Background: The mechanistic action of antitumor agent OSW-1 is not clearly understood.
Results: OSW-1 triggers a calcium-dependent cell death through inhibition of sodium-calcium exchanger 1 (NCX1) and mitochondrial calcium overload.
Conclusion: Potency and efficacy of OSW-1 in eliminating leukemia cells are dependent on homeostatic calcium disruption.
Significance: New insights on the role of calcium in the mechanism of OSW-1 reveal potential in therapeutics.
Keywords: Calcium, ER Stress, Leukemia, Mitochondrial Apoptosis, Sodium-Calcium Exchange, Anticancer Agents, GRP78, NCX1, OSW-1
Abstract
3β,16β,17α-Trihydroxycholest-5-en-22-one 16-O-(2-O-4-methoxybenzoyl-β-d-xylopyranosyl)-(1→3)-2-O-acetyl-α-l-arabinopyranoside (OSW-1) is a natural product with potent antitumor activity against various types of cancer cells, but the exact mechanisms of action remain to be defined. In this study, we showed that OSW-1 effectively killed leukemia cells at subnanomolar concentrations through a unique mechanism by causing a time-dependent elevation of cytosolic Ca2+ prior to induction of apoptosis. A mechanistic study revealed that this compound inhibited the sodium-calcium exchanger 1 on the plasma membrane, leading to an increase in cytosolic Ca2+ and a decrease in cytosolic Na+. The elevated cytosolic Ca2+ caused mitochondrial calcium overload and resulted in a loss of mitochondrial membrane potential, release of cytochrome c, and activation of caspase-3. Furthermore, OSW-1 also caused a Ca2+-dependent cleavage of the survival factor GRP78. Inhibition of Ca2+ entry into the mitochondria by the uniporter inhibitor RU360 or by cyclosporin A significantly prevented the OSW-1-induced cell death, indicating the important role of mitochondria in mediating the cytotoxic activity. The extremely potent activity of OSW-1 against leukemia cells and its unique mechanism of action suggest that this compound may be potentially useful in the treatment of leukemia.
Introduction
The natural saponin molecule 3β,16β,17α-trihydroxycholest-5-en-22-one 16-O-(2-O-4-methoxybenzoyl-β-d-xylopyranosyl)-(1→3)-2-O-acetyl-α-l-arabinopyranoside (OSW-1),3 originally isolated from the bulbs of Ornithogalum saundersiae by Mimaki and co-workers (1) in 1992, has shown potent antitumor activity in various cancer cell lines including leukemia. Several studies of OSW-1 have focused on its isolation, cytotoxic tests, chemical synthesis, and preparation of synthetic analogues to understand the structure-activity relationships (2–18). However, the mechanism by which OSW-1 exerts antitumor activity is not well delineated. An early study proposed that OSW-1 had a direct inhibitory action on gene expression of the steroidal enzyme and on the proliferation of granulosa cells in the ovary (19). Because of its structural similarity to cardiac glycosides, it was speculated that OSW-1 and cardiac glycosides might have a similar mechanistic action through inhibition of the Na+/K+-ATPase (19). It has also been reported that OSW-1 causes activation of caspase-8, leading to cleavage of Bcl-2 and a mitochondrion-mediated cell death (20). In our previous study, we showed the cytotoxic effects of OSW-1 in various cancer cells including leukemia with greater toxicity in malignant cells than in normal cells (21). OSW-1 also killed primary leukemia cells from patients whose disease was refractory to fludarabine and led to a Ca2+-dependent cell death. Interestingly, cells with mitochondrial defects were less sensitive to this compound (21). This suggested that Ca2+ and mitochondria played a key role in the cytotoxic effects of OSW-1 in leukemia, but the mechanism by which OSW-1 disrupts the Ca2+ homeostasis remains unclear. A recent report suggested that OSW-1 may target the oxysterol-binding protein (OSBP) and OSBP-related protein 4L (ORP4L) (22). These proteins are known to be involved in lipid metabolism, signaling, vesicular traffic, and nonvesicular sterol transport (23–26). However, it is not clear whether or how OSBP and ORP4L are involved in calcium regulation.
In the present study, we assessed the role of mitochondria, endoplasmic reticulum (ER), and sodium-calcium exchanger (NCX) in causing Ca2+ elevations in leukemia cells in an effort to delineate the mechanism of action and mode of cell death induced by OSW-1. We found that early mitochondrial Ca2+ elevations were essential for cell death but that the ER was not the source of Ca2+ elevation. OSW-1 led to cytosolic Na+ decreases with simultaneous Ca2+ increases, suggesting that inhibition of the NCX may be a key mechanism by which OSW-1 exerts its cytotoxic effect in leukemia cells.
EXPERIMENTAL PROCEDURES
Cell Lines and Reagents
All human leukemia cells were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum at 37 °C in 5% CO2. HL-60, Raji, and K-562 cells were obtained from American Type Culture Collection (Manassas, VA). The KBM5 cell line was derived from a female chronic myeloid leukemia patient in blast crisis as described previously (27–29). The human myeloblastic leukemia cell line ML-1 containing wild-type p53 was a kind gift from Dr. Michael B. Kastan (St. Jude Children's Research Hospital, Memphis, TN). The compound OSW-1 was generously provided by Dr. Zhendong Jin from the University of Iowa (Iowa City, IA). Stock OSW-1 was dissolved in sterile dimethyl sulfoxide (DMSO) and further diluted in medium. The following fluorescent dyes were obtained from Invitrogen: Calcium Green AM for cytosolic calcium detection, CoroNa Green AM for sodium detection, fluo-3 AM for cytosolic calcium detection, and rhodamine 123 for mitochondrial transmembrane potential (ΔΨm) detection. The following antibodies were purchased from the indicated sources: caspase-3 and cytochrome c (Cell Signaling Technology, Danvers, MA), β-actin (Calbiochem), NCX f(ABR Affinity BioReagents, Golden, CO), NCX isoforms 1 and 3 (Thermo Scientific Pierce Antibodies, Rockford, IL and Abgent, Inc., San Diego, CA), and GRP78 (Santa Cruz Biotechnology, Santa Cruz, CA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), propidium iodide (PI), cyclosporin A (CsA), thapsigargin (TG), and ruthenium red were purchased from Sigma-Aldrich. RU360 and the InnoCyte flow cytometric cytochrome c release kit were purchased from Calbiochem, the active caspase-3 kit was from BD Biosciences, and the annexin V-FITC kit was purchased from BD Pharmingen.
Cell Viability Analysis by MTT Assay
To determine the effect of OSW-1 on cell viability, leukemia cells were seeded in 96-well plates at concentrations of 2 × 104 cells/well (HL-60, Raji, and ML-1) and 1 × 104 cells/well (KBM5 and K-562). Following overnight incubation, cells were treated with several concentrations of OSW-1 and incubated at 37 °C for 72 h. Cell viability was determined by the MTT assay as described previously (21). Each assay was conducted in triplicate and repeated three times.
Annexin V-FITC/PI Labeling for Apoptosis Detection
For annexin V-FITC/PI labeling, after the indicated treatment times (Fig. 1), leukemia cells were harvested, washed once with phosphate-buffered saline (PBS), and resuspended in binding buffer containing 10 mm Hepes (pH 7.4), 140 mm NaCl, and 2.5 mm CaCl2. The cells were then stained with FITC-conjugated annexin V/PI and immediately analyzed for apoptosis using a FACSCalibur flow cytometer (BD Biosciences). Data were analyzed using the BD Biosciences CellQuest Pro software.
