Abstract
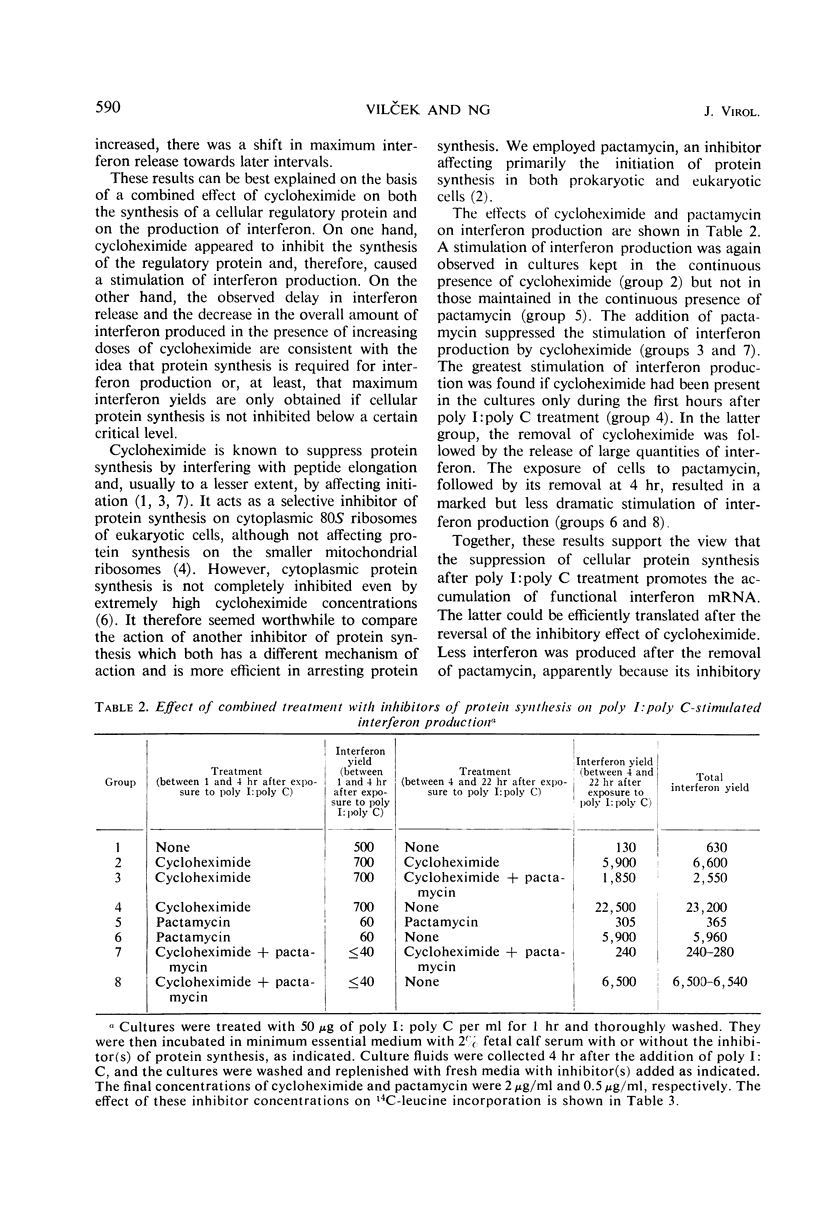
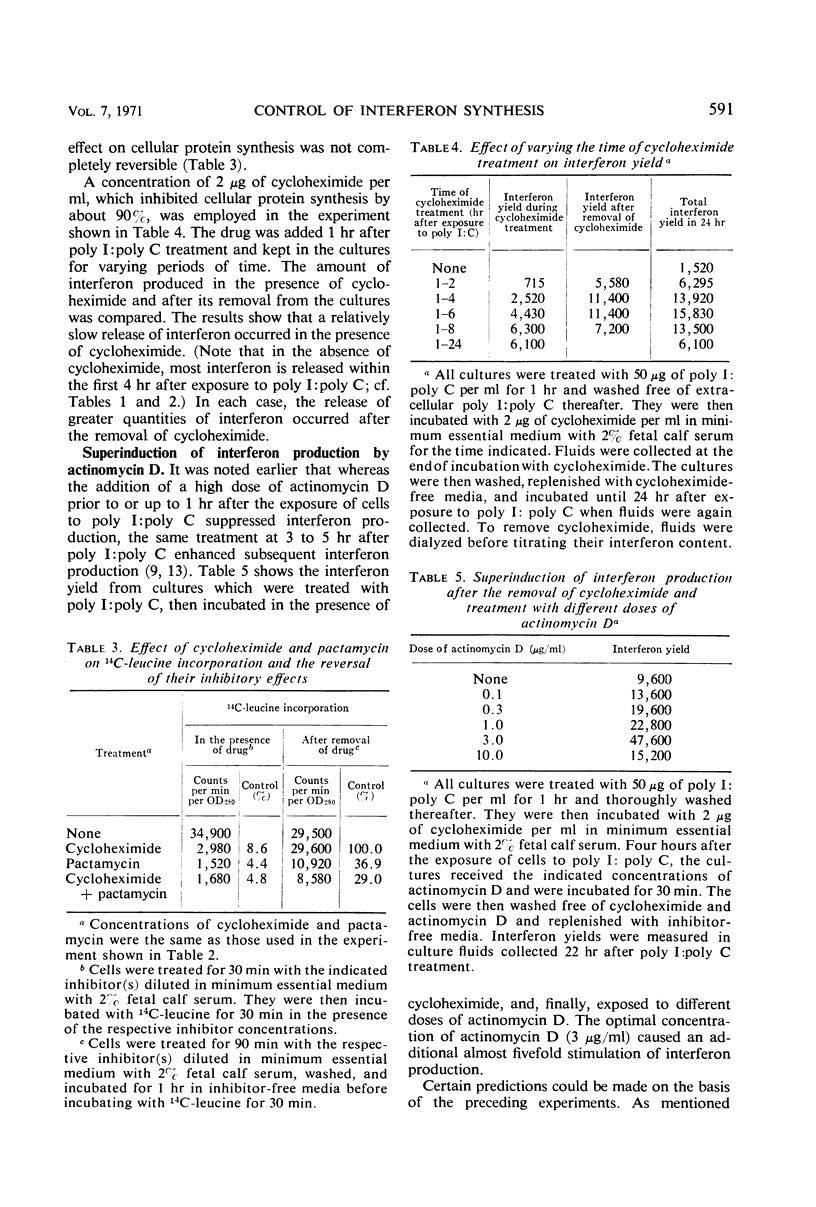
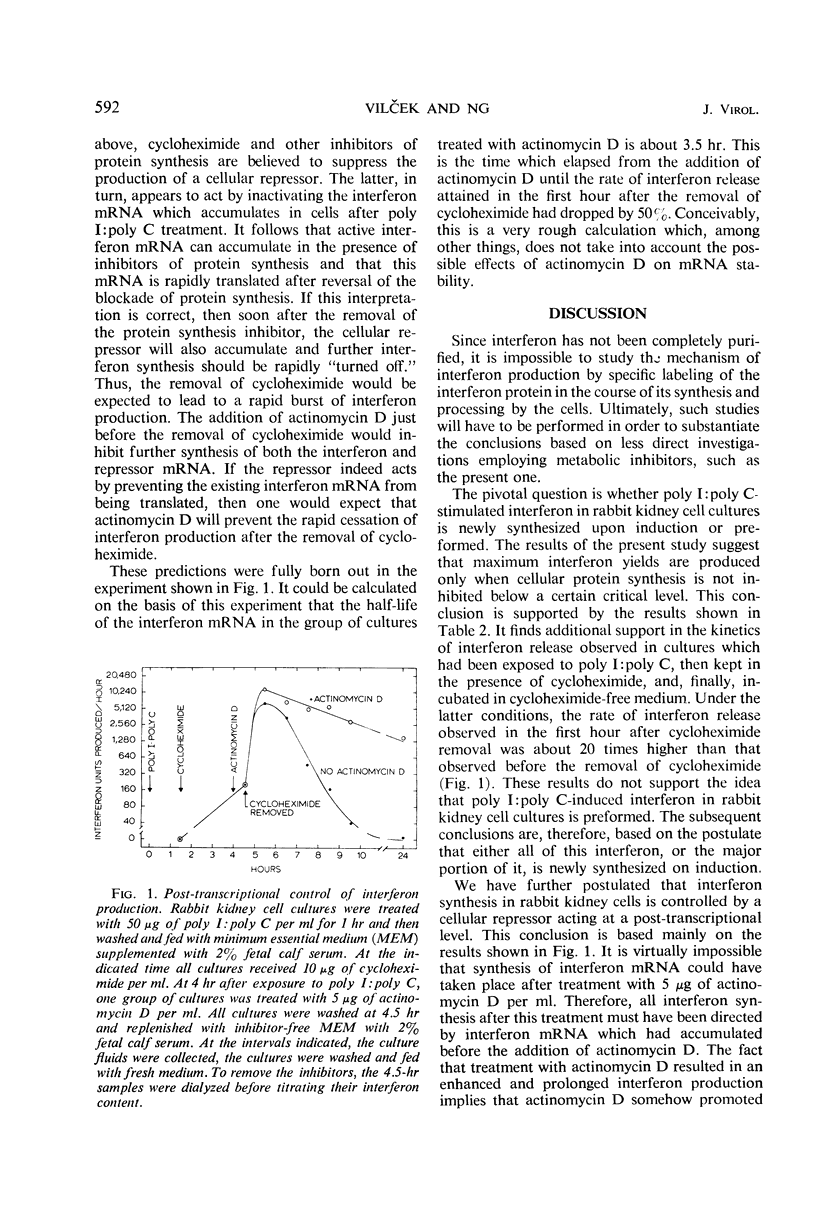
Low to moderate doses of cycloheximide had a stimulatory effect on interferon production in rabbit kidney cell cultures treated with double-stranded polyinosinate-polycytidylate (poly I:poly C). A very marked stimulation occurred in the presence of a dose of cycloheximide inhibiting amino acid incorporation into total cellular protein by about 75%. Higher doses of cycloheximide caused a shift in interferon release towards later intervals and a gradual decrease in the overall degree of stimulation. An even greater increase in the amount of interferon produced was observed if cells were treated with cycloheximide for only 3 to 4 hr immediately after their exposure to poly I:poly C. Under the latter conditions, a rapid burst of interferon production occurred after the reversal of cycloheximide action. Treatment with a high dose of actinomycin D before the reversal of cycloheximide action caused a further increase and a marked prolongation of interferon production. It is postulated that inhibitors of protein synthesis suppress the accumulation of a cellular regulatory protein (repressor) which interacts with the interferon messenger ribonucleic acid mRNA and thereby prevents its translation. Therefore, active interferon mRNA can apparently accumulate in rabbit kidney cells which, after exposure to poly I:poly C, are kept in the presence of an inhibitor of protein synthesis. Some of this accumulated interferon mRNA can be translated during a partial block of cellular protein synthesis, but its most efficient translation occurs after the reversal of the action of the protein synthesis inhibitor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baliga B. S., Pronczuk A. W., Munro H. N. Mechanism of cycloheximide inhibition of protein synthesis in a cell-free system prepared from rat liver. J Biol Chem. 1969 Aug 25;244(16):4480–4489. [PubMed] [Google Scholar]
