Abstract
The subgranular layers (layers 5 and 6) of primary sensory cortex provide corticofugal output to thalamus and they also project to the appropriate secondary sensory cortices. Here we injected two combinations of different color retrograde fluorescent markers in the thalamic and cortical targets of these layers from the three primary sensory cortices (somatosensory, auditory, and visual) in mice to examine the degree of overlap between corticothalamic and interareal corticocortical cells in the subgranular layers. We found that, for all three primary sensory cortices, double-labeled cells were extremely rare, indicating that corticothalamic and interareal corticocortical cells in the subgranular layers represent largely independent populations.
INDEXING TERMS: corticothalamic, corticocortical, fluorescence, retrograde labeling, corticofugal
Primary sensory cortices, such as the primary somatosensory, visual, or auditory cortex, receive information from their respective peripheral organs via the thalamus. They then feed this information both to secondary cortical areas as well as back to the thalamus itself. Corticocortical communication between primary and secondary cortices arise from virtually all cortical layers and it is reciprocal (Felleman and Van Essen, 1991; Covic and Sherman, 2011). Corticothalamic projections of primary sensory cortices, however, arise only from the subgranular layers. More specifically, those from layer 6 mainly target their afferent, first-order thalamic nuclei and are thus organized in a largely feedback manner. On the other hand, layer 5 projections of a primary sensory cortical area innervate higher-order thalamic nuclei (among other subcortical targets), which project to secondary sensory cortices and are thus organized in a feedforward manner (Ojima, 1994; Bourassa et al., 1995; see also Guillery, 1995, for a review). A question that emerges from this anatomical configuration is whether the corticothalamic and interareal corticocortical projections of the subgranular layers emerge as branches of single axons or originate from separate cell populations.
Existing evidence from studies in the developing secondary somatosensory cortex (S2) of the rat suggests that layer 6 cells that project to the thalamus and layer 6 cells that project intracortically emerge at different times in development (Arimatsu and Ishida, 2002). In addition, during embryonic and postnatal development only corticocortical cells express latexin, a carboxypeptidase-A inhibitor that is absent in corticothalamic cells (Arimatsu et al., 1999). This evidence suggests that there is perhaps a trend for segregation between corticothalamic and corticocortical cells that could also apply to primary somatosensory cortex (S1). Evidence from S1 itself also supports such a segregation. In a single-axon tracing study, Zhang and Deschênes (1997) used juxtacellular biocytin labeling of a small number of layer 6 cells in rat S1 to show that there was no overlap between the identified corticothalamic and corticocortical projecting cells. Moreover, these two populations of cells appeared to possess different morphological and physiological features (see also Molnár and Cheung, 2006; Hattox and Nelson, 2007).
Here we sought to examine how much overlap, if any, there is between corticothalamic and corticocortical cells of primary sensory cortices. Instead of being limited to layer 6 of S1, we chose to investigate the above question in both layers 5 and 6 of three primary sensory cortices; the primary visual cortex (V1), the primary auditory cortex (A1), and S1. By injecting different color retrograde fluorescent markers in the thalamic and cortical afferents of these cortices, we show that, with extremely rare exceptions, corticothalamic and interareal corticortical cells constitute independent populations.
MATERIALS AND METHODS
Surgical injection of fluorescent tracers
All procedures used in this study were in accordance with the guidelines of the Institutional Animal Care and Use Committee at the University of Chicago. BALB/c mice (age 20–50 days) of both sexes were anesthetized with a ketamine (100 mg/kg) / xylazine (3 mg/kg) mixture and were placed in a stereotaxic apparatus (Kopf, Tujunga, CA). Depth of anesthesia was monitored frequently by tail and toe pinching and maintenance doses were administered as necessary. Special care was taken to maintain aseptic conditions throughout the surgical procedures. Each animal received a unilateral cortical injection (in the second somatosensory area, S2, the ventral portion of the secondary auditory cortex, A2v, or the medial or lateral portion of the secondary visual cortex, V2) and a large unilateral injection on the same side of an appropriate thalamic region: an area covering the ventral posterior medial and lateral nuclei plus the posterior medial nucleus paired with S2 injections; the medial geniculate body paired with A2v injections; and an area that includes the lateral geniculate nucleus and the lateral posterior nucleus paired with V2 injections. The V2 areas we directed our injections to correspond approximately to areas PM, LM, P, MM, AL, RL, and A as described in Wang and Burkhalter (2007) or areas 18a and 18b described in Caviness (1975) and Wagor et al. (1980). In each case different color fluorescent tracers were used for the cortical and thalamic injections. We used two combinations of fluorescent tracers; either red and green Retrobeads IX (Lumafluor, Naples, FL) or Fluoro-Gold and Fluoro-Ruby (Fluoroschrome, Denver, CO). The color of the fluorescent tracer used for the thalamic and cortical injections of each animal was counterbalanced within each sensory system, meaning that roughly half the animals received green Retrobeads in thalamus and red Retrobeads in cortex, while the other half received red Retrobeads in thalamus and green Retrobeads in cortex, and the same counterbalancing applied to Fluoro-Gold and Fluoro-Ruby injections. A few animals also received injections of a 50:50 mixture of red and green Retrobeads or Fluoro-Gold and Fluoro-Ruby.
