Introduction
In clinical practice, diseases of the neuromuscular system, particularly those which are of primary muscle origin, are evaluated with history and examination, electrodiagnostic testing (nerve conduction testing and electromyography), genetic testing and muscle biopsy. Recently, there has been increasing interest in non-invasive imaging modalities, particularly muscle MRI, for the diagnosis and assessment of disease progression for a number of neuromuscular diseases, including Duchenne muscular dystrophy (DMD).
Muscle Imaging
Muscle Ultrasound
The use of muscle ultrasound in the assessment of muscle disease began to gain interest in the 1980’s (Heckmatt, Dubowitz, & Leeman, 1980; Heckmatt, Leeman, & Dubowitz, 1982). This technique is still used clinically to identify muscle involvement (Mercuri et al., 2007) and to aid in the selection of muscles for biopsy. However, the utility of muscle ultrasound is limited by the fact that this technique is highly operator-dependent and not all muscles can be adequately assessed.
Muscle MRI and MRS
Magnetic Resonance Imaging (MRI) is a noninvasive imaging method, without ionizing radiation, which has the ability to resolve muscle, fat, connective tissue and bone. MRI has several advantages over muscle ultrasound, including that MRI has minimal operator-dependence and allows for excellent visualization of all muscles. Magnetic resonance spectroscopy (MRS) is a related noninvasive biochemical sampling technique, has been used in conjunction with MRI, to quantify lipid fraction and metabolic products within muscle (Prompers et al., 2006).
Duchenne Muscular Dystrophy
DMD is the most common muscular dystrophy affecting children. It is an X-linked recessive disorder with an incidence of 1 in 3,300 live male births (Emery, 2002). The disease is caused by mutations in the gene coding for the dystrophin protein (Hoffman, Brown, & Kunkel, 1987). The dystrophin protein is a member of the dystrophin glycoprotein complex an essential component of the muscle membrane (Watkins, Hoffman, Slayter, & Kunkel, 1988) which provides a link to the extracellular matrix. Mutations in the gene leading to abnormal or absent dystrophin protein lead to absence of localization of dystrophin to the dystrophin-associated glycoprotein complex as seen in muscle biopsy specimens. Disruption of the dystrophin glycoprotein complex and its linkage to the extracellular matrix, leads to muscle membrane fragility (Petrof, Shrager, Stedman, Kelly, & Sweeney, 1993), and renders the myofiber more susceptible to contraction-induced injury. Repeated cycles of myofiber degeneration and necrosis ultimately lead to fatty and connective tissue replacement (Dubowitz, 1995).
Clinical Course
Boys with DMD typically come to clinical attention before age 5, presenting with delayed motor milestone acquisition, abnormal gait and difficulty rising from the floor or ascending stairs. Patients experience progressive weakness, beginning in the proximal hip and shoulder girdle muscles, and later progressing to involve more distal muscles. Loss of ambulatory function is usually observed between the age of 10 and 15 years, and by the late teens or early twenties most patients die of cardiopulmonary complications.
Diagnosis
The diagnosis of dystrophin-related muscular dystrophy currently is made by history, physical examination, markedly elevated serum creatine kinase level and confirmed by genetic analysis of the dystrophin gene looking for deletions, duplications or sequence variations.
Current Treatment
Glucocorticoids, a class of corticosteroids, are the only current pharmaceutical agent found to be beneficial in slowing disease progression in boys with DMD and are recommended as standard of care once boys enter the plateau or decline phases of the disease (Moxley et al., 2005). Steroid therapy has been shown to benefit boys with DMD, with improved muscle strength, prolonged ambulation, stabilized pulmonary and cardiac function, and a reduced incidence of scoliosis (Angelini, 2007; Manzur, Kuntzer, Pike, & Swan, 2008; Markham et al., 2005; Moxley et al., 2005). Despite known clinical benefit, the cellular mechanism by which steroid agent stabilizes muscle function has not been clearly elucidated. While glucocorticoids have been shown to reduce the number of cytotoxic T cells (Kissel, Burrow, Rammohan, & Mendell, 1991), the immunosuppressant action of this drug is likely not the only mechanism protecting muscle in boys with DMD (Griggs et al., 1993).
