Abstract
OBJECTIVES:
Although intestinal dysbiosis is well established in Crohn's disease (CD), little is known about the microbial metabolic activity of CD patients. In this study, we compared the metabolite patterns of the CD patients with profiles from healthy controls (HCs) and correlated them to disease activity and bacterial composition. In addition, the influence of the prebiotic oligofructose-enriched inulin (OF-IN) on the CD metabolites profile was evaluated.
METHODS:
Sixty-seven inactive and moderately active CD patients were included in a double-blinded randomized placebo controlled trial (RCT). Patients consumed either 10 g OF-IN or 10 g placebo twice per day for 4 weeks. They collected a fecal sample before the start of the study (baseline) and after the treatment period. In addition, fecal samples were obtained from 40 HCs. The metabolite profile was assessed using gas chromatography–mass spectrometry.
RESULTS:
The number of fecal metabolites was significantly higher in HCs than in CD patients (P<0.001). Forty compounds differed between CD patients and HCs. When correlating the metabolite levels to disease activity, significantly lower levels of butyrate, pentanoate, hexanoate, heptanoate, and p-cresol were found in active patients as compared with HCs. In the RCT, no significant changes in the metabolite pattern were found in patients randomized to placebo. In patients receiving OF-IN (per protocol; n=21), the relative levels of acetaldehyde (P=0.0008) and butyrate (P=0.0011) were significantly increased as compared with baseline.
CONCLUSIONS:
We identified medium chain fatty acids and p-cresol as differentiating metabolites toward CD disease status and as compared with HCs. In addition, OF-IN intake primarily increased the carbohydrate fermentation metabolites butyrate and acetaldehyde.
INTRODUCTION
Although the etiology of Crohn's disease (CD) is incompletely understood, a role of the intestinal microbiota, in addition to genetic factors, in the inititation and perpetuation of inflammatory processes is well established.1, 2 Using molecular culture-independent techniques, a general dysbiosis has been described.3, 4, 5 This dysbiosis is characterized by lower amounts of bifidobacteria6 and firmicutes,7, 8 excess of mucosal bacteria, Enterobacteria, and a larger proportion of Gram–negative bacteria.4, 9, 10 Our group identified five disproportionate bacterial species, namely Dialister invisus, an uncharacterized species of Clostridium cluster XIVa, Faecalibacterium prausnitzii, Bifidobacterium adolescentis, and Ruminococcus gnavus, that characterize CD-associated dysbiosis in the predominant fecal microbiota of a large cohort of CD patients, their unaffected relatives, and unrelated controls.11
Strategies to influence the composition of the colon microbiota may be an attractive therapeutic option in CD patients. One emerging strategy consists of the consumption of prebiotics such as inulin-type fructans.12, 13 As several in vitro and in vivo (animal and human) trials have demonstrated that prebiotics are capable of modulating the intestinal microbiota in healthy subjects,14, 15, 16 resulting in the predominance of beneficial Lactobacillus and Bifidobacterium species, they may also render a clinical benefit for CD patients.17 Indeed, we recently demonstrated that the CD-associated dysbiosis can be modified by oligofructose-enriched inulin (OF-IN) supplementation in inactive and mild to moderately active CD patients. We found a promising correlation between improvement in disease activity and increase in fecal number of Bifidobacterium longum.18 In contrast, Benjamin et al.19 found no impact of OF-IN on disease activity nor on the fecal bifidobacteria counts in active CD patients.
Nowadays, analysis of the microbial diversity of the intestinal ecosystem has been complemented with the functional analysis of the colonic microbiota.20 Untargeted metabolic profiling combined with multivariate statistical methods, so-called metabonomics, has gained increasing interest as a means to elucidate subtle changes in metabolic activity.21 Functional analysis at the metabolome level provides information on the metabolites that have effectively been produced, rather than on the ability of the microbiota to perform metabolic activities. Until now, metabonomics in inflammatory bowel diseases (IBD) has mainly been utilized as a classification tool to distinguish IBD patients from healthy subjects and to identify characteristic metabolites in the feces or urine specific for CD and ulcerative colitis (UC). Marchesi et al.22 applied metabolic profiling using 1H-nuclear magnetic resonance on fecal water samples from IBD patients and controls. In an identical twin study with healthy subjects and CD patients, differentiating metabolites in fecal samples were found in the metabolism of amino acids, fatty acids, bile acids, and arachidonic acid.23 In another study, urine samples from IBD patients were analyzed using 1H-nuclear magnetic resonance.24 So far, the effect of a dietary strategy such as prebiotics on the metabolite profile in CD patients has not been performed.
In this study, we analyzed the metabolite profiles of the fecal samples of the CD patients before and after the intervention with OF-IN. In addition, fecal samples from a healthy control (HC) group were analyzed to characterize the differences between CD patients and controls. Changes in metabolite patterns due to the OF-IN intervention were related to changes in microbiota composition.
MATERIALS AND METHODS
Ethics statement
The study was approved by the University Hospital Ethics Committee (Leuven, Belgium), and informed consent was obtained before any study-related procedures from all participating subjects. The clinical trial was registered at ClinicalTrial.gov (NCT 01487759).
Subjects
Healthy controls
Fecal samples were obtained from 40 HCs (28 females/12 males) after informed consent. None of the subjects had a history of gastrointestinal or metabolic disease or previous surgery (apart from appendectomy) nor had they used antibiotics or any other medical treatment influencing gut transit or intestinal microbiota during the preceding 3 months. An aliquot of the fecal samples was immediately frozen at −20° for metabolite profiling.
CD patients
Sixty-seven patients with inactive and mild to moderately active CD (Harvey-Bradshaw index25 (HBI) 0–12)) were recruited and randomized after informed consent for inclusion in the prebiotic intervention study. Exclusion criteria included severe CD (HBI>12), pregnancy, history of total colectomy, use of antibiotics in the 4 weeks before the start of the study, use of sulfapyridine, dose adjustments in the CD maintenance therapy during the past 4 weeks, and the use of commercially available pre- and probiotics.
Randomized controlled trial in CD patients: study design
The trial was conducted as a prospective, randomized controlled pilot study with a parallel group design conducted in line with CONSORT recommendations (http://www.consort-statement.org/). The study was double-blind, and the randomization codes were only unblinded after all analyses were finished. Randomization was performed by an independent researcher using Random Allocation Software Version 1.0. Patients randomized to the control group continued their standard medication supplemented with placebo twice a day, patients in the OF-IN group continued their maintenance medication supplemented with 10 g of OF-IN twice a day as adjuvant therapy. The treatment regime was not disclosed to the patients, the clinicians nor to the researchers who carried out the analyses. The dose of OF-IN and the duration of the trial was chosen based on previous studies in hemodialysis patients26 and in healthy subjects.15
A fecal sample was collected from each subject on the day before the start of the intervention (day 0) and on the day after the last intake of OF-IN or placebo (day 29) and an aliquot was immediately frozen at –20° for metabolite profiling. The clinical disease activity (HBI) was assessed at enrollment and at the end of the 4-week trial period. Patients that completed the 4-week intervention according to the protocol were included in the per protocol (PP) analysis. If patients discontinued the intervention precociously, they were asked to still provide a fecal sample and HBI assessment. Patients that succeeded were included in the intention-to-treat (ITT) population.
Prebiotic substrate
OF-IN (ORAFTISynergy-1) is a 1:1 mixture of inulin and oligofructose.13, 27 Maltodextrin was used as placebo. Both substrates were kindly provided by Beneo-Orafti (Tienen, Belgium) and were wrapped in identical packings.
Analysis of volatile organic compounds (VOCs) in fecal samples
Chemicals
2-Ethylbutyrate (at least 99% purity), supplied by Merck (München, Germany), was used as an internal standard. Sodium sulfate (99%) was purchased from Acros organics (Geel, Belgium) and sulfuric acid (99%) from Sigma-Aldrich (Steinheim, Germany).
