Abstract
Purpose
There is a need to develop predictive tests that would allow identifying cancer patients with a high risk of developing side effects to radiotherapy. We compared the predictive value of three functional assays: the G0 aberration assay, the G2 aberration assay and the alkaline comet assay in lymphocytes of breast cancer and gynaecological cancer patients.
Material and methods
Peripheral blood was collected from 35 patients with breast cancer and 34 patients with gynaecological cancer before the onset of therapy. Chromosomal aberrations were scored in lymphocytes irradiated in the G0 or G2 phase of the cell cycle. DNA repair kinetics was performed with the alkaline comet assay following irradiation of unstimulated lymphocytes. The results were compared with the severity of early and late side effects to radiotherapy.
Results
No correlation was observed between the results of the assays and the severity of side effects. Moreover, each assay identified different patients as radiosensitive.
Conclusions
There is no simple correlation between the in vitro sensitivity of lymphocytes and the risk of developing early and late side effects.
Keywords: breast cancer, gyneacological cancer, comet assay, HDR brachytherapy, predictive testing, normal tissue response
Purpose
Radiotherapy patients show different degrees of adverse side effects and it is assumed that the risk of developing side effects is related to the genetically determined, intrinsic sensitivity of the patient [1–3]. From the perspective of the radiation protection of the patient it would be desirable to develop tests predicting the individual risk of developing side effects. In the 1990s numerous studies have been performed with the aim of finding a genetic fingerprint of intrinsic radiosensitivity (reviewed in [2]). More recently it was realised that the genetics behind the intrinsic radiosensitivity is very complex and its characterisation requires a systems biology approach rather than the search for genetic changes in a few loci [4].
An alternative approach in the search for a marker of individual radiosensitivity is a functional assay. Here, surrogate normal tissue – most often peripheral blood lymphocytes (PBL) – is drawn from a patient and the in vitro radiosensitivity is determined by assays that allow quantifying the level of DNA damage or the capacity of DNA repair. Notwithstanding some promising results [5], the bulk of data is controversial and the reasons for the inconsistency are not understood [6–8]. Also, it is clear that a single assay will not have a satisfactory predictive value [9]. But will combining different assays solve the problem? Do different functional assays complement each other?
The present study was undertaken in order to compare three functional assays: the G0 aberration assay, the G2 aberration assay and the alkaline comet assay. The assays were compared using PBL of patients treated by external beam radiotherapy for breast cancer and gynaecological cancers. PBL were collected before radiotherapy, exposed in vitro to a single dose of radiation and assessed for the in vitro sensitivity. The results were correlated with early and late side effects. Although the in vitro sensitivity of PBL showed marked inter-individual variabilities, no correlations were detected, neither between the assays nor with the extent of side effects.
Material and methods
Cancer patients
Breast cancer (BC) patients: the group consisted of 35 patients with breast cancer staged IIA to IIIB (median age 57). All patients underwent radical mastectomy and chemotherapy prior to radiotherapy. Conformal radiotherapy with a 3D planning system was performed using the modified inverse hockey stick technique [10].
Gynaecological cancer (GC) patients: the group consisted of 34 patients with cancer of endometrium (18 patients) and cervix (16 patients) of different stages (median age 63). All patients with endometrial cancer underwent surgery and were subsequently treated by external beam radiotherapy and high dose rate (HDR) brachytherapy without chemotherapy. Patients with cervix cancer received different forms of therapy: 5 patients underwent surgery, 12 received chemotherapy and all were treated by external beam radiotherapy plus HDR brachytherapy. The radiotherapy of gynaecological cancers was based on a 2D planning system. The patients were treated with the isocentric 4 field box technique.
The study was approved by the local ethical committee.
Lymphocyte collection and in vitro irradiation
All blood samples were collected in the Holy Cross Cancer Centre from patients before the onset of radiotherapy, immediately aliquoted in 1.5 ml Eppendorf tubes and cooled in ice water (0.8°C) in order to maintain reproducible irradiation conditions. All irradiations were carried out at the Holy Cross Cancer Centre using 6 MeV photons generated by a Siemens therapeutic linear accelerator. Blood samples were always irradiated at a distance of 80 cm from the source in a cold paraffin bolus to achieve electron equilibrium. A blood sample from one donor was always processed for all three assays.