Potential Uses of MRI in DMD
MRI Findings in DMD
Numerous studies have shown the ability of MRI to detect alterations in skeletal muscle structure and composition in patients with muscular dystrophy (Huang et al., 1994; Kuriyama et al., 1989; Marden, Connolly, Siegel, & Rubin, 2005; Matsumura et al., 1989). Initial studies used T1- weighted images and post-contrast imaging to document structural alterations in patients with muscular dystrophy and provide a qualitative assessment of skeletal muscle (Marden et al., 2005; Matsumura et al., 1988; Matsumura et al., 1989; Sookhoo, Mackinnon, Bushby, Chinnery, & Birchall, 2007). Mercuri et al developed a four-point grading system to categorize disease severity, based on visual inspection of fatty tissue infiltration (Mercuri et al., 2002; Mercuri et al., 2005). This strategy was recently used to screen DMD subjects in a clinical trial involving injection with antisense oligonucleotides (van Deutekom et al., 2007). However, there is considerable interest in utilizing quantitative imaging to monitor disease progression and efficacy of treatment strategies, which is the focus of the remainder of this article.
T1-weighted imaging
Quantitative evaluation using T1 imaging has been used to evaluate MRI changes in DMD. T1 values in DMD subjects are high early in the course of disease and fall with increasing clinical severity, associated with increasing fatty replacement of muscle (Matsumura et al., 1988). More recently, Garrood et al., showed that in eight of nine muscles analyzed, there was a statistically significant difference in the median muscle signal intensities on T1-weighted imaging between DMD subjects treated with steroids and control subjects (Garrood et al., 2009).
T1-weighted imaging and Gadolinium enhancement
Gadolinium contrast is used in MRI to better visualize vascular structures. In animal models of muscular dystrophy, gadolinium which in normal muscle remains extracellular, is taken up into muscle fibers with damaged membranes (Amthor et al., 2004; Thibaud et al., 2007). Given that membrane damage is present since birth, some hypothesize that this technique may be more appropriate for evaluating DMD patients early in the course of disease, before fatty infiltration is significant. In a recent study, Garrood et al. showed a significant increase in gadolinium uptake in the tibialis anterior muscles after stepping exercise in subjects with DMD, but not in the six other muscles studied and no change in the T2 value (Garrood et al., 2009).
Muscle cross-sectional area
A number of studies have utilized various MRI approaches to measure muscle size in DMD. Muscle cross-sectional area is a quantitative measure, typically calculated from manually tracing individual muscles in axial slices from T1-weighted images (Figure 1). Recently, Mathur et al. used muscle crosssectional area to compare boys with DMD to age matched controls (Mathur et al., 2010). They found that muscle cross-sectional area of the posterior calf muscles (soleus, medial and lateral gastrocnemius) was approximately 60% greater in boys with DMD compared with controls, but this finding was not found in the two other muscles tested (tibialis anterior and quadriceps). Further, they showed that there was an age-dependent relationship between the muscle cross-sectional area of the quadriceps muscle, with larger cross-sectional area in boys with DMD compared with controls under the age of 10 years with a reversal of this association in boys aged 11 years and older. Therefore, the rate of progression of disease is dependent on muscle group, and an advantage of MRI is that numerous muscles can be evaluated at the same time.
Figure 1.