Pressure injections were performed using a 1-µl Hamilton syringe (Reno, NV) and the volumes per injection varied between 250 and 800 nl. The stereotaxic coordinates of the injections were determined using the Franklin and Paxinos (2008) mouse brain atlas (all distances are mm from bregma, unless stated otherwise): injections of the ventral posterior medial, ventral posterior lateral, and posterior medial nuclei (AP: −2.05, ML: ± 1.5, DV: −3.3); medial geniculate body injections (AP: −3.3, ML: ± 2.1, DV: −3.4); lateral geniculate and lateral posterior nuclei injections (AP: −1.8, ML: ± 1.7, DV: −2.5); medial V2 injections (AP: −2.5, ML: ± 1.7, DV: −0.5); and lateral V2 injections (AP: −2.5, ML: ± 3.1, DV: −1.1). For the S2 and A2v injections, the head of the animal had to be rotated 90° on the anterior–posterior axis to allow an easier needle insertion that would also minimize the potential spread of the fluorescent tracers in undesired areas of cortex. Prior to rotating the head, we used bregma as the landmark to determine the anterior–posterior and medial–lateral coordinates and marked the location on the skull where we would drill. After rotating the head we used the surface of the brain to determine the depth of the injection: S2 injections (from bregma: AP: −0.1, ML: ± 4.1; from brain surface: DV: −0.5); A2v injections (from bregma: AP: −2.1, ML: ± 4.4; from brain surface: DV: −0.5). All injections were performed at a rate of 7–15 nl per minute. Following an injection the needle was left in place for 10–15 minutes and was retracted 0.7 mm every 10–15 minutes to minimize the upward suction of the fluorescent tracer. Following the injections, animals were treated locally with Lidocaine Hydrochloride jelly (Akorn, Buffalo Grove, IL) and Vetropolycin antibiotic ointment (Dechra, Overland Park, KS), and were allowed to recover for 3–5 days. During that period, animals received analgesic doses (0.1 mg/kg) of Buprenex (Reckitt Benckiser Healthcare, Hull, UK) every 12 hours.
Tissue processing
Following the recovery period, animals were transcardially perfused with phosphate-buffered saline (PBS) (pH 7.4) followed by 4% paraformaldehyde in PBS. The brains were then placed in 10% and subsequently 30% sucrose-paraformaldehyde solution until saturated. Next, brains were placed in a sliding microtome for sectioning. Thirty-five-µm-thick sections were cut and then mounted onto gelatin-coated slides. Finally, the sections were dehydrated and cover-slipped. Due to the light sensitivity of the fluorescent tracers, special care was taken not to expose the slices to light, unless necessary.
Cell counting
Brain sections were examined under a microscope using a 100W mercury lamp with fluorescence optics (Leica Microsystems, Wetzlar, Germany) and photomicrographs of the areas of interest were taken at various magnifications using a Retiga 2000 monochrome CCD camera and Q Capture Pro software (QImaging, Surrey, BC). Leica TX2 filter cubes (excitation 560 nm, emission 645 nm, dichroic 595 nm) were used to visualize red Retrobeads and Fluoro-Ruby, Leica L5 filter cubes (excitation 480 nm, emission 527 nm, dichroic 505 nm) were used to visualize green Retrobeads, and Leica E4 filter cubes (excitation 436 nm, emission 470 nm, dichroic 455 nm) were used to visualize Fluoro-Gold labeling. Q Capture Pro software was used to adjust brightness and contrast of individual photos and to overlay images of the red and green Retrobead (or Fluoro-Gold and -Ruby) labeling to create the overlaid (double-labeled) images.
Photomicrographs used for counting the labeled neurons were taken at 20× magnification and were focused exclusively on areas of overlap between the two tracers, areas that were selected by visual inspection. After a photomicrograph was taken for the purpose of cell counting, identified patterns of labeled neurons at the edges of the photomicrograph were used as landmarks for transitioning to directly adjacent areas within the region of interest before the next photomicrograph was taken, ensuring that labeled neurons did not appear in multiple photomicrographs. AxioVision software (Carl Zeiss Instruments) was used to analyze the overlaid images and count the number of single- and double-labeled neurons. CorelDraw (Corel, Ottawa, ON) was used for the production of figures.
Nissl-stained sections were used to aid in the identification of boundaries between cortical layers and areas. These sections, acquired from two, age-matched animals, were dehydrated in descending gradients of alcohol, rehydrated in distilled water, stained for 2 minutes in cresyl violet (1.25 g of cresyl violet acetate, 0.75 ml of glacial acetic acid, and 250 cc of warm water), quenched in distilled water, dehydrated in ascending gradients of alcohol, and finally rinsed with Histoclear (National Diagnostics, Charlotte, NC) for 5 minutes before coverslipped. We used anatomical landmarks and established cytoarchitectonic features to identify specific cortical or thalamic areas and assess the accuracy of our injections.
Due to the proximity of primary and secondary sensory cortical areas in all three sensory systems, it was of particular importance to ensure that no primary sensory cortical area received a direct injection of tracers. S2 injections were directed on an area adjacent to the easily identifiable barrel field of S1, which allowed us to assess the containment of our injections and reject any animals with tracers spread into S1. A1 was distinguished from A2 on the basis of its greater cell density and better-defined laminar organization (see Covic and Sherman, 2011). Finally, V1 was distinguished from V2 on the basis of its more prominent layer 4 and considerably narrower layers 2/3 (Caviness, 1975). The identification of the thalamic nuclei is a considerably more straightforward process and was carried out with the help of the Franklin and Paxinos (2008) mouse brain atlas.
RESULTS
In total, we injected 30 animals. We injected green and red Retrobeads in 20 animals: five in the somatosensory system, six in the auditory system, six in the visual system, and in addition we injected a 50:50 mixture of green and red Retrobeads to one animal for each sensory system (these injections were done either in cortex or thalamus). Ten animals were injected with Fluoro-Gold and Fluoro-Ruby; three animals were used for each sensory system and an additional animal was injected with a 50:50 mixture of Fluoro-Gold and Fluoro-Ruby in V1.
