Abstract
Aims
Current knowledge on the prognosis of metabolically healthy but obese phenotype is limited due to the exclusive use of the body mass index to define obesity and the lack of information on cardiorespiratory fitness. We aimed to test the following hypotheses: (i) metabolically healthy but obese individuals have a higher fitness level than their metabolically abnormal and obese peers; (ii) after accounting for fitness, metabolically healthy but obese phenotype is a benign condition, in terms of cardiovascular disease and mortality.
Methods and results
Fitness was assessed by a maximal exercise test on a treadmill and body fat per cent (BF%) by hydrostatic weighing or skinfolds (obesity = BF% ≥25 or ≥30%, men or women, respectively) in 43 265 adults (24.3% women). Metabolically healthy was considered if meeting 0 or 1 of the criteria for metabolic syndrome. Metabolically healthy but obese participants (46% of the obese subsample) had a better fitness than metabolically abnormal obese participants (P < 0.001). When adjusting for fitness and other confounders, metabolically healthy but obese individuals had lower risk (30–50%, estimated by hazard ratios) of all-cause mortality, non-fatal and fatal cardiovascular disease, and cancer mortality than their metabolically unhealthy obese peers; while no significant differences were observed between metabolically healthy but obese and metabolically healthy normal-fat participants.
Conclusions
(i) Higher fitness should be considered a characteristic of metabolically healthy but obese phenotype. (ii) Once fitness is accounted for, the metabolically healthy but obese phenotype is a benign condition, with a better prognosis for mortality and morbidity than metabolically abnormal obese individuals.
Keywords: Cardiovascular diseases, Heart diseases, Metabolic syndrome, Mortality, Obesity, Physical fitness
See page 330 for the editorial comment on this article (doi:10.1093/eurheartj/ehs237)
Introduction
Obesity is a major public health concern in most developed and developing countries. It is well known that obesity is related to a large number of chronic diseases and metabolic abnormalities. However, a subset of obese people who seem to be protected against obesity-related metabolic complications has been identified.1 These individuals are described as metabolically healthy but obese, having uncomplicated obesity, or having metabolically benign obesity.2 This concept should not be mixed with a different concept and opposite health condition, the so-called metabolically obese but normal weight, which is defined as a subset of individuals who are not obese on the basis of height and weight, but who, like people with overt obesity, are hyperinsulinaemic, insulin resistant, and predisposed to type 2 diabetes, hypertriglyceridaemia, and premature coronary heart disease.3 This study is focused on the metabolically healthy but obese phenotype.
Primeau et al.4 have recently reviewed the existing literature and reported a number of characteristics of the metabolically healthy but obese phenotype, including lower visceral fat accumulation, higher birth weight, adipose cell size, and gene expression-encoding markers of adipose cell differentiation. However, the authors did not consider that differences in the metabolic profile of obese individuals might be partially explained by differences in cardiorespiratory fitness (hereinafter fitness). There is strong evidence indicating that higher fitness levels are associated with fewer metabolic complications and lower prevalence of metabolic syndrome at any age and across different weight status groups.4–9 Further research is needed to understand whether higher fitness is a common characteristic in metabolically healthy but obese individuals.
The extent to which metabolically healthy but obese people are at a lower risk for diseases or have a lower risk for mortality, compared with the rest of obese people, is currently under debate. While some studies observed a low risk for cardiovascular disease (CVD) incidence and mortality in this group of people (similar to that observed in metabolically healthy normal-weight people),10–14 others suggested that obesity per se rather than the metabolic profile increased the risk of morbidity and mortality.15,16 In this study, we refer to CVD in broad terms not meaning only atherosclerotic outcomes.
Obesity is internationally defined based on the body mass index (BMI); however, BMI is criticized for its lack of sensitivity to distinguish between the fat and lean mass. A recent meta-analysis has shown that BMI failed to identify half of the people with excess body fat per cent (BF%).17 To better understand the metabolically healthy but obese phenotype and its prognosis, further research using more accurate measures of adiposity, e.g. BF% derived from skinfold thicknesses and/or hydrostatic weighing, is warranted. To the best of our knowledge, the long-term effect of the metabolically healthy but obese phenotype on mortality has not been examined using BF% to define obesity. In addition, only one previous study conducted in men by our group has considered fitness, a strong and well-known predictor of mortality,18 as a potential confounder in the analyses of obesity and health outcomes.19 The present study aimed to add to our previous study by: (i) including the measure of BF% in addition to BMI, (ii) doubling the sample size for obese people, which will allow us to have higher statistical power after dividing the sample into metabolically healthy vs. metabolically unhealthy obese; (iii) adding women to the study; (iv) increasing the follow-up period; (v) adding non-fatal CVD major events, in addition to CVD mortality, as outcomes; and (vi) adding cancer mortality as an outcome.
The present study aimed to test the following hypotheses: (i) metabolically healthy but obese individuals have a higher fitness level than their metabolically abnormal and obese peers; (ii) if the metabolically healthy but obese phenotype is a benign condition, these people should have lower risk of CVD and mortality compared with the ‘theoretically unhealthiest group’, i.e. metabolically abnormal and obese individuals, and a similar risk as the ‘theoretically healthiest group’, i.e. metabolically healthy and normal-weight individuals. We examined these hypotheses after controlling for relevant confounders, including fitness, and using both BMI and BF% to define obesity.
Methods
Study cohort
The Aerobics Center Longitudinal Study (ACLS) is a prospective epidemiological investigation of adult men and women.20–22 The ACLS participants are mostly Caucasian (98%), well educated, and worked in executive or professional positions.23 All participants completed a detailed questionnaire and underwent an extensive clinical evaluation, including a physical examination, fasting blood chemistry analyses, personal and family health history, body composition, smoking and alcohol use, and a maximal exercise treadmill test between 1979 and 2003. All participants provided written informed consent, and the study protocol was approved annually by the Institutional Review Board of the Cooper Institute.
Inclusion criteria for the present analysis were: (i) no existing CVD or cancer at baseline; (ii) achieving 85% or more of the individual's age-predicted maximal heart rate during the graded modified Balke treadmill exercise testing (considered a maximal test); (iii) BMI ≥18.5 kg/m2, (iv) ≥1 year of follow-up; (v) complete data on BMI, BF%, fitness, metabolic syndrome traits, mortality outcomes, and all the confounders included in the study. A total of 43 265 participants (24.3% women) aged 20 years or older at baseline met all these criteria and were therefore included in the study.