- Cohen L. B., Herner A. E., Goldberg I. H. Inhibition by pactamycin of the initiation of protein synthesis. Binding of N-acetylphenylalanyl transfer ribonucleic acid and polyuridylic acid to ribosomes. Biochemistry. 1969 Apr;8(4):1312–1326. doi: 10.1021/bi00832a004. [DOI] [PubMed] [Google Scholar]
- Fan H., Penman S. Regulation of protein synthesis in mammalian cells. II. Inhibition of protein synthesis at the level of initiation during mitosis. J Mol Biol. 1970 Jun 28;50(3):655–670. doi: 10.1016/0022-2836(70)90091-4. [DOI] [PubMed] [Google Scholar]
- Lamb A. J., Clark-Walker G. D., Linnane A. W. The biogenesis of mitochondria. 4. The differentiation of mitochondrial and cytoplasmic protein synthesizing systems in vitro by antibiotics. Biochim Biophys Acta. 1968 Jul 23;161(2):415–427. [PubMed] [Google Scholar]
- Muramatsu M., Shimada N., Higashinakagawa T. Effect of cycloheximide on the nucleolar RNA synthesis in rat liver. J Mol Biol. 1970 Oct 14;53(1):91–106. doi: 10.1016/0022-2836(70)90047-1. [DOI] [PubMed] [Google Scholar]
- Perlman S., Penman S. Mitochondrial protein synthesis: resistance to emetine and response to RNA synthesis inhibitors. Biochem Biophys Res Commun. 1970 Aug 24;40(4):941–948. doi: 10.1016/0006-291x(70)90994-0. [DOI] [PubMed] [Google Scholar]
- Stanners C. P. The effect of cycloheximide on polyribosomes from hamster cells. Biochem Biophys Res Commun. 1966 Sep 8;24(5):758–764. doi: 10.1016/0006-291x(66)90390-1. [DOI] [PubMed] [Google Scholar]