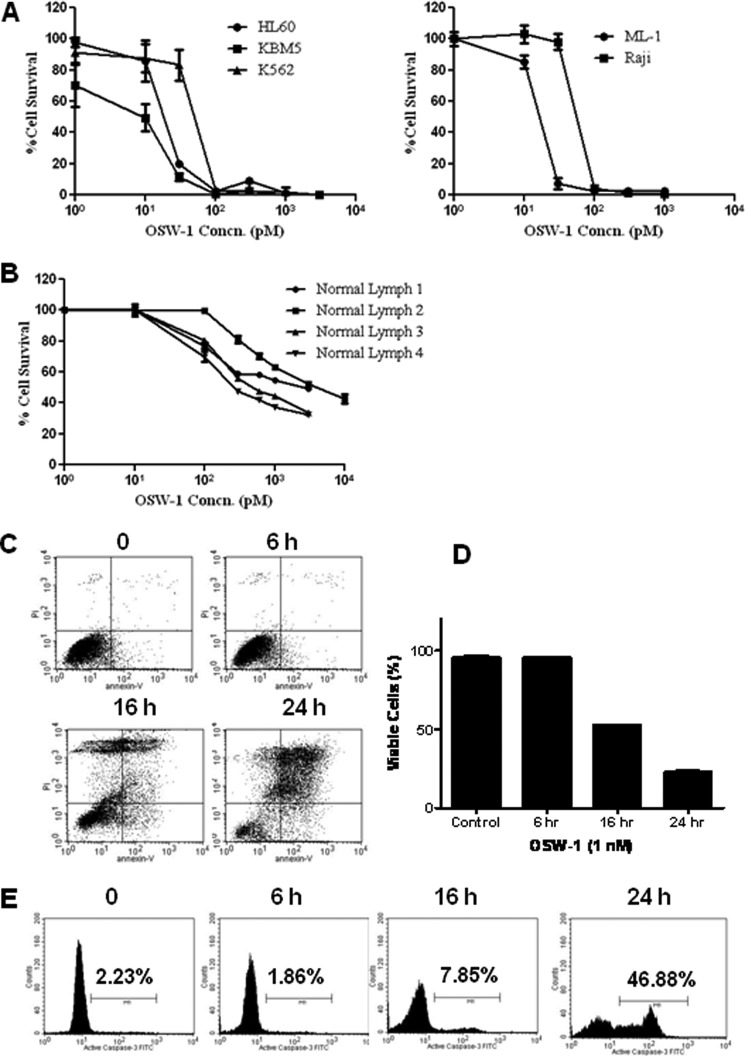
FIGURE 1.
OSW-1 caused a time-dependent death in leukemia and lymphoma cells. A and B, leukemia cells and normal lymphocytes were plated in 96-well plates at concentrations of 2 × 104 (HL-60, Raji, ML-1, and normal lymphocytes) and 1 × 104 (KBM5 and K-562) cells/well and treated with varying concentrations (Concn.) of OSW-1 for 72 h, and the cytotoxic effect of OSW-1 was determined by MTT assay. C, HL-60 cells were seeded at a concentration of 2 × 105 cells/ml and grown overnight. Cells were treated with 1 nm OSW-1 for 6, 16, and 24 h, and apoptosis was detected using annexin V-FITC and PI as described under “Experimental Procedures.” D, the bar graph represents cell viability data post-OSW-1 treatment from three independent experiments by annexin V/PI staining. E, HL-60 cells were plated and treated with 1 nm OSW-1 for 6, 16, and 24 h as indicated, and the induction of apoptosis was measured by caspase-3 activation using the caspase-3 activity kit and flow cytometry. The population of cells with active caspase-3 was gated to the right of the control peak and is displayed as a percentage (%). Error bars represent S.E.
Determination of Caspase-3 Activation
A caspase-3 activation assay was used to determine the levels of activated caspase-3 as an indicator of apoptosis induction. Briefly, 1 × 106 cells/sample were fixed on ice for 20 min using the Cytofix/Cytoperm reagent provided in the kit. After fixation, the cells were washed twice using the provided Perm/Wash Buffer, stained with FITC-conjugated anti-active caspase-3 monoclonal antibody, and analyzed using a FACSCalibur flow cytometer.
Analysis of Cytochrome c Release from Mitochondria
The cytochrome c release assay kit was used according to the manufacturer's recommended procedures to measure the loss of mitochondrial cytochrome c. Briefly, HL-60 cells treated with OSW-1 were harvested, permeabilized, and fixed with 8% paraformaldehyde in PBS for 20 min. The cells were then washed three times and blocked for 1 h in the blocking buffer followed by incubation with anti-cytochrome c and anti-IgG-FITC antibodies. The cells were resuspended in the wash buffer and analyzed using a FACSCalibur flow cytometer.
Measurement of Mitochondrial Transmembrane Potential
The HL-60 cells were incubated with 1 μm rhodamine 123 for 60 min at 37 °C before the end of the incubation with OSW-1 for the indicated treatment times (Fig. 3). The cells were washed twice with PBS and then analyzed by flow cytometry.
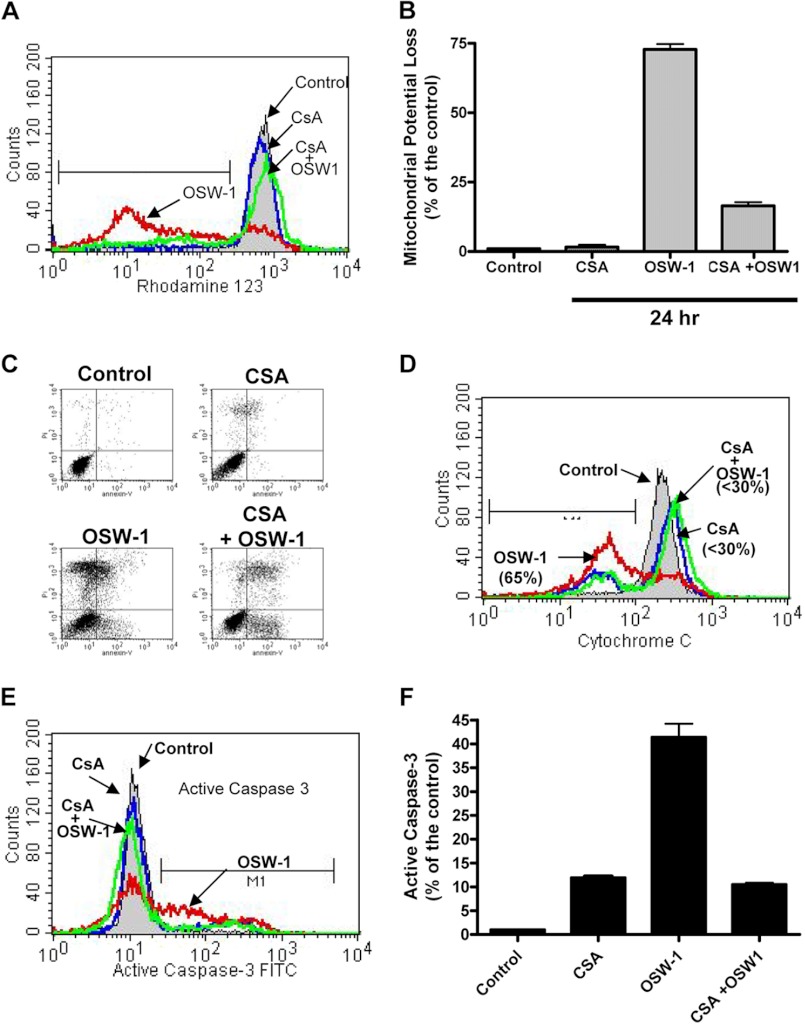
FIGURE 3.
CsA prevented loss of MMP, cytochrome c release, and caspase-3 activation induced by OSW-1. A, HL-60 cells were incubated for 24 h with 10 μm CsA and 1 nm OSW-1 alone and in combination by adding the CsA 0.5 h prior to the addition of OSW-1. Loss of MMP was detected by the fluorescent probe rhodamine 123 as described under “Experimental Procedures.” B, the histogram represents the loss of MMP as a percentage of the control for the different conditions for 24 h with 10 μm CsA and/or 1 nm OSW-1 treatment. C, HL-60 cells at a concentration of 2.0 × 105 cells/ml were plated and incubated with either 10 μm CsA or 1 nm OSW-1 alone or in combination by adding CsA 0.5 h prior to the addition of OSW-1. Annexin V-FITC and PI staining was performed 24 h post-treatment as described under “Experimental Procedures.” D, cytochrome c was detected in cells treated with OSW-1 and CsA for 24 h using a cytochrome c kit as described under “Experimental Procedures.” An increase in the population to the left of the control indicates the population of cells in which there was a release of cytochrome c from the mitochondria. E and F, CsA prevented loss of caspase-3. Active caspase-3 levels were detected in cells using the caspase-3 activation assay kit following incubation with OSW-1 and cyclosporin A either alone or in combination for 24 h. F, OSW-1 activates caspase-3 (population to the right of the control), which was suppressed in the presence of CsA. Error bars represent S.E.
Immunoblotting
HL-60 cells pretreated with OSW-1 or other compounds were lysed using a lysis buffer containing 50 mm Tris-HCl (pH 6.8), 10% glycerol, 2% SDS, 0.025% bromphenol blue, and 2.5% β-mercaptoethanol. Cell lysates were then loaded directly onto the gel, separated using SDS-polyacrylamide gel electrophoresis (PAGE), transferred to a nitrocellulose membrane, and probed for various proteins using the indicated antibodies. The SuperSignal West Pico Chemiluminescent Substrate System (Pierce) was used to visualize the proteins.
Measurement of Mitochondrial Calcium Levels
Mitochondrial calcium changes were monitored using rhod-2 (Invitrogen), a mitochondrial calcium-specific dye, and analyzed by flow cytometry. Briefly, cells were incubated in 1 μm rhod-2 for 45 min at 37 °C at the end of the drug incubation. The cells were then washed three times in PBS and analyzed with a FACSCalibur flow cytometer. The BD CellQuest Pro software was used to quantitate the shift in fluorescence.
