Background: The two-metal-ion mechanism of the nucleotidyl transfer reaction by RNA/DNA polymerases has not been adequately elucidated due to lack of temporal resolution.
Results: Soak-trigger-freeze x-ray crystallography revealed structures of natural, time-resolved intermediates of transcript initiation.
Conclusion: First structural evidence shows that catalytic metal binds after the nucleotide and nucleotide-binding metal and right before reaction chemistry.
Significance: Time-dependent soak-trigger-freeze x-ray crystallography can yield functionally relevant high resolution information to study enzyme reactions.
Keywords: Bacteriophage, RNA Polymerase, Structural Biology, Transcription, X-ray Crystallography, Bacteriophage N4, Nucleotidyl Transfer Reaction, Soak-trigger-freeze X-ray Crystallography
Abstract
The challenge for structural biology is to understand atomic-level macromolecular motions during enzymatic reaction. X-ray crystallography can reveal high resolution structures; however, one perceived limitation is that it reveals only static views. Here we use time-dependent soak-trigger-freeze X-ray crystallography, namely, soaking nucleotide and divalent metal into the bacteriophage RNA polymerase (RNAP)-promoter DNA complex crystals to trigger the nucleotidyl transfer reaction and freezing crystals at different time points, to capture real-time intermediates in the pathway of transcription. In each crystal structure, different intensities and shapes of electron density maps corresponding to the nucleotide and metal were revealed at the RNAP active site which allow watching the nucleotide and metal bindings and the phosphodiester bond formation in a time perspective. Our study provides the temporal order of substrate assembly and metal co-factor binding at the active site of enzyme which completes our understanding of the two-metal-ion mechanism and fidelity mechanism in single-subunit RNAPs. The nucleotide-binding metal (MeB) is coordinated at the active site prior to the catalytic metal (MeA). MeA coordination is only temporal, established just before and dissociated immediately after phosphodiester bond formation. We captured these elusive intermediates exploiting the slow enzymatic reaction in crystallo. These results demonstrate that the simple time-dependent soak-trigger-freeze X-ray crystallography offers a direct means for monitoring enzymatic reactions.
Introduction
The phosphoryl transfer reactions including the nucleotidyl transfer play a fundamental role in genome maintenance and gene expression (6, 7). Nucleotidyl transferases, DNA polymerase (DNAP)2 and RNA polymerase, catalyze the nucleophilic attack of the 3′-O oxyanion of the primer terminus onto the 5′-α-phosphate of the incoming nucleotide. The reaction proceeds via a “two-metal-ion mechanism” involving two or three conserved aspartates that position two divalent cations in the active site; a catalytic metal (MeA) is a Lewis acid to reduce the pKa of the primer 3′-OH bound at the P-site (+1 nucleotide in this study, Fig. 1d) whereas a nucleotide-binding metal (MeB) plays roles in coordinating the triphosphate groups of the nucleotide at the N-site (+2 nucleotide in this study, Fig. 1d) and in stabilizing the pentacovalent phosphate intermediate (4–6, 8). These enzymes are characterized by the flexibility of substrate utilization: DNAP uses four different deoxynucleotide triphosphates, and RNAP uses four different nucleotide triphosphates, combined with DNA sequence-dependent correct nucleotide addition (9, 10).
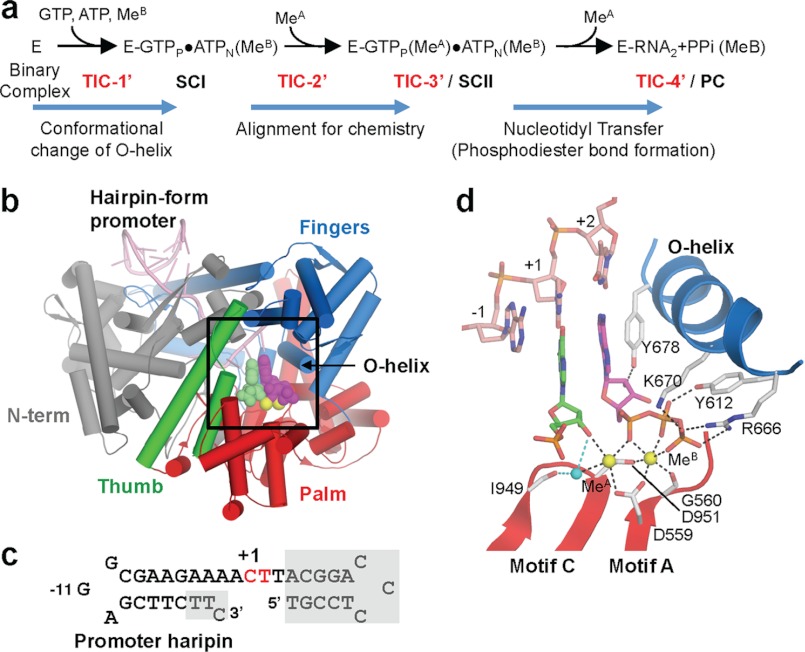
FIGURE 1.
