Background: Deregulation of AR signaling may lead to prostate cancer.
Results: PAK6 promotes AR ubiquitin-mediated degradation through phosphorylation of AR and Mdm2, resulting in inhibition of prostate cancer growth in vivo.
Conclusion: PAK6 suppresses prostate cancer growth via regulating AR homeostasis.
Significance: Modulating PAK6 kinase activity is a therapeutic strategy for AR-positive and hormone-sensitive prostate cancer.
Keywords: Androgen Receptor, Phosphorylation, Prostate Cancer, Tumor Suppressor Gene, Ubiquitination, Mdm2, PAK6, Homeostasis
Abstract
The androgen receptor (AR) signaling pathway plays a crucial role in the development and growth of prostate malignancies. Regulation of AR homeostasis in prostate tumorigenesis has not yet been fully characterized. In this study, we demonstrate that p21-activated kinase 6 (PAK6) inhibits prostate tumorigenesis by regulating AR homeostasis. First, we demonstrated that in normal prostate epithelium, AR co-localizes with PAK6 in the cytoplasm and translocates into the nucleus in malignant prostate. Furthermore, AR phosphorylation at Ser-578 by PAK6 promotes AR-E3 ligase murine double minute-2 (Mdm2) association, causing AR degradation upon androgen stimuli. We also showed that PAK6 phosphorylates Mdm2 on Thr-158 and Ser-186, which is critical for AR ubiquitin-mediated degradation. Moreover, we found that Thr-158 collaborates with Ser-186 for AR-Mdm2 association and AR ubiquitin-mediated degradation as it facilitates PAK6-mediated AR homeostasis. PAK6 knockdown promotes prostate tumor growth in vivo. Interestingly, we found a strong inverse correlation between PAK6 and AR expression in the cytoplasm of prostate cancer cells. These observations indicate that PAK6 may be important for the maintenance of androgen-induced AR signaling homeostasis and in prostate malignancy, as well as being a possible new therapeutic target for AR-positive and hormone-sensitive prostate cancer.
Introduction
The androgen receptor (AR),2 a well known transcription factor, generally regulates gene expression through a ligand-dependent mechanism. The androgen receptor signaling pathway plays an essential role in the development, function, and balance of the androgen response system, especially for the growth and development of normal prostate and cancer cells, as well as in recurrent prostate cancer after androgen ablation therapy (1–4). Moreover, animal and human models showed that AR expression positively correlated with progression of primary and metastatic prostate cancer (5, 6). AR signaling homeostasis needs to be tightly controlled to maintain diverse cell functions. Deregulation of this homeostasis promotes defects in androgen responses and promotes prostate cancer (7).
Androgen stimulation promotes AR translocation from the cytoplasm to the nucleus and initiates transcriptional regulation of downstream target genes associated with a distinct profile of cofactors. Also, it is well known that excessive AR expression in the nucleus leads to prostate cancer (8). AR undergoes post-translational modifications, such as phosphorylation, sumoylation, acetylation, and ubiquitination (9). In particular, AR is a substrate for many protein kinases, such as Aurora-A, PKC, and Akt (10–12), which modulate AR activity via phosphorylation. AR phosphorylation also leads to its ubiquitination and degradation by E3 ligases, such as murine double minute 2 protein (Mdm2) (13–15) and carboxyl terminus of Hsc70-interacting protein (CHIP) (16).
As a well known negative regulator of AR, PAK6 is of great concern for the inhibition of prostate cancer growth. As a serine/threonine kinase, PAK6 was initially cloned from prostate cancer cells as an AR-interacting protein (17, 18). It is a member of the p21-activated kinase (PAKs) family, which is the major effector of RhoGTPases Cdc42 and Rac (19). Upon upstream signals, PAK proteins are involved in a variety of cellular functions, including gene regulation, cell motility, and cell survival (20–22). The PAK family contains six members, subdivided into two groups: PAK1–3 (group I) and PAK4–6 (group II) (22–24). PAK6 exists primarily in the cytoplasm, no matter whether AR is present or not, whereas AR down-regulation is dependent on PAK6 kinase activity but is not related to AR cofactors (25). However, the mechanism of AR modulation by PAK6 remains unclear. Although PAK6 is a tumor suppressor, its expression was increased in primary and metastatic prostate cancer compared with normal prostate epithelium (26). Also, no correlation between PAK6 and AR was observed in normal prostatic tissue and prostate cancer. Understanding how PAK6 regulates AR is vital for understanding prostate tumorigenesis.
In this present study, we aimed to assess how PAK6 regulates AR ubiquitin-mediated degradation through phosphorylation of AR and Mdm2 and its implication in the inhibition of prostate cancer growth in vivo.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfections
HEK293 and COS7 cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS). The prostate cell line CWR22Rv1 cells were cultured in RPMI 1640 medium containing 10% FBS, 10% charcoal-stripped serum (Hyclone, Thermo Fisher Scientific). Lipofectamine 2000 (Invitrogen) was used for transfection. Cells at ∼60% confluence were transfected for 6 h and incubated with phenol red-free medium containing 10% charcoal-stripped serum for 16 h. Cell extracts were prepared following another 16 h treatment with 10 nm dihydrotestosterone (DHT).
Plasmids and Materials
pcDNA-EGFP-PAK6 wild-type (WT)/K436A (KA; kinase-dead) were gifts from Dr. G. M. Bockoh (The Scripps Research Institute). Wild-type Mdm2 (Mdm2 WT) and C464A were from Dr. A. G. Jochemsen (Leiden University Medical Centre, The Netherlands) and Dr. B. M. Burgering (University Medical Centre Utrecht, Molecular Cancer Research, The Netherlands). AR and ARE-luc were gifts from Dr. Y. Zhao (China Medical University). His/Myc/GST-tagged PAK6 and Mdm2 were constructed by PCR and subcloned into pcDNA3.1-Myc-HisA, pcDNA3.1-HisC (Invitrogen) and pGEX-5X-1/2 (GE Healthcare) vectors, respectively. AR/Mdm2 deletions were obtained using PCR and subcloned into pGEX-5X-2/1. Site-directed mutagenesis was generated from AR/Mdm2 WT using the QuikChange kit (Stratagene) according to the manufacturer's instruction. PKC inhibitor (sc-3007) was from Santa Cruz Biotechnology (Santa Cruz). Cycloheximide (CHX), DHT, and MG132 were from Sigma.
GST Pulldown Assay
The GST pulldown assays were performed by incubating equal amounts of GST and GST fusion proteins immobilized by GST-Sepharose beads (GE Healthcare) with in vitro-translated protein prepared by TnT-coupled translation system (Promega). Bound proteins were incubated and washed with binding buffer (20 mm Tris, pH 7.5, 50 mm NaCl, 10% glycerol, 1% Nonidet P-40) and stained by Ponceau or Coomassie Brilliant Blue. Bound proteins were then visualized by Western blot.
Western Blot Analysis
Cells were lysed in RIPA lysis buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, 1 mm EDTA, and protease inhibitor mixture). Denatured proteins were analyzed by SDS-PAGE and transferred to a PVDF membrane (Millipore). Samples were incubated and detected with the indicated antibodies. Anti-AR, anti-prostate-specific antigen, and anti-poly(ADP-ribose) polymerase (PARP)/paxillin antibodies were from Neomarkers, Thermo Fisher Scientific. Anti-PAK6 antibody was from Abcam (Cambridge, MA), anti-FLAG antibody was from Shanghai Genomics, Inc. (Shanghai, China), and anti-GAPDH antibody was from Shanghai KangChen (Shanghai, China). Remaining antibodies were purchased from Santa Cruz Biotechnology.