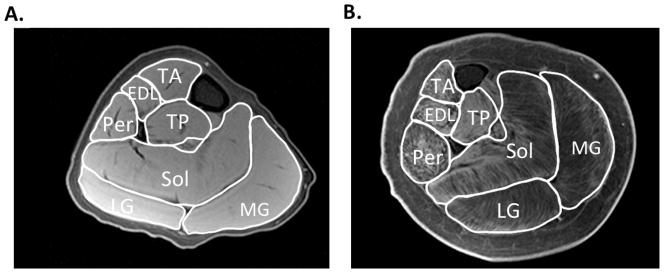
Representative T1-weighted fat suppressed images of the lower leg of a boy with DMD that appears to have relatively little involvement (A) and in a boy that the disease is more progressed (B). This MR sequence can be utilized to determine maximal cross sectional area in various lower leg muscles, including the tibialis anterior (TA), extensor digitorum longus (EDL), tibialis posterior (TP), peroneaus (Per), soleus (Sol), lateral gastrocnemius (LG), and medial gastrocnemius (MG).
While the calculation of muscle cross-sectional is valuable, it does not discriminate between muscle tissue and fatty tissue/edema. These two components can be separated to calculate the muscle contractile and non-contractile tissue within an individual muscle. A recent study used this technique to compare 28 boys with DMD and 10 control subjects (Akima et al., 2011). They showed that boys with DMD had a significantly greater proportion of non-contractile tissue when compared with control subjects and that the proportion of non-contractile tissue increased significantly with age. Further, the proportion of non-contractile tissue correlated with a number of functional tests, including time to walk 30 ft, rise from the floor (supine up), and ascend four steps (Akima et al., 2011).
T2-weighted imaging
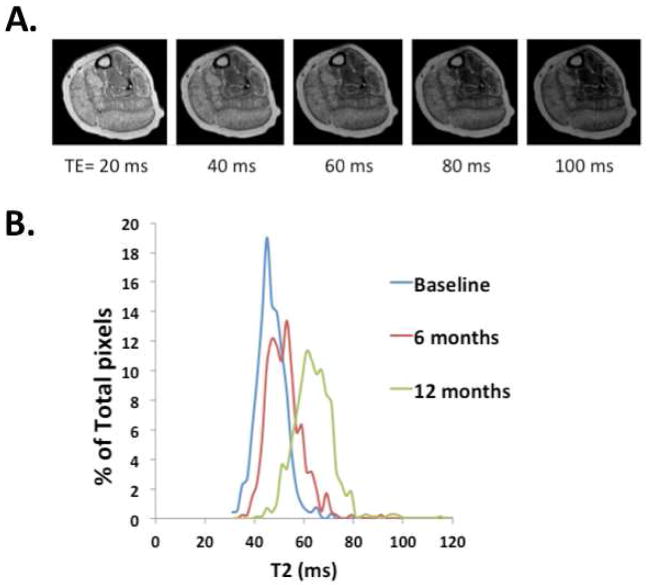
Other investigators have used T2-weighted imaging to reflect muscle composition, including damage, inflammation and lipid composition. T2-weighted imaging has been used to visualize dystrophic lesions in animals, and T2 mapping enables a quantifiable measure that enables more direct comparisons over time and at different sites. Kim et al recently used a T2 mapping strategy to correlate mean T2 values with both the non-quantitative MRI grading scale developed by Mercuri et al (Mercuri et al., 2007) and with clinical measures of disease severity (Kim et al., 2010). They found that the mean T2 of the gluteus maximus muscle showed significant correlation with the nonquantitative MRI score for fatty infiltration. Further, they showed a significant positive correlation between the mean T2 value for the gluteus maximus muscle and several clinical measures, including patient age, clinical function scale, timed Gower score and time to run 30 feet (Kim et al., 2010). In addition, distribution of T2 values in a region of- interest has been examined on a pixel-by-pixel basis to provide information about muscle heterogeneity (Huang et al., 1994), and an example of how this may be utilized to monitor progression of disease is shown in Figure 2.
Figure 2.
Representative T2-weighted images acquired with a TE of 20, 40, 60, 80, and 100 ms (A). Using these images A T2 map can be created, then the pixels plotted within a region of interest to create a histogram (B). Note, the rightward shift of the histogram indicating progression of disease in the soleus over a one year period in this subject.