Structure of the transcript initiation complex. a, schematic of the transcript initiation. The RNAP and promoter DNA complex is depicted as E. The appearance of intermediates, from TIC-1′ to TIC-4′, isolated in this study as well as SCI, SCII, and PC identified in the previous study (17) are indicated. b, overall structure of the TIC-3′. Each domain and the O-helix of RNAP are denoted by a unique color and labeled. c, sequence of the P2_7c DNA used for crystallization (red, +1 and +2 nucleotide binding sites; gray boxes, disordered in the crystal structures). The position of promoter hairpin is indicated. d, TIC-3′ active site. The amino acid residues involved in nucleotides and metal binding are shown. Nucleotides of +1 (green) and +2 (magenta) are shown. Mn2+ and water are depicted by yellow and cyan spheres, respectively.
Stopped-flow kinetics (9–12) and x-ray crystallographic studies of the A-family polymerases, including bacterial DNAP I Klenow fragment (13, 14) and bacteriophage DNAP (15) and RNAPs (16, 17), have elucidated the kinetic scheme of the nucleotide addition cycle and provided structural perspectives on nucleotide incorporation and selection. These studies proposed the presence of at least two prechemistry steps as the nucleotide selectivity checkpoints, including conformational change of the Fingers O-helix and another unidentified step preceding phosphodiester bond formation (9, 10).
One of the strategies for trapping elusive intermediate of nucleic acid polymerases is the use of a 3′-deoxynucleotide analog (13–15, 18), which lacks the essential O3′-nucleophile for the phosphodiester bond formation. The intermediate structures captured by using this nucleotide analog revealed the rotation of Fingers subdomain including the O-helix, which is known as the “closing of Fingers,” upon nucleotide binding at the active site and proposed that the DNA rearrangement plus the closing of Fingers is the early fidelity checkpoint of the nucleotide selection. However, the 3′-deoxynucleotide analog is not able to coordinate MeA, resulting in misalignment of the reactive groups. Another actively used substrate analog is the nonreactive NMPCPP, which allows the coordination of the critical MeA but prevents the phosphodiester bond formation due to the nonhydrolyzable methylene substitution on its triphosphate. Using this analog, intermediates in the T7 RNAP elongation and bacteriophage N4 RNAP initiation pathways were trapped and revealed the prechemistry events (16, 17, 19). These structures showed that substrate and metal binding induced the closing of the Fingers to engage basic residues on the O-helix to interact with the incoming nucleotide triphosphates and thus provided the structural basis of the primary substrate selection mechanism. Our recent studies of transcript initiation by the central domain of N4 phage virion-encapsulated RNAP (mini-vRNAP), thus presented the substrate complex (SCII) structure in transcript initiation, containing all atoms at the active site, including MeA, MeB, and reactive groups of nucleotides, poised for the nucleotidyl transfer reaction (17). We also determined two other crystal structures of transcript initiation by soaking natural nucleotides into the binary complex crystals that yielded a prechemistry substrate complex (SCI) and a postchemistry product complex (PC), which provide a complete set of snapshots during the transcript initiation process from the nucleotide and metal bindings to the phosphodiester bond formation (Fig. 1a). Transition from a binary RNAP-promoter DNA complex (BC) to the ternary SCI mainly involved the closing of Fingers and template DNA rearrangement. Although the closing of Fingers is a common early conformational change seen in all A-family polymerases upon substrate binding, the extent of movements is not the same in all. The rotation of the Fingers is more modest in T7 RNAP (16, 20) with respect to DNAP I (14, 15) and further subtle in the N4 mini-vRNAP (17). Comparison of the two precatalytic intermediates SCI and SCII identified critical realignment of the substrates to a catalysis-competent conformation brought about by the addition of catalytic MeA in SCII. We thus proposed that the MeA binding is the last step before the phosphodiester bond formation. The same conclusion was also proposed by the stopped-flow kinetics of DNAP I (11). The kinetic scheme of the same N4 mini-vRNAP transcript initiation process in crystallo was investigated by Raman crystallography and revealed that the DNA rearrangement and the closing of Fingers are completed immediately after soaking nucleotide and metal into RNAP-DNA complex crystals whereas the metal binding and phosphodiester bond formation occurred at a later stage of the reaction (21). This work showed that the enzyme remains active in crystallo and found that the single-turnover nucleotidyl transfer reaction in crystallo proceeds substantially slower (seconds to minutes) compared with the one in solution 300/min (17). On the other hand, it raised an intriguing question: what is the temporal order of these events? To answer this question, in this study, we used the time-dependent soak-trigger-freeze X-ray crystallography to film the transcription at atomic resolution. Highly concentrated RNAP (5 mm) in the small volume of crystals (0.3 nl, see “Experimental Procedures”) allows complete diffusion of nucleotide-metal to all binary complexes in the crystal before the reaction starts. This unique and ideal environment of enzyme in crystallo could synchronize the reaction of all molecules and capture some hitherto undetected and unstable intermediates by freezing crystals at different time steps during the reaction.
EXPERIMENTAL PROCEDURES
Transcript initiation complexes were prepared as follows. BC crystals (17) were sequentially transferred through cryoprotectant solutions containing the mother liquor with increasing concentrations of PEG 3350 (30, 35, and 40%). Then crystals were transferred to the soak solution containing 5 mm GTP, 5 mm ATP, and 10 mm MnCl2 in addition to the final cryosolution composition. Crystals were harvested from the soaking solution at specific time points and flash frozen in liquid nitrogen. Crystallographic data were processed with HKL2000 (22) (Table 1). The BC structure (23) was used as an initial model for the rigid body and TLS refinements by using Phenix (24). Nucleotides were modeled into the respective Fo − Fc electron density maps by Coot (25). Final coordinates and structure factors were submitted to the Protein Data Bank; IDs codes are listed in Table 1.