Experimental procedure
Immediately before analysis, fecal aliquots were thawed and 0.25 g fecal sample was suspended in 4870 μl water. 2-Ethylbutyrate (40 μl; 250 mg/100 ml) was added as internal standard. A magnetic stirrer, a pinch of sodium sulfate, and 130 μl sulfuric acid were added to the sample to salt out and acidify the solution, respectively. To prevent crossover from one sample to another, water samples were extracted after each sample.
The VOCs were analyzed on a gas chromatography–mass spectrometry type time of flight (Trace GC, Thermoquest, Rodano, Italy and Tempus II, Thermo Electron, San Jose, CA, USA), which was coupled on-line to a purge-and-trap system (Velocity, Teledyne Tekmar, Mason, OH, USA). The optimal procedure for analysis of fecal VOC has been described previously.28
Briefly, the metabolites were purged out the sample for 20 min with a helium flow rate of 40 ml/min, carried over a dry flow column (Trap Tenax tbv Velocity, Interscience, Louvain-la-Neuve, Belgium) for 3 min to control moisture transfer, and concentrated on a second polar trap column (Trap Vocarb tbv Velocity Interscience). Consequently, the VOCs were desorbed from the trap by raising the trap temperature to 250 °C for 5 min. After desorption, the trap temperature was further raised to 270 °C for 10 min to remove any contamination of tailing compounds. The desorbed compounds were conducted via the transfer line (175 °C) to the injector of the gas chromatograph to the gas chromatography (GC) column. The analytical column was a 30 × 0.25 mm internal diameter, 0.25 μm film thickness, AT Aquawax DA (Grace, Deerfield, IL, USA). Helium GC grade was used as carrier gas with a constant flow of 10 ml/min. The oven temperature was maintained at 35 °C (isothermal for 2 min) and increased with 5 °C/min to 100 °C and to 240 °C with 10 °C/min. This final temperature was held for 5 min. Time-of-flight-mass spectrometry was performed in full scan mode from 30 to m/z 500 at two scans/s. Xcalibur software (Thermo Scientific, Breda, The Netherlands) was used for the automatization of the GS–mass spectrometry and for data acquisition.
The resulting chromatograms were processed using AMDIS version 2.1 provided by the National Institute of Standards and Technology (Gaithersburg, MD, USA). Identification of the analytes in the samples was achieved by comparing the mass spectra of unknown peaks with the National Institute of Standards and Technology library. Compounds showing mass spectra with match factors ≥90% were positively identified. All compounds were relatively quantified compared with 2-ethylbutyrate. The resulting metabolite profiles were organized in a three-dimensional data matrix using sample names (observations), identified metabolite (variables), and normalized peak intensity (variable indices).
Analysis of the microbiota composition
The real-time-PCR data of the microbiota of the same sample set in CD patients were reported previously18 as well as a detailed analysis of the real-time-PCR data.11, 29
Statistics
Evaluation of the study population characteristics
Descriptive statistics (median and interquartile range (IQR)) were performed with SPSS Statistics 17.0 (SPSS, Chicago, IL, USA) using the Wilcoxon signed-rank test, Mann– U-test, and Fisher exact test. P-values below 0.05 were considered significant.
Statistical processing of the VOC profiles
Subject-specific VOCs, i.e., compounds that were detected in only one person, were discarded from statistical analysis because they do not exert any discriminatory power due to their low occurrence rate and introduce noise if implemented into the classification model.30
Multivariate statistical analysis
Exploratory data analysis: Principal component analysis was used to detect outliers and to obtain an overview of the variation among the groups. Partial least square analysis discriminant analysis (PLS-DA) is a supervised learning technique. This statistical technique was applied to cluster observations with similar metabolite profiles and to identify VOCs accounting for discrimination between CD and healthy state or for the prebiotic effect in CD. The predictions of the multivariate modeling were validated using full cross-validation. Predictions were tested for each group in turn on samples excluded from the sample set. Using the cross-validated PLS-DA analysis, the number of correctly predicted samples, and from this, the sensitivity and specificity were tested for HC and CD samples. Analyses were performed using Unscrambler Version 9.7 (CAMO A/S, Trondheim, Norway).
Statistical analysis: Data were compared by nonparametric tests (Wilcoxon test or Kruskal–Wallis+Mann–Whitney U test). Correction for multiple testing was performed using the Benjamini and Hochberg31 false discovery rate method. For correlation analysis, Spearman's rank correlation coefficient was used.
Correlation of metabolites with microbiota data
The baseline metabolic activity was correlated to the baseline levels of B. longum, B. adolescentis, F. prausnitzii, and R. gnavus using Spearman's ρ correlation. Furthermore, the correlation between changes in metabolic activity and the changes in number of bacteria per species was also assessed with Spearman's ρ correlation. We therefore calculated a delta matrix where values before and after treatment were replaced by their difference, i.e., each measurement before and after treatment was replaced by its remainder, the delta value. This enabled us to correlate actual changes per parameter.
RESULTS
Study population
The CD patients were significantly older than the healthy subjects (42 years (IQR 31–52) vs. 27 years (IQR 21–36), P<0.001). No significant difference in gender between CD patients and HCs was found.
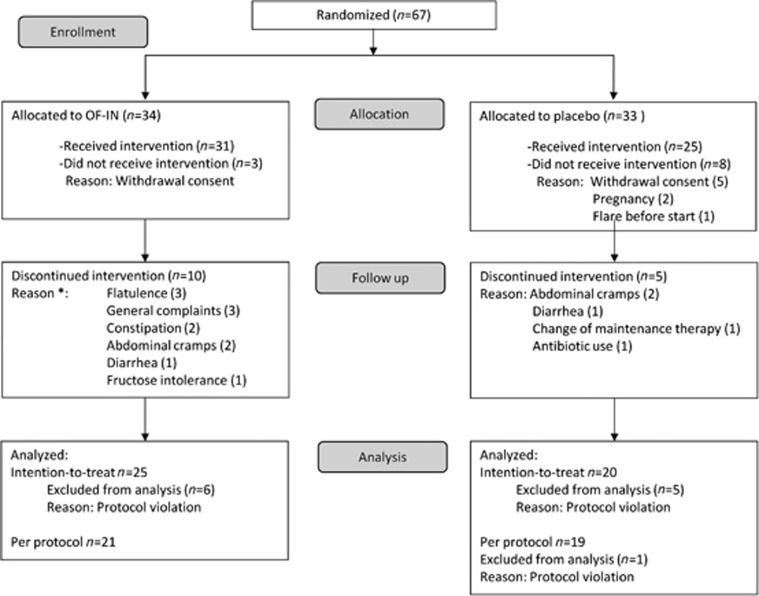
Of the 67 randomized CD patients, 56 subjects effectively started the intervention (31 to OF-IN, 25 to placebo; Figure 1). In the group that received OF-IN, 10/31 patients discontinued the study due to side effects, vs. 3/25 in the placebo group (one-sided Fisher exact: P=0.07). Forty patients (60%) completed the study according to the study protocol (PP population): 19 in the placebo group and 21 in the OF-IN group. Some patients who discontinued the study still provided a fecal sample (n=25 in OF-IN and n=20 in placebo) and were included in the ITT population. The median disease activity at the time of inclusion was 4 (2–6) in both groups (P=NS), and there were no significant demographic differences between groups (Table 1). The majority of the CD patients in the ITT population received drug treatment: 15 received anti-tumor necrosis factor alone or combined with immunomodulators (n=3) or with aminosalicylates or sulfasalazine (grouped as 5-aminosalicylic acid (5-ASA); n=2). Others received 5-ASA alone (n=7) or along with immunomodulators (n=2). In total, 10 patients received other gastrointestinal medication, whereas 6 patients did not receive any medication.
Figure 1.
Flow diagram of Crohn's disease patients included in the study (*two patients had >1 complaint).