Measurement of Cytosolic Calcium (Ca2+) Levels
HL-60 cells were incubated with the fluorescent dye Calcium Green AM for 30 min at 37 °C before the end of the OSW-1 incubation period. The cells were then washed in PBS, and the fluorescence shift indicative of cytosolic calcium level changes was analyzed with a FACSCalibur flow cytometer using the FL-1 x axis.
Measurement of Cytosolic Sodium (Na+) Levels
To test the possibility that OSW-1 may target the NCX on the plasma membrane, the cytosolic Ca2+ and Na+ levels were detected post-OSW-1 treatment. Briefly, the drug-treated cells were washed with Hanks' balanced salt solution once and incubated with 1 μm CoroNa Green AM for 45 min at 37 °C. After 45 min, the cells were washed, reconstituted in PBS, and analyzed by flow cytometry.
Measurement of Calcium Release from the ER
To examine the effect of OSW-1 on ER calcium ATPase, OSW-1- or thapsigargin-treated HL-60 cells were incubated for 1 h with 5 μm fluo-3 AM (1 × 106) in Ca2+-free Hanks' balanced salt solution containing 1% bovine serum albumin. The cells were washed twice with Hanks' balanced salt solution and resuspended in Hanks' balanced salt solution containing 2 mm Ca2+. Calcium changes were measured by flow cytometry, and the kinetic analysis of fluo-3 versus time as the mean of the individual cells was performed using FlowJo version 7.6.1 software (Tree Star, Inc., Ashland, OR).
Statistical Analysis
The histograms from flow cytometry were obtained and analyzed using the CellQuest Pro software. Bar graphs and plots were produced using GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, CA). Data were expressed as mean ± 95% confidence interval. Statistical analysis was done to compare experimental values for two samples, and a p value less than 0.05 or less than 0.01 was considered significant. Student's two-tailed t test was used to evaluate the experimental values.
RESULTS
OSW-1 Causes a Time-dependent Cell Death with Caspase-3 Activation in Human Leukemia Cells
We reported previously that OSW-1 demonstrated potent anticancer activity in various cancer cells with the half-maximal inhibitory concentration (IC50) in the subnanomolar range (21). To investigate the effect of OSW-1 on cell viability, we tested several leukemia cell lines using the MTT assay. As shown in Fig. 1A, OSW-1 exhibited cytotoxicity in all five human leukemia cell lines with IC50 values in the picomolar range, ranging from 6 to 55 pm. However, it induced minimal toxicity in normal lymphocytes at the same concentrations (Fig. 1B). Furthermore, the ratio of the average IC50 value of normal lymphocytes (1.64 nm) over leukemia cells (0.019 nm) was ∼86, thus indicating that OSW-1 has substantial sensitivity toward leukemia cells in vitro. Furthermore, apoptosis analysis of HL-60 cells exposed to 1 nm OSW-1 for 16 and 24 h showed a 50 and 77% loss in cell viability (Fig. 1, C and D), demonstrating the time-dependent cell inhibitory activity of OSW-1.
Caspase-3 activation is a characteristic of apoptotic cells following both the intrinsic and extrinsic apoptosis pathways (30). Analysis of caspase-3 by flow cytometry in HL-60 cells treated with OSW-1 demonstrated a time-dependent caspase-3 activation with an almost 47% caspase-3 increase 24 h post-OSW-1 incubation (Fig. 1E). The loss in cell viability induced by OSW-1 directly correlated with the activation of caspase-3. Taken together, these results show that OSW-1 is a potent anticancer agent that reduces cell viability in various leukemia cell lines and causes a time-dependent cell death via activation of caspase-3.
OSW-1 Triggers Cytosolic and Mitochondrial Calcium Elevations
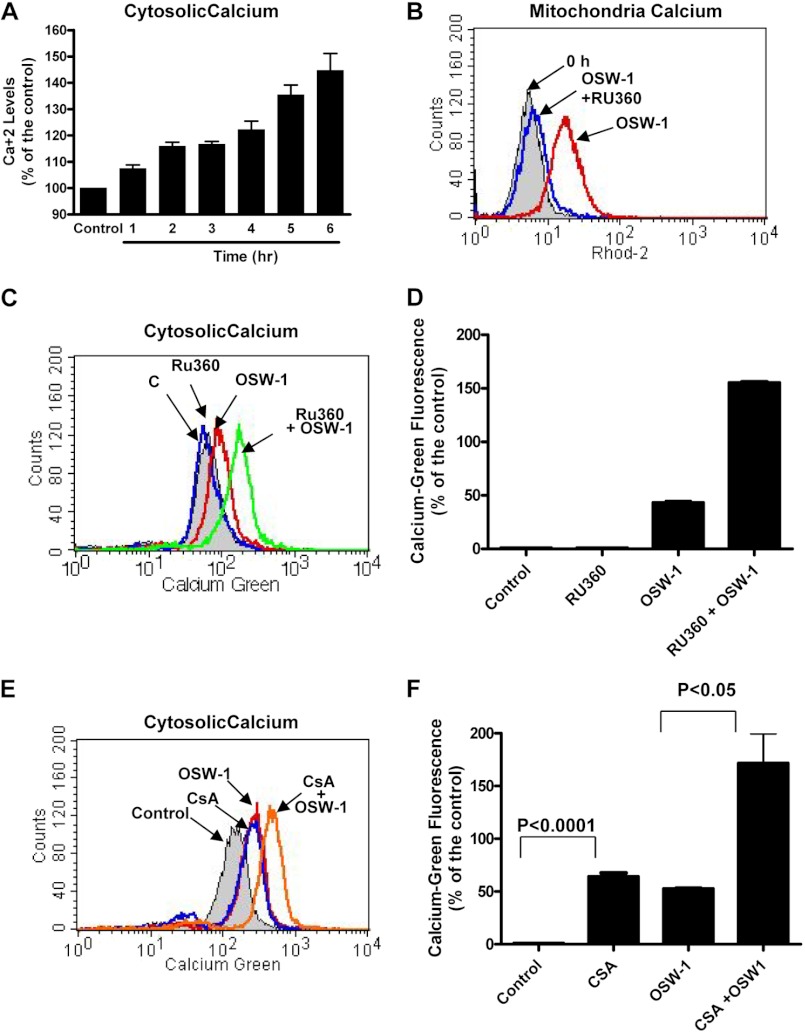
Because our previous study showed that intracellular calcium played a role in OSW-1-induced cell death (21), we monitored calcium elevations in the cytosol and mitochondria. Interestingly, although cell death was not observed until 16 h post-treatment (Fig. 1C), OSW-1 caused increases in cytosolic calcium as early as 6 h (Fig. 2A). This measurement of change in cellular calcium levels was conducted at earlier time points because at later time points other factors due to activation of cell death affected the cellular Ca2+ level readings (data not shown). In addition to the ER being the major intracellular store for calcium, mitochondria play a key role in the uptake of excess cytosolic calcium (30, 31). The uniporter is the main route of Ca2+ uptake by the mitochondria and is dependent on the mitochondrial membrane potential (MMP). Therefore, we used the mitochondrion-specific calcium dye rhod-2 to test the effect of OSW-1 on the calcium levels in the mitochondria. As shown in Fig. 2B, treatment of HL-60 cells with OSW-1 caused a significant increase in fluorescence, indicating an elevation of mitochondrial calcium. To further test the hypothesis that OSW-1 caused cytosolic calcium elevations that triggered subsequent uptake by the mitochondria, we used RU360, a ruthenium red derivative specific for blocking mitochondria uniporters, to prevent excess calcium from entering the mitochondria. As shown in Fig. 2C, OSW-1 by itself caused an elevation in cytosolic Ca2+. In contrast, RU360 alone did not significantly increase the cytosolic Ca2+. However, when RU360 was combined with OSW-1, there was a dramatic increase in the level of Ca2+ as demonstrated by an elevation of the Calcium Green fluorescence in the cytosol (Fig. 2D). A similar effect was observed using CsA, a cyclophilin D inhibitor, which also blocked mitochondrial calcium entry (Fig. 2, E and F). Taken together, these data suggest that mitochondria play a direct role in the uptake of cytosolic Ca2+ when treated with OSW-1.
FIGURE 2.