TABLE 1.
Data collection and refinement statistics of the N4 mini-vRNAP transcript initiation complexes
Data sets were collected at MacCHESS-F1, Ithaca, NY. Highest resolution shell is shown in parentheses.
| Complex | TIC-1′ | TIC-2′ | TIC-3′ | TIC-4′ |
|---|---|---|---|---|
| Crystal parameters | ||||
| PDB code | 4FF1 | 4FF2 | 4FF3 | 4FF4 |
| Space group | P212121 | P212121 | P212121 | P212121 |
| Cell dimensions | ||||
| a (Å) | 82.163 | 81.908 | 82.019 | 82.398 |
| b (Å) | 111.500 | 111.437 | 111.652 | 111.779 |
| c (Å) | 277.103 | 275.760 | 276.145 | 276.894 |
| Data collection | ||||
| Resolution (Å) | 50–2.46 | 50–2.00 | 50–2.00 | 50–2.00 |
| Rsym | 0.116 (0.426) | 0.186 (0.806) | 0.105 (0.546) | 0.127 (0.583) |
| I/σ | 15.6 (2.9) | 7.4 (1.2) | 15.8 (2.6) | 11.2 (1.4) |
| Completeness (%) | 95.4 (93.0) | 90.3 (75.4) | 89.0 (79.0) | 95.1 (84.6) |
| Redundancy | 5.0 (3.6) | 4.4 (3.4) | 3.8 (3.7) | 3.3 (2.4) |
| Refinement | ||||
| Resolution (Å) | 47.8–2.46 | 47.7–2.00 | 42.4–2.00 | 43.9–2.03 |
| No. reflections | 87,771 | 154,842 | 153,225 | 156,753 |
| Rwork/Rfree | 15.3/21.1 | 20.8/25.5 | 18.4/21.6 | 19.1/23.9 |
| Root mean square deviations | ||||
| Bond length (Å) | 0.007 | 0.004 | 0.004 | 0.006 |
| Bond angles (°) | 1.075 | 0.831 | 0.820 | 0.929 |
Final concentration, c, of the N4 phage polymerase-DNA complex in a single crystal was calculated using Equation 1,
where n is the number of molecules of complex per unit cell of the crystal; v is the volume of a unit cell, and NA is Avogadro's number. The BC crystals contain two RNAP-DNA complexes per asymmetric unit, and in the P212121 space group each unit cell has four asymmetric units. Thus there are n = 8 molecules/unit cell. From the unit cell parameters (82 Å × 112 Å × 276 Å), v is calculated to be 2.54 × 10−12 nl. Solving the Equation 1 concentration of complex in crystal, c = 5.1 mm. The volume, V, of a typical crystal was calculated from its dimensions (25 μm × 100 μm × 125 μm) = 0.3125 × 106 μm3 = 0.31 nl.
RESULTS
The BC crystal contains N4 mini-vRNAP bound to its promoter DNA (P2_7c, 36 bases, Fig. 1c), which has the template DNA bases of C(+1) and T(+2). Addition of GTP and ATP plus divalent cations allows formation of the transcript initiation complex (TIC) accommodating GTP at +1 position (P-site) and ATP at +2 position (N-site) that, upon phosphodiester bond formation, yields the 5′-pppGpA-3′ 2-mer RNA and pyrophosphate (Fig. 1). The BC and all TIC structures resemble a “cupped right hand” (Fig. 1b), and the active site located at the bottom of this cup is not involved in crystal packing. Although the N4 mini-vRNAP is active in crystallo (17, 21), the crystal packing prevents a next round of reaction, which provides an ideal environment for observing the single-turnover reaction. The intermediates were trapped by freezing crystals at different time points from 1 to 4 min (Fig. 2a) after transfer of the BC crystals to the soaking solution containing GTP, ATP, and Mn2+; their structures are indicated as TIC-1′, TIC-2′, TIC-3′, and TIC-4′. In each TIC structure, different intensities and shapes of Fo − Fc electron density maps, corresponding to nucleotides and metals, were revealed at their active sites (Fig. 2, b–e). These unbiased Fo − Fc maps allowed true evaluation of the occupancies of nucleotides and metals and the phosphodiester bond formation during the course of reaction.
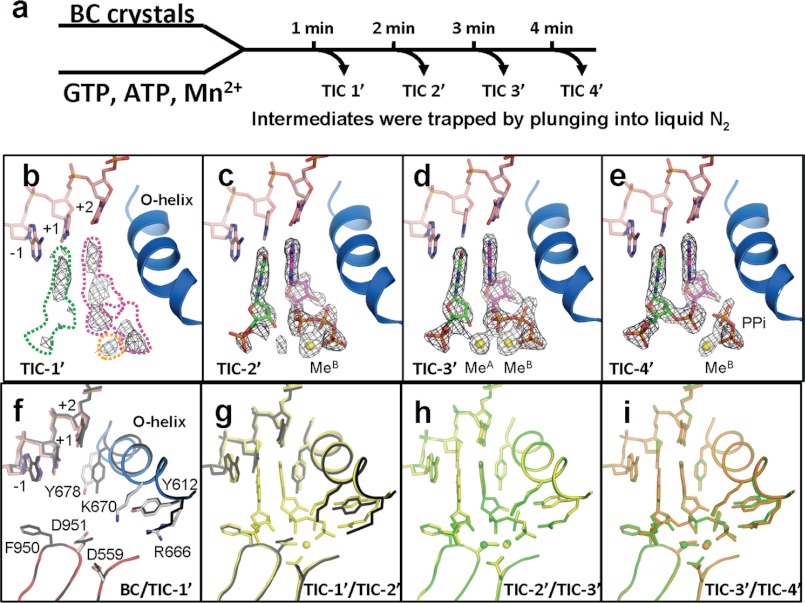
FIGURE 2.
