Abstract
Regulation of medullary blood flow (MBF) is essential in maintaining normal kidney function. Blood flow to the medulla is supplied by the descending vasa recta (DVR), which arise from the efferent arterioles of juxtamedullary glomeruli. DVR are composed of a continuous endothelium, intercalated with smooth muscle-like cells called pericytes. Pericytes have been shown to alter the diameter of isolated and in situ DVR in response to vasoactive stimuli that are transmitted via a network of autocrine and paracrine signalling pathways. Vasoactive stimuli can be released by neighbouring tubular epithelial, endothelial, red blood cells and neuronal cells in response to changes in NaCl transport and oxygen tension. The experimentally described sensitivity of pericytes to these stimuli strongly suggests their leading role in the phenomenon of MBF autoregulation. Because the debate on autoregulation of MBF fervently continues, we discuss the evidence favouring a physiological role for pericytes in the regulation of MBF and describe their potential role in tubulo-vascular cross-talk in this region of the kidney. Our review also considers current methods used to explore pericyte activity and function in the renal medulla.
Keywords: blood flow, cross-talk, medulla, pericyte
Renal blood flow is regionally specific, with cortical blood flow being tightly regulated by well-documented mechanisms (Kriz 1981, Pallone et al. 2003). Conversely, medullary blood flow (MBF) regulation is less well understood and remains highly controversial (Pallone et al. 1998, 2003). There is contradictory evidence regarding whether or not MBF is autoregulated, and numerous studies have focused on delineating the potential mechanisms that might be involved in this process (Pallone 1994, Pallone et al. 1998, Crawford et al. 2012). Our own findings on regulation of MBF have arisen from studies utilizing the in situ live kidney slice model, developed in our laboratory, which uniquely permits access to the intact inner and outer medullary regions of the kidney, and facilitates the examination of both vascular and tubular structures and cell function in parallel (Pallone 1994, Crawford et al. 2011). The application of this novel live slice model has facilitated detailed investigations in to the potential mechanisms underlying MBF regulation and tubulo-vascular cross-talk to be performed and has enabled us to characterize the key role that pericytes play in this phenomenon. We have demonstrated that in situ pericytes can respond to a variety of endogenous stimuli and in so doing play an important role in regulating vasa recta diameter. In this review, we discuss our experimental findings on the physiological role(s) pericytes may play in regulating MBF in the context of the wider literature in the field.
To help contextualize the role pericytes play in physiological regulation of MBF, we begin by (i) describing the structure of the medullary vasculature and how this lends itself to regulation of MBF, and (ii) describing the role pericytes play in regulating medullary microvessels and discuss the significance of tightly regulated MBF.
Physiological regulation of medullary blood flow
Blood flow to the renal medulla is via vasa recta capillaries, which branch from efferent arterioles at juxtamedullary glomeruli, and run in parallel to the loops of Henlé and collecting ducts (Pallone et al. 2003). A second peritubular capillary bed, which branches from the efferent arterioles at cortical nephrons, surrounds the tubular structures in the cortex. Renal blood flow is constant, despite variations in arterial perfusion pressure. Changes in mean arterial blood pressure within the autoregulatory range (i.e. 90–200 mmHg) have little effect on renal blood flow or glomerular filtration rate. Renal blood flow is also regionally specific, with blood flow in the cortex tightly regulated. Experiments using the isolated in vitro blood-perfused juxtamedullary nephron technique have demonstrated autoregulation of afferent arteriolar blood flow (Takenaka et al. 1994, Harrison-Bernard & Navar 1996). Regulation of medullary blood flow is less well understood and initially thought to be passively regulated; more recent studies suggest renal medullary blood flow can be autoregulated independently of total renal blood flow (O'Connor et al. 2006, Rajapakse & Mattson 2011, Ahmeda & Johns 2012). Medullary blood flow regulation is important, because it must satisfy the conflicting demands of preserving the cortico-medullary gradients of NaCl and urea, while maintaining adequate oxygen and nutrient delivery, as well as metabolic clearance in the medulla. The vasa recta form capillary bundles in the outer and inner stripes of the outer medulla, entering as the descending vasa recta (DVR) that penetrate deep into the medulla before forming the ascending vasa recta (AVR). DVR are composed of a continuous endothelium, with pericytes juxtaposed at regular intervals along the microvessel. As the DVR advance to the inner medulla, pericytes become less frequent and diminish nearer the tip of the papilla (Sims 1986). Beyond this point, the endothelium becomes discontinuous, giving rise to the ascending vasa recta (AVR), which are characterized by their fenestrated endothelium (Kriz 1981, Pallone et al. 1998, 2003).
The parallel arrangement of descending and ascending limbs allows the vasa recta capillaries to form a countercurrent exchange system that maintains the cortico-medullary osmotic gradient established from countercurrent multiplication by the loops of Henlé, which is crucial for urine concentration (Jamison & Kriz 1982, Michel 1995, Cowley 1997, Pallone et al. 1998). Moreover, this parallel arrangement has a key role in regulating regional perfusion between the outer versus inner medulla (Cowley 1997). Contraction of the DVR results in the redirection of blood to the outer medullary inter-bundle capillaries and vice versa on dilation (Pallone et al. 1998). A consequence of this structural arrangement is low oxygen tension in the medulla: medullary partial pressure of oxygen is reported to be between 10 and 20 mmHg compared with 50 mmHg in the cortex (Brezis et al. 1991, Rosen et al. 1992, Liss et al. 1998). This creates a relatively hypoxic environment, (Eckardt et al. 2005), and failure to regulate it may result in severe hypoxia and ischaemic injury.
Medullary blood flow is <10% of total renal blood flow (RBF), and so tight regulation of the medullary microcirculation is essential for the preservation of normal kidney function. An increase in net MBF would result in ‘washout’ of the cortico-medullary osmotic gradient (Cowley 1997) and impaired urinary concentrating ability, whereas a sustained decrease in MBF would inevitably lead to ischaemia, which, depending on the severity and duration, could result in tissue (papillary) necrosis, scarring and chronic kidney injury (Fine et al. 2000, Nangaku 2006, Norman 2006).
It has been suggested previously that pericyte-mediated changes in DVR diameter can regulate MBF and alter its distribution in response to changes in active NaCl transport and the oxygen demands of renal tubular cells (Pallone 1994, Pallone & Mattson 2002, Crawford et al. 2011, 2012). Pericyte-mediated regulation of capillary diameter is achieved by pericyte contraction and relaxation, and it has also been demonstrated in other tissues, such as the central nervous system (CNS), with a similar functional significance attributed to pericytes (Rucker et al. 2000, Wu et al. 2003, Peppiatt et al. 2006).
Experimental investigations of medullary blood flow
To date, the notion that MBF is regulated independently of arterial perfusion pressure remains controversial, despite the many experimental studies that have been undertaken to examine this concept. Several different experimental approaches have been adopted in various experimental animal models, and species and inter-strain differences have been reported.
Studies utilizing imaging techniques based on the dual-slit method [measurement of red blood cell (RBC) velocity at two separate points on the same capillary], to determine blood flow, velocity and vasa recta diameter in the exposed rat papilla concluded that MBF is regulated within a limited arterial pressure range (125–130 mmHg) and that regulation of MBF is governed upstream by renal arterial pressure (Cohen et al. 1983). Imaging studies focusing specifically on juxtamedullary blood flow similarly conclude that renal sensitivity to endogenous vasoactive mediators is confined to afferent and efferent arterioles and that downstream vasa recta were insensitive (Harrison-Bernard & Carmines 1994, 1995).