Baseline examination
Previous reports have described the baseline examination in detail.20,20 Briefly, height and weight were measured using a stadiometer and a standard scale. The waist circumference was obtained at the level of the umbilicus with a plastic anthropometric tape. The BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2), and participants were classed as normal weight (BMI = 18.5–24.9 kg/m2), overweight (BMI = 25.0–29.9 kg/m2), and obese (BMI ≥30 kg/m2). Body fat per cent was assessed by hydrostatic weighing or the sum of seven skinfold measures, following standardized protocols.24,25 Some participants chose to go through the underwater weighing assessment for hydrostatically estimated body density with a mathematical conversion to BF%, whereas other participants received a skinfold estimate of BF%. Standardized protocols used and specific procedures for the ACLS assessment of BF% were published elsewhere.21,24,26,27 The correlations between hydrostatically estimated BF% and skinfold estimated BF% were >0.90 for participants who had both measurements.26,27 When available, hydrostatically estimated per cent BF was always used in the analysis, i.e. hydrostatic weighing was available on 18 104 participants (42%) and the sum of seven skinfold measures in 25 161 participants (58%). Body fat per cent was further dichotomized based on standard clinical definitions for men (normal fat <25%, overfat ≥25%) and for women (normal fat <30%, overfat ≥30%),17 which has shown to be associated with a higher risk for all-cause and CVD mortality.21,24
Cardio respiratory fitness was defined as the total time of a symptom-limited maximal treadmill exercise test, using a modified Balke protocol.20,28 Total time of the test on this protocol correlates highly with measured maximal oxygen uptake (VO2max) in both men (r = 0.92)29 and women (r = 0.94).30 The test endpoint was volitional exhaustion or when the physician stopped it for medical reasons. VO2max was calculated from the final treadmill speed and grade.31 Fitness was also expressed in metabolic equivalents (METs, 3.5 mL/kg/min of oxygen uptake). Information on smoking (never, former, and current smoker), alcohol consumption (≥5 drinks/week or <5 drinks/week), and parental history of CVD was obtained from a standardized medical history questionnaire.
Systolic and diastolic blood pressures were obtained with a mercury sphygmomanometer and auscultory methods following the American Heart Association protocol.32 A fasting blood sample was obtained by venipuncture, and serum triglycerides, HDL cholesterol, and plasma glucose were assayed with automated techniques at the Cooper Clinic Laboratory, which participates in and meets the quality control standards of the U.S. Centers for Disease Control and Prevention Lipid Standardization Programme.
Definition of metabolically healthy
We have followed the metabolic syndrome definition proposed in the latest joint statement by the International Diabetes Federation Task Force on Epidemiology and Prevention, National Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society, and the International Association for the Study of Obesity.33 Based on this definition, participants who met 0 or 1 of the criteria were classified as metabolically healthy. Consistent with previous literature on this topic,4,16 the waist circumference was excluded as a criterion, since our main purpose was to study the prognosis of obese people who regardless of their adiposity levels (both total and central) have a better metabolic profile, i.e. metabolically healthy but obese phenotype. In the present study, >80% of the obese participants had a high waist circumference, i.e. ≥102 and ≥88 cm for men and women, respectively.
The rest of criteria used were: high blood pressure (≥130/85 mmHg), high triglycerides (≥150 mg/dL), low HDL cholesterol (<40 and 50 mg/dL in men and women, respectively), and high fasting glucose level (≥100 mg/dL).
Participants with normal blood pressure or fasting plasma glucose at the clinical evaluation who indicated a history of physician-diagnosed hypertension (n = 1571) or diabetes (n = 482) were also coded as positive for these risk factors, which increased the number of participants with metabolic syndrome by 635 (n = 15 648 vs. n = 16 283). The results obtained when these participants were excluded from all analyses were virtually identical to those reported here.
Assessment of outcomes
The participants were followed from the baseline examination until the date of death or 31 December 2003 (for mortality outcomes). Mortality surveillance was based on the national death index (NDI). The underlying cause of death was determined from the NDI report or by a nosologist's review of official death certificates obtained from the department of vital records in the decedent's state of residence. Cardiovascular disease mortality was defined by International Classification of Diseases, Ninth Revision (ICD-9) codes 390 to 448.9 before 1999 and Tenth Revision (ICD-10) codes I00 to I78 during 1999–2003.34 Cancer mortality was defined by ICD-9 codes 140 to 208 and ICD-10 codes C00 to C97.34
Data on non-fatal CVD events were additionally ascertained in a subsample of 18 430 individuals (77.5% men). Non-fatal CVD events were ascertained from responses to mail-back health surveys in 1982, 1999, and 2004. The aggregate survey response rate across all survey periods in the ACLS is ∼65%. Non-response bias is a concern in epidemiological surveillance, and this issue has been investigated in the ACLS.35 Baseline health histories and clinical measures were mostly similar between responders and non-responders and between early and late responders. Total mortality rates also have been similar between responders and non-responders (data not shown). We observed similar response rates between metabolically healthy but obese participants and metabolically abnormal obese participants (data not shown). On the other hand, metabolically healthy normal-fat participants had a 5% higher response rate than both obese groups (data not shown). This finding is consistent with previous epidemiological studies that reported increasing responding rates as BMI decreases.36,37
Non-fatal CVD endpoints were defined as diagnosis by a physician of myocardial infarction, stroke, or a coronary revascularization procedure (coronary artery bypass graft or percutaneous coronary intervention). In participants reporting multiple events, the first event was used for analysis. The primary outcome was all CVD events. Secondary outcomes were coronary heart disease events (myocardial infarction, coronary revascularization) and myocardial infarction and stroke as separate endpoints. In a random sample of these endpoints, we applied a standard definition for defining and adjudicating myocardial infarction, revascularization, and stroke.38,39 The percentage of agreement between reported events and participants' medical records was 88, 100, and 89% for myocardial infarction, revascularization, and stroke, respectively.40
Statistical analysis
All statistical analyses were performed using PASW (Predictive Analytics Soft Ware, formerly SPSS), version 18.0 SPSS, Inc., Chicago, IL, USA. The level of significance was set at <0.05 for all the analyses. The characteristics of the study sample are presented as means and standard deviations or as frequencies and percentages, as appropriate. For the purposes of the present study, we focused the analyses on 3 × 2 study groups: the metabolically healthy but obese, metabolically abnormal obese (‘unhealthiest group’) and metabolically healthy normal weight/fat (‘healthiest group’), using both BMI and BF% to define obesity. Since we aimed to compare the metabolically healthy but obese phenotype with the two ‘extreme’ groups defined above, ‘middle’ groups were not included in the analyses, i.e. overweight participants (n = 17 453) and metabolically abnormal normal weight (n = 4161) /fat (n = 9383) participants.
