1. Introduction
Visual pigments are photon-absorbing molecules that enable rod and cone photoreceptors to produce electrical signals in response to light. They consist of a protein called opsin and a chromophore, in vertebrates usually 11-cis retinal. In contrast to other G protein-coupled receptors, the ligand (retinal) in the visual pigment is covalently attached to the protein and functions both as reverse agonist in the dark (11-cis configuration), and as an agonist upon absorption of a photon (all-trans configuration). The relative ease of delivering light stimuli of known strength and duration allows the detailed characterization of the function of visual pigments in intact photoreceptors. As a result, it is possible to use physiological measurements from single rod and cone photoreceptors to investigate the interaction between opsin and chromophore, as well as the relation between the properties of visual pigments and the function of photoreceptors.
There are two complementary approaches to the study of visual pigments under physiological conditions. The first involves the modification of the chromophore by substituting the native form with a retinoid analog. The second is the transgenic expression of exogenous opsin in place of, or in addition to, the native opsin.
The first approach is based on the light-induced decay of the photoactivated (bleached) visual pigment. Following photon absorption, the activated complex decays into free opsin and all-trans retinal (1). All-trans retinal is then reduced to all-trans retinol by a retinol dehydrogenase and is translocated from the photoreceptors to the retinal pigment epithelium (RPE), where it is converted back into 11-cis retinal. The recycled 11-cis retinal is then sent back to the photoreceptors where it recombines covalently with opsin to form the ground-state visual pigment molecule (2). However, experimental detachment of the retina from the RPE interrupts this visual cycle and prevents the recycling of chromophore and regeneration of the bleached visual pigment. As a result, after exposure of the isolated retina or isolated photoreceptors to bright light, most of the visual pigment is converted to free opsin which is now available for pigment regeneration (3, 4). Application of exogenous retinoid analogs to such bleached photoreceptors allows investigating the noncovalent and covalent binding properties of chromophore to opsin by physiological techniques.
The second approach is based on the tremendous progress in the techniques of molecular biology that has occurred during the last two decades, which has made possible the expression of mutant or foreign opsins in photoreceptors. These techniques allow studies of the effects of opsin mutations on the signaling properties of the visual pigments. Furthermore, as rods and cones use distinct forms of opsin but share the same chromophore (11-cis retinal) (5), transgenic expression of rod and cone opsins allows examination of the differences in sensitivity and response kinetics that derive from differences in opsin structure.
1.1 Animal models
Salamander (Ambystoma tigrinum) has been the animal of choice for single-cell recordings from rod and cone photoreceptors to investigate different aspects of the interaction between retinoid and opsin using retinoid analogs (6, 7). This well-established preparation offers large and abundant rods (Fig. 1A) and cones that can be easily dissociated and maintained in culture. Physiological recordings from salamander photoreceptors are stable over hours and allow extended and rigorous experimental protocols. This greatly facilitates experimental approaches involving replacement of the native chromophore, because the decay of photoactivated pigments and their subsequent regeneration with exogenous chromophore can take as much as 1–2 hours.
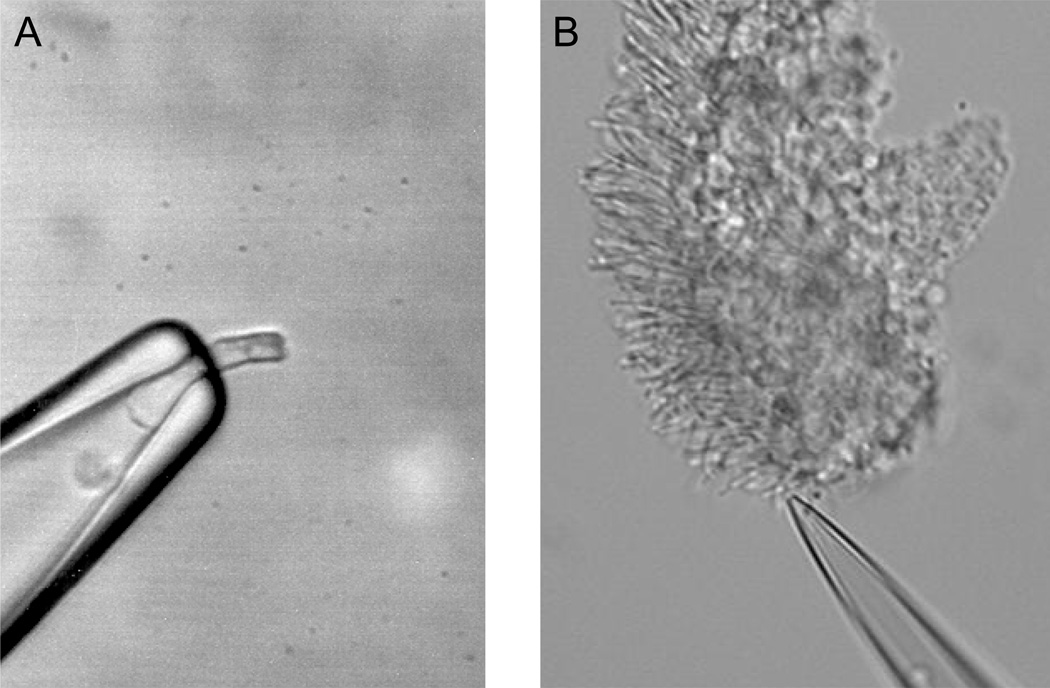
Figure 1.
Suction electrode configuration for recording from single photoreceptors. A, Dissociated salamander rod with its inner segment drawn in the suction electrode and the outer segment protruding out of it. B, Mouse rod with its outer segment drawn in the suction electrode and the inner segment still attached to a piece of retina.
The animals of choice for studies of transgenic opsins have been Xenopus laevis and mouse. Xenopus photoreceptors are relatively large and provide stable and reproducible recordings (8). In addition, the high yield of transgenic Xenopus animals produced by oocyte injection makes unnecessary the breeding and maintenance of transgenic lines for extended periods of time. However, the native chromophore in Xenopus (11-cis 3,4-dehydroreltinal, or A2) is slightly different from the native chromophore in most mammals, including mouse and human (11-cis retinal, or A1). As a result, pigment properties that depend on the chromophore are likely to differ in Xenopus and mammalian photoreceptors. Another drawback of this preparation has been the lack of developed tools for deleting endogenous genes. This has limited Xenopus studies to the transgenic expression of exogenous opsin genes.
Mice, on the other hand, are amenable to both transgenic and gene knockout manipulations. In addition, mouse studies can take advantage of the wide and continuously increasing number of genetically modified lines including transgenic and knockout animals. Finally, mouse rod recordings (see Fig. 1B) have been used routinely for over a decade to investigate rod phototransduction (9). Although the methods developed for regenerating salamander visual pigments with exogenous retinoid analogs are yet to be widely used in mouse photoreceptors, recent studies indicate that the same methods might be applicable there (10–12). Finally, although mouse cone recordings have been challenging, the recent development of genetically modified mice and the creative use of single-cell and electroretinographic (ERG) recording techniques have proven successful (13, 14). This indicates the feasibility of physiological studies of mouse cone pigment properties in their native environment.
1.2 Chromophore Analog Studies
Pigment regeneration takes place in two steps. Initially, the chromophore binds noncovalently in a hydrophobic pocket in the core of opsin (15). Then the aldehyde group located at the end of its polyene chain forms a Schiff-base covalent bond with a lysine residue of opsin (16). As the amount of free 11-cis retinal in the retina (17) and specifically in photoreceptors (18) is minimal, bright light exposure can remove a large fraction of the chromophore from opsin, provided the photoreceptors have been removed from the retina and isolated from other cells and the RPE. This allows the application of various exogenous retinoids to photoreceptors to investigate the interactions between opsin and chromophore and the role of specific parts of the retinoid molecule in the function of visual pigment.
With this approach, the non-covalent interaction between opsin and chromophore can be investigated with analogs of 11-cis retinal having a shortened or modified side chain that are capable of binding in the chromophore pocket of opsin but not of forming a covalent bond with it (19). Studies with such retinoids have revealed that in rods, the non-covalent binding of retinoid to opsin upregulates its activity and results in activation of the rod phototransduction cascade, producing desensitization of the rod and acceleration of its flash response (7, 20–22). In contrast, in red cones the binding of retinoid inactivates opsin and relieves adaptation, thus increasing cone sensitivity and slowing the time course of its flash response (4, 21, 23). As a result, in rods the noncovalent binding of retinoid, including the native 11-cis retinal, to opsin slows down dark adaptation. In red cones, however, the noncovalent binding of retinoid to cone opsin reverses the effects of bleaching adaptation and accelerates cone recovery from a bleach even before the pigment can be regenerated (24). This non-covalent interaction between cone opsin and its chromophore is believed to be one of the mechanisms contributing to the faster dark adaptation of cones compared to rods.