Conversely, when the same technique was used to investigate autoregulation of blood flow in the DVR of anti-diuretic rats, regulation of MBF was observed over a broad range of perfusion pressures (Cupples & Marsh 1988). Subsequent studies performed in alternative rat strains also reported regulated MBF, albeit over varying perfusion pressure ranges: 101–132 mmHg in Munich Wistar rats compared with 104–126 mmHg in spontaneously hypertensive rats, and 90–114 mmHg in Sprague Dawley rats (Farrugia et al. 1992, Larson & Lockhart 1995). The inability to effectively assess autoregulation in these studies was attributed to animals being exposed to varying and inconsistent ranges in renal perfusion pressures (Cupples & Braam 2007). Studies that have utilized in vivo laser Doppler flowmetry (LDF) techniques to measure regional red blood cell flux (in rats, rabbits and dogs) have also failed to provide any clear answers (Stern et al. 1979, Takezawa et al. 1987, Roman et al. 1988, Mattson et al. 1993, Huang et al. 1994, Strick et al. 1994, Lerman et al. 1995, Harrison-Bernard & Navar 1996, Majid & Navar 1996, Majid et al. 1997, 1998, 1999, 2001, Nafz et al. 1998), perhaps due in part to the inconsistent perfusion pressure ranges applied in each study (Roman et al. 1988, Huang et al. 1994). LDF studies carried out on larger mammals have provided more consistent evidence favouring regulated MBF (Majid et al. 1997, Eppel et al. 2004, Cupples & Braam 2007). Studies performed in rabbits reported a constant MBF, despite increasing renal arterial pressure (Eppel et al. 2003).
Collectively, these studies not only serve to emphasize the potential for species and inter-strain differences in regulation of MBF, but also reveal limitations of the different techniques used. Erythrocyte velocity for example is thought to be less well regulated at the papilla than in the medulla and cortex (Cohen et al. 1983, Roman et al. 1988, Farrugia et al. 1992), and papillary autoregulation is deemed to be highly sensitive to changes in volume expansion (Roman et al. 1988); thus, imaging experiments performed on the exposed papilla must be interpreted with caution. LDF studies rely on accurate probe calibration and precise placement of probes in the renal parenchyma, which is difficult especially in smaller animals; moreover, kidney size may vary between animals further hampering consistent localization of probes in the renal parenchyma. Lastly, it is possible that the experimental discrepancies reported regarding regulation of MBF could arise from differential regulation of blood flow in the outer and inner medulla, as proposed in Pallone's hypothesis of redistribution of flow in the medulla (Pallone & Silldorff 2001). If blood flow is redistributed from the papilla towards the outer medulla, this would explain the apparent decrease in autoregulatory efficiency observed in the papilla. While redistribution of medullary blood flow has yet to be demonstrated experimentally, it provides a tantalizing explanation for the conflicting data on autoregulation of MBF and merits further investigation.
Renal pericytes
Despite the emerging evidence in favour of regulated MBF (Cupples & Marsh 1988, Pallone 1994, Pallone & Silldorff 2001, Crawford et al. 2011, 2012) and evidence describing regulated capillary blood flow in other organs and tissues (Krogh 1929, Wu et al. 2003, Peppiatt et al. 2006), the dogma that capillary blood flow is passive and driven by upstream arteriolar control of perfusion pressure has been a widely accepted mechanism until recently. Historically, this assumption was based on the anatomical observation that arteries and arterioles are encircled by contractile smooth muscle cells, critical for vasoconstriction and vasodilation, and that capillaries apparently are not. The presence of pericytes along microvessels and their contractile capabilities was ‘however’ revealed in the late 19th and early 20th centuries (Rouget 1879, Krogh 1929), although this was largely overlooked until more recently (Pallone 1994, Pallone & Silldorff 2001, Pallone & Mattson 2002, Kawamura et al. 2004, Peppiatt et al. 2006, Puro 2007, Crawford et al. 2011, 2012).
Pericytes are singular smooth muscle-like cells residing on the abluminal side of the endothelium. As their name implies, pericytes are perivascular cells consisting of a cell body with numerous claw-like processes that branch from the cell body and ‘wrap around’ microvessels, including arterioles, capillaries and venules (Shepro & Morel 1993, Hirschi & D'Amore 1996, Bergers & Song 2005). NG2 is an extracellular proteoglycan expressed by pericytes, and the anti-NG2 antibody has been used to identify pericytes in many organs, including vasa recta capillaries of the kidney, (Ozerdem et al. 2001, Peppiatt et al. 2006, Virgintino et al. 2007, Huang et al. 2010, Crawford et al. 2011, 2012, Mogensen et al. 2011), revealing a complex network of processes that extend from the cell body along the axis of, and wrap around, the capillary (Fig. 1a). In NG2-DsRed BAC transgenic mice, cells that express NG2 fluoresce red (Zhu et al. 2008, Hamilton et al. 2010), thus facilitating the identification of pericytes throughout the whole organism, including the kidney (Fig. 1b). Pericytes have been identified in all organs and tissues in a wide range of species [for review see (Sims 1986)] and have a variety of contrasting functional roles such as vessel stabilization, endothelial cell regulation, angiogenesis and phagocytosis. Pericytes have also been described as mesenchymal stem cells, because of their potential to differentiate into different cell types (Dore-Duffy et al. 2006, Crisan et al. 2011, Montemurro et al. 2011). Of particular interest in this review is the role of pericytes in the regulation of capillary blood flow (Sims 1986, Shepro & Morel 1993, Hirschi & D'Amore 1996, Bergers & Song 2005). Pericytes typically express smooth muscle α-actin (α-SMA), which provides the contractile machinery necessary for the regulation of vessel diameter, and it has been shown to be expressed by renal pericytes (Park et al. 1997).
Figure 1.
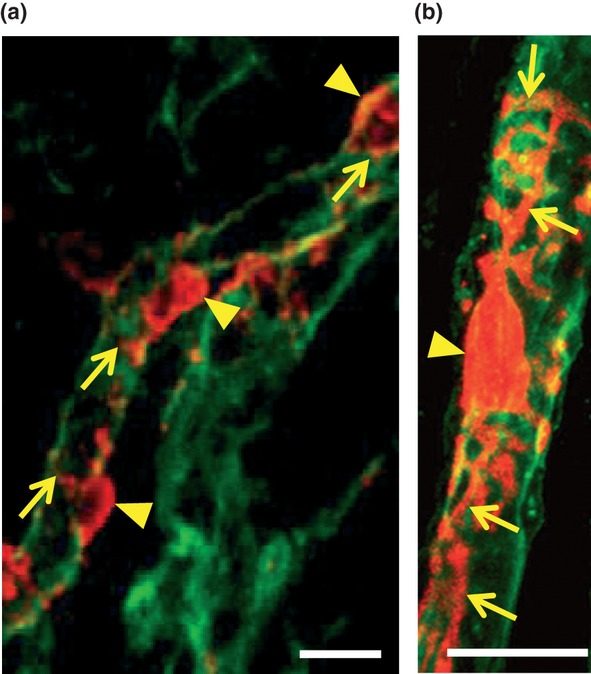
Identification of pericytes in the renal medulla. Kidney tissue slices (200 μm, from adult, male Sprague Dawley rats) were labelled with Alexa-488-conjugated IB4 and anti-NG2 (probed with Alexa 555 secondary antibody) to identify vasa recta capillaries and pericytes respectively. Pericytes (red) are identified on vasa recta capillaries (green; a). Pericyte cell bodies (arrowheads; a) are located on the abluminal side of the capillary. Finger-like processes (arrows; a) extend from the cell body to run along and wrap around the capillary [image adapted from (Crawford et al. 2012)]. Kidney slices (200 μm) obtained from NG2 Ds-Red Bac transgenic mice, in which pericytes express fluorescent Ds-Red (red; b) and vasa recta capillaries were labelled with Alexa-488-conjugated IB4 (green; b). Processes (arrows; b) are shown extend from the cell body (arrowhead; B). Primary processes are seen to extend along the length of the capillary, and secondary processes wrap around the vessel (Crawford C and Peppiatt-Wildman CM, unpublished data). Scale bar = 10 μm.