Table 1. Baseline characteristics of the patients per group (ITT population).
| OF-IN group | Placebo group | P-value | |
|---|---|---|---|
| Male/female | 16 F/9 M | 13 F/7 M | NS |
| Median (IQR) age in years | 46 (31–51) | 37 (30–50) | NS |
| Median (IQR) age at diagnosis in years | 24 (19–35) | 22 (17–30) | NS |
| Median (IQR) disease duration | 11 (6–23) | 13 (3–20) | NS |
| Disease location (ileocolitis/colitis/ileitis) | 15/6/4 | 13/5/2 | NS |
| Percentage active smokers | 12% | 10% | NS |
| Median (IQR) baseline HBI | 4 (2–6) | 4 (2–6) | NS |
Abbreviations: F, female; HBI; Harvey-Bradshaw index; IQR, interquartile range; M, male; NS, not significant; OF-IN, prebiotic oligofructose-enriched inulin.
Clinical outcome
Median HBI remained unchanged in the placebo group (4 (IQR 2–6) at baseline vs. 4 (2–5) after placebo intake), but significantly decreased after intervention with OF-IN: from 4 (IQR 2–6) to 3 (IQR 0–5), P=0.048.
When considering only patients with mild to moderately active disease (HBI>4), 4 out of 10 (40%) patients (ITT) and 3 out of 6 (50%) patients (PP) randomized to OF-IN achieved remission (HBI≤4). The median HBI decreased from 7 to 5 in mild to moderately active CD patients (P=0.02).
Metabolite profiles in CD patients before intervention and HCs
Assignment of VOCs
A total of 200 different VOCs were identified, of which 56 compounds were subject specific. The number of VOCs was significantly higher in HCs than in CD patients (HC: 60 (56–66) vs. CD: 48 (44–54); P<0.001). Eight compounds (acetate, propionate, butyrate, pentanoate, 2-methyl propionate, dimethyl disulfide, and benzaldehyde) were found in all subjects (Supplementary Table 1).
Classification and prediction of fecal metabolite profiles in HC and CD
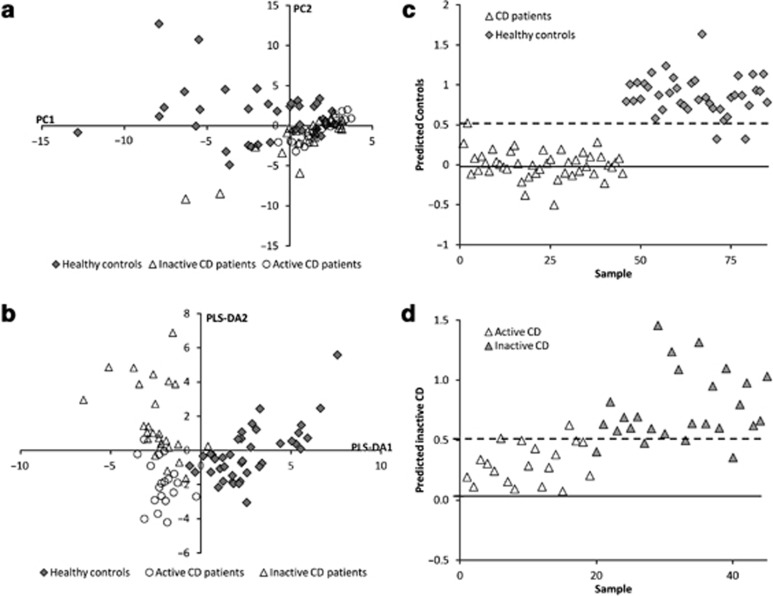
For classification and prediction analysis, the metabolite levels of CD patients were grouped and arranged according to disease activity (HBI≤4 (remission) and HBI>4 (not in remission))25 and compared with the levels of the HCs. In all, 19 patients had mild to moderately active CD, whereas 26 patients were in remission (HBI≤4). Using principal component analysis, complete discrimination between HC, active CD, and inactive CD was not achieved (Figure 2a). By utilizing group information in PLS-DA analysis, systematic differences could be observed between the three groups (Figure 2b).
Figure 2.
(a) Principal component analysis score plot and (b) the corresponding partial least square analysis discriminant analysis (PLS-DA) cross-validated score plot generated from the gas chromatography–time-of-flight–mass spectrometry spectra of fecal samples from healthy controls (HCs) (♦) and Crohn's disease (CD) patients in remission (Harvey-Bradshaw index (HBI)≤4, Δ) and not in remission (HBI>4, ○), and cross-validated prediction from the PLS-DA model for (c) HCs vs. CD patients and (d) CD patients in remission vs. not in remission. In (c–d), the predicted group has a target value of y=1, the other two groups have target value y=0, and the discriminant threshold (y∼0.5) is the dashed line.
For prediction analysis (Figure 2c,d), samples with scores above the dashed line in Figure 2c,d were classified as true positives if they belong to the class indicated on the y axis, but as false positives if they belong to either of the other classes. It is clear that the samples are almost perfectly classified as HC vs. CD samples (prediction rate HC vs. CD: sensitivity=98% and specificity=95%). The prediction performance was less good between the remission CD and mild to moderately active CD samples, indicating that is more difficult to discriminate between a CD profile of a patient in remission or with moderately active disease based on this model (prediction rate HC vs. CD: sensitivity=85% and specificity=89%).
Metabolites discriminating between CD and HCs
After correction for multiple testing, the levels of 40 compounds were significantly different between CD patients and HCs (Table 2). The relative levels of benzeneacetaldehyde, 1-methoxy-4-methylbenzene, phenol, 1-ethyl-3-methylbenzene, 2-methyl-2-propenylbenzene, carbon disulfide, 2-methylpropanal, 3-methyl-2-pentene, 5-methyl-2-(1-methylethyl)-cyclohexanol, furan, hexanal, 2-methyl-2-furancarboxaldehyde, acetone, and hydrogen sulfide were significantly higher in CD patients as compared with HCs, whereas the levels of 26 other compounds were significantly lower in CD patients (Table 2). Remarkably, levels of the medium chain fatty acids (pentanoate, hexanoate, heptanoate, and octanoate) were decreased in CD. Also, the levels of some S-containing compounds (methanethiol, dimethyl sulfide, methyl propyl sulfide, and methyl-2-propenyl disulfide) were lower in CD.
Table 2. Relative levels of VOCs differentially expressed in CD patients and healthy subjects (median (IQR)).
