Background: NAMPT catalyzes the rate-limiting reaction in converting nicotinamide to NAD+ in cancers.
Results: NAMPT inhibition attenuates glycolysis at the glyceraldehyde 3-phosphate dehydrogenase step, resulting in perturbing metabolic pathways related to glycolysis.
Conclusion: The metabolic basis of NAMPT inhibition is the attenuation of glycolysis by reducing NAD+ available to glyceraldehyde 3-phosphate dehydrogenase.
Significance: This study sheds new light on how NAMPT regulates cancer metabolism.
Keywords: DNA Repair, Drug Discovery, Glycolysis, Metabolism, NAD, NAD Biosynthesis, Tumor Metabolism
Abstract
Nicotinamide phosphoribosyltransferase (NAMPT) catalyzes the first rate-limiting step in converting nicotinamide to NAD+, essential for cellular metabolism, energy production, and DNA repair. NAMPT has been extensively studied because of its critical role in these cellular processes and the prospect of developing therapeutics against the target, yet how it regulates cellular metabolism is not fully understood. In this study we utilized liquid chromatography-mass spectrometry to examine the effects of FK866, a small molecule inhibitor of NAMPT currently in clinical trials, on glycolysis, the pentose phosphate pathway, the tricarboxylic acid (TCA) cycle, and serine biosynthesis in cancer cells and tumor xenografts. We show for the first time that NAMPT inhibition leads to the attenuation of glycolysis at the glyceraldehyde 3-phosphate dehydrogenase step due to the reduced availability of NAD+ for the enzyme. The attenuation of glycolysis results in the accumulation of glycolytic intermediates before and at the glyceraldehyde 3-phosphate dehydrogenase step, promoting carbon overflow into the pentose phosphate pathway as evidenced by the increased intermediate levels. The attenuation of glycolysis also causes decreased glycolytic intermediates after the glyceraldehyde 3-phosphate dehydrogenase step, thereby reducing carbon flow into serine biosynthesis and the TCA cycle. Labeling studies establish that the carbon overflow into the pentose phosphate pathway is mainly through its non-oxidative branch. Together, these studies establish the blockade of glycolysis at the glyceraldehyde 3-phosphate dehydrogenase step as the central metabolic basis of NAMPT inhibition responsible for ATP depletion, metabolic perturbation, and subsequent tumor growth inhibition. These studies also suggest that altered metabolite levels in tumors can be used as robust pharmacodynamic markers for evaluating NAMPT inhibitors in the clinic.
Introduction
The NAD+ cofactor is essential for a number of enzymes and regulatory proteins involved in a variety of cellular processes. In mammals, NAD+ can be synthesized from nicotinamide, nicotinic acid, or tryptophan (1–4). The in vivo concentration of nicotinic acid is extremely low due to its rapid excretion and metabolism, so the utilization of nicotinic acid for NAD+ biosynthesis as compared with that of nicotinamide is limited in mammals (2). The de novo biosynthesis of NAD+ from tryptophan mainly occurs in the liver and under certain stressed conditions (3). Therefore, the two-step salvage pathway that converts nicotinamide to NAD+ represents the major route to NAD+ biosynthesis in mammals (5–7).
Nicotinamide phosphoribosyl transferase (NAMPT),3 originally identified as a pre-B-cell colony enhancing factor (8), is the rate-limiting enzyme that catalyzes the first step in the biosynthesis of NAD+ from nicotinamide (9, 10). Recent studies have demonstrated that NAMPT-mediated NAD+ biosynthesis in cancer cells plays a crucial role in several physiological processes including metabolism, energy generation, survival, apoptosis, DNA repair, and inflammation (1, 11–13). NAMPT is overexpressed in several types of tumors including breast, colorectal, gastric, lung, prostate, and other carcinomas (14–17), and its expression appears to be associated with tumor progression (18). The down-regulation of NAMPT suppresses tumor cell growth in vitro and in vivo, and sensitizes cells to oxidative stress and DNA damaging agents (7, 14, 17, 19–21). The inhibition of NAMPT also leads to the attenuation of tumor growth and induction of apoptosis due to NAD+ depletion (7, 20–23). Taken together, NAMPT represents a promising therapeutic target for the development of potential novel cancer drugs.
NAD+ is a substrate for dehydrogenases, poly(ADP-ribose) polymerases, sirtuins, mono ADP-ribosyl transferases, and ADP-ribosyl cyclases (1, 3, 11). In most cancer cells, poly(ADP-ribose) polymerase, a key protein required for DNA repair and also involved in apoptosis, is activated due to DNA damage and genome instability (1, 24–26). The activation of poly(ADP-ribose) polymerase leads to NAD+ depletion in cancer cells (1, 7, 24–26). As a result, the down-regulation of NAMPT sensitizes cancer cells to DNA damaging agents and apoptosis (10, 21). Similarly, sirtuin also serves as a key downstream effector of NAMPT that regulates a variety of cellular functions including survival and inflammation (27–29). Recent studies have demonstrated that sirtuins regulate cytokine production (29) and that reducing NAD+ levels through the inhibition of NAMPT attenuates TNF-α and IL-6 production (19, 29). Furthermore, a NAMPT inhibitor has demonstrated anti-inflammatory effects in animal models of inflammation (19, 29). Finally, a number of studies have shown that the inhibition of NAMPT leads to ATP depletion in cancer cells (7, 17, 20). However, the underlying metabolic basis for ATP depletion is not fully understood, and how modulating NAMPT activity in cancer cells affects their cellular metabolism also remains unknown.
FK866, a small molecule inhibitor of NAMPT, has been the subject of extensive studies (1, 20, 30). The molecule has been co-crystallized with and found to be bound to the nicotinamide binding pocket of NAMPT, thereby demonstrating its mechanism as a competitive inhibitor of NAMPT with respect to nicotinamide (31). Several studies also suggest that FK866 specifically inhibits NAMPT in the cell and exhibits anti-tumor activity in preclinical tumor models (10, 13, 19, 29, 31, 32).
Thus, FK866 appears to be an ideal tool molecule for assessing the physiological function of NAMPT in the cell. In addition, FK866 and also GMX1777, another NAMPT inhibitor, have been tested in the clinic for potential anticancer indications (5, 7, 11, 33). Yet, how to assess the pharmacodynamic (PD) effects of the molecules in the clinic was unknown (1, 5, 7, 33).
In this study we investigated the effects of pharmacological inhibition of NAMPT by FK866 on glycolysis, the pentose phosphate pathway, serine biosynthesis, and the TCA cycle in human cancer cells. We show that the inhibition of NAMPT by FK866 results in the attenuation of glycolysis at the glyceraldehyde 3-phosphate dehydrogenase step. The attenuation of glycolysis in cancer cells leads to reduced serine biosynthesis and TCA cycle, and altered pentose phosphate pathway activities. These results, therefore, demonstrate that NAMPT plays an important role in multiple physiological processes essential for energy as well as intermediary and amino acid metabolism and that the blockade of glycolysis at the glyceraldehyde 3-phosphate dehydrogenase step is the central mechanism responsible for ATP depletion and metabolic perturbation in the cell. The findings from this study may have clinical implications, as the metabolic changes observed in tumors in response to NAMPT inhibition can be measured in the clinic via direct tumor biopsy, thereby used as robust PD markers for assessing effects of NAMPT inhibitors on the intended tumor target in the clinic.
EXPERIMENTAL PROCEDURES
Assay for NAD+ Levels in Cancer Cells
A2780 (NCI DCTD), an ovarian cancer cell line, and HCT116 (ATCC), a colorectal cancer cell line, were cultured in RPMI 1640 (Invitrogen 30-2001) and McCoys 5a (Hyclone SH30200), respectively, in the presence of 10% FBS. Cells were seeded into a 96-well culture plate at a density of 8 × 104 cells per well and incubated at 37 °C in 5% CO2 for 4 h and then treated with FK866 at various concentrations for 24 h. All treatments were conducted in duplicate. FK866 was synthesized as described (34, 35). To assess NAD+ levels in these cells, a method described (36) was used with modifications. Cells grown in 96-well plates were lysed with radioimmune precipitation assay buffer (Pierce) followed by the addition of 50 μl of 0.2 n HCl. The resulting cell lysates were incubated at 60 °C for 10 min and neutralized with 50 μl of 0.2 n NaOH. After centrifugation at 2000 × g for 15 min, the supernatants (50 μl) were mixed with 20 μl of 0.2 n KOH and acetophenone and incubated at 90 °C for 10 min followed by the addition of 90 μl of formic acid. After incubation at 90 °C for 10 min, the preparations were measured for fluorescence at 360 (excitation) and 420 nm (emission) (CytoFluor reader). NAD+ levels were also measured by LC-MS. For rescue purposes, cells were grown and treated with nicotinic acid (10 μm) and FK866 for 24 h before measuring NAD+ levels.
The LC-MS analysis of NAD+ levels was performed on an HPLC system coupled to a Thermo Quantum Ultra triple quadrupole mass spectrometer operated in positive heated electrospray mode with selected reaction monitoring detection. For cell extracts, 50 μl of extract and 10 μl of 10 μm internal standard solution were transferred to a 96-well plate, dried under nitrogen and reconstituted in 50 μl of water. For tissue extracts, 10 μl of extract and 10 μl of internal standard solution were dried and reconstituted in 50 μl water. The internal standard solution contained 10 μm nicotinamide-d4 (C/D/N Isotopes), nicotinic acid-d4 (C/D/N Isotopes), nicotinamide mononucleotide-d4 (prepared by custom synthesis), and nicotinamide 1, N6-ethenoadenine dinucleotide in methanol. The metabolites were separated on a Waters Atlantis T3 column (2.1 × 50 mm, 3 μm) with an injection volume of 20 μl and a flow rate of 0.6 ml/min using 10 mm ammonium acetate for mobile phase A and methanol for mobile phase B. The gradient was as follows: 0 min, 0% B; 1.5 min, 30% B; 1.51 min, 95% B; 1.8 min, 95% B; 1.81 min, 0% B, 3 min, 0% B.
