Background: Oxidant-induced elevation in tissue factor activity increases thrombosis risk.
Results: Tissue factor can form a disulfide bridge with human cytosolic thioredoxin and be reduced by thioredoxin-thioredoxin reductase-NADPH along with a decrease in its activity.
Conclusion: Thioredoxin/thioredoxin reductase is involved in orchestration of tissue factor-dependent events.
Significance: Revealing novel modulators can be exploited for therapeutic benefits in tissue factor-related disorders.
Keywords: Coagulation Factors, Redox Regulation, Thioredoxin, Thioredoxin Reductase, Tissue Factor, Procoagulation
Abstract
Abnormally enhanced tissue factor (TF) activity is related to increased thrombosis risk in which oxidative stress plays a critical role. Human cytosolic thioredoxin (hTrx1) and thioredoxin reductase (TrxR), also secreted into circulation, have the power to protect against oxidative stress. However, the relationship between hTrx1/TrxR and TF remains unknown. Here we show reversible association of hTrx1 with TF in human serum and plasma samples. The association is dependent on hTrx1-Cys-73 that bridges TF-Cys-209 via a disulfide bond. hTrx1-Cys-73 is absolutely required for hTrx1 to interfere with FVIIa binding to purified and cell-surface TF, consequently suppressing TF-dependent procoagulant activity and proteinase-activated receptor-2 activation. Moreover, hTrx1/TrxR plays an important role in sensing the alterations of NADPH/NADP+ states and transducing this redox-sensitive signal into changes in TF activity. With NADPH, hTrx1/TrxR readily facilitates the reduction of TF, causing a decrease in TF activity, whereas with NADP+, hTrx1/TrxR promotes the oxidation of TF, leading to an increase in TF activity. By comparison, TF is more likely to favor the reduction by hTrx1-TrxR-NADPH. This reversible reduction-oxidation reaction occurs in the TF extracellular domain that contains partially opened Cys-49/-57 and Cys-186/-209 disulfide bonds. The cell-surface TF procoagulant activity is significantly increased after hTrx1-knockdown. The response of cell-surface TF procoagulant activity to H2O2 is efficiently suppressed through elevating cellular TrxR activity via selenium supplementation. Our data provide a novel mechanism for redox regulation of TF activity. By modifying Cys residues or regulating Cys redox states in TF extracellular domain, hTrx1/TrxR function as a safeguard against inappropriate TF activity.
Introduction
Tissue factor (TF)2 is found on cell-surface of many cell lines and also in blood, so-called blood-borne TF (1). As a cell surface receptor, TF binds and activates coagulation factor VII (FVII) to activated FVIIa (2). The formed TF·FVIIa complex in turn binds coagulation factor X (FX) to generate activated FXa. The latter in the presence of calcium catalyzes the conversion of prothrombin to thrombin, leading to platelet activation and fibrin formation and ultimately forming thrombus (3). Enhanced assembly of TF·FVIIa complex on cell-surface is correlated to thrombosis risk and involved in efficiently activating protease-activated receptor-2 (PAR2), which is relevant to cancer angiogenesis and inflammatory balance (4, 5).
The majority of TF molecules located on the cell surface are in a low procoagulant (cryptic) state and require “decryption” to show fully procoagulant activity (6). Although the mechanism underlying the process of TF “encryption/decryption” remains unclear, the influence of redox equilibria on TF-mediated blood coagulation has been suggested for decades (7). The ability of HgCl2 (8), phenylarsonic acid (9), protein disulfide isomerase, and glutathione (10) to modify TF procoagulant activity has indicated the importance of Cys residues in TF function. Relevant evidence has been provided by the detailed characterization of the Cys-186/-209 pair (11). Yet there still remains the challenge for the Cys-186/-209 pair to contribute to TF activity (12) and for the formation of the disulfide bond to be responsible for the increased TF activity (13). Some paradoxical results came from TF activation by not only oxidizing agent HgCl2 but also reducing agents GSH and DTT especially at 50 and 100 mmol/liter (13). It seems to be overlooked that a high concentration of GSH/DTT has side effects, including that DTT may induce apoptosis (14) and GSH has prooxidant potential (15).
The subject of this study is the relationship between TF and thioredoxin (Trx)/thioredoxin reductase (TrxR). Compared with protein disulfide isomerase, Trx/TrxR had a high negative redox potential. Mammalian Trx/TrxR can react with protein disulfide isomerase (16) and is also present in blood (17) as well as in cell membrane. Trx, TrxR, and NADP(H) form the Trx system. Within the Trx system, electrons transfer between NADP(H) and Trx via TrxR (18). The reduced form of Trx may reduce a broad range of substrates (19), including the proteins related to coagulation, such as β2-glycoprotein 1 (20) and human factor VIII complex (21). Dysfunction of Trx system is related to many disease processes (22).
Human cytosolic Trx (hTrx1) is a 12-kDa protein that can secrete into the extracellular environment (23) and translocate into the nucleus (24) and plasma membrane (25). hTrx1 contains active-site residues Cys-32 and Cys-35, which are essential for transferring reducing equivalent between TrxR and substrate (26). hTrx1 also contains three structural Cys residues at positions 62, 69, and 73. Of them, Cys-62 and Cys-69 may form a second disulfide under oxidative conditions (27), and Cys-73 located on the surface can form intermolecular disulfide bond.
Given that the Cys-186/-209 pair in TF extracellular domain is redox active (9) and the interaction of FVIIa with TF involves a labile disulfide in FVIIa (28), we reasoned that TF would be a good target for Trx/TrxR regulation. Particularly, the location of hTrx1 partially overlaps with TF, hTrx1-Cys-73 can engage in thiol-disulfide exchange reaction, and Trx-TrxR-NADPH has power to catalyze the reduction of disulfide bond in a wide range of substrates. Thus, previously unrecognized effect of hTrx1 or Trx system on TF activity most likely exists.
In this study we demonstrate that hTrx1 is associated with TF in human blood, and hTrx1/TrxR mainly acts as a negative regulator of TF activity. A new mechanism for regulating TF-mediated events is therefore proposed.
EXPERIMENTAL PROCEDURES
Reagents
Mouse anti-human TF monoclonal antibodies, FVIIa, FX, and 6-amino-1-naphthalenesulfonamide-based fluorogenic substrates were from Hematological Technologies Inc. (Essex Junction, VT). Mouse anti-human Trx1 monoclonal antibody, anti-human TrxR monoclonal antibody, and anti-human PAR2 monoclonal antibody were purchased from Santa Cruz Biotechnology, Inc. Horseradish peroxidase-conjugated secondary antibodies were from Invitrogen. Dylight 488 amine-reactive dye and EZ-Link iodoacetyl-PEG2-biotin were from Pierce. Streptavidin-agarose (Dynabeads M-280 Streptavidin) was from Invitrogen. Other reagents were purchased from Sigma unless otherwise noted.
Thioredoxin and Thioredoxin Reductase
Calf liver TrxR and recombinant hTrx1 were prepared following the methods described previously (29, 30). Activity of cellular Trx or TrxR was determined using the modified super-insulin assay (17).
Construction and Expression of Recombinant Proteins
As the potential pitfalls using a bacterial expression system to express a truncated version of human TF have been carefully excluded by other researchers (31), a similar system was used in this study to synthesize a larger amount of truncated human TF. Complementary DNA (cDNA) for truncated human TF (residues 1–243, including extracellular and transmembrane domains) was cloned into pET43.1a vector (Novagen, WI). To replace Cys-186 of TF with Ala, the forward primer was 5′-GGA TAA AGG AGA AAA CTA CGC GTT CAG T-3′, and the reverse primer was 5′-TCA CTG CTT GAA CAC TGA ACG CGT AGT T-3′. To mutate Cys-209 of TF to Ala, the forward primer was 5′-TAG AGG CTA TGG GCC AGG AG-3′, and the reverse primer was 5′-ATA GCC TCT ACC GGG CTG TC-3′. The QuikChange Site-directed Mutagenesis kit (Agilent Technologies, Inc.) was used to make point mutations. Escherichia coli BL21 (DE3) was used to express the recombinant proteins. When the cell density reached an A600 of ∼1.0, protein expression was induced with 0.5 mmol/liter isopropyl 1-thio-β-d-galactopyranoside followed by shifting temperature from 37 °C to 18 °C and incubating for another 24 h. The resulting poly-His-tagged TF was purified by Ni-iminodiacetic acid resin, then by an anti-TF immunoaffinity column. The phospholipids used for re-lipidating the purified recombinant TF consisted of 30% 1,2-dioleoyl-sn-glycero-3-phosphatidylserine and 70% 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (Avanti Polar Lipids). The purified recombinant truncated human TF (referred as rtTF) migrates as a single band on SDS-PAGE with molecular mass of ∼32 kDa (Fig. 1B). This protein specifically reacts with an anti-TF monoclonal antibody and is both active and soluble, suggesting that it folds properly. The amino acid sequence of this protein is identical with human TF (residues 1–243) (Figs. 5D and 6B).
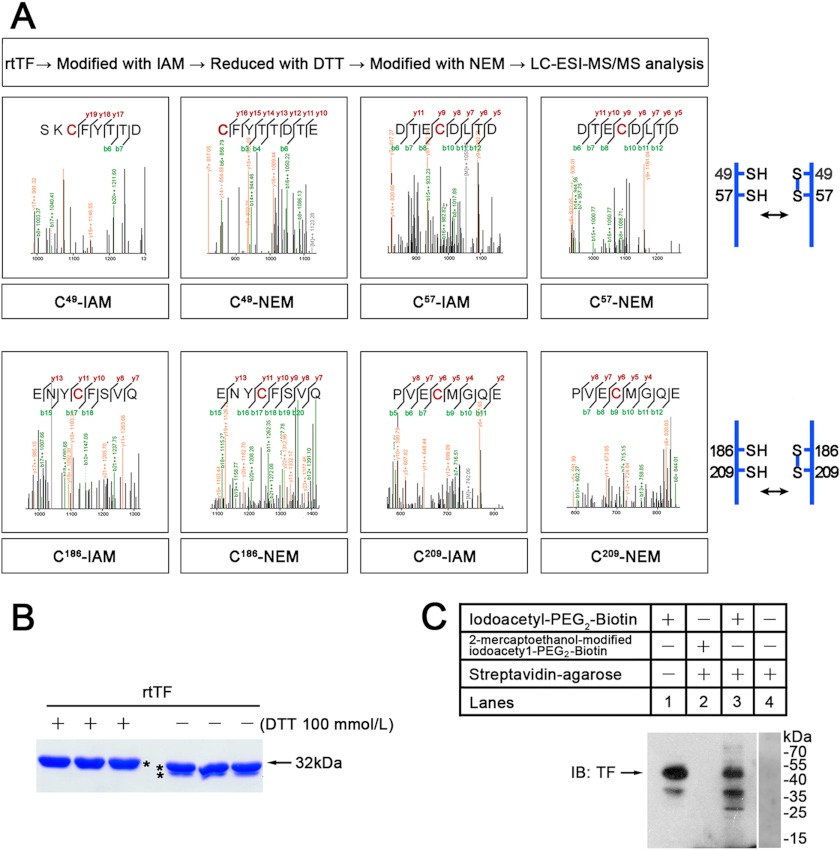
FIGURE 1.