| Metabolite | CD patients | Detected (n/45) | HC | Detected (n/40) | FDR-adjusted P-value |
|---|---|---|---|---|---|
| Benzeneacetaldehyde | 0.004 (0.001–0.006) | 40 | 0.000 (0.000–0.000) | 7 | <0.0001 |
| Dimethyl sulfide | 0.000 (0.000–0.001) | 5 | 0.026 (0.004–0.045) | 31 | <0.0001 |
| 2-Butanone | 0.000 (0.000–0.000) | 10 | 0.325 (0.157–0.472) | 37 | <0.0001 |
| 1-Methoxy-4-methylbenzene | 0.001 (0.000–0.002) | 24 | ND | 0 | <0.0001 |
| 2-Methylpropanal | 0.370 (0.235–0.520) | 45 | 0.098 (0.025–0.289) | 35 | <0.0001 |
| Phenol | 0.003 (0.000–0.018) | 44 | 0.000 (0.000–0.001) | 27 | <0.0001 |
| 3-Methyl-1H-indole | 0.000 (0.000–0.001) | 11 | 0.016 (0.001–0.045) | 33 | <0.0001 |
| 1-Ethyl-2,4-dimethylbenzene | <0.000 | 1 | 0.001 (0.000–0.013) | 19 | <0.0001 |
| Heptanoate | 0.000 (0.000–0.002) | 19 | 0.054 (0.001–0.241) | 35 | <0.0001 |
| 1-Hexanol | 0.000 (0.000–0.000) | 3 | 0.001 (0.000–0.002) | 20 | <0.0001 |
| Hexanoate | 0.009 (0.003–0.057) | 45 | 0.230 (0.030–0.809) | 39 | <0.0001 |
| Methyl propyl disulfide | 0.000 (0.000–0.000) | 6 | 0.001 (0.000–0.003) | 25 | <0.0001 |
| 2,5-Dimethyl furan | 0.000 (0.000–0.000) | 6 | 0.007 (0.000–0.013) | 23 | <0.0001 |
| 2-Pentyl furan | 0.000 (0.000–0.000) | 2 | 0.001 (0.000–0.002) | 18 | <0.0001 |
| Nonyl-cyclopropane | ND | 0 | 0.001 (0.000–0.005) | 14 | <0.0001 |
| 1-Methyl-4-(1-methylethenyl)-benzene | 0.000 (0.000–0.001) | 9 | 0.002 (0.000–0.006) | 22 | <0.001 |
| 1-Ethyl-3-methyl-benzene | 0.026 (0.018–0.043) | 45 | 0.008 (0.001–0.028) | 39 | <0.001 |
| Hexane | 0.000 (0.000–0.000) | 2 | 0.002 (0.000–0.540) | 15 | <0.001 |
| α-Terpinene | ND | 0 | 0.001 (0.000–0.002) | 11 | <0.001 |
| Methyl alcohol | <0.000 | 1 | 0.000 (0.000–0.051) | 13 | 0.001 |
| Octanoate | 0.000 (0.000–0.003) | 17 | 0.004 (0.000–0.042) | 30 | 0.002 |
| 2-Methyl-2-propenyl-benzene | 0.000 (0.000–0.001) | 15 | <0.000 | 1 | 0.002 |
| 5-Methyl-2-(1-methylethyl)-cyclohexanol | 0.000 (0.000–0.005) | 14 | <0.000 | 1 | 0.004 |
| Furan | 0.057 (0.039–0.083) | 44 | 0.033 (0.023–0.053) | 39 | 0.005 |
| 2,2,4-Trimethyl-pentane | ND | 0 | 0.000 (0.000–0.001) | 9 | 0.005 |
| Carbon disulfide | 0.022 (0.011–0.046) | 35 | 0.005 (0.000–0.017) | 28 | 0.005 |
| 2-Methylbutyrate | 0.300 (0.102–0.482) | 44 | 0.656 (0.337–1.189) | 36 | 0.007 |
| Methyl 2-propenyl disulfide | ND | 0 | 0.000 (0.000–0.001) | 8 | 0.009 |
| Methanethiol | 0.000 (0.000–0.000) | 9 | 0.009 (0.000–0.029) | 22 | 0.009 |
| Pentanoate | 0.248 (0.019–0.426) | 45 | 0.427 (0.240–0.606) | 40 | 0.011 |
| 3-Methyl-2-pentene | 0.000 (0.000–0.000) | 9 | ND | 0 | 0.014 |
| α-Methylstyrene | <0.000 | 1 | 0.000 (0.000–0.000) | 9 | 0.018 |
| Hexanal | 0.000 (0.000–0.021) | 23 | 0.000 (0.000–0.001) | 11 | 0.024 |
| 5-Methyl-2-furancarboxaldehyde | 0.003 (0.002–0.005) | 45 | 0.002 (0.000–0.004) | 35 | 0.024 |
| Cyclohexanone | 0.000 (0.000–0.001) | 16 | 0.001 (0.000–0.007) | 20 | 0.027 |
| 6-Methyl-3,5-heptadiene-2-one | <0.000 | 1 | 0.000 (0.000–0.000) | 8 | 0.027 |
| Acetone | 1.062 (0.631–2.031) | 45 | 0.699 (0.572–1.022) | 39 | 0.030 |
| Acetophenone | 0.002 (0.000–0.009) | 30 | 0.007 (0.002–0.018) | 33 | 0.030 |
| Hydrogen sulfide | 0.005 (0.000–0.009) | 27 | 0.000 (0.000–0.002) | 13 | 0.044 |
| 3-Phenyl-2-propenal | 0.000 (0.000–0.000) | 2 | 0.000 (0.000–0.001) | 9 | 0.045 |
Abbreviations: CD, Crohn's disease; FDR, false discovery rate; HC, healthy control; IQR, interquartile range; n, number out of the total number of individuals/group; ND, non-detectable; VOC, volatile organic compound.
Pattern of volatile metabolites in relation to disease activity
After correction for multiple testing, 36 metabolites differed according to disease activity (Table 3). The relative levels of heptanoate were significantly lower in patient with mild to moderately active CD as compared with CD patients with inactive disease. The levels of acetophenone, p-cresol, butyrate, pentanoate, and octanoate were reduced in CD patients in remission, but a statistical significant reduction was only found in mild to moderately active CD as compared with HCs. Significantly higher levels were found in patients with mild to moderately active disease, but not in patients in remission, for 5-methyl-2-(1-methylethyl)-cyclohexanol as compared with HCs.
Table 3. Relative levels of VOCs differentially expressed in CD patients according to disease activity (HBI) and healthy subjects (median (IQR)).
| Metabolite | CD HBI≤4 | Detected (n/26) | CD HBI>4 | Detected (n/19) | HC | Detected (n/40) | FDR-adjusted P-value |
|---|---|---|---|---|---|---|---|
| Benzeneacetaldehyde | 0.005 (0.001–0.006)a | 22 | 0.004 (0.003–0.006)a | 18 | 0.000 (0.000–0.000)a | 7 | <0.0001 |
| Dimethyl sulfide | 0.000 (0.000–0.001)a | 3 | 0.000 (0.000–0.001)a | 2 | 0.026 (0.004–0.045)a | 31 | <0.0001 |
| 2-Butanone | 0.000 (0.000–0.000)a | 7 | 0.000 (0.000–0.000)a | 3 | 0.325 (0.157–0.472)a | 37 | <0.0001 |
| 1-Methoxy-4-methylbenzene | 0.001 (0.000–0.002)a | 16 | 0.000 (0.000–0.001)a | 8 | ND | 0 | <0.0001 |