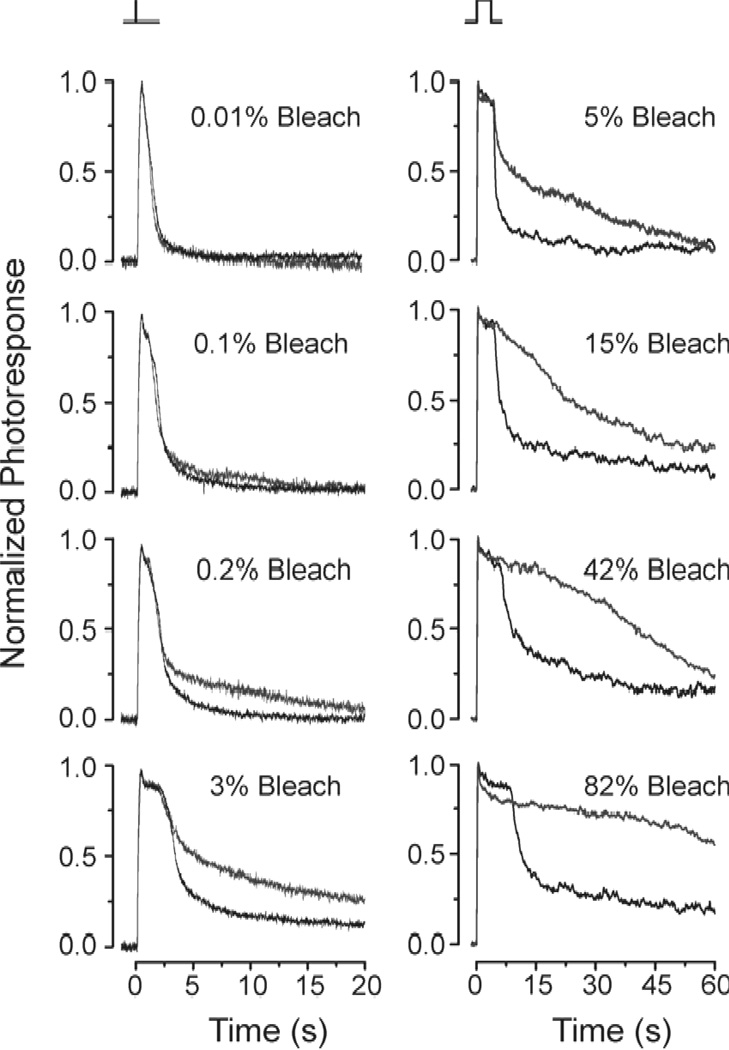
A similar experimental approach has been used successfully to investigate the role of the methyl group at position 9 on the polyene side chain of retinal. Removal of that group produces 9-demethyl retinal which can form a 9-demethyl visual pigment in both rods and cones (25, 26). In rods, 9-demethyl visual pigment produces a quantal response that is about 30 times smaller and decays 5 times slower than that of the native pigment (27). These results reveal that the 9-methyl group is critical for controlling the activation of the G-protein transducin by the rod visual pigment as well as for the inactivation of the rod visual pigment by rhodopsin kinase and arrestin. In cones, on the other hand, 9-demethyl visual pigment produces a quantal response with an amplitude and kinetics that are identical to those of the native pigment (28). However, for flashes activating more than ~0.2% of the 9-demethyl cone pigment, response inactivation with increasing flash intensity is progressively slower than that of the native cone pigment (Fig. 2). These results are consistent with the slower decay of the physiologically-active meta II state of 9-demethyl visual pigment (25) and indicate that, in bright light, the inactivation of cone photoresponses may be rate-limited by cone meta II decay.
Figure 2.
Comparison of response termination in red salamander cones with pigment containing 11-cis retinal (black traces) and 9-demethyl retinal (red traces). Responses produced by the two pigments were identical for low flash strengths (<0.1% bleach). For brighter flashes, termination of 9-demethyl retinal cone pigment responses became progressively slower than the termination of 11-cis retinal cone pigment responses. Reprinted with permission from (28).
Other physiological studies using the technique of chromophore replacement in salamander rods have been performed to examine the role of the chromophore in modulating the spontaneous thermal activation of the visual pigment. Vertebrate visual pigments incorporate either 11-cis retinal (A1, e.g. mouse, bovine, human) or 11-cis 3,4-dehydroreltinal (A2, e.g. Xenopus, bullfrog) as their chromophore. The salamander presents a curious case because its pigment undergoes a shift from the A2 to A1 chromophore type as the animal metamorphoses from the larval to adult stage (29, 30). Larval salamander rods with predominantly A2 as their native chromophore can be bleached and then regenerated with A1 chromophore. Studies of this kind have revealed that A1 rod pigment is at least 36 times more stable than the corresponding A2 pigment (31). As a result, spontaneous activation of salamander rod A2 pigment occurs more frequently than that of the corresponding A1 pigment and may produce adaptation of the rod. Although the rate of thermal activation for cone pigments is significantly higher than that of rods, the stability of human L-cone A1 pigment has also been estimated to be 40-fold higher than that of the corresponding A2 pigment (32). The effect of cone pigment thermal activation on cone physiology is discussed below.
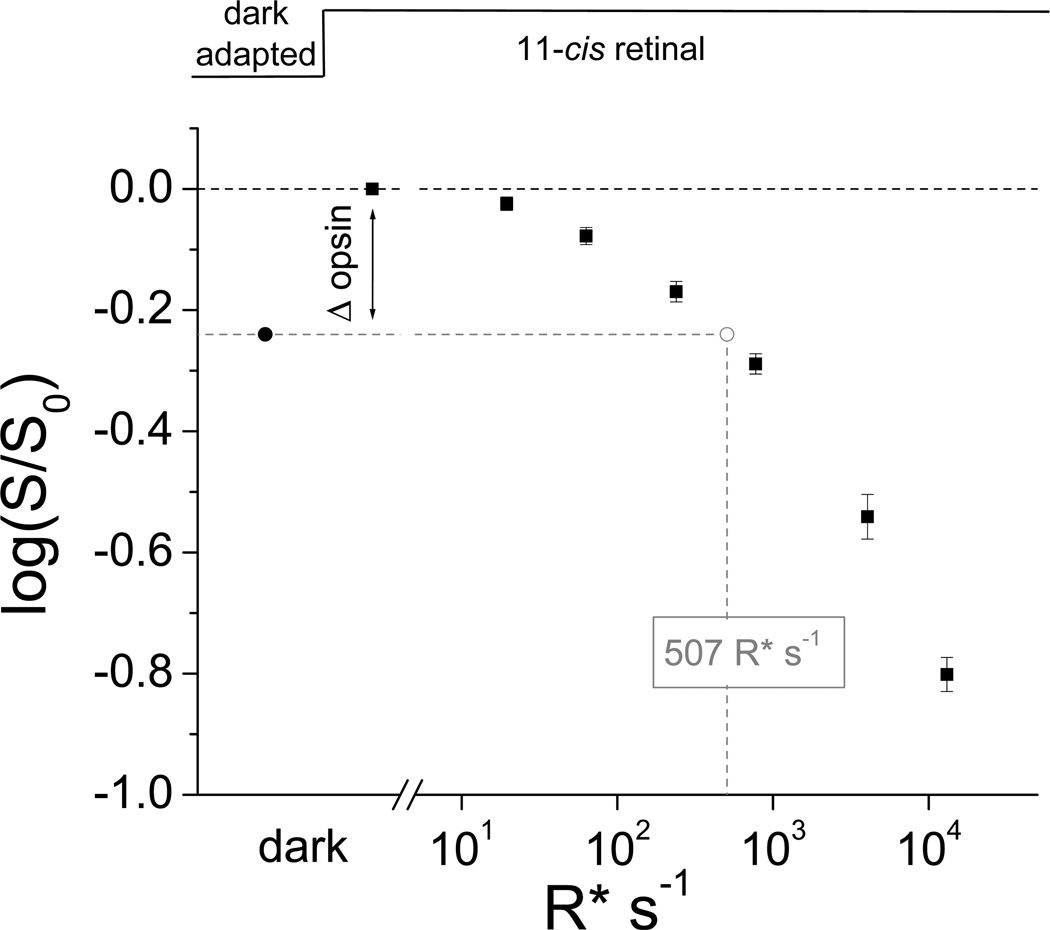
Finally, physiological recordings have also allowed the investigation of the stability of the covalent bond between opsin and 11-cis retinal in rod and cone visual pigments. Biochemical studies had suggested that the formation of the covalent bond between 11-cis retinal and opsin is reversible in cones but irreversible in rods (33, 34). These results predicted the presence of a dynamic equilibrium between chromophore-bound and free opsin in dark-adapted cones, which should be shifted in opposite directions by applying excess chromophore or chromophore-binding proteins. In addition, excess exogenous chromophore would be expected to displace gradually the native chromophore in cone pigment even in darkness. Indeed, application of exogenous 9-cis retinal to dark-adapted isolated salamander red cones results in a gradual blue spectral shift consistent with the gradual replacement of the native 11-cis retinal with 9-cis retinal as the cone chromophore (18). Furthermore, treatment of isolated cones with the 11-cis specific chromophore-binding protein CRALBP results in their gradual desensitization which can be reversed by application of 11-cis retinal (18). Pigment dissociation into opsin and 11-cis retinal has been observed in salamander red and blue cones as well as in green rods, but not in red rods. Notably, the equilibrium between chromophore-bound and free opsin in dark-adapted cones is such that ~10 % of opsin is in the apo-protein state. Despite the very weak catalytic activity of opsin, this surprisingly large fraction of free opsin in dark adapted cells produces substantial activation of the cone phototransduction cascade. Hence, in salamander the reversibility of the covalent bond formation in cone, but not rod, visual pigment contributes to the difference in sensitivity between rods and cones. In red cones, the resulting desensitization is equivalent to that produced by background light activating ~500 pigment molecules per second (Fig. 3) (18). Notably, this level of activity is comparable to the effect of thermal activation by red cone pigment (35, 36). Thus, in salamander red cones the dissociation of cone pigment and its thermal activation produce comparable effects on cone sensitivity. However, unlike thermal activation, the opsin desensitization can be removed by applying excess 11-cs retinal to the dark-adapted salamander cones (18).