Structures of RNAP active site, DNA, nucleotides, and metals during the phosphodiester bond formation. a, scheme of TIC preparations and structure determinations by the time-dependent soak-trigger-freeze X-ray crystallography. b–e, electron density (Fo − Fc, σ cutoff = 4) maps showing nucleotides, 2-mer RNA, pyrophosphate, and metals found in the TIC-1′–TIC-4′ structures. These maps were calculated using the native amplitudes and the phase derived from the final structures without nucleotides, metals, 2-mer RNA, and/or pyrophosphate. In b, the positions of GTP(+1), ATP(+2), and MeB binding sites, which are based on the TIC-3′ structure (d), are indicated by green, magenta, and yellow dashed lines, respectively. f–i, conformational changes of RNAP and DNA during the transcript initiation showing superposition of the BC versus TIC-1′ (f), TIC-1′ versus TIC-2′ (g), TIC-2′ versus TIC-3′ (h), and TIC-3′ versus TIC-4′ (i). BC is colored as in Fig. 1d, and TIC-1′, TIC-2′, TIC-3′, and TIC-4′ are colored in black, yellow, green, and orange, respectively.
After 1 min of soaking (TIC-1′, Fig. 2b), the active site shows a discontinuous Fo − Fc map corresponding to GTP, ATP, and faint density for MeB, indicating the presence of nucleotides and metal with partial occupancies. However, the O-helix has almost completed the conformational change and residue Y-678 in the helix has swung back to make a space for ATP(+2) binding (Fig. 2f). The electron density maps for Y-678 and DNA template from −1 to +2 positions are not solid (Fig. 3b) and were also observed in the BC structure (Fig. 3a), and the B-factor of the Y-678 side chain is substantially high (59.5), suggesting ongoing conformation changes of the O-helix.
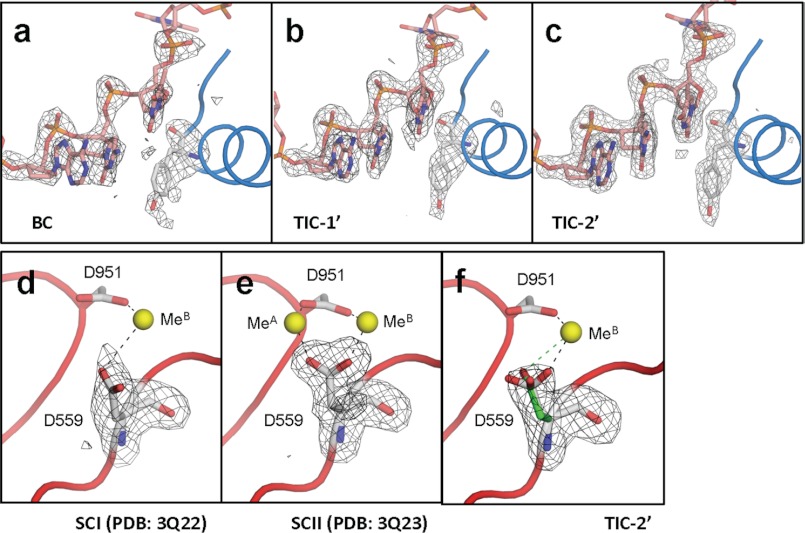
FIGURE 3.
Structural transitions of the active site. a–c, Fo − Fc maps (black net, σ cutoff = 3) showing Y-678 and DNA template from −1 to +2 positions superimposed on the final models (sticks and tubes) of BC (a), TIC-1′ (b), and TIC-2′ (c). d–f, Fo − Fc maps (black net, σ cutoff = 3) showing D-559 superimposed on the final models (sticks, tubes, and spheres) of SCI (d), SCII (e), and TIC-2′ (f). Two conformers of D-559 found in the TIC-2′ are shown (green, MeB coordinating form; white, both MeA and MeB coordinating form).