Assays for Cell Death and ATP Levels in Cancer Cells
A2780 cells (2 × 103/well) were grown in a 96-well plate overnight and treated with FK866 ± nicotinic acid (10 μm) in duplicate as described before. Cell death was determined by using an assay kit (CellTiter-FluorTM, Promega) per the manufacturer's instructions. ATP levels were determined by using an assay system (ATPlite, PerkinElmer Life Sciences) per the manufacturer's instructions.
Preparation of Lysates for LC-MS Measurement of Metabolite Levels in Cancer Cells
Cells (5 × 104/well) were grown as described above and treated with or without FK866 or nicotinic acid (10 μm) in 100 μl of DMEM supplemented with 10% FBS (dialyzed) and 25 mm glucose (Sigma). After 24 h of treatment, the medium was removed, and 200 μl of 80% methanol was added to each well. After incubation at room temperature for 15 min, the resulting extracts were transferred to 96 deep-well plates (Analytical), washed twice with 200 μl of 80% methanol, and stored at −80 °C or dried and reconstituted in 90% of acetonitrile, which was used for LC-MS analysis (10 μl each).
For labeling studies, cells (105/well) were seeded in 24-well plates and grown overnight as described above. Then the culture medium was replaced with DMEM supplemented with the following: ± FK866, ± 2 mm glutamine, 10% dialyzed FBS, and 25 mm d-[1,6-13C]glucose (Cambridge Isotope Laboratories). All treatments were conducted in triplicate. After 24 h, the culture medium was carefully removed by aspiration. Intracellular metabolites were extracted as described above for LC-MS analysis.
Preparation of Lysates for LC-MS Measurement of Metabolite Levels in Tumors
HCT116 cells (ATCC) were grown as described before. The cells (106/animal) were mixed with Matrigel (1:1) and implanted subcutaneously into the rear flank of the mice (female CB17 SCID, Charles River). The implanted tumor cells grew as solid tumors. The animals (7/group) were dosed intraperitoneally with 8, 15, and 20 mg/kg of FK866 in 20% of Captisol® and 25 mm phosphate buffer, pH 2, twice a day after tumors reached 500 mm3 for about 10 days. The tumor volume and body weight were measured twice a week. Tumor samples (≈50 mg each) were homogenized using lysing matrix A (MP Biomedicals) and 1 ml of ice cold extraction solution (7.5 ml chloroform, 67.5 ml methanol, and 25 ml water) for 30 s (Bio101 Fastprep P120). The preparations were centrifuged at 4000 × g for 2 min, and the supernatants (0.5 ml each) were collected and mixed with chloroform (0.5 ml each) by vortexing. The preparations were centrifuged at 14,000 × g for 2 min. The aqueous phase was collected (0.2 ml) for LC-MS analysis.
Detection and Quantitation of Metabolites by LC-MS
Chromatographic separations were performed with an HPLC system (Shimadzu Prominence, Schimadzu) that was coupled to a mass spectrometer (Qtrap 5500) (Applied Biosystems). Analytes with phosphates were analyzed as follows. The analytes in the cell extracts were separated on a Phenomenex Luna amino HPLC column (2.1 × 50 mm 3 μm) with 100% acetonitrile as mobile phase A and 100% 10 mm ammonium acetate, pH 10, as the mobile phase B. The gradient (1 ml/min) was as follows: 0–1 min, 10% B; 1–3 min, 10–50% B; 3–6 min, 50% B; 6–10 min, 10% B. The mass spectrometer was operated under negative ESI MRM mode. For organic acids, the cell extracts were mixed with 100 μl of 1 m 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide and 10 μl of 0.1 m O-benzylhydroxylamine for 1 h at room temperature. The resulting mixture was extracted twice with ethyl acetate. The organic phase was dried and reconstituted in 100 μl of 50% methanol. The analytes were separated using a C18 HPLC column (Xbridge 2.1 × 50 mm, 3.5 μm) under the following conditions: 0.1% formic acid/water as mobile phase A and methanol as mobile phase B. The gradient (0.6 ml/min) was as follows: 0–0.5 min, 20% B; 0.5–2.5 min, 20–98% B; 2.5–3.0 min, 98% B; 3–4.5 min, 20% B.
RESULTS
FK866 as a Tool Molecule for Evaluating the Effects of NAMPT Inhibition on Cancer Cell Metabolism
As a first step toward a better understanding of how NAMPT regulates cellular metabolism in cancer cells, we characterized FK866 with respect to its selectivity, specificity, and effects on cellular NAD+ and ATP levels. As the substrates and product of NAMPT contain components similar to nucleotides, we biochemically evaluated FK866 at 20 μm against a panel of human kinases (≈100) (Cerep) and found that FK866 was inactive against all of the kinases tested (data not shown) except Alk, Chk1, Jak2, Musk, and Rsk with estimated IC50 values of 4.2, 2.0, 2.0, 3.3, and 5.1 μm, respectively. As shown below, FK866 exhibits IC50 values of ≈0.5 and ≈1.0 nm in NAD+ formation and cell proliferation assays, respectively, when tested in A2780 and HCT116. Therefore, the activity of FK866 against these kinases described above is physiologically not relevant. To further assess its selectivity, we tested whether FK866 inhibited glucose 6-phosphate dehydrogenase, alcohol dehydrogenase, and glyceraldehyde 3-phosphate dehydrogenase, representing enzymes requiring NADP(H) or NAD(H) as cofactors. FK866 did not exhibit any significant inhibitory activity against these dehydrogenases when tested at up to 20 μm (data not shown). Together, these results demonstrate that FK866 is a specific NAMPT inhibitor.
To further characterize FK866, we treated A2780 and HCT116 cells with the molecule for 24 and 72 h and assessed its effects on NAD+ formation and cell proliferation. We showed that FK866 potently inhibited NAD+ formation in and proliferation of both cell lines with the IC50 values of 0.5 and 1.4 nm (for A2780) and 0.5 and 3.0 nm (for HCT116), respectively. It is known that some cancer cells, such as A2780, only use nicotinamide for NAD+ biosynthesis, but others, like HCT116, can also utilize nicotinic acid via the alternative nicotinic acid phosphoribosyltransferase-mediated pathway to produce NAD+ (7). If FK866 is a specific NAMPT inhibitor, adding nicotinic acid to the growth medium should abolish FK866 inhibitory activity against HCT116 but not A2780. This is indeed the case. The addition of nicotinic acid totally abolished FK866 inhibitory activities against HCT116 with regard to NAD+ formation and proliferation (IC50 values: >500 and >500 nm, respectively) but not A2780 cells (IC50 values: <1.0 and 2.0 nm, respectively). Furthermore, the addition of nicotinamide or nicotinamide mononucleotide to the growth medium of both cell lines also abolished FK866 inhibitory activities (data not shown) as expected, as FK866 is a competitive inhibitor of NAMPT with respect to nicotinamide, and nicotinamide mononucleotide is a direct reaction product of NAMPT, which can bypass NAMPT function. Together these results further confirm the utility of FK866 as a selective and potent tool molecule.
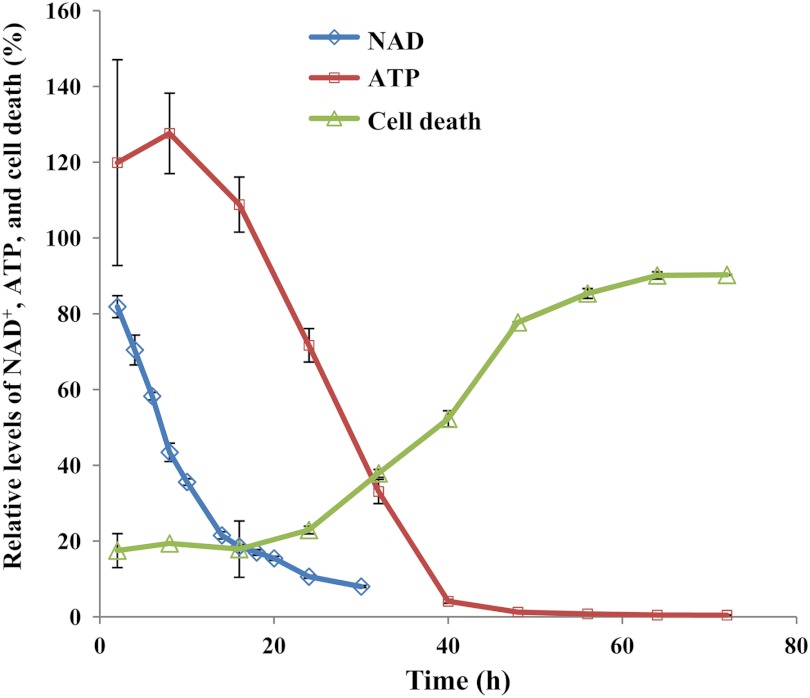
FK866 was initially identified as a slow-acting agent inducing a delayed cell death of human cancer cells (20). To confirm its effects on NAD+ and ATP levels and cell death, we carried out a time course study. As shown in Fig. 1, NAD+ and ATP levels were depleted after 20–30 and 40–50 h of the treatment, respectively. Cell death reached the maximum after >60 h (Fig. 1). Thus, NAMPT inhibition results in NAD+ depletion followed by ATP depletion and eventual cell death (7, 47).