Figure 3.
Desensitization of salamander red cones by free opsin produced in pigment dissociation and by pigment activation. The desensitization produced by free opsin (Δ opsin, black circle) was measured by applying 100 µM 11-cis retinal to dark-adapted salamander red cones. To obtain the equivalent level of pigment activation, the cells were next exposed to a series of background lights of increasing intensity and the corresponding desensitization was measured (black squares). By interpolation, the desensitization produced by free opsin was equivalent tot that produced by the photoactivation of ~500 cone pigments per second. This level of pigment activation is comparable to that produced by spontaneous thermal activation of salamander red cone pigment in darkness. Reprinted with permission from (18).
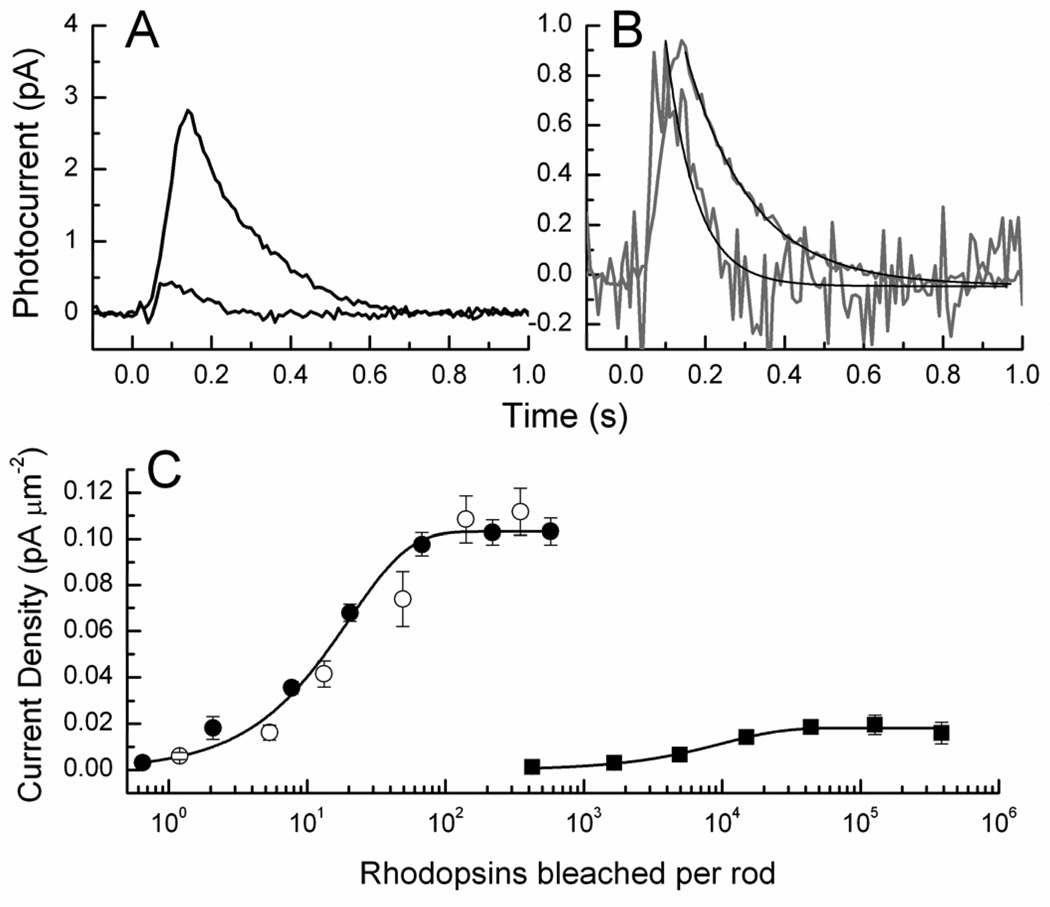
So far, chromophore analog studies as those listed above have been done exclusively in amphibian photoreceptors. However, recent work showing that exogenous retinal can be successfully incorporated into mouse rods (12) indicates the feasibility of such studies in mammalian photoreceptors as well. A useful preparation for studies of this kind is the Rpe65−/− mouse (37), which lacks the gene for the retinal isomerase, the enzyme in the RPE that converts all-trans retinyl ester to 11-cis retinol (38). Rods from Rpe65−/− mice have very little chromophore, and their outer segments contain mostly bare opsin. Since rod opsin is noisy in darkness (3), the rods are desensitized (see Fig. 4) and behave as if in the presence of a continuous background light (39), with a reduced circulating current and greatly accelerated response decay to a brief stimulus. When lipid vesicles containing 11-cis retinal are added to the rods, the time course of response recovery (Fig. 4A, B) and the sensitivity (Fig. 4C) recover to values indistinguishable from those of dark-adapted wild-type rods. The dark adaptation of Rpe65−/− rods with exogenous 11-cis retinal demonstrates that retinoids can be successfully added to mouse rods lacking chromophore so as to completely regenerate the pigment and relieve the adaptation produced by the constitutively active opsin. The question still remains whether pigment regeneration with exogenous chromophore is possible for wild-type and other mouse strains in experimental conditions where the native chromophore has been removed by bleaching light.
Figure 4.
Addition of vesicles containing 11-cis retinal to Rpe65−/− rods. A, Responses of Rpe65−/− rods before (blue) and after (red) 30–60 min incubation with vesicles containing 11-cis retinal. Responses in both cases have been averaged from 17 rods. Intensities of flashes were 4.9 × 104 photons µm−2 (blue) and 43 photons µm−2 (red). B, Traces in A were normalized cell by cell to the maximum response amplitude of the flash and then averaged. Black lines are fits to single exponential decay function with time constants of 75 ms (blue) and 164 ms (red). C, Response-intensity curves of Rpe65−/− rods before (blue) and after (red) addition of vesicles containing 11-cis retinal. Current densities were calculated by dividing by OS surface area. Intensities in rhodopsins bleached were calculated from light intensities in photons µm−2 and collecting areas, estimated for Rpe65−/− rods to be 0.10 before addition of vesicles and 0.31 after vesicle addition. Difference in collecting area before and after is result of different absorption peak and quantum efficiency of 9-cis based isorhodopsin pigment. Black symbols and curve are for WT rods shown for comparison. Reproduced with permission from (12).