Blood samples for the G0 assay and comet assay were irradiated with 2 Gy of photons shortly after collection and transported in an ice box back to the laboratory (located in a different part of the town) where they was immediately processed for the assays as described below. Blood for the G2 assay was not irradiated, but transported to the laboratory in an ice box and cultured as described below. After 69 hours cultures were cooled 0.8°C (melting ice), transported in an ice box to the Holy Cross Cancer Centre and irradiated with a dose of 1 Gy. Cooled cultures were transported back to the laboratory, placed back into the incubator and processed for the chromosomal aberration G2 assay as described below.
Chromosomal aberration assay
0.5 ml of whole blood was added to 4.5 ml of complete culture medium as described in [11]. 5-bromo-2-deoxyuridine (BrdU) was used only for the G0 aberration assay. Three test tubes were set up from each patient: one with blood irradiated for the G0 aberration assay, one with blood to be irradiated for the G2 aberration assay and one with unexposed blood. Test tubes were incubated at 37°C (5% CO2, 95% humidity). For the G0 aberration assay colcemid (Gibco 15212-046, 0.08 µg/ml) was added after 45 hours of culture time and cells were harvested 3 hours later. For the G2 aberration assay colcemid was added one hour after placing the culture back into the incubator (corresponding to about 70th hour of culture time) and cells were harvested 2 hours later.
Cells were harvested and fixed as described in [11]. Slides for the G0 aberration assay were stained with the fluorescence plus Giemsa (FPG) technique that allows to identify cells in the first postirradiation division [12]. Slides for the G2 assay were stained for 10 minutes with a 10% Giemsa solution. No cell proliferation control was needed because of the short time between exposure and harvest.
For metaphase analysis the slides were coded and the aberrations scored randomly in 100 mitoses. All aberrations (G0 assay: mainly dicentrics, rings and acentric fragments; G2 assay: mainly acentric fragments) were scored and expressed as sum of aberrations. Gaps (chromosome arm discontinuities that are shorter than the width of the chromosome arm) were not included in the analysis. Spontaneous aberrations were scored on slides harvested after 72 hours of culture.
Comet assay
Comet assay was performed according to the method of Singh et al. [13] and as described in detail in [14]. 50 cells per point were analysed for the Olive tail moment (OTM) using the CASP software [14]. Repair capacity was assessed by two ways. 1: the level of damage after 0 min repair was considered as 100% and the residual damage after specific times of repair were expressed as percent of the initial level. Each repair kinetic curve was used to calculate the area under the curve (AUC) by fitting the curve to the exponential decay function y = ae-bx with the help of SigmaPlot (Systat Software Inc., USA®). AUC was calculated with the help of GraphCalc (http://www.graphcalc.com). 2: For each repair time point an OTM value was classified as high (being in the top 33.3% of values), intermediate (middle 33.3% of values) and low (bottom 33.3% of values) with respect to pooled values from all patients (per patient group). This approach allowed constructing cluster heat maps, similarly as used for analysis of gene expression patterns [15].
Clinical classification of normal tissue reactions
Acute and late normal tissue reactions to radiotherapy were scored using the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer (EORTC/RTOG) grading system. Early reactions were scored during the treatment or immediately thereafter. Late reactions were scored periodically, starting six months post treatment. A lack of reaction was classified as grade 0, a mild reactions as grade 1, a moderate reaction as grade 2 and severe reaction as grade 3 or 4. For breast cancer patients reactions of the skin, larynx, lung and heart were recorded. For gynaecological patients reactions were scored in skin, colon, bladder and the peripheral blood (leucopoenia).
Statistical methods
The Mann-Whitney Rank Sum Test was used to compare the frequencies of aberrations and the AUC values between groups of patients. Relationships between the data were analysed by the Pearson product moment correlation. Relation between the aberration frequencies and the characteristics of the DNA repair kinetics were analysed by constructing cluster heat maps [15].