Mitochondrial calcium increased after treatment with OSW-1 in leukemia cells, whereas inhibition of the mitochondrial uniporter by RU360 blocked OSW-1-induced calcium elevations. A, HL-60 cells were treated with 1 nm OSW-1 for 1–6 h, and the cytosolic calcium changes were determined by Calcium Green AM staining and subsequent flow cytometry fluorescence analysis. B and C, HL-60 cells were treated with 1 nm OSW-1 alone, 1 μm RU360 alone, or a combination of 1 nm OSW-1 and 1 μm RU360 for 5 h. Calcium changes in the mitochondria and cytoplasm were detected using the fluorescent dyes Calcium Green AM and rhod-2 as described under “Experimental Procedures.” D, the bar graph depicts the OSW-1-induced elevation in cytosolic calcium as determined by staining with Calcium Green AM. Three independent experiments were performed to confirm the effect of OSW-1 on cellular calcium; the results were statistically significant (p < 0.0001). E and F, HL-60 cells were treated with 10 μm CsA, a cyclophilin D inhibitor, either alone or in combination with 1 nm OSW-1 for 5 h, and the changes in cytosolic calcium were detected using the fluorescent probe Calcium Green AM. F, the results from independent experiments of OSW-1 with and without CsA are presented as histograms. The results were statistically significant at a confidence interval of 95%. Error bars represent S.E.
OSW-1 Kills Leukemia Cells by Inducing Apoptosis through a Mitochondrion-mediated Mechanism
It is well established that apoptotic stimuli can lead to the opening of the mitochondrial permeability transition pore, causing membrane permeability and loss of apoptotic factors such as cytochrome c and Second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI (Smac/DIABLO) (32). Treatment of the cells with CsA alone did not cause a loss of MMP. In contrast, OSW-1 triggered a loss of ∼75% of MMP at 24 h post-treatment (Fig. 3A). Interestingly, preincubation with CsA markedly reduced the percentage of cells showing a loss of MMP from 75 to ∼20% (Fig. 3B). Consistent with this observation, analysis of apoptosis by annexin V/PI showed a similar pattern with OSW-1 causing cell death by 24 h post-treatment that was markedly rescued by combining OSW-1 with CsA (Fig. 3C). As indicated in Fig. 3D, leukemia cells treated with OSW-1 showed a massive decrease in mitochondrial cytochrome c. Additionally, as shown in Fig. 3E, OSW-1 alone caused caspase-3 activation that was blocked by combining OSW-1 with CsA. Altogether, these data indicated that the loss of MMP is an important event during cell death induced by OSW-1.
Effect of OSW-1 on ER Calcium and Chaperone GRP78
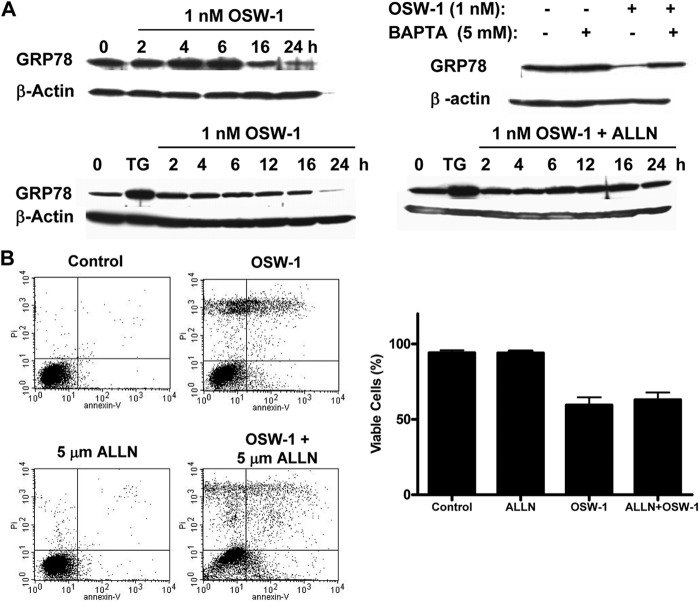
Several studies have shown that GRP78 is important for the survival of cancer cells and that the down-regulation of GRP78 can be an effective way of inhibiting cancer cell growth (33, 34). As shown in Fig. 4A, incubation of HL-60 with OSW-1 resulted in a time-dependent reduction of GRP78. This was in marked contrast to the effect of TG, which caused a substantial increase in GRP78 (Fig. 4A), consistent with the previous observation that TG causes leakage of Ca2+ from the ER to the cytosol by inhibiting the ATPase and leads to up-regulation of GRP78 as an indication of ER stress (35). Thus, it seems OSW-1 did not cause ER stress as its primary mechanism of action.
FIGURE 4.
OSW-1 caused a decrease of ER chaperone GRP78 through a calpain-mediated mechanism. A, HL-60 cells were treated with OSW-1 for various time points, cells were lysed, and GRP78 levels were detected by Western blotting. OSW-1 caused a time-dependent decrease in GRP78 expression in contrast to TG (24-h incubation), an ER calcium ATPase pump inhibitor. This reduction in GRP78 by OSW-1 was not observed in the presence of either the calcium chelator BAPTA (24-h incubation) or the calpain inhibitor ALLN. B, simultaneously, annexin V-FITC/PI staining was performed in cells treated with 1 nm OSW-1 for 16 h both in the presence and absence of 5 μm ALLN. Error bars represent S.E.
Because calcium played an important role in the cytotoxicity of OSW-1, we used BAPTA-AM, a cytosolic calcium chelator, to test the potential role of calcium in OSW-1-induced change of GRP78. As shown in Fig. 4A, preincubation of cells with BAPTA prior to the addition of OSW-1 effectively prevented a reduction in the GRP78 protein level, suggesting that calcium plays a major role in the degradation of GRP78. Because calpains are intracellular proteases involved in the degradation of many proteins and are activated by cytosolic calcium (36), we further investigated whether calpains were responsible for the reduction in GRP78 protein levels by using a calpain inhibitor, Ac-Leu-Leu-Nle-H (ALLN), prior to OSW-1 treatment. As shown in Fig. 4A, OSW-1 caused a time-dependent reduction in GRP78 protein levels, whereas TG caused a significant increase in GRP78. Conversely, co-incubation of cells with ALLN and OSW-1 showed that ALLN effectively prevented the degradation of GRP78, suggesting that calpains might play a role in causing the reduction of GRP78. However, co-incubation of ALLN with OSW-1 did not prevent cell death as shown in Fig. 4B. These data provide evidence that OSW-1 induced the calcium increase, leading to the activation of cytosolic calpains and subsequent degradation of GRP78.
Source of Ca2+ Elevations Is Not from the ER, but Extracellular Ca2+ Plays a Role
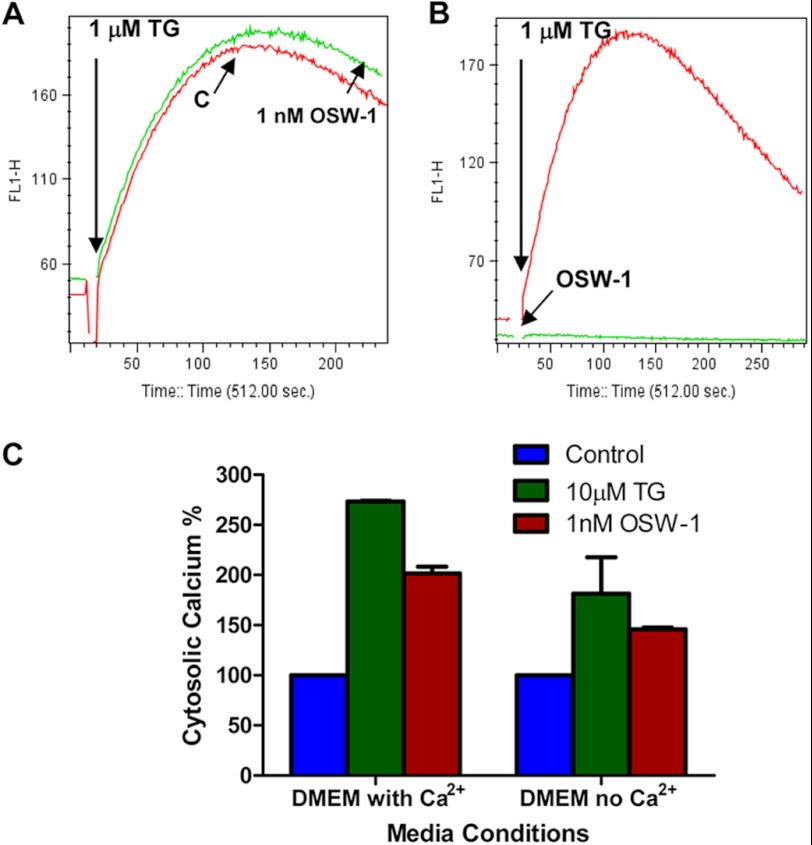
The ER is known to have the highest concentration of Ca2+ within the cells with a micromolar concentration range in the hundreds (37). The early increases in cytosolic and mitochondrial calcium prompted us to further investigate the source of Ca2+. TG, a well known inhibitor of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), was used as a positive control. OSW-1 did not appear to have an effect on the ER Ca2+ concentration because emptying the ER calcium reserve by TG treatment did not show major differences between the untreated control and the cells pretreated with OSW-1 (Fig. 5A).