Fat suppression sequences
Implementing a fat suppression procedure during a spin echo sequence (e.g., Short-tau inversion recovery imaging (STIR)) suppresses the signal from adipose tissue, and thus the resulting signal is more sensitive to increased extracellular or unbound water content, consistent with edema or inflammation. This may be a particularly useful technique in younger boys with DMD in whom inflammatory changes with edema may be better markers than fatty infiltration as the latter change occurs later in the disease process. This is supported by a study by Marden et al (Marden et al., 2005), using STIR, which found regions of increased signal intensity or muscle inflammation in dystrophic muscles of young boys with DMD in the absence of fatty tissue infiltration.
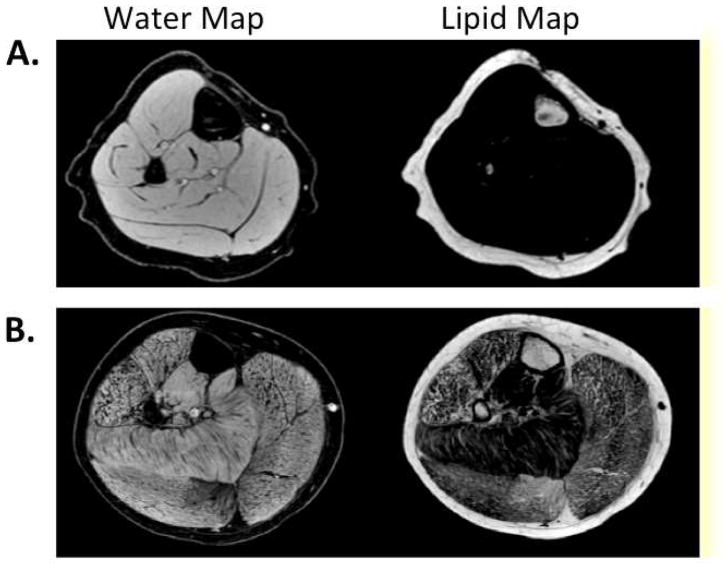
Three-point Dixon technique
In standard MRI sequences, the signal intensity for each voxel (the unit of measurement) is determined based on both the fat and water signal intensities within that voxel. The three-point Dixon technique allows separation of MRI signal intensity into separate values for the individual contributions of fat and water in each voxel of tissue (Dixon, 1984). This results in high resolution water and fat maps (Figure 3), and enables quantifying fat fractions of individual muscles. In addition, this sequence has the advantage of correcting for MR inhomogeneities (Glover & Schneider, 1991). Recently, Wren et al (Wren, Bluml, Tseng-Ong, & Gilsanz, 2008) implemented a three-point Dixon MR technique to quantify the amount of lipid infiltration in the thigh muscles of nine boys with DMD and showed that quantitative measures of muscle adiposity correlate better with disease severity than strength measures.
Figure 3.
Example water and lipid maps of the lower leg of a control (A) and a boy with Duchenne muscular dystrophy (B) using 3pt Dixon.
Similarly, preliminary data from our multi-site study of the use of MRI in boys with DMD quantified the intramuscular lipid accumulation in the soleus muscle of twenty-four ambulatory boys with DMD. We found a strong correlation between the amount of intramuscular lipid assessed by volume localized 1Hspectroscopy and measures of functional ability, including the Brooke Lower Extremity (LE) score (R2=0.78) and the time to walk 30 feet (R2=0.80) (unpublished data).
