Abstract
Background
An individual’s propensity to engage in adaptive health and rehabilitation behaviors may account for variation in postsurgical outcome.
Purpose
To determine the psychometric properties and construct validity of the recently developed Patient Activation Measure (PAM) (previously unused in spine research) in persons undergoing elective lumbar spine surgery.
Methods
We prospectively used the PAM to assess activation in 283 patients undergoing elective lumbar spine surgery. Reliability statistics were computed using repeated assessment (baseline and 1-week follow-up) before surgery. Additional psychological attributes were assessed at baseline and correlated with patient activation. Factor analysis was used to confirm the theoretical structure of patient activation.
Results
Repeat PAM administrations had an intraclass correlation coefficient of 0.85. The PAM showed positive correlation with optimism (r = 0.75), hope (r = 0.73), self-efficacy (r = 0.65), and internal locus of control (r = 0.65) but no correlation with comorbidity (r = 0.01). Confirmatory factor analysis of the PAM items indicated reasonable fit between observed data and a three-factor patient activation model.
Conclusions
The PAM is a reliable, valid measure of patient activation for individuals undergoing elective lumbar spine surgery and may have clinical utility in identify those at risk for poor engagement in postsurgical rehabilitation.
Keywords: Patient Activation Measure, Validation, Positive psychology, Lumbar spine surgery
Introduction
Variability in outcome after lumbar spine surgery is well-documented [1], and a meta-analysis of the literature found that satisfactory clinical outcomes ranged from 16% to 95% [2]. Variability has also been observed across multiple patient-reported outcome domains (e.g., reduction in pain severity and improvement in quality of life) and other clinical outcomes (e.g., change in spinal flexion, improvement in muscle power, or change in neurological status). Previous work, which focused on identifying patient and surgical/medical management factors that may contribute to this variation in outcomes, found there were several relevant biological factors (e.g., increased age [3-6], minority race/ethnicity [7-12], and comorbid conditions [13-15]) and social factors (e.g., low socioeconomic status [16,17] and poor social support [18]). The presence of depressive symptoms has also been related to poor recovery. For example, among individuals undergoing surgery for disc herniation, those with elevated depressive symptoms on the Beck Depression Inventory had diminished surgical success [19]. Similar associations have been seen between increased depressive symptoms and (1) decreased patient satisfaction [20,21] and (2) decreased rate of return to work [22,23].
However, neither differences in demographic and social characteristics [24,25] nor use of different surgical techniques and medical management [26,27] adequately explain the observed variation. In a recently published study to investigate the predictors of multidimensional outcome after spinal surgery, the authors reported that individual psychosocial variables, such as depression or social support, account for approximately 8.4% of the variance in outcome, whereas medical variables, such as surgical technique, account for approximately 4.5% of that variance [28]. These models adjusted for differences in age, gender, and baseline pain and disability. Thus, to date, the most comprehensive study addressing both individual and treatment variables accounted for 33.9% of the variation in outcome [28].
The chronic disease literature has identified psychological factors and personal competencies that contribute to a person being engaged in their health care and has found that those factors are related to patient outcomes [29]. However, similar research has not been performed in acute settings, such as recovery after spine surgery. Because evolving healthcare models emphasize patient-centered care and require the patient to take a central role, a greater understanding of the engaged, activated patient is critical. Having a method that reliably assesses patient activation is an important step in understanding how these characteristics can be positively influenced.
Patient activation has been conceptualized as having an impact on six dimensions: (1) self-management of symptoms, (2) engagement in activities to maintain function, (3) involvement in healthcare decisions, (4) collaboration with healthcare providers, (5) informed choices of provider based on quality, and (6) navigation of the healthcare system. These dimensions are also influenced by a number of other factors, such as healthcare system structure, financial constraints regarding insurance coverage, and mutable factors. We have chosen to focus on mutable factors, that is, psychological factors such as optimism, hope, self-efficacy, and locus of control on health behavior [30]. The concept of patient activation emerges as an integration of these psychological factors, but it also incorporates personal competency components, such as condition-specific knowledge and communication skills [30]. Patient activation is conceptualized as having a hierarchical structure: an individual moves from believing an active role is important, to having confidence and knowledge to take action, to taking action, and finally to staying the course under stress. Studies have shown that individuals who are highly activated experience better health outcomes than individuals who are less activated [31,32].
Because of similar health behaviors, such as self-care and treatment adherence, patient activation may contribute to our understanding of the variability in outcome after lumbar spine surgery. A clinically relevant instrument has been developed to measure patient activation among individuals with chronic diseases: the Patient Activation Measure (PAM) [30]. In a recently published study, we showed a relationship between increased patient activation and adherence to physical therapy [33]. Although these results are promising, the PAM must be fully tested in such a population.
The objectives of our study were to determine the psychometric properties and construct validity of the PAM in a cohort of individuals undergoing lumbar spine surgery. We hypothesized that: (1) scores on the PAM would be stable and reproducible; (2) the measure of patient activation would exhibit positive correlation with the measures of the psychological factors of optimism, hope, self-efficacy, and internal locus of control, and would exhibit negative correlation with a measure of depressive symptoms; (3) patient activation would not be correlated with a measure of severity of comorbid conditions; and (4) a factor analysis of the items of the PAM would provide evidence to support the stages of activation as presented by Hibbard et al. [30].
Methods
The current study was reviewed and approved by our institutional review board.