At 2 min (TIC-2′, Fig. 2c), the electron density maps for Y-678 and DNA template are well ordered (Fig. 3c), and the Y-678 side chain B-factor is substantially low (22.6), indicating completion of the O-helix movement (Fig. 2g). Three Fingers residues, Y-612, R-666, and K-670, which play a role in the +2 nucleotide binding also change their positions (Fig. 2g). Concomitantly, continuous and complete Fo − Fc maps corresponding to GTP(+1) and ATP(+2) are observed. The electron densities for the β and γ phosphate groups of GTP(+1) are not well resolved due to their flexibility; therefore, GMP was modeled at the +1 position. In addition to nucleotides, a strong Fo − Fc map for MeB is found at the active site. The Fo − Fc map around the MeA binding site is weaker than the one for MeB, indicating the presence of Mn2+ with partial occupancy. In a previous study, we found that the Mn2+ coordinating D-559 changes its conformation upon MeA binding (17). Accordingly, the D-559 electron density map of TIC-2′ (Fig. 3f) is distinct from the ones observed from SCI (coordinating MeB, Fig. 3d) and SCII (coordinating MeA and MeB, Fig. 3e) (17), suggesting the presence of alternative conformers of the D-559 side chain and supporting the presence of Mn2+ with partial occupancy. After 2 min of soaking, some molecules contain GTP(+1), ATP(+2), and MeB corresponding to the SCI structure, and some molecules contain GTP(+1), ATP(+2), MeA and MeB corresponding to the SCII structure determined in our previous study (Fig. 1a) (17). TIC-2′ is an intermediate where the two nucleotides and MeB are loaded at the active site and it is waiting for the MeA binding to form the catalytically competent complex.
At 3 min (TIC-3′, Fig. 2d), a strong Fo − Fc map for MeA appears at the active site, and its intensity is equivalent to the one of MeB. Except the D-559 side chain orientation, loading of MeA does not trigger any other conformational change of RNAP and DNA (Fig. 2h). After 3 min of soaking, the active site contains GTP(+1), ATP(+2), MeA and MeB, corresponding to SCII isolated in our previous study (Fig. 1a) (17). However, in this study, we solved this elusive intermediate structure with natural substrate. TIC-3′ is a fully reactive, naturally existing enzyme-substrate-cofactor complex, the closest to the pentacovalent phosphate intermediate during the nucleotidyl transfer reaction.
At 4 min (TIC-4′, Fig. 2e), a strong Fo − Fc map for the +1 and +2 nucleotides is found, but there is continuous density between O3′(+1) and αP(+2) and a gap between the αP(+2) and βP(+2) groups, indicating that this map corresponds to the 2-mer RNA (5′-pppGpA-3′). The pyrophosphate (PPi) is still coordinated by residues R-666, K-670, and Y-612 and is observed with MeB; however, the Fo − Fc map for MeA is absent, indicating that the MeA dissociates from the active site immediately after phosphodiester bond formation. Accordingly, D-559 rotates its side chain (Fig. 2i) to approximately the same position found in the BC and SCI structures, indicating its coordination to single MeB (17). After 4 min of soaking, the active site contains the nascent 2-mer RNA, PPi, and MeB, corresponding to the PC isolated in our previous study (Fig. 1a) (17). The high B-factors for PPi (82.1) and MeB (92.1) suggest their dissociation from the active site.
DISCUSSION
In this study, we successfully captured four intermediate structures, from the nucleotide/metal bindings at the active site to the phosphodiester bond formation, of the N4 phage vRNAP transcript initiation (Figs. 1a and 2). Nucleotide soaking triggers the O-helix closing at early stage of reaction. After 1 min of soaking (TIC-1′, Fig. 2b), a weak but distinct Fo − Fc map for MeB is observed with fragmented +2 nucleotide electron density supporting a widely accepted but unproved prediction that MeB comes to the active site with nucleotide. After 2 min of soaking (TIC-2′, Fig. 2c), both +1 and +2 nucleotides as well as MeB bindings are completed but not MeA, which is only completed at 3 min of soaking (TIC-3′, Fig. 2d). This is a clear proof that MeB is loaded to the active site prior to MeA, which is the penultimate step before phosphodiester bond formation. The presence of MeA at the active site is only temporal during the reaction. After 4 min of soaking (TIC-4′, Fig. 2e), the active site possesses a 2-mer RNA, PPi, and MeB but not MeA, indicating that MeA dissociates from the active site immediately after the bond formation. Therefore, leaving MeA from the active site is the first step after bond formation and is preparing for a next round of reaction. Only a temporal presence of MeA at the active site is beneficial to prevent a reverse reaction pyrophosphorysis.
We established a kinetic scheme of N4 vRNAP transcript initiation (Fig. 1a) by using a soak-trigger-freeze x-ray crystallography experiment (Fig. 2a). The kinetic scheme is based on structural snapshots from multiple crystals; however, this scheme reflects the temporal order of events in single crystal because it is consistent with results from a Raman crystallography experiment, which uses a single crystal to trace the entire reaction. Furthermore, our scheme consists of the one proposed by the fluorescence-based assays and stopped flow kinetics for Escherichia coli DNAP I (11).
Why does MeA come to the active site just before the bond formation? The MeA is a key element of the nucleotidyl transfer reaction and plays multiple roles in catalysis. When the MeA coordination is established, it aligns the reactive groups of 3′-OH(+1) and αP(+2), and the active site proceeds to the SN2 reaction. The A-family polymerase is not able to coordinate MeA without properly oriented 3′-OH and αP groups from two different nucleotides. This requirement satisfies two essential functions of nucleic acid polymerase, catalytic efficiency and fidelity of nucleotide selection, by using a single atom at the active site. The presence of MeA is essential for catalysis and thereby the MeA is a great sensor of Watson-Crick base pairing between DNA template and nucleotide although base pairings are 10–12 Å away from the catalytic site. Whereas the A-family polymerase uses the O-helix movement as an early checkpoint of nucleotide selection, this additional kinetic checkpoint right before the bond formation is able to greatly enhance the fidelity (10).