Aim 1
Differences in baseline fitness among the three study groups were examined by one-way analysis of covariance, after adjustment for age, sex, examination year, smoking, and alcohol consumption.
Aim 2
We used Cox proportional hazards regression (two-sided tests) to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) according to exposure categories (aforementioned three study groups). The metabolically healthy but obese group was used as referent in the analyses. The main study outcomes were: all-cause mortality, CVD mortality, non-fatal CVD events, and cancer mortality. In secondary analyses, we also studied non-fatal coronary heart disease, myocardial infarction, and stroke separately. All the models were adjusted for age, sex, examination year, smoking, alcohol consumption, and fitness. Parental history of CVD was additionally included in all the models, except when the outcome was cancer. Proportionality assumption for Cox regression analyses was met in all the models studied.
Results
The baseline characteristics of the study sample are shown in Table 1. A total of 5649 (13.1%) and 12 829 (29.7%) participants were obese according to the standard BMI and BF% criteria, respectively. Within the obese participants, 30.8 and 46.3% of them, for BMI-based obesity and BF%-based obesity, respectively, were metabolically healthy. Metabolically abnormal obese participants had higher BMI, BF%, and waist circumference than metabolically healthy but obese participants, regardless of the set of confounders (Table 2). The differences were attenuated after additional adjustment for fitness (data not shown).
Table 1.
Baseline characteristics of the study sample
| All (n = 43 265) | Men (32 764) | Women (10 501) | |
|---|---|---|---|
| Age (years) | 44.2 (9.9) | 44.3 (9.6) | 44.0 (10.5) |
| BMI (kg/m2) | 25.8 (4.0) | 26.5 (3.7) | 23.5 (4.0) |
| BF% | 22.8 (6.8) | 21.5 (6.3) | 26.9 (6.8) |
| Waist circumference (cm)a | 87.3 (19.3) | 92.0 (16.5) | 70.1 (19.3) |
| Triglycerides (mg/dL) | 121.4 (73.5) | 130.5 (76.6) | 93.2 (53.9) |
| HDL cholesterol (mg/dL) | 50.0 (14.5) | 46.1 (11.9) | 62.2 (15.0) |
| Glucose (mg/dL) | 98.4 (15.6) | 99.9 (15.9) | 93.8 (13.4) |
| Systolic blood pressure (mmHg) | 119.0 (14.0) | 121.0 (13.3) | 112.6 (14.3) |
| Diastolic blood pressure (mmHg) | 79.9 (9.8) | 81.2 (9.5) | 75.9 (9.5) |
| VO2 max (mL/kg/min) | 39.8 (8.9) | 41.7 (8.5) | 34.0 (7.4) |
| Maximal METs | 11.4 (2.5) | 11.9 (2.4) | 9.7 (2.1) |
| Cigarette smoking, n (%) | |||
| Never | 31 378 (72.5) | 23 176 (70.7) | 8202 (78.1) |
| Former | 5541 (12.8) | 4185 (12.8) | 1356 (12.9) |
| Current | 6346 (14.7) | 5403 (16.5) | 943 (9.0) |
| Alcohol consumption, n (%) of ≥5 drinks/week | 19 328 (44.7) | 16 058 (49.0) | 7231 (68.9) |
| Parental history of CVD, n (%) | 11 765 (27.2) | 8954 (27.3) | 2811 (26.8) |
| BMI-based obesity, n (%) | 5649 (13.1) | 4907 (15.0) | 742 (7.1) |
| BF%-based obesity, n (%)b | 12 829 (29.7) | 9343 (28.5) | 3516 (33.5) |
| Metabolically healthy, n (%)b | 26 982 (62.4) | 18 287 (55.8) | 8695 (82.8) |
| Metabolically healthy among the BMI-based obese sample, n (%)b | 1738 (30.8) | 1381 (28.1) | 357 (48.1) |
| Metabolically healthy among the BF%-based obese sample, n (%)b | 5959 (46.3) | 3548 (38.0) | 1105 (31.4) |
Data are means (standard deviations), unless otherwise indicated. BF%, percent body fat; BMI, body mass index; HDL, high-density lipoproteins; VO2max, maximal oxygen consumption; METs, metabolic equivalents, 1 MET = 3.5 mL of oxygen uptake/ kg/min.
aThis variable has 2979 missing values; for the rest of variables data are complete.
bBMI-based obesity was defined as BMI ≥30 kg/m2; BF%-based obesity was defined as BF% ≥25% in men and BF% ≥30% in women; ‘Metabolically healthy’ was defined as meeting 0 or 1 of the criteria for metabolic syndrome.33
Table 2.