Participants
From August 2005 through July 2008, we recorded demographic and clinical data for all patients who presented to our service to undergo lumbar spine surgery for degenerative disc disease. Criteria for inclusion in our study were age >18 years and the ability to provide informed consent (Mini-Mental Status Examination score >18/30) and speak English. Because recovery of function after revision surgery has a markedly different clinical course than that after primary surgery [34], we excluded individuals with previous spine surgery.
We approached 300 eligible individuals for inclusion in the study; 17 individuals refused participation and 283 agreed to participate, and the latter were enrolled in our prospective study (there were no discernable demographic or clinical differences between those agreeing and those refusing participation). Our participants were predominantly non-Hispanic white (87%) and female (56%) with a mean age of 59 years (standard deviation [SD] = 16 years; range, 18--86 years) (Table 1). The demographic and clinical characteristics of this participant sample are consistent with those of our surgical practice and of large national cohorts presented elsewhere [23,35].
Table 1.
Descriptive characteristics of the participant cohort
| Characteristic | Total (n = 283) | Sub-set (n = 65) |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 59.0 (15.7) | 58.0 (15.4) |
| Range | 18--86 | 18--86 |
| <45 | 54 (19.1%) | 11 (16.9%) |
| 45--65 | 132 (46.6%) | 33 (50.8%) |
| >65 | 97 (34.3%) | 21 (32.3%) |
| Gender (female) | 158 (55.8%) | 38 (58.4%) |
| Marital status | ||
| Married/living with spouse | 224 (79.2%) | 52 (80.0%) |
| Living with partner | 12 (4.2%) | 3 (4.6%) |
| Separated, divorced, or widowed | 17 (6.0%) | 4 (6.1%) |
| Never married | 30 (10.6%) | 6 (9.3%) |
| Race | ||
| White | 251 (88.7%) | 58 (89.2%) |
| Non-White | 32 (11.3%) | 7 (10.8%) |
| Ethnicity | ||
| Hispanic | 8 (2.8%) | 2 (3.1%) |
| Non-Hispanic | 275 (97.2%) | 63 (96.9%) |
| Household income | ||
| <$30,000 | 31 (11.0%) | 7 (10.8%) |
| $30,000--$50,000 | 127 (44.9%) | 28 (43.1%) |
| >$50,000 | 101 (35.7%) | 24 (36.9%) |
| Not reported | 24 (8.5%) | 6 (9.2%) |
| Education | ||
| <College | 131 (46.3%) | 29 (44.6%) |
| College | 99 (35.0%) | 23 (35.4%) |
| >College | 6 (18.7%) | 13 (20.0%) |
Procedures
All study assessments took place in a private research room. At the baseline preoperative visit, the research staff provided the participant with an assessment packet consisting of questions to elicit (in addition to or repetition of information obtained at presentation) demographic factors (age, gender, race/ethnicity), presence of comorbid disease, social factors (household income and highest level of education attained), and psychological factors (optimism, hope, self-efficacy, and locus of control), as well as the measure of patient activation. After completion of this baseline assessment, a subset of 65 participants was asked to complete a second administration of the patient activation measure in the following week and to mail it to the research office. For forms not received within 1 week of the baseline assessment, a reminder telephone call was made to the participant. All 65 forms were returned.
Measuring patient activation
Patient activation was measured using the PAM, which has been shown to be a reliable and well-validated tool for assessing patient activation among healthy respondents and individuals with chronic disease. In developing this measure, key psychological factors and personal competencies that are important in self-management of chronic health conditions were identified. This process resulted in a scale that was shown to be internally consistent (Cronbach’s alpha, 0.87) and to have good test-retest reliability. Criterion validity was measured through correlation of the scores on the PAM and assessment of patient activation by three independent judges after an open-ended, semi-structured interview. The correlation between the preliminary PAM and each of the individual judges was high (Cronbach’s alpha, 0.90, 0.80, and 0.80, respectively). Patient activation was revealed to be a hierarchical construct, with movement through the stages of activation occurring sequentially, dependent on an individual’s current level of activation.
We used a shortened version of the original PAM. In the development of the 22-item PAM, the authors conceded that its clinical use may be limited because of its length [36]. Thus, the 13-item PAM was created to increase the feasibility of measuring activation in a clinical setting. Based on an iterative examination of the reliability statistics from the original telephone survey of 1,515 respondents, a research team identified nine items that could be removed from the scale [36]. Removal of these items did not appreciably diminish the reliability or construct validity of the PAM [36]. The 13-item PAM scale is a participant-completed questionnaire that addresses key psychological factors (e.g., self-efficacy) and specific personal competencies (e.g., condition-specific knowledge and skills). From an individual’s responses on this questionnaire, a continuous activation score can be computed ranging from 0 (no activation) to 100 (high activation). As revealed during scale development, the PAM is a multistage scale in which each successive stage requires greater activation. The stages are: believing active role important (items 1 and 2); having confidence and knowledge to take action (items 3--8); taking action (items 9--11); and staying the course under stress (items 12 and 13). A previous report of this questionnaire, in populations of those with chronic disease and in healthy adults, has indicated that observed scores range between 40 and 80 points (average, 55 points) [30].
Psychological measures
To examine the construct validity of the PAM, its correlation with optimism, hope, self-efficacy, and locus of control were measured.