Results
Chromosomal aberrations
Spontaneous aberrations were all of chromatid type and the mean aberrations frequency was for each group was 1.5. The mean G0 aberration frequency in lymphocytes of BC patients was 40.7 and in lymphocytes of GC patients 32.4. This difference is statistically significant. The mean G2 aberrations frequencies were 37.5 for the BC patients and 30.6 for GC patients, the difference not being significant.
Comet assay
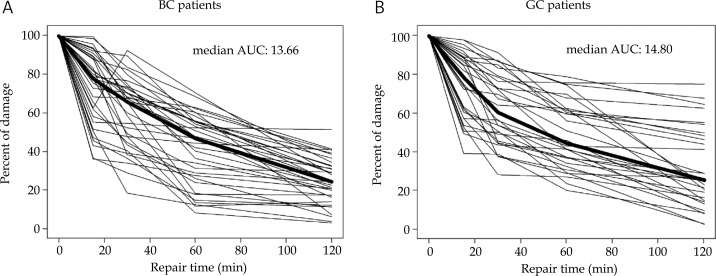
The results of the comet assay are shown as individual repair kinetic curves in Fig. 1. The median AUC values were 13.66 for BC patients and 14.80 for GC patients. This difference is not statistically significant. From the curves shown in Fig. 1 it is clear that the level of residual damage showed a stronger individual heterogeneity in lymphocytes of GC patients than in lymphocytes of BC patients.
Fig. 1.
Individual repair kinetic curves obtained with the comet assay. A) Breast cancer patients, B) gynaecological cancer patients. AUC: area under curve. Thick line represents the median curve
Correlation between aberrations and comet assay
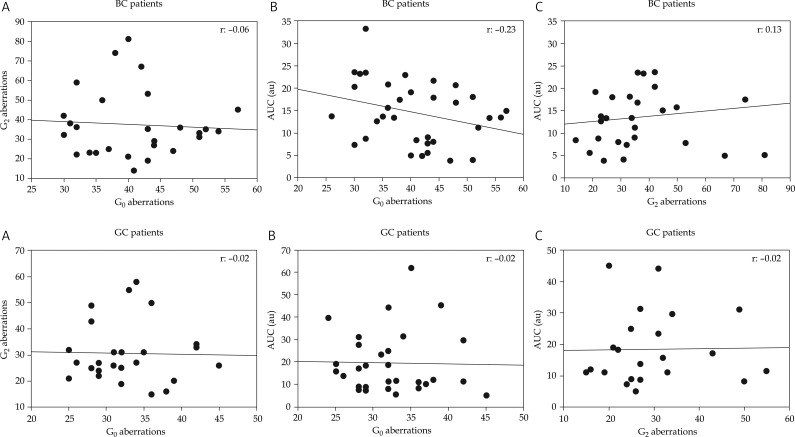
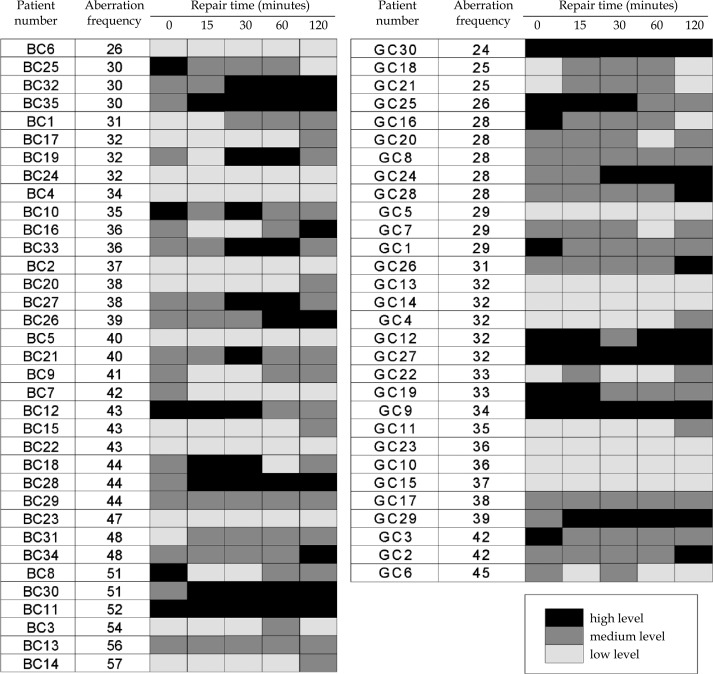
The correlation analyses between the different endpoints are shown in Fig. 2. No correlations were observed between G0 and G2 aberrations (panels A), G0 aberrations and AUC (panels B) and G2 aberrations and AUC (panels C). In order to analyse in more detail the relationship between aberrations and the kinetics of DNA repair we constructed cluster heat maps where the OTM values per repair time point and patient group were stratified into three groups: high, medium and low. A grey-scale hue was assigned to each level group. The alignments of grey scale patterns and sorted G0 aberration frequencies are shown in Fig. 3. If a given level of chromosomal aberrations correlated with a particular pattern of grey scale hues, this would be optically discernible. However, neither for the BC nor for the GC patients could a pattern be identified. The same situation was seen for G2 aberrations (not shown).