Spectroscopy
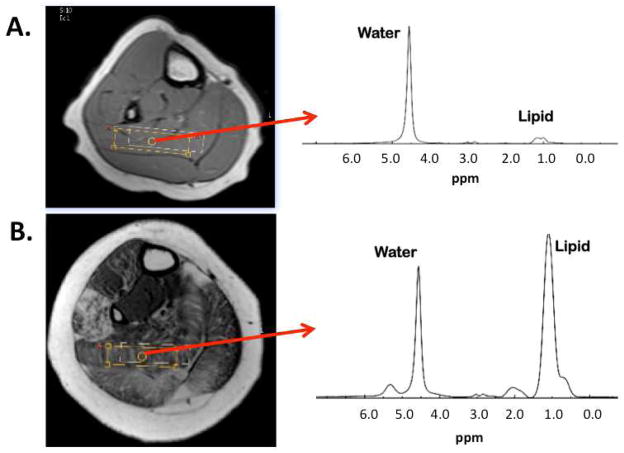
In addition to MRI, various approaches of MRS have been utilized to study muscle involvement in muscular dystrophy, including proton spectroscopy (1H-MRS) and 31phosphorus-MRS (31P-MRS) (Kan et al., 2010) (Torriani et al., In press). While spectroscopy provides limited spatial information, it has proven to accurately quantify muscle metabolites and does not suffer from partial volume filling (Huang et al., 1994). One approach is to acquire a spectrum from a single voxel that is maximized in size within an individual muscle in order to obtain a large representation of the relative lipid concentration (Figure 4). Recently Torriani et al. (In press) used a similar approach to evaluate lipid composition in children with DMD in the soleus and tibialis anterior. In that study relationships between lipid fraction and functional measures were observed. Alternatively, Hsieh et al used MRS to show that the trimethylamines-to-muscle total creatine ratio (TMA/tCr) is significantly reduced in boys with DMD and correlates negatively with function (Hsieh et al., 2009). Therefore, there are a variety of MRS measures that show promise in measuring disease progression in DMD.
Figure 4.
Example spectrum acquired from the soleus of a control (A) and a boy with Duchenne muscular dystrophy (B). Lipid peak includes a composite of intramyocellular (IMCL) and extramyocelluar (EMCL) lipid.
MRI as a Biomarker for Clinical Trials
With a number of potential therapeutic interventions for DMD under development, and some in phase I/II clinical trials, there is an immediate need for such a non-invasive biomarker and MRI appears to be an enticing option. However, few MR studies have presented a robust quantitative approach to monitor disease progression in DMD. Therefore, while MR is a promising non-invasive method, further longitudinal studies are required to validate the sensitivity of the measures to monitor disease progression and treatment.
MRI compared to muscle biopsy
Currently, muscle biopsy, used to document the restoration of the dystrophin protein to the muscle membrane, is the gold standard for assessing benefit of potential therapeutic interventions for DMD. Use of muscle biopsy is based on its use in the diagnosis of DMD and its use for drug development and preclinical studies in model systems of DMD. In addition, a recent study by Kinali et al showed a correlation between MRI changes (based on subjective scoring system) and histopathologic changes on muscle biopsy (Kinali et al., 2011). They showed that there was a good correlation between MRI severity score and a categorical assessment of muscle involvement on standard histological staining.
Despite its common use as the “gold standard” there are several concerns with the use of muscle biopsy in clinical trials. First, it is an invasive procedure that involves taking repeated samples from individuals who may already have limited muscle mass. Second, it is susceptible to considerable human error, particularly with specimen processing. Further, recent studies have suggested considerable variability of muscle involvement in DMD, even within a single muscle (e.g.,. rectus femoris), and therefore a muscle biopsy may not provide a true representation of the overall disease progression or therapeutic response.
MRI correlation with functional outcome measures
Functional measures including timed performance tests (six-minute walk, 30 meter walk, time to ascend 4 stairs, etc) and quantitative strength measures have also been routinely used as outcome measures in DMD treatment trials. The study by Wren et al and our preliminary data have shown correlation of various MRI modalities with standard functional measures (Wren et al., 2008).
Treatment response
Notably, several recent treatment trials have included MRI as part of the study protocol. For example, a recent study implemented MRI to compare the T2 relaxation times of the myocardium and sternocleidomastoid muscle (accessory respiratory muscle) of boys chronically treated with Deflazacort (treatment duration of at least 7 years) and a younger group of untreated boys (Mavrogeni et al., 2009). In addition, several studies have used MRI to quantify changes in skeletal muscle volume following treatment with, either a neutralizing antibody to myostatin (Wagner et al., 2008) or following myoblast transplants (Miller et al., 1997).