FIGURE 1.
Inhibition of NAMPT by FK866 led to NAD+ and ATP depletion and subsequent cell death. A2780 cells were grown and treated with FK866 (100 nm) in duplicate as described (“Experimental Procedures”). After the treatment, cells were processed for the analysis of NAD+ by LC-MS and the fluorescence assays, ATP by ATPlite and cell death by CellTiter-Fluor™ as described (“Experimental Procedures”). Error bars represent S.D.
Inhibition of NAMPT Led to Attenuation of Glycolysis and Depletion of ATP Levels in Cancer Cells
To elucidate the metabolic basis of NAMPT inhibition, we hypothesized that NAD+ depletion caused by NAMPT inhibition would result in a blockade of glyceraldehyde 3-phosphate dehydrogenase, a key enzyme in glycolysis dependent on NAD+ for activity. As a result, glycolysis, the main energy source for many cancer cells, would be attenuated at the glyceraldehyde 3-phosphate dehydrogenase step. This would lead to an accumulation in glycolytic intermediates before and at the glyceraldehyde 3-phosphate dehydrogenase step and a decrease in intermediates after the glyceraldehyde 3-phosphate dehydrogenase step. To test this hypothesis, we developed a LC-MS methodology (“Experimental Procedures” and supplemental Fig. S1) for detecting glycolytic intermediates in cancer cells and tumor tissues and used the methodology to measure glycolytic intermediate levels in A2780 and HCT116 cells treated with 25 nm FK866 for 24 h. We observed a significant increase in glucose 6-phosphate and fructose 6-phosphate, fructose 1,6-bisphosphate, and glyceraldehyde 3-phosphate and dihydroxyacetone phosphate levels, and a decrease in 1,3-bisphosphoglycerate, 2-phosphoglycerate and 3-phosphoglycerate, and phosphoenolpyruvate levels (data not shown). Because these three pairs of metabolites (glucose 6-phosphate and fructose 6-phosphate, glyceraldehyde 3-phosphate and dihydroxyacetone phosphate, and 2-phosphoglycerate and 3-phosphoglycerate) could not be separated chromatographically and also because they have identical molecular masses, the exact amount of each individual species (glucose 6-phosphate versus fructose 6-phosphate for example) could not be determined by LC-MS. As a result, the total amount of the isomers from each pair was determined. To show that the observed metabolic changes were due to NAMPT inhibition, we treated both cell lines with FK866 at different concentrations for 24 h in the presence or absence of nicotinic acid and analyzed glycolytic intermediate levels as described above. As shown in Fig. 2, FK866 alone caused a dose-dependent increase in glucose 6-phosphate and fructose 6-phosphate, fructose 1,6-bisphosphate, and glyceraldehyde 3-phosphate and dihydroxyacetone phosphate levels and a decrease in 2-phosphoglycerate and 3-phosphoglycerate, and phosphoenolpyruvate levels, as well as 1, 3-bisphosphoglycerate levels (data not shown) in both cell lines. The addition of nicotinic acid completely abolished these effects observed in HCT116 but not in A2780 (Fig. 2) as HCT116, but not A2780, can use nicotinic acid to produce NAD+ (6, 7). Furthermore, we have also ruled out the possibility that the blockade at the glyceraldehyde 3-phosphate dehydrogenase step is due to the direct inhibition of the enzyme by FK866, as no inhibitory activity against the enzyme was observed when FK866 was tested at 20 μm, 800–840,000-fold higher than that used for the LC-MS studies as described above. Together, these studies demonstrate that NAMPT inhibition attenuates glyceraldehyde 3-phosphate dehydrogenase activity, profoundly affecting glycolysis and its downstream pathway activities in cancer cells.
FIGURE 2.
Inhibition of NAMPT by FK866 led to attenuation of glycolysis in cancer cells. A2780 (A and C) and HCT116 (B and D) cells were grown and treated in triplicate with FK866 at various concentrations and ± nicotinic acid (10 μm) as described (“Experimental Procedures”). After the treatment, cells were processed for the analysis of glucose 6-phosphate and fructose 6-phosphate (G6P and F6P), fructose 1,6-bisphosphate (FBP), and glyceraldehyde 3-phosphate and dihydroxyacetone phosphate (G3P and DHAP) (A and B) and 2-phosphoglycerate and 3-phosphoglycerate (2-PG and 3-PG), and phosphoenolpyruvate (PEP) (C and D) levels by LC-MS as described (Experimental Procedures). Because the three pairs of metabolites, glucose 6-phosphate and fructose 6-phosphate, glyceraldehyde 3-phosphate and dihydroxyacetone phosphate, 2-phosphoglycerate and 3-phosphoglycerate, could not be separated chromatographically and they have identical molecular masses, the amount determined by LC-MS represented the total amount of both species in the cell. Error bars represent S.D. *, p ≤ 0.001 versus control.
Inhibition of NAMPT Led to Alteration of the Pentose Phosphate Pathway Activity
Many cancer cells rely on glycolysis for ATP production to fuel their rapid growth and proliferation (7, 21). Glycolysis also plays an important role in providing intermediates to other metabolic pathways for generating building blocks for cancer cells. For example, glucose 6-phosphate, fructose 6-phosphate, and glyceraldehyde 3-phosphate are used by the pentose phosphate pathway to generate pentose 5-phosphate for nucleotide biosynthesis and NADPH for other metabolic and red/ox processes. To assess the effects of NAMPT inhibition on the pentose phosphate pathway, we treated A2780 and HCT116 cells with FK866 and analyzed 6-phosphogluconate, pentose phosphates (ribulose, ribose, or xylulose 5-phosphate), erythrose 4-phosphate, and sedoheptulose 7-phosphate. Because the pentose phosphates are isomers, they could not be distinguished individually by LC-MS and thereby quantified as a group of pentose phosphates. As shown in Fig. 3, the treatment with FK866 alone led to a significant increase in pentose phosphate and sedoheptulose 7-phosphate levels as well as erythrose 4-phosphate levels (data not shown) in these cells. The addition of nicotinic acid abolished these effects in HCT116 but not in A2780 (Fig. 3). This study demonstrates that NAMPT inhibition results in carbon overflow from glycolysis to the pentose phosphate pathway.
FIGURE 3.

Inhibition of NAMPT by FK866 resulted in alteration of pentose phosphate pathway intermediate levels in cancer cells. A2780 (A) and HCT116 (B) cells were grown and treated in triplicate with FK866 at various concentrations and ± nicotinic acid (10 μm) as described (“Experimental Procedures”). After the treatment, cells were collected and processed for the analysis of 6-phosphogluconate (Gn6P), pentose phosphates (R5P), and sedoheptulose 7-phosphate (S7P) levels by LC-MS (“Experimental Procedures”). Error bars represent S.D. *, p ≤ 0.001 versus control.
The effects of FK866 on 6-phosphogluconate levels appeared different (Fig. 3). An increase in FK866 concentrations (up to ≈5 nm) initially caused a decrease in 6-phosphogluconate levels, but a further increase in FK866 concentrations (≥ 5 nm) appeared to cause a reversal of its levels (Fig. 3). The initial decrease in 6-phosphogluconate levels may be due to a drop in NADP levels. Because NADP depletion appears to be delayed (supplemental Table S1), a further increase in FK866 concentrations caused a much higher accumulation of glucose 6-phosphate levels. The increase in glucose 6-phosphate levels might have driven the pathway forward, thereby compensating for the drop in NADP levels.
To further investigate how NAMPT inhibition affects the contribution of each branch of the pentose phosphate pathway to intermediate accumulation, we carried out a labeling study using [1,6-13C]glucose. If sedoheptulose 7-phosphate is mainly derived from glucose 6-phosphate via the oxidative branch, one carbon (C7) of sedoheptulose 7-phosphate should be primarily labeled when [1,6-13C]glucose is used. If sedoheptulose 7-phosphate is mainly derived from fructose 6-phosphate and glyceraldehyde 3-phosphate/erythrose 4-phosphate via the non-oxidative branch, two carbons (C1,7) of sedoheptulose 7-phosphate should be labeled when [1,6-13C]glucose is used. Therefore, the amount of sedoheptulose 7-phosphate with one (M1) or two labels (M2) may give clues as to the role that each branch plays in sedoheptulose 7-phosphate formation. The amount of sedoheptulose 7-phosphate (M2) was about 2.5-fold (9.4/3.8) higher than sedoheptulose 7-phosphate (M1) in untreated A2780 cells (Table 1), indicating that ≈70% of sedoheptulose 7-phosphate is derived from the non-oxidative branch. The treatment of A2780 cells with FK866 led to a 1.2–17.2-fold increase in sedoheptulose 7-phosphate (M2) formation and a 1.0–4.5-fold increase in sedoheptulose 7-phosphate (M1) formation (Table 1), thus indicating that NAMPT inhibition preferentially increased the activity of the non-oxidative branch. The amount of pentose phosphates (M1) was about 3.6-fold (18.0/5.0) higher than pentose phosphates (M2), indicating that ≈78% of pentose phosphates in untreated cells was derived from the oxidative branch (Table 1). The treatment of the cells with FK866 led to a 1.0–3.1-fold increase in pentose phosphate (M2) formation and a 1.0–1.6-fold increase in pentose phosphate (M1) formation (Table 1). Together, these results show that NAMPT inhibition preferentially increases the activity of the non-oxidative branch of the pentose phosphate pathway. The impact of increased pentose phosphate pathway intermediates on other cellular metabolic pathways such as nucleotide biosynthesis deserves further investigation.