Immunoprecipitation
Cells were washed with PBS prior to cell lysis in 500 μl of IP lysis buffer (25 mm Tris, pH 7.4, 150 mm NaCl, 1% Nonidet P-40, 1 mm EDTA), supplemented with 1 mm PMSF and protease inhibitor mixture (Roche Applied Science). Cell lysates were collected, washed, and incubated at 4 °C. Then, 30 μl of protein A-agarose slurry (GE Healthcare) preloaded with antibodies or normal IgG was added to equal amounts of cell extracts and incubated overnight at 4 °C. Washed precipitated proteins were analyzed by Western blot.
PAK6 Kinase Assay
Commercialized PAK6 kinase (Invitrogen) or immunoprecipitated synthesized PAK6 kinase was used for kinase assay in kinase buffer (50 mm HEPES, pH 7.5, 10 mm MgCl2, 2 mm MnCl2, and 0.2 mm DTT) added to 10 μCi of [γ-32P]ATP (5,000 Ci/mmol) and 2.5 μm cold ATP for 30 min at 30 °C. Phosphorylated proteins were analyzed by SDS-PAGE followed by autoradiography. Histone H3/H4 (Invitrogen) was used as the positive control. Ponceau staining indicated the loading amounts of GST fusion proteins.
Ubiquitination Assay
COS7 cells were transfected using plasmids with or without Myc-ubiquitin for 16 h in charcoal-stripped serum and treated with 10 nm DHT and 5 μm MG132 for 6 h. Cells were harvested in immunoprecipitation lysis buffer and denatured with 1% SDS. AR proteins were immunoprecipitated with anti-FLAG or anti-AR antibodies and subjected to SDS-PAGE, followed by immunoblotting with anti-Myc, anti-FLAG, or anti-AR antibodies after stripping.
Immunofluorescence Staining
Cells were fixed in 4% paraformaldehyde and then blocked with normal goat serum. Cells were incubated with the primary antibody for 1 h at 25 °C, washed with PBS with 0.1% Triton X-100, and subsequently incubated with secondary antibody conjugated with green or red dye. DNA was stained using To-PRO-3 (Molecular Probes. Invitrogen) or DAPI (Roche Applied Science). Confocal scanning analysis was performed using a Leika/Olympus laser confocal scanning microscope.
Lentivirus Production and Infection
hu6-MCS-CMV-puromycin enhanced GFP-tagged vector was used to construct RNAi lentivirus system. Silence RNA sequences targeting PAK6 was produced by the GENECHEM company (Shanghai, China). shRNA PAK6 sequences is 5′-GCAGGCUAUUCCGAAGCAUTT-3′ and shRNA control sequences is 5′-UUCUCCGAACGUGUCACGU-3′.
Commercial control silencing/shPAK6 virus was used to infect CWR22Rv1 cells in a 12-well plate with 3 mg/ml Polybrene. Infected CWR22Rv1 cells were then subjected to sorting by enhanced GFP expression.
Tissue Specimens and Analysis
Among our tissue specimens, 24 specimens were from the PR481 tissue microarray purchased from Alenabio Co. (Beijing, China), and 13 specimens were procured from surgical specimens of patients with prostate cancer for which complete information on clinical metastatic status was available. All human tissues were collected and used according to protocols approved by the Ethics Committee of the China Medical University Health Science Center. After antigen retrieval, specimens were subjected to normal immunohistochemical and immunofluorescence staining. PAK6 and AR co-localization score or AR localization score were determined by intensity (0 to 3) and fraction of stained cells (0 to 4). A total score (ranging from 0 to 12) was obtained by multiplying the staining intensity and fraction scores (27).
Tumor Growth in Human Prostate Carcinoma Xenografts
0.2 ml of CWR22Rv1 cells (2 × 106) in Matrigel (BD Biosciences) was inoculated into the dorsal side of 6- to 7-week-old NOD SCID nude male mice with an average weight of 20 to 25 g. Tumor size was measured using a caliper and recorded twice a week. At the end of the treatment, mice were sacrificed, and tumors were removed, photographed, and weighed. Standard tumor was a subcutaneous nodule ≥0.5 cm.
Cell Counting Assay
5 × 105 shRNA control/PAK6 lentivirus-infected CWR22Rv1 cells were plated in a 12-well plate. Cell amounts in each sample was measured daily in triplicates.
Statistical Analysis
Student's t test and one-way analysis of variance were performed to determine the statistical significance among values for in vitro experiments. Data derived from the immunostaining analysis of human prostate tissue specimens were analyzed using the unpaired t test. Comparisons were performed using two-tailed paired t test. All continuous data are presented as the mean values ± standard error of the mean.
RESULTS
PAK6 Involvement in AR Localization and Expression
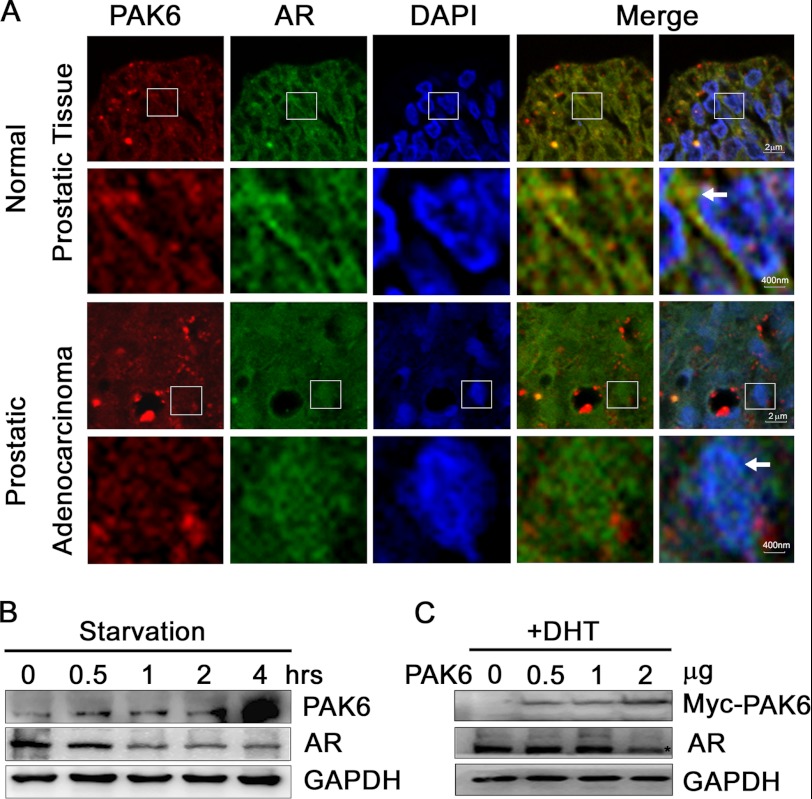
PAK6 and AR are highly expressed in prostate cancer (2, 8, 26). To investigate the relationship between PAK6 and AR in prostate and cancer tissues, 14 normal prostatic tissues and 23 prostatic adenocarcinoma samples were analyzed by immunofluorescence staining. There was a remarkable co-localization of PAK6 and AR in cytoplasm in normal prostate epithelium, although no co-localization in malignant prostate was observed. AR translocated into the nucleus in malignant prostate cells (Fig. 1A). Because nutrient depletion is a common event during tumor growth and PAK6 is increased in primary and metastatic prostate cancer (26), we treated CWR22Rv1 cells with serum starvation to mimic nutrient depletion and found that PAK6 was increased, and AR was decreased (Fig. 1B). This result indicates that nutrient deprivation induces PAK6 expression and inhibits AR expression. To further assess the relationship between PAK6 and AR, CWR22Rv1 cells were transfected with Myc-PAK6, and AR protein levels were reduced upon DHT stimulation (Fig. 1C). In this regard, PAK6 might suppress AR expression in cytoplasm.