Cys redox states of TF. A, shown is identification of Cys redox states in rtTF by LC-ESI-MS/MS. Free thiols are labeled with IAM, and DTT-induced thiols are labeled with NEM. B, shown are SDS-PAGE and the effect of DTT on electrophoretic mobility of rtTF. C, shown is a Western blot (IB) with anti-TF monoclonal antibody. To analyze free thiols in extracellular domain of cell-surface TF, intact cells were modified with membrane-impermeable thiol-reactive biotinylating reagent. Then the biotinylated proteins were separated using streptavidin-agarose beads. Lane 1, cell lysate control; lane 2, reagent control; lane 3, eluted proteins from the agarose; lane 4, agarose control. These results are representative of at least two independent experiments.
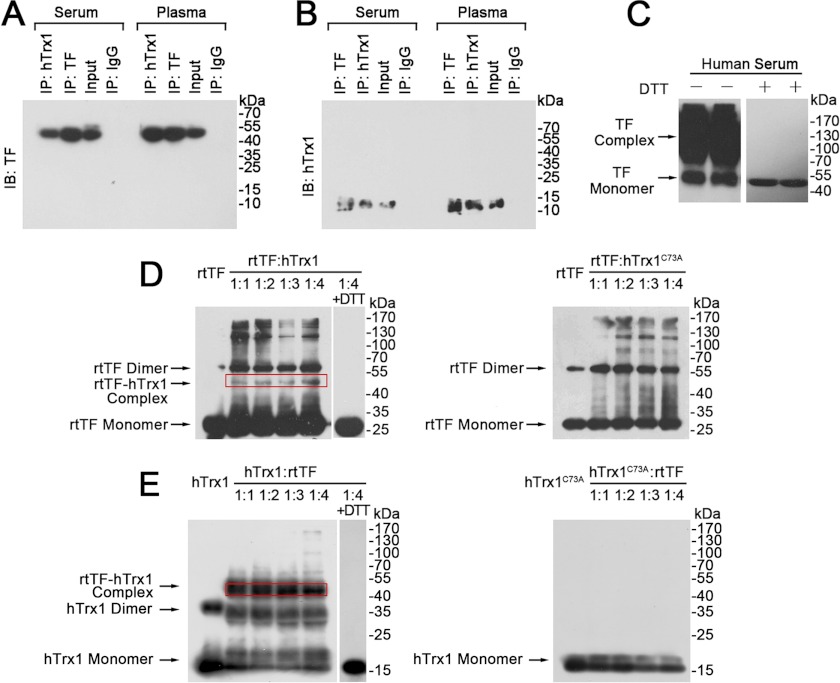
FIGURE 5.
Association of hTrx1 with TF. TF (A) and hTrx1 (B) were detected by Western blotting (IB) after co-immunoprecipitation (IP) from human serum and plasma. C, shown is a Western blot of human serum TF. The same amount of serum protein (140 μg) was loaded per well and separated on SDS-PAGE under non-reducing (left, without DTT) or reducing (right, with DTT) conditions. D, shown is Western blotting analysis of rtTF status in rtTF-hTrx1/hTrx1C73A mixture under non-reducing condition. The immunoblots were detected with anti-TF monoclonal antibody. E, shown is Western blotting analysis of hTrx1 or hTrx1C73A status in rtTF-hTrx1/hTrx1C73A mixture under non-reducing condition. The immunoblots were detected with anti-hTrx1 monoclonal antibody. These results are representative of at least three independent experiments.
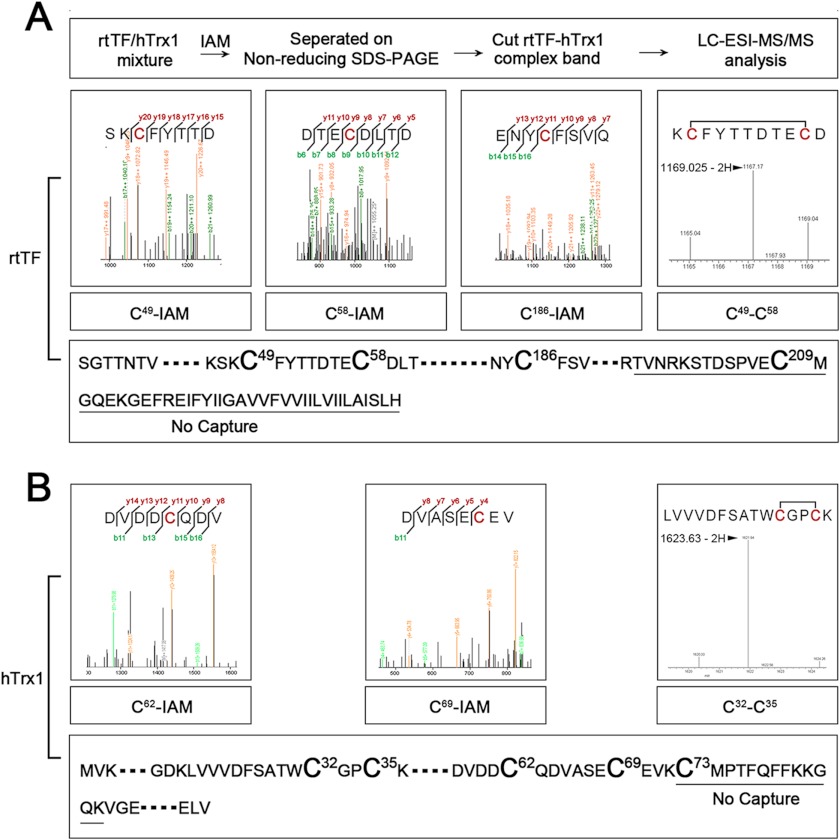
FIGURE 6.
hTrx1-Cys-73 bridges rtTF-Cys-209. The mixture of hTrx1 and rtTF was incubated with IAM to block free thiols and then separated by non-reducing SDS-PAGE. The protein band corresponding to TF-hTrx1 complex was cut out for in-gel tryptic digestion and LC-ESI-MS/MS analysis. A, MS/MS signals showed those corresponding to IAM-labeled Cys-47, Cys-59, and Cys-189 from rtTF. MS signals revealed a fragment containing Cys-47/-59 disulfide of rtTF. A peptide containing rtTF-Cys-209 was missing. B, MS/MS signals showed those corresponding to IAM-labeled Cys-62 and Cys-69 from hTrx1. MS signals contained a fragment containing Cys-32/-35 disulfide of hTrx1. A peptide containing Cys-73 was missing.
PAR2 cleavage reporter plasmid was constructed by fusing the cDNA encoding for secreted human placental alkaline phosphatase (SEAP or ALP) to the extracellular domain Asn-30 of human PAR2. The fusion region contains the amino acid sequence: UAAH PGUL QDY KDD DDV DAT LDP RNR SSK GR↓S LIG KVD, where the ALP sequence is underlined, the linker sequence in the junction between ALP and PAR2 is shown in bold, and the downward arrow indicates the FVIIa/trypsin cleavage site. The resulting ALP/PAR2 fusion cDNA, referred as ALP-PAR2, was then cloned into HindIII/NotI cloning sites of mammalian expression vector pcDNA3.1(+) (Invitrogen) (32).
Cell Culture and Selenium Supplementation
MDA-MB-231 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 1% penicillin/streptomycin and 10% fetal bovine serum (FBS) in a 37 °C, 5% CO2 humidified incubator. To supplementation selenium, the cells were cultured in the above medium plus 1 μmol/liter sodium selenite for 5 days, referred to as Se+ cells. The cells without sodium selenite supplementation are called as Se0 cells. Before other treatments were performed, Se+/Se0 cells were moved to 24-well plates and grown until confluence. Then the cells were washed with phosphate-buffered saline (PBS) and cultured in serum-free medium overnight.
RNA Interference
Transient knockdown of hTrx1 in MDA-MB-231 cells was achieved by using pGeneclip U1 Hairpin cloning systems (Promega). shRNAs bear fold-back stem-loop structures that were transcribed from the U1 promoter. Two DNA oligonucleotides were TrxiA (5′-TCT CAG ATG TGG ATG ACT GTC AGG ACT TCC TGT CAT CCT GAC AGT CAT CCA CAT CTC T-3′) and TrxiB (5′-CTG CAG AGA TGT GGA TGA CTG TCA GGA TGA CAG GAA GTC CTG ACA GTC ATC CAC ATC T-3′). These oligonucleotides form a DNA insert that contains the shRNA targeting sequence shown in bold type. The ends of this insert are compatible with the ends of pGeneclip vector and get ligated together. For transient transfection, 2 × 105 cells per well were seeded in a 6-well plate and grown for 1 day, then transfected with 4 μg of the shRNA plasmid in 10 μl of Lipofectamine 2000 (Invitrogen). The latter was diluted into 250 μl of Opti-MEM (Invitrogen). The same methods were used to construct control pGeneclip plasmid. Two DNA oligonucleotides were A (5′-TCT CTT CTC CGA ACG TGT CAC GTC TTC CTG TCA ACG TGA CAC GTT CGG AGA ACT-3′) and B (5′-CTG CAG TTC TCC GAA CGT GTC ACG TTG ACA GGA AGA CGT GAC ACG TTC GGA GAA-3′). The cells transfected with the control plasmid were used as negative controls. After 72 h, knockdown efficiency was evaluated by Western blotting.