1.3 Transgenic Cone Pigment Studies
The small number of cones as a percentage of the total photoreceptor population (3–5%) in most mammalian species, including mouse and human, has limited the biochemical studies of cone phototransduction proteins, including cone visual pigments. As a result, the properties of cone pigments are not as well understood as those of rods. The use of transgenic animals has allowed the expression of cone opsins in rods in order to investigate their signaling properties. In addition, the use of genetically-modified mice has allowed the investigation of the mechanisms by which mutations in opsins lead to retinal diseases.
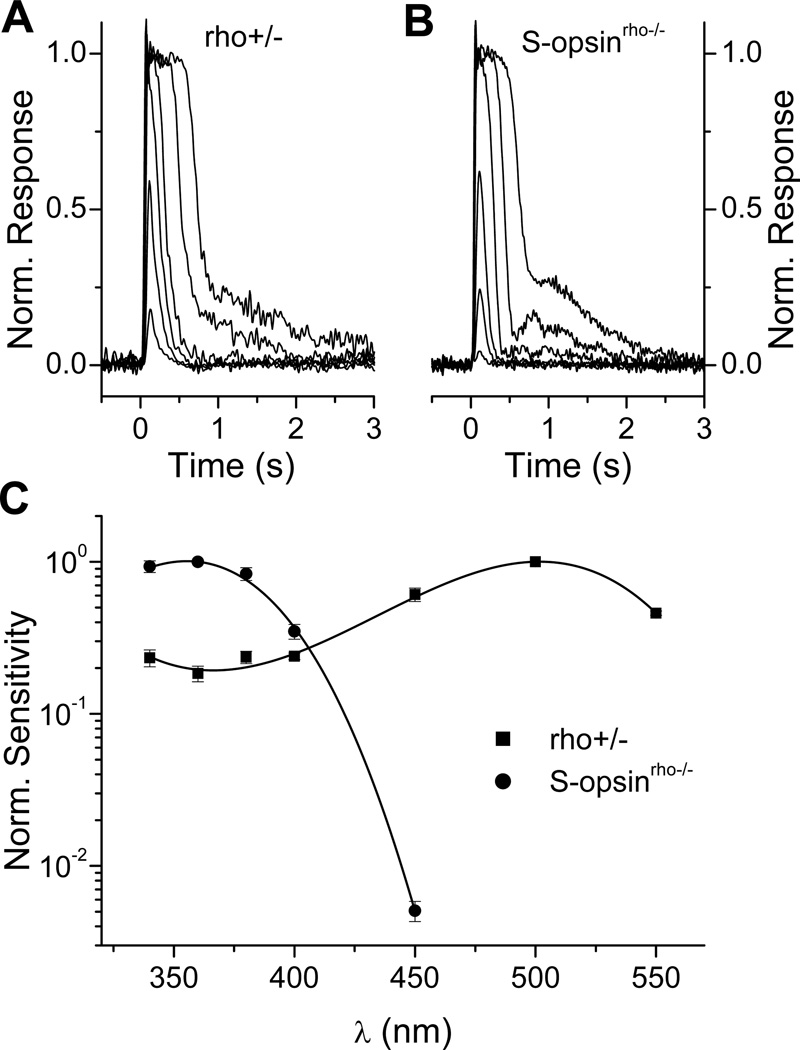
It has been long known that cones are 30–100 fold less sensitive than rods (40, 41), although the mechanisms for this difference in sensitivity had been poorly understood. One possible explanation for this lower sensitivity of cones is the faster decay of the physiologically active meta II of cone pigments compared to rod pigment (42–44). The generation of transgenic animals co-expressing rod and cone opsins has made possible the testing of this hypothesis. With transgenic animals, rod and cone opsins can be coexpressed in the same photoreceptor allowing direct comparison of their signaling properties. Such studies from transgenic Xenopus (35) and mice (32, 45–47) expressing various cone opsins have revealed that, surprisingly, cone pigments produce rod-like responses when expressed in rods. For instance, the expression of mouse cone S-opsin in mouse rods that lack their native rod pigment (rho−/−) results in a dramatic spectral shift in rod sensitivity and renders the rods most sensitive to ultraviolet light (Fig. 5). Nevertheless, the flash responses produced by cone S-opsin are comparable to these produced by the native rod pigment (Fig. 5A, B). Similarly, rod pigment produces cone-like responses when expressed in transgenic Xenopus cones (35). These results indicate that rod and cone visual pigments have similar signaling properties. Thus, rod and cone pigments activate rod transducin and are inactivated by rod rhodopsin kinase and arrestin with similar efficiencies. These results also demonstrate that meta II decay is not the rate-limiting step for the inactivation of the photoresponse under dark adapted conditions.
Figure 5.
Functional expression of mouse cone S-opsin in mouse rods. A, Photoresponses from a control mouse rod heterozygous for rhodopsin (rho+/−). B, Photoresponses from a mouse rod expressing exclusively transgenic mouse cone S-opsin (S-opsinrho−/−). Note the similarity in response wave forms produced by the rod and cone pigments. C, Spectral sensitivities of mouse rods expressing native rhodopsin (rho+/−, squares) or cone opsin (S-opsinrho−/−, circles). For each cell type, sensitivity was normalized at the peak of their pigment’s respective absorption spectrum (500 nm for rhodopsin and 360 nm for S-cone pigment) and fit with a third-order polynomial (solid lines). Error bars indicate SEM. Reprinted with permission from (47).
The studies of transgenic Xenopus and mouse rods expressing cone opsins have also revealed an interesting dichotomy between the function of cone pigments in these two species. In Xenopus rods, the thermal activation of transgenic red cone pigment is some 10,000-fold higher than that of the rod pigment (35). The high molecular rate of thermal activation of red cone pigment is consistent with the estimate of noise from thermal pigment activation in salamander red cones (36, 48) and indicates that in animals using A2 11-cis retinal, thermal activation of red cone pigment contributes significantly to the low sensitivity of cones compared to rods. In contrast, in transgenic mouse rods, the same red cone pigment, while still 600 times more thermally active than rod pigment (32), has a substantially lower rate of thermal activation than would be expected from the Xenopus and salamander studies. This result indicates that in animals using A1 11-cis retinal, such as mouse and human, thermal activation of cone pigment is not significant enough to affect cone sensitivity.
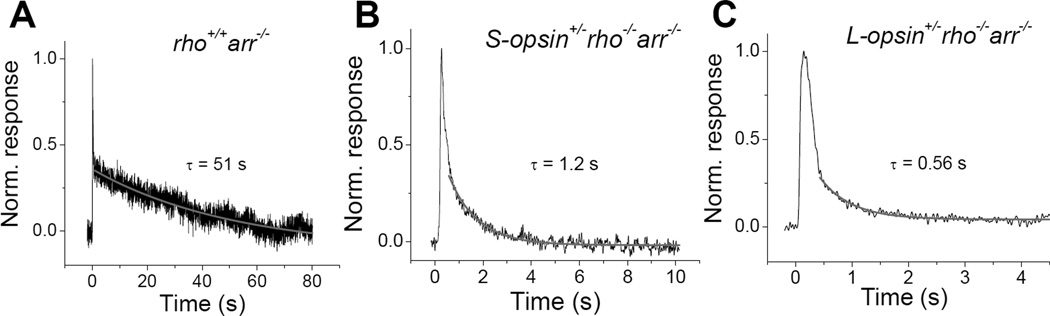
The transgenic expression of cone opsins in rods has also allowed the measurement of the rate of decay of the meta II state of cone pigments under physiological conditions. Whereas in vitro meta II decays spontaneously, in intact photoreceptors the photoactivated visual pigment is rapidly inactivated, initially by phosphorylation by rhodopsin kinase and then by the binding of arrestin (49). In arrestin-deficient (arr1−/−) mouse rods, the response displays a biphasic shutoff, with normal initial inactivation by rhodopsin kinase, followed by a slow tail that represents the decay of rod meta II (Fig. 6A) (50). The same method can be used to observe the decay of cone meta II. The deletion of arrestin in transgenic rods expressing cone opsin removes the inactivation of phosphorylated cone pigment. This renders the decay of the active cone meta II state the rate-limiting step for photoresponse inactivation, which can be observed experimentally. Such experiments reveal that whereas the time constant of the rod meta II decay is ~50 s (Fig. 6A), mouse S-cone meta II decays in ~1.3 s (Fig. 6B) (47) and human L-cone meta II in only 0.6 s (Fig. 6C) (32). These measurements were done from intact photoreceptors at 37 °C and most likely reflect accurately the in vivo decay rates of S and L cone meta II.
Figure 6.