Optimism
Optimism is a general sense of confidence based on expectancies of attaining a goal and the value placed on that goal [37]. Evidence shows that individuals who are optimistic are less likely to experience stress when faced with difficulties [37-39]. Optimism was measured using the Life Orientation Test – Revised (LOT-R), a six-item questionnaire designed to assess optimism on a continuous scale [37,39]. Using this scale, participants are presented with positive and negative statements regarding their expectancies to reach positive goals and to avoid negative goals. The LOT-R has been shown to be valid across several populations [37], and the items on the LOT-R are internally consistent (Cronbach’s alpha, 0.76). This measure has previously been shown to be stable over time in assessing optimism [40].
Hope
Theory and research indicate that hope has two components: pathway (the ability to find workable pathways to goals) and agency (the motivation to take action to achieve those goals) [41]. The theory predicts that individuals who are more likely to identify multiple pathways to attain a given goal are more likely to overcome adversity. The Trait Hope Scale [42] consists of four agency, four pathway, and four distracter items. Individuals are asked to respond to individual statements using a 4-point Likert scale. The Trait Hope Scale provides a stable measure of hope by asking respondents to consider themselves across time and situational contexts. This scale has been shown to be a reliable (test-retest r range 0.73--0.82) and valid measure of hope [42,43].
Self-efficacy
Self-efficacy is the belief that an individual is able to perform specific tasks or activities. It has been shown that state-dependent customized measures of self-efficacy are useful in predicting behavior [44-50]. Self-efficacy to participate in physical therapy is a customized instrument (four items) based on the Arthritis Self-efficacy Scale [51] to assess an individual’s confidence to perform required exercises/tasks.
Locus of control
Locus of control refers to the extent to which a person believes his or her outcomes, in this case his or her health, are controlled by personal action (internal) versus outside forces (external) [52]. These expressions of control may be prognostic of better functional recovery and adherence [53]. In one study, adolescents with an internal locus of control experienced a more complete and timely recovery from spine surgery, which was thought to be a result of improved use of coping strategies [54]. The Multidimensional Health Locus of Control Scale is a reliable and well-validated 18-item scale [55] that provides estimates of assignment of control to internal and external (chance occurrence, powerful others, doctors, and other people) forces. Reliability statistics of the Multidimensional Health Locus of Control Scale range between 0.83 and 0.86 across a variety of clinical populations [55].
Depression
The PRIME-MD [56] is a nine-item brief screening tool designed to identify the presence of depressive symptoms. This tool was developed using the diagnostic criteria from the Diagnostic and Statistical Manual of Mental Disorders. In a psychometric study of the PRIME-MD compared with structured clinical interviews, the PRIME-MD was both sensitive (0.73) and specific (0.89) for the diagnosis of major depression [56].
Health status
The Charlson Comorbidity Index is a well-validated means of risk adjustment for in-hospital complications and mortality [57]. This index provides a weighted score based on the severity of present comorbid medical conditions. Individual participants are asked to indicate whether a physician or other healthcare provider has informed them that they have any of a series of 16 conditions, such as hypertension [58].
Data analysis
Type I error rate of 0.05 was used to determine statistical significance.
Test-retest reliability
A subset of 65 participants completed a second administration of the PAM within one week of the initial presentation, and the responses were correlated [59] with those of the original baseline PAM. The Shrout Fleiss Intraclass Correlation Coefficient was used to estimate agreement between these two administrations of the PAM [60]. Intraclass correlation coefficients of at least 0.80 are indicative of acceptable test-retest reliability [61]. In addition to test-retest reliability, internal consistency of the PAM was estimated using split-half scores from the baseline PAM. A correlation coefficient between these split-half scores was then computed using the Spearman-Brown formula [59] for the underestimation of true internal consistency. A Spearman-Brown adjusted measure of at least 0.80 is generally considered good evidence of internal consistency [62].
Construct validity
Support for the construct validity of the PAM was built through the use of convergent and divergent evidence using response data from the entire set of 283 participants [63]. Convergent evidence was obtained through observation of relatively high correlation coefficients between the PAM and measures to assess optimism, hope, self-efficacy to participate in physical therapy, and locus of control. An observed correlation between two variables of 0.60 or less indicates poor convergent evidence [64].
Divergent evidence was obtained through observation of relatively low correlation coefficients between the PAM and scales to measure unrelated constructs, such as measures of comorbid conditions [36]. An a priori rule for divergent validity between PAM and these measures was an observed correlation of less than 0.40 [62].
Confirmatory factor analysis
Confirmatory factor analysis was used to evaluate whether the prespecified four-factor structure for patient activation proposed by Hibbard et al. [30] provided a good fit to the observed data. We used the CALIS procedure from the SAS statistical package (SAS Institute, Inc., Cary, NC) on the variance-covariance matrix. Confirmatory factor analysis assesses goodness of fit based on the variance remaining after the factors have been taken into account [65]. Goodness of fit was tested using the Goodness of Fit Index (GFI), adjusted GFI, Bentler’s comparative fit index [66], and the Root Mean Square Error of Approximation. Scores on these indices of more than 0.90 on the GFI, adjusted GFI, and Bentler’s comparative fit index [66] were considered evidence of fit between the model and the data. Regarding the Root Mean Square Error of Approximation, values less than 0.08 are considered to indicate an adequate fit and those less than 0.05 are considered to indicate a good fit between then model and the data.