Anthropometric and metabolic parameters according to study groups
| BMI-based groups |
P-value* | BF%-based groups |
P-value* | |||||
|---|---|---|---|---|---|---|---|---|
| Metabolically healthy and normal weight (n = 16 002) | Metabolically healthy but obese (n = 1738) | Metabolically abnormal and obese (n = 3911) | Metabolically healthy and normal fat (n = 21 023) | Metabolically healthy but obese (n = 5959) | Metabolically abnormal and obese (n = 6900) | |||
| Mean (SEM) | Mean (SEM) | Mean (SEM) | Mean (SEM) | Mean (SEM) | Mean (SEM) | |||
| BMI (kg/m2) | 22.6 (0.0) | 32.4 (0.1) | 33.0 (0.0) | <0.001 | 23.8 (0.0) | 28.1 (0.0) | 30.0 (0.0) | <0.001 |
| BF% | 19.3 (0.0) | 31.6 (0.1) | 31.9 (0.1) | <0.001 | 19.4 (0.0) | 29.9 (0.1) | 30.5 (0.1) | <0.001 |
| Waist circumference (cm)a | 77.6 (0.1) | 99.5 (0.4) | 103.0 (0.3) | <0.001 | 81.3 (0.1) | 92.4 (0.2) | 97.9 (0.2) | <0.001 |
| Triglycerides (mmol/L) | 83.3 (0.4) | 106.9 (1.2) | 191.2 (0.8) | <0.001 | 86.8 (0.4) | 102.3 (0.7) | 182.3 (0.6) | <0.001 |
| HDL cholesterol (mmol/L) | 57.0 (0.1) | 50.3 (0.3) | 41.7 (0.2) | <0.001 | 55.4 (0.1) | 52.1 (0.1) | 42.4 (0.1) | <0.001 |
| Glucose (mmol/L) | 93.6 (0.1) | 95.3 (0.3) | 107.6 (0.2) | <0.001 | 94.3 (0.1) | 95.0 (0.2) | 106.3 (0.2) | <0.001 |
| Systolic blood pressure (mmHg) | 113.5 (0.1) | 118.4 (0.3) | 126.3 (0.2) | <0.001 | 114.8 (0.1) | 116.6 (0.2) | 125.7 (0.1) | <0.001 |
| Diastolic blood pressure (mmHg) | 75.9 (0.1) | 80.5 (0.2) | 86.2 (0.1) | <0.001 | 76.7 (0.1) | 79.0 (0.1) | 85.2 (0.1) | <0.001 |
BF%, percent body fat; BMI, body mass index; HDL, high-density lipoproteins; SEM, standard error of the mean.
aThis variable has 2979 missing values; for the rest of variables data are complete.
*The model (one-way analysis of covariance) was adjusted for age, sex, examination year, smoking and alcohol consumption. Pairwise comparisons were adjusted for the Bonferroni correction and were significant among all the groups (P< 0.001).
The median (25–75th percentiles) follow-up period for mortality was 14.3 (6.0–19.8) years and for non-fatal CVD incidence was 7.9 (2.9–16.2) years. Frequency and percentage of deaths and incidence of non-fatal CVD events are shown in Table 2. A total of 1779 (4.1%) participants died during the study period; 546 of the deaths were caused by CVD and 698 by cancer. The incidence of non-fatal CVD events was 5.8%, being 1.7% due to myocardial infarction and 1.3% to stroke.
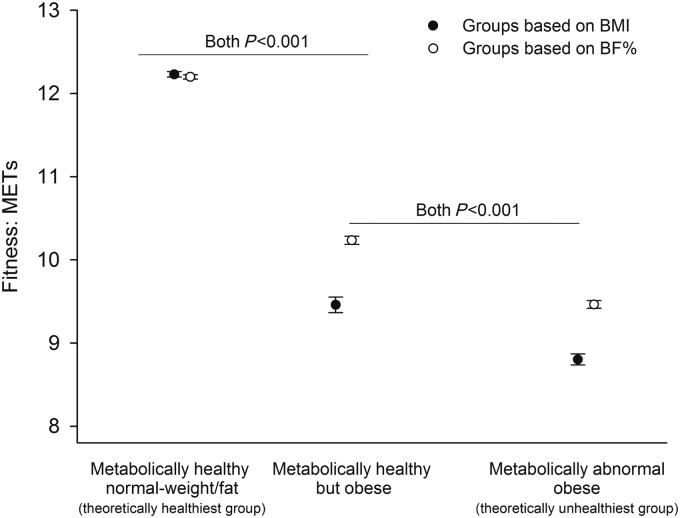
Figure 1 shows that metabolically healthy but obese participants had a better baseline fitness level than metabolically abnormal obese participants, after adjustment for the set of confounders, and when using either BMI or BF% to define obesity (P < 0.001). This difference was consistent in men and women, particularly when using BF%-based obesity (P < 0.001, data not shown). The results did not materially change when excluding class 3 obesity, i.e. BMI ≥ 40 kg/m2, or when excluding participants with abnormal electrocardiogram (data not shown).
Figure 1.
Differences in baseline fitness among the study groups using either body mass index or percent body fat to define obesity. The circled points and error bars represent adjusted means and 95% confidence intervals, respectively. The model (one-way analysis of covariance) was adjusted for age, sex, examination year, smoking, and alcohol consumption. Pairwise comparisons were adjusted for the Bonferroni correction. METs, metabolic equivalents, 1 MET = 3.5 mL of oxygen uptake/kg/min.
Hazards ratios and 95% CI of all-cause mortality, CVD mortality and non-fatal CVD incidence, and cancer mortality in the study groups are shown in Tables 3–5, respectively. When obesity was defined using BF% and the models were fully adjusted by the set of confounders including fitness, metabolically healthy but obese individuals had a 38% lower risk of all-cause mortality than their metabolically unhealthy obese peers, while no significant difference was observed between metabolically healthy but obese and metabolically healthy normal-fat participants (Table 3). This result was consistent for CVD mortality and incident non-fatal CVD (Table 4), as well as for cancer mortality (Table 5). Further analyses focused on coronary heart disease, myocardial infarction and stroke also showed similar results (data not shown). The risk difference between metabolically healthy but obese and metabolically abnormal obese patients ranged from 32 to 51% (Tables 3–5). Comparisons between models adjusted and non-adjusted for fitness allow exploring the role of fitness in these associations (Tables 3–5). The results did not materially change when excluding class 3 obesity, i.e. BMI ≥40 kg/m2, or when excluding participants with abnormal electrocardiogram (data not shown).
Table 3.
Hazard ratios of all-cause mortality in metabolically healthy but obese individuals compared with metabolically abnormal obese and metabolically healthy normal-weight individuals, using both body mass index and per cent body fat to define obesity
| BMI-based obesity |
BF%-based obesity |
|||||
|---|---|---|---|---|---|---|
| Cases (total) | HR (95% CI)a | Fitness-adjusted HR (95% CI)b | Cases (total) | HR (95% CI)a | Fitness-adjusted HR (95% CI)b | |
| All-cause mortality | ||||||
| Metabolically abnormal obesec | 218 (3911) | 1.61 (1.19–2.18) | 1.44 (1.06–1.96) | 436 (6900) | 1.57 (1.32–1.85) | 1.38 (1.16–1.63) |
| Metabolically healthy but obese | 52 (1738) | 1 (Ref.) | 1 (Ref.) | 216 (5959) | 1 (Ref.) | 1 (Ref.) |
| Metabolically healthy normal-weight/fatd | 449 (16 002) | 0.65 (0.48–0.87) | 0.91 (0.67–1.24) | 584 (21 023) | 0.84 (0.72–0.99) | 1.10 (0.92–1.30) |
CI, confidence interval.
aModel adjusted for age, sex, examination year, smoking, alcohol consumption and parental history of CVD.
bAdditional adjustment for fitness.
cTheoretically unhealthiest group.
dTheoretically healthiest group.