Analysis for hTrx1/TrxR to Mediate Electron Transfer between rtTF and NADP(H)
The ability for Trx system to catalyze oxidation or reduction of rtTF was determined by following an increase or a decrease in absorbance at 340 nm due to the formation or consumption of NADPH. For measuring the formation of NADP(H), 500 μl of reaction mixtures contained 5 μmol/liter hTrx1, 0.2 mmol/liter NADP+/NADPH, 20 nmol/liter CL-TrxR1, and 1m mol/liter EDTA in 0.1 mol/liter potassium phosphate buffer, pH 7.4. The reaction was started by the addition of TF. Absorbance measurements were carried out in a Double Beam UV-visible spectrophotometer (UV-8500).
Assay of TF Procoagulant Activity
The activity was measured by following TF-dependent generation of FXa. To analyze cell-surface TF activity, confluent monolayers of the cells in a 96-well plate were washed twice with buffer A (10 mmol/liter HEPES, 0.15 mol/liter NaCl, 4 mmol/liter KCl, and 1mmol/liter glucose, pH 7.4) then incubated with 10 nmol/liter FVIIa and 175 nmol/liter FX in 25 μl of buffer B (buffer A plus 5 mmol/liter CaCl2 and 1 mg/ml BSA) at 37 °C for 30 min. The reaction was stopped by adding 50 μl of stopping buffer (20 mmol/liter Tris-HCl, 0.15 mol/liter NaCl, 1 mg/ml BSA, and 10 mmol/liter EDTA). A 50-μl aliquot from each well was withdrawn and added to a cuvette containing 250 μl of stopping buffer. The amount of generated FXa was determined based on its ability to cleave 6-amino-1-naphthalenesulfonamide-based fluorogenic substrates (final concentration of 33 μmol/liter). An increase in fluorescence intensity was measured (excitation at 352 nm and emission at 470 nm) using a HITACHI 4700 fluorescence spectrophotometer (HITACHI).
To analyze the effect of hTrx1-Cys-73 on purified rtTF activity, the relipidated rtTF (700 nmol/liter) in 50 mmol/liter Tris-HCl, pH 7.4, was mixed, respectively, with hTrx1 or hTrx1C73A from 1:1 to 1:4 molar ratios at room temperature. After 1 h, 0.25 μl of the mixture was added to 25 μl of buffer B containing 10 nmol/liter FVIIa and 175 nmol/liter FX, which was incubated at 37 °C. After 30 min, 25 μl of the reaction mixture was added to a cuvette containing 275 μl of stopping buffer. The amount of generated FXa was determined as stated above.
Dylight 488 Amine-Reactive Dye Labeling of FVIIa
Purified FVIIa (40 μg) was incubated with 10 μg of Dylight 488 amine-reactive dye in 100 μl of reaction buffer (0.1 mol/liter sodium phosphate, 0.15 mol/liter NaCl, pH 7.4) at room temperature for 1 h. Then the excess dye was removed using a dye removal column (Zeba Micro Spin Desalting Columns, 7000 molecular weight cutoff, Thermo Scientific Pierce®).
Flow Cytometry Analysis of FVIIa Binding to Cell-surface TF
The cells were cultured in 10-mm dish to 90% confluence. After washing twice with PBS, the cells were detached from the dish with 0.25% trypsin. The collected cells were washed twice with PBS to remove any residual trypsin, then suspended in 1.2 ml of binding buffer (10 mmol/liter HEPES, pH 7.4, 0.15 mol/liter NaCl, 4 mmol/liter KCl, 5 mmol/liter CaCl2, and 1 mmol/liter glucose), which were then divided equally into 6 vials. In each vial the cells were treated with 10 μmol/liter hTrx1 or hTrx1C73A at room temperature for 30 min, then FVIIa-Dylight 488 was added reaching a final concentration of 0.2 μmol/liter followed by incubation at room temperature for 30 min in the dark. The vials without FVIIa-Dylight 488 were used as background controls. After 3 washes in PBS, the cells were fixed in 1% paraformaldehyde for 10 min. Then the cells were washed 3 times with PBS again, resuspended in 200 μl of PBS, and analyzed using a FACSCalibur flow cytometer (BD Biosciences). Fluorescence data from 15,000 cells were collected and analyzed by FACSCalibur software.
Co-immunoprecipitation
Serum or plasma sample from healthy persons was diluted to 10 mg/ml protein concentration. In a preclearing step, the diluted sample (1 ml) was incubated with 50 μl of protein A/G-agarose beads at 4 °C for 2 h then centrifuged. The resulting supernatant was collected and was, respectively, incubated with 40 μg of anti-hTrx1, anti-TF monoclonal antibody, or normal mouse IgG at 4 °C overnight with shaking followed by incubation with 50 μl of Protein A/G beads at 4 °C for 2 h. The incubated mixture was centrifuged at 1000 rpm for 5 min to collect the beads. The bound proteins were eluted by resuspending the beads in 50 μl of 1 × SDS-PAGE sample buffer and heating at 95 °C for 10 min. A 20-μl aliquot from the eluted proteins was subjected to SDS-PAGE and Western blot analysis.
Preparation of rtTF-hTrx1 Complex
Purified recombinant hTrx1 (200 μmol/liter) and rtTF (20 μmol/liter) were mixed in 20 mmol/liter Tris-HCl buffer, pH 7.4, containing 150 mmol/liter NaCl. The total final volume was 500 μl. The mixture was incubated at 4 °C for 12 h. To block free thiol groups, iodoacetamide (IAM) was added to the incubation mixture with a final concentration of 10 mmol/liter for 1 h at room temperature in the dark. Then the incubation mixture was concentrated to 150 μl using centrifugal filter tube (molecular mass cut-off 10 kDa, Millipore) and separated by non-reducing SDS-PAGE. The protein band corresponding to rtTF-hTrx1 complex was cut out and used for in-gel tryptic digestion and liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) analysis.
Two-step Labeling of Cys Residues in Purified rtTF
To modify free thiol groups, purified rtTF (20 μmol/liter) was incubated with 10 mmol/liter IAM in 20 mmol/liter Tris-HCl buffer, pH 7.4, containing 150 mmol/liter NaCl at room temperature for 30 min in the dark. A total final volume was 500 μl. After dialyzing to remove excess IAM, 20 mmol/liter DTT was added to reduce disulfide bonds. The nascent Cys thiols were modified by 50 mmol/liter N-ethylmaleimide (NEM). Then the modified rtTF was separated by SDS-PAGE, and the protein band was cut out for in-gel tryptic digestion and LC-ESI-MS/MS analysis.
LC-ESI-MS/MS Analysis
The modified Cys residues were identified by LC-ESI-MS/MS using a Biobasic-18 column (0.18 × 150 mm) (Surveyor HPLC System, Thermal Thermo Inc.) coupled with an ion trap mass spectrometer (LCQ DECA Xp plus; ThermoFinnigan, Inc.). The instrument was run in the data-dependent mode with cycling between one full MS scan from m/z 400–2000 and MS/MS scans of the five most abundant ions using dynamic exclusion. The normalized collision energy was set to 35% to promote fragmentation. The MS and MS/MS data were used to search the target protein sequence using the pFind software Version 2.6.0.1. The search parameters were set to detect four differential modifications: +57 atomic mass units (amu) on cysteine for carboxyamidomethylation, +71 amu on cysteine for propionamide, +125 amu on cysteine for NEM, and +16 amu on methionine for oxidation. The decoy sequence (reverse sequence) was used in searching, and false discovery rate was set ≤1%.
Detection of Free Thiol(s) on Cell-Surface TF
A thiol-reactive biotinylation reagent, EZ-Link iodoacetyl-PEG2-Biotin (Pierce) was used in this experiment which is water-soluble and membrane-impermeable. A 2-mercaptoethanol-modified EZ-Link iodoacety1-PEG2-Biotin was used as a reagent control, which was prepared by incubating 2-mercaptoethanol (final concentration of 1 mmol/liter) with 2 ml of 0.5 mg/ml EZ-Link iodoacety1-PEG2-Biotin for 30 min in the dark.
The cells were grown in 100-mm dish till 90% confluence. After 3 washes in PBS, the cells were incubated with 2 ml of 0.5 mg/ml EZ-Link iodoacetyl-PEG2-Biotin or 2-mercaptoethanol modified EZ-Link iodoacety1-PEG2-Biotin at room temperature for 30 min in the dark. Then the cells were washed once with culture medium and twice with PBS to completely remove excess thiol-reactive reagent. The modified cells were lysed in 500 μl of lysis buffer (50 mmol/liter Tris-HCl, pH 7.4, 250 mmol/liter NaCl, 0.1% Nonidet P-40, 0.1% SDS, 0.1% sodium deoxycholate, 5 mmol/liter EDTA, and 1 mmol/liter PMSF). The resulting cell extracts were incubated with 50 μl of streptavidin-agarose beads at 4 °C for 12 h. The bound proteins were eluted by washing the beads 3 times with PBS containing 0.1% Nonidet P-40, suspending the beads in 100 μl of 1 × SDS-sample buffer, and boiling for 10 min. The eluted proteins were subjected to SDS-PAGE and Western blotting analysis.
Analysis of PAR2 Cleavage
The cells at 90% confluence in 24-well plates were transiently transfected with plasmid pcDNA3.1(+) containing the ALP-PAR2 sequence (0.8 μg/well) by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. After 3 days of incubation at 37 °C in 5% CO2, the cells were cultured in UltraCULTURE serum-free medium (Lonza, Walkersville, MD) overnight. The medium was then replaced by 100 μl of serum-free medium containing 100 nm FVIIa. After 1 h, culture medium was collected in which ALP activity was determined using the Great EscAPe SEAP Fluorescence Detection kit (Clontech Laboratories, Inc.).
Statistical Analysis
The unpaired Student's t test was used to compare the means of two groups. p values of 0.05 or less were considered to be statistically significant.