FIGURE 1.
PAK6 involvement in AR localization and expression. A, PAK6 and AR are co-localized in the cytoplasm of normal prostate cells, and AR is localized in the nucleus in prostate cancer cells. Tissue specimens were subjected to immunohistochemical analysis as usual. After antigen retrieval, specimens were fixed and incubated with anti-PAK6 antibody followed by Alexa 594 Fluor (red) antibody and anti-AR antibody followed by Alexa 488 Fluor (green) antibody. Nucleus was stained by DAPI (blue). The 2nd and 4th lines are the 25-fold enlarged pictures of the 1st and 3rd lines, respectively. The 4th row was merged with red and green images, and 5th row was merged with red, green, and blue images. The white arrows in the 2nd line indicate AR co-localizes with PAK6 in the cytoplasm, and arrows in 4th line indicate AR translocates in the nucleus. B, starvation experiment showing that PAK6 expression increased and AR decreased upon starvation. CW22Rv1 cells were starved with Hanks' buffered saline solution (Invitrogen) at different time points as indicated. Western blot analysis was performed, and endogenous proteins were detected with the indicated antibodies. C, CWR22Rv1 cells transfected with Myc-PAK6 show decreased AR levels upon DHT activation. CWR22Rv1 cells were transfected with indicated amounts of pcDNA3.1-Myc-His-PAK6. Endogenous AR and exogenous PAK6 were detected with indicated antibodies.
PAK6 Regulates AR Localization through Phosphorylation of AR on Ser-578
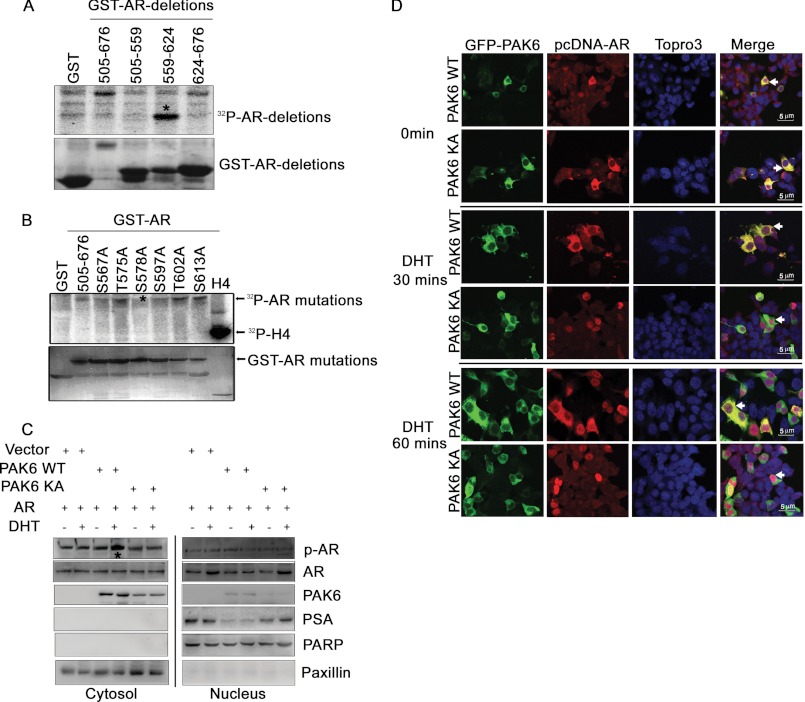
To elucidate how PAK6 negatively modulates AR, we focused on PAK6 kinase activity. Multiple lines of evidence indicate PAK6 negatively regulates AR through phosphorylation (supplemental Fig. S1) (25). It has been reported that PAK6 phosphorylates AR in its DNA binding domain (amino acids 505–676) (25). GST-AR deletion constructs were used to map the shorter phosphorylation domain, which is between amino acids 559 and 624 of AR, by in vitro kinase assay (Fig. 2A). Six candidate phosphorylation sites in this short region were chosen for single-site mutation from serine/threonine to alanine, which is resistant to phosphorylation. Further in vitro kinase assays screened out Ser-578 as a novel phosphorylation site for PAK6 (Fig. 2B). As the Ser-578 site is also a PKC phosphorylation site on the AR (11), a PKC inhibitor (sc-3007) was used in subsequent experiments to avoid nonspecific results.
FIGURE 2.
PAK6 regulates AR localization through phosphorylation of AR on Ser-578. A, PAK6 phosphorylates AR between amino acids 559 and 624. HEK293 cells were transfected with pcDNA3.1Myc-His-PAK6 and lysed for immunoprecipitation with anti-Myc antibody, and the immunoprecipitated PAK6 kinase was incubated with GST-AR deletions (505–559, 559–624, 624–676, and 505–676 amino acids) for in vitro kinase assay. B, AR Ser-578 is the phosphorylation site by PAK6. In vitro kinase assay was performed using commercialized PAK6 kinase and GST-AR WT (amino acids 505–676) or GST-AR single-site mutants as indicated. Histone H4 served as a positive control. C, wild-type PAK6 phosphorylated AR in the cytoplasm and reduced its nuclear translocation, leading to decreased prostate-specific antigen (PSA) levels. HEK293 cells were co-transfected with pcDNA3.1-His-PAK6 WT/KA and pcDNA3.1-His-AR in the presence or absence of DHT, and cytoplasmic and nuclear proteins were subjected to SDS-PAGE separately. Western blot analysis was performed as indicated. p-AR is phosphorylated AR Ser-578 antibody. Poly(ADP-ribose) polymerase (PARP) served as a nuclear loading control, and paxillin served as a cytoplasmic loading control. D, inhibition of AR nuclear translocation by PAK6 relied on Ser-578 phosphorylation. HEK293 cells were co-transfected with pcDNA-EGFP-PAK6 WT/KA and pcDNA3.1-His-AR. Cells were starved with steroid hormone for 16 h, stimulated with 10 nm DHT for 0, 30, and 60 min as indicated, fixed, and incubated with anti-AR antibody followed by Alexa Fluor 546 (red) antibody. Nucleus was stained with Topro3 (blue). The white arrows in the 4th rows indicate the subcellular co-localization of PAK6 WT/KA and AR.