Due to highly conserved active site architecture, the reaction scheme of nucleotidyl transfer reaction of the N4 vRNAP should be universal to all A-family polymerase, including bacterial DNAP I (13, 14), bacteriophage DNAP (15), and also pol γ in eukaryotes involved in the mitochondrial DNA replication (26). In contrast, the order of MeA and MeB bindings at the active site is different in multisubunit RNAP. The MeA is coordinated by three Asp residues in the absolutely conserved DFDGD motif in the largest subunit (27), and the MeA is coordinated at the active site without primer 3′-OH or nucleotide. This difference is consistent with the fact that the A-family polymerase carries out only the nucleotidyl transfer reaction whereas the multisubunit RNAP is capable of not only nucleotidyl transfer but also RNA hydrolysis, which plays a role in the transcription proofreading (28).
In each TIC structure, distinct shapes and intensities of Fo − Fc electron density maps corresponding to nucleotides and metals were revealed at their active sites (Fig. 2, b–e) although the times of soaking for each TIC preparation differ on the minutes time scale. Emerging evidence supports a hypothesis that the protein flexibility plays an important role in enhancing the enzyme catalysis speed (1, 29). In crystals, due to the crystal packing, protein flexibilities are restricted, therefore the nucleotidyl transfer reaction proceeds at a substantially slower rate (minutes) than in solution (milliseconds to seconds) (17, 21). Moreover, the RNAP-DNA complex is highly concentrated (5 mm) in small volume (0.3 nl) (“Experimental Procedures”). This unique environment allows diffusion of nucleotide plus metal to all binary complexes in short period of time and synchronizing the reaction of all molecules in crystal to reveal some hitherto undetected intermediates by freezing crystals sequentially and solving their high resolution crystal structures.
The mechanism of phosphoryl transfer reaction is explained by the two-metal-ion mechanism, which was proposed two decades ago (4, 5, 8). This model has been an important framework for explaining many reactions of nucleic acid enzymes, including DNAP, RNAP, self-splicing introns, and nucleases (6). Our study provided a new basis, chronology of substrate and metal bindings and protein conformational change, for the two-metal-ion mechanism. We demonstrated that the MeA binds after MeB and nucleotides and does so immediately before the chemistry step, phosphodiester bond formation. The MeA is a Lewis acid to reduce the pKa of the primer 3′-OH and aligns the reactive groups of 3′-OH and α-phosphate to proceed through the SN2 reaction. The MeA also satisfies the fidelity of nucleotide selection. The MeA loading depends on presence of the primer 3′-OH group and other ligand positions (9, 30), and both reactive groups, 3′-OH and α-phosphate, are involved in the MeA coordination. Therefore, the MeA can be a great sensor to monitor correct base pairing between DNA and nucleotide; once the Watson-Crick base pairs are established, the MeA binds at the active site and completes the reaction. Our structures provide evidence that the MeA binding is the final checkpoint of the nucleotide selection of A-family polymerase.
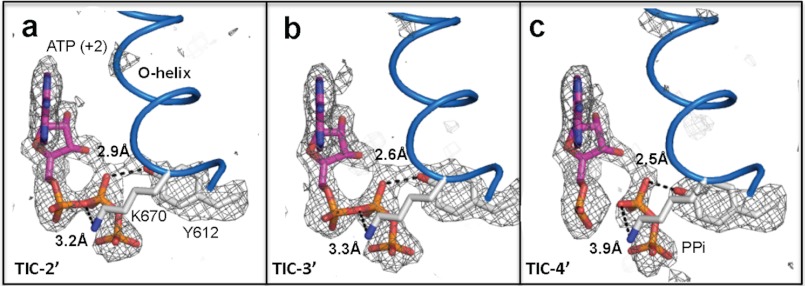
The proton transfer mechanism in the nucleotidyl transfer reaction has a longstanding question regarding the identification of a general base that accepts the proton to generate the 3′O nucleophile and a general acid, which provides a proton to stabilize the PPi leaving group. Although a conserved lysine residue at the O-helix has been proposed as a general acid in the reaction (31), K-670 in the N4 mini-vRNAP transcript initiation complex structures does not lie close to a bridging oxygen of the βP of ATP(+2) (Fig. 4). However, the -OH group of residue Y-612 is at a closer distance from the nonbridging oxygen at the βP (+2) (Fig. 4). We thus ask the question if this Y-612 was lending its hydrogen to stabilize the negative charge developed on the PPi leaving group and serving the general acid in the reaction. A mutation Y571F at this position of T7 RNAP reduces its catalytic activity (32), which supports the proposed role of this conserved tyrosine as a general acid. In case of A-family DNAP, histidine instead of tyrosine is found at this position. A substitution of histidine (H932Y) reduced the specificity constant kpol/KD of the human mitochondrial DNAP (pol γ) by ∼150-fold, and this effect is largely because of slowing down PPi release (33). Near neutral pKa of histidine imidazole group is a more efficient general acid, which might be a reason why DNAP is >10 times faster than RNAP for nucleotidyl transfer reaction. However, such a conclusion should await an ultra higher resolution structure or complementary studies capable of locating the proton between the Y-612 and the phosphate.
FIGURE 4.