Table 4.
Hazard ratios of cardiovascular disease mortality and incidence in metabolically healthy but obese individuals compared with metabolically abnormal obese and metabolically healthy normal-weight individuals, using both body mass index and body fat percentage to define obesity
| BMI-based obesity |
BF%-based obesity |
|||||
|---|---|---|---|---|---|---|
| Cases (total) | HR (95% CI)a | Fitness-adjusted HR (95% CI)b | Cases (total) | HR (95% CI)a | Fitness-adjusted HR (95% CI)b | |
| CVD mortality | ||||||
| Metabolically abnormal obesec | 81 (3911) | 1.77 (1.05–2.99) | 1.48 (0.87–2.52) | 153 (6900) | 1.76 (1.31–2.37) | 1.44 (1.06–1.95) |
| Metabolically healthy but obese | 17 (1738) | 1 (Ref.) | 1 (Ref.) | 64 (5959) | 1 (Ref.) | 1 (Ref.) |
| Metabolically healthy normal-weight/fatd | 98 (16 002) | 0.41 (0.24–0.70) | 0.73 (0.42–1.28) | 144 (21 023) | 0.74 (0.54–1.00) | 1.13 (0.82–1.56) |
| Non-fatal CVD eventse | ||||||
| Metabolically abnormal obesec | 107 (1300) | 1.44 (0.96–2.17) | 1.39 (0.92–2.10) | 231 (2598) | 1.61 (1.29–2.01) | 1.51 (1.20–1.89) |
| Metabolically healthy but obese | 30 (544) | 1 (Ref.) | 1 (Ref.) | 123 (2340) | 1 (Ref.) | 1 (Ref.) |
| Metabolically healthy normal-weight/fatd | 261 (7001) | 0.58 (0.39–0.86) | 0.78 (0.52–1.18) | 353 (9263) | 0.78 (0.63–0.96) | 0.95 (0.76–1.20) |
CI, confidence interval.
aModel adjusted for age, sex, examination year, smoking, alcohol consumption and parental history of CVD.
bAdditional adjustment for fitness.
cTheoretically unhealthiest group.
dTheoretically healthiest group.
eNon-fatal CVD events include: myocardial infarction, stroke, and coronary revascularization (i.e. bypass, coronary angioplasty); data available in a subsample of 18 430 participants.
Table 5.
Hazard ratios of cancer mortality in metabolically healthy but obese individuals compared with metabolically abnormal obese and metabolically healthy normal-weight individuals, using both body mass index and body fat percentage to define obesity
| BMI-based obesity |
BF%-based obesity |
|||||
|---|---|---|---|---|---|---|
| Cases (total) | HR (95% CI)a | Fitness-adjusted HR (95% CI)b | Cases (total) | HR (95% CI)a | Fitness-adjusted HR (95% CI)b | |
| Cancer mortality | ||||||
| Metabolically abnormal obesec | 74 (3911) | 1.19 (0.75–1.89) | 1.17 (0.73–1.88) | 171 (6900) | 1.40 (1.08–1.80) | 1.32 (1.02–1.71) |
| Metabolically healthy but obese | 24 (1738) | 1 (Ref.) | 1 (Ref.) | 100 (5959) | 1 (Ref.) | 1 (Ref.) |
| Metabolically healthy normal-weight/fatd | 194 (16 002) | 0.56 (0.36–0.86) | 0.61 (0.38–0.97) | 237 (21 023) | 0.76 (0.60–0.97) | 0.84 (0.65–1.09) |
CI, confidence interval.
aModel adjusted for age, sex, examination year, smoking, alcohol consumption.
bAdditional adjustment for fitness.
cTheoretically unhealthiest group.
dTheoretically healthiest group.
If a more strict definition of ‘metabolically healthy’ was applied, i.e. meeting 0 metabolic syndrome criteria, the metabolically healthy but obese sample was reduced to one-third of the original sample (n = 5959 vs. n = 2197, 37%). When the same analyses were repeated using these new groups, almost identical findings were observed for all-cause mortality. Similar trends were also observed for CVD mortality and cancer mortality, though the differences between the metabolically healthy but obese group and the metabolically abnormal obese group were not significant, presumably due to the 63% reduction in the sample size and consequently reduced number of cases (deaths) of the main study group, i.e. metabolically healthy but obese (data not shown).
Discussion
The results of the present study suggest that: (i) metabolically healthy but obese individuals have better fitness than their metabolically abnormal obese peers, both in men and women and using BMI or BF% to define obesity; (ii) for a given fitness level, the metabolically healthy but obese phenotype is a benign condition, since these individuals had a lower risk for mortality and morbidity than that observed in metabolically abnormal obese individuals. In addition, we did not observe differences in the prognosis between metabolically healthy but obese individuals and metabolically healthy normal-fat individuals. These findings are consistent for all the outcomes studied, i.e. all-cause mortality, CVD mortality and morbidity, and cancer mortality. Although the prevalence of metabolically healthy but obese phenotype differs depending on the definition of metabolically healthy used and no standard definition is currently available,4,41 the prevalence of metabolically healthy but obese participants observed in our study (∼30%, based on BMI definition) is in agreement with that previously observed in US adults42 and in other populations,43–46 and supports the notion that this phenotype is a frequent condition.
The prevalence of obesity in the present study was 13% as defined by BMI and 30% as defined by BF%. This difference is in agreement with the findings from a recent meta-analysis that concluded that BMI (≥30 kg/m2) failed to identify half of the individuals with excess BF% (standard cut points used in the present study).17 Based on this, the ‘actual’ number of obese people would be two times higher than that identified by BMI, which would result in a similar percentage observed in this study when using BF% (i.e. ∼30%). In the remaining discussion, we focus on the BF%-based results rather than BMI, since it is a more accurate measure of adiposity and a major contribution of this study to the previous literature.