To test the function of PAK6-mediated AR phosphorylation, phospho-AR Ser-578 antibody was used, and its quality and availability were verified (supplemental Fig. S2). Because AR and PAK6 are highly expressed in prostate cancer, AR and PAK6 constructs were transfected into HEK293 cells, which have no endogenous expression of AR and PAK6. Because AR translocation from the cytoplasm to the nucleus is the key step to initiate transcription of its downstream target genes, Western blot analysis was performed with cytoplasmic and nuclear protein separately. It was demonstrated that wild-type PAK6 (PAK6 WT) but not kinase-dead (PAK6 KA) PAK6 phosphorylated AR mainly in the cytoplasm and reduced nuclear AR translocation, leading to down-regulation of the AR downstream target gene prostate-specific antigen (PSA) (Fig. 2C). These results were obtained upon androgen stimulation, indicating that PAK6-mediated AR regulation is androgen-dependent and that PAK6 might obstruct AR nuclear translocation. PAK6 and AR co-localization was further examined by immunofluorescence staining. DHT-induced AR was shown to be retained in the cytoplasm by PAK6 WT but not by PAK6 KA (Fig. 2D). Inhibition of AR nuclear translocation by PAK6 relied on Ser-578 phosphorylation (Fig. 2, C and D). Because the PAK6 kinase activity inhibitory effect on AR is triggered by DHT, the following results were obtained in the presence of androgen. It was demonstrated that pcDNA3.1-His-PAK6 localized in the cytoplasm, regardless of the presence of androgen, avoiding the obstructive effect of the GFP tag on PAK6 nuclear translocation (supplemental Fig. S3). These results reveal that PAK6 inhibits AR nuclear translocation through Ser-578 phosphorylation.
PAK6-mediated AR Phosphorylation Promotes Its Ubiquitin-mediated Degradation
Because the inhibition of androgen-induced AR nuclear translocation by PAK6 contributes to accumulation of phosphorylated AR in the cytoplasm (Fig. 2C), it became very interesting to further investigate the fate of AR in the cytoplasm. The amount of AR protein levels in cells is the result of equilibrium between synthesis and degradation. By using cycloheximide to inhibit protein synthesis (28), PAK6 induced a reduction in AR protein levels upon DHT stimulation (Fig. 3A). Because phosphorylation plays a crucial role in proteasome-mediated degradation of proteins, such as the cyclin E and p27Kip1 protein (29, 30), we explored the possibility that PAK6-mediated phosphorylation regulates AR degradation. We showed that PAK6 induced a reduction of the wild-type AR (AR WT) rather than S578A mutant protein levels under DHT stimulation (Fig. 3B).
FIGURE 3.
PAK6-mediated AR phosphorylation promotes its ubiquitin-mediated degradation. A, PAK6 reduced AR levels under DHT stimulation. HEK293 cells were transfected with pcDNA3.1-Myc-His-PAK6 and pcDNA 3.1-His-AR and treated with 10 nm DHT and 10 μm cycloheximide at the indicated times. PAK6 and AR protein levels were measured by Western blot analysis as indicated. B, PAK6 reduced wild-type AR under DHT stimulation, while AR S578A mutant was unaffected. HEK293 cells were co-transfected with pcDNA-FLAG-AR wild-type/S578A (505–919 amino acids) (including DNA binding domain and ligand binding domain), and pcDNA3.1-Myc-His-PAK6 was treated with 10 nm DHT and 10 μm cycloheximide. C, ubiquitination assay showed that PAK6 markedly enhanced AR WT but not AR S578A ubiquitination. HEK293 cells were transfected with Myc-ubiquitin and pcDNA-FLAG-AR WT/S578A (505–919 amino acids) in combination with pcDNA3.1-Myc-His PAK6/vector-treated or untreated with 5 μm MG132 for 6 h in the presence of 10 nm DHT. 30 μg of total lysates were set aside as input; equal amounts lysates of protein were then harvested for ubiquitination assay. IP, immunoprecipitation; IB, immunoblot. D, stable knockdown of PAK6 by shRNA in CWR22Rv1 cells showed impairment of the endogenous ubiquitination of AR when treated with MG132 and DHT (7th lane from left). CWR22Rv1 cells infected with lentiviruses harboring shRNA control/PAK6 were treated with or without 5 μm MG132 for 6 h in the presence/absence of 10 nm DHT. Equal amounts lysates of protein were then harvested for ubiquitination assay.
To validate whether PAK6-dependent phosphorylation mediates AR proteasome-dependent degradation, we used MG132, a 26 S proteasome inhibitor (31). Ubiquitination assay showed that PAK6 markedly enhanced AR WT but not AR S578A ubiquitination (Fig. 3C). Because PAK6 regulates AR and Estrogen Receptor (ER) (18), CWR22Rv1 cells were used, which express only AR but not ER (32). Stable knockdown of PAK6 by shRNA in CWR22Rv1 cells showed impairment of the endogenous ubiquitination of AR when treated with MG132 and DHT (7th lane from left, Fig. 3D). Interestingly, PAK6-mediated AR ubiquitination is androgen-dependent, indicating that PAK6 modulates endogenous ubiquitination of AR in vivo upon androgen stimulation. The above data indicate that the accumulated AR in cytoplasm was further degraded in a proteasome pathway prompted by PAK6-mediated phosphorylation of AR.
PAK6-mediated AR Phosphorylation Enhances AR-Mdm2 Association
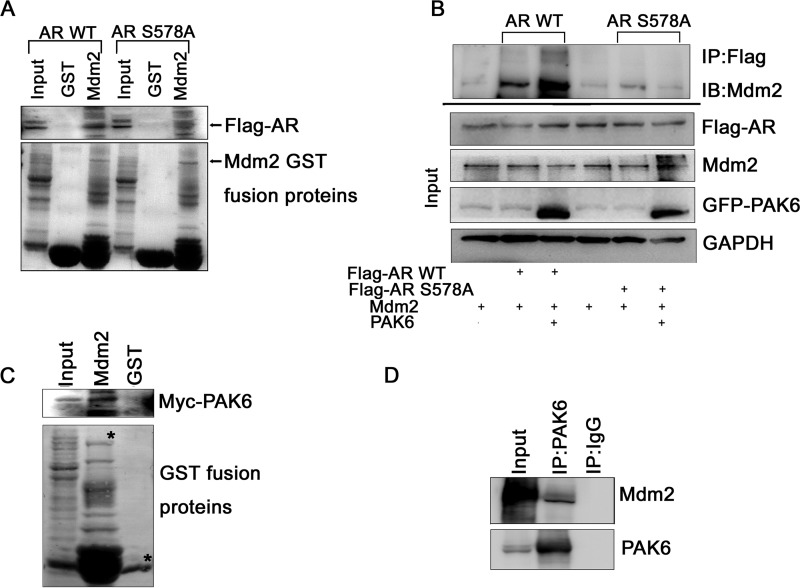
E3 ligase is necessary for protein degradation, but PAK6 is not an E3 ligase. We examined several E3 ligases targeting AR for degradation, such as Mdm2 and CHIP, and Mdm2 seems effective in AR degradation. To test whether E3 ligase participation was affected by AR Ser-578 phosphorylation, we compared Mdm2 interaction with AR WT and AR S578A mutant in vitro. As shown in Fig. 4A, Mdm2 interacted strongly with AR WT but much more weakly with AR S578A mutant. This result is also validated by immunoprecipitation in vivo (Fig. 4B). Mdm2 associated stronger with AR WT than AR S578A mutant in the presence of PAK6 (Fig. 4B), indicating that PAK6-mediated phosphorylation of AR is involved in the recruitment of Mdm2 to AR and further enhances the AR-Mdm2 association.
FIGURE 4.