Summary
A number of studies have shown promise in using various approaches of MRI and MRS to evaluate skeletal muscle involvement in DMD. However, these studies have mainly been performed using a cross-sectional design, and the correlation of these MRI changes with disease progression and disease severity has not be fully elucidated. Overall, skeletal muscle MRI is a powerful and sensitive technique in the evaluation of muscle disease, and its use as a biomarker for disease progression or therapeutic response in clinical trials deserves further study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akima H, Lott D, Senesac C, Deol J, Germain S, Arpan I, et al. Relationships of thigh muscle contractile and non-contractile tissue with function, strength, and age in boys with duchenne muscular dystrophy. Neuromuscular Disorders: NMD. 2011 doi: 10.1016/j.nmd.2011.06.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor H, Egelhof T, McKinnell I, Ladd ME, Janssen I, Weber J, et al. Albumin targeting of damaged muscle fibres in the mdx mouse can be monitored by MRI. Neuromuscular Disorders: NMD. 2004;14(12):791–796. doi: 10.1016/j.nmd.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Angelini C. The role of corticosteroids in muscular dystrophy: A critical appraisal. Muscle & Nerve. 2007;36(4):424–435. doi: 10.1002/mus.20812. [DOI] [PubMed] [Google Scholar]
- Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153(1):189–194. doi: 10.1148/radiology.153.1.6089263. [DOI] [PubMed] [Google Scholar]
- Dubowitz V. Muscle disorders in children. 2. UK: Kidlington; 1995. [Google Scholar]
- Emery AE. The muscular dystrophies. Lancet. 2002;359(9307):687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- Garrood P, Hollingsworth KG, Eagle M, Aribisala BS, Birchall D, Bushby K, et al. MR imaging in duchenne muscular dystrophy: Quantification of T1-weighted signal, contrast uptake, and the effects of exercise. Journal of Magnetic Resonance Imaging: JMRI. 2009;30(5):1130–1138. doi: 10.1002/jmri.21941. [DOI] [PubMed] [Google Scholar]
- Glover GH, Schneider E. Three-point dixon technique for true water/fat decomposition with B0 inhomogeneity correction. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1991;18(2):371–383. doi: 10.1002/mrm.1910180211. [DOI] [PubMed] [Google Scholar]
- Griggs RC, Moxley RT, 3rd, Mendell JR, Fenichel GM, Brooke MH, Pestronk A, et al. Duchenne dystrophy: Randomized, controlled trial of prednisone (18 months) and azathioprine (12 months) Neurology. 1993;43(3 Pt 1):520–527. doi: 10.1212/wnl.43.3_part_1.520. [DOI] [PubMed] [Google Scholar]
- Heckmatt JZ, Dubowitz V, Leeman S. Detection of pathological change in dystrophic muscle with B-scan ultrasound imaging. Lancet. 1980;1(8183):1389–1390. doi: 10.1016/s0140-6736(80)92656-2. [DOI] [PubMed] [Google Scholar]
- Heckmatt JZ, Leeman S, Dubowitz V. Ultrasound imaging in the diagnosis of muscle disease. The Journal of Pediatrics. 1982;101(5):656–660. doi: 10.1016/s0022-3476(82)80286-2. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: The protein product of the duchenne muscular dystrophy locus. Cell. 1987;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hsieh TJ, Jaw TS, Chuang HY, Jong YJ, Liu GC, Li CW. Muscle metabolism in duchenne muscular dystrophy assessed by in vivo proton magnetic resonance spectroscopy. Journal of Computer Assisted Tomography. 2009;33(1):150–154. doi: 10.1097/RCT.0b013e318168f735. [DOI] [PubMed] [Google Scholar]