| Heptanoate | 0.001 (0.000–0.050)a | 16 | 0.000 (0.000–0.000)a | 3 | 0.054 (0.001–0.241)a | 35 | <0.0001 |
| 3-Methyl-1H-indole | 0.000 (0.000–0.004)a | 10 | <0.000a | 1 | 0.016 (0.001–0.045)a | 33 | <0.0001 |
| Phenol | 0.001 (0.000–0.005)a | 26 | 0.014 (0.002–0.040)a | 18 | 0.000 (0.000–0.001)a | 27 | <0.0001 |
| Hexanoate | 0.028 (0.004–0.347)a | 26 | 0.004 (0.001–0.012)a | 19 | 0.230 (0.030–0.809)a | 39 | <0.0001 |
| 2-Methylpropanal | 0.396 (0.242–0.505)a | 26 | 0.303 (0.239–0.516)a | 19 | 0.098 (0.025–0.289)a | 35 | <0.0001 |
| 1-Ethyl-2,4-dimethylbenzene | <0.000a | 1 | ND | 0 | 0.001 (0.000–0.013)a | 19 | <0.0001 |
| 1-Hexanol | <0.000a | 1 | 0.000 (0.000–0.000)a | 2 | 0.001 (0.000–0.002)a | 20 | <0.0001 |
| Methyl propyl disulfide | 0.000 (0.000–0.000)a | 5 | <0.000a | 1 | 0.001 (0.000–0.003)a | 25 | <0.0001 |
| 2,5-Dimethyl furan | 0.000 (0.000–0.000)a | 5 | <0.000a | 1 | 0.007 (0.000–0.013)a | 23 | <0.0001 |
| 2-Pentyl furan | <0.000a | 1 | <0.000a | 1 | 0.001 (0.000–0.002)a | 18 | 0.001 |
| Nonyl-cyclopropane | ND | 0 | ND | 0 | 0.001 (0.000–0.005) | 14 | 0.001 |
| 5-Methyl-2-(1-methylethyl)-cyclohexanol | 0.000 (0.000–0.000)a | 5 | 0.000 (0.000–0.010)a | 9 | <0.000a | 1 | 0.002 |
| Octanoate | 0.000 (0.000–0.004)a | 11 | 0.000 (0.000–0.000)a | 6 | 0.004 (0.000–0.042)a | 30 | 0.003 |
| 1-Methyl-4-(1-methylethenyl)-benzene | 0.000 (0.000–0.000)a | 5 | 0.000 (0.000–0.000)a | 4 | 0.002 (0.000–0.006)a | 22 | 0.003 |
| Hexane | 0.000 (0.000–0.000)a | 2 | ND | 0 | 0.022 (0.000–0.540)a | 15 | 0.003 |
| 1-Ethyl-3-methyl-benzene | 0.027 (0.019–0.047)a | 26 | 0.026 (0.019–0.036)a | 19 | 0.008 (0.001–0.028)a | 39 | 0.003 |
| Furan | 0.065 (0.048–0.085)a | 26 | 0.045 (0.024–0.071)a | 18 | 0.033 (0.023–0.053)a | 39 | 0.004 |
| 2-Methyl-2-propenyl-benzene | 0.000 (0.000–0.000)a | 7 | 0.000 (0.000–0.000)a | 8 | <0.000a | 1 | 0.004 |
| Methyl alcohol | ND | 0 | <0.000a | 1 | 0.000 (0.000–0.051)a | 13 | 0.004 |
| Acetone | 1.562 (0.919–2.771)a | 26 | 0.770 (0.454–1.090)a | 19 | 0.699 (0.572–1.022) | 39 | 0.004 |
| α-Terpinene | ND | 0 | ND | 0 | 0.001 (0.000–0.002) | 11 | 0.005 |
| Pentanoate | 0.267 (0.111–0.500)a | 26 | 0.118 (0.004–0.325)a | 19 | 0.427 (0.240–0.606)a | 40 | 0.012 |
| 3-Methyl-2-pentene | 0.000 (0.000–0.005)a | 7 | 0.000 (0.000–0.000)a | 2 | ND | 0 | 0.014 |
| Carbon disulfide | 0.027 (0.000–0.046)a | 19 | 0.020 (0.013–0.077)a | 16 | 0.005 (0.000–0.017)a | 28 | 0.018 |
| 2,2,4-Trimethyl-pentane | ND | 0 | ND | 0 | 0.000 (0.000–0.001) | 9 | 0.018 |
| Acetophenone | 0.004 (0.000–0.015)a | 19 | 0.001 (0.000–0.003)a | 11 | 0.007 (0.002–0.018)a | 33 | 0.020 |
| 2-Methylbutyrate | 0.255 (0.092–0.454)a | 26 | 0.347 (0.215–0.498)a | 18 | 0.656 (0.337–1.189)a | 36 | 0.023 |
| Methyl 2-propenyl disulfide | ND | 0 | ND | 0 | 0.000 (0.000–0.001) | 8 | 0.033 |
| Methanethiol | 0.000 (0.000–0.000)a | 5 | 0.000 (0.000–0.000)a | 4 | 0.009 (0.000–0.029)a | 22 | 0.035 |
| Butyrate | 0.696 (0.361–0.913)a | 26 | 0.352 (0.126–0.492)a | 19 | 0.721 (0.442–1.084)a | 40 | 0.038 |
| Phenol, 4-methyl- | 0.111 (0.042–0.407)a | 25 | 0.035 (0.001–0.183)a | 17 | 0.212 (0.103–0.367)a | 40 | 0.047 |
| 5-Methyl-2-furancarboxaldehyde | 0.004 (0.002–0.006)a | 26 | 0.003 (0.002–0.004)a | 19 | 0.002 (0.001–0.003)a | 35 | 0.050 |
Abbreviations: CD, Crohn's disease; FDR, false discovery rate; HBI, Harvey-Bradshaw index; IQR, interquartile range; n, number out of the total number of individuals/group; ND, non-detectable; VOC, volatile organic compound.
Different subscript letters indicate groups that differed significantly (FDR-adjusted P-value <0.05).
Effects of OF-IN in CD patients
Assignment of VOCs
A total of 130 different VOCs were identified, with a median of 48 (44–54) VOCs per sample (Supplementary Table 2). All VOCs identified in the PP group are presented in descending order of occurrence in Supplementary Table 3.
Metabolite profile
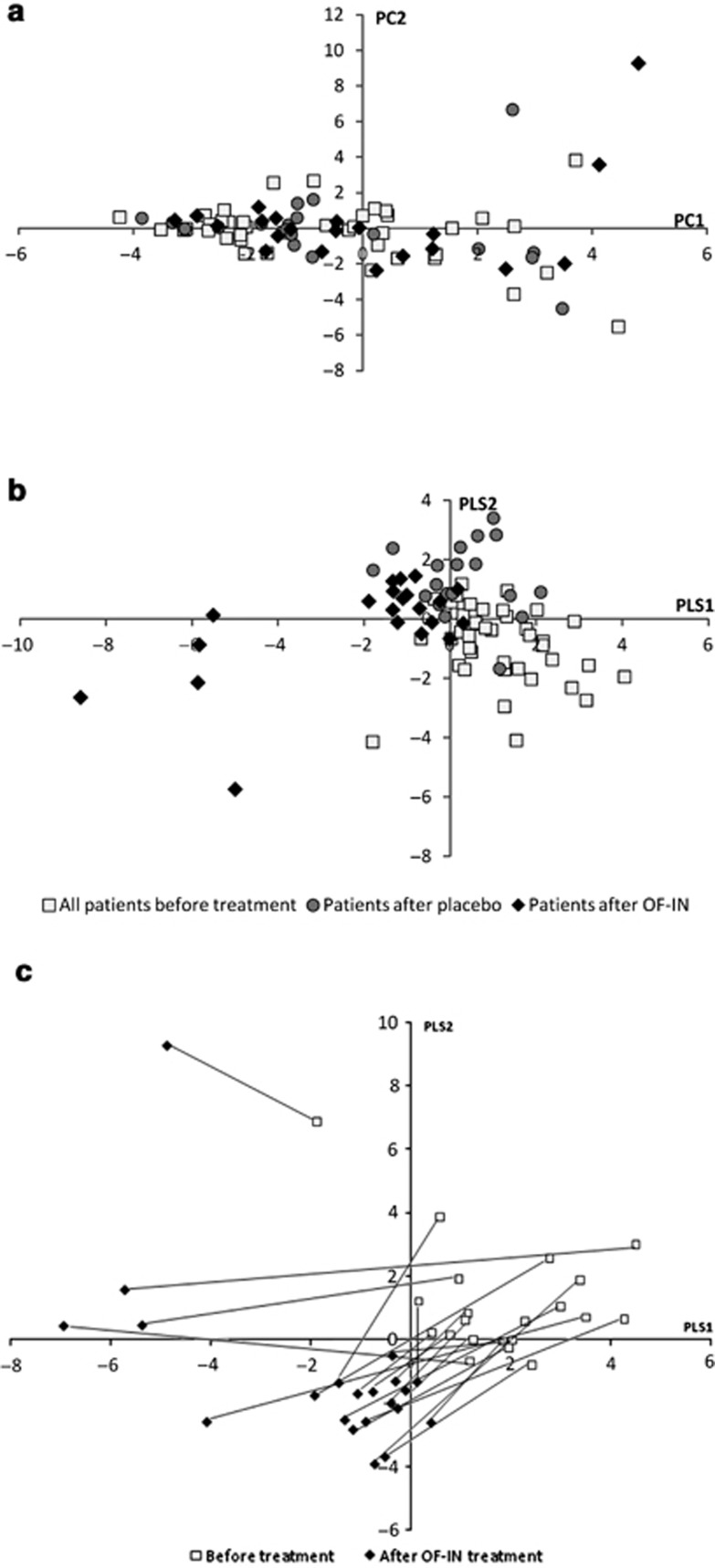
The resulting principal component analysis score plot (Figure 3a) showed no clear differences between the baseline samples, samples after placebo, and samples after OF-IN. By utilizing group information in PLS-DA analysis, samples before and after OF-IN treatment were separated, whereas no clear separation was observed before and after placebo (Figure 3b). PLS-DA pairwise comparison between before and after OF-IN treatment suggested a change in bacterial metabolism.
Figure 3.
(a) Principal component analysis score plot and (b) the corresponding partial least square analysis discriminant analysis (PLS-DA) cross-validated score plot generated from the gas chromatography–time-of-flight–mass spectrometry spectra of fecal samples from Crohn's disease (CD) patients (PP) before treatment (□), after placebo (•), and after prebiotic (prebiotic oligofructose-enriched inulin (OF-IN)) intake (♦). (c) PLS-DA score plot of the pairwise comparison between before and after OF-IN treatment.