The precise location of pericytes along a vessel wall is thought to be determined by the functional demand of the tissue being serviced (Sims 2000, Bergers & Song 2005). The density of pericytes in different tissues is known to vary, pericyte density being greater in the kidney than in many other tissues, such as the CNS and retina (Sims 1986, Bergers & Song 2005, Crawford et al. 2012). Within the kidney, pericyte density also varies, the density being greater in the outer medulla compared with the inner medulla (Crawford et al. 2012). In fitting with our findings regarding pericyte density in the renal medulla, it has previously been reported that pericyte density in the kidney increases to meet the metabolic demands of the tissue region in which they reside (Park et al. 1997).
Given the complexity of MBF and its role in facilitating urine concentration, the need for tight local control of blood flow, independent of changes in cortical blood flow, is perhaps not surprising. Regulation of vasa recta blood flow and the role of pericytes in regulating vasa recta capillary diameter have been investigated extensively by Pallone and colleagues who utilized the isolated perfused vasa recta model. This experimental model proved to be pivotal in identifying which agents were mediating vasa recta constriction/dilation and in attributing a functional role to contractile pericytes. Vasa recta pericytes were shown to evoke both vasoconstriction and dilation of isolated perfused DVR in response to a number of different agonists, many of which are endogenous to the medulla: angiotensin-II (Ang-II), endothelin-1 (ET-1), nitric oxide (NO), adenosine and prostaglandin E2 (PGE2) (Pallone 1994, Pallone & Silldorff 2001).
Endogenous vasoactive agents and their effect on renal pericytes
There are many vasoactive agents endogenous to the kidney that might physiologically regulate MBF. The pericyte-mediated vascular responses to these agents are summarized in Tables 1 and 2. Both vasa recta endothelial cells and tubular epithelial cells are established sources of medullary vasoactive signals [ET-1, Ang-II, PGE2, NO, adenosine, adenosine triphosphate (ATP),] as are interstitial cells (PGE2) and sympathetic nerves [noradrenaline (NA), ATP] (de Nucci et al. 1988, Pallone 1994, Silldorff et al. 1995, 1996, Yang et al. 1995, Pallone & Mattson 2002, Peppiatt et al. 2006, Crawford et al. 2011, Edwards et al. 2011). Anatomical studies have identified sympathetic nerves in the renal medulla (Eppel et al. 2004), predominantly in the outer medullary DVR (OMDVR) (Yang et al. 1995), and we have elaborated on these studies by specifically identifying sympathetic nerves in close apposition to pericytes in the outer medulla [Fig. 2 (Crawford et al. 2012)]. Given that pericytes are known to respond to acetylcholine (ACh), NA and ATP (Eglen et al. 1994, Yang et al. 1995, Crawford et al. 2011, 2012), and their close proximity to sympathetic nerve terminals, it is likely that neuronal release of NA and ATP contributes to the regulation of MBF.
Table 1.
Endogenous stimuli that evoke a pericyte-mediated vasoconstriction of vasa recta
| Stimulus | Source of vasoactive agent | Receptor activated | Receptor location | References |
|---|---|---|---|---|
| Acetylcholine | Parasympathetic nerves | Muscarinic | Functional evidence for Mus Rs on pericytes, no gene expression studies | Eglen et al. 1994, Yang et al. 1995, |
| Angiotensin-II | Endothelial cells | AT1 | AT1: vasa recta bundles, interstitial cells and collecting duct epithelium | Mujais et al. 1986, Zhuo et al. 1992, Edwards & Aiyar 1993, Seldin & Geibisch 2008, Crawford et al. 2012, |
| Adenosine triphosphate (ATP) | Tubular epithelium, endothelial cells, RBCs | P2 receptors | DVR, loop of Henle and collecting duct epithelium | Sprague et al. 1996, Jans et al. 2002, Unwin et al. 2003, Praetorius et al. 2005, Wildman & King 2008, Crawford et al. 2011, |
| Endothelin-1 | Endothelial cells and collecting duct epithelium | ETA | Collecting duct epithelium, vascular bundles, RMIC | de Nucci et al. 1988, Silldorff et al. 1995, Crawford et al. 2012, |
| Noradrenaline | Sympathetic nerves | α1-adreno-receptors | OM vasa recta | Yang et al. 1995, DiBona & Kopp 1997, Crawford et al. 2012, |
| UTP | Tubular epithelium, endothelium, plasma | P2 receptors | DVR, loop of Henle and collecting duct epithelium | Lazarowski & Boucher 2001, Jans et al. 2002, Unwin et al. 2003, Praetorius et al. 2005, Wildman & King 2008, Crawford et al. 2011, |
| Vasopressin | Hypothalamus (circulation) | V1a | V1: medullary vasculature, thin ascending limbs and OM collecting duct | Ostrowski et al. 1993, Nielsen et al. 1995, Turner & Pallone 1997, Cowley 2000 |
ATP, Adenosine triphosphate; RBC, Red blood cells; RMIC, renal medullary interstitial cells; UTP, uridine triphosphate.
Table 2.
Endogenous stimuli that evoke a pericyte-mediated vasodilation of vasa recta
| Stimulus | Source of vasoactive agent | Receptor activated | Receptor location | References |
|---|---|---|---|---|
| Acetylcholine (NO mediated) | Parasympathetic nerves | Muscarinic | Functional evidence for Mus Rs on pericytes | Eglen et al. 1994, Yang et al. 1995, |
| Adenosine | mTAL | A1 and A2a, A2b, sub-types | A1: DVR, loops of Henle and collecting duct epithelium. | Silldorff et al. 1996, Kreisberg et al. 1997, Guan et al. 2007, Crawford et al. 2011, Silldorff & Pallone 2001, |
| A2a: collecting duct epithelium. | ||||
| A2b: loops of Henle | ||||
| Angiotensin-II (NO mediated) | Endothelial cells | AT2 (dilation- NO mediated) | AT2: renal arteries (higher expression in foetal and neonatal renal tissues) | Zhuo et al. 1992, Edwards & Aiyar 1993, Pallone 1994, Seldin & Geibisch 2008, Crawford et al. 2012, |
| ATP (concentration dependent, NO mediated) | Tubular epithelium, endothelial cells, RBCs | P2 receptors | DVR, loop of Henle and collecting duct epithelium | Burnstock 1987, Sprague et al. 1996, Jans et al. 2002, Unwin et al. 2003, Praetorius et al. 2005, Wildman & King 2008, Erlinge & Burnstock 2008, Crawford et al. 2011, |
| Nitric Oxide | RBCs, endothelial cells, collecting duct epithelium | (Freely diffuses into cells) | – | Mattson & Higgins 1996, Wu et al. 1999, Dickhout et al. 2002, Cao et al. 2010, Edwards et al. 2011, Crawford et al. 2012, |
| PGE2 | RMIC, collecting duct epithelium | EP2, EP4 | EP2: descending thin loop and OM vasa recta. | Pallone 1994, Jensen et al. 2001, Crawford et al. 2012, |
| EP4: Collecting duct epithelium, OM vasa recta | ||||
| Vasopressin | Hypothalamus (circulation) | V2 | V2: Collecting Duct | Ostrowski et al. 1993, Nielsen et al. 1995, Turner & Pallone 1997, Cowley 2000 |
ATP, Adenosine triphosphate; DVR, Descending vasa recta; RBC, Red blood cells; RMIC, renal medullary interstitial cells.