FIGURE 5.
Comparison of effect of OSW-1 and TG on calcium homeostasis in leukemia cells. A, either HL-60 cells (1 × 106 cells) pretreated with 1 nm OSW-1 for 6 h or control cells were loaded with 5 μm fluo-3 for 1 h. Cells were then treated with 1 μm TG to deplete ER calcium reserves and activate capacitative calcium entry. The free Ca2+ in the cells was measured by recording fluo-3 fluorescence using flow cytometry as described under “Experimental Procedures.” B, 1 nm OSW-1 or 1 μm TG was added to HL-60 cells prestained with fluo-3 10 s after detecting their basal calcium levels, and the calcium changes triggered by OSW-1 or TG were recorded by flow cytometry. C, HL-60 cells grown in either regular (with calcium) or calcium-free DMEM were treated with either 10 μm TG or 1 nm OSW-1 for 6 h, and the cytosolic calcium levels were measured by Calcium Green AM staining. The bar graph shown is representative of three independent measurements of cytosolic calcium in either the OSW-1- or TG-treated samples. Error bars represent S.E.
Because TG inhibits ER calcium ATPase, we presumed that if OSW-1 worked similarly to TG then treating cells with OSW-1 would produce the same results as those produced with TG. As expected, TG caused an immediate increase in cytosolic calcium, indicating the inhibition of ER ATPase (Fig. 5B). In contrast, OSW-1 did not trigger an instantaneous increase, suggesting a different mode of action not involving the ER calcium pumps. These results together with data showing the lack of GRP78 up-regulation in the ER (Fig. 4A) suggest that OSW-1 increases cytosolic calcium without causing calcium release from the ER.
Extracellular Ca2+ Affects OSW-1-induced Calcium Elevations
To evaluate the role of extracellular calcium in OSW-1-induced elevation of cellular calcium, experiments were performed using media with and without Ca2+. As shown in Fig. 5C, a calcium increase of about 100% was seen in OSW-1-treated cells in DMEM with calcium. In contrast, OSW-1 caused a nearly 50% increase of cellular calcium when the cells were cultured in calcium-free DMEM. Because extracellular Ca2+ is important for refilling ER calcium stores by the store-operated calcium channels when the ER experiences low calcium levels, TG emptying of the ER in HL-60 cells growing in calcium-free DMEM showed reduced cytoplasmic calcium levels because the outside levels were also lower. It should also be noted that NCX functions both at the plasma and mitochondrial membranes, and consequently, inhibition of NCX could activate other counterbalancing mechanisms like the SERCA and release of calcium from mitochondrial stores to maintain cellular calcium homeostasis. This could explain the increase of calcium in cells despite the absence of extracellular calcium in calcium-free DMEM.
OSW-1 Induces Increase in Cytosolic Ca2+ by Inhibiting NCX
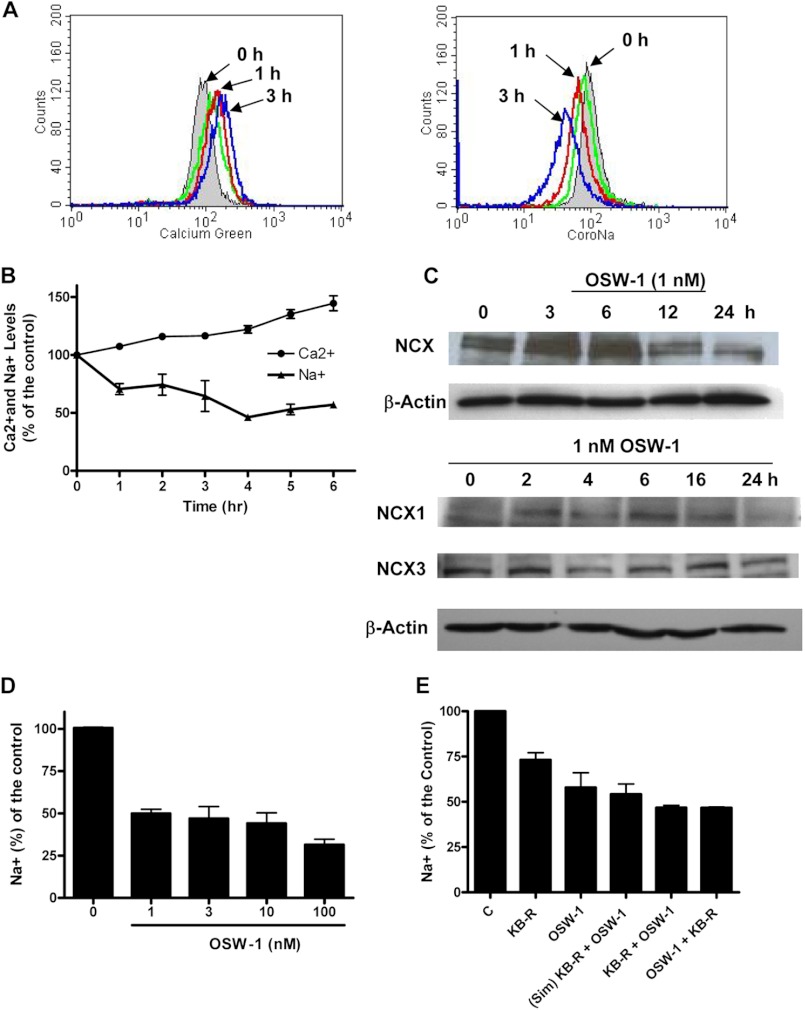
The above observations led us to test the possibility that OSW-1 might inhibit the NCX. As shown in Fig. 6, A and B, OSW-1 caused a notable increase in the cytosolic Ca2+ and a simultaneous decrease in the Na+ concentration levels in HL-60 cells in a time-dependent fashion. Similar results were also observed with KB-R7943, a known NCX inhibitor. Thus, it is likely that OSW-1 disrupted calcium/sodium homeostasis by causing a decrease in NCX function. Western blot analysis showed that OSW-1 treatment reduced the expression of NCX in HL-60 cells, suggesting that NCX might be a target of OSW-1 (Fig. 6C). Further study demonstrated that OSW-1 inhibited the expression of NCX isoform 1 (NCX1) in HL-60 cells at 16–24 h post-treatment with OSW-1, whereas NCX3 levels remained relatively unchanged (Fig. 6C).
FIGURE 6.
Effect of OSW-1 on cytosolic Ca2+ and Na+ levels and NCX expression. A, an increase in cytosolic Ca2+ and decrease in Na+ in human leukemia HL-60 cells up to 3 h after treatment with 1 nm OSW-1 was measured by flow cytometry using the fluorescent dyes Calcium Green AM and CoroNa Green AM. KB-R7943 (10 μm; shown in green), incubated for 3 h, was used as a positive control for NCX inhibition. B, the simultaneous increase of Ca2+ and decrease in Na+ in HL-60 cells up to 6 h after OSW-1 treatment was measured by flow cytometry. Experiments were performed in triplicate, and results are displayed as a percentage of the control. C, the effect of OSW-1 on NCX was determined by assaying the protein extracts from the control and HL-60 cells treated with 1 nm OSW-1 for the times indicated by Western blot for NCX, NXC1, and NCX3. β-Actin was used as a loading control. D, flow cytometry analysis of cytosolic Na+ levels in HL-60 cells using CoroNa Green AM at various concentrations of OSW-1 for 5 h was performed as indicated. Results are displayed as a percentage of the control. E, HL-60 cells were incubated for 5 h with 1 nm OSW-1 or 10 μm KB-7943 alone or in combination by adding KB-7943 either 1 h prior to OSW-1 treatment, simultaneously (Sim), or 1 h after OSW-1 treatment; cells were then stained with CoroNa Green AM and measured by flow cytometry. Results are from two independent experiments and are displayed as a percentage of the control. Error bars represent S.E.
Furthermore, increasing concentrations of OSW-1 (from 1 to 100 nm) did not cause a dramatic drop in Na+ concentration levels, indicating that 1 nm OSW-1 was sufficient to exert an effect on the cells (Fig. 6D). We also studied the combination of OSW-1 and KB-R7943. We postulated that if the combination caused a further decrease in Na+ when compared with individual agents then it would suggest that OSW-1 and KB-R7943 act on different targets. However, as shown in Fig. 6E, the combination of OSW-1 and KB-R7943 did not produce a synergistic or additive effect. In fact, OSW-1 alone caused a higher reduction in cytosolic sodium (42%) than did the combination of KB-R7943 and OSW-1 (56%), thus suggesting potential competitive inhibition of NCX by OSW-1 and KB-R7943.