PAK6-mediated phosphorylation of AR enhances AR association with Mdm2. A, Mdm2 interacted strongly with AR WT but much more weakly with AR S578A mutant. In vitro translated pcDNA-FLAG-AR WT/S578A (505–919 amino acids) was incubated with GST-Mdm2 as indicated and analyzed with anti-FLAG antibody. GST served as control. B, Mdm2 associated stronger with AR WT than AR S578A mutant in the presence of PAK6. COS7 cells were co-transfected with pcDNA-FLAG-AR WT/S578A (505–919 amino acids) in combination with pcDNA-EGFP-PAK6 and Mdm2 as indicated, treated with 10 nm DHT. 30 μg of total lysates were set aside as input; equal amounts of lysates of protein were immunoprecipitated (IP) with anti-FLAG antibody and immunoblotted (IB) with anti-Mdm2 antibody. C, GST pulldown assay showed that PAK6 and Mdm2 interact with each other in vitro. In vitro translated pcDNA3.1-Myc-His-PAK6 was incubated with GST-Mdm2 and analyzed with anti-Myc antibody. GST served as control. D, immunoprecipitation assay confirmed that PAK6 associates with Mdm2 in CWR22Rv1 cells. Equal amounts of CWR22Rv1 cell lysates were immunoprecipitated with anti-PAK6 or IgG. Endogenous PAK6 and Mdm2 were immunoblotted with anti-PAK6 and anti-Mdm2 antibodies.
This finding led us to presume that Mdm2 may be associated with PAK6 and forms a complex with AR, thus targeting AR for ubiquitin-mediated degradation. GST pulldown assay showed that PAK6 and Mdm2 interact with each other in vitro (Fig. 4C). Immunoprecipitation assay confirmed that PAK6 associates with Mdm2 in CWR22Rv1 cells (Fig. 4D), which express endogenous PAK6, Mdm2, and AR. Because Mdm2 has been shown to interact with AR (14), our results indicate that PAK6, Mdm2, and AR form a complex. Therefore, these results suggest that PAK6 associates with Mdm2 to recruit AR for its ubiquitin-mediated degradation.
PAK6 Phosphorylates Mdm2 on Thr-158 and Ser-186
As PAK6 is a protein kinase and interacts with Mdm2, Mdm2 is a potential phosphorylated substrate of PAK6. The in vitro kinase assay verified this postulation and showed that amino acids 121–300 were the phosphorylation domain by PAK6 (Fig. 5, A and B). In this region, eight candidate mutants were constructed. Two novel sites in Mdm2, Thr-158 and Ser-186, were identified by in vitro kinase assay as the phosphorylation sites by PAK6 (Fig. 5C).
FIGURE 5.
PAK6 phosphorylates Mdm2 on Thr-158 and Ser-186. A, PAK6 phosphorylates Mdm2. HEK293 cells were transfected with pcDNA3.1-Myc-His-PAK6 and lysed for immunoprecipitated with anti-Myc antibody; the immunoprecipitated PAK6 kinase was incubated with GST-Mdm2 and analyzed by autoradiography. Histone H3 served as positive control. B, PAK6 phosphorylates Mdm2 between amino acids (aa) 121 and 300. In vitro kinase assay was performed using commercialized PAK6 kinase and GST-Mdm2 full-length and deletions as indicated and analyzed by autoradiography (auto). C, Mdm2 Thr-158 and Ser-186 are the phosphorylation sites by PAK6. In vitro kinase assay was performed using commercialized PAK6 kinase and GST-Mdm2 (121–300 amino acids) or GST-Mdm2 single-site mutants as indicated and analyzed by autoradiography. Histone H3 (H3) served as a positive control.
Functional Roles of Mdm2 Thr-158 and Ser-186 in AR Degradation
Because PAK6-mediated AR phosphorylation was shown to enhance AR-Mdm2 interaction (Fig. 4, A and B), we postulated that PAK6 phosphorylates AR in the cytoplasm. Subsequently, PAK6 phosphorylates and recruits Mdm2 to the PAK6-AR complex, leading to AR ubiquitin-mediated degradation. To validate whether Mdm2 phosphorylation was related to AR proteasome degradation, ubiquitination assay was performed upon DHT stimulation in COS7 cells, which have no endogenous expression of PAK6 and AR and low levels of Mdm2 expression. As expected, AR ubiquitination only occurred in the presence of Myc-Ub (2nd lane from left, Fig. 6A). To study the function of Mdm2 Thr-158 and Ser-186, both sites were mutated into an alanine to abolish the phosphorylation. We compared the effect of wild-type Mdm2 (Mdm2 WT) and mutants on AR ubiquitination and degradation. When treated with MG132, the co-existence of PAK6 and Mdm2 WT enhanced AR ubiquitination remarkably (4th lane from left, Fig. 6A). However, the other three Mdm2 mutants, including Mdm2 T158A, S186A, and T158A/S186A mutants, did not induce AR ubiquitination (Fig. 6A). This result is consistent with Mdm2 C464A mutant, a RING finger mutant that failed to induce ubiquitination of substrate (33). The faint ubiquitylated AR bands in Fig. 6A, 6th to 8th lanes, suggest that phosphorylation of Mdm2 by PAK6 is critical for AR ubiquitination and degradation.
FIGURE 6.
Functional roles of Mdm2 Thr-158 and Ser-186 in AR degradation. A, PAK6 induces AR ubiquitination dependent on Mdm2 phosphorylation. COS7 cells were transfected with pcDNA3.1-His-AR in combination with vector, pcDNA3.1-His-PAK6, or pcDNA3.1-His-Mdm2 mutants in the presence or absence of Myc-ubiquitin (Myc-Ub) in 10% charcoal-stripped medium for 16 h, followed by treatment with 10 nm DHT for 16 h. The cells were then harvested for ubiquitination assay. IP, immunoprecipitation; IB, immunoblot. B, AR interacted strongly with Mdm2 WT and T158A but weakly with Mdm2 S186A mutant. In vitro translated pcDNA3.1-His-AR was incubated with GST-Mdm2 mutants as indicated and analyzed with anti-AR antibody. GST served as control. C, PAK6-mediated phosphorylation of Mdm2 Thr-158 promotes Ser-186 association with AR. In vitro translated pcDNA3.1-His-AR was incubated with equal amounts of GST-Mdm2 S186A or T158A mutant. Meanwhile, increasing amounts of in vitro translated T158A or S186A (5, 10, and 20 μl) were added and analyzed with anti-AR antibody. GST served as control. D, Mdm2 S186A but not T158A mutant weakened PAK6-AR association. In vitro translated pcDNA3.1-His-AR was incubated with equal amounts of GST-PAK6; meanwhile, increasing amounts of in vitro translated Mdm2 mutants (5, 10, and 20 μl) were added and analyzed with anti-His and anti-AR antibodies. GST served as control.
What is the function of Mdm2 Thr-158 and Ser-186? Is there any collaboration between them? Fig. 6B showed that AR interacted strongly with Mdm2 WT and T158A, much more weakly with Mdm2 S186A mutant, and did not interact at all with Mdm2 T158A/S186A mutant, indicating that phosphorylation of Mdm2 Ser-186 by PAK6 is essential for AR-Mdm2 association. Furthermore, GST pulldown assay was used to judge the effect of PAK6-mediated phosphorylation of Mdm2 on AR-Mdm2 association. Increasing the Mdm2 T158A mutant resulted in lowering S186A association with AR with equal amounts of the S186A mutant. However, increasing the Mdm2 S186A mutant did not diminish T158A association with AR with equal amounts of the T158A mutant. Collectively, these results suggest that PAK6-mediated phosphorylation of Mdm2 Thr-158 promotes Ser-186 association with AR (Fig. 6C). To elucidate the effect of PAK6-mediated phosphorylation of Mdm2 on PAK6-AR association, we showed (Fig. 6D) that Mdm2 S186A but not T158A mutant weakened PAK6-AR association. Moreover, Mdm2 T158A/S186A mutant vigorously abolished PAK6-AR association with equal amounts of PAK6 protein. This further demonstrates that Mdm2 Thr-158 acts on AR through modulating Mdm2 Ser-186 association with AR as it facilitates PAK6-regulated AR ubiquitin degradation. Thus, phosphorylation of Mdm2 Thr-158 by PAK6 collaborates with phosphorylated Ser-186 for AR degradation.