TABLE 1.
Effects of the inhibition of NAMPT by FK866 on the levels (ng/ml) of major isotope-labeled metabolites derived from [1,6-13C]glucose in A2780 cells
A2780 cells were grown in the presence of [1,6-13C]glucose, treated with FK866, and processed for the analysis of metabolites by LC-MS as described (“Experimental Procedures”). Only the major 13C-labeled metabolites are reported. S7P, sedoheptulose 7-phosphate; Gn6P, 6-phosphogluconate; R5P, ribose 5-phosphate, ribulose 5-phosphate, and xylulose 5-phosphate; G6P, glucose 6-phosphate; FBP, fructose 1,6-bisphosphate; G3P, glyceraldehyde 3-phosphate; PG, 2-phosphoglycerate and 3-phosphoglycerate; PEP, phosphoenolpyruvate. M2, 2 carbons of the metabolites were 13C-labeled. M1, 1 carbon of the metabolites was 13C-labeled.
| FK866 (nm) | Metabolite levelsa (ng/ml) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S7P(M1) | S7P(M2) | Gn6P(M1) | Gn6P(M2) | R5P(M1) | R5P(M2) | G6P(M2) | FBP(M2) | G3P(M1) | PG(M1) | PEP(M1) | |
| 0.0 | 3.8 ± 0.8 | 9.4 ± 0.6 | 0.7 ± 0.2 | 6.5 ± 0.6 | 18 ± 1.4 | 5 ± 0.6 | 63 ± 8.4 | 213 ± 32 | 44.5 ± 7.5 | 141 ± 1.8 | 17.7 ± 1.1 |
| 0.33 | 3.8 ± 0.8 | 11 ± 1.7 | 0.6 ± 0.1 | 4.9 ± 1 | 17.2 ± 4.3 | 5 ± 1 | 66 ± 11.3 | 237 ± 43 | 50 ± 11.3 | 129 ± 2.8 | 13.9 ± 1.9 |
| 3.3 | 10.7 ± 0.1 | 95 ± 2.1 | 0.7 ± 0.1 | 5.6 ± 0.7 | 23 ± 1.1 | 12.6 ± 0.5 | 368 ± 13 | 1833 ± 184 | 336 ± 51 | 47 ± 7 | 3.1 ± 0.3 |
| 33 | 17.2 ± 0.7 | 162 ± 6.2 | 1.1 ± 0.1 | 7.6 ± 0.8 | 28 ± 4.2 | 15.3 ± 1.4 | 543 ± 35 | 2947 ± 114 | 571 ± 46 | 52 ± 0.2 | 3.3 ± 0.2 |
a Tumor metabolite levels (means ± S.E.).
Inhibition of NAMPT Led to Attenuation of Serine Biosynthesis
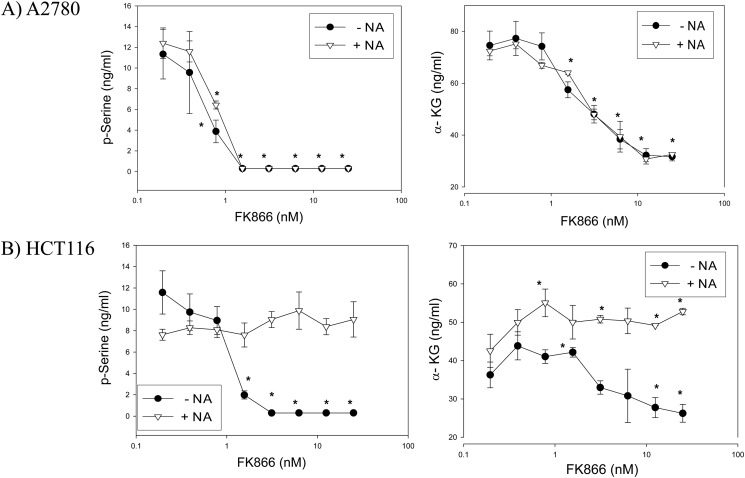
Serine is used as an obligate precursor for the biosynthesis of nucleotides in all organisms. Consistent with its essential role in the cell, the biosynthetic pathway for this important amino acid has been preserved in the mammalian systems. The de novo biosynthesis of serine begins with the 3-phosphoglycerate dehydrogenase-catalyzed NAD+-dependent oxidation of 3-phosphoglycerate to 3-phosphohydroxypyruvate. 3-Phosphoglycerate dehydrogenase is overexpressed in certain types of cancer cells due to a genomic amplification of the gene (37, 38). In these cancer cells, overexpressed 3-phosphoglycerate dehydrogenase diverts a large amount of 3-phosphoglycerate from glycolysis to produce α-ketoglutarate (37–38). In light of these findings, we tested whether NAMPT inhibition attenuates 3-phosphoglycerate dehydrogenase-mediated serine biosynthesis in cancer cells. We showed that the treatment with FK866 caused a reduction in phosphoserine and α-ketoglutarate levels in A2780 and HCT116 cells (Fig. 4). Due to technical difficulties, we were unable to detect 3-phosphohydroxypyruvate by LC-MS. The dose-dependent reduction of phosphoserine and α-ketoglutarate in response to FK866 treatment was diminished in HCT116, but not A2780, upon the addition of nicotinic acid to the growth medium (Fig. 4), demonstrating that these effects were specifically due to NAMPT inhibition. The changes in serine intermediate levels show that a restricted carbon flow from glycolysis along with a reduced NAD+ level contributed to the attenuation of serine and α-ketoglutarate formation in these cancer cells.
FIGURE 4.
Inhibition of NAMPT resulted in attenuation of serine biosynthesis in cancer cells. A2780 (A) and HCT116 (B) cells were grown and treated in duplicate with FK866 at various concentrations and ± nicotinic acid (10 μm) as described (“Experimental Procedures”). After the treatment, cells were collected and processed for the analysis of phosphoserine (p-Serine) and α-ketoglutarate (α-KG) levels by LC-MS (“Experimental Procedures”). Error bars represent S.D. *, p ≤ 0.001 versus control.
Inhibition of NAMPT Led to Attenuation of the TCA Cycle
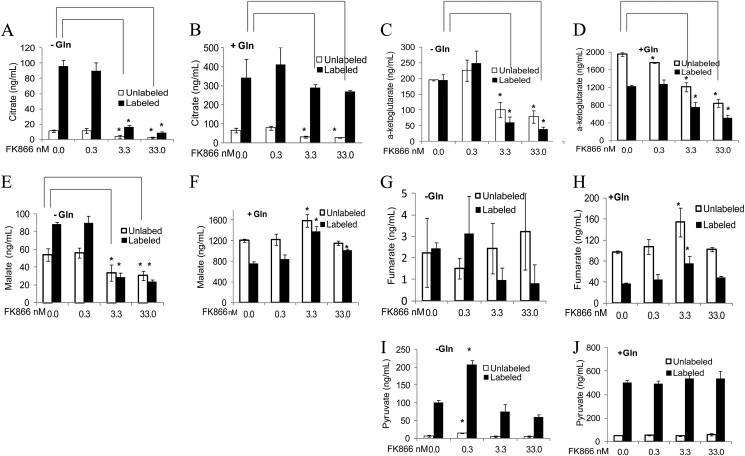
NAMPT inhibition attenuates glycolysis at the glyceraldehyde 3-phosphate dehydrogenase step. This in turn could reduce the flow of pyruvate to the TCA cycle. The conversion of pyruvate to acetyl-CoA represents the first step in the entry of pyruvate into the TCA cycle in which multiple steps require NAD+. Therefore, we tested whether reduced carbon flow from glycolysis and NAD+ levels also attenuated the TCA cycle by measuring fumarate, malate, citrate, oxaloacetate, and α-ketoglutarate levels in A2780 cells treated with FK866 as described above. The treatment with FK866 caused a decrease in citrate and α-ketoglutarate levels but not other intermediate levels in A2780 cells grown in the presence of glutamine (Fig. 5 and unlabeled metabolites). Because the overall effects of FK866 on the TCA cycle (Fig. 5 and unlabeled metabolites) appear to be less pronounced than those on glycolysis, serine biosynthesis, and the pentose phosphate pathway when tested under the same conditions (Figs. 2–4), we reasoned that the reduced carbon flow from glycolysis to the TCA cycle was compensated for by the flux of glutamine intermediates into the TCA cycle, thereby reducing the effects of NAMPT inhibition on the TCA cycle. To test this, we carried out a [1,6-13C]glucose labeling study with A2780 cells treated with FK866 in the presence or absence of glutamine and analyzed TCA cycle intermediates. The FK866 treatment mainly caused a reduction in citrate, malate, fumarate, and α-ketoglutarate levels, especially those 13C-labeled, in the absence of glutamine (Fig. 5). Similar results were obtained for pyruvate (Fig. 5). The reduction of 13C-labeled pyruvate and several TCA cycle intermediate levels by FK866 under the glutamine limiting conditions suggests that reduced carbon flow from glycolysis to the TCA cycle leads to the attenuation of the TCA cycle in the cell.