The four-factor model proposed by Hibbard et al. [30] consisted of: (1) believes taking an active role is important; (2) has confidence and knowledge to take action; (3) takes action; and (4) stays the course under pressure. The fit of this four-factor structure was compared with the fit to the data of alternate models. Three models were considered as alternates: one-factor, two-factor, and three-factor models. In the one-factor model, all 13 items of the PAM were specified to constitute one general factor. In the two-factor model, the first eight items (related to beliefs, confidence, and knowledge) were specified to identify with the first factor, whereas the last five items (related to action and perseverance) were specified to identify with the second factor. In the three-factor model, the first two items (related to beliefs) were specified to identify with the first factor, the middle six items (related to confidence and knowledge) were specified to identify with the second factor, and the last five items (related to action and perseverance) were specified to identify with the third factor.
Results
Distribution of scores
At baseline, mean patient activation was 63.67 (SD = 18.06; range, 20.9--100.0). The distribution of scores approximated a normal distribution (Shapiro-Wilk W = 0.982, P = 0.273). The range of these scores was similar to that reported in the literature [30,36].
Test-retest reliability
There was no statistically significant difference between the patient activation scores at baseline and the following week (paired t-test = 1.25, P = 0.212). This measure showed strong evidence of agreement between the two administrations of the PAM (Shrout Fleiss Intraclass Correlation Coefficient = 0.84).
The internal consistency of the PAM as measured by split-half reliability was 0.92. This exceeds the a priori rule of internal consistency of 0.80 to show internal consistency of the PAM.
Construct validity
Relatively high correlation between the PAM and other theoretically related measures provided convergent evidence of the construct’s validity (Table 2). The PAM showed high correlation with measures of optimism (LOT-R) (r = 0.75), of hope (Trait Hope Scale) (r = 0.73), of self-efficacy to participate in physical therapy (r = 0.65), and of internalized locus of control (Multidimensional Health Locus of Control Scale) (r = 0.66). The PAM showed a small but consistently negative correlation with measures of externalized control. We observed a negative correlation between presence of depressive symptoms and patient activation (r = -0.13), indicating that individuals with greater depressive symptoms endorse lower levels of patient activation. The hypotheses that patient activation would be relatively unrelated to measures of comorbidity were confirmed. Measures of comorbid disease severity were not significantly correlated with patient activation (r = 0.01).
Table 2.
Correlation between the Patient Activation Measure and individual psychological assessment scales
| Psychological Assessment Scale | r | P-value |
|---|---|---|
| Life Orientation Test – Revised | 0.754 | <0.001 |
| Trait Hope Scale | ||
| Agency | 0.681 | <0.001 |
| Pathway | 0.535 | <0.001 |
| Total | 0.731 | <0.001 |
| Self efficacy for physical therapy | 0.650 | <0.001 |
| Multi-Dimensional Health Locus of Control | ||
| Internal | 0.659 | <0.001 |
| Chance | -0.114 | 0.055 |
| Powerful others | -0.35 | <0.001 |
| Doctors | -0.391 | <0.001 |
| Other people | 0.137 | 0.021 |
| Depression, PRIME-MD | -0.128 | 0.032 |
| Charlson Comorbidity Index | 0.007 | 0.904 |
Confirmatory factor analysis
Fit values for the four-factor model and the three alternate models are presented in Table 3. In examining the goodness of fit statistics, it is clear that neither the one-factor nor the two-factor models are particularly suited to explain the variability in the data. The three-factor model (Fig. 1) showed a better fit relative to the four-factor model (Chi-square change = 160.06, df = 1, P < 0.001). When examining the fit statistics for the three-factor model, the overall fit was reasonable. All parameters from the manifested indicators to the latent variables were statistically significant (t > 1.96, P < 0.05).
Table 3.
Fit indices for the Confirmatory Factor Analysis models
| Model | GFI | Adjusted GFI | RMSR | CFI | Parsimonious NFI | Chi-square | df | ECVI (90% CI) |
|---|---|---|---|---|---|---|---|---|
| 1-factor | 0.736 | 0.631 | 0.090 | 0.772 | 0.625 | 525.77 | 65 | 2.059 (1.807, 2.338) |
| 2-factor | 0.830 | 0.759 | 0.076 | 0.852 | 0.679 | 362.39 | 64 | 1.487 (1.284, 1.718) |
| 3-factor | 0.869 | 0.807 | 0.064 | 0.896 | 0.691 | 273.32 | 62 | 1.186 (1.014, 1.3860 |
| 4-factor | 0.841 | 0.762 | 0.272 | 0.816 | 0.621 | 433.38 | 61 | 1.761 (1.535, 2.015 |
GFI, Goodness of Fit Index; Adjusted GFI, GFI adjusted for degrees of freedom; RMSR, Root Mean Square Residual; CFI, Bentler’s Comparative Fit Index; Parsimonious NFI, Normed Fit Index; df, degrees of freedom; ECVI, Expected Cross-Validation Index; CI, Confidence Interval.
Fig. 1.