Figure 2.
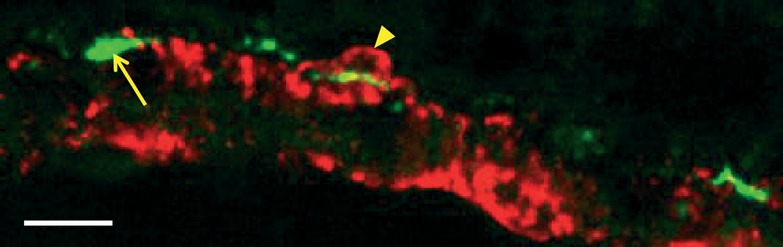
Co-localization of pericytes and sympathetic nerves in the renal medulla. Kidney tissue slices (200 μm, from adult, male Sprague Dawley rats) were labelled with anti-NG2 (probed with Alexa 555 secondary antibody) to identify vasa recta pericytes (red, arrowhead). Sympathetic nerve varicosities were identified with an anti-tyrosine hydroxylase antibody, amplified with a biotinylated secondary antibody that was probed with FITC-conjugated tertiary antibody (green, arrow). Confocal image shows pericytes (red) on a vasa recta capillary, co-localized and in close proximity to sympathetic nerves (green) (Crawford et al. 2012). Scale bar = 10 μm.
In addition to peptide and hormonal vasoactive agents, free radicals such as NO and reactive oxygen species (ROS) are also known to be potent regulators of MBF (Cao et al. 2010, Edwards et al. 2011). The renal medulla is known to have a far greater capacity for NO production than the cortex (Biondi & Romero 1990, Moridani & Kline 1996, Mattson & Wu 2000), and NO has been intrinsically linked to changes in DVR lumen and solute transport along the nephron (Plato & Garvin 1999, Pallone & Mattson 2002). All three isoforms of nitric oxide synthase (NOS) – neuronal NOS (nNOS), inducible NOS (iNOS) and endothelial NOS (eNOS) – have been identified on vascular and tubular structures in the kidney (Bachmann & Mundel 1994, Mattson & Higgins 1996). NO bioavailability is also governed by reactive oxygen species (ROS). ROS are generated by one and two electron reductions of O2, resulting in the formation of either superoxide ion (O2−.), hydrogen peroxide (H2O2) or hydroxyl free radical (OH) (Fridovich 1995, Thannickal & Fanburg 2000). ROS favour vasoconstriction and are increasingly being associated with hypertension and diabetic nephropathy (Schnackenberg et al. 1998, Touyz 2003). ROS-evoked vasoconstriction is mediated following peroxynitrite formation in response to superoxide interacting with locally released NO. In comparison with NO, peroxynitrite evokes a weak vasodilation, thus resulting in a net reduction in MBF (Rubanyi & Vanhoutte 1986, Pallone & Mattson 2002). ROS-mediated vasoconstriction is known to be attenuated by antioxidants, such as superoxide dismutase (SOD), and by the SOD mimetic TEMPOL, both of which have been shown to increase MBF by as of yet undetermined mechanisms (Schnackenberg et al. 1998, Zou et al. 2001).
Functional studies using N5-[imino(nitroamino)methyl]-L-ornithine, methyl ester, monohydrochloride (L-NAME), a NO inhibitor, have demonstrated that inhibition of endogenous NO in live kidney slices shows pericyte-mediated vasoconstriction of vasa recta capillaries (Crawford et al. 2012), whereas the application of S-nitroso-N-acetylpenicillamine (SNAP), a NO donor, to live kidney slices evoked a pericyte-mediated vasodilation of in situ vasa recta (Crawford et al. 2012).
Vasoactive stimuli are released in the medulla by autocrine or paracrine mechanisms and act at their respective receptors expressed on DVR pericytes to mediate their effects. It has been suggested that feedback of vasoactive stimuli to juxtamedullary resistance vessels may provide the medulla with an intrinsic feedback loop, which could allow the medulla to control its own perfusion (Pallone & Silldorff 2001). In principle, this feedback system could operate on the basis that stimuli leaving the medulla via the AVR would be in close proximity to pericytes on the DVR at the outer medullary vascular bundles; thus, stimuli with a sufficiently long half-life could exert their vasoactive effects a second time. This concept is in keeping with the hypothesis of locally controlled MBF, and it merits further investigation.
Tubulo-vascular cross-talk
The close proximity of tubular and vascular structures in the medulla provides the ideal setting for tubulo-vascular cross-talk mechanisms to operate. We propose that pericyte cells bridge the gap between tubular and endothelial cell signalling mechanisms and that they detect vasoactive stimuli released from the epithelial, interstitial and endothelial cells and respond by regulating vasa recta diameter and consequently MBF (Crawford et al. 2011, 2012). The actual source of the vasoactive signal could be tubular, endothelial or neuronal, and both autocrine and paracrine pathways might be responsible for its release (Fig. 3).
Figure 3.
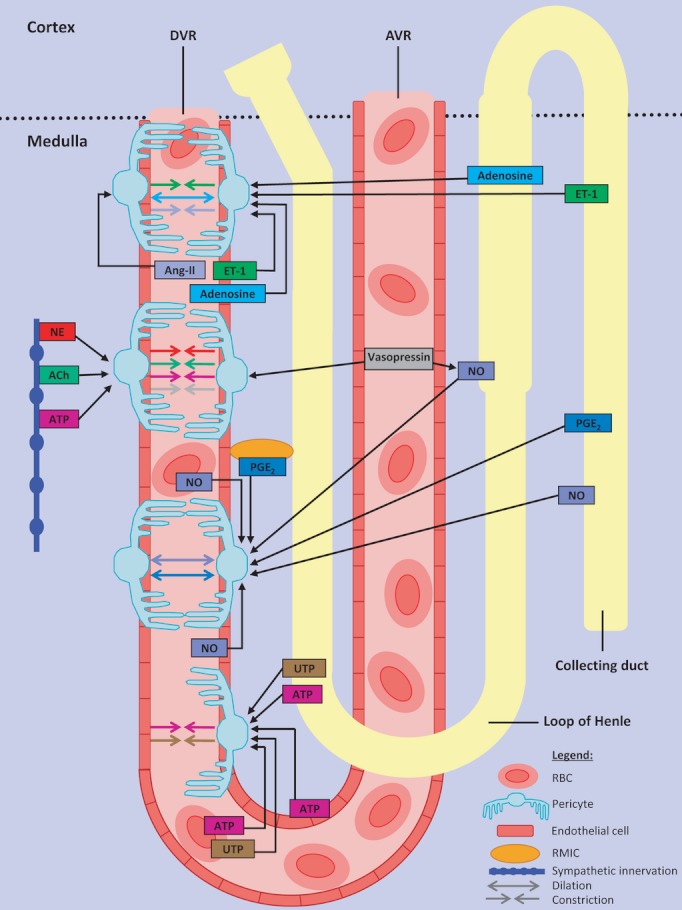
Proposed signalling mechanisms involved in pericyte-mediated regulation of descending vasa recta (DVR) diameter. Various endogenous vasoactive mediators are released from neighbouring tubular epithelium, vascular endothelium, red blood cells (RBC) and renal medullary interstitial cells (RMIC) of the renal medulla. These compounds signal to pericytes residing on DVR capillaries to cause pericyte-mediated vasoconstriction (arrows→←, endothelin-1 [ET-1], prostaglandin E2 [PGE2], nitric oxide [NO], adenosine, uridine triphosphate [UTP], adenosine triphosphate [ATP], acetylcholine [Ach] and circulating hormones: angiotensin [Ang-II], vasopressin, noradrenaline [NA]) or vasodilation (arrows ↔, NO and PGE2).