FIGURE 5.
Inhibition of NAMPT by FK866 resulted in attenuation of the TCA cycle in cancer cells. A2780 cells, labeled with 1,6-13C-labeled glucose, were grown in the absence (A, C, E, G, and I) or presence (B, D, F, H, and J) of glutamine (Gln) and treated in triplicate with FK866 at various concentrations as described (“Experimental Procedures”). After the treatment, cells were collected and processed for the analysis of 13C-labeled and unlabeled citrate (A and B), α-ketoglutarate (C and D), malate (E and F), fumarate (G and H), and pyruvate (I and J) levels by LC-MS (“Experimental Procedures:). Error bars represent S.D. *, p ≤ 0.001 versus control.
Citrate is mainly derived from glucose in A2780 cells cultured with or without glutamine (Fig. 5). Contrary to the findings with other cell lines (39–41), glutamine does not significantly contribute to citrate formation in A2780 cells under the conditions tested. The addition of glutamine enhanced glycolysis and the TCA cycle, as their intermediate levels (labeled and unlabeled) were significantly increased (Fig. 5). The addition of glutamine also appeared to diminish the effects of FK866 on pyruvate and several TCA cycle intermediates (labeled and unlabeled) except α-ketoglutarate (Fig. 5). Thus, the effects of NAMPT inhibition on the TCA cycle are less pronounced than on glycolysis and the pentose phosphate pathway in cancer cells, especially under the conditions where glutamine is not limiting.
Inhibition of NAMPT Led to Similar Metabolic Changes in Other Types of Cancer Cell Lines
We have assessed the metabolic consequences of NAMPT inhibition in A2780 and HCT116 cells. To further investigate whether NAMPT inhibition leads to similar metabolic changes in other types of cancer cells, we treated the following cancer cell lines with FK866 at 25 nm for 24 h and analyzed their glycolytic, pentose phosphate pathway, and TCA cycle intermediate levels as described above: HT20, DLD-1, and SW480 (colon cancer); HCC1937, MDA-MB231, MDA-MB468, and SUM149 (breast cancer); U87MG (glioma); BXPC-3 (pancreatic cancer). The inhibition of NAMPT by FK866 in these cells also led to metabolic changes similar to those observed in A2780 and HCT116 (supplemental Fig. S2). These results indicate that the physiological function of NAMPT is conserved in different types of cancer cells and that NAMPT inhibitors may have clinical utility for treating a variety of different types of cancers.
Inhibition of NAMPT Led to Similar Metabolic Changes in Tumor Xenografts
To confirm that the effects of NAMPT inhibition on cancer cell metabolism observed in vitro also occur in tumors in vivo, we analyzed FK866-treated tumor xenografts derived from HCT116. As shown in Table 2, the treatment of animals bearing tumors with FK866 alone led to a significant inhibition of tumor growth, whereas the treatment with FK866 along with nicotinic acid did not result in any significant inhibition of tumor growth, consistent with the finding that nicotinic acid can rescue the NAMPT inhibitory activity of FK866 in vivo (5, 7, 27). Similarly, the treatment with FK866 alone resulted in an increase in glucose 6-phosphate and fructose 6-phosphate, fructose 1,6-bisphosphate, glyceraldehyde 3-phosphate and dihydroxyacetone phosphate, pentose phosphates, and sedoheptulose 7-phosphate levels (Table 2) and a decrease in NAD(H), NADP, pyruvate, lactate, succinate, and α-ketoglutarate levels (Table 2 and supplemental Table S1). But, the treatment with FK866 along with nicotinic acid did not lead to significant metabolic changes in tumors (Table 2 and supplemental Table S1). Consistent with the labeling study, effects of NAMPT inhibition on the TCA cycle are less pronounced (supplemental Table S1). Similar results were also obtained from an analysis of metabolite levels in FK866-treated A2780 tumor xenografts (data not shown). Taken together, these in vivo and in vitro studies show that NAMPT inhibition attenuates glycolysis by the blockade of glyceraldehyde 3-phosphate dehydrogenase activity, a key mechanism responsible for ATP depletion, metabolic perturbation, and eventual tumor growth inhibition.
TABLE 2.
The inhibition of NAMPT by FK866 led to attenuation of HCT116 tumor growth and alteration of tumor glycolytic and pentose phosphate pathway metabolite levels
Tumor xenografts derived from HCT116 were grown and treated with FK866 and processed for the analysis of metabolites by LC-MS as described (“Experimental Procedures”). NA, nicotinic acid; G6P, glucose 6-phosphate; F6P, fructose 6-phosphate; FBP, fructose 1,6-bisphosphate; G3P, glyceraldehydes 3-phosphate; DHAP, dihydroxyacetone phosphate; 2(3)PG, 2-phosphoglycerate and 3-phosphoglycerate; PEP, phosphoenolpyruvate; R5P, ribose 5-phosphate, ribulose 5-phosphate, and xylulose 5-phosphate; Gn6P, 6-phosphogluconate; and S7P, sedoheptulose 7-phosphate.
| Treatment groupsa |
||||||||
|---|---|---|---|---|---|---|---|---|
| FK (0.0) | FK (8.0) | FK (15.0) | FK (20.0) | FK (0.0) + NA | FK (8.0) + NA | FK(15.0) + NA | FK (20.0) + NA | |
| Tumor growth inhibition (%)b | 0.0 ± 13 | 40.3 ± 8.3 | 64 ± 3.7 | 64.2 ± 6.9 | 0.0 ± 9.7 | −4 ± 9.1 | 13 ± 8.9 | −4 ± 9.4 |
| Metabolite levels (ng/mg)c | ||||||||
| G6P and F6P | 9.9 ± 1.5 | 121 ± 16 | 84 ± 13 | 124 ± 22 | 15.2 ± 4.2 | 8.7 ± 1.7 | 13.9 ± 3.4 | 14.6 ± 2.6 |
| FBP | 81 ± 12.6 | 1114 ± 140 | 636 ± 115 | 719 ± 238 | 96 ± 25 | 68 ± 8 | 101 ± 22 | 77 ± 14 |
| G3P and DHAP | 0.3 ± 0.1 | 14.8 ± 2.6 | 5.9 ± 1 | 8.4 ± 2.3 | 0.5 ± 0.2 | 0.04 ± 0.0 | 0.1 ± 0.03 | 0.4 ± 0.3 |
| 2(3)PG | 53 ± 5.3 | 89 ± 8.6 | 41 ± 8.5 | 63 ± 18.8 | 45 ± 6 | 50 ± 9.7 | 44 ± 5.9 | 78 ± 11.1 |
| PEP | 4.4 ± 0.4 | 4.7 ± 0.4 | 2.5 ± 0.5 | 3.2 ± 0.9 | 3.4 ± 0.2 | 3.7 ± 0.5 | 3.5 ± 0.2 | 5.3 ± 0.9 |
| R5P | 16.7 ± 2 | 32.1 ± 4.3 | 21.4 ± 5.2 | 22 ± 3.9 | 18.8 ± 1.8 | 19.5 ± 1.2 | 14 ± 1.7 | 24 ± 2.4 |
| Gn6P | 7.3 ± 1.5 | 16.5 ± 5 | 6.8 ± 1.7 | 5.9 ± 1.5 | 6.6 ± 1.0 | 2.9 ± 0.4 | 4.7 ± 0.7 | 3.2 ± 0.7 |
| S7P | 18 ± 4.6 | 208 ± 31.6 | 100 ± 17.8 | 104 ± 28.3 | 27 ± 4.2 | 21 ± 2.5 | 25 ± 5.5 | 27 ± 4.7 |
| NAD+ | 63 ± 43 | 4.2 ± 4.4 | 3.9 ± 4.9 | 3.7 ± 5.3 | 31 ± 19.7 | 40 ± 15 | 36 ± 17 | 19 ± 10.6 |
a Treatment groups: FK (0.0) = FK866 (0.0 mg/kg), FK (8.0) = FK866 (8.0 mg/kg), FK (15.0) = FK866 (15.0 mg/kg), FK (20.0) = FK866 (20.0 mg/kg), FK (0.0) + NA = FK866 (0.0 mg/kg) + nicotinic acid (75 mg/kg), FK (8.0) + NA = FK866 (8.0 mg/kg) + nicotinic acid (75 mg/kg), FK(15.0) + NA = FK866 (15.0 mg/kg) + nicotinic acid (75 mg/kg), and FK (20.0) + NA = FK866 (20.0 mg/kg) + nicotinic acid (75 mg/kg).
b Tumor grow inhibition (means ± S.E. of the mean) was calculated at the end of the study when compared to the vehicle (untreated) control groups.
c Tumor metabolite levels (means ± S.E. of the mean).