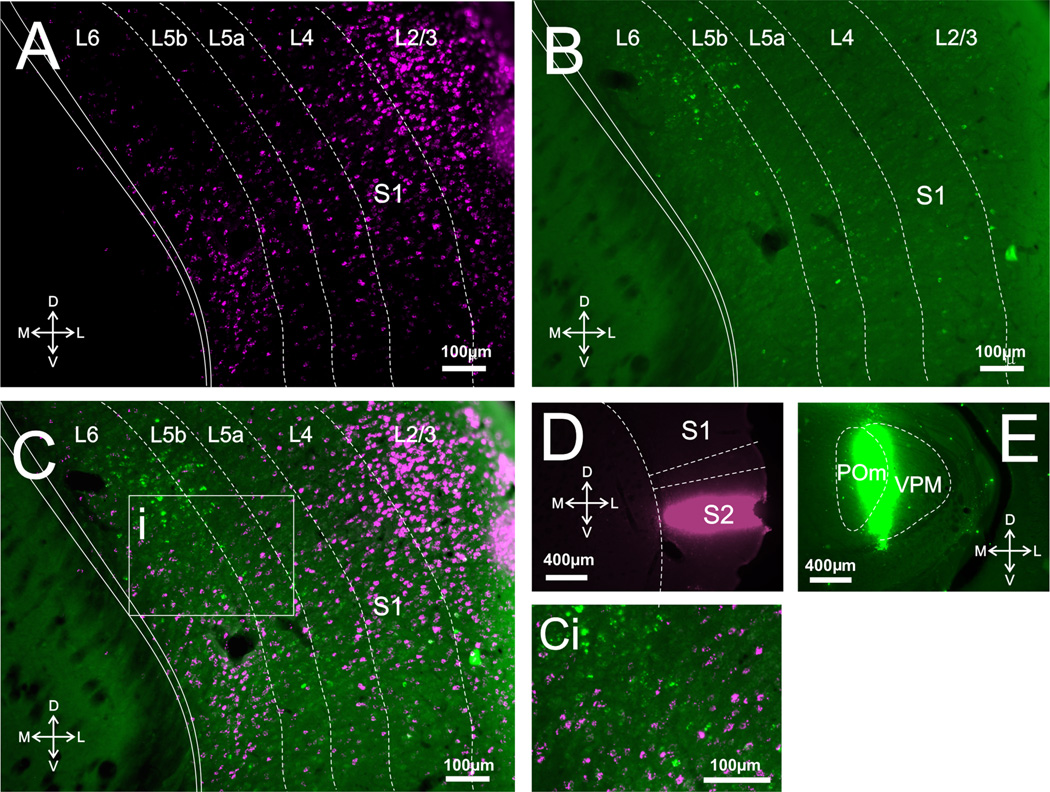
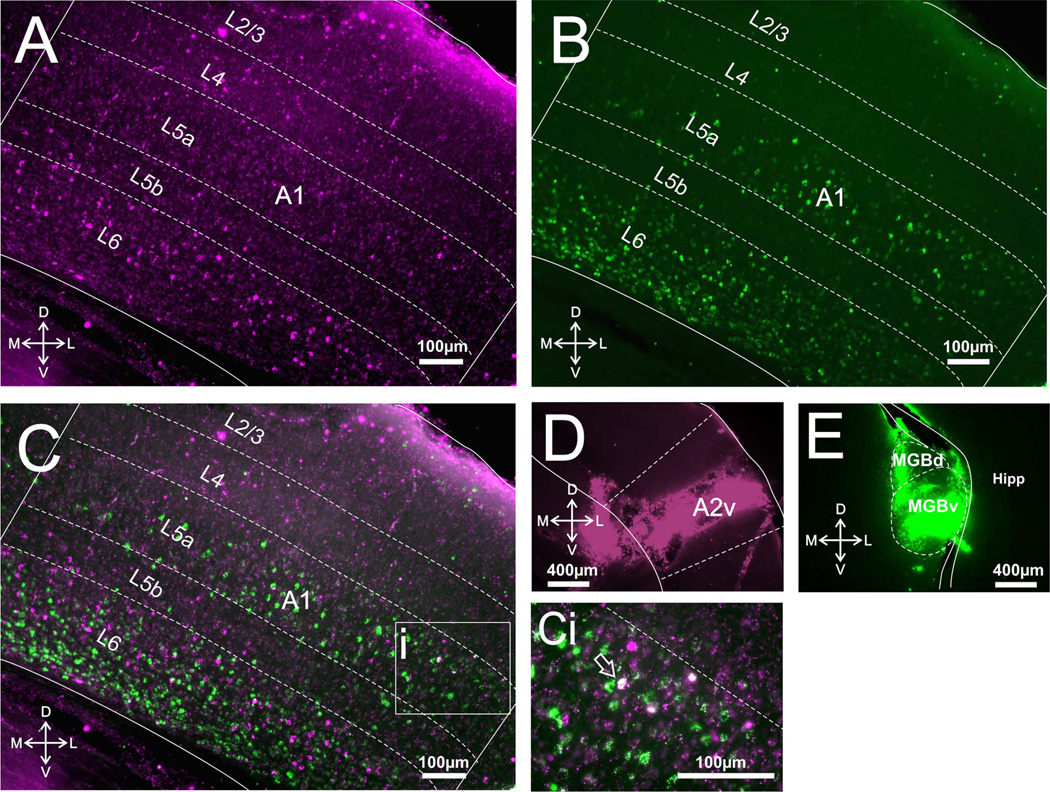
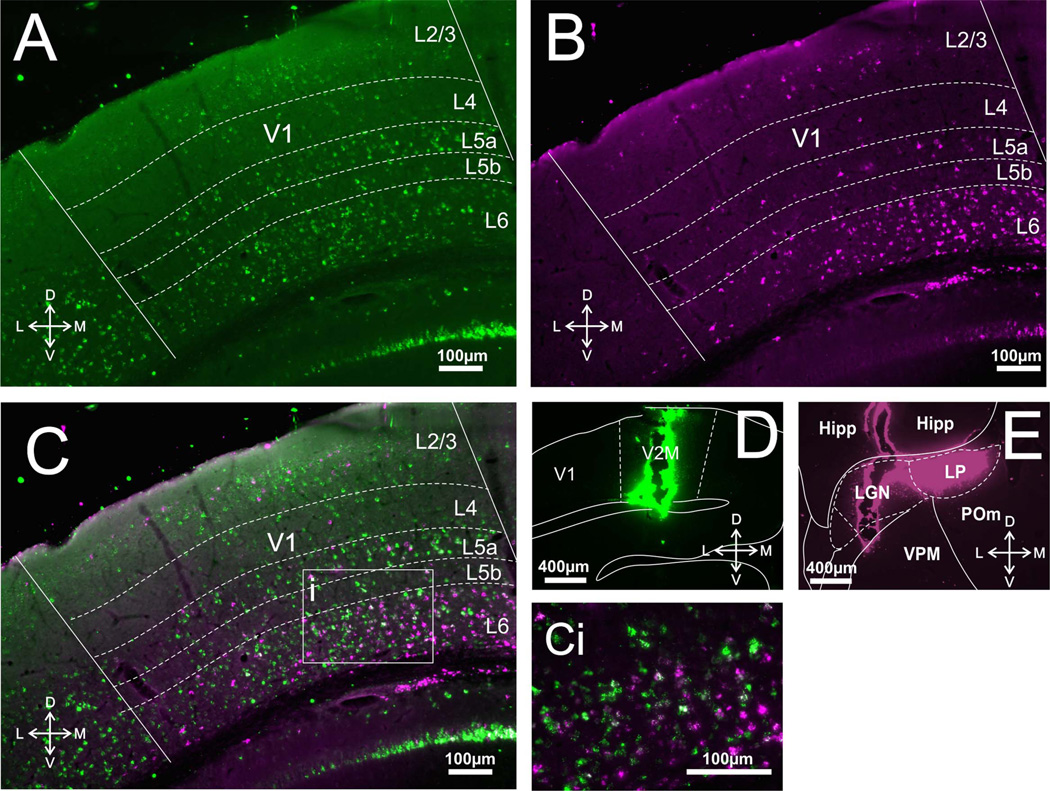
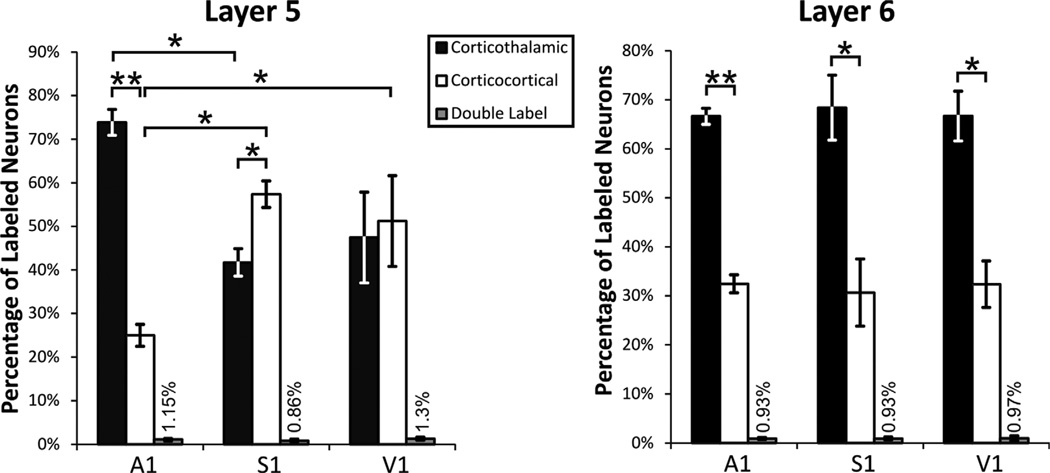
Only animals with injection sites of sufficient size and with no, or very little, spread to nontarget areas were included in the analyses (for examples, see Figs. 1D,E, 2D,E, 3D,E). Inspection of the images taken under the appropriate filters revealed that cortical injections resulted in retrograde labeling in virtually all layers of S1, A1, or V1 (Figs. 1A, 2A, 3A), while thalamic injections resulted in retrograde labeling only in layers 5 and 6 of S1, A1, or V1 (Figs. 1B, 2B, 3B). We counted ≈250 cells per layer per animal, focusing in regions of overlap between cells retrogradely labeled from thalamic and cortical injections; these regions were restricted to layers 5 and 6, because only these layers have cells projecting subcortically. Within these regions we saw a considerably larger proportion of retrogradely labeled cells in layer 5 of A1 following thalamic injections compared to cortical injections. These values are (mean ± SE): thalamic, 73.86% ± 2.95%; cortical, 24.99% ± 2.52% (Bonferroni-adjusted Mann–Whitney test, P < 0.001). On the other hand, within these regions of overlap cortical injections produced more retrogradely labeled cells in layer 5 of S1 than did thalamic injections: cortical, 57.4% ± 3.03%; thalamic, 41.73% ± 3.13% (P < 0.05). For layer 5 of V1 these proportions were relatively balanced: thalamic, 47.46% ± 10.42%; cortical, 51.23% ± 10.41% (P > 0.05; see Fig. 4, left panel).