Potential examples of pericyte-mediated tubulo-vascular cross-talk might be inferred from studies not primarily focused on investigating this mechanism directly. Here, we provide some examples that demonstrate the potential role of pericytes in reciprocally coordinating medullary nephron function and blood flow demands. In the medulla, for example, adenosine acts as a vasodilator via pericytes (Silldorff & Pallone 2001), therefore increasing MBF (Agmon et al. 1993). Medullary oxidative stress arising from a pathological insult can cause adenosine release from the medullary thick ascending limb (mTAL) (Beach & Good 1992), which can dilate neighbouring vasa recta by its action at pericytes (Crawford et al. 2011), thereby increasing oxygen supply and simultaneously reducing oxygen consumption by direct inhibition of NaCl reabsorption in the mTAL (Agmon et al. 1993, Zou et al. 1999, Pallone et al. 2003).
ET-1 constricts isolated and in situ DVR (Silldorff et al. 1995, Crawford et al. 2012) and inhibits absorption of sodium and water in the mTAL (Plato et al. 2000). ET-1 is synthesized by both collecting duct (CD) epithelial cells and endothelial cells, and once it is released it acts locally, unless it is taken up into the circulation (de Nucci et al. 1988). The mTAL, CD and vasa recta are all closely apposed; therefore, locally released ET-1 (epithelial or endothelial) would act via pericytes to reciprocally regulate vasa recta diameter (Crawford et al. 2011). We have demonstrated that Ang-II and NO exhibit their vasoactive effects specifically via pericytes (Crawford et al. 2012), and Ang-II-mediated constriction of DVR in medullary ray tissue strips is attenuated by NO released from neighbouring mTAL epithelium (Dickhout et al. 2002).
Similarly, the constrictive effects of both ET-1 and Ang-II are subject to attenuation by vasodilatory PGE2, which is released by renal medullary interstitial cells (RMIC) and collecting duct epithelial cells (Pallone 1994). The ensuing increase in blood flow brought about by PGE2-mediated vasodilation is thought to increase NaCl excretion (Silldorff et al. 1995), and PGE2 itself may modulate solute absorption along the nephron through its direct action at pericytes. Crawford et al. (2011) have demonstrated ATP- and uridine triphosphate (UTP)-mediated constriction and dilation of in situ vasa recta, specifically at pericyte sites (Crawford et al. 2011). The most likely sources of these extracellular nucleotides are endothelial cells, red blood cells, tubular epithelial cells and sympathetic nerves (Sprague et al. 1996, Jans et al. 2002, Praetorius et al. 2005). Most recently, it has been demonstrated that exposing live kidney slices to hypotonic insult, so as to release ATP, results in pericyte-mediated vasodilation of in situ vasa recta, which is direct evidence for tubulo-vascular cross-talk in the medulla (Crawford et al. 2012). Here, contractile pericytes act by sensing a change in the concentration of extracellular nucleotides and respond by relaying this to the vasculature in order to fine-tune MBF. In all examples described here, it appears that pericytes are acting as a biological transducer and are pivotal in ultimately determining vessel diameter and blood flow.
In support of this key role for pericytes in the kidney, there are similar studies describing pericyte communication or interactions with adjacent cell types in other systems. For example, retinal and cerebellar pericytes respond to changes in the metabolic demands of the surrounding neurones and alter capillary diameter accordingly; however, the exact mechanism by which this occurs is still unclear (Wu et al. 2003, Peppiatt & Attwell 2004, Peppiatt et al. 2006).
Role of pericytes in pathophysiology: renal fibrosis
In the retina, it is well established that loss of pericyte function is an instrumental factor in the development of pathological conditions such as diabetic retinopathy (Sakagami et al. 1999, Cai & Boulton 2002, Tu et al. 2011). Indeed, pericyte dysfunction is implicated in other pathological conditions such as tumour angiogenesis and atherosclerosis (Benjamin et al. 1999, Morikawa et al. 2002, Yamagishi & Imaizumi 2005), and more recently in Alzheimer's disease (Bell et al. 2010).
In the kidney, a role for pericytes in the pathogenesis of renal fibrosis and progression of chronic kidney disease has been proposed (Lin et al. 2008, Duffield & Humphreys 2011, Schrimpf & Duffield 2011, Smith et al. 2012). It has been suggested that detachment and migration of pericytes from renal microvessels and their differentiation to myofibroblasts (defined as fibroblasts with contractile capabilities) cause vessel destabilization, capillary rarefaction and loss. Loss of vessels ultimately leads to ischaemia and promotes a pro-fibrotic environment that is accelerated by the transformation of pericytes to myofibroblasts. The enhanced myofibroblast population is known to result in increased production of extracellular matrix components (ECM), a key factor in the development of interstitial fibrosis (Kida & Duffield 2011, Liu 2011). The initiators of, and signalling mechanisms involved in, pericyte detachment, migration and differentiation are not well defined, although genes regulating proteolytic activity and angiogenesis have been implicated (Schrimpf et al. 2012). The recent evidence identifying pericytes as the key cell type in the progression of renal fibrosis has met with some controversy (Zeisberg & Duffield 2010). It is well established that myofibroblasts are important in the pathogenesis of fibrosis, although myofibroblasts are thought to arise from a number of different sources, including differentiation of resident fibroblasts (Liu 2006, 2011, Wada et al. 2007). Epithelial cells undergoing epithelial to mesenchymal transition (EMT) were thought to contribute to the myofibroblast pool; however, the occurrence of EMT in vivo is still heavily debated (Wada et al. 2007, Humphreys et al. 2010, Zeisberg & Duffield 2010, Kriz et al. 2011, Liu 2011). Recent genetic ‘fate-mapping’ studies indicate that epithelial cells are unable to migrate from the endothelial compartment, and instead, they implicate pericytes as the myofibroblast progenitor (Lin et al. 2008, Humphreys et al. 2010). Unfortunately, this is difficult to demonstrate in vivo or ex vivo using cell markers because alpha-SMA, a commonly used myofibroblast marker, is also expressed by pericytes (Park et al. 1997, Strutz & Zeisberg 2006). Moreover, the anti-NG2 antibody used to identify pericytes has also been used to identify myofibroblasts in other tissues (Terada et al. 2006). The lack of specific markers for pericytes and myofibroblasts has hindered researchers' efforts to clarify the origin of myofibroblasts (Strutz & Zeisberg 2006). More selective markers of these cells, or transgenic animal models, may help to determine the role of pericytes in renal fibrosis.
Methods for assessing pericyte activity and future directions
A variety of experimental techniques from in vitro cell culture approaches to isolated vessel preparations and LDF have been employed to further our understanding of MBF regulation. To make significant advances, the technical problem of renal medulla inaccessibility must first be overcome. As already discussed, early approaches to the investigation of MBF regulation based on the LDF technique or the dual-slit imaging method have been inconclusive. To date, the best-established technique for studying pericyte-mediated regulation of vasa recta is the isolated perfused DVR method (Pallone 1994). Although this model has provided much of our knowledge about the vasoactivity of these cells, it does not allow tubulo-vascular cross-talk mechanisms to be explored. Studies performed on microtissue strips of medulla have alluded to ‘tubular-vascular cross-talk’ in experiments that demonstrated that Ang-II-evoked constriction in isolated vasa recta is buffered by locally produced NO in adjacent tubules (Dickhout et al. 2002). This finding is consistent with the hypothesis of tubulo-vascular cross-talk in vivo, and it is at least consistent with our proposal that pericytes may be key mediators. The in vitro blood-perfused juxtamedullary technique (Casellas & Navar 1984) has also been used extensively to investigate afferent and efferent arteriolar blood flow (Harrison-Bernard & Carmines 1994, 1995, Takenaka et al. 1994), and the application of certain elements of this technique may indeed improve current methodologies, that is, perfusion of DVR within slices.