Measurement of the physiologically active meta II state of rod and cone pigments expressed in rods lacking arrestin. A, Normalized dim flash response of mouse rod lacking arrestin (rho+/+ arr−/−). The slow tail of the response inactivation is fit by a single exponential decay function with τ = 51 s which represents the decay of rod pigment meta II. B, Normalized dim flash response of mouse rod that expresses exclusively S-opsin and lacks arrestin (S-opsin+/− rho−/− arr−/−) showing that τ = 1.2 s for Sopsin meta II. C, Normalized dim flash response of mouse rod that expresses exclusively L-opsin and lacks arrestin (L-opsin+/− rho−/− arr−/−) showing that τ = 0.56 s for L-opsin meta II. Reprinted with permission from (47) and (32).
1.4 Rhodopsin Mutation Studies
Transgenic expression of mutant rod opsin has also been used to investigate the mechanism by which certain rhodopsin mutations lead to congenital night blindness. Patients with the rhodopsin G90D mutation and several other rhodopsin mutations have been shown to have a persistent loss of rod sensitivity, produced by a mechanism that is similar if not identical to that produced by steady background light (51, 52). Two possible explanations have been given for why the G90D mutation of opsin might result in constitutive activation of the rods. According to one model, the G90D mutation produces opsin without chromophore with a high constitutive activity (53). Several studies have shown that G90D opsin in vitro is very effective in stimulating the G-protein transducin (54). According to the second model, it is G90D rhodopsin rather than G90D opsin that produces spontaneous activity of the cascade, whether by a high rate of spontaneous conversion of G90D rhodopsin to meta II (51, 52) or by some other mechanism (12).
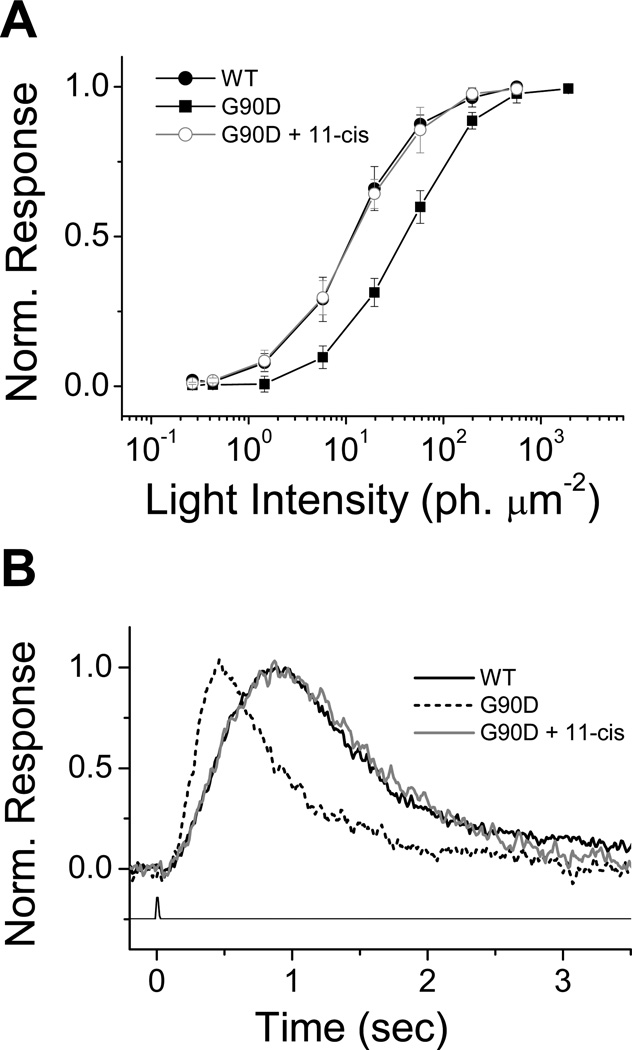
The validity of these two hypotheses has been tested by introducing the mutant G90D rhodopsin into rods of two species, Xenopus and mouse. In transgenic Xenopus rods (55), expression of the mutant G90D opsin produces constitutive activity and results in desensitization and acceleration of the decay of the flash response (55). Normal sensitivity (Fig. 7A) and response kinetics (Fig. 7B) can be restored with the application of exogenous 11-cis retinal. This result shows that in dark-adapted Xenopus rods the spontaneous activation responsible for the reduction in sensitivity is produced by the high constitutive activity of G90D opsin, which can be removed upon the covalent attachment of the reverse agonist 11-cis retinal.
Figure 7.
Desensitization in night blindness mutant G90D and phenotypic rescue with 11-cis retinal in Xenopus rod photoreceptors. Intensity response curves (A) and dim flash responses (B) were measured in single cells isolated from retinae of wild type frogs and transgenic frogs containing the rhodopsin EGFP gene with the G90D mutation. Normalized curves plotted in (A) were constructed from averages of peak flash response amplitude elicited from different isolated rods at different flash intensities: wild type, n=6; G90D, n=7; G90D+11-cis retinal, n=8. Error bars indicate standard deviation. Dim flash responses plotted in B are averaged from rods from different animals: wild type, n=6; G90D, n=7; G90D + 11-cis retinal, n=6. Reprinted with permission from (55).
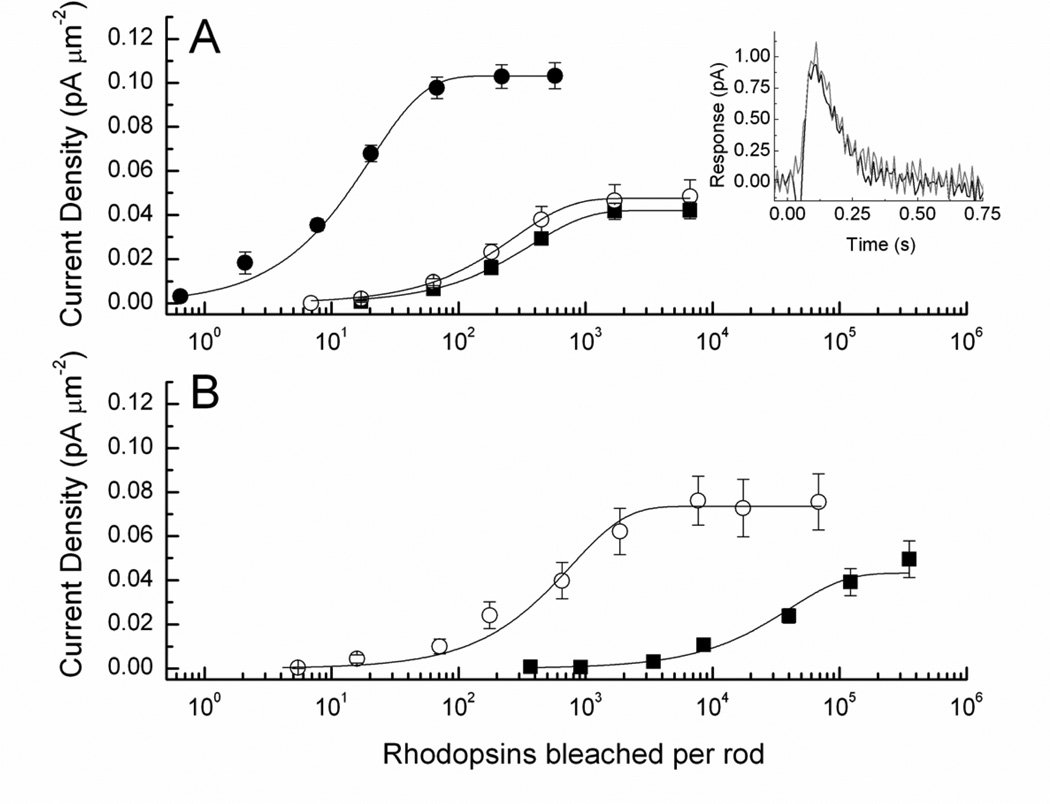
In transgenic mouse rods, expression of the mutant G90D opsin also produces constitutive activity, which results in decreased circulating current and lower sensitivity, as well as an accelerated decay of the flash response (12, 51, 52). However, the desensitization produced by mammalian G90D pigment expressed in a mammal is 200–3000 times smaller than that produced when the pigment is expressed at the same concentration in Xenopus (12). In addition, unlike in Xenopus, normal sensitivity (Fig. 8A) and response kinetics (Fig. 8A, inset) in a mouse rod cannot be restored by the addition of exogenous 11-cis retinal (12). If 11-cis retinal is added to an Rpe65−/− mouse containing the normal rhodopsin gene, the sensitivity of the rod recovers to that in a wildtype animal, as we have seen (Fig. 4). But if 11-cis retinal is added to rods from an Rpe65−/− mouse with G90D pigment and no wild-type pigment, the sensitivity recovers only to that of a G90D rod with its normal complement of chromophore (Fig. 8B). Thus, the lack of recovery of G90D-expressing mouse rods can not be attributed to the failure of exogenous chromophore to promote G90D pigment regeneration. Instead, these experiments show that in a dark-adapted mouse the spontaneous activation responsible for the elevation of threshold is produced by G90D rhodopsin bound to chromophore and not by G90D opsin.