Electron density of Y-612 suggesting proton transfer mechanism. a–c, Fo − Fc maps (black net, σ cutoff = 3) of Y-612 and ATP (+2) superimposed on final models (sticks, Y-612, K-670, and ATP (+2); scheme, O-helix) of TIC-2′ (a), TIC-3′ (b), and TIC-4′ (c). Distances between atoms (black dotted lines) are labeled.
Time-resolved structure determination techniques have been developed to film biological processes at atomic or near-atomic resolutions (1–3). Nuclear magnetic resonance (NMR) spectroscopy has a clear advantage and is able to reveal the time scale of protein motions with atomic resolution structure (34, 35), but it is applicable to only well behaved smaller proteins and is not able to monitor the reaction over a time course. Kinetic x-ray crystallography, Laue diffraction, is able to deliver unprecedented detail of enzymatic reaction (2, 3, 36); however, it demands on ultrafast triggering of the reaction, largely restricting the application to light-induced reactions. Due to these obvious restrictions, complex reactions such as DNA replication and transcription have been excluded from the scope of time-resolved structural study. A simplistic time-resolved soak-trigger-freeze approach used in this study is able to trace the reaction pathway at ambient temperatures without any modification of enzyme and substrate and without any specific equipment for x-ray data collection. The time-dependent soak-trigger-freeze x-ray crystallography can be applicable to other systems and become a general method to look directly at biological reactions (37).
Acknowledgments
We thank the staff at F1 of the MacCHESS for support crystallographic data collection; P. R. Carey and Y. Chen for discussions; and L. B. Rothman-Denes, P. C. Bevilacqua, S. J. Benkovic, and R. Yajima for critical reading of the manuscript. Figures were prepared using PyMOL.
This work was supported, in whole or in part, by National Institutes of Health Grant AI12575.
The atomic coordinates and structure factors (codes 4FF1, 4FF2, 4FF3, and 4FF4) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- DNAP
- DNA polymerase
- BC
- binary RNAP-promoter DNA complex
- MeA
- catalytic metal
- MeB
- nucleotide-binding metal
- NMPCPP
- 5′-[(α,β)-methyleno]nucleotide triphosphate
- PC
- product complex
- RNAP
- RNA polymerase
- SC
- substrate complex
- TIC
- transcript initiation complex
- vRNAP
- virion-encapsulated RNAP.
REFERENCES
- 1. Henzler-Wildman K., Kern D. (2007) Dynamic personalities of proteins. Nature 450, 964–972 [DOI] [PubMed] [Google Scholar]
- 2. Bourgeois D., Royant A. (2005) Advances in kinetic protein crystallography. Curr. Opin. Struct. Biol. 15, 538–547 [DOI] [PubMed] [Google Scholar]
- 3. Schmidt M. (2008) in Ultrashort Laser Pulses in Biology and Medicine (Braun M., Gilch P., Zinth W., eds) pp. 201–241, Springer, Berlin [Google Scholar]
- 4. Beese L. S., Steitz T. A. (1991) Structural basis for the 3′-5′ exonuclease activity of Escherichia coli DNA polymerase I: a two metal ion mechanism. EMBO J. 10, 25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steitz T. A., Steitz J. A. (1993) A general two-metal-ion mechanism for catalytic RNA. Proc. Natl. Acad. Sci. U.S.A. 90, 6498–6502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang W., Lee J. Y., Nowotny M. (2006) Making and breaking nucleic acids: two-Mg2+-ion catalysis and substrate specificity. Mol. Cell 22, 5–13 [DOI] [PubMed] [Google Scholar]
- 7. Lassila J. K., Zalatan J. G., Herschlag D. (2011) Biological phosphoryl-transfer reactions: understanding mechanism and catalysis. Annu. Rev. Biochem. 80, 669–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steitz T. A. (1998) A mechanism for all polymerases. Nature 391, 231–232 [DOI] [PubMed] [Google Scholar]
- 9. Johnson K. A. (2010) The kinetic and chemical mechanism of high-fidelity DNA polymerases. Biochim. Biophys. Acta 1804, 1041–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Joyce C. M., Benkovic S. J. (2004) DNA polymerase fidelity: kinetics, structure, and checkpoints. Biochemistry 43, 14317–14324 [DOI] [PubMed] [Google Scholar]
- 11. Bermek O., Grindley N. D., Joyce C. M. (2011) Distinct roles of the active-site Mg2+ ligands, Asp-882 and Asp-705, of DNA polymerase I (Klenow fragment) during the prechemistry conformational transitions. J. Biol. Chem. 286, 3755–3766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joyce C. M., Potapova O., Delucia A. M., Huang X., Basu V. P., Grindley N. D. (2008) Fingers-closing and other rapid conformational changes in DNA polymerase I (Klenow fragment) and their role in nucleotide selectivity. Biochemistry 47, 6103–6116 [DOI] [PubMed] [Google Scholar]