DISCUSSION
In this study, we demonstrated that OSW-1 effectively kills leukemia cells by triggering elevations in cytosolic and mitochondrial calcium followed by activation of apoptotic factors. We previously reported that calcium played a role in OSW-1-induced cell death and that OSW-1 treatment led to morphological changes of the mitochondria through a yet unknown mechanism (21). In this study, the ionic changes were observed prior to the onset of apoptosis, indicating that this was likely an upstream event. We showed that cytosolic calcium elevations occurred as early as 6 h, which coincided with the early mitochondrial swelling observed in our previous study (21). Calcium is known to play an important role during the cell death process in that overwhelming the mitochondrial calcium capacity can trigger the release of apoptotic factors (38, 39). By blocking the mitochondrial permeability transition pore and mitochondria uniporters using cyclosporin A and RU360 prior to OSW-1 treatment, we demonstrated the importance of calcium entry into the mitochondria in OSW-1-induced apoptosis because the activation of apoptotic factors was prevented by suppression of calcium entry into the mitochondria.
Because various factors that regulate cellular calcium are found in the plasma membrane, ER, and mitochondria (38), we tested the ability of OSW-1 to affect calcium regulators such as the ER-associated ATPases, mitochondrial uniporters, and plasma membrane-associated Ca2+/Na+ exchanger. The ER ATPases are ATP-consuming pumps that transfer calcium from the cytosol to the ER to maintain a relatively low level of calcium in the cytosol, and inhibition of ER ATPases can lead to increased cytosolic calcium levels (40). We observed that unlike the pharmacological agent TG, which inhibited ER ATPase and caused an immediate increase in cytosolic calcium, OSW-1 did not exert such effect. Furthermore, GRP78, an ER chaperone involved in the stress response, is often up-regulated or overexpressed following ER stress such as in the case of inhibition of ER ATPase (41–43). In contrast, OSW-1 treatment led to a decrease in GRP78 levels, suggesting that OSW-1 may not be interacting with the ER ATPase. Nonetheless, we found this of particular importance as it has been suggested that down-regulation of GRP78 may be an effective way to compromise cancer cell viability because GRP78 has been shown previously to be important in the survival of cancer cells (33, 34). Interestingly, we observed that inhibition of calpain by ALLN prevented GRP78 protein degradation, suggesting that calpain may be the calcium-dependent enzyme that mediates GRP78 degradation induced by OSW-1.
It has been suggested that OSW-1 may work similarly to cardiac glycosides because of structural similarities with both having a steroidal aglycon attached to a carbohydrate unit (19). Cardiac glycosides are plant-derived compounds that have been used in the treatment of congested heart failure and are known to function by inhibition of the Na+/K+-ATPase. Cardiac glycosides such as oleandrin and digitoxin have been suggested as potent compounds that can inhibit cancer cell proliferation (44, 45). Na+/K+-ATPase pumps are located on the plasma membrane, and their function allows K+ entry into the cells while pumping Na+ out of the cells against its concentration gradient. Inhibition of the Na+/K+-ATPase leads to sodium increases in the cytosol, and consequently, the high level of Na+ leads to the reversal of the NCX and an increase of Ca2+ in the cytosol. In marked contrast to a Na+ increase, we observed that the cytosolic Na+ level decreased in a time-dependent manner after cells were incubated with OSW-1. Thus, it is unlikely that this compound inhibits Na+/K+-ATPase.
Our study showed that OSW-1 caused a time-dependent decrease in cytosolic Na+ coupled with a time-dependent increase in cytosolic Ca2+ in a fashion similar to the NCX inhibitor KB-R7943 (Fig. 6), suggesting that OSW-1 may function as an inhibitor of NCX. The NCX is an antiporter expressed in all mammalian cells that can exchange Na+ and Ca2+ in either direction depending on the transmembrane electrical gradients and the cytosolic Na+ and Ca2+ concentrations (46–48). Furthermore, the co-incubation of cells with OSW-1 and KB-R7943 did not induce additional decreases in Na+, suggesting that both compounds might act upon the same ion transporter. If OSW-1 and KB-R7943 affect different targets, co-incubation of the two compounds would be expected to cause an additional reduction of Na+, which did not occur. Interestingly, Western blot analysis showed that OSW-1 caused an initial increase of NCX protein, which was subsequently decreased substantially (Fig. 6C), suggesting a possibility that OSW-1 might bind to NCX, inhibit its function, and affect its stability or turnover rate. Earlier work by Yokoyama (49) provided evidence that OSW-1 has a strong attractive interaction with a ganglioside GM3 monolayer using atomic force microscopy. With this surface imaging technique, which is capable of nanometer-scale lateral resolution, structural changes in GM3 were noted upon OSW-1 binding. Interestingly, recent work showed a high affinity association between GM1, another ganglioside, and NCX1 in the nuclear envelope of neurons that regulate subcellular calcium dynamics (50). Because gangliosides are known to be ubiquitously expressed in virtually all vertebrate cells, investigation of this potential interaction among OSW-1, NCX1, and the specific ganglioside involved and its impact on calcium regulation is an area that deserves further investigation. Harley et al. (51) demonstrated that inhibition of NCX1.1 and sodium-proton exchanger 1 (NHE1) led to cell death in malignant gliomas. To our knowledge, this is the first report that illustrates the role of NCX in leukemia cell survival. This inhibitory effect of OSW-1 is cancer cell-specific with minimal effect on normal lymphocytes, a result in concurrence with the earlier study by Harley et al. (51) who found that the NCX inhibitors selectively kill malignant glioma cell lines but not primary astrocytes. Moreover, recent work by Burgett et al. (22) indicated that OSW-1 and other related compounds such as cephalostatin 1, ritterazine B, and schweinfurthin A may physically interact with the OSBP and ORP4L. Because OSW-1 contains a steroidal aglycon structure, it is not surprising that this compound may have a high affinity to bind OSBP and ORP4L. However, it is unclear whether such binding could lead to cytotoxicity. Moreover, shRNA knockdown of OSBP did not exhibit a similar cytotoxic effect as OSW-1, suggesting that OSBP might not be the key target of OSW-1 in term of cytotoxicity. Interestingly, Rodriguez et al. (52) demonstrated that RACK1, a protein kinase C anchoring protein homologous to G protein β complex, interacted with the pleckstrin homology domain of OSBP, whereas a report by Liu et al. (53) showed that calcium was essential for the interaction of calmodulin with G protein β and γ subunits. Therefore, it is plausible that calcium might play a role in the regulation of OSBP expression, and the binding of OSW-1 with OSBP might be associated with molecules involved in calcium regulation. These mechanisms merit further investigation.
Because of the ubiquity of Ca2+ signaling, targeting Ca2+ regulators to kill cancer cells may have potential concern in terms of selectivity. However, growing evidence suggests that some Ca2+-mediated signaling pathways are implicated in metastasis, invasion, and angiogenesis and that alterations in the expression and function of Ca2+ regulators such as plasma membrane Ca2+ channels and exchangers likely have a role in the process of tumorigenesis (54–56). For instance, altered expression of Ca2+ pumps and channels has been observed in numerous cancer cell lines and tumors (54). Up-regulation of the plasma membrane Ca2+ pump PMCA4 is associated with differentiation of a human colon cancer cell line, whereas the ER Ca2+ pump SERCA3 is reduced or lost in colon carcinomas (57, 58). It has also been reported that a decrease in SERCA2b expression as the androgen-sensitive prostate cancer epithelial cells differentiate to become androgen-insensitive cells and that alteration in the Ca2+ homeostasis led to an increased apoptotic resistance (59). Therefore, a differential expression or altered function of the calcium regulators may potentially provide a basis for selectivity of OSW-1 against cancer cells. In fact, OSW-1 was shown to exert selective anticancer activity in various cancer types (21), suggesting that this compound is potentially useful as a novel agent in cancer treatment.
In summary, our study delineates the mechanisms behind the highly potent cytotoxic activity of OSW-1 in leukemia. Treatment of HL-60 cells with OSW-1 leads to disruption of cellular calcium homeostasis through inhibition of NCX1, loss of mitochondrial membrane potential, and subsequent cell death. More importantly, our finding that inhibition of NCX1 causes cell death in leukemia cells is novel and significant and suggests that NCX1 is a potential therapeutic target in leukemia.
Acknowledgments
We thank Helene Pelicano and Li Feng for technical assistance and support. Our gratitude is also extended to Lovell A. Jones and Richard A. Hajek from The Dorothy B. Height Center for Health Equity and Evaluation Research (DH-CHEER) at The University of Texas M. D. Anderson Cancer Center for support in the completion of this work.