- Sugiyama T. Translational control of MS2 RNA cistrons. Cold Spring Harb Symp Quant Biol. 1969;34:687–694. doi: 10.1101/sqb.1969.034.01.078. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Armstrong J. A., Ke Y. H., Ho M. Regulation of cellular interferon production: enhancement by antimetabolites. Proc Natl Acad Sci U S A. 1970 Sep;67(1):464–471. doi: 10.1073/pnas.67.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins G. M., Gelehrter T. D., Granner D., Martin D., Jr, Samuels H. H., Thompson E. B. Control of specific gene expression in higher organisms. Expression of mammalian genes may be controlled by repressors acting on the translation of messenger RNA. Science. 1969 Dec 19;166(3912):1474–1480. doi: 10.1126/science.166.3912.1474. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Rossman T. G., Varacalli F. Differential effects of actinomycin D and puromycin on the release of interferon induced by double stranded RNA. Nature. 1969 May 17;222(5194):682–683. doi: 10.1038/222682a0. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Hallum J. V. Interferon production in mice by double-stranded synthetic polynucleotides: induction or release? Virology. 1968 May;35(1):177–179. doi: 10.1016/0042-6822(68)90320-6. [DOI] [PubMed] [Google Scholar]
- Youngner J. S. Influence of inhibitors of protein synthesis on interferon formation in mice. II. Comparison of effects of glutarimide antibiotics and tenuazonic acid. Virology. 1970 Feb;40(2):335–343. doi: 10.1016/0042-6822(70)90410-1. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Stinebring W. R., Taube S. E. Influence of inhibitors of protein synthesis on interferon formation in mice. Virology. 1965 Dec;27(4):541–550. doi: 10.1016/0042-6822(65)90179-0. [DOI] [PubMed] [Google Scholar]