RESULTS
Redox States of Cys Residues in Purified rtTF and Cell-surface TF
The extracellular domain of TF contains Cys residues at positions 49, 58, 186, and 209 (8), but the way by which redox regulation occurs remains unclear. To investigate their redox state, we used rtTF as a study model that contains extracellular and transmembrane domains with procoagulant activity. The rtTF exhibited a molecular mass of ∼32 kDa (Fig. 1B). Cell-surface TF had a molecular mass of ∼45 kDa (Fig. 1C). On SDS-PAGE gels, DTT-treated rtTF migrated as a single band; DTT-untreated rtTF had a faster mobility than DTT-treated rtTF and contained a second faint band (Fig. 1B). This indicates that redox states of the Cys residues in freshly purified rtTF are not identical under non-reducing condition. After analyzing the double-labeled rtTF, i.e. free thiols labeled with IAM and DTT-induced thiols labeled with NEM, we found that both the Cys-186/-209 pair and Cys-49/-57 pair were modified with not only IAM but also NEM (Fig. 1A). TF was thought to have two disulfide bonds between Cys-49/-57 and Cys-186/-209 (33), but our results suggest that they are present partly in free thiols and partly in disulfide bonds.
To test whether the extracellular domain of cell-surface TF contains free thiol(s), the cells were incubated with the membrane-impermeable EZ-Link iodoacety1-PEG2-Biotin that was completely washed away before lysing the cells. The biotin-labeled proteins were collected via streptavidin-agarose. The bound proteins were eluted and then subjected to Western blotting analysis with anti-TF monoclonal antibody. As shown in lane 3 of Fig. 1C, TF monomer (∼45 kDa) was detected in the eluate of streptavidin-agarose. The 2-mercaptoethanol-modified iodoacety1-PEG2-Biotin and streptavidin-agarose alone, used as reagent controls, did not capture TF (lanes 2 and 4 of Fig. 1C). This result agrees with the existence of free thiol in TF extracellular domain under non-reducing conditions.
Redox Regulation of rtTF by Trx System
We next tested the ability of hTrx1/TrxR to mediate electron transfer between NADP(H) and rtTF. The molar extinction coefficient 6220 m−1cm−1 (or 6.22 × 10−3 ml/nmol·cm) for NADPH at 340 nm was used for calculating the amount of NADPH consumed or formed. The formula is x nmol of NADPH/ml = ΔA/0.00622 ml/nmol.
When hTrx1/TrxR transferred electrons from DTT-prereduced rtTF to NADP+, an increase in absorbance at 340 nm due to the formation of NADPH appeared in a TF concentration- and time-dependent manner. As the reaction proceeded for 10 min, the amount of generated disulfides was 0.23 per mole of rtTF (Fig. 2A). Upon mutation of TF-Cys-189 and Cys-209 to Ala, the amount of generated disulfides per minute was reduced from 1.125 to 0.354 with 20 mol of rtTF (Fig. 2C). This indicates that the Cys-189/-209 pair seems more sensitive than the Cys-49/-57 pair to regulation by Trx system.
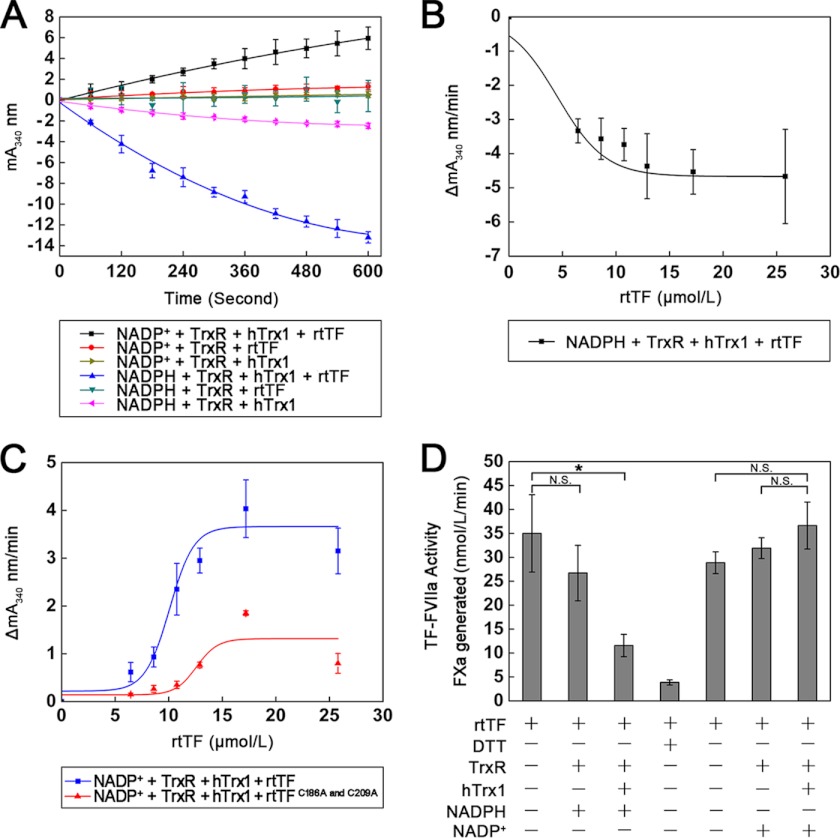
FIGURE 2.
Effect of Trx system on rtTF redox states and activity. A, hTrx1/TrxR-mediated oxidation and reduction of rtTF by NADP(H) is shown. The ratio of hTrx1/rtTF was ∼0.6:1. The formation or consumption of NADPH was measured by following an increase or a decrease in absorbance at 340 nm. B, shown is reduction of rtTF by hTrx1-TrxR-NADPH in a TF concentration-dependent manner. C, shown is oxidation of prereduced rtTF by hTrx1-TrxR-NADP+. The rtTF was treated with 10 mmol/liter DTT followed by dialysis to remove DTT before assay of NADPH formation. D, shown is the effect of hTrx1-TrxR-NADP(H) on rtTF-precoagulant activity. rtTF was incubated with hTrx1-TrxR-NADP(H) at room temperature for 2 h before assay for TF activity. The ratio of hTrx1/rtTF was ∼7:1. All data points in A–D are expressed as the mean ± S.D. (n = 3). *, p < 0.05; N.S., not significant.
Conversely, TrxR/hTrx1 readily transferred electrons from NADPH to rtTF, leading to a decrease in absorbance at 340 nm in a TF concentration- and time-dependent manner (Fig. 2, A and B). As the reaction was followed for 10 min, the amount of reduced disulfides was 0.46 per mole of rtTF (Fig. 2A). The amount of reduced disulfides per minute was 1.286 with 20 mol of rtTF (Fig. 2B).
These redox changes in TF were closely coupled with the alterations of TF procoagulant activity. As presented, hTrx1-TrxR-NADPH significantly decreased TF activity, whereas hTrx1-TrxR-NADP+ could increase TF activity (Fig. 2D) in which hTrx1 was an essential component. By comparison, TF was most likely to favor reduction by hTrx1-TrxR-NADPH over oxidation by hTrx1-TrxR-NADP+. A disulfide reducing agent, DTT, also inhibited TF activity (Fig. 2D). The inhibitory efficiency caused by 10 mmol/liter DTT could be achieved through 5 μmol/liter hTrx1, 20 nmol/liter TrxR, and 0.2 mmol/liter NADPH.
Regulation of Cell-surface TF Procoagulant Activity by Cellular hTrx1/TrxR
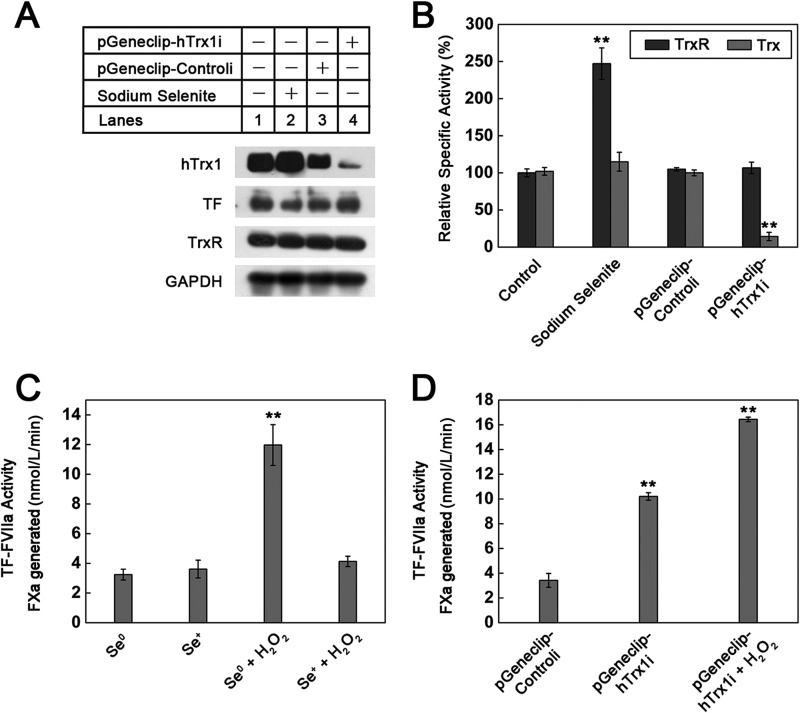
To examine the effect of cellular hTrx1/TrxR on cell-surface TF procoagulant activity, we used RNA interference to turn hTrx1 gene off or selenium supplementation to increase TrxR activity. Efficient knockdown of hTrx1 was confirmed by Western blotting (Fig. 3A) and Trx activity assay (Fig. 3B). hTrx1 knockdown caused a significant increase in cell-surface TF procoagulant activity that could be further enhanced by 1 mmol/liter H2O2 (Fig. 3D). This result demonstrates the suppressive effect of cellular hTrx1 on TF activity and suggests the action of H2O2 independent of hTrx1. In Se+ cells, protein levels of hTrx1, TrxR, and TF did not change significantly (Fig. 3A), but TrxR activity increased by 2.4-fold, and Trx activity increased slightly compared with those in Se0 cells (Fig. 3B). Without H2O2, basal TF procoagulant activity for both Se+ and Se0 cells was similar (Fig. 3C). After exposure to H2O2, Se0 cells showed much higher TF procoagulant activity than Se+ cells (Fig. 3C), indicating the specific suppression of TrxR on H2O2 action. Although selenium supplementation increases not only TrxR but also other selenoprotein activity, the results together with hTrx1 knockdown data may confirm a critical role of Trx system in controlling TF activity.
FIGURE 3.