Figure 1.
Corticothalamic and corticocortical cells in S1. A: Photomicrograph of retrogradely labeled cells in S1 following injections of red Retrobeads in S2 (all red Retrobeads here and elsewhere are shown in magenta). B: Photomicrograph of retrogradely labeled cells in S1 following injections of green Retrobeads in somatosensory thalamus. C: Overlay of A,B. Ci: Highlighted area in C magnified showing the lack of double-labeled cells. D,E: Photomicrographs of injection sites in S2 and POm/VPM, respectively.
Figure 2.
Corticothalamic and corticocortical cells in A1. A: Photomicrograph of retrogradely labeled cells in A1 following injections of red Retrobeads in A2v. B: Photomicrograph of retrogradely labeled cells in A1 following injections of green Retrobeads in auditory thalamus. C: Overlay of A,B. Ci: Highlighted area in C magnified showing an example of a double-labeled cell in focus, seen in white (arrow). Two more double-labeled cells are also visible but outside the plane of focus. D,E: Photomicrographs of injection sites in A2v and MGB, respectively. MGBv, ventral medial geniculate body; MGBd, dorsal medial geniculate body; Hipp, hippocampus.
Figure 3.
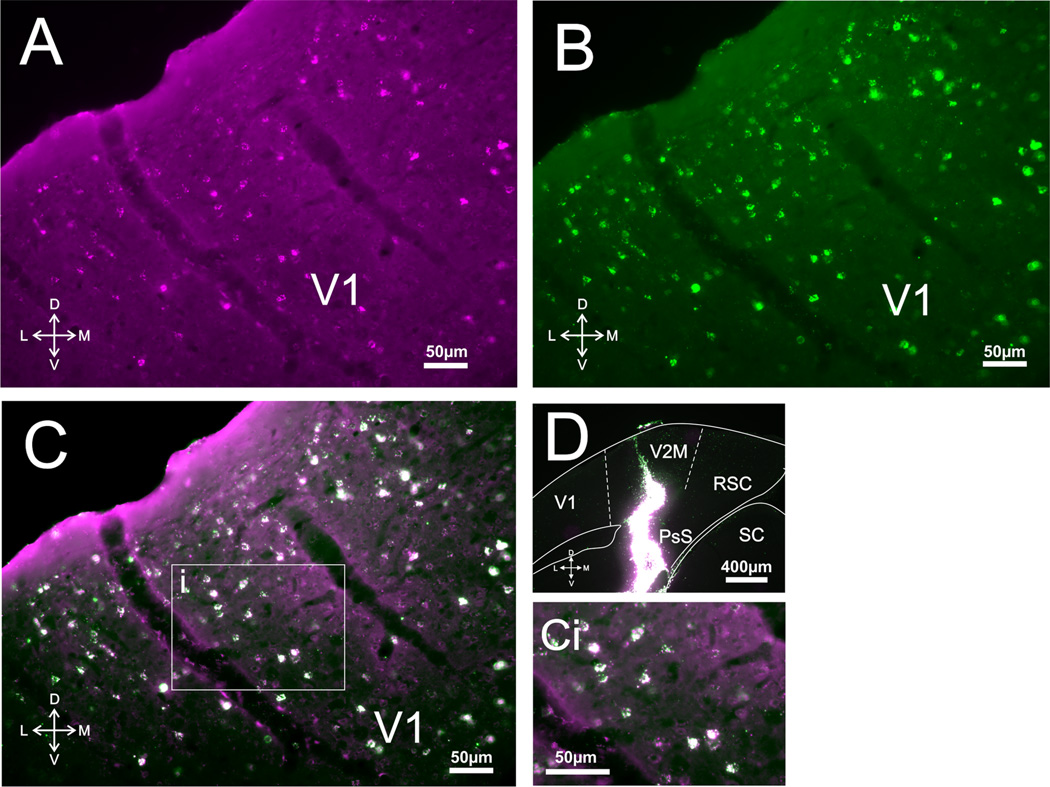
Corticothalamic and corticocortical cells in V1. A: Photomicrograph of retrogradely labeled cells in V1 following injections of red Retrobeads in medial V2. B: Photomicrograph of retrogradely labeled cells in V1 following injections of green Retrobeads in visual thalamus. C: Overlay of A,B. Ci: Highlighted area in C magnified showing the lack of double-labeled cells. D,E: Photomicrographs of injection sites in medial V2 and LGN/LP, respectively. Hipp, hippocampus.
Figure 4.
Percentage of corticothalamic, interareal corticocortical, and double-labeled cells in areas of overlapping labeling in layers 5, and 6 of A1, S1 and V1. Error bars represent standard error of the mean. Significance tests: *P < 0.05; **P < 0.001.
Regarding retrograde labeling in layer 6, thalamic injections produced approximately twice as many retrogradely labeled cells within the areas of overlap compared with cortical injections in all three systems. For the auditory system the values are: thalamic, 66.62% ± 1.64%, and cortical, 32.45% ± 1.83% (P < 0.001); for the somatosensory system, thalamic, 68.4% ± 6.61%, and cortical, 30.67% ± 6.85% (P < 0.05); for the visual system, thalamic, 66.67% ± 5.05%), and cortical, 32.35% ± 4.74% (P < 0.01; see Fig. 4, right panel). These proportions were the same for both types (or colors) of fluorescent tracer injected in thalamus or cortex.