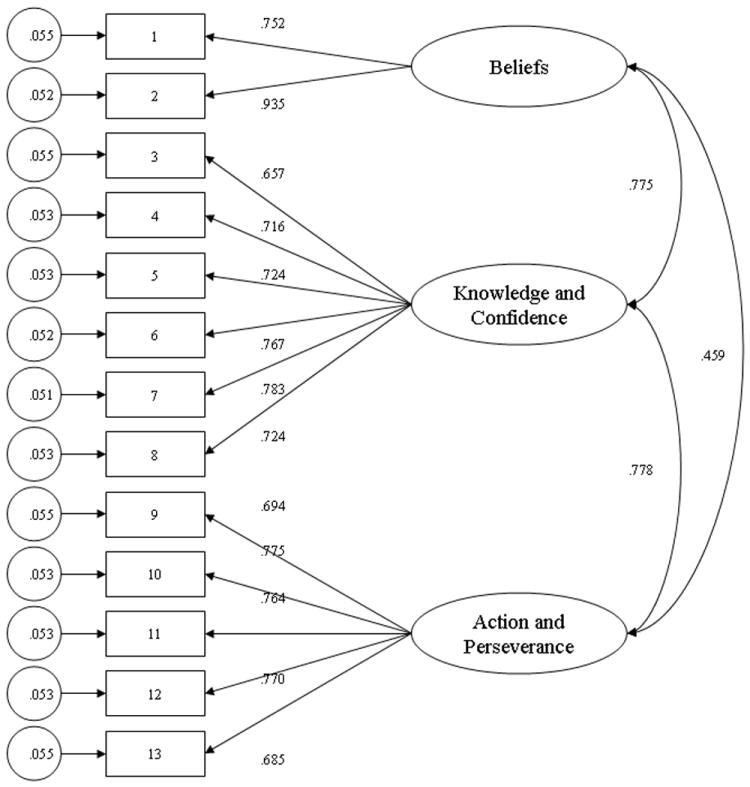
Three-factor model for patient activation. This model indicates the item to factor relationships as well as the relationships among the factors.
Discussion
We investigated the psychometric properties of the PAM in a population of individuals undergoing elective spine surgery. We have shown that the PAM is a reliable and stable measure in this population, with high test-retest reliability. This conclusion was further supported by good internal consistency of the individual items of the scale, shown when using a split-half reliability assessment.
The correlation data provide convergent evidence for the construct validity of the PAM. The data show strong positive correlations between scores on the PAM and scores on measures of optimism, hope, self-efficacy, and internalized locus of control. These findings support our hypothesis that highly activated individuals would also report higher levels of positive psychological resources that may be marshaled when faced with adversity, such as recovery from surgery. There was moderate evidence for construct validity in the form of negative correlations between patient activation and externalized locus of control, indicating that highly activated individuals are less likely to assign influence over their health state to external sources. The PAM assesses personal responsibility and confidence, and does not specifically ask an individual to ascribe a locus of control for his or her health to doctors or other people. Therefore, a modest negative correlation is to be expected. The negative correlation between patient activation and depressive symptoms supports the hypothesis that there would be a divergent relationship between these two constructs. The observed weak correlation between patient activation and presence of comorbid disease supports the notion that patient activation is unrelated to the number of comorbid conditions.
In developing the PAM, Hibbard et al. [30] posited a multistage theoretical model for patient activation. The Stages of Change (SOC) model assumes variation in the degree to which individuals are prepared to change their behavior [67]. This model suggests that readiness to change is situation specific, i.e. an individual’s readiness to engage in a weight loss program may be different than his or her readiness to engage in a smoking cessation program. The patient activation model is hypothesized to be trans-situational, i.e. patient activation is believed to be relatively stable across health care behaviors. The SOC model provides a theoretical basis for the PAM and for the organization of the scale items in a hierarchical fashion. Both the SOC and PAM may be used to guide tailored interventions. Using responses from the PAM, a healthcare provider can design a treatment plan that takes into account that individual’s level of activation and the psychological factors and personal competencies that contribute to it. Our confirmatory factor analysis provided evidence that patient activation is a multistage construct; however, it diverged slightly from the theoretical model described by Hibbard et al. [30]. The first two factors (Beliefs, and Confidence and Knowledge) corresponded to the structure outlined in the development of the theory of patient activation. The final factor (Action and Perseverance) was a combination of the two final stages of activation observed by Hibbard et al. [30]. Additional work is needed to determine if the structure observed in this study is unique to this sample or may reflect a difference that exists between chronic and acute medical populations.
Additional investigation is needed to understand what facilitates patient activation and the factors that determine how individuals move from one stage to another. Development of patient activation and transition from one stage to the next is likely to depend on a complex interplay of specific psychological factors and the mastery of certain personal competencies. The role of these psychological factors and personal competencies may change, depending on an individual’s current stage of patient activation. Although considerable work is necessary to understand the determinants and process by which patient activation develops, the PAM has potential as a single measure that captures multiple factors that contribute to patient’s engagement in their health care. Rather than measuring multiple dimensions, the PAM may be useful in identifying individuals in chronic and surgical settings who have low activation and thus are at risk for poor follow through on medical or rehabilitation recommendations. Scarce resources for improving adherence and self-care behaviors can then be targeted for these individuals.
Our study has certain limitations that should be taken into account when interpreting these findings. First, our sample was relatively small when compared with the reported samples on which the PAM was initially developed; however, the sample is representative of a cross-section of individuals who undergo elective spine surgery. This observation is supported by the similarity of the demographic characteristics of our study to those of other published reports in this population [23,35]. Second, our sample was drawn from a single institution, which may hinder the ability to generalize these findings to other settings or types of institutions. However, our sample was recruited from two hospitals (an academic teaching hospital and a community hospital). Nevertheless, additional testing at various types of institutions would extend the validation process. Third, we made extensive use of self-reported information for the presence of comorbid conditions and depressive symptoms. It is possible that respondent answers concerning past medical history were subject to poor recall, lack of information, or discomfort with self-disclosure regarding medical/psychiatric diagnoses and symptoms.