Effects of endogenous hTrx1/TrxR on cell-surface TF-procoagulant activity. A, a Western blot tests the effects of hTrx1 knockdown and selenium supplementation on the expression of hTrx1, TrxR, and TF. GAPDH was used as an internal control. B, shown are specific activities of cellular TrxR and Trx. Cell extracts had a protein concentration of 3 mg/ml, 20 μl of which was used in measuring TrxR/Trx activity via super-insulin assay. C, shown is the effect of elevating TrxR activity by selenium supplementation on the basal activity of cell-surface TF as well as on the response of cell-surface TF activity to H2O2. The concentration of H2O2 was 1 mmol/liter, and the treatment time of H2O2 was 15 min. D, shown is the effect of hTrx1 knockdown on cell-surface TF-procoagulant activity. All data points in B–D are expressed as the means ± S.D., n = 5. **, p < 0.01.
Regulation of Cell-surface TF Procoagulant Activity by Extracellular Trx System
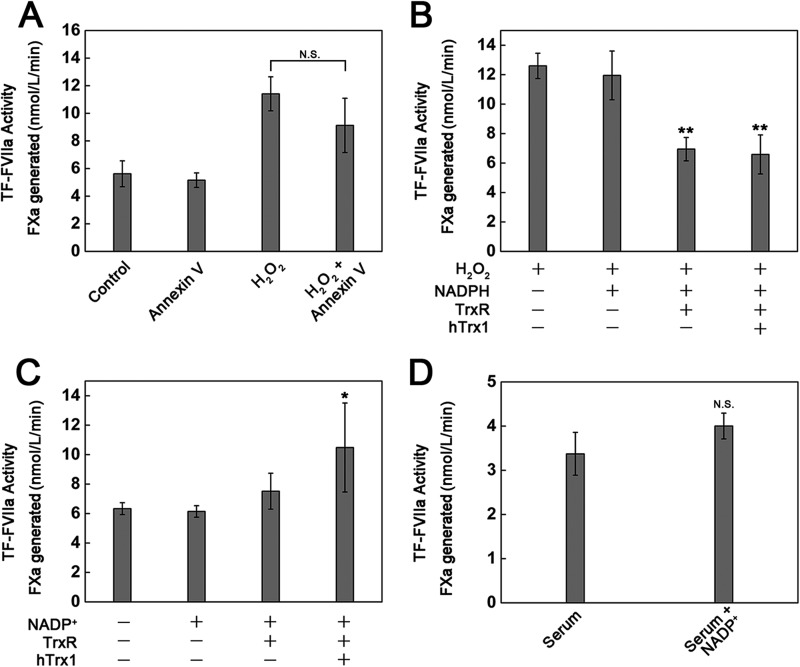
As H2O2-induced phosphatidylserine exposure at cell surface (34) is a potent accelerator of TF-initiated coagulation in vitro (35), we thus analyzed the contribution of H2O2-induced phosphatidylserine exposure to H2O2-stimulated TF procoagulant activity using Annexin-V that binds to negative charged phospholipids. Once exposed to H2O2 the cells that were treated with Annexin-V showed 20% lower cell-surface TF procoagulant activity than the untreated cells (Fig. 4A). Attenuation of H2O2-induced increase in cell-surface TF procoagulant activity by hTrx1-TrxR-NADPH or TrxR-NADPH was even more significant (Fig. 4B). These data indicate that the formation of disulfide bonds other than phosphatidylserine exposure also contributes to H2O2-induced increase in cell-surface TF procoagulant activity.
FIGURE 4.
Redox-related changes in cell-surface TF-procoagulant activity. A, shown is the effect of Annexin-V on H2O2-stimulated cell-surface TF activity. The cells were exposed to 200 nmol/liter Annexin-V for 15 min after they were treated with 1 mmol/liter H2O2 for 15 min. B, shown is the effect of hTrx1-TrxR-NADPH on the response of cell-surface TF activity to H2O2. The cells were pretreated with 1 mmol/liter H2O2 for 15 min followed by washing twice with PBS, then incubated with 5 μmol/liter hTrx1, 20 nmol/liter TrxR, and 0.2 mmol/liter NADPH at 37 °C for 1 h, which was washed away before the assay of TF activity. C, shown is the effect of hTrx1-TrxR-NADP+ on cell-surface TF activity. The cells were treated with 5 μmol/liter hTrx1, 20 nmol/liter TrxR, and 0.2 mmol/liter NADP+ at 37 °C for 1 h, which was washed away before assay TF activity. D, shown is the effect of human serum with elevated NADP+ levels on cell-surface TF activity. The cells were incubated with 200 μl of human serum or serum plus 0.2 mmol/liter NADP+ for 1 h. All data points in A–D are expressed as the means ± S.D., n = 5. *, p < 0.05; **, p < 0.01; N.S., not significant.
Under oxidative stress, the level of NADP+ was increased. We thus tested a potential role of NADP+ in TF activity. Treatment of the cells with PBS buffer containing hTrx1-TrxR-NADP+ enhanced the cell-surface TF procoagulant activity by 50%, but NADP+ alone did not exhibit such potential (Fig. 4C). Moreover, TF procoagulant activity on the surface of the cells treated with serum of healthy persons was not significantly different from that of the cells treated with the serum plus NADP+ (Fig. 4D). These results further indicate that hTrx1/TrxR sense and transduce the changes in NADPH/NADP+ states into the difference of TF activity.
Interaction between hTrx1 and TF
To extend the above findings to human, we tested the relationship between hTrx1 and TF in serum and plasma samples from healthy persons. First, the association of hTrx1 with TF was examined using co-immunoprecipitation with Western blotting analysis. hTrx1 co-immunoprecipitated with TF and vice versa in both serum and plasma samples. No binding of hTrx1 or TF was observed when the samples were incubated with normal mouse IgG, indicating that there was no observed background binding to the anti-hTrx1/TF monoclonal antibody (Fig. 5, A and B). These results confirm that hTrx1 is associated with TF in human blood. Then we analyzed redox forms of serum TF by Western blotting with anti-TF monoclonal antibody. Without DTT, TF migrated either as high molecular weight smears or as one distinct band at the expected molecular weight of TF monomer. In the presence of DTT, the smears disappeared, and TF migrated as a single band matching the molecular weight of TF monomer (Fig. 5C). Thus, the smears are more likely to reflect TF-TF or TF-proteins complexes that are linked by intermolecular disulfide bonds.
We reasoned that hTrx1-Cys-73 located on the molecular surface (26) could link to TF via disulfide bond under a non-reducing environment such as serum. To confirm this hypothesis, the purified rtTF was incubated with hTrx1 or hTrx1C73A in different molar ratios at 37 °C for 4 h. Then, the incubated mixture was separated by non-reducing SDS-PAGE, and the protein bands were detected by Western blotting. Using anti-TF monoclonal antibody, the Western blotting showed that the relative amount of rtTF formed dimers with a molecular mass of ∼64 kDa, and a fraction of rtTF was associated with hTrx1 (a weak band matching theoretical mass of ∼44 kDa). The latter was not observed when rtTF was incubated with hTrx1C73A (Fig. 5D) but was positively detected by anti-hTrx1 monoclonal antibody (Fig. 5E). Thus, hTrx1-Cys-73 is most likely involved in disulfide bond connecting to rtTF.
Identification of Cys Residues Participating in the Mixed Disulfide between hTrx1 and rtTF
To find the Cys residue connecting rtTF to hTrx1-Cys-73, the purified rtTF was first mixed with hTrx1 followed by incubation with IAM to block free thiols. The incubation mixture was then separated by non-reducing SDS-PAGE. The protein band corresponding to hTrx1-rtTF complex was cut out, digested with trypsin, and subjected to LC-ESI-MS/MS analysis. The resulting peptide sequence covered 69% rtTF sequence and 65% hTrx1 sequence. Fragmentation of rtTF peptides identified IAM-modified Cys-49, Cys-57, and Cys-186 residues. A peak with an m/z value of 1167.17, which matched the theoretical mass of TF Cys-49/-57 disulfide-containing peptide, was detected in the sample (Fig. 6A). Fragmentation of hTrx1 peptides identified IAM-modified Cys-62 and Cys-69 residues. A peak with a m/z value of 1621.94, which matched the theoretical mass of hTrx1 Cys-32/-35 disulfide-containing peptide, was detected in the sample (Fig. 6B). However, rtTf-Cys-209-containing peptide and hTrx1-Cys-73-containing peptide were not detected in the sample. These results indicate that the mixed disulfide could not involve other Cys residues except for hTrx1-Cys-73 and rtTF-Cys-209.
Inhibitory Effect of hTrx1 Itself on TF Procoagulant Activity
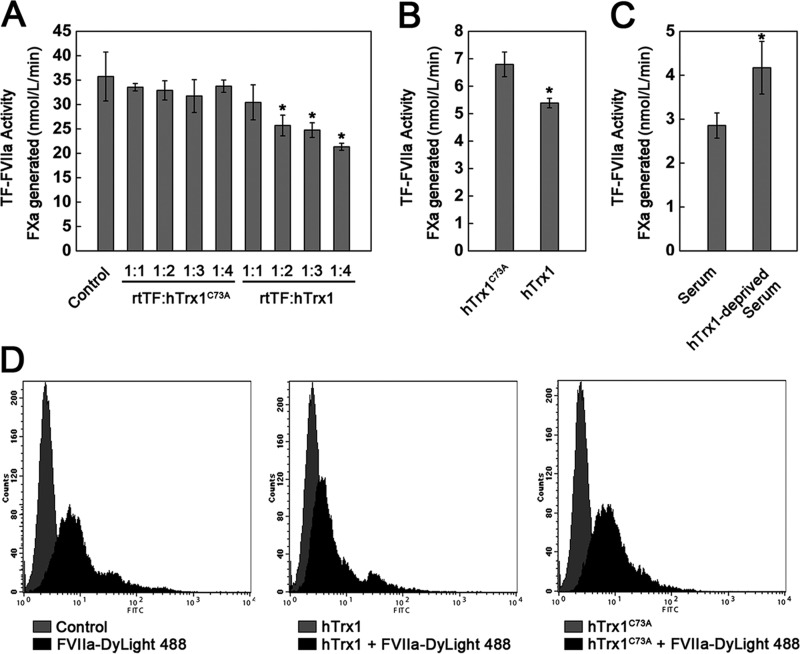
Given that hTrx1-Cys-73 can form a stable mixed disulfide with TF, we postulated that hTrx1, but not hTrx1C73A, would interfere with the binding of FVIIa to TF, leading to loss of TF activity. To prove this hypothesis, we treated rtTF (700 nmol/liter) with hTrx1 or hTrx1C73A from 1:1 to 1:4 molar ratios at room temperature for 1 h. The results indeed showed that hTrx1C73A caused no effect on TF activity, but hTrx1 decreased TF procoagulant activity in a concentration-dependent manner (Fig. 7A). Similarly, the cells treated with (1 μmol/liter) hTrx1 displayed lower cell-surface TF procoagulant activity than that of the cells treated with the same amount of hTrx1C73A (Fig. 7B). Considering the concentration of hTrx1 in human serum is about ∼ 2.1 nmol/liter (17), we examined the potential effect of serum hTrx1 on TF in vivo. After the cells were treated with human serum or hTrx1-deprived human serum for 30 min, hTrx1-deprived human serum exhibited a potential to promote cell-surface TF procoagulant activity (Fig. 7C).