Effect of OF-IN vs. placebo on the metabolite pattern
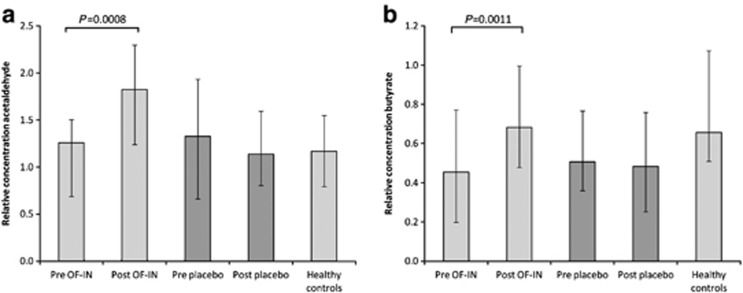
At baseline, fecal concentrations of the VOCs were not different between the placebo and treatment groups (P=NS). Similarly, the metabolite profiles of the patients randomized to the placebo group were not different after the intervention as compared with baseline (P=NS). In contrast, in patients treated with OF-IN, a Wilcoxon test revealed significant differences between pre and post treatment in the metabolite profiles due to significant changes in 9 (ITT) and 11 VOCs (PP; Table 4). After correction for multiple testing, the levels acetaldehyde and butyrate remained significantly increased by OF-IN intake (Figure 4). Interestingly, butyrate levels after OF-IN treatment achieved similar levels as in HCs. Nevertheless, the increase in acetaldehyde or butyrate levels in CD patients after receiving OF-IN was not related to a decrease in HBI (P=0.535 and P=0.445, respectively).
Table 4. Compounds significantly affected by OF-IN intervention as compared with baseline.
| Compound |
P-value |
Effect OF-IN | |
|---|---|---|---|
| ITT | PP | ||
| Acetaldehyde | 0.003 | 0.0008* | Increase |
| Butyrate | 0.003 | 0.0011* | Increase |
| 2-Methyl-2-propenylbenzene | 0.006 | 0.009 | Increase |
| 1-Octanol | NS | 0.017 | Decrease |
| Indole | 0.039 | 0.022 | Increase |
| 2,5-Dimethyl furan | 0.027 | 0.027 | Increase |
| 5-Methyl-2-furancarboxaldehyde | 0.021 | 0.032 | Increase |
| 3,3-Dimethyl-2-butanone | NS | 0.035 | Increase |
| Methyl propyl disulfide | 0.043 | 0.043 | Decrease |
| 2-Butanone | 0.026 | 0.046 | Increase |
| 1-Ethyl-3-methylbenzene | 0.021 | 0.049 | Increase |
Abbreviations: FDR, false discovery rate; NS, not significant; OF-IN, oligofructose-enriched inulin.
*FDR <0.1
Figure 4.
Relative concentration of acetaldehyde (a) and butyrate (b) before and after treatment in Crohn's disease patients (PP), and in healthy controls. The bars represent the median metabolite level and the whiskers represent the interquartile ranges.
Correlation of metabolite levels to microbiota composition
Analysis of the microbiota composition by denaturing gradient gel electrophoresis and real-time PCR of the samples from the intervention study was reported previously.18 In patients receiving OF-IN, a significant increase in the number of B. longum was found (ITT (n=25): P=0.03), whereas R. gnavus decreased after OF-IN intake (PP (n=21): P=0.03). Furthermore, a positive correlation between improvement in disease activity and increase in number of B. longum was found in patients receiving OF-IN (Spearman's ρ=0,894, P=0.02). In patients randomized to placebo, no significant changes in microbiota or clinical activity were found after 4-week intervention.
Correlation of the baseline levels
At baseline, F. prausnitzii counts were positively correlated to the butyrate levels (Spearman's ρ=0.444, P=0.006), whereas acetaldehyde levels were significantly correlated to B. longum counts (Spearman's ρ=0.356, P=0.031). B. adolescentis and R. gnavus counts were not correlated with acetaldehyde and butyrate levels.
Correlation of the changes before and after OF-IN treatment
Changes in acetaldehyde and butyrate levels were not correlated to changes in counts of, respectively, B. longum and F. prausnitzii.
DISCUSSION
In this study, fecal metabolite profiles were analyzed using a GC–mass spectrometry-based analytical platform. In view of the growing evidence that the colonic microbiota is involved in the pathogenesis of IBD, we aimed to characterize the luminal microbial metabolome. As compared with urine samples that rather reflect human metabolism or microbial–human co-metabolism, fecal samples are more appropriate to analyze microbial influences. By choosing a gas chromatography analytical technique, we focussed on the volatile part of the metabolome. Most information on microbial VOC arises from environmental studies that used those compounds as indicators of biocontamination. Since the 1990s, microbial VOCs have been analyzed in indoor air to detect hidden microbial growth and have been related to health risks such as eye and upper respiratory tract irritation.32 However, surprisingly little is known about the intestinal microbial VOC production.
As previously observed in healthy subjects, total acids, benzenoids, aldehydes, heterocyclics, ketones, and S-containing compounds also constituted the predominant chemical classes of the volatile metabolite profiles in CD patients.30 However, as compared with healthy subjects, the fecal samples of CD patients manifested differences in the levels of 40 fecal metabolites. Previously, the depletion of butyrate has been identified as a prominent feature of UC patients when compared with healthy subjects.22 Butyrate is considered as the major energy source for the colonic mucosa. It is oxidized through the fatty acid β-oxidation and tricarboxylic acid cycle pathways in the mitochondria, which provides up to 70% of the energetic needs.33 The effects of butyrate are diverse and complex and involve several distinct mechanisms that go beyond the classical impact as an energy source for colonic epithelial cells. The inhibition of colonic carcinogenesis, inflammation and oxidative stress, and reinforcing various components of the colonic defense barrier are some of the effects butyrate exerts.34 Butyrate promotes the growth of colonocytes in animal models and appears to lower the risk of malignant transformation in the colon. Furthermore, butyrate modulates the transcription of numerous genes through its ability to inhibit histone deacetylase activity. Suppression of nuclear factorκB activation, which may result from the inhibition of histone deacetylase activity, is the most frequently studied anti-inflammatory effect of butyrate.35 In this study, we identified a link between disease activity and the levels of butyrate. Butyrate levels were lower in active CD, which confirms results previously reported in several UC studies.22
Consumption of OF-IN resulted in a stimulation of the saccharolytic fermentation, which was evident from the significantly increased butyrate levels. More importantly, an upregulation of butyrate to the levels of HCs was observed after OF-IN intake. An increased short chain fatty acid production upon prebiotic administration has often been described in animal and in vitro studies, but results of studies in healthy human subjects have been less consistent. Increased fecal short chain fatty acid concentrations were found after consumption of resistant starch, whereas other fermentable carbohydrates did not reveal changes in short chain fatty acid concentrations.36 Interestingly, although a significant correlation was found between baseline butyrate levels and F. prausnitzii, no association was found between butyrate and F. prausnitzii changes in response to OF-IN treatment, which is most likely due to a lack of effect of F. prausnitzii numbers. As demonstrated in several studies, inulin-type fructans are selectively fermented by the colon microbiota, in particular bifidobacteria.14, 15 It is clear that bifidobacteria do not produce butyrate.37 Probably, bacterial cross-feeding mechanisms lay at the basis of butyrate production in the colon. Although cross-feeding between B. longum and F. prausnitzii has been demonstrated in vitro, probably also other butyrate-producing bacteria such as Roseburia spp attribute to the increase in butyrate seen in this study.37
Another indication for increased carbohydrate fermentation after OF-IN supplementation was found in higher levels of acetaldehyde. This compound has previously been described in fecal samples.30, 38 Acetaldehyde production in the colon results from the oxidation of ethanol, a reaction catalyzed by bacterial or colonic mucosal alcohol dehydrogenase or microbial catalase. In a subsequent reaction, acetaldehyde is oxidized by colonic mucosal or bacterial aldehyde dehydrogenase to acetate,39 which levels were not influenced by OF-IN intake in this study. Contrary to our results, Zidi et al.40 showed reduced intraluminal acetaldehyde concentrations in the colon of rats fed lactulose for 14 days and reported this as beneficial effect because it has been shown that acetaldehyde is potentially a local carcinogen in humans. Results from micronucleus assays with acetaldehyde have clearly shown that it is a DNA-damaging agent and produces DNA damage at much lower doses than ethanol.41 In our study, acetaldehyde levels were higher after OF-IN intake than in controls, suggesting a detrimental effect of the prebiotic. However, as acetate levels were slightly lower in CD than in controls, the oxidation reaction of acetaldehyde to acetate by mucosal or bacterial aldehyde dehydrogenase might be impaired in CD. More studies are necessary to evaluate the impact of prebiotics on acetaldehyde levels in the colon in patients and controls.