Differences in fitness between metabolically healthy but obese and metabolically abnormal obese individuals
In spite of the strong evidence indicating that higher fitness levels are related to lower metabolic complications and lower prevalence of metabolic syndrome,5–9 a recent review4 characterizing the metabolically healthy but obese phenotype did not mention the possibility that the better metabolic profile observed in this subset of obese people could be, at least in part, explained by fitness. Our study conducted on thousands of obese people supports that metabolically healthy but obese individuals have a better fitness level than the rest of obese individuals, regardless of a set of relevant confounders and using BF% to define obesity. Messier et al.47 observed a higher fitness level (VO2 peak) in metabolically healthy but obese women when using a definition based on hyperinsulinaemic–euglycaemic clamp, but not when using other definitions for metabolically healthy profile (n = 113). Brochu et al.48 also observed a higher fitness level (VO2 peak) in metabolically healthy (based on insulin sensitivity) but obese post-menopausal women compared with their metabolically abnormal obese peers, yet the difference was not significant, maybe due to the small sample size (n = 43 split into two groups) and consequent low statistical power. Similar findings, i.e. higher but not significantly higher fitness (VO2 peak) in the metabolically healthy (based on hyperinsulinaemic–euglycaemic clamp) but obese group, were observed in another study conducted on sedentary post-menopausal women (n = 42) by Karelis et al.43 In a later study from the same authors that included a larger sample (n = 137) of overweight and obese sedentary post-menopausal women, the investigators observed a significantly higher fitness (VO2 peak) in the metabolically healthy group, as well as a higher physical activity level in this group. Likewise, data from the National Health and Nutrition Examination Surveys 1999–2004 showed a higher physical activity level (fitness was not studied) in the metabolically healthy but overweight/obese group, compared with the metabolically abnormal overweight/obese group (n = 1664 obese participants).42
Long-term prognosis in metabolically healthy but obese individuals: role of fitness
Our study focused on two comparisons: (i) metabolically healthy but obese vs. metabolically abnormal obese (‘theoretically unhealthiest group’) individuals; and (ii) metabolically healthy but obese vs. metabolically healthy normal-weight/fat (‘theoretically the healthiest group’) individuals. The first comparison addressed in the present study provides for the first time evidence that metabolically healthy but obese individuals have a significantly better prognosis, in terms of mortality and morbidity, than the rest of obese individuals, after adjustment for fitness. The fact that metabolically healthy but obese participants have slightly lower levels of adiposity might have contributed to explain their better prognosis. The available literature from prospective studies has focused on the second comparison and provided mixed findings. Our results agree with those from three prospective studies that observed a similar risk between metabolically healthy but obese individuals and metabolically healthy normal-weight/fat individuals for: (i) incident ischaemic heart disease (n = 1824);11 (ii) incident fatal and non-fatal CVD (n = 2902)12; and (iii) all-cause, CVD, and cancer mortality (n = 2011).10 In contrast, two previous studies have suggested that compared with metabolically healthy normal-weight individuals, metabolically healthy but obese individuals have a higher risk of: (i) all-cause mortality (n = 6011)16; and (ii) all-cause mortality, CVD mortality and morbidity and cancer incidence (n = 1758).15 Note that none of these studies included fitness as a potential confounder in the analyses, which could partially explain the discrepancies with the present study findings. Indeed, our non-adjusted for fitness models concur with the results observed in the two mentioned studies, but once fitness is accounted for, the metabolically healthy but obese group had no longer a higher risk compared with their normal-fat peers. This finding supports a major role of fitness in these associations and suggests that fitness should be included in future research on this topic as a relevant confounder.
Comparisons between models adjusted and non-adjusted for fitness allow for the further exploration of the role of fitness in these associations. We observed that fitness eliminated the risk for mortality and morbidity (all outcomes) associated with obesity, i.e. comparison of metabolically healthy but obese vs. metabolically healthy normal weight, in accordance with previous studies.24,49 On the other hand, fitness attenuated but did not eliminate the risk of mortality and morbidity associated with an abnormal metabolic profile (all outcomes), i.e. comparison of metabolically healthy but obese vs. metabolically abnormal obese groups. An explanation could be that obesity plus metabolic syndrome is so adverse condition that cannot be fully counteracted by fitness. Nevertheless, it is important to note that adjusting for fitness lowered the risk of mortality and morbidity by 10–30%, indicating a beneficial effect also in this subgroup.
Limitations and strengths
A complex issue in the study of the metabolically healthy but obese phenotype is how to define the ‘metabolically healthy’ phenotype. Multiple definitions have been used, and can be classed into two groups: (i) those based on insulin resistance/sensitivity measurements or (ii) those based on metabolic syndrome criteria (excluding waist).4,41 In the present study, we considered a participant as being ‘metabolically healthy’ if he/she met 0 or 1 of the updated criteria for metabolic syndrome.33 Unfortunately, data on insulin resistance were not available in the ACLS sample. Nevertheless, we believe it is unlikely that the results could be largely influenced by using a different definition for metabolically healthy, since previous prospective studies observed consistent results using both insulin resistance and metabolic syndrome definitions.12,15,16 Markers of systemic inflammation or detailed dietary measurements on the entire cohort were not available. Another limitation is the lack of information on the duration of obesity. Data from the NHANES have shown that not only the current BMI, but also the duration of obesity is an important predictor of metabolic risk in adults.50 Note that the number of female participants is much lower than the number of male participants (24.3% of the participants were women), which precludes separate analyses for men and women. The participants were 98% Caucasian, well educated and with high professional positions, so we cannot know to which extent the present findings apply to other populations. The fact that measurement error for skinfolds is larger in obese population should be acknowledged.
Strengths of the present study are: (i) the inclusion of an accurate measure of BF% to define obesity; (ii) the inclusion of fitness and the study of its role in these associations; (iii) the complete set of major outcomes studied, i.e. all-cause mortality, CVD mortality and morbidity (total non-fatal CVD events, coronary heart disease events, myocardial infarction and stroke were analyzed separately) and cancer mortality; and (iv) the large sample size, particularly the large number of obese participants allowed power analyses on the obese subgroups.