FIGURE 7.
hTrx1 is specifically involved in interfering with FVIIa binding to TF. A, the inhibitory effect of hTrx1 on rtTF activity was absent using hTrx1C73A mutant instead of wild-type hTrx1. hTrx1 (or hTrx1C73A) and rtTF were incubated at room temperature for 1 h. B, hTrx1-Cys-73 was required for hTrx1 to inhibit cell-surface TF activity. C, a positive effect of hTrx1-deprived serum on cell-surface TF activity shown is shown. The cells were treated with human serum or hTrx1-deprived serum at 37 °C for 30 min. D, shown is flow cytometry analysis. hTrx1-Cys-73 was implicated in reducing FVIIa binding to cell-surface TF. These results are representative of three independent experiments. The results shown in A–C are expressed as the means ± S.D., n = 5. *, p < 0.05.
To gain quantitative insight into the interference effect of hTrx1-Cys-73 on TF binding to FVIIa, we conducted flow cytometry analysis using Dylight 488 amine-reactive dye-labeled FVIIa that provides green fluorescence. After treatment with 10 μmol/liter hTrx1 or hTrx1C73A for 30 min, respectively, the cells were incubated with the FVIIa-Dylight 488. The results showed that hTrx1, but not hTrx1C73A, could decrease the number of the cells with high intensity green fluorescence (Fig. 7D).
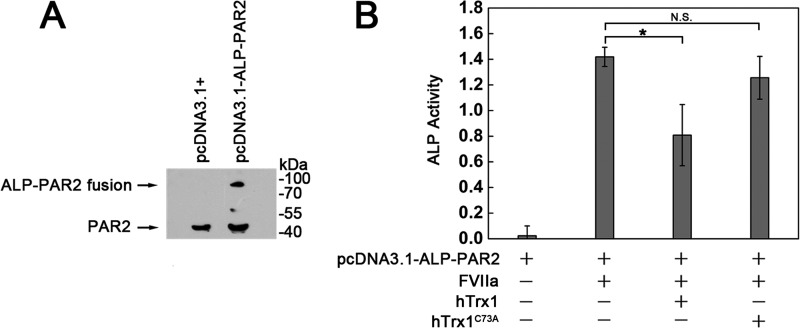
Inhibitory Effect of hTrx1 Itself on TF·FVIIa-dependent PAR2 Activation
In view of the fact that FVIIa·TF can activate PAR2 by cleavage of an N-terminal peptide bond (36), we thereby tested the inhibitory effect of hTrx1 on the capacity of TF·FVIIa to cleave PAR2. We constructed ALP-PAR2 plasmid that directs the expression of chimeric protein in which ALP is fused to the extracellular domain of PAR-2 with a region-containing protease cleavage site. This plasmid was transiently transfected into the cells. The expression of ALP-PAR2 was confirmed by Western blotting (Fig. 8A). The resulting protein tethers ALP to the cell surface via the PAR-2 N-terminal extracellular domain, so that TF·FVIIa cleavage of that domain releases ALP into the culture medium. After incubated with 1 μmol/liter hTrx1 or hTrx1PC73A at 37 °C for 30 min, respectively, the cells were incubated with 100 nmol/liter FVIIa at 37 °C for 60 min. The amount of ALP shed from the cell surface was determined by measuring ALP activity present in the culture media. The results showed that hTrx1, but not hTrx1PC73A, significantly decreased the amount of ALP shed from the cell surface (Fig. 8B). Background ALP activity from FVIIa-untreated cells was used as control.
FIGURE 8.
hTrx1-Cys-73 is required for hTrx1 to inhibit TF·FVIIa-dependent activation of PAR2. A, Western blotting analysis of PAR2 expression is shown. Transient expression of ALP-PAR2 fusion protein in MDA-MB-231 cells was examined using anti-PAR2 monoclonal antibody. B, shown is determination of PAR2 cleavage (activation) by measuring increased ALP activity in culture medium. The cells bearing ALP-PAR2 were treated with serum-free medium containing hTrx1 or hTrx1C73A. Then the cells were washed once with PBS followed by the addition of FVIIa. After 1 h of incubation, the cell culture medium was collected and used for analyzing ALP activity. The results are expressed as the means ± S.D., n = 5. *, p < 0.05; N.S., not significant.
DISCUSSION
Early studies demonstrated that TF antigen was detectable in human plasma sample (1). Our study extends this observation by showing the association of hTrx1 with TF in human serum and plasma (Fig. 5, A and B). The TF that co-immunoprecipitates with hTrx1 either from serum or from plasma migrates at ∼45–48 kDa, which matches the molecular mass of full-length TF. Western blotting with the serum sample and anti-TF monoclonal antibody reveals that in the absence of DTT, TF exhibits not only a monomer but also a broad smearing in the high molecular weight region (>70 kDa); in the presence of DTT that reduces disulfide bond, TF shows only a distinct monomer band (Fig. 5C). This result suggests that Cys residues in TF can participate in the formation of intermolecular disulfide bonds.
To test whether the high molecular weight smear observed in the non-reduced serum sample are homomultimers or heteromultimers of TF, the purified rtTF (∼32 kDa) was mixed with hTrx1 (∼12 kDa) at 37 °C. Under non-reducing condition, a Western blot of this mixture displays four major bands that are detected by anti-TF monoclonal antibody. These protein bands match theoretical molecular mass of rtTF monomers, rtTF homodimers, rtTF-hTrx1 complex, and rtTF tetramers (∼128 kDa), respectively (Fig. 5D). Among them, the rtTF-hTrx1 complex is detectable with anti-hTrx1 monoclonal antibody (Fig. 5E). The formation of these rtTF-rtTF or rtTF-hTrx1 complexes is completely prevented in the presence of DTT. These data suggest that TF may form not only self-associated complexes but also hTrx1-associated and other proteins-associated complexes via disulfide bond. The physiological significance of the association between hTrx1 and TF is highlighted with the finding that hTrx1-deprived serum shows potential to promote cell-surface TF procoagulant activity (Fig. 7C).
As inflammatory and other pathophysiological factors may induce TF expression (37, 38), MDA-MB-231 breast cancer cell line with high TF expression (39) was chosen as a model. Regarding the effect of extracellular hTrx1 on TF activity, our initial concern was about their concentrations in vivo. Several studies address this issue indicating that healthy individuals have ∼2.1 nmol/liter serum hTrx1 (17), 1∼2.3 nmol/liter plasma Trx (40, 41), and ∼ 4.2 pmol/liter of TF plasma antigen (42) (molecular mass of 45 kDa is used for calculating the molar concentration of TF). It means that the level of hTrx1 is about 500-fold higher than that of TF in human plasma. Considering a relatively high ratio of hTrx1 to TF and intravenous dosage (40 μg) of hTrx injected in mouse (43), 1 or 10 μmol/liter of hTrx1 was thus used to treat the cells. The results show that hTrx1, but not hTrx1C73A, leads to a decrease in FVIIa binding to TF (Fig. 7D), TF procoagulant activity (Fig. 7A), and TF·FVIIa-dependent activation of PAR2 (Fig. 8B). The exclusive requirement of hTrx1-Cys-73 in its inhibitory action suggests that this Cys residue is the site binding to TF. The following results support this suggestion. One is the formation of hTrx1-rtTF complex in a hTrx1-Cys-73-dependent manner (Fig. 5, D and E); the other is the mixed disulfide between rtTF-Cys-209 and hTrx1-Cys-73 (Fig. 6, A and B). This mixed disulfide should be quite stable because it survives despite the presence of free thiols (Fig. 1A). We thus hypothesize that the association between extracellular hTrx1 and cell-surface TF constrains the TF in the state of limited FVIIa binding (Fig. 7D), which enables cell-surface TF activity to remain low (Fig. 7A).
Similarly, cellular hTrx1/TrxR also function as negative regulators of cell-surface TF activity. The cells with hTrx1 knockdown have significant higher levels of cell-surface TF procoagulant activity than control cells (Fig. 3D). Se+ cells that contain elevated TrxR activity are endowed with a low response of cell-surface TF procoagulant activity to H2O2 (Fig. 3C). However, the molecular basis underlying the inhibitory action of cellular hTrx1/TrxR should be different from extracellular hTrx1/TrxR because extracellular hTrx1/TrxR is present in a dominantly oxidized, whereas cellular hTrx1/TrxR is present mainly in reduced form. Our data suggest the following models for elucidating the role of hTrx1/TrxR in regulating TF functions.
Using purified proteins, we found that the reduction of disulfide bonds by 10 mmol/liter DTT led to inactivation of rtTF. This mechanism is similar to the hTrx1-TrxR-NADPH catalytic mechanism and contrasts with the hTrx1-TrxR-NADP+ catalytic model involving disulfide formation of TF (Fig. 2A). Nevertheless, hTrx1-TrxR-NADPH is much more effective than DTT in reducing TF disulfide bond. A similar effect was achieved using the amount of hTrx1, TrxR, and NADPH at 2,000, 50,000, and 50 times lower than DTT (Fig. 2D). Moreover, even if thiols coexist with disulfide bonds in the purified rtTF (Fig. 1), the reduction of rtTF by hTrx1-TrxR-NADPH is kinetically favorable than its oxidation by hTrx1-TrxR-NADP+. As shown in Fig. 2A, when the reaction is followed for 10 min, the amount of reduced disulfide is 0.46, whereas the amount of formed disulfide is 0.23 mol per mol of rtTF. This situation is even more evident at rtTF concentrations below 5 μmol/liter (Fig. 2, B and C). These data reveal a predominant role of Trx system in suppressing TF activity.