As is apparent from the above, thalamic injections resulted in significantly different percentages of retrogradely labeled cells in layer 5 of the three sensory systems (Kruskal–Wallis test, P < 0.05). Following thalamic injections of tracers, the percentage of retrogradely labeled cells in layer 5 of A1 was significantly larger than that in layer 5 of S1 (Bonferroni-adjusted Mann–Whitney test, P < 0.01) but not in V1 (P > 0.05). The percentage of retrogradely labeled cells in layer 5 of S1 and V1 following thalamic injections did not differ (P > 0.05)
Similarly, cortical injections resulted in significantly different percentages of retrogradely labeled cells in layer 5 of the three sensory systems: Kruskal–Wallis: P < 0.01. Following cortical injections of tracers, the percentage of retrogradely labeled cells in layer 5 of A1 was significantly smaller than that in layer 5 of S1 (Bonferroni-adjusted Mann–Whitney test, P < 0.01) and V1 (P < 0.05). The percentage of retrogradely labeled cells in layer 5 of S1 and V1 following cortical injections did not differ (P > 0.05).
With regard to layer 6, the percentage of retrogradely labeled cells was similar for all three sensory systems, both after thalamic injections (Kruskal–Wallis, P > 0.05) and after cortical injections (P > 0.05).
For any given slice, overlaying the images captured under the two filters (Figs. 1C, 2C, 3C) revealed that, while there was an extensive overlap in the regions where retrogradely labeled cells from the thalamus and cortex could be found, double-labeled cells were extremely rare (see Fig. 2Ci) or, for the majority of the slices, completely nonexistent. More specifically, for both layer 5 and layer 6, the cumulative percentage of double-labeled cells for each sensory system amounted to less than 1% (see Fig. 4 for details).
Finally, to demonstrate that retrograde double labeling was possible in the pathways that we examined with the fluorescent tracers that we used, we performed injections of mixed-color tracers. Injections of the 50:50 mixture of red and green Retrobeads or Fluoro-Gold and Fluoro-Ruby in thalamus or a secondary sensory cortical area resulted in the double-labeling of the vast majority of retrogradely labeled cells in a primary sensory cortical area (Fig. 5). More specifically, injections of mixed tracers in thalamus resulted in double-labeling in 95.9% of retrogradely labeled cells in the subgranular layers of a primary sensory cortical area, while injections of mixed tracers in a secondary sensory cortical area resulted in double-labeling in 86.9% of retrogradely labeled cells in a primary sensory cortical area.
Figure 5.
Examples of double-labeled cells in V1 after a large injection of mixed red and green Retrobeads in V2M, restrosplenial cortex, and postsubiculum. A: Photomicrograph of retrogradely labeled cells in V1 taken with the TX2 filter to visualize the red beads. B: Photomicrograph of retrogradely labeled cells in V1 taken with the L5 filter to visualize the green beads. C: Overlay of A,B. Ci: Highlighted area in C magnified, showing a close-up view of the double-labeled cells seen in white. D: Photomicrograph of the injection site. PsS, postsubiculum; RSC, retrosplenial cortex; SC, superior colliculus.
DISCUSSION
For each sensory system that we examined (somatosensory, auditory, and visual), we found that, following the injection of different color retrograde tracers in the thalamic and secondary sensory cortical targets of a primary sensory cortical area, double-labeled cells in that area were extremely rare. These results were confirmed using two combinations of different color fluorescent tracers. Our findings suggest that in layers 5 and 6 of a mouse primary sensory cortical area, cells that project to a secondary sensory area and cells that project to thalamus effectively represent separate cell populations and that there is virtually no branching of their axons to thalamic and interareal cortical targets, respectively. These data confirm and extend existing evidence that postulated a segregation between interareal or callosal corticocortical cells of the subgranular layers and corticofugal cells in these layers that innervate subcortical targets (Fries et al., 1985; Hübener and Bolz, 1988; Kasper et al., 1994; Zhang and Deschênes, 1997; Hallman et al., 1998; Mercer et al., 2005; Molnár and Cheung, 2006; West et al., 2006; Hattox and Nelson, 2007; Brown and Hestrin, 2009; also see Thomson, 2010, for a review).
Despite the differences in their internal organization (see Woolsey, 1967; Smith and Populin, 2001; Kaas and Collins, 2001; Linden and Schreineer, 2003; Anderson et al., 2009), it is worth noting that the primary cortices of the three sensory systems were very similar with regard to the amount of double-labeled cells in layers 5 and 6 and also the overall proportions of layer 6 cells that were labeled with the different color fluorescent tracers following the thalamic and cortical injections. We thus found that the number of corticothalamic cells was almost twice that of corticocortical cells in layer 6 of all three cortices, which contrasts with previous reports describing equal percentages for these two cell populations in S1 of the rat (Zhang and Deschênes, 1997). This discrepancy could be perhaps attributed to the different species or methodology used in the two studies. Our measurements were focused on areas of overlap between the two retrogradely labeled populations and could have therefore underestimated the total number of retrogradely labeled corticocortical cells. With regard to areas of overlap in layer 5, the three cortices contained very different proportions of corticothalamic and corticocortical cells. While layer 5 of V1 contained an equal proportion of the two types of cells, S1 had a considerably larger proportion of corticocortical layer 5 cells, and A1 an even larger proportion of corticothalamic layer 5 cells. These differences, which were consistent across animals and tracers used, could potentially reflect a difference in the output pattern of this layer for the three sensory systems we investigated. In any case, the functional significance of this is unclear.
The double-labeled cells that we saw were extremely few, yet their proportions were very similar for both layers and all three sensory cortices. It is possible that these cells represent isolated exceptions and may even represent occasional errors in development of connections. Similar patterns have been observed elsewhere. For example, previous reports investigating the degree of overlap between corticothalamic and corticotrigeminal projecting cells in layer 5 of the mouse S1 found it to be about 1% (Hattox and Nelson, 2007), a percentage very similar to what we report here for the corticothalamic and interareal corticocortical projection cells.