Figure 8.
Addition of 11-cis retinal to G90D mouse rods. A, Response-intensity curves for 30 D+/+ rods homozygous for the G90D mutation before treatment with retinal (black squares) and 15 D+/+ rods after 1–3 hours of vesicle incubation (red circles). Measurements were made from rods from the same retinas. Current densities were calculated by dividing by outer segment area of 99 µm2; collecting area was 0.40. Small shift in curves was not statistically significant. Filled circles and curve are for WT rods shown for comparison. Inset, mean unnormalized responses to 20 ms flash of 1.5 × 105 photons µm−2 from D+/+ rods homozygous for the G90D mutation, averaged from 24 rods before treatment (black trace) and 20 rods after 70–140 min treatment with phospholipid vesicles containing 11-cis retinal (red trace). B, Addition of vesicles to D+/−;Rh−/− rods with or without the RPE65 isomerase. Black squares are mean responses from 24 D+/−;Rh−/−;Rpe65−/− rods before addition of vesicles. Current density was calculated from outer segment area of 40.5 µm2; collecting area was 0.06 (see 13). Red circles are mean responses from 24 D+/−;Rh−/−;Rpe65−/− rods after addition of vesicles containing 11-cis retinal. Outer segment area was 69 µm2 and collecting area 0.28.
Because the two studies in Xenopus and mouse used identical recording methods, the most likely explanation for the discrepancy is that mammalian G90D pigment behaves differently in Xenopus and in mouse rods. Possible factors for this difference include differences in the interaction of G90D opsin with A2 (Xenopus) and A1 (mouse) chromophore, the different body temperature of amphibians and mammals, differences in the lipid composition of the outer segment membrane, or different modification of the opsin molecule in Xenopus and mouse rods. Although unlikely, it is also possible that the bovine opsin, used in the Xenopus study, and the mouse opsin, used in the mouse study, are affected differently by the G90D mutation. Regardless of the exact mechanism, the discrepancy between these two studies emphasizes the need for caution when extrapolating animal studies to the function of human visual pigments. These studies also indicate that mammalian pigments may respond differently when expressed in a mammal than when expressed in an amphibian.
1.5 Epilogue
Studies of amphibian rods and cones and transgenic Xenopus and mouse rods have yielded a wealth of information about the interactions between chromophore and opsin, the signaling properties of visual pigments, and the modulation of photoreceptor function by visual pigments. Future studies are likely to focus on two areas. First, the methods of chromophore replacement by bleaching and regeneration developed in salamander photoreceptors need to be extended to mouse photoreceptors. Previous work has shown that chromophore can be successfully introduced into rods lacking their normal complement of photopigment, as for example in Rpe65−/− mice (see Fig. 4), but it would be useful to be able to bleach and replace pigment in photoreceptors from wild-type or other genetically modified animals. This would allow the combination of chromophore replacement and transgenic opsin modifications to provide a powerful set of tools to study the interactions between retinoid and opsin in mammalian visual pigments. Second, the recent development of methods for recording from mouse cone photoreceptors will allow investigating the properties of cone visual pigments in their native environment and how these properties modulate the function of mammalian cones. It will also make possible experiments investigating the role of cone pigment properties for cone function not only in darkness but also in bright light or during dark adaptation, both critical for the photoreceptors that mediate our daytime, acute, and color vision.
2 Materials
2.1 Perfusion solutions
Salamander perfusion solution: 110 mM NaCl, 2.5 mM KCl, 1.6 mM MgCl2, 1.0 mM CaCl2, 10 mM dextrose, 10 mM HEPES, pH 7.8, and bovine serum albumin (100 mg/l) (3).
Mouse perfusion solution: 112.5 mM NaCl, 3.6 mM KCl, 2.4 mM MgCl2, 1.2 mM CaCl2, 10 mM HEPES (pH 7.4), 20 mM NaHCO3, 3 mM Na succinate, 0.5 mM Na glutamate, 0.02 mM EDTA, and 10 mM glucose. The solution is bubbled with 95% O2/5% CO2, and warmed to 36–38 °C (see Note 1).
Mouse recording electrode solution: 140 mM NaCl, 3.6 mM KCl, 2.4 mM MgCl2, 1.2 mM CaCl2, 3 mM HEPES(pH 7.4), 0.02 mM EDTA, and 10 mM glucose (47).
2.2 Preparation and application of retinoid solution
The application of retinoids to photoreceptors is complicated by their high hydrophobicity. The two most practical approaches to preparing retinoid solution include suspending the retinoid in lipid vesicles or dissolving it in ethanol and diluting that solution in Ringer (6, 12).
Aliquots containing 300 µg dry retinoid are prepared by dissolving the retinoid in ethanol to 10 mg/ml, aliquoting 30 µl solution in individual small conical vials, drying under gentle stream of nitrogen (see Note 2), and then storing in darkness at −80 °C until use.
Phospholipid vesicles are prepared by placing 25 mg L-α-phosphatidylcholine (type V-E; Sigma) in a glass scintillation vial and evaporating the solvent to dryness under a stream of nitrogen. Ringer solution (15 ml) is then added to the vial and sonicated for 20 min in an ice bath (1 sec on/ 1 sec off) at 45 W using a 1.0-cm probe. Fresh vesicle solution is prepared daily. One hour before the beginning of the experiment, 1.5 ml vesicle solution is added to a vial of dry retinoid in darkness and sonicated for 60 sec on low power using a tapered microtip. The retinoid content in the solution is determined from the absorption of a small aliquot, diluted 10-fold in ethanol, using the molar extinction coefficient for the particular retinoid.
An alternative method for dissolving the retinoid in Ringer solution involves using ethanol. Initially, 4 µl ethanol is added to the bottom of the vial containing the dry retinoid. The solution is stirred gently using the pipet tip and then Ringer is gradually added to the vial to a final volume of 4 ml. The resulting 0.1% ethanol solution can then be applied to the photoreceptor preparation. Although not as efficient as the lipid vesicle method, the ethanol solution offers the advantage that it can be prepared very quickly, typically after the photoreceptors have been bleached. With the perfusion off, a small volume of retinoid solution (~0.5 ml) can be added to the recording chamber, and the photoreceptors can be incubated for ~2 min before turning the perfusion on again. The chamber should be rinsed with ethanol between experiments to ensure that it is clean of retinoid.
3 Methods
The single-cell suction electrode technique allows recordings from individual rod and cone photoreceptors without penetrating their plasma membrane. The method, developed by Yau, Lamb, and Baylor (56) is based on the polar nature of photoreceptors with inward current flowing at their outer segment, matched by outward current at their inner segment. Drawing the photoreceptor outer segment (see Note 3) into a tightly-fitting glass electrode (Fig. 1) allows collection of that current, which can then be amplified, digitized, and stored on a computer for analysis. Exposure of the photoreceptor to a test flash activates the phototransduction cascade and results in closure of the cGMP transduction channels in the outer segment which can be observed as a decrease in the current recorded from the outer segment. Inversed, but otherwise identical, photoresponses can be recorded if the inner segment of the photoreceptor is drawn into the suction electrode instead.
Experiments are typically performed after animals are dark-adapted overnight.
The animal is euthanized and the eyes are removed and hemisected under dim red light illumination (see Note 4). All subsequent manipulations are performed in infrared light with the help of infrared image converters.
The retina is torn free of the pigment epithelium and chopped into small pieces with a razor blade. Overdoing this step can result in a preparation with many broken outer segments but few intact cells. Gently drawing and releasing the solution containing retinal pieces through a transfer pipette also helps in dissociating photoreceptors.
A fraction of the resulting suspension is transferred to a recording chamber fit on the stage of an inverted microscope (see Note 5).
Recordings can be performed either from dissociated photoreceptors of from the outer segments of cells protruding from a piece of retina. As salamander photoreceptors are rather sturdy and continue to produce robust photoresponses for hours after dissociation from the retina, suction recordings are typically done from dissociated cells. As dissociated mouse rods do not usually yield robust and lasting responses, mouse rod recordings are typically performed from the outer segments of cells protruding from a piece of retina.