PAK6 Inhibits Prostate Cancer Growth in Vivo
To test the inhibitory effect of PAK6 on tumor growth, cell counting assay was first used. Stable knockdown of PAK6 by shRNA in CWR22Rv1 cells showed a significant enhancement of proliferation in vitro (Fig. 7A). The proliferating marker gene cyclin D1 in AR signaling was up-regulated in shRNA PAK6 group upon DHT stimuli (Fig. 7B). Furthermore, the growth-inhibitory role of PAK6 was tested in vivo. NOD SCID nude male mice were subcutaneously injected with 2 × 106 CWR22Rv1 cells infected with lentiviruses harboring shRNA PAK6/Control. Four weeks after shRNA control/PAK6 CWR22Rv1 cells implantation, subcutaneous nodules became measurable. From the 4th to 7th week, shRNA PAK6-injected group on the right dorsal side developed rapidly and significantly larger tumors than controls on the left dorsal side (Fig. 7C). The ratio of tumor development of the shRNA PAK6 group was markedly higher than in shRNA control group. Meanwhile, the final tumor weight of shRNA PAK6 was much heavier than shRNA control cells. These data indicate that PAK6 may suppress tumor growth in human prostate cancer.
FIGURE 7.
PAK6 inhibits prostate cancer growth in vivo. A, stable knockdown of PAK6 by shRNA in CWR22Rv1 cells showed a significant enhancement of proliferation in vitro. CWR22Rv1 cells infected with lentiviruses harboring shRNA control/PAK6 were plated in 12-well plates with equal amounts and counted every day for 1 week. B, proliferating marker gene cyclin D1 in AR signaling was up-regulated in shRNA PAK6 group upon DHT stimuli. shRNA control/PAK6 in CWR22Rv1 cells was treated with or without DHT for 16 h. Equal amounts of protein lysates were then harvested for Western blot with antibodies as indicated. C, effect of shRNA control (left dorsal side) or shRNA PAK6 (right dorsal side) on the growth of CWR22Rv1 cells inoculated into nude mice. NOD SCID nude male mice were subcutaneously injected with 2 × 106 CWR22Rv1 cells infected with lentiviruses harboring shRNA PAK6/control. Tumor volume was monitored over time as indicated, and the tumor was excised and weighed after 49 days. PAK6 depletion causes a high ratio of tumor development (table) and an increase in tumor volume and weight (graphs).
Relationship between PAK6 and AR in Prostate Cancer
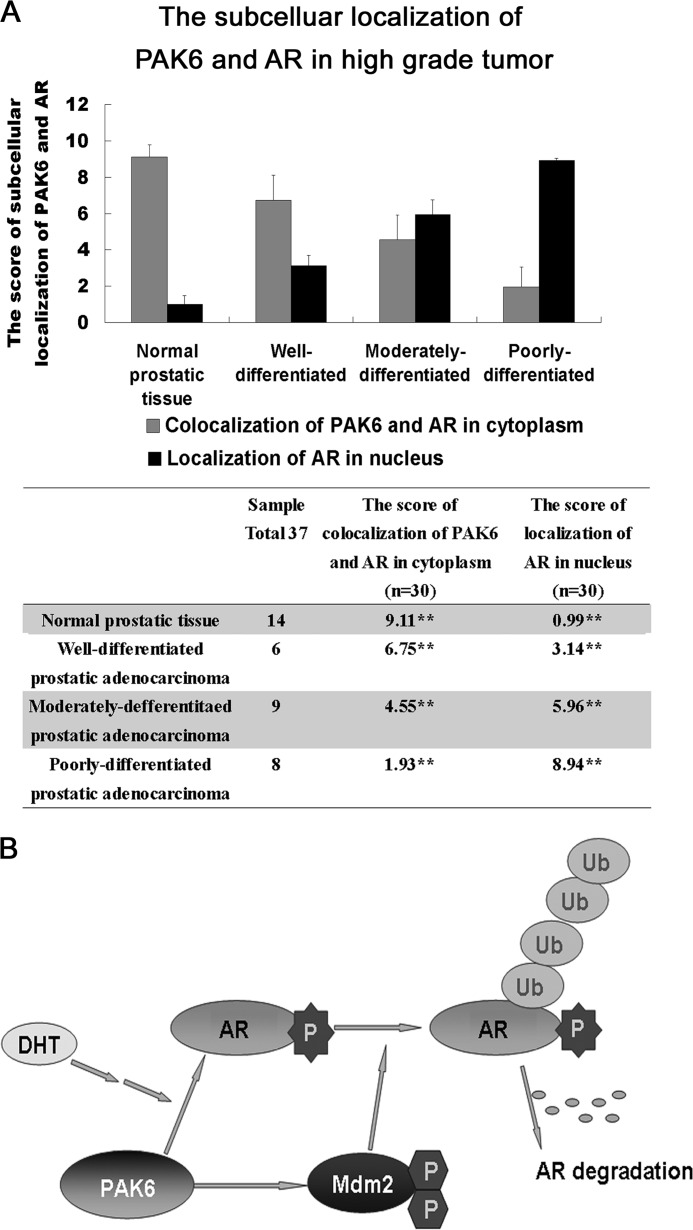
To further demonstrate the clinical relevance between PAK6 and AR, the correlation of PAK6 and AR in prostate cancer was investigated, and a significant decrease of co-localization of PAK6 and AR in the cytoplasm and an increased AR nuclear translocation associated with high grade tumor was observed, indicating a strong inverse correlation between expression of PAK6 and AR in cytoplasm (Fig. 8A and supplemental Fig. S4). This may point out important implications for PAK6 in the maintenance of AR signaling homeostasis and in prostate malignancy, as well as in a therapeutic strategy for AR-positive and hormone-sensitive prostate cancer by modulating PAK6 activity.
FIGURE 8.
A, relationship between PAK6 and AR in prostate cancer. 14 normal prostate, 6 well differentiated, 9 moderately differentiated, and 8 poorly differentiated prostate tumor specimens were collected and subjected to immunofluorescence analysis. The score of immunostaining co-localization of PAK6 and AR in cytoplasm or localization of AR in nucleus was counted from 30 independent visions and determined by intensity (0 to 3) and fraction of stained cells (0 to 4). A total score (ranging from 0 to 12) was obtained by multiplying the staining intensity and fraction scores. ** means p value less than or equal to 0.001 was considered statistically significant according to unpaired t test. B, proposed mechanism for the relation between PAK6, AR, Mdm2, and ubiquitin-proteasome degradation. Upon DHT stimulation, PAK6 obstructs AR nuclear translocation through phosphorylation on AR Ser-578; this phosphorylation enhances the binding between AR and Mdm2. The further phosphorylation of Mdm2 by PAK6 promotes proteasome to recognize the polyubiquitylated AR, leading to AR degradation.