This work was supported, in whole or in part, by National Institutes of Health Grants CA085563, CA100428, CA109041, CA16672, and P60MD000503.
- OSW-1
- 3β,16β,17α-trihydroxycholest-5-en-22-one 16-O-(2-O-4-methoxybenzoyl-β-d-xylopyranosyl)-(1→3)-2-O-acetyl-α-l-arabinopyranoside
- NCX
- sodium-calcium exchanger
- MMP
- mitochondrial membrane potential
- OSBP
- oxysterol-binding protein
- ORP4L
- OSBP-related protein 4L
- ER
- endoplasmic reticulum
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PI
- propidium iodide
- CsA
- cyclosporin A
- TG
- thapsigargin
- BAPTA
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- AM
- acetoxymethyl ester
- ALLN
- Ac-Leu-Leu-Nle-H where Nle is norleucine
- SERCA
- sarco/endoplasmic reticulum Ca2+-ATPase
- GM3
- NeuAcα2,3Galβ1,4Glc-ceramide
- GM1
- Galβ1,3GalNAcβ1,4(NeuAcα2,3)Galβ1,4Glc-ceramide.
REFERENCES
- 1. Kubo S., Mimaki Y., Terao M., Sashida Y., Nikaido T., Ohmoto T. (1992) Acylated cholestase glycosides from the bulbs of Ornithogalum saundersiae. Phytochemistry 31, 3969–3973 [Google Scholar]
- 2. Tschamber T., Adam S., Matsuya Y., Masuda S., Ohsawa N., Maruyama S., Kamoshita K., Nemoto H., Eustache J. (2007) OSW-1 analogues: modification of the carbohydrate moiety. Bioorg. Med. Chem. Lett. 17, 5101–5106 [DOI] [PubMed] [Google Scholar]
- 3. Wojtkielewicz A., Długosz M., Maj J., Morzycki J. W., Nowakowski M., Renkiewicz J., Strnad M., Swaczynová J., Wilczewska A. Z., Wójcik J. (2007) New analogues of the potent cytotoxic saponin OSW-1. J. Med. Chem. 50, 3667–3673 [DOI] [PubMed] [Google Scholar]
- 4. Tang P., Mamdani F., Hu X., Liu J. O., Yu B. (2007) Synthesis of OSW saponin analogs with modified sugar residues and their antiproliferative activities. Bioorg. Med. Chem. Lett. 17, 1003–1007 [DOI] [PubMed] [Google Scholar]
- 5. Shi B., Tang P., Hu X., Liu J. O., Yu B. (2005) OSW saponins: facile synthesis toward a new type of structures with potent antitumor activities. J. Org. Chem. 70, 10354–10367 [DOI] [PubMed] [Google Scholar]
- 6. Guo C., Fuchs P. L. (1998) The first synthesis of the aglycone of the potent anti-tumor steroidal saponin OSW-1. Tetrahedron Lett. 39, 1099–1102 [Google Scholar]
- 7. Deng S., Yu B., Lou Y., Hui Y. (1999) First total synthesis of an exceptionally potent antitumor saponin, OSW-1. J. Org. Chem. 64, 202–208 [DOI] [PubMed] [Google Scholar]
- 8. Ma X., Yu B., Hui Y., Miao Z., Ding J. (2001) Synthesis of OSW-1 analogues and a dimer and their antitumor activities. Bioorg. Med. Chem. Lett. 11, 2153–2156 [DOI] [PubMed] [Google Scholar]
- 9. Ma X., Yu B., Hui Y., Miao Z., Ding J. (2001) Synthesis of steroidal glycosides bearing the disaccharide moiety of OSW-1 and their antitumor activities. Carbohydr. Res. 334, 159–164 [DOI] [PubMed] [Google Scholar]
- 10. Ma X., Yu B., Hui Y., Xiao D., Ding J. (2000) Synthesis of glycosides bearing the disaccharide of OSW-1 or its 1→4-linked analogue and their antitumor activities. Carbohydr. Res. 329, 495–505 [DOI] [PubMed] [Google Scholar]
- 11. Yu W., Jin Z. (2001) A new strategy for the stereoselective introduction of steroid side chain via α-alkoxy vinyl cuprates: total synthesis of a highly potent antitumor natural product OSW-1. J. Am. Chem. Soc. 123, 3369–3370 [DOI] [PubMed] [Google Scholar]
- 12. Yu W., Jin Z. (2002) Total synthesis of the anticancer natural product OSW-1. J. Am. Chem. Soc. 124, 6576–6583 [DOI] [PubMed] [Google Scholar]
- 13. Morzycki J. W., Wojtkielewicz A. (2002) Synthesis of a cholestane glycoside OSW-1 with potent cytostatic activity. Carbohydr. Res. 337, 1269–1274 [DOI] [PubMed] [Google Scholar]
- 14. Morzycki J. W., Wojtkielewicz A., Wołczyński S. (2004) Synthesis of analogues of a potent antitumor saponin OSW-1. Bioorg. Med. Chem. Lett. 14, 3323–3326 [DOI] [PubMed] [Google Scholar]
- 15. Zheng D., Zhou L., Guan Y., Chen X., Zhou W., Lei P. Synthesis of cholestane glycosides bearing OSW-1 disaccharide or its 1→4-linked analogue and their antitumor activities. Bioorg. Med. Chem. Lett. 20, 5439–5442 [DOI] [PubMed] [Google Scholar]
- 16. Zheng D., Guan Y., Chen X., Xu Y., Chen X., Lei P. (2011) Synthesis of cholestane saponins as mimics of OSW-1 and their cytotoxic activities. Bioorg. Med. Chem. Lett. 21, 3257–3260 [DOI] [PubMed] [Google Scholar]
- 17. Guan Y., Zheng D., Zhou L., Wang H., Yan Z., Wang N., Chang H., She P., Lei P. (2011) Synthesis of 5(6)-dihydro-OSW-1 analogs bearing three kinds of disaccharides linking at 15-hydroxy and their antitumor activities. Bioorg. Med. Chem. Lett. 21, 2921–2924 [DOI] [PubMed] [Google Scholar]
- 18. Maj J., Morzycki J. W., Rárová L., Oklest'ková J., Strnad M., Wojtkielewicz A. (2011) Synthesis and biological activity of 22-deoxo-23-oxa analogues of saponin OSW-1. J. Med. Chem. 54, 3298–3305 [DOI] [PubMed] [Google Scholar]
- 19. Tamura K., Honda H., Mimaki Y., Sashida Y., Kogo H. (1997) Inhibitory effect of a new steroidal saponin, OSW-1, on ovarian functions in rats. Br. J. Pharmacol. 121, 1796–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu J., Xiong L., Yu B., Wu J. (2005) Apoptosis induced by a new member of saponin family is mediated through caspase-8-dependent cleavage of Bcl-2. Mol. Pharmacol. 68, 1831–1838 [DOI] [PubMed] [Google Scholar]
- 21. Zhou Y., Garcia-Prieto C., Carney D. A., Xu R. H., Pelicano H., Kang Y., Yu W., Lou C., Kondo S., Liu J., Harris D. M., Estrov Z., Keating M. J., Jin Z., Huang P. (2005) OSW-1: a natural compound with potent anticancer activity and a novel mechanism of action. J. Natl. Cancer Inst. 97, 1781–1785 [DOI] [PubMed] [Google Scholar]
- 22. Burgett A. W., Poulsen T. B., Wangkanont K., Anderson D. R., Kikuchi C., Shimada K., Okubo S., Fortner K. C., Mimaki Y., Kuroda M., Murphy J. P., Schwalb D. J., Petrella E. C., Cornella-Taracido I., Schirle M., Tallarico J. A., Shair M. D. (2011) Natural products reveal cancer cell dependence on oxysterol-binding proteins. Nat. Chem. Biol. 7, 639–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yan D., Lehto M., Rasilainen L., Metso J., Ehnholm C., Ylä-Herttuala S., Jauhiainen M., Olkkonen V. M. (2007) Oxysterol binding protein induces upregulation of SREBP-1c and enhances hepatic lipogenesis. Arterioscler. Thromb. Vasc. Biol. 27, 1108–1114 [DOI] [PubMed] [Google Scholar]
- 24. Wang P. Y., Weng J., Anderson R. G. (2005) OSBP is a cholesterol-regulated scaffolding protein in control of ERK 1/2 activation. Science 307, 1472–1476 [DOI] [PubMed] [Google Scholar]
- 25. Ngo M. H., Colbourne T. R., Ridgway N. D. (2010) Functional implications of sterol transport by the oxysterol-binding protein gene family. Biochem. J. 429, 13–24 [DOI] [PubMed] [Google Scholar]
- 26. Schulz T. A., Choi M. G., Raychaudhuri S., Mears J. A., Ghirlando R., Hinshaw J. E., Prinz W. A. (2009) Lipid-regulated sterol transfer between closely apposed membranes by oxysterol-binding protein homologues. J. Cell Biol. 187, 889–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beran M., Pisa P., O'Brien S., Kurzrock R., Siciliano M., Cork A., Andersson B. S., Kohli V., Kantarjian H. (1993) Biological properties and growth in SCID mice of a new myelogenous leukemia cell line (KBM-5) derived from chronic myelogenous leukemia cells in the blastic phase. Cancer Res. 53, 3603–3610 [PubMed] [Google Scholar]