Test flashes as well as bleaching and background lights are provided from a calibrated dual-beam optical stimulator (57). Flash intensity and wavelength are controlled by a set of neutral density filters and narrow band interference filters, respectively. Test flash duration, typically 10–20 ms, is controlled by computer-driven shutters.
The fraction of bleached pigment can be estimated from the relation: F = 1 − exp(−IPt), where F is the fraction of bleached pigment, I is the light intensity in photons µm−2 s−1, and t is the duration of light exposure in seconds. The value used for the photosensitivity of the cell P is 6.2 × 10−9 µm2 for rods (58) and 6.0 × 10−9 µm2 for cones (59).
Footnotes
The perfusion solution can be brought to 36–38 °C by running it through a ceramic resistor (e.g. SBCHE633RJ, Tyco Electronics) under small DC voltage via stainless steel tubing. The heater should be located as close to the recording chamber as possible, ideally at the stage of the microscope. To prevent bubbles from forming in the tubing, the solution should be pre-heated to 36–38 °C when bubbling with 95% O2/5% CO2.
When preparing retinoid aliquots by drying retinoid ethanol solution, the gas stream should be low enough to prevent the solution from splashing along the walls of the vial. This makes resuspending the retinoid easier and more efficient.
A simple suction device can be constructed by connecting tubing partially filled with mineral oil to the side-port of the electrode holder. The other end of the tubing is connected to a small reservoir of mineral oil (1cc syringe works well). Raising or lowering the reservoir applies positive or negative pressure to the tip of the electrode. Suction for drawing the cell in the electrode can be applied by mouth through tubing and a needle drawn through the piston of the syringe.
An easy method to achieve clean and fast eye hemisection involves the use of two matching plastic blocs. The bottom one has a row of round semi-spherical wells of different diameters, matching the size of eyeballs of different species (e.g. fish, mouse, salamander). The eyeball is placed in the matching well, iris facing up, and then clamped with the second plastic block that has holes matching the wells in the bottom block. A clean cut of the eye can be achieved by slicing a razor blade between the two blocks. Usually, the lens is pushed out through the hole in the top block, leaving a clean-cut eyecup in the well of the bottom block.
Stopping the perfusion for ~2 minutes right after adding the cell suspension to the recording chamber allows most cells to settle on the bottom of the chamber and not get washed away by the solution flow. If necessary, a bypass tubing can be added to prevent the chamber from drying while the perfusion is off.
References
- 1.Ebrey T, Koutalos Y. Vertebrate photoreceptors. Prog Retin Eye Res. 2001;20:49–94. doi: 10.1016/s1350-9462(00)00014-8. [DOI] [PubMed] [Google Scholar]
- 2.Saari JC. Biochemistry of visual pigment regeneration: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2000;41:337–348. [PubMed] [Google Scholar]
- 3.Cornwall MC, Fain GL. Bleached pigment activates transduction in isolated rods of the salamander retina. J Physiol. 1994;480(Pt 2):261–279. doi: 10.1113/jphysiol.1994.sp020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornwall MC, Matthews HR, Crouch RK, Fain GL. Bleached pigment activates transduction in salamander cones. J Gen Physiol. 1995;106:543–557. doi: 10.1085/jgp.106.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokoyama S. Molecular evolution of vertebrate visual pigments. Prog Retin Eye Res. 2000;19:385–419. doi: 10.1016/s1350-9462(00)00002-1. [DOI] [PubMed] [Google Scholar]
- 6.Cornwall MC, Jones GJ, Kefalov VJ, Fain GL, Matthews HR. Electrophysiological methods for measurement of activation of phototransduction by bleached visual pigment in salamander photoreceptors. Methods Enzymol. 2000;316:224–252. doi: 10.1016/s0076-6879(00)16726-6. [DOI] [PubMed] [Google Scholar]
- 7.Crouch RK, Kefalov V, Gartner W, Cornwall MC. Use of retinal analogues for the study of visual pigment function. Methods Enzymol. 2002;343:29–48. doi: 10.1016/s0076-6879(02)43126-6. [DOI] [PubMed] [Google Scholar]
- 8.Xiong WH, Yau KW. Rod sensitivity during Xenopus development. J Gen Physiol. 2002;120:817–827. doi: 10.1085/jgp.20028702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lem J, Makino CL. Phototransduction in transgenic mice. Curr Opin Neurobiol. 1996;6:453–458. doi: 10.1016/s0959-4388(96)80049-3. [DOI] [PubMed] [Google Scholar]
- 10.Fan J, Woodruff ML, Cilluffo MC, Crouch RK, Fain GL. Opsin activation of transduction in the rods of dark-reared Rpe65 knockout mice. J Physiol. 2005;568:83–95. doi: 10.1113/jphysiol.2005.091942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo DG, Yau KW. Rod sensitivity of neonatal mouse and rat. J Gen Physiol. 2005;126:263–269. doi: 10.1085/jgp.200509342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dizhoor AM, Woodruff ML, Olshevskaya EV, Cilluffo MC, Cornwall MC, Sieving PA, Fain GL. Night blindness and the mechanism of constitutive signaling of mutant G90D rhodopsin. J Neurosci. 2008;28:11662–11672. doi: 10.1523/JNEUROSCI.4006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikonov SS, Kholodenko R, Lem J, Pugh EN., Jr Physiological features of the S- and M-cone photoreceptors of wild-type mice from single-cell recordings. J Gen Physiol. 2006;127:359–374. doi: 10.1085/jgp.200609490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heikkinen H, Nymark S, Koskelainen A. Mouse cone photoresponses obtained with electroretinogram from the isolated retina. Vision Res. 2008;48:264–272. doi: 10.1016/j.visres.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto H, Yoshizawa T. Existence of a beta-ionone ring-binding site in the rhodopsin molecule. Nature. 1975;258:523–526. doi: 10.1038/258523a0. [DOI] [PubMed] [Google Scholar]
- 16.Bownds D. Site of attachment of retinal in rhodopsin. Nature. 1967;216:1178–1181. doi: 10.1038/2161178a0. [DOI] [PubMed] [Google Scholar]
- 17.Lyubarsky AL, Pugh EN., Jr Over 98% of 11-cis Retinal in the Dark-Adapted Mouse Eye Is Bound to Rod and Cone Opsins. Invest. Ophthalmol. Vis. Sci. 2007;48 3246- [Google Scholar]
- 18.Kefalov VJ, Estevez ME, Kono M, Goletz PW, Crouch RK, Cornwall MC, Yau KW. Breaking the covalent bond--a pigment property that contributes to desensitization in cones. Neuron. 2005;46:879–890. doi: 10.1016/j.neuron.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crouch RK. Studies of rhodopsin and bacteriorhodopsin using modified retinals. Photochem Photobiol. 1986;44:803–807. doi: 10.1111/j.1751-1097.1986.tb05540.x. [DOI] [PubMed] [Google Scholar]
- 20.Kefalov VJ, Carter Cornwall M, Crouch RK. Occupancy of the chromophore binding site of opsin activates visual transduction in rod photoreceptors. J Gen Physiol. 1999;113:491–503. doi: 10.1085/jgp.113.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corson DW, Kefalov VJ, Cornwall MC, Crouch RK. Effect of 11-cis 13-demethylretinal on phototransduction in bleach-adapted rod and cone photoreceptors. J Gen Physiol. 2000;116:283–297. doi: 10.1085/jgp.116.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isayama T, Chen Y, Kono M, Degrip WJ, Ma JX, Crouch RK, Makino CL. Differences in the pharmacological activation of visual opsins. Vis Neurosci. 2006;23:899–908. doi: 10.1017/S0952523806230256. [DOI] [PubMed] [Google Scholar]
- 23.Jin J, Crouch RK, Corson DW, Katz BM, MacNichol EF, Cornwall MC. Noncovalent occupancy of the retinal-binding pocket of opsin diminishes bleaching adaptation of retinal cones. Neuron. 1993;11:513–522. doi: 10.1016/0896-6273(93)90155-k. [DOI] [PubMed] [Google Scholar]