- Huang Y, Majumdar S, Genant HK, Chan WP, Sharma KR, Yu P, et al. Quantitative MR relaxometry study of muscle composition and function in duchenne muscular dystrophy. Journal of Magnetic Resonance Imaging: JMRI. 1994;4(1):59–64. doi: 10.1002/jmri.1880040113. [DOI] [PubMed] [Google Scholar]
- Kan HE, Klomp DW, Wong CS, Boer VO, Webb AG, Luijten PR, et al. In vivo 31P MRS detection of an alkaline inorganic phosphate pool with short T1 in human resting skeletal muscle. NMR in Biomedicine. 2010;23(8):995–1000. doi: 10.1002/nbm.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Laor T, Horn PS, Racadio JM, Wong B, Dardzinski BJ. T2 mapping in duchenne muscular dystrophy: Distribution of disease activity and correlation with clinical assessments. Radiology. 2010;255(3):899–908. doi: 10.1148/radiol.10091547. [DOI] [PubMed] [Google Scholar]
- Kinali M, Arechavala-Gomeza V, Cirak S, Glover A, Guglieri M, Feng L, et al. Muscle histology vs MRI in duchenne muscular dystrophy. Neurology. 2011;76(4):346–353. doi: 10.1212/WNL.0b013e318208811f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissel JT, Burrow KL, Rammohan KW, Mendell JR. Mononuclear cell analysis of muscle biopsies in prednisone-treated and untreated duchenne muscular dystrophy. CIDD study group. Neurology. 1991;41(5):667–672. doi: 10.1212/wnl.41.5.667. [DOI] [PubMed] [Google Scholar]
- Kuriyama M, Hayakawa K, Konishi Y, Konishi K, Sudo M, Nakamura K, et al. MR imaging of myopathy. Computerized Medical Imaging and Graphics: The Official Journal of the Computerized Medical Imaging Society. 1989;13(4):329–333. doi: 10.1016/0895-6111(89)90210-3. [DOI] [PubMed] [Google Scholar]
- Manzur AY, Kuntzer T, Pike M, Swan A. Glucocorticoid corticosteroids for duchenne muscular dystrophy. Cochrane Database of Systematic Reviews (Online) 2008;(1):CD003725. doi: 10.1002/14651858.CD003725.pub3. [DOI] [PubMed] [Google Scholar]
- Marden FA, Connolly AM, Siegel MJ, Rubin DA. Compositional analysis of muscle in boys with duchenne muscular dystrophy using MR imaging. Skeletal Radiology. 2005;34(3):140–148. doi: 10.1007/s00256-004-0825-3. [DOI] [PubMed] [Google Scholar]
- Markham LW, Spicer RL, Khoury PR, Wong BL, Mathews KD, Cripe LH. Steroid therapy and cardiac function in duchenne muscular dystrophy. Pediatric Cardiology. 2005;26(6):768–771. doi: 10.1007/s00246-005-0909-4. [DOI] [PubMed] [Google Scholar]
- Mathur S, Lott DJ, Senesac C, Germain SA, Vohra RS, Sweeney HL, et al. Age-related differences in lower-limb muscle cross-sectional area and torque production in boys with duchenne muscular dystrophy. Archives of Physical Medicine and Rehabilitation. 2010;91(7):1051–1058. doi: 10.1016/j.apmr.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K, Nakano I, Fukuda N, Ikehira H, Tateno Y, Aoki Y. Proton spin-lattice relaxation time of duchenne dystrophy skeletal muscle by magnetic resonance imaging. Muscle & Nerve. 1988;11(2):97–102. doi: 10.1002/mus.880110202. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Nakano I, Fukuda N, Ikehira H, Tateno Y, Aoki Y. Duchenne muscular dystrophy carriers. proton spin-lattice relaxation times of skeletal muscles on magnetic resonance imaging. Neuroradiology. 1989;31(5):373–376. doi: 10.1007/BF00343858. [DOI] [PubMed] [Google Scholar]
