon C., Wicksteed B. L., Rhodes C. J. (2006) Specific regulation of IRS-2 expression by glucose in rat primary pancreatic islet beta-cells. J. Biol. Chem. 281, 15884–15892 [DOI] [PubMed] [Google Scholar]
- 40. Park S., Dong X., Fisher T. L., Dunn S., Omer A. K., Weir G., White M. F. (2006) Exendin-4 uses Irs2 signaling to mediate pancreatic beta cell growth and function. J. Biol. Chem. 281, 1159–1168 [DOI] [PubMed] [Google Scholar]
- 41. Wang H. W., Mizuta M., Saitoh Y., Noma K., Ueno H., Nakazato M. (2011) Glucagon-like peptide-1 and candesartan additively improve glucolipotoxicity in pancreatic β-cells. Metab. Clin. Exp. 60, 1081–1089 [DOI] [PubMed] [Google Scholar]
- 42. Kubota N., Tobe K., Terauchi Y., Eto K., Yamauchi T., Suzuki R., Tsubamoto Y., Komeda K., Nakano R., Miki H., Satoh S., Sekihara H., Sciacchitano S., Lesniak M., Aizawa S., Nagai R., Kimura S., Akanuma Y., Taylor S. I., Kadowaki T. (2000) Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes 49, 1880–1889 [DOI] [PubMed] [Google Scholar]
- 43. Withers D. J., Gutierrez J. S., Towery H., Burks D. J., Ren J. M., Previs S., Zhang Y., Bernal D., Pons S., Shulman G. I., Bonner-Weir S., White M. F. (1998) Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391, 900–904 [DOI] [PubMed] [Google Scholar]
- 44. Lingohr M. K., Dickson L. M., Wrede C. E., McCuaig J. F., Myers M. G., Jr., Rhodes C. J. (2003) IRS-3 inhibits IRS-2-mediated signaling in pancreatic beta-cells. Mol. Cell. Endocrinol. 204, 85–99 [DOI] [PubMed] [Google Scholar]
- 45. Schuppin G. T., Pons S., Hügl S., Aiello L. P., King G. L., White M., Rhodes C. J. (1998) A specific increased expression of insulin receptor substrate 2 in pancreatic beta-cell lines is involved in mediating serum-stimulated beta-cell growth. Diabetes 47, 1074–1085 [DOI] [PubMed] [Google Scholar]
- 46. Withers D. J., Burks D. J., Towery H. H., Altamuro S. L., Flint C. L., White M. F. (1999) Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat. Genet. 23, 32–40 [DOI] [PubMed] [Google Scholar]
- 47. Sun X. J., Wang L. M., Zhang Y., Yenush L., Myers M. G., Jr., Glasheen E., Lane W. S., Pierce J. H., White M. F. (1995) Role of IRS-2 in insulin and cytokine signalling. Nature 377, 173–177 [DOI] [PubMed] [Google Scholar]
- 48. Lee Y. H., White M. F. (2004) Insulin receptor substrate proteins and diabetes. Arch. Pharm. Res. 27, 361–370 [DOI] [PubMed] [Google Scholar]
- 49. Burks D. J., White M. F. (2001) IRS proteins and β-cell function. Diabetes 50, Suppl. 1, S140–S145 [DOI] [PubMed] [Google Scholar]
- 50. Costes S., Longuet C., Broca C., Faruque O., Hani E. H., Bataille D., Dalle S. (2004) Cooperative effects between protein kinase A and p44/p42 mitogen-activated protein kinase to promote cAMP-responsive element binding protein activation after beta cell stimulation by glucose and its alteration due to glucotoxicity. Ann. N. Y. Acad. Sci. 1030, 230–242 [DOI] [PubMed] [Google Scholar]
- 51. Stork P. J. S., Schmitt J. M. (2002) Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 12, 258–266 [DOI] [PubMed] [Google Scholar]
- 52. Farilla L., Hui H., Bertolotto C., Kang E., Bulotta A., Di Mario U., Perfetti R. (2002) Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology 143, 4397–4408 [DOI] [PubMed] [Google Scholar]
- 53. Trümper A., Trümper K., Hörsch D. (2002) Mechanisms of mitogenic and anti-apoptotic signaling by glucose-dependent insulinotropic polypeptide in beta(INS-1)-cells. J. Endocrinol. 174, 233–246 [DOI] [PubMed] [Google Scholar]
- 54. Buteau J., Accili D. (2007) Regulation of pancreatic beta-cell function by the forkhead protein FoxO1. Diabetes Obes. Metab. 9, Suppl. 2, 140–146 [DOI] [PubMed] [Google Scholar]
- 55. Tuttle R. L., Gill N. S., Pugh W., Lee J.-P., Koeberlein B., Furth E. E., Polonsky K. S., Naji A., Birnbaum M. J. (2001) Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBα. Nat. Med. 7, 1133–1137 [DOI] [PubMed] [Google Scholar]
- 56. Jones D. C., Lakatos A., Rogge G. A., Kuhar M. J. (2009) Regulation of cocaine- and amphetamine-regulated transcript mRNA expression by calcium-mediated signaling in GH3 cells. Neuroscience 160, 339–347 [DOI] [PubMed] [Google Scholar]
- 57. Rogge G. A., Jones D. C., Green T., Nestler E., Kuhar M. J. (2009) Regulation of CART peptide expression by CREB in the rat nucleus accumbens in vivo. Brain Res. 1251, 42–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Goehring I., Sauter N. S., Catchpole G., Assmann A., Shu L., Zien K. S., Moehlig M., Pfeiffer A. F., Oberholzer J., Willmitzer L., Spranger J., Maedler K. (2011) Identification of an intracellular metabolic signature impairing beta cell function in the rat beta cell line INS-1E and human islets. Diabetologia 54, 2584–2594 [DOI] [PubMed] [Google Scholar]
- 59. Maedler K., Schulthess F. T., Bielman C., Berney T., Bonny C., Prentki M., Donath M. Y., Roduit R. (2008) Glucose and leptin induce apoptosis in human beta-cells and impair glucose-stimulated insulin secretion through activation of c-Jun N-terminal kinases. FASEB J. 22, 1905–1913 [DOI] [PubMed] [Google Scholar]
- 60. Xie T., Chen M., Weinstein L. S. (2010) Pancreas-specific Gsα deficiency has divergent effects on pancreatic alpha- and beta-cell proliferation. J. Endocrinol. 206, 261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Persaud S. J., Liu B., Sampaio H. B., Jones P. M., Muller D. S. (2011) Calcium/calmodulin-dependent kinase IV controls glucose-induced Irs2 expression in mouse beta cells via activation of cAMP response element-binding protein. Diabetologia 54, 1109–1120 [DOI] [PubMed] [Google Scholar]
- 62. Liu B., Barbosa-Sampaio H., Jones P. M., Persaud S. J., Muller D. S. (2012) The CaMK4/CREB/IRS-2 cascade stimulates proliferation and inhibits apoptosis of β-cells. PLoS One 7, e45711. [DOI] [PMC free article] [PubMed] [Google Scholar]