Extracellular hTrx1/TrxR are found to influence cell-surface TF activity in an unique fashion. The ability of extracellular TrxR-NADPH to suppress H2O2-stimulated cell-surface TF activity is similar to that of hTrx1-TrxR-NADPH (Fig. 4B). This phenomenon indicates the effective role of membrane Trx or the direct role of TrxR-NADPH in eliminating H2O2. By contrast, extracellular hTrx1 is an indispensable component for hTrx1-TrxR-NADP+ to promote cell-surface TF activity (Fig. 4C). This result stresses that membrane Trx is ineffective in transferring electrons from TF to NADP+ via TrxR despite the presence of a thiol group(s) in TF extracellular domain (Fig. 4C). Thereby, membrane Trx seems to act as an electron receptor specifically transducing reducing potential from TrxR-NADPH to TF.
Regulation of cell-surface TF activity by Trx system shows the dynamic process in response to changes in NADPH/NADP+ levels (Fig. 2A). The latter dictates the redox states and activity of Trx/TrxR. Under normal metabolic conditions, NADPH/NADP+ ratio is usually high (44); thus hTrx1-TrxR-NADPH may play a dominant role in reducing TF activity. Oxidative stress can lead to a decline of NADPH level and increase serum NADP+/NADPH ratio (45), which can favor the positive effect of hTrx1-TrxR-NADP+ on TF activity. This may partially explain why cells with increased reactive oxygen species exhibit prothrombotic state (46–50).
Cellular hTrx1/TrxR are supposed to regulate cell-surface TF activity via three ways. First, the specific suppression of TrxR on H2O2-stimulated TF activity (Figs. 3C and 4B) most likely results from the ability of TrxR to eliminate H2O2 (29, 51, 52). This function of cellular TrxR can be easily conceived in view of fact that the stimulatory effect of H2O2 on TF activity is basically eliminated in Se+ cells. The latter have significantly higher TrxR activity than Se0 cells (Fig. 3B) but have the levels of Trx activity (Fig. 3B), TF protein (Fig. 3A), and TF basal activity (Fig. 3C) similar to Se0 cells. Second, cell membrane Trx is most likely involved in communication of TrxR with cell-surface TF. In the absence of hTrx1, TrxR-NADPH is unable to reduce oxidized form of purified rtTF (Fig. 2A) and inhibit its activity efficiently (Fig. 2D) but can significantly decrease cell-surface TF activity (Fig. 4B). Although we do not yet know how endogenous/cell membrane Trx reacts with cell-surface TF, it cannot be ruled out that reduction of oxidized TF by electrons is direct or indirect from cell membrane Trx. TrxR is the only known enzyme that catalyzes the reduction of Trx by NADPH. Third, TrxR may work together with protein disulfide isomerase that is a substrate of mammalian TrxR (16), suggesting a previously unrecognized coupling among TF, protein disulfide isomerase (11, 54), and TrxR.
Furthermore, the hTrx1 or Trx system may serve as a barrier to PAR2-mediated events by two means. At the cell surface, extracellular hTrx1 inhibits TF·FVIIa-dependent PAR2 activation (Fig. 8B). Inside the cells Trx/TrxR are involved in eliminating reactive oxygen species (55) that is an activator of phospholipase Cγ (56, 57). The latter partially mediates PAR2 effects (58).
Our data demonstrate that hTrx1 and TrxR are endogenous regulators of TF actions. A new redox-dependent mechanism is therefore proposed that can directly couple hTrx1/TrxR to TF activity. This mechanism may be of particular relevance to certain conditions, such as coronary artery disease. Serum hTrx1 levels of coronary artery disease patients are negatively associated with disease severity (17). Because TF plays a critical role in thrombus formation in vivo (59), and enrichment of circulating TF in human atherosclerotic plaques is linked to plaque disruption (53), proper suppression of TF activity by hTrx1 or Trx system should have a favorable effect on human health.
Acknowledgment
We thank Professor Dexi Chen, Beijing YouAn Hospital for kindly providing pSEAP2-Control plasmid.
This work was supported by National Natural Science Foundation of China Grants 30970629 and 31170764, Main Direction Program of Knowledge Innovation of Chinese Academy of Sciences Grant KSCX2-EW-J-29, Special Science and Technology Projects for Outstanding Young Persons in Life Sciences Grant KSCX2-EW-Q-19, and Ministry of Science and Technology of the People's Republic of China Grants 2009ZX09103-432 and 2012ZX09103-101-063.
- TF
- tissue factor
- rtTF
- recombinant truncated human TF
- FX
- factor X
- APL
- alkaline phosphatase
- Trx
- thioredoxin
- TrxR
- Trx reductase
- hTrx1
- human cytosolic Trx
- IAM
- iodoacetamide
- ESI
- electrospray ionization
- NEM
- N-ethylmaleimide
- PAR2
- protease-activated receptor 2
- ALP
- alkaline phosphatase.
REFERENCES
- 1. Cimmino G., Golino P., Badimon J. J. (2011) Pathophysiological role of blood-borne tissue factor. Should the old paradigm be revisited? Intern. Emerg. Med. 6, 29–34 [DOI] [PubMed] [Google Scholar]
- 2. Rao L. V., Rapaport S. I. (1988) Activation of factor VII bound to tissue factor. A key early step in the tissue factor pathway of blood coagulation. Proc. Natl. Acad. Sci. U.S.A. 85, 6687–6691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Riewald M., Ruf W. (2003) Science review. Role of coagulation protease cascades in sepsis. Crit. Care 7, 123–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Riewald M., Ruf W. (2001) Mechanistic coupling of protease signaling and initiation of coagulation by tissue factor. Proc. Natl. Acad. Sci. U.S.A. 98, 7742–7747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belting M., Ahamed J., Ruf W. (2005) Signaling of the tissue factor coagulation pathway in angiogenesis and cancer. Arterioscler. Thromb. Vasc. Biol. 25, 1545–1550 [DOI] [PubMed] [Google Scholar]
- 6. Bach R. R. (2006) Tissue factor encryption. Arterioscler. Thromb. Vasc. Biol. 26, 456–461 [DOI] [PubMed] [Google Scholar]
- 7. Bayele H. K., Murdock P. J., Perry D. J., Pasi K. J. (2002) Simple shifts in redox/thiol balance that perturb blood coagulation. FEBS Lett. 510, 67–70 [DOI] [PubMed] [Google Scholar]
- 8. Chen V. M., Ahamed J., Versteeg H. H., Berndt M. C., Ruf W., Hogg P. J. (2006) Evidence for activation of tissue factor by an allosteric disulfide bond. Biochemistry 45, 12020–12028 [DOI] [PubMed] [Google Scholar]
- 9. Liang H. P., Brophy T. M., Hogg P. J. (2011) Redox properties of the tissue factor Cys-186/-209 disulfide bond. Biochem. J. 437, 455–460 [DOI] [PubMed] [Google Scholar]
- 10. Reinhardt C., von Brühl M. L., Manukyan D., Grahl L., Lorenz M., Altmann B., Dlugai S., Hess S., Konrad I., Orschiedt L., Mackman N., Ruddock L., Massberg S., Engelmann B. (2008) Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J. Clin. Invest. 118, 1110–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ahamed J., Versteeg H. H., Kerver M., Chen V. M., Mueller B. M., Hogg P. J., Ruf W. (2006) Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc. Natl. Acad. Sci. U.S.A. 103, 13932–13937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kothari H., Nayak R. C., Rao L. V., Pendurthi U. R. (2010) Cys-186/-209 disulfide bond is not essential for the procoagulant activity of tissue factor or for its de-encryption. Blood 115, 4273–4283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pendurthi U. R., Ghosh S., Mandal S. K., Rao L. (2007) Tissue factor activation. Is disulfide bond switching a regulatory mechanism? Blood 110, 3900–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tartier L., McCarey Y. L., Biaglow J. E., Kochevar I. E., Held K. D. (2000) Apoptosis induced by dithiothreitol in HL-60 cells shows early activation of caspase 3 and is independent of mitochondria. Cell Death Differ. 7, 1002–1010 [DOI] [PubMed] [Google Scholar]
- 15. Solov'eva M. E., Solov'ev V. V., Faskhutdinova A. A., Kudriavtsev A. A., Akatov V. S. (2007) Prooxidant and cytotoxic action of N-acetylcysteine and glutathione combined with vitamin Bl2b. Tsitologiia 49, 70–78 [PubMed] [Google Scholar]
- 16. Lundström J., Holmgren A. (1990) Protein disulfide-isomerase is a substrate for thioredoxin reductase and has thioredoxin-like activity. J. Biol. Chem. 265, 9114–9120 [PubMed] [Google Scholar]
- 17. Wu Y., Yang L., Zhong L. (2010) Decreased serum levels of thioredoxin in patients with coronary artery disease plus hyperhomocysteinemia is strongly associated with the disease severity. Atherosclerosis 212, 351–355 [DOI] [PubMed] [Google Scholar]
- 18. Zhong L., Arnér E. S., Holmgren A. (2000) Structure and mechanism of mammalian thioredoxin reductase. The active site is a redox-active selenolthiol/selenenylsulfide formed from the conserved cysteine-selenocysteine sequence. Proc. Natl. Acad. Sci. U.S.A. 97, 5854–5859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arnér E. S., Holmgren A. (2000) Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 267, 6102–6109 [DOI] [PubMed] [Google Scholar]
- 20. Passam F. H., Rahgozar S., Qi M., Raftery M. J., Wong J. W., Tanaka K., Ioannou Y., Zhang J. Y., Gemmell R., Qi J. C., Giannakopoulos B., Hughes W. E., Hogg P. J., Krilis S. A. (2010) Redox control of β2-glycoprotein I-von Willebrand factor interaction by thioredoxin-1. J. Thromb. Haemost. 8, 1754–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hessel B., Jörnvall H., Thorell L., Söderman S., Larsson U., Egberg N., Blombäck B., Holmgren A. (1984) Structure-function relationships of human factor VIII complex studied by thioredoxin-dependent disulfide reduction. Thromb. Res. 35, 637–651 [DOI] [PubMed] [Google Scholar]