Our study has provided evidence for the use of the PAM as a preoperative measure for assessing psychological factors and personal competencies in individuals undergoing spine surgery. With this instrument, healthcare providers can assess an individual’s readiness to engage in adaptive health behaviors that may, in turn, lead to improved outcome. Using responses from the PAM, a healthcare provider may able to design a treatment plan that takes into account that individual’s level of activation and the psychological factors and personal competencies that contribute to it.
The importance that patient activation places on the individual patient as an agent of change follows closely with recommendations from the Institute of Medicine [68] in designing patient-centered care to improve the quality of healthcare. Focusing on an individual’s readiness to engage in self-management of his or her health moves the spotlight from the technological aspects of healthcare to methods of empowering an individual to care for his or her own health. Through the establishment of well-validated and reliable measures in this new population, we will be able to investigate further the role of patient activation in explaining postoperative health behavior, such as adherence to physical therapy and the variation that has been reported in functional recovery after spine surgery.
Acknowledgments
This project was supported by grant number 1 R03 HS016106 from the Agency for Healthcare Research and Quality.
References
- 1.Junge A, Frohlich M, Ahrens S, Hasenbring M, Sandler AJ, Grob D, et al. Predictors of bad and good outcome of lumbar spine surgery: a prospective clinical study with 2 years’ follow-up. Spine. 1996;21(9):1056–1064. doi: 10.1097/00007632-199605010-00013. [DOI] [PubMed] [Google Scholar]
- 2.Turner JA, Ersek M, Herron L, Deyo R. Surgery for lumbar spinal stenosis. Attempted meta-analysis of the literature. Spine. 1992;17(1):1–8. doi: 10.1097/00007632-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Shabat S, Leitner Y, Nyska M, Berner Y, Fredman B, Gepstein R. Surgical treatment of lumbar spinal stenosis in patients aged 65 years and older. Arch Gerontol Geriatr. 2002;35(2):143–152. doi: 10.1016/s0167-4943(02)00016-x. [DOI] [PubMed] [Google Scholar]
- 4.Goldman L. Cardiac risks and complications of noncardiac surgery. Ann Intern Med. 1983;98(4):504–513. doi: 10.7326/0003-4819-98-4-504. [DOI] [PubMed] [Google Scholar]
- 5.Johnson JC. Surgical assessment in the elderly. Geriatrics. 1988;43(Suppl):83–89. discussion 90. [PubMed] [Google Scholar]
- 6.Ergina PL, Gold SL, Meakins JL. Perioperative care of the elderly patient. World J Surg. 1993;17(2):192–198. doi: 10.1007/BF01658926. [DOI] [PubMed] [Google Scholar]
- 7.Navarro V. Race or class versus race and class: mortality differentials in the United States. Lancet. 1990;336(8725):1238–1240. doi: 10.1016/0140-6736(90)92846-a. [DOI] [PubMed] [Google Scholar]
- 8.Braithwaite RL, Taylor SE, editors. Health Issues in the Black Community. San Francisco: Jossey-Bass; 1992. [Google Scholar]
- 9.Furino A. Health Policy and the Hispanic. Boulder (CO): Westview Press; 1992. [Google Scholar]
- 10.Livingston IL, editor. Handbook of Black American Health: the Mosaic of Conditions, Issues, Policies, and Prospects. Westport (CT): Greenwood Press; 1994. [Google Scholar]
- 11.Selim AJ, Fincke G, Ren XS, Deyo RA, Lee A, Skinner K, et al. Racial differences in the use of lumbar spine radiographs. Results from the Veterans Health Study. Spine. 2001;26(12):1364–1369. doi: 10.1097/00007632-200106150-00021. [DOI] [PubMed] [Google Scholar]
- 12.Grant MD, Herman S. Ethnic and geographic variation in gastrostomy placement among hospitalized older patients. J Natl Med Assoc. 2004;96(10):1346–1349. [PMC free article] [PubMed] [Google Scholar]
- 13.Andreshak TG, An HS, Hall J, Stein B. Lumbar spine surgery in the obese patient. J Spinal Disord. 1997;10(5):376–379. [PubMed] [Google Scholar]
- 14.Telfeian AE, Reiter GT, Durham SR, Marcotte P. Spine surgery in morbidly obese patients. J Neurosurg Spine. 2002;97(1):20–24. doi: 10.3171/spi.2002.97.1.0020. [DOI] [PubMed] [Google Scholar]
- 15.Olsen MA, Mayfield J, Lauryssen C, Polish LB, Jones M, Vest J, et al. Risk factors for surgical site infection in spinal surgery. J Neurosurg. 2003;98(2):149–155. [PubMed] [Google Scholar]
- 16.Adler NE, Boyce WT, Chesney MA, Folkman S, Syme SL. Socioeconomic inequalities in health. No easy solution. JAMA. 1993;269(24):3140–3145. [PubMed] [Google Scholar]
- 17.Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, et al. Socioeconomic status and health. The challenge of the gradient. Am Psychol. 1994;49(1):15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 18.Laxton AW, Perrin RG. The relations between social support, life stress, and quality of life following spinal decompression surgery. Spinal Cord. 2003;41(10):553–558. doi: 10.1038/sj.sc.3101432. [DOI] [PubMed] [Google Scholar]