Clinical implications
The results from the current study have important clinical implications. Our data suggest that accurate BF% and fitness assessment can contribute to properly define a subset of obese individuals who do not have an elevated risk of CVD or cancer. Fatness and fitness assessment provide useful information to clinicians about the severity of obesity and health-related problems. In this context, the Edmonton Obesity Staging System proposed by Sharma and Kushner51 provide information on the presence or the extent of comorbidities or functional limitations that would guide clinicians' decision-making. Based on the findings of the present and other studies, staging systems should consider the inclusion of fitness as an informative measure of the severity of obesity and functional limitations.
Conclusion
Our results suggest that: (i) better fitness should be considered a characteristic of metabolically healthy but obese phenotype. (ii) Once fitness is duly accounted for and an accurate measure of adiposity is used, the metabolically healthy but obese phenotype is a benign condition, with a better prognosis (30–50% lower risk) for mortality and morbidity than metabolically abnormal obese people. Interestingly, no difference in the prognosis is observed between metabolically healthy but obese individuals and metabolically healthy normal-fat individuals once fitness is accounted for, suggesting a key role of fitness in these associations.
Funding
This study is supported by National Institutes of Health grants AG06945, HL62508, and R21DK088195, an unrestricted research grant from The Coca-Cola Company, by the Spanish Ministry of Science and Innovation (RYC-2010-05957, RYC-2011-09011) and the Swedish Heart-Lung Foundation (20090635). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest: none declared.
Acknowledgements
We thank the Cooper Clinic physicians and technicians for collecting the baseline data, and staff at the Cooper Institute for data entry and data management.
References
- 1.McAuley PA, Blair SN. Obesity paradoxes. J Sports Sci. 2011;29:1–10. doi: 10.1080/02640414.2011.553965. [DOI] [PubMed] [Google Scholar]
- 2.Karelis AD. Metabolically healthy but obese individuals. Lancet. 2008;372:1281–1283. doi: 10.1016/S0140-6736(08)61531-7. [DOI] [PubMed] [Google Scholar]
- 3.Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47:699–713. doi: 10.2337/diabetes.47.5.699. [DOI] [PubMed] [Google Scholar]
- 4.Primeau V, Coderre L, Karelis AD, Brochu M, Lavoie ME, Messier V, Sladek R, Rabasa-Lhoret R. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 2011;35:971–981. doi: 10.1038/ijo.2010.216. [DOI] [PubMed] [Google Scholar]
- 5.Church TS, Finley CE, Earnest CP, Kampert JB, Gibbons LW, Blair SN. Relative associations of fitness and fatness to fibrinogen, white blood cell count, uric acid and metabolic syndrome. Int J Obes Relat Metab Disord. 2002;26:805–813. doi: 10.1038/sj.ijo.0802001. [DOI] [PubMed] [Google Scholar]
- 6.Katzmarzyk PT, Church TS, Blair SN. Cardiorespiratory fitness attenuates the effects of the metabolic syndrome on all-cause and cardiovascular disease mortality in men. Arch Intern Med. 2004;164:1092–1097. doi: 10.1001/archinte.164.10.1092. [DOI] [PubMed] [Google Scholar]
- 7.LaMonte MJ, Barlow CE, Jurca R, Kampert JB, Church TS, Blair SN. Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: a prospective study of men and women. Circulation. 2005;112:505–512. doi: 10.1161/CIRCULATIONAHA.104.503805. [DOI] [PubMed] [Google Scholar]
- 8.Lee S, Kuk JL, Katzmarzyk PT, Blair SN, Church TS, Ross R. Cardiorespiratory fitness attenuates metabolic risk independent of abdominal subcutaneous and visceral fat in men. Diabetes Care. 2005;28:895–901. doi: 10.2337/diacare.28.4.895. [DOI] [PubMed] [Google Scholar]
- 9.Messier V, Malita FM, Rabasa-Lhoret R, Brochu M, Karelis AD. Association of cardiorespiratory fitness with insulin sensitivity in overweight and obese postmenopausal women: a Montreal Ottawa New Emerging Team study. Metabolism. 2008;57:1293–1298. doi: 10.1016/j.metabol.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 10.Calori G, Lattuada G, Piemonti L, Garancini MP, Ragogna F, Villa M, Mannino S, Crosignani P, Bosi E, Luzi L, Ruotolo G, Perseghin G. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: the Cremona Study. Diabetes Care. 2011;34:210–215. doi: 10.2337/dc10-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St-Pierre AC, Cantin B, Mauriege P, Bergeron J, Dagenais GR, Despres JP, Lamarche B. Insulin resistance syndrome, body mass index and the risk of ischemic heart disease. CMAJ. 2005;172:1301–1305. doi: 10.1503/cmaj.1040834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D'Agostino RB. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 13.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, Bonadonna RC, Muggeo M. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: the Bruneck study. Diabetes Care. 2007;30:318–324. doi: 10.2337/dc06-0919. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin T, Abbasi F, Lamendola C, Reaven G. Heterogeneity in the prevalence of risk factors for cardiovascular disease and type 2 diabetes mellitus in obese individuals: effect of differences in insulin sensitivity. Arch Intern Med. 2007;167:642–648. doi: 10.1001/archinte.167.7.642. [DOI] [PubMed] [Google Scholar]
- 15.Arnlov J, Ingelsson E, Sundstrom J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation. 2010;121:230–236. doi: 10.1161/CIRCULATIONAHA.109.887521. [DOI] [PubMed] [Google Scholar]
- 16.Kuk JL, Ardern CI. Are metabolically normal but obese individuals at lower risk for all-cause mortality? Diabetes Care. 2009;32:2297–2299. doi: 10.2337/dc09-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, Lopez-Jimenez F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond) 2010;34:791–799. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 18.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 19.Katzmarzyk PT, Church TS, Janssen I, Ross R, Blair SN. Metabolic syndrome, obesity, and mortality: impact of cardiorespiratory fitness. Diabetes Care. 2005;28:391–397. doi: 10.2337/diacare.28.2.391. [DOI] [PubMed] [Google Scholar]