DISCUSSION
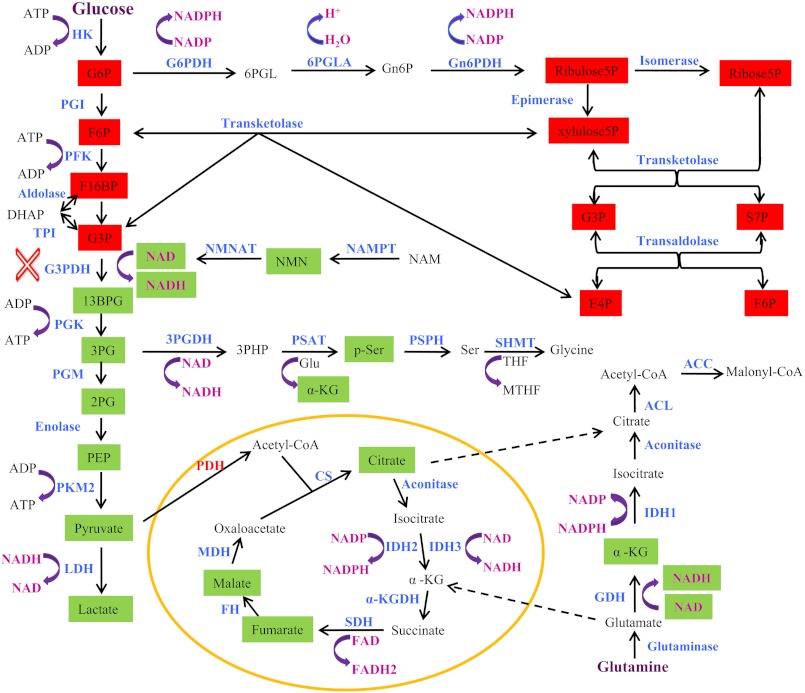
In this study we have elucidated the metabolic basis of NAMPT inhibition and shown that NAMPT plays an important role in several aspects of cellular metabolism and that the central metabolic basis of NAMPT inhibition is the attenuation of glycolysis, resulting in ATP depletion and metabolic perturbation in cancer cells (Fig. 6). The profound effects on cancer cell metabolism observed due to NAMPT inhibition could be used as potential PD markers for effectively evaluating effects of NAMPT inhibitors on their target in tumors in the clinic.
FIGURE 6.
Metabolic basis of NAMPT inhibition in cancer cells. NAMPT inhibition affects glycolysis, the pentose phosphate, serine biosynthesis, and the TCA cycle. The affected metabolites are highlighted as either red (increased) or green (decreased). The blockade of the glyceraldehyde 3-phosphate dehydrogenase (G3PDH) step is indicated (red X). The abbreviations used are as follows: HK, hexokinase; PGI, phosphoglucose isomerase; PFK, phosphofructose kinase; TPI, triose phosphate isomerase; PGK, phosphoglycerate kinase; PGM, phosphoglycerate mutase; PKM2, pyruvate kinase 2; LDH, lactate dehydrogenase; G6PDH, glucose 6-phosphate (G6P) dehydrogenase; 6PGLA, 6-phosphogluconolactonase; Gn6PDH, 6-phosphogluconate (Gn6P) dehydrogenase; NMNAT, nicotinamide mononucleotide adenine (NMN) transferase; 3PGDH, 3-phosphoglycerate (3PG) dehydrogenase; PSAT, phosphoserine (p-ser) aminotransferase; PSPH, phosphoserine phosphatase; SHMT, serine hydroxymethyltransferase; ACC, acetyl-CoA carboxylase; ACL, ATP citrate lyase; CS, citrate synthase; IDH, isocitrate dehydrogenase; GDH, glutamate dehydrogenase; α-KGDH, α-ketoglutarate (α-KG) dehydrogenase; SDH, succinate dehydrogenase; FH, fumarate hydratase; MDH, malate dehydrogenase; PDH, pyruvate dehydrogenase; F6P, fructose 6-phosphate; F16BP, fructose 1,6-bisphosphate; 13BPG, 1,3-bisphosphoglycerate; 2PG, 2-phosphoglycerate; PEP, phosphoenolpyruvate; 6PGL, 6-phosphogluconolactone; E4P, erythrose 4-phosphate; xylulose5P, xylulose 5-phosphate; ribose5P, ribose 5-phosphate; ribulose5P, ribulose 5-phosphate; THF, tetrahydrofolate; MTHF, 5,10-methylenetetrahydrofolate; 3PHP, 3-phosphohydroxypyruvate; NAM, nicotinamide.
The central role that NAD(H) play in cellular metabolism and other physiological processes is well documented (1, 3–4, 11). The regulation of NAD(H) levels in the cell is tightly controlled (1, 3, 4, 11). NAMPT appears to play a key role in maintaining cellular NAD(H) levels. The discovery and clinical exploration of NAMPT inhibitors, FK866 and GMX1777, have validated the important role of NAMPT in the regulation of cellular NAD(H) levels and also its potential as a target for developing novel drugs (7, 20). Yet, little is known about the metabolic basis of NAMPT inhibition and the mechanism of action of these NAMPT inhibitors (7). In this study we demonstrate that NAMPT inhibition primarily results in a blockade of glycolysis at the glyceraldehyde 3-phosphate dehydrogenase step (Fig. 6). This blockade leads to an accumulation in glycolytic intermediates before and at the glyceraldehyde 3-phosphate dehydrogenase step with a corresponding decrease in intermediates after the glyceraldehyde 3-phosphate dehydrogenase step. As a result, a major portion of glycolysis is significantly inhibited. On one hand, the blockade of glycolysis at the glyceraldehyde 3-phosphate dehydrogenase step promotes an overflow of the carbon from glucose 6-phosphate, fructose 6-phosphate, and glyceraldehyde 3-phosphate into the pentose phosphate pathway mainly via the non-oxidative branch of the pathway. On the other hand, this blockade restricts a carbon flow into serine biosynthesis, the TCA cycle, and probably other downstream pathways. The attenuation of glycolysis in conjunction with the reduction of NAD+ levels causes a decrease in carbohydrate and amino acid metabolism and an eventual depletion in ATP levels in the cell. Therefore, the primary metabolic basis for NAMPT inhibition in cancer cells is the attenuation of glycolysis (Fig. 6).
The effects of NAMPT inhibition on the cancer cell TCA cycle warrant further investigation. Glutamine appears to be crucial to the effectiveness of NAMPT activity in regulating the TCA cycle as glutamine can compensate for a reduced carbon flow from glycolysis to the TCA cycle resulting from NAMPT inhibition. Thus, the lack of significant effects of NAMPT inhibition on the TCA cycle suggests that the carbon restriction to the TCA cycle does not appear to be a major mechanism of NAMPT inhibition, especially under the conditions where glutamine is not limiting. In the presence of glutamine, the TCA cycle appeared to be fully functional even after 24 h of treatment with FK866. This suggests that NAMPT inhibition did not lead to significantly reduced NAD+ levels in the mitochondria. How NAD+ is generated in the mitochondria of mammalian cells remains controversial. Yang et al. (21) suggest that both NAMPT and NMNAT are present in the mitochondria of HEK293 cells and that the formation of NAD+ in the organelles is sensitive to FK866 inhibition. Later, the studies by Nikiforov et al. (50) and Pittelli et al. (30) suggest that NAMPT is absent in the organelles. Furthermore, Nikiforov et al. (50) suggest that NAD+ levels in mitochondria are high and sensitive to FK866 inhibition. On the contrary, Pittelli et al. (30) suggest that the mitochondrial pool of NAD+ is insensitive to NAMPT inhibition by FK866. Our study appears to support the conclusion by Pittelli et al. (30). Because cancer cells may possess relatively few mitochondria compared with highly metabolically active cells such as hepatocytes (21, 50), NAD+ levels in mitochondria may only contribute a small fraction of the total NAD+ pool in the cell. Thus, this suggests that NAMPT inhibition mainly affects the cytoplasmic pool of NAD+. Together, the less pronounced effects of NAMPT inhibition on the TCA cycle, especially under the normal growth conditions, are probably due to the lack of significant effects of NAMPT inhibition on the mitochondrial pool of NAD+ and also the flux of glutamine intermediates into mitochondria, compensating for a reduced carbon flow from glycolysis.
The effects of NAMPT inhibition and glutamine supplementation on α-ketoglutarate levels in the cell are worth noting. The inhibition of NAMPT by FK866 consistently leads to a reduction in 13C-labeled or unlabeled α-ketoglutarate levels regardless of whether glutamine is supplemented. This along with the less pronounced effects on the TCA cycle suggests that the reduction of α-ketoglutarate levels by FK866 occurs mainly in the cytoplasm probably through the attenuation of 3-phosphoglycerate dehydrogenase and glutamate dehydrogenase, two major sources of α-ketoglutarate in the cell. As discussed earlier, the NAD+ pool in the cytoplasm appears to be depleted after 24 h of treatment with FK866. Like the NAD+ pool in the mitochondria, the amount of α-ketoglutarate in the organelles probably contributes a small fraction of the total α-ketoglutarate pool in the cell. The 3-phosphoglycerate dehydrogenase-mediated serine biosynthetic pathway is a major source of α-ketoglutarate in certain cancer cells (37, 38). NAMPT inhibition, resulting in the restriction of carbon flow from glycolysis and the reduction of NAD+ levels, significantly attenuates 3-phosphoglycerate dehydrogenase-mediated serine biosynthetic activity and thereby α-ketoglutarate formation in the cell. In humans, glutamate dehydrogenase 1 encoded by GLUD1 is also involved in α-ketoglutarate formation using NAD(H) and NADP(H) (42–45). GLUD1, localized in the mitochondria and cytoplasm (43, 45), appears present in certain cancer cells (46). Thus, the attenuation of 3-phosphoglycerate dehydrogenase and GLUD1 activities in the cytoplasm may be the key mechanism of NAMPT inhibition responsible for α-ketoglutarate reduction.