Fig. 2.
Correlations between the frequencies of G0 aberrations, G2 aberrations and AUC values. Top panels: breast cancer patients, bottom panels: gynaecological cancer patients
Fig. 3.
Relationship between the G0 aberration frequency in lymphocytes of breast cancer patients (left panel) and gynaecological cancer patients (right panel) and the kinetic of DNA repair measured with the comet assay. The grey hue is indicative of a high, medium or low value of OTM (Olive tail moment) with respect to pooled values per patient group and repair time point. Patients are sorted according to increasing aberration frequency
Correlation between aberrations, comet assay and side effects of therapy
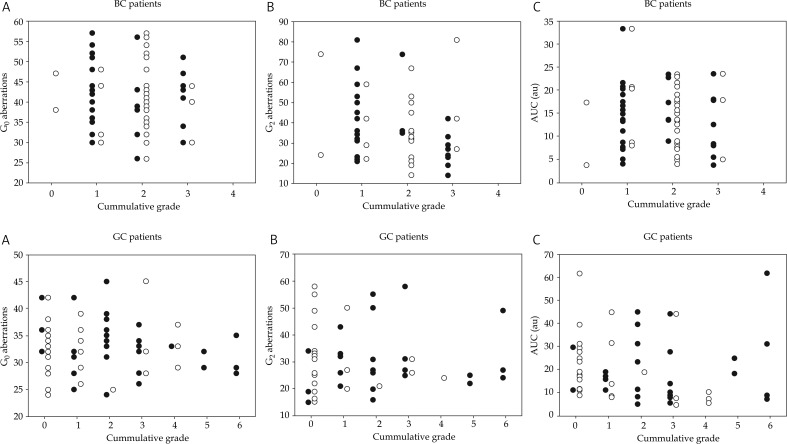
The levels of chromosomal aberrations and the AUC values were plotted as function of the degree of severity of side effects. Side effects observed in different tissues were pooled and expressed as cumulative grades. The results are presented in Fig. 4 for early and late effects and both patient groups. Due to a larger number of tissues/organs at risk, the values of the cumulative grades were higher for GC patients as compared to BC patients. The severity of neither early not late effects correlated with the frequency of the aberrations or the AUC.
Fig. 4.
Relationships between radiation-induced aberration frequencies plus AUC values and early (black symbols) and late (open symbols) reactions to radiotherapy, expressed as cumulative grades. Top panels: breast cancer patients, bottom panels: gynaecological cancer patients. Panels A: G0 aberrations, panels B: G2 aberrations, panels C: AUC
Discussion
The search for predictive tests for acute and late reactions to radiotherapy is based on the assumption that the major factor associated with a high risk of developing side effects is the genetically determined capacity to detect and repair DNA damage [1]. Although methods are now available to directly analyse genes and proteins involved in biological responses to radiation [16, 17], we chose to use three functional assays in PBL of patients because they integrate the influence of many genes that potentially play a role in the cellular radiation sensitivity.