DISCUSSION
PAK6, as a negative regulator of AR, received much attention for inhibiting prostate cancer, but the exact mechanisms had not been determined. In this study, we investigated the mechanism of AR ubiquitin-mediated degradation prompted by PAK6 in the presence of androgen. Both PAK6 and AR are highly expressed in prostate cancer (2, 8, 26), although the correlation between PAK6 and AR in prostate cancer has not yet been reported. To the best of our knowledge, this study is the first to show that AR co-localized with PAK6 in the cytoplasm of prostate epithelial cells and translocated into the nucleus of malignant prostate cells (Fig. 1A). Furthermore, a significant decrease of PAK6 and AR co-localization in the cytoplasm was accompanied by an increase of AR nuclear translocation associated with high grade tumor. These data demonstrate that PAK6 plays an important role in regulating AR nuclear translocation. Androgen-induced AR nuclear translocation is the key step to initiate the transcription of downstream genes involved in diverse cell functions, such as cell proliferation and survival (34). However, excessive AR activation would disrupt its homeostasis and causes disorder, such as prostate cancer. To maintain physiological status, cells use strategies to balance AR expression. In this regard, PAK6 suppressed AR expression and further impaired AR nuclear translocation in the presence of androgen.
Androgen ablation therapy is the main therapy in prostate cancer, is initially effective, and generally leads to disease remission (4). However, recurrent tumors arise within 2–3 years, on average (35). At present, few therapeutic regimens have been described to effectively manage recurrent prostate cancers, which are currently considered as an incurable disease (35). Given the inhibitory effect of PAK6 on AR upon androgen stimuli, exploring the mechanisms of PAK6 down-regulation of AR will provide insight into new eventual prostate cancer therapy. We established that PAK6 plays its inhibitory role through AR phosphorylation on Ser-578, impairing AR translocation from the cytoplasm to the nucleus upon androgen stimulation. It was reported that PKC phosphorylates AR Ser-578 in the presence of EGF, and impairs the Ku-70/80 and nuclear-cytoplasmic shuttling of the AR (11), which partially supports our findings. Our results demonstrated that PAK6 kinase activity is involved in the inhibition of AR nuclear translocation.
As multiple evidence indicates that co-activators such as β-catenin, SRC1, p300, Tip60α, and ARA55 and the SMRT co-repressor did not modify the relative inhibitory effect of wild-type or kinase-dead PAK6 on AR transactivation (25), we speculated that PAK6 might regulate AR through the ubiquitin-proteasome pathway instead of epigenetics. We have provided several lines of evidence to address this issue. On the one hand, PAK6 efficiently promotes AR WT ubiquitination and degradation but not the AR S578A mutant. On the other hand, PAK6 knockdown failed to induce endogenous AR ubiquitin-mediated degradation upon DHT stimulation. These data suggest that PAK6 regulates AR ubiquitination and degradation. This may be viewed in another way that PAK6 expression may relapse after androgen deprivation therapy compared with tumors (26). Therefore, modulating PAK6 kinase activity may inhibit excessive AR nuclear translocation and promote its proteolysis and regulate AR expression homeostasis.
E3 ligase is necessary for the ubiquitin degradation complex. Although Mdm2 and CHIP were reported to interact with AR and induce AR degradation (14, 16), we found that only Mdm2 interacted with the PAK6-AR complex. Moreover, PAK6 mediated AR phosphorylation on Ser-578 and contributed to recruitment of Mdm2 to AR.
To gain further insight into the involvement of Mdm2 in PAK6-mediated AR ubiquitination, Mdm2 Thr-158 and Ser-186 were identified as the PAK6 phosphorylation sites. In this study, Mdm2 T158A, S186A, and T158A/S186A mutants prevented accumulation of AR ubiquitination, indicating that phosphorylation of Mdm2 by PAK6 is indispensable for AR ubiquitin-mediated degradation. Akt mediates AR ubiquitination through phosphorylation of Mdm2 Ser-166 and Ser-186 (14). The impact of phosphorylation of Mdm2 Thr-158 and Ser-186 by PAK6 on AR-Mdm2 association was also investigated. It was demonstrated that phosphorylated Ser-186 is essential for AR-Mdm2 association. Moreover, Mdm2 Thr-158 collaborates with Ser-186 for AR-Mdm2 association and AR ubiquitin degradation, as it facilitates PAK6-regulated AR homeostasis. Increasing PAK6 kinase activity not only suppresses AR nuclear translocation but also recruits Mdm2 to AR ubiquitination complex.
Our in vivo study established that PAK6 inhibits prostate tumor growth via regulating AR proteolysis. However, a previous study (36) described the ability of PAK6 to promote prostate tumor growth and invasive ability. We cannot rule out the reasons for this difference in this study.
The novel model we described demonstrates how PAK6 targets AR for its degradation upon androgen stimulation, which stresses the necessary strategy to balance AR homeostasis to maintain cell physiological status. Interestingly, PAK6 is highly expressed in primary and metastatic prostate cancer (26). We suspect this is a stress-induced increment of PAK6 in response to increased AR in prostate cancer. Once AR homeostasis is disrupted, increasingly uncontrolled AR translocation will lead to prostate cancer. As shown by clinical results, the increase of AR nuclear translocation is associated with high grade tumor. In this regard, PAK6 expression is also increased to inhibit excessive AR expression along with the tumor aggressiveness. This may explain why PAK6 expression is enriched in prostate cancer. Thus, increasing PAK6 kinase activity might be a potential therapeutic strategy for AR-positive and hormone-sensitive prostate cancer.
Overall, this study demonstrated that PAK6 suppresses prostate carcinogenesis by impairing AR nuclear translocation and by promoting recruitment of E3 ligase Mdm2 to AR for its degradation via the ubiquitin-proteasome pathway. These results provide some light on the regulation machinery of AR homeostasis and inspire interventions on PAK6 kinase activity as a potential therapeutic strategy for prostate cancer.
Acknowledgments
We thank Dr. G. M. Bokoch for providing PAK6 plasmids, Dr. A. G. Jochemse and Dr. B. M. Burgering for Mdm2 plasmids, and Dr. Y. Zhao for AR and ARE-luc constructs.
This work was supported by National Natural Science Foundation of China Grants 30771128, 90813038, and 31171360, Ministry of Education of China Doctoral Fund 20102104110016, and Education Department of Liaoning Province Grant LT2011011.

This article contains supplemental Figs. S1–S4.
- AR
- androgen receptor
- PAK6
- p21-activated kinase 6
- DHT
- dihydrotestosterone.