Furthermore, we specifically observed lower levels of medium chain fatty acids (C5–C8) in CD. The levels of pentanoate, hexanoate, and heptanoate were also disease activity dependent. Like butyrate, the metabolic fate of hexanoate and octanoate is β-oxidation to acetyl-CoA, which is oxidized in the tricarboxylic acid cycle or converted to ketone bodies. The odd chained fatty acids, pentanoate, and heptanoate are converted to propionyl-CoA.42 Jorgensen et al.42 showed that the oxidation rate of C5–C8 fatty acids in rat colonocytes is similar to that of butyrate. However, it is also not known whether these medium chain fatty acids also share nonoxidative cellular effects, such as anti-inflammatory and anti-carcinogenic effects, with butyrate.
Besides a disease activity-dependent reduction in a number of saccharolytic fermentation metabolites, a reduction in protein fermentation in CD was evident from reduced levels of p-cresol and, to a lesser extent, a number of S-containing compounds. p-Cresol is a unique bacterial metabolite of tyrosine, which is not formed by human enzymes. It is absorbed from the colon and excreted in urine after sulfate or glucuronide conjugation in the mucosa or liver. Previously, Williams et al.24 described significantly reduced levels of sulfate conjugated p-cresol in urine of CD patients as compared with HCs. In another study, higher amounts of the amino-acid tyrosine were found in fecal samples of CD patients as compared with HCs, suggesting that the degradation of amino acids is impaired.23 An alteration of the microbiota composition may be responsible for a decreased generation of protein fermentation compounds or an impaired bacterial proteolytic activity.
Remarkably, lower levels of several S-containing compounds, except hydrogen sulfide and carbon disulfide, were found in CD patients as compared with healthy subjects. S-containing compounds typically originate from microbial degradation of sulfur-containing amino acids and, dietary and mucinous sulfate by sulfate-reducing bacteria.43 Higher levels of hydrogen sulfide and possibly also other S-containing compounds have previously been implicated in the etiology and/or risk of relapse of UC.44 Also, a number of other metabolites (benzenoids, furan derivatives, ketones, heterocyclic compounds, and (cyclo)alka/enes) were differently expressed in CD patients as compared with controls. Probably, the depletion or augmentation of these microbiota-related fecal metabolites is a consequence of the disruption of the normal bacterial ecology in patients with CD.45
Tolerance to OF-IN was lower in CD patients as compared with healthy subjects (drop-out rate of 0%46). The drop-out rate due to side effects in patients treated with OF-IN (33%) was more than twice as high as in the placebo group (12%). CD patients appeared more vulnerable than healthy subjects to side effects of the prebiotic, a finding previously also observed by Benjamin et al.19 The use of lower doses of OF-IN in future studies might prevent considerable discontinuation.
Only a limited number of studies evaluated the potential therapeutic efficacy of prebiotics in CD patients. A small open-label trial with fructo-oligosaccharides indicated the potential role for inulin-type prebiotics in the treatment of CD. In total, 10 patients with active ileocolitis received 15 g of fructo-oligosaccharides for 3 weeks after which a significant increase of fecal bifidobacteria and a significant reduction in disease activity were found.45 In contrast, a recent double-blind placebo-controlled trial performed Benjamin et al.19 showed that daily consumption of 15 g fructo-oligosaccharides worsened the clinical signs of active CD. In a study with 10 g of lactulose daily as adjuvant therapy for 4 months in eight active CD patients, no significant improvements were found.47 The findings of these studies indicate differential effects of prebiotic treatment, and further evaluation in larger controlled clinical trials is necessary. Furthermore, none of these studies reported on the impact of prebiotic administration on the metabolite profile in CD.
In conclusion, we demonstrated that a metabolomics approach can be used to distinguish CD patients from control individuals. Furthermore, metabolic profiling of the effect of OF-IN in CD patients rendered several important results. OF-IN has the ability to modulate not only the composition of the intestinal microbiota but also its activity in a beneficial way. The increase of the butyrate concentrations, which exhibits immunomodulatory and anti-inflammatory properties, is encouraging for follow-up studies in CD patients with this prebiotic. Butyrate may not only be therapeutic for UC but also for CD.
Study Highlights
Guarantor of the article: Kristin Verbeke, PhD.
Specific author contributions: VDP: concept and design of the study, recruitment of patients, metabolite profiling, statistical analysis and interpretation of the data, and drafting the article; MJ: concept and design of the study, recruitment of patients, analysis and interpretation of the data, and revising the article; VB: design of the study, recruitment of patients, and revising the article; ZS verified the statistical analysis; PR: concept and design of the study, supervised clinical recruitment, and revising the article; SV: supervised clinical recruitment, conception and design, interpretation of the data, and revising the article; KV: concept and design of the study, interpretation of the data, and revising the article.
Financial support: None.
Potential competing interests: None.
Footnotes
Supplementary Information accompanies this paper on the Clinical and TranslationalGastroenterology website (http://www.nature.com/ctg)
Supplementary Material
References
- Rutgeerts P, Geboes K, Peeters M, et al. Effect of fecal stream diversion on recurrence of crohns-disease in the neoterminal ileum. Lancet. 1991;338:771–774. doi: 10.1016/0140-6736(91)90663-a. [DOI] [PubMed] [Google Scholar]
- Seksik P, Sokol H, Lepage P, et al. Review article: the role of bacteria in onset and perpetuation of inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24:11–18. doi: 10.1111/j.1365-2036.2006.03053.x. [DOI] [PubMed] [Google Scholar]
- Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H, Seksik P, Rigottier-Gois L, et al. Specificities of the fecal microbiota in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:106–111. doi: 10.1097/01.MIB.0000200323.38139.c6. [DOI] [PubMed] [Google Scholar]
- Tamboli CP, Neut C, Desreumaux P, et al. Dysbiosis in inflammatory bowel disease. Gut. 2004;53:1–4. doi: 10.1136/gut.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favier C, Neut C, Mizon C, et al. Fecal beta-D-galactosidase production and Bifidobacteria are decreased in Crohn's disease. Dig Dis Sci. 1997;42:817–822. doi: 10.1023/a:1018876400528. [DOI] [PubMed] [Google Scholar]
- Seksik P, Rigottier-Gois L, Gramet G, et al. Alterations of the dominant faecal bacterial groups in patients with Crohn's disease of the colon. Gut. 2003;52:237–242. doi: 10.1136/gut.52.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidsinski A, Ladhoff A, Pernthaler A, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;122:44–54. doi: 10.1053/gast.2002.30294. [DOI] [PubMed] [Google Scholar]
- Frank DN, Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing B, Halfvarson J, Dicksved J, et al. Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn's disease. Inflamm Bowel Dis. 2009;15:653–660. doi: 10.1002/ibd.20783. [DOI] [PubMed] [Google Scholar]
- Joossens M, Huys G, Cnockaert M, et al. Dysbiosis of the faecal microbiota in patients with Crohn's disease and their unaffected relatives. Gut. 2011;60:631–637. doi: 10.1136/gut.2010.223263. [DOI] [PubMed] [Google Scholar]