Nonetheless, it is not the case that corticothalamic and corticocortical cells do not branch at all. Existing evidence suggests that some corticocortical cells branch across the corpus callosum to also innervate contralateral cortical areas (Zhang and Deschênes, 1997) and also that some layer 6 corticothalamic cells branch to target areas in layer 4 or layer 5a of the same cortical area (Herkenham, 1980; Lu and Lin, 1993; Zhang and Deschênes, 1997), whereas layer 5 corticothalamic cells often branch to reach the midbrain tectum, the pontine nuclei, and other areas of the brainstem (Deschênes et al., 1994; Bourassa and Deschênes, 1995). In addition, corticothalamic layer 6 cells branch to innervate the thalamic reticular nucleus and a dorsal thalamic target (Bourassa and Deschênes, 1995).
Corticothalamic projections originating in layers 5 and 6 possess different synaptic features and have thus been associated with different functional properties. More specifically, layer 5 inputs to thalamus exhibit paired-pulse depression, an all-or-none ability to generate excitatory postsynaptic potentials (EPSPs) and do not activate metabotropic glutamate receptors, all features of a “driver” pathway; that is, an information-carrying pathway (Sherman and Guillery, 2006). On the other hand, layer 6 inputs to thalamus show paired-pulse facilitation, a graded EPSP generation pattern (they produce EPSP whose amplitude is monotonically related to the amount of stimulation current), and can activate metabotropic glutamate receptors (Li et al., 2003; Reichova and Sherman, 2004). These synaptic features have been associated with a modulatory pathway, which is not involved in information transfer but can alter various properties of a thalamic relay (Sherman and Guillery, 2006). Similarly, interareal corticocortical projections vary in their synaptic profile depending on the layer of origin and layer of termination. For example, in A1 layers 5b and 6 projections to layers 2/3 of A2v exhibit synaptic properties equivalent to those of a driver pathway, while their projections to layers 4 and 6 exhibit synaptic properties equivalent to these of a modulator pathway (Covic and Sherman, 2011). Given the heterogeneity of the corticocortical projections of layers 5 and 6 one would assume that they also originate from separate populations of cells within each layer, even though the existence of individual cells with branching axons of differing synaptic properties (driver vs. modulator) could not be excluded (Reyes et al., 1998).
In conclusion, our anatomical data support the idea that the subcortical and interareal corticocortical projections of layers 5 and 6 of sensory cortex in the mouse represent relatively independent output circuits. However, we cannot rule out that, via local collaterals, axons of these corticofugal systems contribute indirectly (transynaptically) to interareal corticocortical processing. The outputs from layer 5 are particularly interesting, because recent evidence suggests that communication between S1 and S2 takes place not only directly via corticocortical projections but also indirectly via a cortico-thalamo-cortical pathway that starts in layer 5 of S1 and is relayed through the posterior medial thalamic nucleus to S2 (Theyel et al., 2010). A similar transthalamic pathway from layer 5 of V1 and A1 is thought to innervate V2 and A2v, respectively (Sherman and Guillery, 2006). It appears that these two parallel routes of corticocortical communication are substantially independent of each other.
Acknowledgments
Grant sponsor: National Institute on Deafness and Other Communication Disorders; Grant number: DC008794 (to S.M.S.); Grant sponsor: National Institute of General Medical Sciences Medical Scientist National Research Service Award; Grant number: 5 T32 GM07281 (to A.N.V.).
LITERATURE CITED
- Anderson LA, Christianson GB, Linden JF. Mouse auditory cortex differs from visual and somatosensory cortices in the laminar distribution of cytochrome oxidase and acetylcholinesterase. Brain Res. 2009;1252:130–142. doi: 10.1016/j.brainres.2008.11.037. [DOI] [PubMed] [Google Scholar]
- Arimatsu Y, Ishida M. Distinct neuronal populations specified to form corticocortical and corticothalamic projections from layer VI of developing cerebral cortex. Neuroscience. 2002;114:1033–1045. doi: 10.1016/s0306-4522(02)00201-4. [DOI] [PubMed] [Google Scholar]
- Arimatsu Y, Ishida M, Sato M, Kojima M. Corticocortical associative neurons expressing latexin: specific cortical connectivity formed in vivo and in vitro. Cereb Cortex. 1999;9:569–976. doi: 10.1093/cercor/9.6.569. [DOI] [PubMed] [Google Scholar]
- Bourassa J, Deschênes M. Corticothalamic projections from the primary visual cortex in rats: a single fiber study using biocytin as an anterograde tracer. Neuroscience. 1995;66:253–263. doi: 10.1016/0306-4522(95)00009-8. [DOI] [PubMed] [Google Scholar]
- Bourassa J, Pinault D, Deschênes M. Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats: a single-fibre study using biocytin as an anterograde tracer. Eur J Neurosci. 1995;7:19–30. doi: 10.1111/j.1460-9568.1995.tb01016.x. [DOI] [PubMed] [Google Scholar]
- Brown SP, Hestrin S. Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nat Lett. 2009;457:1133–1137. doi: 10.1038/nature07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS. Architectonic map of neocortex of the normal mouse. J Comp Neurol. 1975;164:247–263. doi: 10.1002/cne.901640207. [DOI] [PubMed] [Google Scholar]