We did not detect any relationship between the severity of acute and late effects and the in vitro sensitivity of PBL as assessed by the G0 aberration test, the G2 aberration test and the alkaline comet assay. This negative result is not totally unexpected in view of the conflicting results published by others. A number of groups have attempted to find a correlation between the chromosomal radiosensitivity of PBL and the reactions of healthy tissues to radiotherapy (reviewed in [6, 8]). A positive relationship was reported in the case of patients with cervix cancer [18–20], head and neck cancer [21], breast cancer [22] and prostate cancer [23]. In contrast, Slonina et al. [24] and Lisowska et al. [11] did not observe any correlation between the frequency of radiation-induced cytogenetic changes in PBL and of normal tissue reactions in patients with head and neck cancer. Similarly negative results were published for breast cancer [25] and prostate cancer [7]. The reasons for the conflicting results are not clear.
The application of the alkaline comet assay as a predictive test yielded similarly disagreeing results. Müller et al. [5] observed a delayed repair of DNA damage in PBL of cancer patients with various tumour types that developed severe side effects to radiotherapy. Similar results were published by Gabelova et al. [26] for cervical cancer and Alapetite et al. [27] for breast cancer. In contrast, Djuzenova et al. [28] did not find an impaired level of initial DNA damage, residual DNA damage and repair kinetics in PBL of breast cancer patients that developed severe side effects. This result is in accordance with our data. It is clear that the comet assay still requires validation as a test to detect individual radiosensitivity.
Our study not only showed a lack of correlation between the in vitro sensitivity of PBL and the severity of side effects, but also between the in vitro assays. That the results of the G0 and the G2 aberration assays do not correlate may not be surprising in view of the fact that different DNA double strand break repair pathways dominate during the G0 and G2 phases of the cell cycle [29]. A more intriguing result appears to be the lack of relationship between the G0 aberrations and the comet assay, since both assays were performed on unstimulated lymphocytes. The relationship was analysed by comparing the frequencies of aberrations with the AUC values. Because no correlation was observed, we performed a more detailed comparison by aligning the aberration frequencies against grey-scale hue diagrams that characterise the level of DNA damage following 0, 15, 30, 60 and 180 minutes of repair. This approach, called a cluster heat map was introduced by [15] to detect possible patterns in complex data sets. The rational for applying the heat map approach here was to test the possibility that an association exists between the aberrations frequency and a particular time point of DNA repair kinetics, measured by the comet assay. Such association would be visible by clustering of a hue in a heat map aligned according to increasing aberration frequency. However, no such association could be detected. An explanation of the lack of correlation between the frequency of aberrations and the comet assay results may be that, as suggested by Speit et al. [30], an enhanced frequency of cytogenetic damage in PBL results from an impaired fidelity of DNA repair rather than the kinetics of damage removal. Changes in the fidelity of DNA repair cannot be visualised by the comet assay.
In conclusion, our study failed to reveal a relationship between the risk of developing side effects to radiotherapy and the in vitro sensitivity of PBL as assessed by three functional assays. Each in vitro test identified different donors as radiosensitive. This could be either due to a different specificity of a test with respect to the DNA damage processing pathway or to other factors such as the physiological state of the blood donor or random experimental fluctuations. Thus, the usefulness of the functional tests for predicting healthy tissue reactions requires further investigation.
Acknowledgement
The work was supported by the Polish Ministry for Education and Science, Project KBN 3P05D 029 25.