- Mavrogeni S, Papavasiliou A, Douskou M, Kolovou G, Papadopoulou E, Cokkinos DV. Effect of deflazacort on cardiac and sternocleidomastoid muscles in duchenne muscular dystrophy: A magnetic resonance imaging study. European Journal of Paediatric Neurology: EJPN: Official Journal of the European Paediatric Neurology Society. 2009;13(1):34–40. doi: 10.1016/j.ejpn.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Mercuri E, Bushby K, Ricci E, Birchall D, Pane M, Kinali M, et al. Muscle MRI findings in patients with limb girdle muscular dystrophy with calpain 3 deficiency (LGMD2A) and early contractures. Neuromuscular Disorders: NMD. 2005;15(2):164–171. doi: 10.1016/j.nmd.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Mercuri E, Pichiecchio A, Allsop J, Messina S, Pane M, Muntoni F. Muscle MRI in inherited neuromuscular disorders: Past, present, and future. Journal of Magnetic Resonance Imaging: JMRI. 2007;25(2):433–440. doi: 10.1002/jmri.20804. [DOI] [PubMed] [Google Scholar]
- Mercuri E, Talim B, Moghadaszadeh B, Petit N, Brockington M, Counsell S, et al. Clinical and imaging findings in six cases of congenital muscular dystrophy with rigid spine syndrome linked to chromosome 1p (RSMD1) Neuromuscular Disorders: NMD. 2002;12(7–8):631–638. doi: 10.1016/s0960-8966(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Miller RG, Sharma KR, Pavlath GK, Gussoni E, Mynhier M, Lanctot AM, et al. Myoblast implantation in duchenne muscular dystrophy: The san francisco study. Muscle & Nerve. 1997;20(4):469–478. doi: 10.1002/(sici)1097-4598(199704)20:4<469::aid-mus10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Moxley RT, 3rd, Ashwal S, Pandya S, Connolly A, Florence J, Mathews K, et al. Practice parameter: Corticosteroid treatment of duchenne dystrophy: Report of the quality standards subcommittee of the american academy of neurology and the practice committee of the child neurology society. Neurology. 2005;64(1):13–20. doi: 10.1212/01.WNL.0000148485.00049.B7. [DOI] [PubMed] [Google Scholar]
- Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(8):3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prompers JJ, Jeneson JA, Drost MR, Oomens CC, Strijkers GJ, Nicolay K. Dynamic MRS and MRI of skeletal muscle function and biomechanics. NMR in Biomedicine. 2006;19(7):927–953. doi: 10.1002/nbm.1095. [DOI] [PubMed] [Google Scholar]
- Sookhoo S, Mackinnon I, Bushby K, Chinnery PF, Birchall D. MRI for the demonstration of subclinical muscle involvement in muscular dystrophy. Clinical Radiology. 2007;62(2):160–165. doi: 10.1016/j.crad.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Thibaud JL, Monnet A, Bertoldi D, Barthelemy I, Blot S, Carlier PG. Characterization of dystrophic muscle in golden retriever muscular dystrophy dogs by nuclear magnetic resonance imaging. Neuromuscular Disorders: NMD. 2007;17(7):575–584. doi: 10.1016/j.nmd.2007.03.013. [DOI] [PubMed] [Google Scholar]
- van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS, Aartsma-Rus A, Bremmer-Bout M, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. The New England Journal of Medicine. 2007;357(26):2677–2686. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Fleckenstein JL, Amato AA, Barohn RJ, Bushby K, Escolar DM, et al. A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Annals of Neurology. 2008;63(5):561–571. doi: 10.1002/ana.21338. [DOI] [PubMed] [Google Scholar]
- Watkins SC, Hoffman EP, Slayter HS, Kunkel LM. Immunoelectron microscopic localization of dystrophin in myofibres. Nature. 1988;333(6176):863–866. doi: 10.1038/333863a0. [DOI] [PubMed] [Google Scholar]
- Wren TA, Bluml S, Tseng-Ong L, Gilsanz V. Three-point technique of fat quantification of muscle tissue as a marker of disease progression in duchenne muscular dystrophy: Preliminary study. AJR American Journal of Roentgenology. 2008;190(1):W8–12. doi: 10.2214/AJR.07.2732. [DOI] [PubMed] [Google Scholar]