Mathematical modelling has also been applied to investigate the regulation of MBF (Zhang & Edwards 2007). This computational model provides a two-dimensional representation of the inner stripe of the outer medulla and was constructed by randomly distributing a number of tubular and vascular structures within a concentric region. The model is adapted from the configuration initially published by Layton and Layton, which was based on data collated from in vivo and in vitro experiments (Layton & Layton 2005, Zhang & Edwards 2007). Although the construction of such a model may be subject to ‘investigator manipulation’, it can provide useful insights into the generation and distribution of endogenous vasoactive stimuli.
A recent development of a live kidney slice model provides an alternative in vitro experimental model in which medullary structures can be investigated in situ. As with most techniques, this model is not without its limitations. For example, it is not possible to simulate the osmotic and oxygen gradients present in vivo, and DVR in the live slice model are not perfused (Crawford et al. 2012). Perfusion of tubular and/or vascular structures within the tissue slice, to more accurately assess the intraluminal signalling pathways, would offer an obvious (yet technically challenging) improvement. Despite these limitations, this method uniquely allows visualization of pericytes in their in situ environment, and data collected using this model have confirmed that in vitro observations regarding pericyte activity are reproducible, and new insights into pericyte activity and regulation are being described (Crawford et al. 2011, 2012). A significant and noteworthy advantage of this model is that the relationship between pericytes and the surrounding interstitial and tubular cells can be investigated, and this has been key to demonstrating the role of pericytes in tubulo-vascular cross-talk (Crawford et al. 2012). Although the live tissue slice model is relatively new in renal blood flow studies, the slice technique per se is a well-established model for investigating brain function and has previously been used to investigate the role of pericytes in regulating the cerebellar microcirculation (Peppiatt et al. 2006). We expect this experimental model will complement existing approaches employed to investigating renal microvascular function and hope that future investigations using this model will facilitate the delineation of MBF regulatory mechanisms.
In summary, there appears to be significant experimental evidence, which favours regulated blood flow in the medulla, and this complements the need for maintained homoeostasis in this region. We propose pericytes are key players in the regulation of renal medullary function, a proposal that is supported not only by their physical location on the vasa recta, but also by evidence describing their ability to respond to endogenous vasoactive agents originating from neighbouring tubular and interstitial cells. There is still much to understand about renal pericyte activity, particularly about their ability to communicate with neighbouring structures and the way in which they transcribe changes in tubular function to changes in blood flow. However, technical advances coupled with increased interest, particularly because of the implication of pericytes in renal disease, should rapidly lead to increased understanding of pericyte activity in renal physiology and pathophysiology.
Conflict of interest
There is no conflict of interest.
References
- Agmon Y, Dinour D, Brezis M. Disparate effects of adenosine A1- and A2-receptor agonists on intrarenal blood flow. Am J Physiol. 1993;265:F802–F806. doi: 10.1152/ajprenal.1993.265.6.F802. [DOI] [PubMed] [Google Scholar]
- Ahmeda AF, Johns EJ. The regulation of blood perfusion in the renal cortex and medulla by reactive oxygen species and nitric oxide in the anaesthetised rat. Acta Physiol (Oxf) 2012;204:443–450. doi: 10.1111/j.1748-1716.2011.02346.x. [DOI] [PubMed] [Google Scholar]
- Bachmann S, Mundel P. Nitric oxide in the kidney: synthesis, localization, and function. Am J Kidney Dis. 1994;24:112–129. doi: 10.1016/s0272-6386(12)80170-3. [DOI] [PubMed] [Google Scholar]
- Beach RE, Good DW. Effects of adenosine on ion transport in rat medullary thick ascending limb. Am J Physiol. 1992;263:F482–F487. doi: 10.1152/ajprenal.1992.263.3.F482. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi M, Romero JC. Nitric oxide-mediated reactions stimulate cGMP in the dog kidney. J Vasc Med Biol. 1990;2:294–298. [Google Scholar]
- Brezis M, Heyman SN, Dinour D, Epstein FH, Rosen S. Role of nitric oxide in renal medullary oxygenation. Studies in isolated and intact rat kidneys. J Clin Invest. 1991;88:390–395. doi: 10.1172/JCI115316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Local control of blood pressure by purines. Blood Vessels. 1987;24:156–160. doi: 10.1159/000158691. [DOI] [PubMed] [Google Scholar]
- Cai J, Boulton M. The pathogenesis of diabetic retinopathy: old concepts and new questions. Eye (Lond) 2002;16:242–260. doi: 10.1038/sj.eye.6700133. [DOI] [PubMed] [Google Scholar]
- Cao C, Edwards A, Sendeski M, Lee-Kwon W, Cui L, Cai CY, Patzak A, Pallone TL. Intrinsic nitric oxide and superoxide production regulates descending vasa recta contraction. Am J Physiol Renal Physiol. 2010;299:F1056–F1064. doi: 10.1152/ajprenal.00070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casellas D, Navar LG. In vitro perfusion of juxtamedullary nephrons in rats. Am J Physiol. 1984;246:F349–F358. doi: 10.1152/ajprenal.1984.246.3.F349. [DOI] [PubMed] [Google Scholar]
- Cohen HJ, Marsh DJ, Kayser B. Autoregulation in vasa recta of the rat kidney. Am J Physiol. 1983;245:F32–F40. doi: 10.1152/ajprenal.1983.245.1.F32. [DOI] [PubMed] [Google Scholar]
- Cowley AW., Jr Role of the renal medulla in volume and arterial pressure regulation. Am J Physiol. 1997;273:R1–R15. doi: 10.1152/ajpregu.1997.273.1.R1. [DOI] [PubMed] [Google Scholar]
- Cowley AW., Jr Control of the renal medullary circulation by vasopressin V1 and V2 receptors in the rat. Exp Physiol. 2000;85:223S–231S. doi: 10.1111/j.1469-445x.2000.tb00027.x. Spec No. [DOI] [PubMed] [Google Scholar]
- Crawford C, Kennedy-Lydon TM, Callaghan H, Sprott C, Simmons RL, Sawbridge L, Syme HM, Unwin RJ, Wildman SS, Peppiatt-Wildman CM. Extracellular nucleotides affect pericyte-mediated regulation of rat in situ vasa recta diameter. Acta Physiol (Oxf) 2011;202:241–251. doi: 10.1111/j.1748-1716.2011.02310.x. [DOI] [PubMed] [Google Scholar]