The findings from this study may have significant clinical implications. To effectively assess a molecule in the clinic, it is essential to have a robust clinical diagnostic assay for identifying appropriate patient populations and a reliable PD biomarker assay for evaluating the effects of the molecule on its intended target in tumors. The altered metabolite levels in tumors including, but not limited to glucose 6-phosphate, fructose 6-phosphate, fructose 1,6-bisphosphate, glyceraldehyde 3-phosphate, pentose phosphates, and sedoheptulose 7-phosphate or 2-phosphoglycerate, 3-phosphoglycerate, phosphoenolpyruvate, phosphoserine, and α-ketoglutarate can be used to assess effects of NAMPT inhibitors on their intended target in the clinic. First, the LC-MS method has been successfully used to detect and quantify many metabolites derived from several metabolic pathways, including glycolysis, the pentose phosphate pathway, the TCA cycle, and glutamine metabolism from fresh, frozen, and formalin-fixed paraffin-embedded human tumors (48, 49). Among the 119 different metabolites analyzed, several of them, including glucose 6-phosphate, fructose 1,6-bisphosphate, glyceraldehyde 3-phosphate, and ribose 5-phosphate, which we show are significantly elevated in FK866-treated cancer cells and tumor xenografts, were reported to be robustly detected from human tumor tissues (48, 49). In addition, the amount (10–15 mg or a 40-μm formalin-fixed paraffin-embedded slice) of materials required for this type of analysis may be obtained via needle biopsy. Because the treatment of tumors with NAMPT inhibitors leads to increased glucose 6-phosphate, fructose 1,6-bisphosphate, glyceraldehyde 3-phosphate, pentose phosphate, and sedoheptulose 7-phosphate levels, the amount of materials required for detection will be even smaller, and the signal will be much higher. In this study we show that only of the 50 mg tumor sample was used for analysis. Therefore, it is possible that a needle-based biopsying approach could obtain a sufficient amount of material for analysis. Finally, the tumor samples used in these two studies were frozen for 3–5 years on average (48, 49), suggesting that these metabolites are stable. Second, the LC-MS method reported is feasible, and the changes in these metabolites represent the most direct and robust response of tumor cells to NAMPT inhibition. Third, the tumor-based biomarkers have been used extensively in the clinic. For example, FDA has recently approved the KRAS PCR kit as a companion diagnostic for identifying patients with metastatic colorectal cancer who are most likely to respond to the chemotherapy of Eli Lilly and Bristol-Myers Squibb's Erbitux in combination with FOLFIRI as first-line treatment of patients whose tumors express EGFR and wild type KRAS. Together, the tumor biopsy-based LC-MS approach is technically sound and feasible. A successful implementation of a methodology such as the tumor biopsy-based LC-MS approach for evaluating these metabolic changes may enhance our understanding of the mechanism of action of NAMPT inhibitors in the clinic and subsequently accelerate their clinical development.
In summary, the results of this study provide further support that NAMPT plays an important role in cancer cells and is an attractive anticancer target with unique characteristics and specificity and that NAMPT inhibitors possess promising therapeutic potential. First, the NAD+ turnover in cancer cells is much higher compared with normal cells due to the activation of poly(ADP-ribose) polymerase activity required for DNA repair and genome stability (1, 24–26). The high dependence of cancer cells on NAD+ levels makes tumor cells more susceptible to NAMPT inhibition than normal cells. Second, NAMPT is the rate-limiting enzyme in the biosynthesis of NAD+ (9, 10) and is overexpressed in different cancer cells (14–17). Third, DNA damage causes NAD+ deletion in cancer cells (21, 26). Fourth, nicotinic acid co-administration with FK866 and GMX1777 rescues toxicity associated with NAMPT inhibition and enhances the therapeutic index (5, 7). Fifth, the profound metabolic effects resulting from NAMPT inhibition could be used as potential PD markers for evaluating effects of NAMPT inhibitors on the target in the clinic.
Acknowledgments
We thank Sucai Dong for help with the LC-MS assay, Yue-wei Qian and He Wang for expression and purification of NAMPT, Jake Starling, Robert Wild, Bharvin Patel, Alfonso De Dios, Jonathan Yingling, and Gregory D. Plowman for support, guidance, and critical review of the manuscript, and Frank C. Dorsey, James E. Thomas, and Raymond Gilmour for discussions and critical review of the manuscript.
This work was supported by Eli Lilly and Company.

This article contains supplemental Table S1 and Figs. S1 and S2.
- NAMPT
- nicotinamide phosphoribosyltransferase
- PD
- pharmacodynamic.
REFERENCES
- 1. Garten A., Petzold S., Körner A., Imai S., Kiess W. (2009) Nampt. Linking NAD+ biology, metabolism, and cancer. Trends Endocrinol. Metab. 20, 130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirkland J. B. (2009) Niacin status, NAD distribution, and ADP-ribose metabolism. Curr. Pharm. Des. 15, 3–11 [DOI] [PubMed] [Google Scholar]
- 3. Magni G., Amici A., Emanuelli M., Orsomando G., Raffaelli N., Ruggieri S. (2004) Enzymology of NAD+ homeostasis in man. Cell. Mol. Life Sci. 61, 19–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sauve A. A. (2008) NAD+ and vitamin B3. From metabolism to therapies. J. Pharmacol. Exp. Ther. 324, 883–893 [DOI] [PubMed] [Google Scholar]
- 5. Olesen U. H., Thougaard A. V., Jensen P. B., Sehested M. (2010) A preclinical study on the rescue of normal tissue by nicotinic acid in high-dose treatment with APO866, a specific nicotinamide phosphoribosyltransferase inhibitor. Mol. Cancer Ther. 9, 1609–1617 [DOI] [PubMed] [Google Scholar]
- 6. Olesen U. H., Christensen M. K., Björkling F., Jäättelä M., Jensen P. B., Sehested M., Nielsen S. J. (2008) Anticancer agent CHS-828 inhibits cellular synthesis of NAD. Biochem. Biophys. Res. Commun. 367, 799–804 [DOI] [PubMed] [Google Scholar]
- 7. Watson M., Roulston A., Bélec L., Billot X., Marcellus R., Bédard D., Bernier C., Branchaud S., Chan H., Dairi K., Gilbert K., Goulet D., Gratton M. O., Isakau H., Jang A., Khadir A., Koch E., Lavoie M., Lawless M., Nguyen M., Paquette D., Turcotte E., Berger A., Mitchell M., Shore G. C., Beauparlant P. (2009) The small molecule GMX1778 is a potent inhibitor of NAD+ biosynthesis. Strategy for enhanced therapy in nicotinic acid phosphoribosyltransferase 1-deficient tumors. Mol. Cell. Biol. 29, 5872–5888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Samal B., Sun Y., Stearns G., Xie C., Suggs S., McNiece I. (1994) Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony enhancing factor. Mol. Cell. Biol. 14, 1431–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Revollo J. R., Grimm A. A., Imai S. (2004) The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 279, 50754–50763 [DOI] [PubMed] [Google Scholar]
- 10. Rongvaux A., Galli M., Denanglaire S., Van Gool F., Drèze P. L., Szpirer C., Bureau F., Andris F., Leo O. (2008) Nicotinamide phosphoribosyl transferase/pre-B cell colony-enhancing factor/visfatin is required for lymphocyte development and cellular resistance to genotoxic stress. J. Immunol. 181, 4685–4695 [DOI] [PubMed] [Google Scholar]
- 11. Imai S. (2009) Nicotinamide phosphoribosyltransferase (NAMPT). A link between NAD biology, metabolism, and diseases. Curr. Pharm. Des. 15, 20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khan J. A., Forouhar F., Tao X., Tong L. (2007) Nicotinamide adenine dinucleotide metabolism as an attractive target for drug discovery. Expert Opin. Ther. Targets. 11, 695–705 [DOI] [PubMed] [Google Scholar]
- 13. Luk T., Malam Z., Marshall J. C. (2008) Pre-B cell colony-enhancing factor (PBEF)/visfatin. A novel mediator of innate immunity. J. Leukoc. Biol. 83, 804–816 [DOI] [PubMed] [Google Scholar]
- 14. Bi T. Q., Che X. M., Liao X. H., Zhang D. J., Long H. L., Li H. J., Zhao W. (2011) Overexpression of Nampt in gastric cancer and chemopotentiating effects of the Nampt inhibitor FK866 in combination with fluorouracil. Oncol. Rep. 26, 1251–1257 [DOI] [PubMed] [Google Scholar]
- 15. Hufton S. E., Moerkerk P. T., Brandwijk R., de Bruïne A. P., Arends J. W., Hoogenboom H. R. (1999) A profile of differentially expressed genes in primary colorectal cancer using suppression subtractive hybridization. FEBS Lett. 463, 77–82 [DOI] [PubMed] [Google Scholar]
- 16. Van Beijnum J. R., Moerkerk P. T., Gerbers A. J., De Bruïne A. P., Arends J. W., Hoogenboom H. R., Hufton S. E. (2002) Target validation for genomics using peptide-specific phage antibodies. A study of five gene products overexpressed in colorectal cancer. Int. J. Cancer 101, 118–127 [DOI] [PubMed] [Google Scholar]