- 22. Holmgren A., Lu J. (2010) Thioredoxin and thioredoxin reductase. Current research with special reference to human disease. Biochem. Biophys. Res. Commun. 396, 120–124 [DOI] [PubMed] [Google Scholar]
- 23. Rubartelli A., Bonifaci N., Sitia R. (1995) High rates of thioredoxin secretion correlate with growth arrest in hepatoma cells. Cancer Res. 55, 675–680 [PubMed] [Google Scholar]
- 24. Patenaude A., Fortin J. S., Deschenes R., Côté M. F., Lacroix J., C-Gaudreault R., Petitclerc E. (2010) Chloroethyl urea derivatives block tumour growth and thioredoxin-1 nuclear translocation. Can J. Physiol. Pharmacol. 88, 1102–1114 [DOI] [PubMed] [Google Scholar]
- 25. World C., Spindel O. N., Berk B. C. (2011) Thioredoxin-interacting protein mediates TRX1 translocation to the plasma membrane in response to tumor necrosis factor-α. A key mechanism for vascular endothelial growth factor receptor-2 transactivation by reactive oxygen species. Arterioscler. Thromb. Vasc. Biol. 31, 1890–1897 [DOI] [PubMed] [Google Scholar]
- 26. Holmgren A. (1995) Thioredoxin structure and mechanism. Conformational changes on oxidation of the active-site sulfhydryls to a disulfide. Structure 3, 239–243 [DOI] [PubMed] [Google Scholar]
- 27. Hashemy S. I., Holmgren A. (2008) Regulation of the catalytic activity and structure of human thioredoxin 1 via oxidation and S-nitrosylation of cysteine residues. J. Biol. Chem. 283, 21890–21898 [DOI] [PubMed] [Google Scholar]
- 28. Higashi S., Matsumoto N., Iwanaga S. (1997) Conformation of factor VIIa stabilized by a labile disulfide bond (Cys-310/-329) in the protease domain is essential for interaction with tissue factor. J. Biol. Chem. 272, 25724–25730 [DOI] [PubMed] [Google Scholar]
- 29. Zhong L., Holmgren A. (2002) Mammalian thioredoxin reductases as hydroperoxide reductases. Methods Enzymol. 347, 236–243 [DOI] [PubMed] [Google Scholar]
- 30. Zhai G, Zhong L. (2010) N-terminal affinity tags may lead to increased sensitivity of human thioredoxin-1 to oxidants. Chinese Journal of Biochemistry and Molecular Biology 26, 243–253 [Google Scholar]
- 31. Stone M. J., Ruf W., Miles D. J., Edgington T. S., Wright P. E. (1995) Recombinant soluble human tissue factor secreted by Saccharomyces cerevisiae and refolded from Escherichia coli inclusion bodies. Glycosylation of mutants, activity, and physical characterization. Biochem. J. 310, 605–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ludeman M. J., Kataoka H., Srinivasan Y., Esmon N. L., Esmon C. T., Coughlin S. R. (2005) PAR1 cleavage and signaling in response to activated protein C and thrombin. J. Biol. Chem. 280, 13122–13128 [DOI] [PubMed] [Google Scholar]
- 33. Bach R., Konigsberg W. H., Nemerson Y. (1988) Human tissue factor contains thioester-linked palmitate and stearate on the cytoplasmic half-cystine. Biochemistry 27, 4227–4231 [DOI] [PubMed] [Google Scholar]
- 34. Hampton M. B., Vissers M. C., Keenan J. I., Winterbourn C. C. (2002) Oxidant-mediated phosphatidylserine exposure and macrophage uptake of activated neutrophils. Possible impairment in chronic granulomatous disease. J. Leukoc. Biol. 71, 775–781 [PubMed] [Google Scholar]
- 35. Bach R., Rifkin D. B. (1990) Expression of tissue factor procoagulant activity. Regulation by cytosolic calcium. Proc. Natl. Acad. Sci. U.S.A. 87, 6995–6999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arora P., Ricks T. K., Trejo J. (2007) Protease-activated receptor signalling, endocytic sorting, and dysregulation in cancer. J. Cell Sci. 120, 921–928 [DOI] [PubMed] [Google Scholar]
- 37. Verhagen H. J., Heijnen-Snyder G. J., Vink T., Pronk A., van Vroonhoven T. J., Eikelboom B. C., Sixma J. J., de Groot P. G. (1995) Tissue factor expression on mesothelial cells is induced during in vitro culture. Manipulation of culture conditions creates perspectives for mesothelial cells as a source for cell seeding procedures on vascular grafts. Thromb. Haemost. 74, 1096–1102 [PubMed] [Google Scholar]
- 38. Kumar A., Koenig K. B., Johnson A. R., Idell S. (1994) Expression and assembly of procoagulant complexes by human pleural mesothelial cells. Thromb. Haemost. 71, 587–592 [PubMed] [Google Scholar]
- 39. Zhou J. N., Ljungdahl S., Shoshan M. C., Swedenborg J., Linder S. (1998) Activation of tissue-factor gene expression in breast carcinoma cells by stimulation of the RAF-ERK signaling pathway. Mol. Carcinog. 21, 234–243 [PubMed] [Google Scholar]
- 40. Nakamura H., Vaage J., Valen G., Padilla C. A., Björnstedt M., Holmgren A. (1998) Measurements of plasma glutaredoxin and thioredoxin in healthy volunteers and during open-heart surgery. Free Radic Biol. Med. 24, 1176–1186 [DOI] [PubMed] [Google Scholar]
- 41. Jekell A., Hossain A., Alehagen U., Dahlström U., Rosén A. (2004) Elevated circulating levels of thioredoxin and stress in chronic heart failure. Eur. J. Heart Fail. 6, 883–890 [DOI] [PubMed] [Google Scholar]
- 42. Song K. S., Kim H. (2004) Plasma levels of tissue factor antigen in patients with non-insulin-dependent diabetes mellitus. Yonsei Med. J. 45, 38–42 [DOI] [PubMed] [Google Scholar]
- 43. Nakamura H., Herzenberg L. A., Bai J., Araya S., Kondo N., Nishinaka Y., Herzenberg L. A., Yodoi J. (2001) Circulating thioredoxin suppresses lipopolysaccharide-induced neutrophil chemotaxis. Proc. Natl. Acad. Sci. U.S.A. 98, 15143–15148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pollak N., Dölle C., Ziegler M. (2007) The power to reduce. Pyridine nucleotides, small molecules with a multitude of functions. Biochem. J. 402, 205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cao X., Liu M., Tuo J., Shen D., Chan C. C. (2010) The effects of quercetin in cultured human RPE cells under oxidative stress and in Ccl2/Cx3cr1 double-deficient mice. Exp. Eye Res. 91, 15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Berk B. C. (1999) Redox signals that regulate the vascular response to injury. Thromb. Haemost. 82, 810–817 [PubMed] [Google Scholar]
- 47. Griendling K. K., FitzGerald G. A. (2003) Oxidative stress and cardiovascular injury. Circulation 108, 2034–2040 [DOI] [PubMed] [Google Scholar]
- 48. Harrison D., Griendling K. K., Landmesser U., Hornig B., Drexler H. (2003) Role of oxidative stress in atherosclerosis. Am. J. Cardiol. 91, 7A–11A [DOI] [PubMed] [Google Scholar]
- 49. Tracy R. P. (2003) Thrombin, Inflammation, and cardiovascular disease. Chest 124, 49S–57S [DOI] [PubMed] [Google Scholar]
- 50. Wedgwood S., Black S. M. (2003) Role of reactive oxygen species in vascular remodeling associated with pulmonary hypertension. Antioxid. Redox Signal 5, 759–769 [DOI] [PubMed] [Google Scholar]
- 51. Björnstedt M., Hamberg M., Kumar S., Xue J., Holmgren A. (1995) Human thioredoxin reductase directly reduces lipid hydroperoxides by NADPH and selenocystine strongly stimulates the reaction via catalytically generated selenols. J. Biol. Chem. 270, 11761–11764 [DOI] [PubMed] [Google Scholar]
- 52. Mitsumoto A., Takanezawa Y., Okawa K., Iwamatsu A., Nakagawa Y. (2001) Variants of peroxiredoxins expression in response to hydroperoxide stress. Free Radic. Biol. Med. 30, 625–635 [DOI] [PubMed] [Google Scholar]
- 53. Mallat Z., Hugel B., Ohan J., Lesèche G., Freyssinet J. M., Tedgui A. (1999) Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques. A role for apoptosis in plaque thrombogenicity. Circulation 99, 348–353 [DOI] [PubMed] [Google Scholar]
- 54. Rehemtulla A., Ruf W., Edgington T. (1991) The integrity of the cysteine 186–cysteine 209 bond of the second disulfide loop of tissue factor is required for binding of factor VII. J. Biol. Chem. 266, 10294–10299 [PubMed] [Google Scholar]
- 55. Zhang H., Zhong L. (2010) Opposing regulation of histamine-induced calcium signaling by sodium selenite and ebselen via alterations of thiol redox status. Eur. J. Pharmacol. 626, 276–282 [DOI] [PubMed] [Google Scholar]
- 56. Xing J., Strange K. (2010) Phosphatidylinositol 4,5-bisphosphate and loss of PLCγ activity inhibit TRPM channels required for oscillatory Ca2+ signaling. Am. J. Physiol. Cell Physiol 298, C274–C282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. González-Pacheco F. R., Caramelo C., Castilla M. A., Deudero J. J., Arias J., Yagüe S., Jiménez S., Bragado R., Alvarez-Arroyo M. V. (2002) Mechanism of vascular smooth muscle cells activation by hydrogen peroxide. Role of phospholipase Cγ. Nephrol. Dial. Transplant. 17, 392–398 [DOI] [PubMed] [Google Scholar]
- 58. van der Merwe J. Q., Moreau F., MacNaughton W. K. (2009) Protease-activated receptor-2 stimulates intestinal epithelial chloride transport through activation of PLC and selective PKC isoforms. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1258–G1266 [DOI] [PubMed] [Google Scholar]
- 59. Biró E., Sturk-Maquelin K. N., Vogel G. M., Meuleman D. G., Smit M. J., Hack C. E., Sturk A., Nieuwland R. (2003) Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J. Thromb. Haemost. 1, 2561–2568 [DOI] [PubMed] [Google Scholar]