- Crawford C, Kennedy-Lydon T, Sprott C, Desai T, Sawbridge L, Munday J, Unwin RJ, Wildman SS, Peppiatt-Wildman CM. An Intact Kidney Slice Model to Investigate Vasa Recta Properties and Function in situ. Nephron Physiol. 2012;120:p17–p31. doi: 10.1159/000339110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Corselli M, Chen CW, Peault B. Multilineage stem cells in the adult: a perivascular legacy? Organogenesis. 2011;7:101–104. doi: 10.4161/org.7.2.16150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupples WA, Braam B. Assessment of renal autoregulation. Am J Physiol Renal Physiol. 2007;292:F1105–F1123. doi: 10.1152/ajprenal.00194.2006. [DOI] [PubMed] [Google Scholar]
- Cupples WA, Marsh DJ. Autoregulation of blood flow in renal medulla of the rat: no role for angiotensin II. Can J Physiol Pharmacol. 1988;66:833–836. doi: 10.1139/y88-133. [DOI] [PubMed] [Google Scholar]
- DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- Dickhout JG, Mori T, Cowley AW., Jr Tubulovascular nitric oxide crosstalk: buffering of angiotensin II-induced medullary vasoconstriction. Circ Res. 2002;91:487–493. doi: 10.1161/01.res.0000035243.66189.92. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- Duffield JS, Humphreys BD. Origin of new cells in the adult kidney: results from genetic labeling techniques. Kidney Int. 2011;79:494–501. doi: 10.1038/ki.2010.338. [DOI] [PubMed] [Google Scholar]
- Eckardt KU, Bernhardt WM, Weidemann A, Warnecke C, Rosenberger C, Wiesener MS, Willam C. Role of hypoxia in the pathogenesis of renal disease. Kidney Int Suppl. 2005;99:S46–51. doi: 10.1111/j.1523-1755.2005.09909.x. [DOI] [PubMed] [Google Scholar]
- Edwards RM, Aiyar N. Angiotensin II receptor subtypes in the kidney. J Am Soc Nephrol. 1993;3:1643–1652. doi: 10.1681/ASN.V3101643. [DOI] [PubMed] [Google Scholar]
- Edwards A, Cao C, Pallone TL. Cellular mechanisms underlying nitric oxide-induced vasodilation of descending vasa recta. Am J Physiol Renal Physiol. 2011;300:F441–F456. doi: 10.1152/ajprenal.00499.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglen RM, Reddy H, Watson N, Challiss RA. Muscarinic acetylcholine receptor subtypes in smooth muscle. Trends Pharmacol Sci. 1994;15:114–119. doi: 10.1016/0165-6147(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Eppel GA, Bergstrom G, Anderson WP, Evans RG. Autoregulation of renal medullary blood flow in rabbits. Am J Physiol Regul Integr Comp Physiol. 2003;284:R233–R244. doi: 10.1152/ajpregu.00061.2002. [DOI] [PubMed] [Google Scholar]
- Eppel GA, Malpas SC, Denton KM, Evans RG. Neural control of renal medullary perfusion. Clin Exp Pharmacol Physiol. 2004;31:387–396. doi: 10.1111/j.1440-1681.2004.04003.x. [DOI] [PubMed] [Google Scholar]
- Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4:1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrugia E, Lockhart JC, Larson TS. Relation between vasa recta blood flow and renal interstitial hydrostatic pressure during pressure natriuresis. Circ Res. 1992;71:1153–1158. doi: 10.1161/01.res.71.5.1153. [DOI] [PubMed] [Google Scholar]
- Fine LG, Bandyopadhay D, Norman JT. Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia. Kidney Int Suppl. 2000;75:S22–S26. [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- Guan Z, Osmond DA, Inscho EW. Purinoceptors in the kidney. Exp Biol Med (Maywood) 2007;232:715–726. [PubMed] [Google Scholar]
- Hamilton N, Vayro S, Wigley R, Butt AM. Axons and astrocytes release ATP and glutamate to evoke calcium signals in NG2-glia. Glia. 2010;58:66–79. doi: 10.1002/glia.20902. [DOI] [PubMed] [Google Scholar]
- Harrison-Bernard LM, Carmines PK. Juxtamedullary microvascular responses to arginine vasopressin in rat kidney. Am J Physiol. 1994;267:F249–F256. doi: 10.1152/ajprenal.1994.267.2.F249. [DOI] [PubMed] [Google Scholar]
- Harrison-Bernard LM, Carmines PK. Impact of cyclo-oxygenase blockade on juxtamedullary microvascular responses to angiotensin II in rat kidney. Clin Exp Pharmacol Physiol. 1995;22:732–738. doi: 10.1111/j.1440-1681.1995.tb01927.x. [DOI] [PubMed] [Google Scholar]
- Harrison-Bernard LM, Navar LG. Renal cortical and medullary microvascular blood flow autoregulation in rat. Kidney Int Suppl. 1996;57:S23–S29. [PubMed] [Google Scholar]
- Hirschi KK, D'Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–698. [PubMed] [Google Scholar]
- Huang C, Davis G, Johns EJ. Effect of nitrendipine on autoregulation of perfusion in the cortex and papilla of kidneys from Wistar and stroke prone spontaneously hypertensive rats. Br J Pharmacol. 1994;111:111–116. doi: 10.1111/j.1476-5381.1994.tb14031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang FJ, You WK, Bonaldo P, Seyfried TN, Pasquale EB, Stallcup WB. Pericyte deficiencies lead to aberrant tumor vascularization in the brain of the NG2 null mouse. Dev Biol. 2010;344:1035–1046. doi: 10.1016/j.ydbio.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison RL, Kriz W. Urinary Concentrating Mechanism: Structure and Function. New York: Oxford University Press; 1982. [Google Scholar]
- Jans D, Srinivas SP, Waelkens E, Segal A, Lariviere E, Simaels J, Van Driessche W. Hypotonic treatment evokes biphasic ATP release across the basolateral membrane of cultured renal epithelia (A6) J Physiol. 2002;545:543–555. doi: 10.1113/jphysiol.2002.026641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen BL, Stubbe J, Hansen PB, Andreasen D, Skott O. Localization of prostaglandin E(2) EP2 and EP4 receptors in the rat kidney. Am J Physiol Renal Physiol. 2001;280:F1001–F1009. doi: 10.1152/ajprenal.2001.280.6.F1001. [DOI] [PubMed] [Google Scholar]
- Kawamura H, Kobayashi M, Li Q, Yamanishi S, Katsumura K, Minami M, Wu DM, Puro DG. Effects of angiotensin II on the pericyte-containing microvasculature of the rat retina. J Physiol. 2004;561:671–683. doi: 10.1113/jphysiol.2004.073098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida Y, Duffield JS. Pivotal role of pericytes in kidney fibrosis. Clin Exp Pharmacol Physiol. 2011;38:467–473. doi: 10.1111/j.1440-1681.2011.05531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisberg MS, Silldorff EP, Pallone TL. Localization of adenosine-receptor subtype mRNA in rat outer medullary descending vasa recta by RT-PCR. Am J Physiol. 1997;272:H1231–H1238. doi: 10.1152/ajpheart.1997.272.3.H1231. [DOI] [PubMed] [Google Scholar]
- Kriz W. Structural organization of the renal medulla: comparative and functional aspects. Am J Physiol. 1981;241:R3–R16. doi: 10.1152/ajpregu.1981.241.1.R3. [DOI] [PubMed] [Google Scholar]
- Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Invest. 2011;121:468–474. doi: 10.1172/JCI44595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A. The Anatomy and Physiology of Capillaries. New Haven: Yale University Press; 1929. [Google Scholar]
- Larson TS, Lockhart JC. Restoration of vasa recta hemodynamics and pressure natriuresis in SHR by L-arginine. Am J Physiol. 1995;268:F907–F912. doi: 10.1152/ajprenal.1995.268.5.F907. [DOI] [PubMed] [Google Scholar]