References
- 1.Alsner J, Andreassen CN, Overgaard J. Genetic markers for prediction of normal tissue toxicity after radiotherapy. Semin Radiat Oncol. 2008;18:126–135. doi: 10.1016/j.semradonc.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Andreassen CN, Alsner J. Genetic variants and normal tissue toxicity after radiotherapy: a systematic review. Radiother Oncol. 2009;92:299–309. doi: 10.1016/j.radonc.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 3.Bentzen SM, Overgaard J. Patient-to-Patient Variability in the Expression of Radiation-Induced Normal Tissue Injury. Semin Radiat Oncol. 1994;4:68–80. doi: 10.1053/SRAO00400068. [DOI] [PubMed] [Google Scholar]
- 4.Andreassen CN, Alsner J, Overgaard J. Does variability in normal tissue reactions after radiotherapy have a genetic basis-where and how to look for it? Radiother Oncol. 2002;64:131–140. doi: 10.1016/s0167-8140(02)00154-8. [DOI] [PubMed] [Google Scholar]
- 5.Müller W-U, Bauch T, Stüben G, et al. Radiation sensitivity of lymphocytes from healthy individuals and cancer patients as measured by the comet assay. Radiat Environ Biophys. 2001;40:83–89. doi: 10.1007/s004110000087. [DOI] [PubMed] [Google Scholar]
- 6.Borgmann K, Hoeller U, Nowack S, et al. Individual radiosensitivity measured with lymphocytes may predict the risk of acute reaction after radiotherapy. Int J Radiat Oncol Biol Phys. 2008;71:256–264. doi: 10.1016/j.ijrobp.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Brzozowska K, Pinkawa M, Eble MJ, et al. In vivo versus in vitro individual radiosensitivity analyzed in healthy donors and in prostate cancer patients with and without severe side effects after radiotherapy. Int J Radiat Oncol Biol Phys. 2012;88:405–413. doi: 10.3109/09553002.2012.666002. [DOI] [PubMed] [Google Scholar]
- 8.Dikomey E, Borgmann K, Peacock J, et al. Why recent studies relating normal tissue response to individual radiosensitivity might have failed and how new studies should be performed. Int J Radiat Oncol Biol Phys. 2003;56:1194–1200. doi: 10.1016/s0360-3016(03)00188-3. [DOI] [PubMed] [Google Scholar]
- 9.Crompton NE, Ozsahin M, Schweizer P, et al. Theory and practice of predictive assays in radiation therapy. Strahlenther Onkol. 1997;173:58–67. doi: 10.1007/BF03038924. [DOI] [PubMed] [Google Scholar]
- 10.Kukolowicz P, Wieczorek A, Selerski B, et al. The modified inverse hockey stick technique for adjuvant irradiation after mastectomy. Nowotwory Journal of Oncology. 2004;54:481–487. [in Polish] [Google Scholar]
- 11.Lisowska H, Lankoff A, Wieczorek A, et al. Enhanced chromosomal radiosensitivity in peripheral blood lymphocytes of larynx cancer patients. Int J Radiat Oncol Biol Phys. 2006;66:1245–1252. doi: 10.1016/j.ijrobp.2006.07.1370. [DOI] [PubMed] [Google Scholar]
- 12.Goto K, Maeda S, Kano Y, et al. Factors involved in differential giemsa-staining of sister chromatids. Chromosoma. 1978;66:351–359. doi: 10.1007/BF00328535. [DOI] [PubMed] [Google Scholar]
- 13.Singh NP, Tice RR, Stephensen RE, et al. A microgel electrophoresis technique for the direct quantitation of DNA damage and repair in individual fibroblasts cultured on microscope slides. Mutat Res. 1991;252:289–296. doi: 10.1016/0165-1161(91)90008-v. [DOI] [PubMed] [Google Scholar]
- 14.Konca K, Lankoff A, Banasik A, et al. A cross platform public domain PC image analysis program for the comet assay. Mutat Res. 2003;534:15–20. doi: 10.1016/s1383-5718(02)00251-6. [DOI] [PubMed] [Google Scholar]