- 13. Johnson S. J., Taylor J. S., Beese L. S. (2003) Processive DNA synthesis observed in a polymerase crystal suggests a mechanism for the prevention of frameshift mutations. Proc. Natl. Acad. Sci. U.S.A. 100, 3895–3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Y., Korolev S., Waksman G. (1998) Crystal structures of open and closed forms of binary and ternary complexes of the large fragment of Thermus aquaticus DNA polymerase I: structural basis for nucleotide incorporation. EMBO J. 17, 7514–7525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doublié S., Tabor S., Long A. M., Richardson C. C., Ellenberger T. (1998) Crystal structure of a bacteriophage T7 DNA replication complex at 2.2 Å resolution. Nature 391, 251–258 [DOI] [PubMed] [Google Scholar]
- 16. Yin Y. W., Steitz T. A. (2004) The structural mechanism of translocation and helicase activity in T7 RNA polymerase. Cell 116, 393–404 [DOI] [PubMed] [Google Scholar]
- 17. Gleghorn M. L., Davydova E. K., Basu R., Rothman-Denes L. B., Murakami K. S. (2011) X-ray crystal structures elucidate the nucleotidyl transfer reaction of transcript initiation using two nucleotides. Proc. Natl. Acad. Sci. U.S.A. 108, 3566–3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kennedy W. P., Momand J. R., Yin Y. W. (2007) Mechanism for de novo RNA synthesis and initiating nucleotide specificity by T7 RNA polymerase. J. Mol. Biol. 370, 256–268 [DOI] [PubMed] [Google Scholar]
- 19. Temiakov D., Patlan V., Anikin M., McAllister W. T., Yokoyama S., Vassylyev D. G. (2004) Structural basis for substrate selection by T7 RNA polymerase. Cell 116, 381–391 [DOI] [PubMed] [Google Scholar]
- 20. Cheetham G. M., Steitz T. A. (1999) Structure of a transcribing T7 RNA polymerase initiation complex. Science 286, 2305–2309 [DOI] [PubMed] [Google Scholar]
- 21. Chen Y., Basu R., Gleghorn M. L., Murakami K. S., Carey P. R. (2011) Time-resolved events on the reaction pathway of transcript initiation by a single-subunit RNA polymerase: Raman crystallographic evidence. J. Am. Chem. Soc. 133, 12544–12555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Otwinowski Z., Minor W. (1997) Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 23. Gleghorn M. L., Davydova E. K., Rothman-Denes L. B., Murakami K. S. (2008) Structural basis for DNA-hairpin promoter recognition by the bacteriophage N4 virion RNA polymerase. Mol. Cell 32, 707–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., Zwart P. H. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Emsley P., Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 26. Lee Y. S., Kennedy W. D., Yin Y. W. (2009) Structural insight into processive human mitochondrial DNA synthesis and disease-related polymerase mutations. Cell 139, 312–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vassylyev D. G., Vassylyeva M. N., Zhang J., Palangat M., Artsimovitch I., Landick R. (2007) Structural basis for substrate loading in bacterial RNA polymerase. Nature 448, 163–168 [DOI] [PubMed] [Google Scholar]
- 28. Cheung A. C., Cramer P. (2011) Structural basis of RNA polymerase II backtracking, arrest and reactivation. Nature 471, 249–253 [DOI] [PubMed] [Google Scholar]
- 29. Bhabha G., Lee J., Ekiert D. C., Gam J., Wilson I. A., Dyson H. J., Benkovic S. J., Wright P. E. (2011) A dynamic knockout reveals that conformational fluctuations influence the chemical step of enzyme catalysis. Science 332, 234–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Batra V. K., Beard W. A., Shock D. D., Krahn J. M., Pedersen L. C., Wilson S. H. (2006) Magnesium-induced assembly of a complete DNA polymerase catalytic complex. Structure 14, 757–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Castro C., Smidansky E. D., Arnold J. J., Maksimchuk K. R., Moustafa I., Uchida A., Götte M., Konigsberg W., Cameron C. E. (2009) Nucleic acid polymerases use a general acid for nucleotidyl transfer. Nat. Struct. Mol. Biol. 16, 212–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rechinsky V. O., Chernov B. K., Dragan S. M., Kostyuk D. A., Tunitskaya V. L., Kochetkov S. N. (1995) Targeted mutagenesis identifies Asp-569 as a catalytically critical residue in T7 RNA polymerase. Mol. Gen. Genet. 247, 110–113 [DOI] [PubMed] [Google Scholar]
- 33. Batabyal D., McKenzie J. L., Johnson K. A. (2010) Role of histidine 932 of the human mitochondrial DNA polymerase in nucleotide discrimination and inherited disease. J. Biol. Chem. 285, 34191–34201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Henzler-Wildman K. A., Lei M., Thai V., Kerns S. J., Karplus M., Kern D. (2007) A hierarchy of timescales in protein dynamics is linked to enzyme catalysis. Nature 450, 913–916 [DOI] [PubMed] [Google Scholar]
- 35. Fraser J. S., Clarkson M. W., Degnan S. C., Erion R., Kern D., Alber T. (2009) Hidden alternative structures of proline isomerase essential for catalysis. Nature 462, 669–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schotte F., Lim M., Jackson T. A., Smirnov A. V., Soman J., Olson J. S., Phillips G. N., Jr., Wulff M., Anfinrud P. A. (2003) Watching a protein as it functions with 150-ps time-resolved x-ray crystallography. Science 300, 1944–1947 [DOI] [PubMed] [Google Scholar]
- 37. Nakamura T., Zhao Y., Yamagata Y., Hua Y. J., Yang W. (2012) Watching DNA polymerase η make a phosphodiester bond. Nature 487, 196–201 [DOI] [PMC free article] [PubMed] [Google Scholar]