- 24.Kefalov VJ, Crouch RK, Cornwall MC. Role of noncovalent binding of 11-cis-retinal to opsin in dark adaptation of rod and cone photoreceptors. Neuron. 2001;29:749–755. doi: 10.1016/s0896-6273(01)00249-5. [DOI] [PubMed] [Google Scholar]
- 25.Das J, Crouch RK, Ma JX, Oprian DD, Kono M. Role of the 9-methyl group of retinal in cone visual pigments. Biochemistry. 2004;43:5532–5538. doi: 10.1021/bi036097u. [DOI] [PubMed] [Google Scholar]
- 26.Ganter UM, Schmid ED, Perez-Sala D, Rando RR, Siebert F. Removal of the 9-methyl group of retinal inhibits signal transduction in the visual process. A Fourier transform infrared and biochemical investigation. Biochemistry. 1989;28:5954–5962. doi: 10.1021/bi00440a036. [DOI] [PubMed] [Google Scholar]
- 27.Corson DW, Cornwall MC, MacNichol EF, Tsang S, Derguini F, Crouch RK, Nakanishi K. Relief of opsin desensitization and prolonged excitation of rod photoreceptors by 9-desmethylretinal. Proc Natl Acad Sci U S A. 1994;91:6958–6962. doi: 10.1073/pnas.91.15.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estevez ME, Ala-Laurila P, Crouch RK, Cornwall MC. Turning cones off: the role of the 9-methyl group of retinal in red cones. J Gen Physiol. 2006;128:671–685. doi: 10.1085/jgp.200609630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harosi FI. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975;66:357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makino CL, Dodd RL. Multiple visual pigments in a photoreceptor of the salamander retina. J Gen Physiol. 1996;108:27–34. doi: 10.1085/jgp.108.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ala-Laurila P, Donner K, Crouch RK, Cornwall MC. Chromophore switch from 11-cis-dehydroretinal (A2) to 11-cis-retinal (A1) decreases dark noise in salamander red rods. J Physiol. 2007;585:57–74. doi: 10.1113/jphysiol.2007.142935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu Y, Kefalov V, Luo DG, Xue T, Yau KW. Quantal noise from human red cone pigment. Nat Neurosci. 2008;11:565–571. doi: 10.1038/nn.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crescitelli F. The gecko visual pigment: the dark exchange of chromophore. Vision Res. 1984;24:1551–1553. doi: 10.1016/s0042-6989(84)80004-8. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto H, Tokunaga F, Yoshizawa T. Accessibility of the iodopsin chromophore. Biochim Biophys Acta. 1975;404:300–308. doi: 10.1016/0304-4165(75)90337-2. [DOI] [PubMed] [Google Scholar]
- 35.Kefalov V, Fu Y, Marsh-Armstrong N, Yau KW. Role of visual pigment properties in rod and cone phototransduction. Nature. 2003;425:526–531. doi: 10.1038/nature01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rieke F, Baylor DA. Origin and functional impact of dark noise in retinal cones. Neuron. 2000;26:181–186. doi: 10.1016/s0896-6273(00)81148-4. [DOI] [PubMed] [Google Scholar]
- 37.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 38.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodruff ML, Wang Z, Chung HY, Redmond TM, Fain GL, Lem J. Spontaneous activity of opsin apoprotein is a cause of Leber congenital amaurosis. Nat Genet. 2003;35:158–164. doi: 10.1038/ng1246. [DOI] [PubMed] [Google Scholar]
- 40.Fain GL, Dowling JE. Intracellular recordings from single rods and cones in the mudpuppy retina. Science. 1973;180:1178–1181. doi: 10.1126/science.180.4091.1178. [DOI] [PubMed] [Google Scholar]
- 41.Schnapf JL, Baylor DA. How photoreceptor cells respond to light. Sci Am. 1987;256:40–47. doi: 10.1038/scientificamerican0487-40. [DOI] [PubMed] [Google Scholar]
- 42.Imai H, Imamoto Y, Yoshizawa T, Shichida Y. Difference in molecular properties between chicken green and rhodopsin as related to the functional difference between cone and rod photoreceptor cells. Biochemistry. 1995;34:10525–10531. doi: 10.1021/bi00033a026. [DOI] [PubMed] [Google Scholar]
- 43.Okada T, Matsuda T, Kandori H, Fukada Y, Yoshizawa T, Shichida Y. Circular dichroism of metaiodopsin II and its binding to transducin: a comparative study between meta II intermediates of iodopsin and rhodopsin. Biochemistry. 1994;33:4940–4946. doi: 10.1021/bi00182a024. [DOI] [PubMed] [Google Scholar]
- 44.Starace DM, Knox BE. Activation of transducin by a Xenopus short wavelength visual pigment. J Biol Chem. 1997;272:1095–1100. doi: 10.1074/jbc.272.2.1095. [DOI] [PubMed] [Google Scholar]
- 45.Imai H, Kefalov V, Sakurai K, Chisaka O, Ueda Y, Onishi A, Morizumi T, Fu Y, Ichikawa K, Nakatani K, Honda Y, Chen J, Yau KW, Shichida Y. Molecular properties of rhodopsin and rod function. J Biol Chem. 2007;282:6677–6684. doi: 10.1074/jbc.M610086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakurai K, Onishi A, Imai H, Chisaka O, Ueda Y, Usukura J, Nakatani K, Shichida Y. Physiological properties of rod photoreceptor cells in green-sensitive cone pigment knock-in mice. J Gen Physiol. 2007;130:21–40. doi: 10.1085/jgp.200609729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi G, Yau KW, Chen J, Kefalov VJ. Signaling properties of a short-wave cone visual pigment and its role in phototransduction. J Neurosci. 2007;27:10084–10093. doi: 10.1523/JNEUROSCI.2211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sampath AP, Baylor DA. Molecular mechanism of spontaneous pigment activation in retinal cones. Biophys J. 2002;83:184–193. doi: 10.1016/S0006-3495(02)75160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Makino CL, Wen XH, Lem J. Piecing together the timetable for visual transduction with transgenic animals. Curr Opin Neurobiol. 2003;13:404–412. doi: 10.1016/s0959-4388(03)00091-6. [DOI] [PubMed] [Google Scholar]
- 50.Xu J, Dodd RL, Makino CL, Simon MI, Baylor DA, Chen J. Prolonged photoresponses in transgenic mouse rods lacking arrestin. Nature. 1997;389:505–509. doi: 10.1038/39068. [DOI] [PubMed] [Google Scholar]
- 51.Sieving PA, Richards JE, Naarendorp F, Bingham EL, Scott K, Alpern M. Dark-light: model for nightblindness from the human rhodopsin Gly-90--> Asp mutation. Proc Natl Acad Sci U S A. 1995;92:880–884. doi: 10.1073/pnas.92.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sieving PA, Fowler ML, Bush RA, Machida S, Calvert PD, Green DG, Makino CL, McHenry CL. Constitutive "light" adaptation in rods from G90D rhodopsin: a mechanism for human congenital nightblindness without rod cell loss. J Neurosci. 2001;21:5449–5460. doi: 10.1523/JNEUROSCI.21-15-05449.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rao VR, Cohen GB, Oprian DD. Rhodopsin mutation G90D and a molecular mechanism for congenital night blindness. Nature. 1994;367:639–642. doi: 10.1038/367639a0. [DOI] [PubMed] [Google Scholar]
- 54.Rao VR, Oprian DD. Activating mutations of rhodopsin and other G protein-coupled receptors. Annu Rev Biophys Biomol Struct. 1996;25:287–314. doi: 10.1146/annurev.bb.25.060196.001443. [DOI] [PubMed] [Google Scholar]
- 55.Jin S, Cornwall MC, Oprian DD. Opsin activation as a cause of congenital night blindness. Nat Neurosci. 2003;6:731–735. doi: 10.1038/nn1070. [DOI] [PubMed] [Google Scholar]
- 56.Yau KW, Lamb TD, Baylor DA. Light-induced fluctuations in membrane current of single toad rod outer segments. Nature. 1977;269:78–80. doi: 10.1038/269078a0. [DOI] [PubMed] [Google Scholar]
- 57.Cornwall MC, Fein A, MacNichol EF., Jr Cellular mechanisms that underlie bleaching and background adaptation. J Gen Physiol. 1990;96:345–372. doi: 10.1085/jgp.96.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones GJ. Light adaptation and the rising phase of the flash photocurrent of salamander retinal rods. J Physiol. 1995;487(Pt 2):441–451. doi: 10.1113/jphysiol.1995.sp020891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones GJ, Fein A, MacNichol EF, Jr, Cornwall MC. Visual pigment bleaching in isolated salamander retinal cones. Microspectrophotometry and light adaptation. J Gen Physiol. 1993;102:483–502. doi: 10.1085/jgp.102.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]