- 28. Zhang H., Trachootham D., Lu W., Carew J., Giles F. J., Keating M. J., Arlinghaus R. B., Huang P. (2008) Effective killing of Gleevec-resistant CML cells with T315I mutation by a natural compound PEITC through redox-mediated mechanism. Leukemia 22, 1191–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wetzler M., Talpaz M., Van Etten R. A., Hirsh-Ginsberg C., Beran M., Kurzrock R. (1993) Subcellular localization of Bcr, Abl, and Bcr-Abl proteins in normal and leukemic cells and correlation of expression with myeloid differentiation. J. Clin. Investig. 92, 1925–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Babcock D. F., Herrington J., Goodwin P. C., Park Y. B., Hille B. (1997) Mitochondrial participation in the intracellular Ca2+ network. J. Cell Biol. 136, 833–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rizzuto R. (2003) Calcium mobilization from mitochondria in synaptic transmitter release. J. Cell Biol. 163, 441–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McConkey D. J., Orrenius S. (1996) The role of calcium in the regulation of apoptosis. J. Leukoc. Biol. 59, 775–783 [PubMed] [Google Scholar]
- 33. Pyrko P., Schönthal A. H., Hofman F. M., Chen T. C., Lee A. S. (2007) The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 67, 9809–9816 [DOI] [PubMed] [Google Scholar]
- 34. Daneshmand S., Quek M. L., Lin E., Lee C., Cote R. J., Hawes D., Cai J., Groshen S., Lieskovsky G., Skinner D. G., Lee A. S., Pinski J. (2007) Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum. Pathol. 38, 1547–1552 [DOI] [PubMed] [Google Scholar]
- 35. Camello C., Lomax R., Petersen O. H., Tepikin A. V. (2002) Calcium leak from intracellular stores—the enigma of calcium signalling. Cell Calcium 32, 355–361 [DOI] [PubMed] [Google Scholar]
- 36. Khorchid A., Ikura M. (2002) How calpain is activated by calcium. Nat. Struct. Biol. 9, 239–241 [DOI] [PubMed] [Google Scholar]
- 37. Ashby M. C., Tepikin A. V. (2001) ER calcium and the functions of intracellular organelles. Semin. Cell Dev. Biol. 12, 11–17 [DOI] [PubMed] [Google Scholar]
- 38. Orrenius S., Zhivotovsky B., Nicotera P. (2003) Regulation of cell death: the calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 4, 552–565 [DOI] [PubMed] [Google Scholar]
- 39. Nicotera P., Orrenius S. (1998) The role of calcium in apoptosis. Cell Calcium 23, 173–180 [DOI] [PubMed] [Google Scholar]
- 40. Berridge M., Lipp P., Bootman M. (1999) Calcium signalling. Curr. Biol. 9, R157–R159 [DOI] [PubMed] [Google Scholar]
- 41. Li J., Lee A. S. (2006) Stress induction of GRP78/BiP and its role in cancer. Curr. Mol. Med. 6, 45–54 [DOI] [PubMed] [Google Scholar]
- 42. Wu Y., Zhang H., Dong Y., Park Y. M., Ip C. (2005) Endoplasmic reticulum stress signal mediators are targets of selenium action. Cancer Res. 65, 9073–9079 [DOI] [PubMed] [Google Scholar]
- 43. Dong D., Ko B., Baumeister P., Swenson S., Costa F., Markland F., Stiles C., Patterson J. B., Bates S. E., Lee A. S. (2005) Vascular targeting and antiangiogenesis agents induce drug resistance effector GRP78 within the tumor microenvironment. Cancer Res. 65, 5785–5791 [DOI] [PubMed] [Google Scholar]
- 44. Pathak S., Multani A. S., Narayan S., Kumar V., Newman R. A. (2000) Anvirzel, an extract of Nerium oleander, induces cell death in human but not murine cancer cells. Anticancer Drugs 11, 455–463 [DOI] [PubMed] [Google Scholar]
- 45. Newman R. A., Kondo Y., Yokoyama T., Dixon S., Cartwright C., Chan D., Johansen M., Yang P. (2007) Autophagic cell death of human pancreatic tumor cells mediated by oleandrin, a lipid-soluble cardiac glycoside. Integr. Cancer Ther. 6, 354–364 [DOI] [PubMed] [Google Scholar]
- 46. Blaustein M. P., Lederer W. J. (1999) Sodium/calcium exchange: its physiological implications. Physiol. Rev. 79, 763–854 [DOI] [PubMed] [Google Scholar]
- 47. Iwamoto T., Kita S. (2006) Topics on the Na+/Ca2+ exchanger: role of vascular NCX1 in salt-dependent hypertension. J. Pharmacol. Sci. 102, 32–36 [DOI] [PubMed] [Google Scholar]
- 48. Lytton J. (2007) Na+/Ca2+ exchangers: three mammalian gene families control Ca2+ transport. Biochem. J. 406, 365–382 [DOI] [PubMed] [Google Scholar]
- 49. Yokoyama S. (2005) Distribution of an antitumor natural product OSW-1 in ganglioside GM3-phospholipid membranes. Mater. Technol. 23, 54–58 [Google Scholar]
- 50. Wu G., Xie X., Lu Z. H., Ledeen R. W. (2009) Sodium-calcium exchanger complexed with GM1 ganglioside in nuclear membrane transfers calcium from nucleoplasm to endoplasmic reticulum. Proc. Natl. Acad. Sci. U.S.A. 106, 10829–10834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Harley W., Floyd C., Dunn T., Zhang X. D., Chen T. Y., Hegde M., Palandoken H., Nantz M. H., Leon L., Carraway K. L., 3rd, Lyeth B., Gorin F. A. (2010) Dual inhibition of sodium-mediated proton and calcium efflux triggers non-apoptotic cell death in malignant gliomas. Brain Res. 1363, 159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rodriguez M. M., Ron D., Touhara K., Chen C. H., Mochly-Rosen D. (1999) RACK1, a protein kinase C anchoring protein, coordinates the binding of activated protein kinase C and select pleckstrin homology domains in vitro. Biochemistry 38, 13787–13794 [DOI] [PubMed] [Google Scholar]
- 53. Liu M., Yu B., Nakanishi O., Wieland T., Simon M. (1997) The Ca2+-dependent binding of calmodulin to an N-terminal motif of the heterotrimeric G protein β subunit. J. Biol. Chem. 272, 18801–18807 [DOI] [PubMed] [Google Scholar]
- 54. Monteith G. R., McAndrew D., Faddy H. M., Roberts-Thomson S. J. (2007) Calcium and cancer: targeting Ca2+ transport. Nat. Rev. Cancer 7, 519–530 [DOI] [PubMed] [Google Scholar]
- 55. Brini M., Carafoli E. (2009) Calcium pumps in health and disease. Physiol. Rev. 89, 1341–1378 [DOI] [PubMed] [Google Scholar]
- 56. Lipskaia L., Hulot J. S., Lompré A. M. (2009) Role of sarco/endoplasmic reticulum calcium content and calcium ATPase activity in the control of cell growth and proliferation. Pflugers Arch. 457, 673–685 [DOI] [PubMed] [Google Scholar]
- 57. Ribiczey P., Tordai A., Andrikovics H., Filoteo A. G., Penniston J. T., Enouf J., Enyedi A., Papp B., Kovács T. (2007) Isoform-specific up-regulation of plasma membrane Ca2+ATPase expression during colon and gastric cancer cell differentiation. Cell Calcium 42, 590–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Aung C. S., Kruger W. A., Poronnik P., Roberts-Thomson S. J., Monteith G. R. (2007) Plasma membrane Ca2+-ATPase expression during colon cancer cell line differentiation. Biochem. Biophys. Res. Commun. 355, 932–936 [DOI] [PubMed] [Google Scholar]
- 59. Vanoverberghe K., Vanden Abeele F., Mariot P., Lepage G., Roudbaraki M., Bonnal J. L., Mauroy B., Shuba Y., Skryma R., Prevarskaya N. (2004) Ca2+ homeostasis and apoptotic resistance of neuroendocrine-differentiated prostate cancer cells. Cell Death Differ. 11, 321–330 [DOI] [PubMed] [Google Scholar]