- Delzenne NM, Williams CM. Prebiotics and lipid metabolism. Curr Opin Lipidol. 2002;13:61–67. doi: 10.1097/00041433-200202000-00009. [DOI] [PubMed] [Google Scholar]
- Roberfroid MB. Introducing inulin-type fructans. Br J Nutr. 2006;93:S13–S25. doi: 10.1079/bjn20041350. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Probert HM, Van Loo J, et al. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- De Preter V, Vanhoutte T, Huys G, et al. Baseline microbiota activity and influence responses to prebiotic initial bifidobacteria counts dosing in healthy subjects. Aliment Pharmacol Ther. 2008;27:504–513. doi: 10.1111/j.1365-2036.2007.03588.x. [DOI] [PubMed] [Google Scholar]
- Kolida S, Tuohy K, Gibson GR. Prebiotic effects of inulin and oligofructose. Br J Nutr. 2002;87:S193–S197. doi: 10.1079/BJNBJN/2002537. [DOI] [PubMed] [Google Scholar]
- Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Joossens M, De Preter V, Ballet V, et al. Effect of oligofructose-enriched inulin (OF-IN) on bacterial composition and disease activity of patients with Crohn's disease: results from a double-blinded randomised controlled trial. Gut. 2012;61:958. doi: 10.1136/gutjnl-2011-300413. [DOI] [PubMed] [Google Scholar]
- Benjamin JL, Hedin CR, Koutsoumpas A, et al. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn's disease. Gut. 2011;60:923–929. doi: 10.1136/gut.2010.232025. [DOI] [PubMed] [Google Scholar]
- Zoetendal EG, Rajilic-Stojanovic M, de Vos WM. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut. 2008;57:1605–1615. doi: 10.1136/gut.2007.133603. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Lindon JC, Holmes E. ‘Metabonomics': understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29:1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- Marchesi JR, Holmes E, Khan F, et al. Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. J Proteome Res. 2007;6:546–551. doi: 10.1021/pr060470d. [DOI] [PubMed] [Google Scholar]
- Jansson J, Willing B, Lucio M, et al. Metabolomics reveals metabolic biomarkers of Crohn's disease. PLoS One. 2009;4:e6386. doi: 10.1371/journal.pone.0006386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams HR, Cox IJ, Walker DG, et al. Characterization of inflammatory bowel disease with urinary metabolic profiling. Am J Gastroenterol. 2009;104:1435–1444. doi: 10.1038/ajg.2009.175. [DOI] [PubMed] [Google Scholar]
- Vermeire S, Schreiber S, Sandborn WJ, et al. Correlation between the Crohn's disease activity and Harvey-Bradshaw indices in assessing Crohn's disease severity. Clin Gastroenterol Hepatol. 2010;8:357–363. doi: 10.1016/j.cgh.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Meijers BK, De Preter V, Verbeke K, et al. p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transplant. 2009;25:219–224. doi: 10.1093/ndt/gfp414. [DOI] [PubMed] [Google Scholar]
- Jenkins DJA, Kendall CWC, V Vuksan. Inulin oligofructose and intestinal function. J Nutr. 1999;129:1431S–1433SS. doi: 10.1093/jn/129.7.1431S. [DOI] [PubMed] [Google Scholar]
- De Preter V, Van Staeyen G, Esser D, et al. Development of a screening method to determine the pattern of fermentation metabolites in faecal samples using on-line purge-and-trap gas chromatographic-mass spectrometric analysis. J Chromatogr A. 2009;1216:1476–1483. doi: 10.1016/j.chroma.2008.12.095. [DOI] [PubMed] [Google Scholar]
- Joossens M, Huys G, Van Steen K, et al. High-throughput method for comparative analysis of denaturing gradient gel electrophoresis profiles from human fecal samples reveals significant increases in two bifidobacterial species after inulin-type prebiotic intake. FEMS Microbiol Ecol. 2011;75:343–349. doi: 10.1111/j.1574-6941.2010.01008.x. [DOI] [PubMed] [Google Scholar]
- De Preter V, Ghebretinsae AH, Abrahantes JC, et al. Impact of the synbiotic combination of Lactobacillus casei shirota and oligofructose-enriched inulin on the fecal volatile metabolite profile in healthy subjects. Mol Nutr Food Res. 2011;55:714–722. doi: 10.1002/mnfr.201000442. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J Royal Stat Soc S B Methodolo. 1995;57:289–300. [Google Scholar]
- Korpi A, Järnberg J, Pasanen A.Microbial volatile organic compounds (MVOC)In: Marklund S, Christmansson M, Holmberg K et al., (eds).National Institute for Working Life: Stockholm, Sweden; 20071–78. [Google Scholar]
- Roediger WEW. Role of anaerobic-bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21:793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer HM, Jonkers D, Venema K, et al. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- Place RF, Noonan EJ, Giardina C. HDAC inhibition prevents NF-kappa B activation by suppressing proteasome activity: down-regulation of proteasome subunit expression stabilizes I kappa B alpha. Biochem Pharmacol. 2005;70:394–406. doi: 10.1016/j.bcp.2005.04.030. [DOI] [PubMed] [Google Scholar]
- De Preter V, Hamer HM, Windey K, et al. The impact of pre- and/or probiotics on human colonic metabolism: does it affect human health. Mol Nutr Food Res. 2011;55:46–57. doi: 10.1002/mnfr.201000451. [DOI] [PubMed] [Google Scholar]
- Falony G, Verschaeren A, De Bruycker F, et al. In vitro kinetics of prebiotic inulin-type fructan fermentation by butyrate-producing colon bacteria: implementation of online gas chromatography for quantitative analysis of carbon dioxide and hydrogen gas production. Appl Environ Microbiol. 2009;75:5884–5892. doi: 10.1128/AEM.00876-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lacy Costello B, Ewen R, Ewer AK, et al. An analysis of volatiles in the headspace of the faeces of neonates. J Breath Res. 2008;2:037023. doi: 10.1088/1752-7155/2/3/037023. [DOI] [PubMed] [Google Scholar]
- Salaspuro MP. Acetaldehyde, microbes, and cancer of the digestive tract. Crit Rev Clin Lab Sci. 2003;40:183–208. doi: 10.1080/713609333. [DOI] [PubMed] [Google Scholar]
- Zidi SH, Linderborg K, Vakevainen S, et al. Lactulose reduces intracolonic acetaldehyde concentration and ethanol elimination rate in rats. Alcohol Clin Exp Res. 2003;27:1459–1462. doi: 10.1097/01.ALC.0000086061.31875.C8. [DOI] [PubMed] [Google Scholar]
- Kayani MA, Parry JM. The in vitro genotoxicity of ethanol and acetaldehyde. Toxicol In Vitro. 2010;24:56–60. doi: 10.1016/j.tiv.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Jorgensen JR, Clausen MR, Mortensen PB. Oxidation of short and medium chain C2-C8 fatty acids in Sprague-Dawley rat colonocytes. Gut. 1997;40:400–405. doi: 10.1136/gut.40.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GR, Macfarlane GT, Cummings JH. Sulfate reducing bacteria and hydrogen metabolism in the human large-intestine. Gut. 1993;34:437–439. doi: 10.1136/gut.34.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J, Ellis CJ, Furne JK, et al. Fecal hydrogen sulfide production in ulcerative colitis. Am J Gastroenterol. 1998;93:83–87. doi: 10.1111/j.1572-0241.1998.083_c.x. [DOI] [PubMed] [Google Scholar]
- Lindsay JO, Whelan K, Stagg AJ, et al. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn's disease. Gut. 2006;55:348–355. doi: 10.1136/gut.2005.074971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Preter V, Vanhoutte T, Huys G, et al. Effects of Lactobacillus casei Shirota, Bifidobacterium breve, and oligofructose-enriched inulin on colonic nitrogen-protein metabolism in healthy humans. Am J Physiol Gastrointest Liver Physiol. 2007;292:G358–G368. doi: 10.1152/ajpgi.00052.2006. [DOI] [PubMed] [Google Scholar]
- Hafer A, Kramer S, Duncker S, et al. Effect of oral lactulose on clinical and immunohistochemical parameters in patients with inflammatory bowel disease: a pilot study. BMC Gastroenterol. 2007;7:36. doi: 10.1186/1471-230X-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.