- 19.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4(6):561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 20.Katz JN, Stucki G, Lipson SJ, Fossel AH, Grobler LJ, Weinstein JN. Predictors of surgical outcome in degenerative lumbar spinal stenosis. Spine. 1999;24(21):2229–2233. doi: 10.1097/00007632-199911010-00010. [DOI] [PubMed] [Google Scholar]
- 21.Kjellby-Wendt G, Styf JR, Carlsson SG. The predictive value of psychometric analysis in patients treated by extirpation of lumbar intervertebral disc herniation. J Spinal Disord. 1999;12(5):375–379. [PubMed] [Google Scholar]
- 22.Schade V, Semmer N, Main CJ, Hora J, Boos N. The impact of clinical, morphological, psychosocial and work-related factors on the outcome of lumbar discectomy. Pain. 1999;80(1-2):239–249. doi: 10.1016/s0304-3959(98)00210-3. [DOI] [PubMed] [Google Scholar]
- 23.Trief PM, Grant W, Fredrickson B. A prospective study of psychological predictors of lumbar surgery outcome. Spine. 2000;25(20):2616–2621. doi: 10.1097/00007632-200010150-00012. [DOI] [PubMed] [Google Scholar]
- 24.Dvorak J, Gauchat MH, Valach L. The outcome of surgery for lumbar disc herniation. I. A 4-17 years’ follow-up with emphasis on somatic aspects. Spine. 1988;13(12):1418–1422. doi: 10.1097/00007632-198812000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Dvorak J, Valach L, Fuhrimann P, Heim E. The outcome of surgery for lumbar disc herniation. II. A 4-17 years’ follow-up with emphasis on psychosocial aspects. Spine. 1988;13(12):1423–1427. doi: 10.1097/00007632-198812000-00016. [DOI] [PubMed] [Google Scholar]
- 26.White AH, Rothman RH, Ray CD, editors. Lumbar Spine Surgery. Techniques and Complications. St. Louis: C.V. Mosby Co; 1987. [Google Scholar]
- 27.Fritzell P, Hagg O, Wessberg P, Nordwall A Swedish Lumbar Spine Study Group. Chronic low back pain and fusion: a comparison of three surgical techniques. A prospective multicenter randomized study from the Swedish Lumbar Spine Study Group. Spine. 2002;27(11):1131–1141. doi: 10.1097/00007632-200206010-00002. [DOI] [PubMed] [Google Scholar]
- 28.Mannion AF, Elfering A, Staerkle R, Junge A, Grob D, Dvorak J, et al. Predictors of multidimensional outcome after spinal surgery. Eur Spine J. 2007;16(6):777–786. doi: 10.1007/s00586-006-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Von Korff M, Gruman J, Schaefer J, Curry SJ, Wagner EH. Collaborative management of chronic illness. Ann Intern Med. 1997;127(12):1097–1102. doi: 10.7326/0003-4819-127-12-199712150-00008. [DOI] [PubMed] [Google Scholar]
- 30.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the patient activation measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39(4):1005–1026. doi: 10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams GC, McGregor HA, Zeldman A, Freedman ZR, Deci EL. Testing a self-determination theory process model for promoting glycemic control through diabetes self-management. Health Psychol. 2004;23(1):58–66. doi: 10.1037/0278-6133.23.1.58. [DOI] [PubMed] [Google Scholar]
- 32.Williams GC, McGregor H, Zeldman A, Freedman ZR, Deci EL, Elder D. Promoting glycemic control through diabetes self-management: evaluating a patient activation intervention. Patient Educ Couns. 2005;56(1):28–34. doi: 10.1016/j.pec.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Skolasky RL, MacKenzie EJ, Wegener ST, Riley LH., III Patient activation and adherence to physical therapy in persons undergoing spine surgery. Spine. 2008;33(21):E784–E791. doi: 10.1097/BRS.0b013e31818027f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eichholz KM, Ryken TC. Complications of revision spinal surgery. Neurosurg Focus. 2003;15(3):E1–E4. doi: 10.3171/foc.2003.15.3.1. [DOI] [PubMed] [Google Scholar]
- 35.Fritzell P, Hagg O, Nordwall A Swedish Lumbar Spine Study Group. Complications in lumbar fusion surgery for chronic low back pain: comparison of three surgical techniques used in a prospective randomized study. A report from the Swedish Lumbar Spine Study Group. Eur Spine J. 2003;12(2):178–189. doi: 10.1007/s00586-002-0493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the Patient Activation Measure. Health Serv Res. 2005;40(6):1918–1930. doi: 10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carver CS, Scheier MF. Optimism. In: Snyder CR, Lopez SJ, editors. Handbook of Positive Psychology. New York: Oxford University Press; 2005. pp. 231–243. [Google Scholar]
- 38.Taylor SE, Kemeny ME, Aspinwall LG, Schneider SG, Rodriguez R, Herbert M. Optimism, coping, psychological distress, and high-risk sexual behavior among men at risk for acquired immunodeficiency syndrome (AIDS) J Pers Soc Psychol. 1992;63(3):460–473. doi: 10.1037//0022-3514.63.3.460. [DOI] [PubMed] [Google Scholar]
- 39.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67(6):1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 40.Scheier MF, Carver CS. Optimism, coping, and health: assessment and implications of generalized outcome expectancies. Health Psychol. 1985;4(3):219–247. doi: 10.1037//0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- 41.Snyder CR, Rand KL, Sigmon DR. Hope theory. A member of the positive psychology family. In: Snyder CR, Lopez SJ, editors. Handbook of Positive Psychology. New York: Oxford University Press; 2005. pp. 257–276. [Google Scholar]