REFERENCES
- 1. Agoulnik I. U., Weigel N. L. (2008) Androgen receptor coactivators and prostate cancer. Adv. Exp. Med. Biol. 617, 245–255 [DOI] [PubMed] [Google Scholar]
- 2. Buchanan G., Irvine R. A., Coetzee G. A., Tilley W. D. (2001) Contribution of the androgen receptor to prostate cancer predisposition and progression. Cancer Metastasis Rev. 20, 207–223 [DOI] [PubMed] [Google Scholar]
- 3. Hobisch A., Culig Z., Radmayr C., Bartsch G., Klocker H., Hittmair A. (1996) Androgen receptor status of lymph node metastases from prostate cancer. Prostate 28, 129–135 [DOI] [PubMed] [Google Scholar]
- 4. Ruizeveld de Winter J. A., Janssen P. J., Sleddens H. M., Verleun-Mooijman M. C., Trapman J., Brinkmann A. O., Santerse A. B., Schröder F. H., van der Kwast T. H. (1994) Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am. J. Pathol. 144, 735–746 [PMC free article] [PubMed] [Google Scholar]
- 5. Saraon P., Jarvi K., Diamandis E. P. (2011) Molecular alterations during progression of prostate cancer to androgen independence. Clin. Chem. 57, 1366–1375 [DOI] [PubMed] [Google Scholar]
- 6. Hobisch A., Culig Z., Radmayr C., Bartsch G., Klocker H., Hittmair A. (1995) Distant metastases from prostatic carcinoma express androgen receptor protein. Cancer Res. 55, 3068–3072 [PubMed] [Google Scholar]
- 7. Quigley C. A., De Bellis A., Marschke K. B., el-Awady M. K., Wilson E. M., French F. S. (1995) Androgen receptor defects. Historical, clinical, and molecular perspectives. Endocr. Rev. 16, 271–321 [DOI] [PubMed] [Google Scholar]
- 8. Heinlein C. A., Chang C. (2004) Androgen receptor in prostate cancer. Endocr. Rev. 25, 276–308 [DOI] [PubMed] [Google Scholar]
- 9. Faus H., Haendler B. (2006) Post-translational modifications of steroid receptors. Biomed. Pharmacother. 60, 520–528 [DOI] [PubMed] [Google Scholar]
- 10. Shu S. K., Liu Q., Coppola D., Cheng J. Q. (2010) Phosphorylation and activation of androgen receptor by Aurora-A. J. Biol. Chem. 285, 33045–33053 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11. Ponguta L. A., Gregory C. W., French F. S., Wilson E. M. (2008) Site-specific androgen receptor serine phosphorylation linked to epidermal growth factor-dependent growth of castration-recurrent prostate cancer. J. Biol. Chem. 283, 20989–21001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin H. K., Yeh S., Kang H. Y., Chang C. (2001) Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proc. Natl. Acad. Sci. U.S.A. 98, 7200–7205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gaughan L., Logan I. R., Neal D. E., Robson C. N. (2005) Regulation of androgen receptor and histone deacetylase 1 by Mdm2-mediated ubiquitylation. Nucleic Acids Res. 33, 13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin H. K., Wang L., Hu Y. C., Altuwaijri S., Chang C. (2002) Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J. 21, 4037–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ha S., Ruoff R., Kahoud N., Franke T. F., Logan S. K. (2011) Androgen receptor levels are up-regulated by Akt in prostate cancer. Endocr. Relat. Cancer 18, 245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rees I., Lee S., Kim H., Tsai F. T. (2006) The E3 ubiquitin ligase CHIP binds the androgen receptor in a phosphorylation-dependent manner. Biochim. Biophys. Acta 1764, 1073–1079 [DOI] [PubMed] [Google Scholar]
- 17. Yang F., Li X., Sharma M., Zarnegar M., Lim B., Sun Z. (2001) Androgen receptor specifically interacts with a novel p21-activated kinase, PAK6. J. Biol. Chem. 276, 15345–15353 [DOI] [PubMed] [Google Scholar]
- 18. Lee S. R., Ramos S. M., Ko A., Masiello D., Swanson K. D., Lu M. L., Balk S. P. (2002) AR and ER interaction with a p21-activated kinase (PAK6). Mol. Endocrinol. 16, 85–99 [DOI] [PubMed] [Google Scholar]
- 19. Jaffer Z. M., Chernoff J. (2002) p21-activated kinases: three more join the Pak. Int. J. Biochem. Cell Biol. 34, 713–717 [DOI] [PubMed] [Google Scholar]
- 20. Kumar R., Gururaj A. E., Barnes C. J. (2006) p21-activated kinases in cancer. Nat. Rev. Cancer 6, 459–471 [DOI] [PubMed] [Google Scholar]
- 21. Li X., Liu F., Li F. (2010) PAK as a therapeutic target in gastric cancer. Expert Opin. Ther. Targets 14, 419–433 [DOI] [PubMed] [Google Scholar]
- 22. Whale A., Hashim F. N., Fram S., Jones G. E., Wells C. M. (2011) Signaling to cancer cell invasion through PAK family kinases. Front. Biosci. 16, 849–864 [DOI] [PubMed] [Google Scholar]
- 23. Molli P. R., Li D. Q., Murray B. W., Rayala S. K., Kumar R. (2009) PAK signaling in oncogenesis. Oncogene 28, 2545–2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou H., Kramer R. H. (2005) Integrin engagement differentially modulates epithelial cell motility by RhoA/ROCK and PAK1. J. Biol. Chem. 280, 10624–10635 [DOI] [PubMed] [Google Scholar]
- 25. Schrantz N., da Silva Correia J., Fowler B., Ge Q., Sun Z., Bokoch G. M. (2004) Mechanism of p21-activated kinase 6-mediated inhibition of androgen receptor signaling. J. Biol. Chem. 279, 1922–1931 [DOI] [PubMed] [Google Scholar]
- 26. Kaur R., Yuan X., Lu M. L., Balk S. P. (2008) Increased PAK6 expression in prostate cancer and identification of PAK6 associated proteins. Prostate 68, 1510–1516 [DOI] [PubMed] [Google Scholar]
- 27. Hörnberg E., Ylitalo E. B., Crnalic S., Antti H., Stattin P., Widmark A., Bergh A., Wikström P. (2011) Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration resistance and short survival. PLoS ONE 6, e19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang L. G., Liu X. M., Chiao J. W. (2006) Repression of androgen receptor in prostate cancer cells by phenethyl isothiocyanate. Carcinogenesis 27, 2124–2132 [DOI] [PubMed] [Google Scholar]
- 29. Carrano A. C., Eytan E., Hershko A., Pagano M. (1999) SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1, 193–199 [DOI] [PubMed] [Google Scholar]
- 30. Koepp D. M., Schaefer L. K., Ye X., Keyomarsi K., Chu C., Harper J. W., Elledge S. J. (2001) Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science 294, 173–177 [DOI] [PubMed] [Google Scholar]
- 31. Lee D. H., Goldberg A. L. (1998) Proteasome inhibitors. Valuable new tools for cell biologists. Trends Cell Biol. 8, 397–403 [DOI] [PubMed] [Google Scholar]
- 32. Hartel A., Didier A., Pfaffl M. W., Meyer H. H. (2003) Characterisation of gene expression patterns in 22RV1 cells for determination of environmental androgenic/antiandrogenic compounds. J. Steroid Biochem. Mol. Biol. 84, 231–238 [DOI] [PubMed] [Google Scholar]
- 33. Weissman A. M. (2001) Themes and variations on ubiquitylation. Nat. Rev. Mol. Cell Biol. 2, 169–178 [DOI] [PubMed] [Google Scholar]
- 34. Zhou Z. X., Sar M., Simental J. A., Lane M. V., Wilson E. M. (1994) A ligand-dependent bipartite nuclear targeting signal in the human androgen receptor. Requirement for the DNA-binding domain and modulation by NH2-terminal and carboxyl-terminal sequences. J. Biol. Chem. 269, 13115–13123 [PubMed] [Google Scholar]
- 35. Higano C. S., Crawford E. D. (2011) New and emerging agents for the treatment of castration-resistant prostate cancer. Urol. Oncol. 29, S1–S8 [DOI] [PubMed] [Google Scholar]
- 36. Wen X., Li X., Liao B., Liu Y., Wu J., Yuan X., Ouyang B., Sun Q., Gao X. (2009) Knockdown of p21-activated kinase 6 inhibits prostate cancer growth and enhances chemosensitivity to docetaxel. Urology 73, 1407–1411 [DOI] [PubMed] [Google Scholar]