- 15.Eisen MB, Spellman PT, Brown PO, et al. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nuyten DSA, van de Vijer MJ. Using microarray analysis as a prognostic and predictive tool in oncology: focus on breast cancer and normal tissue toxicity. Sem Radiat Oncol. 2008;18:105–114. doi: 10.1016/j.semradonc.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Rodningen OK, Overgaard J, Alsner J, et al. Microarray analysis of the transcriptional response to single or multiple doses of ionizing radiation in human subcutaneous fibroblasts. Radiother Oncol. 2005;77:231–240. doi: 10.1016/j.radonc.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 18.De Ruyck K, Van Eijkeren M, Claes K, et al. Radiation-induced damage to normal tissues after radiotherapy in patients treated for gynecologic tumors: association with single nucleotide polymorphisms in XRCC1, XRCC3, and OGG1 genes and in vitro chromosomal radiosensitivity in lymphocytes. Int J Radiat Oncol Biol Phys. 2005;62:1140–1149. doi: 10.1016/j.ijrobp.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 19.Widel M, Jedrus S, Lukaszczyk B, et al. Radiation-induced micronucleus frequency in peripheral blood lymphocytes is correlated with normal tissue damage in patients with cervical carcinoma undergoing radiotherapy. Radiat Res. 2003;159:713–721. doi: 10.1667/0033-7587(2003)159[0713:rmfipb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Widel M, Kolosza Z, Jedrus S, et al. Micronucleus assay in vivo provides significant prognostic information in human cervical carcinoma; the updated analysis. Int J Radiat Oncol Biol Phys. 2001;77:631–636. doi: 10.1080/09553000110035558. [DOI] [PubMed] [Google Scholar]
- 21.Borgmann K, Roper B, El Awady R, et al. Indicators of late normal tissue response after radiotherapy for head and neck cancer: fibroblasts, lymphocytes, genetics, DNA repair, and chromosome aberrations. Radiother Oncol. 2002;64:141–152. doi: 10.1016/s0167-8140(02)00167-6. [DOI] [PubMed] [Google Scholar]
- 22.Hoeller U, Borgmann K, Bonacker M, et al. Individual radiosensitivity measured with lymphocytes may be used to predict the risk of fibrosis after radiotherapy for breast cancer. Radiother Oncol. 2003;69:137–144. doi: 10.1016/j.radonc.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Lee TK, Allison RR, O'Brien KF, et al. Lymphocyte radiosensitivity correlated with pelvic radiotherapy morbidity. Int J Radiat Oncol Biol Phys. 2003;57:222–229. doi: 10.1016/s0360-3016(03)00411-5. [DOI] [PubMed] [Google Scholar]
- 24.Slonina D, Klimek M, Szpytma T, et al. Comparison of the radiosensitivity of normal-tissue cells with normal-tissue reactions after radiotherapy. Int J Radiat Oncol Biol Phys. 2000;76:1255–1264. doi: 10.1080/09553000050134483. [DOI] [PubMed] [Google Scholar]
- 25.Barber JB, Burril W, Spreadburrough AR, et al. Relationship between in vitro chromosomal radiosensitivity of peripheral blood lymphocytes and the expression of normal tissue damage following radiotherapy for breast cancer. Radiother Oncol. 2000;55:93–94. doi: 10.1016/s0167-8140(99)00158-9. [DOI] [PubMed] [Google Scholar]
- 26.Gabelova A, Farkasova T, Gurska S, et al. Radiosensitivity of peripheral blood lymphocytes from healthy donors and cervical cancer patients; the correspondence of in vitro data with the clinical outcome. Neoplasma. 2008;55:182–191. [PubMed] [Google Scholar]
- 27.Alapetite C, Thirion P, de la RA, Cosset JM, et al. Analysis by alkaline comet assay of cancer patients with severe reactions to radiotherapy: defective rejoining of radioinduced DNA strand breaks in lymphocytes of breast cancer patients. Int J Cancer. 1999;83:83–90. doi: 10.1002/(sici)1097-0215(19990924)83:1<83::aid-ijc16>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 28.Djuzenova CS, Muhl B, Fehn M, et al. Radiosensitivity in breast cancer assessed by the Comet and micronucleus assays. Br J Cancer. 2006;94:1194–1203. doi: 10.1038/sj.bjc.6603005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothkamm K, Kruger I, Thompson LH, et al. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol Cell Biol. 2003;23:5706–5715. doi: 10.1128/MCB.23.16.5706-5715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Speit G, Trenz K, Schutz P, et al. Mutagen sensitivity of human lymphoblastoid cells with a BRCA1 mutation in comparison to ataxia telangiectasia heterozygote cells. Cytogenet Cell Genet. 2000;91:261–266. doi: 10.1159/000056855. [DOI] [PubMed] [Google Scholar]