- 42.Snyder CR, Harris C, Anderson JR, Holleran SA, Irving LM, Sigmon ST, et al. The will and the ways: development and validation of an individual-differences measure of hope. J Pers Soc Psychol. 1991;60(4):570–585. doi: 10.1037//0022-3514.60.4.570. [DOI] [PubMed] [Google Scholar]
- 43.Babyak MA, Snyder CR, Yoshinobu L. Psychometric properties of the Hope Scale: a confirmatory factor analysis. J Res Pers. 1993;27:154–169. [Google Scholar]
- 44.Strecher VJ, DeVellis BM, Becker MH, Rosenstock IM. The role of self-efficacy in achieving health behavior change. Health Educ Q. 1986;13(1):73–92. doi: 10.1177/109019818601300108. [DOI] [PubMed] [Google Scholar]
- 45.Sitharthan T, Kavanagh DJ. Role of self-efficacy in predicting outcomes from a programme for controlled drinking. Drug Alcohol Depend. 1990;27(1):87–94. doi: 10.1016/0376-8716(91)90091-c. [DOI] [PubMed] [Google Scholar]
- 46.Johnson JA. Self-efficacy theory as a framework for community pharmacy-based diabetes education programs. Diabetes Educ. 1996;22(3):237–241. doi: 10.1177/014572179602200307. [DOI] [PubMed] [Google Scholar]
- 47.Fontaine KR, Cheskin LJ. Self-efficacy, attendance, and weight loss in obesity treatment. Addict Behav. 1997;22(4):567–570. doi: 10.1016/s0306-4603(96)00068-8. [DOI] [PubMed] [Google Scholar]
- 48.Colon RM, Wiatrek DE, Evans RI. The relationship between psychosocial factors and condom use among African-American adolescents. Adolescence. 2000;35(139):559–569. [PubMed] [Google Scholar]
- 49.Fortenberry JD, Brizendine EJ, Katz BP, Orr DP. The role of self-efficacy and relationship quality in partner notification by adolescents with sexually transmitted infections. Arch Pediatr Adolesc Med. 2002;156(11):1133–1137. doi: 10.1001/archpedi.156.11.1133. [DOI] [PubMed] [Google Scholar]
- 50.Zebracki K, Drotar D. Outcome expectancy and self-efficacy in adolescent asthma self-management. Child Health Care. 2004;33(2):133–149. [Google Scholar]
- 51.Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989;32(1):37–44. doi: 10.1002/anr.1780320107. [DOI] [PubMed] [Google Scholar]
- 52.Thompson SC. The role of personal control in adaptive functioning. In: Snyder CR, Lopez SJ, editors. Handbook of Positive Psychology. New York: Oxford University Press; 2005. pp. 202–213. [Google Scholar]
- 53.O’Hea EL, Grothe KB, Bodenlos JS, Boudreaux ED, White MA, Brantley PJ. Predicting medical regimen adherence: the interactions of health locus of control beliefs. J Health Psychol. 2005;10(5):705–717. doi: 10.1177/1359105305055330. [DOI] [PubMed] [Google Scholar]
- 54.LaMontagne LL, Hepworth JT, Cohen F, Salisbury MH. Adolescents’ coping with surgery for scoliosis: effects on recovery outcomes over time. Res Nurs Health. 2004;27(4):237–253. doi: 10.1002/nur.20026. [DOI] [PubMed] [Google Scholar]
- 55.Wallston KA, Wallston BS, DeVellis R. Development of the Multidimensional Health Locus of Control (MHLC) scales. Health Educ Monogr. 1978;6(2):160–170. doi: 10.1177/109019817800600107. [DOI] [PubMed] [Google Scholar]
- 56.Spitzer RL, Williams JBW, Kroenke K, Linzer M, deGruy FV, III, Hahn SR, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. J A M A. 1994;272(22):1749–1756. [PubMed] [Google Scholar]
- 57.O’Connell RL, Lim LL. Utility of the Charlson comorbidity index computed from routinely collected hospital discharge diagnosis codes. Methods Inf Med. 2000;39(1):7–11. [PubMed] [Google Scholar]
- 58.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 59.Campbell DT, Stanley JC. Experimental and Quasi-experimental Designs for Research. Boston: Houghton Mifflin Co; 1963. [Google Scholar]
- 60.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 61.Kerlinger FN. Foundations of Behavioral Research. New York: Holt, Rinehart and Winston, Inc; 1986. [Google Scholar]
- 62.Gliner JA, Morgan GA. Research Methods in Applied Settings: An Integrated Approach to Design and Analysis. Mahwah (NJ): Lawrence Erlbaum Associates; 2000. [Google Scholar]
- 63.Campbell DT, Fiske DW. Convergent and discriminant validation by the multitrait-multimethod matrix. Psychol Bull. 1959;56(2):81–105. [PubMed] [Google Scholar]
- 64.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Measure. 1960;20:37–46. [Google Scholar]
- 65.Floyd FJ, Widaman KF. Factor analysis in the development and refinement of clinical assessment instruments. Psychol Assess. 1995;7(3):286–299. [Google Scholar]
- 66.Bentler PM, Bonett DG. Significance tests and goodness-of-fit in the analysis of covariance structures. Psychol Bull. 1980;88(3):588–606. [Google Scholar]
- 67.Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol. 1992;47(9):1102–1114. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- 68.Committee on Quality Health Care in America, Institute of Medicine. Crossing the Quality Chasm. A New Health System for the 21st Century. Washington, D.C.: National Academy Press; 2001. [Google Scholar]