- 20.Blair SN, Kohl HW, III, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 21.Sui X, LaMonte MJ, Laditka JN, Hardin JW, Chase N, Hooker SP, Blair SN. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007;298:2507–2516. doi: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hooker SP, Sui X, Colabianchi N, Vena J, Laditka J, LaMonte MJ, Blair SN. Cardiorespiratory fitness as a predictor of fatal and nonfatal stroke in asymptomatic women and men. Stroke. 2008;39:2950–2957. doi: 10.1161/STROKEAHA.107.495275. [DOI] [PubMed] [Google Scholar]
- 23.Cheng YJ, Macera CA, Addy CL, Sy FS, Wieland D, Blair SN. Effects of physical activity on exercise tests and respiratory function. Br J Sports Med. 2003;37:521–528. doi: 10.1136/bjsm.37.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999;69:373–380. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- 25.Jackson AW, Lee DC, Sui X, Morrow JR, Jr, Church TS, Maslow AL, Blair SN. Muscular strength is inversely related to prevalence and incidence of obesity in adult men. Obesity (Silver Spring) 2010;18:1988–1995. doi: 10.1038/oby.2009.422. [DOI] [PubMed] [Google Scholar]
- 26.Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40:497–504. doi: 10.1079/bjn19780152. [DOI] [PubMed] [Google Scholar]
- 27.Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Med Sci Sports Exerc. 1980;12:175–181. [PubMed] [Google Scholar]
- 28.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J. 1959;10:675–688. [PubMed] [Google Scholar]
- 29.Pollock ML, Bohannon RL, Cooper KH, Ayres JJ, Ward A, White SR, Linnerud AC. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92:39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- 30.Pollock ML, Foster C, Schmidt D, Hellman C, Linnerud AC, Ward A. Comparative analysis of physiologic responses to three different maximal graded exercise test protocols in healthy women. Am Heart J. 1982;103:363–373. doi: 10.1016/0002-8703(82)90275-7. [DOI] [PubMed] [Google Scholar]
- 31.American College of Sports Medicine. ACSM's Guidelines For Exercise Testing And Prescription. 8th ed. Philadelphia: Lippincott Williams and Wilkins; 2009. [DOI] [PubMed] [Google Scholar]
- 32.Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern BZ. Human Blood Pressure Determination by Sphymomanometry. Dallas, TX: American Heart Association; 2001. [DOI] [PubMed] [Google Scholar]
- 33.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 34.Anderson RN, Minino AM, Hoyert DL, Rosenberg HM. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. Natl Vital Stat Rep. 2001;49:1–32. [PubMed] [Google Scholar]
- 35.Macera CA, Jackson KL, Davis DR, Kronenfeld JJ, Blair SN. Patterns of non-response to a mail survey. J Clin Epidemiol. 1990;43:1427–1430. doi: 10.1016/0895-4356(90)90112-3. [DOI] [PubMed] [Google Scholar]
- 36.Sonne-Holm S, Sorensen TI, Jensen G, Schnohr P. Influence of fatness, intelligence, education and sociodemographic factors on response rate in a health survey. J Epidemiol Community Health. 1989;43:369–374. doi: 10.1136/jech.43.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Childs JD, Teyhen DS, Van Wyngaarden JJ, Dougherty BF, Ladislas BJ, Helton GL, Robinson ME, Wu SS, George SZ. Predictors of web-based follow-up response in the Prevention Of Low Back Pain In The Military Trial (POLM) BMC Musculoskelet Disord. 2011;12:132. doi: 10.1186/1471-2474-12-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly-Hayes M, Robertson JT, Broderick JP, Duncan PW, Hershey LA, Roth EJ, Thies WH, Trombly CA. The American Heart Association Stroke Outcome Classification. Stroke. 1998;29:1274–1280. doi: 10.1161/01.str.29.6.1274. [DOI] [PubMed] [Google Scholar]
- 39.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 40.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness as a predictor of nonfatal cardiovascular events in asymptomatic women and men. Am J Epidemiol. 2007;165:1413–1423. doi: 10.1093/aje/kwm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pataky Z, Bobbioni-Harsch E, Golay A. Open questions about metabolically normal obesity. Int J Obes (Lond) 2010;34(Suppl. 2):S18–23. doi: 10.1038/ijo.2010.235. [DOI] [PubMed] [Google Scholar]
- 42.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 43.Karelis AD, Faraj M, Bastard JP, St-Pierre DH, Brochu M, Prud'homme D, Rabasa-Lhoret R. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90:4145–4150. doi: 10.1210/jc.2005-0482. [DOI] [PubMed] [Google Scholar]
- 44.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Targher G, Alberiche M, Bonadonna RC, Muggeo M. Prevalence of insulin resistance in metabolic disorders: the Bruneck Study. Diabetes. 1998;47:1643–1649. doi: 10.2337/diabetes.47.10.1643. [DOI] [PubMed] [Google Scholar]
- 45.Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR) J Clin Invest. 1997;100:1166–1173. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab. 2004;89:2569–2575. doi: 10.1210/jc.2004-0165. [DOI] [PubMed] [Google Scholar]
- 47.Messier V, Karelis AD, Prud'homme D, Primeau V, Brochu M, Rabasa-Lhoret R. Identifying metabolically healthy but obese individuals in sedentary postmenopausal women. Obesity (Silver Spring) 2010;18:911–917. doi: 10.1038/oby.2009.364. [DOI] [PubMed] [Google Scholar]
- 48.Brochu M, Tchernof A, Dionne IJ, Sites CK, Eltabbakh GH, Sims EA, Poehlman ET. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab. 2001;86:1020–1025. doi: 10.1210/jcem.86.3.7365. [DOI] [PubMed] [Google Scholar]
- 49.Farrell SW, Cortese GM, LaMonte MJ, Blair SN. Cardiorespiratory fitness, different measures of adiposity, and cancer mortality in men. Obesity (Silver Spring) 2007;15:3140–3149. doi: 10.1038/oby.2007.374. [DOI] [PubMed] [Google Scholar]
- 50.Janssen I, Katzmarzyk PT, Ross R. Duration of overweight and metabolic health risk in American men and women. Ann Epidemiol. 2004;14:585–591. doi: 10.1016/j.annepidem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Sharma AM, Kushner RF. A proposed clinical staging system for obesity. Int J Obes (Lond) 2009;33:289–295. doi: 10.1038/ijo.2009.2. [DOI] [PubMed] [Google Scholar]