- 17. Wang B., Hasan M. K., Alvarado E., Yuan H., Wu H., Chen W. Y. (2011) NAMPT overexpression in prostate cancer and its contribution to tumor cell survival and stress response. Oncogene 30, 907–921 [DOI] [PubMed] [Google Scholar]
- 18. Nakajima T. E., Yamada Y., Hamano T., Furuta K., Gotoda T., Katai H., Kato K., Hamaguchi T., Shimada Y. (2009) Adipocytokine levels in gastric cancer patients. Resistin and visfatin as biomarkers of gastric cancer. J. Gastroenterol. 44, 685–690 [DOI] [PubMed] [Google Scholar]
- 19. Busso N., Karababa M., Nobile M., Rolaz A., Van Gool F., Galli M., Leo O., So A., De Smedt T. (2008) Pharmacological inhibition of nicotinamide phosphoribosyltransferase/visfatin enzymatic activity identifies a new inflammatory pathway linked to NAD. PLoS One 3, e2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hasmann M., Schemainda I. (2003) FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 63, 7436–7442 [PubMed] [Google Scholar]
- 21. Yang H., Yang T., Baur J. A., Perez E., Matsui T., Carmona J. J., Lamming D. W., Souza-Pinto N. C., Bohr V. A., Rosenzweig A., de Cabo R., Sauve A. A., Sinclair D. A. (2007) Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 130, 1095–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Drevs J., Löser R., Rattel B., Esser N. (2003) Antiangiogenic potency of FK866/K22.175, a new inhibitor of intracellular NAD biosynthesis, in murine renal cell carcinoma. Anticancer Res. 23, 4853–4858 [PubMed] [Google Scholar]
- 23. Muruganandham M., Alfieri A. A., Matei C., Chen Y., Sukenick G., Schemainda I., Hasmann M., Saltz L. B., Koutcher J. A. (2005) Metabolic signatures associated with a NAD synthesis inhibitor-induced tumor apoptosis identified by 1H-decoupled 31P magnetic resonance spectroscopy. Clin. Cancer Res. 11, 3503–3513 [DOI] [PubMed] [Google Scholar]
- 24. Du L., Zhang X., Han Y. Y., Burke N. A., Kochanek P. M., Watkins S. C., Graham S. H., Carcillo J. A., Szabó C., Clark R. S. (2003) Intra-mitochondrial poly(ADP-ribosylation) contributes to NAD+ depletion and cell death induced by oxidative stress. J. Biol. Chem. 278, 18426–18433 [DOI] [PubMed] [Google Scholar]
- 25. Pillai J. B., Isbatan A., Imai S., Gupta M. P. (2005) Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2α deacetylase activity. J. Biol. Chem. 280, 43121–43130 [DOI] [PubMed] [Google Scholar]
- 26. Yu S. W., Wang H., Poitras M. F., Coombs C., Bowers W. J., Federoff H. J., Poirier G. G., Dawson T. M., Dawson V. L. (2002) Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297, 259–263 [DOI] [PubMed] [Google Scholar]
- 27. Luo J., Nikolaev A. Y., Imai S., Chen D., Su F., Shiloh A., Guarente L., Gu W. (2001) Negative control of p53 by Sir2α promotes cell survival under stress. Cell 107, 137–148 [DOI] [PubMed] [Google Scholar]
- 28. Saunders L. R., Verdin E. (2007) Sirtuins. Critical regulators at the crossroads between cancer and aging. Oncogene 26, 5489–5504 [DOI] [PubMed] [Google Scholar]
- 29. Van Gool F., Gallí M., Gueydan C., Kruys V., Prevot P. P., Bedalov A., Mostoslavsky R., Alt F. W., De Smedt T., Leo O. (2009) Intracellular NAD levels regulate tumor necrosis factor protein synthesis in a sirtuin-dependent manner. Nat. Med. 15, 206–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pittelli M., Formentini L., Faraco G., Lapucci A., Rapizzi E., Cialdai F., Romano G., Moneti G., Moroni F., Chiarugi A. (2010) Inhibition of nicotinamide phosphoribosyltransferase. Cellular bioenergetics reveals a mitochondrial insensitive NAD pool. J. Biol. Chem. 285, 34106–34114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khan J. A., Tao X., Tong L. (2006) Molecular basis for the inhibition of human NMPRTase, a novel target for anticancer agents. Nat. Struct. Mol. Biol. 13, 582–588 [DOI] [PubMed] [Google Scholar]
- 32. Nahimana A, Attinger A, Aubry D, Greaney P, Ireson C, Thougaard AV, Tjørnelund J, Dawson KM, Dupuis M, Duchosal MA. (2009) The NAD biosynthesis inhibitor APO866 has potent antitumor activity against hematologic malignancies. Blood 113, 3276–3286 [DOI] [PubMed] [Google Scholar]
- 33. Holen K., Saltz L. B., Hollywood E., Burk K., Hanauske A. R. (2008) The pharmacokinetics, toxicities, and biologic effects of FK866, a nicotinamide adenine dinucleotide biosynthesis inhibitor. Invest. New Drugs 26, 45–51 [DOI] [PubMed] [Google Scholar]
- 34. Galli U., Ercolano E., Carraro L., Blasi Roman C. R., Sorba G., Canonico P. L., Genazzani A. A., Tron G. C., Billington R. A. (2008) Synthesis and biological evaluation of isosteric analogues of FK866, an inhibitor of NAD salvage. Chem. Med. Chem. 3, 771–779 [DOI] [PubMed] [Google Scholar]
- 35. Tong L., Tao X., Khan J. A. (January 24, 2008) U. S. Patent 0020413
- 36. Putt K. S., Hergenrother P. J. (2004) An enzymatic assay for poly(ADP-ribose) polymerase-1 (PARP-1) via the chemical quantitation of NAD+. Application to the high-throughput screening of small molecules as potential inhibitors. Anal. Biochem. 326, 78–86 [DOI] [PubMed] [Google Scholar]
- 37. Locasale J. W., Grassian A. R., Melman T., Lyssiotis C. A., Mattaini K. R., Bass A. J., Heffron G., Metallo C. M., Muranen T., Sharfi H., Sasaki A. T., Anastasiou D., Mullarky E., Vokes N. I., Sasaki M., Beroukhim R., Stephanopoulos G., Ligon A. H., Meyerson M., Richardson A. L., Chin L., Wagner G., Asara J. M., Brugge J. S., Cantley L. C., Vander Heiden M. G. (2011) Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat. Genet. 43, 869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Possemato R., Marks K. M., Shaul Y. D., Pacold M. E., Kim D., Birsoy K., Sethumadhavan S., Woo H. K., Jang H. G., Jha A. K., Chen W. W., Barrett F. G., Stransky N., Tsun Z. Y., Cowley G. S., Barretina J., Kalaany N. Y., Hsu P. P., Ottina K., Chan A. M., Yuan B., Garraway L. A., Root D. E., Mino-Kenudson M., Brachtel E. F., Driggers E. M., Sabatini D. M. (2011) Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476, 346–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. DeBerardinis R. J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C. B. (2007) Beyond aerobic glycolysis. Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. U.S.A. 104, 19345–19350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Filipp F. V., Scott D. A., Ronai Z. A., Osterman A. L., Smith J. W. (2012) Reverse TCA cycle flux through isocitrate dehydrogenases 1 and 2 is required for lipogenesis in hypoxic melanoma cells. Pigment. Cell Melanoma Res. 25, 375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scott D. A., Richardson A. D., Filipp F. V., Knutzen C. A., Chiang G. G., Ronai Z. A., Osterman A. L., Smith J. W. (2011) Comparative metabolic flux profiling of melanoma cell lines. beyond the Warburg effect. J. Biol. Chem. 286, 42626–42634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Olson J. A., Anfinsen C. B. (1952) The crystallization and characterization of L-glutamic acid dehydrogenase. J. Biol. Chem. 197, 67–79 [PubMed] [Google Scholar]
- 43. Rosso L., Marques A. C., Reichert A. S., Kaessmann H. (2008) Mitochondrial targeting adaptation of the hominoid-specific glutamate dehydrogenase driven by positive darwinian selection. PLoS Genet. 4, e1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spanaki C., Plaitakis A. (2012) The role of glutamate dehydrogenase in mammalian ammonia metabolism. Neurotox. Res. 21, 117–127 [DOI] [PubMed] [Google Scholar]
- 45. Spanaki C., Zaganas I., Kleopa K. A., Plaitakis A. (2010) Human GLUD2 glutamate dehydrogenase is expressed in neural and testicular supporting cells. J. Biol. Chem. 285, 16748–16756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang C., Sudderth J., Dang T., Bachoo R. M., Bachoo R. G., McDonald J. G., DeBerardinis R. J. (2009) Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 69, 7986–7993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bruzzone S., Fruscione F., Morando S., Ferrando T., Poggi A., Garuti A., D'Urso A., Selmo M., Benvenuto F., Cea M., Zoppoli G., Moran E., Soncini D., Ballestrero A., Sordat B., Patrone F., Mostoslavsky R., Uccelli A., Nencioni A. (2009) Catastrophic NAD+ depletion in activated T lymphocytes through Nampt inhibition reduces demyelination and disability in EAE. PLoS One. 4, e7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kelly A. D., Breitkopf S. B., Yuan M., Goldsmith J., Spentzos D., Asara J. M. (2011) Metabolomic profiling from formalin-fixed, paraffin-embedded tumor tissue using targeted LC/MS/MS. Application in sarcoma. PLoS One 6, e25357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yuan M., Breitkopf S. B., Yang X., Asara J. M. (2012) A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 7, 872–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nikiforov A., Dölle C., Niere M., Ziegler M. (2011) Pathways and subcellular compartmentation of NAD biosynthesis in human cells. From entry of extracellular precursors to mitochondrial NAD generation. J. Biol. Chem. 286, 21767–21778 [DOI] [PMC free article] [PubMed] [Google Scholar]