- Covic EN, Sherman SM. Synaptic properties of connections between the primary and secondary auditory cortices in mice. Cereb Cortex. 2011;21:2425–2441. doi: 10.1093/cercor/bhr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschênes M, Bourassa J, Pinault D. Corticothalamic projections from layer V cells in rat are collaterals of long-range corticofugal axons. Brain Res. 1994;664:215–219. doi: 10.1016/0006-8993(94)91974-7. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 2008. [Google Scholar]
- Fries W, Keizer K, Kuypers HGJM. Large layer VI cells in macaque striate cortex (Meynert cells) project to both superior colliculus and prestriate visual area V5. Exp Brain Res. 1985;58:613–616. doi: 10.1007/BF00235878. [DOI] [PubMed] [Google Scholar]
- Guillery RW. Anatomical evidence concerning the role of the thalamus in corticocortical communication: a brief review. J Anat. 1995;187:583–592. [PMC free article] [PubMed] [Google Scholar]
- Hallman LE, Schofield BR, Lin CS. Dendritic morphology and axon collaterals of corticotectal, corticopontine, and callosal neurons in layer V of primary visual cortex of the hooded rat. J Comp Neurol. 1998;272:149–160. doi: 10.1002/cne.902720111. [DOI] [PubMed] [Google Scholar]
- Hattox AM, Nelson SB. Layer V neurons in mouse cortex projecting to different targets have distinct physiological properties. J Neurophysiol. 2007;98:3330–3340. doi: 10.1152/jn.00397.2007. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Laminar organization of thalamic projections to the rat neocortex. Science. 1980;207:532–535. doi: 10.1126/science.7352263. [DOI] [PubMed] [Google Scholar]
- Hübener M, Bolz J. Morphology of identified projection neurons in layer 5 of rat visual cortex. Neurosci Lett. 1988;94:76–81. doi: 10.1016/0304-3940(88)90273-x. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Collins CE. The organization of sensory cortex. Curr Opin Neurobiol. 2001;11:498–504. doi: 10.1016/s0959-4388(00)00240-3. [DOI] [PubMed] [Google Scholar]
- Kasper EM, Larkman AU, Lübke J, Blakemore C. Pyramidal neurons in layer 5 of the rat visual cortex. I. Correlation among cell morphology, intrinsic electrophysiological properties, and axon targets. J Comp Neurol. 1994;339:459–474. doi: 10.1002/cne.903390402. [DOI] [PubMed] [Google Scholar]
- Li J, Guido W, Bickford ME. Two distinct types of corticothalamic EPSPs and their contribution to short-term synaptic plasticity. J Neurophysiol. 2003;90:3429–3440. doi: 10.1152/jn.00456.2003. [DOI] [PubMed] [Google Scholar]
- Linden JF, Schreiner CE. Columnar transformations in auditory cortex? A comparison to visual and somatosensory cortices. Cereb Cortex. 2003;13:83–89. doi: 10.1093/cercor/13.1.83. [DOI] [PubMed] [Google Scholar]
- Lu SM, Lin RC. Thalamic afferents of the rat barrel cortex: a light- and electron-microscopic study using Phaseolus vulgaris leucoagglutinin as an anterograde tracer. Somatosens Mot Res. 1993;10:1–16. doi: 10.3109/08990229309028819. [DOI] [PubMed] [Google Scholar]
- Mercer A, West DC, Morris OT, Kirchhecker S, Kerkhoff JE, Thomson A. Excitatory connections made by presynaptic cortico-cortical pyramidal cells in layer 6 of the neocortex. Cereb Cortex. 2005;15:1485–1496. doi: 10.1093/cercor/bhi027. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Cheung AF. Towards the classification of subpopulations of layer V pyramidal projection neurons. Neurosci Res. 2006;55:105–115. doi: 10.1016/j.neures.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Ojima H. Terminal morphology and distribution of corticothalamic fibers originating from layers 5 and 6 of cat primary auditory cortex. Cereb Cortex. 1994;4:646–663. doi: 10.1093/cercor/4.6.646. [DOI] [PubMed] [Google Scholar]
- Reichova I, Sherman SM. Somatosensory corticothalamic projections: distinguishing drivers from modulators. J Neurophysiol. 2004;92:2185–2197. doi: 10.1152/jn.00322.2004. [DOI] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov R, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the thalamus. Cambridge, MA: MIT Press; 2006. [Google Scholar]
- Smith PH, Populin LC. Fundamental differences between the thalamocortical recipient layers of the cat auditory and visual cortices. J Comp Neurol. 2001;436:508–519. doi: 10.1002/cne.1084. [DOI] [PubMed] [Google Scholar]
- Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat Neurosci. 2010;13:84–88. doi: 10.1038/nn.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM. Neocortical layer 6, a review. Front Neuroanat. 2010;4:1–14. doi: 10.3389/fnana.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagor E, Mangini NJ, Pearlman AL. Retinotopic organization of striate and extrastriate visual cortex in the mouse. J Comp Neurol. 1980;193:187–202. doi: 10.1002/cne.901930113. [DOI] [PubMed] [Google Scholar]
- Wang Q, Burkhalter AH. Area map of mouse visual cortex. J Comp Neurol. 2007;502:339–357. doi: 10.1002/cne.21286. [DOI] [PubMed] [Google Scholar]
- West DC, Mercer A, Kirchhecker S, Morris OT, Thomson A. Layer 6 cortico-thalamic pyramidal cells preferentially innervate interneurons and generate facilitating EPSPs. Cereb Cortex. 2006;16:200–211. doi: 10.1093/cercor/bhi098. [DOI] [PubMed] [Google Scholar]
- Woolsey TA. Somatosensory, auditory and visual cortical areas of the mouse. Johns Hopkins Med J. 1967;121:91–112. [PubMed] [Google Scholar]
- Zhang ZW, Deschênes M. Intracortical axonal projections of lamina VI cells of the primary somatosensory cortex in the rat: a single-cell labeling study. J Neurosci. 1997;17:6365–6379. doi: 10.1523/JNEUROSCI.17-16-06365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]