- Layton AT, Layton HE. A region-based mathematical model of the urine concentrating mechanism in the rat outer medulla. I. Formulation and base-case results. Am J Physiol Renal Physiol. 2005;289:F1346–F1366. doi: 10.1152/ajprenal.00346.2003. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC. UTP as an extracellular signaling molecule. News Physiol Sci. 2001;16:1–5. doi: 10.1152/physiologyonline.2001.16.1.1. [DOI] [PubMed] [Google Scholar]
- Lerman LO, Bentley MD, Fiksen-Olsen MJ, Strick DM, Ritman EL, Romero JC. Pressure dependency of canine intrarenal blood flow within the range of autoregulation. Am J Physiol. 1995;268:F404–F409. doi: 10.1152/ajprenal.1995.268.3.F404. [DOI] [PubMed] [Google Scholar]
- Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss P, Nygren A, Erikson U, Ulfendahl HR. Injection of low and iso-osmolar contrast medium decreases oxygen tension in the renal medulla. Kidney Int. 1998;53:698–702. doi: 10.1046/j.1523-1755.1998.00811.x. [DOI] [PubMed] [Google Scholar]
- Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid DS, Navar LG. Medullary blood flow responses to changes in arterial pressure in canine kidney. Am J Physiol. 1996;270:F833–F838. doi: 10.1152/ajprenal.1996.270.5.F833. [DOI] [PubMed] [Google Scholar]
- Majid DS, Godfrey M, Navar LG. Pressure natriuresis and renal medullary blood flow in dogs. Hypertension. 1997;29:1051–1057. doi: 10.1161/01.hyp.29.4.1051. [DOI] [PubMed] [Google Scholar]
- Majid DS, Omoro SA, Chin SY, Navar LG. Intrarenal nitric oxide activity and pressure natriuresis in anesthetized dogs. Hypertension. 1998;32:266–272. doi: 10.1161/01.hyp.32.2.266. [DOI] [PubMed] [Google Scholar]
- Majid DS, Said KE, Omoro SA. Responses to acute changes in arterial pressure on renal medullary nitric oxide activity in dogs. Hypertension. 1999;34:832–836. doi: 10.1161/01.hyp.34.4.832. [DOI] [PubMed] [Google Scholar]
- Majid DS, Said KE, Omoro SA, Navar LG. Nitric oxide dependency of arterial pressure-induced changes in renal interstitial hydrostatic pressure in dogs. Circ Res. 2001;88:347–351. doi: 10.1161/01.res.88.3.347. [DOI] [PubMed] [Google Scholar]
- Mattson DL, Higgins DJ. Influence of dietary sodium intake on renal medullary nitric oxide synthase. Hypertension. 1996;27:688–692. doi: 10.1161/01.hyp.27.3.688. [DOI] [PubMed] [Google Scholar]
- Mattson DL, Wu F. Control of arterial blood pressure and renal sodium excretion by nitric oxide synthase in the renal medulla. Acta Physiol Scand. 2000;168:149–154. doi: 10.1046/j.1365-201x.2000.00647.x. [DOI] [PubMed] [Google Scholar]
- Mattson DL, Lu S, Roman RJ, Cowley AW., Jr Relationship between renal perfusion pressure and blood flow in different regions of the kidney. Am J Physiol. 1993;264:R578–R583. doi: 10.1152/ajpregu.1993.264.3.R578. [DOI] [PubMed] [Google Scholar]
- Michel CC. Renal medullary microcirculation: architecture and exchange. Microcirculation. 1995;2:125–139. doi: 10.3109/10739689509146761. [DOI] [PubMed] [Google Scholar]
- Mogensen C, Bergner B, Wallner S, Ritter A, d'Avis S, Ninichuk V, Kameritsch P, Gloe T, Nagel W, Pohl U. Isolation and functional characterization of pericytes derived from hamster skeletal muscle. Acta Physiol (Oxf) 2011;201:413–426. doi: 10.1111/j.1748-1716.2010.02206.x. [DOI] [PubMed] [Google Scholar]
- Montemurro T, Andriolo G, Montelatici E, Weissmann G, Crisan M, Colnaghi MR, Rebulla P, Mosca F, Peault B, Lazzari L. Differentiation and migration properties of human foetal umbilical cord perivascular cells: potential for lung repair. J Cell Mol Med. 2011;15:796–808. doi: 10.1111/j.1582-4934.2010.01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moridani BA, Kline RL. Effect of endogenous L-arginine on the measurement of nitric oxide synthase activity in the rat kidney. Can J Physiol Pharmacol. 1996;74:1210–1214. [PubMed] [Google Scholar]
- Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujais SK, Kauffman S, Katz AI. Angiotensin II binding sites in individual segments of the rat nephron. J Clin Invest. 1986;77:315–318. doi: 10.1172/JCI112293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafz B, Berger K, Rosler C, Persson PB. Kinins modulate the sodium-dependent autoregulation of renal medullary blood flow. Cardiovasc Res. 1998;40:573–579. doi: 10.1016/s0008-6363(98)00194-1. [DOI] [PubMed] [Google Scholar]
- Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17:17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA. 1995;92:1013–1017. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JT. Protecting the microvasculature: a Tie-ght connection to ameliorating chronic kidney disease? J Am Soc Nephrol. 2006;17:2353–2355. doi: 10.1681/ASN.2006070741. [DOI] [PubMed] [Google Scholar]
- de Nucci G, Thomas R, D'Orleans-Juste P, Antunes E, Walder C, Warner TD, Vane JR. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci USA. 1988;85:9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor PM, Kett MM, Anderson WP, Evans RG. Renal medullary tissue oxygenation is dependent on both cortical and medullary blood flow. Am J Physiol Renal Physiol. 2006;290:F688–F694. doi: 10.1152/ajprenal.00275.2005. [DOI] [PubMed] [Google Scholar]
- Ostrowski NL, Young WS, III, Knepper MA, Lolait SJ. Expression of vasopressin V1a and V2 receptor messenger ribonucleic acid in the liver and kidney of embryonic, developing, and adult rats. Endocrinology. 1993;133:1849–1859. doi: 10.1210/endo.133.4.8404628. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- Pallone TL. Vasoconstriction of outer medullary vasa recta by angiotensin II is modulated by prostaglandin E2. Am J Physiol. 1994;266:F850–F857. doi: 10.1152/ajprenal.1994.266.6.F850. [DOI] [PubMed] [Google Scholar]
- Pallone TL, Mattson DL. Role of nitric oxide in regulation of the renal medulla in normal and hypertensive kidneys. Curr Opin Nephrol Hypertens. 2002;11:93–98. doi: 10.1097/00041552-200201000-00014. [DOI] [PubMed] [Google Scholar]
- Pallone TL, Silldorff EP. Pericyte regulation of renal medullary blood flow. Exp Nephrol. 2001;9:165–170. doi: 10.1159/000052608. [DOI] [PubMed] [Google Scholar]
- Pallone TL, Silldorff EP, Turner MR. Intrarenal blood flow: microvascular anatomy and the regulation of medullary perfusion. Clin Exp Pharmacol Physiol. 1998;25:383–392. doi: 10.1111/j.1440-1681.1998.tb02220.x. [DOI] [PubMed] [Google Scholar]
- Pallone TL, Zhang Z, Rhinehart K. Physiology of the renal medullary microcirculation. Am J Physiol Renal Physiol. 2003;284:F253–F266. doi: 10.1152/ajprenal.00304.2002. [DOI] [PubMed] [Google Scholar]
- Park F, Mattson DL, Roberts LA, Cowley AW., Jr Evidence for the presence of smooth muscle alpha-actin within pericytes of the renal medulla. Am J Physiol. 1997;273:R1742–R1748. doi: 10.1152/ajpregu.1997.273.5.R1742. [DOI] [PubMed] [Google Scholar]
- Peppiatt C, Attwell D. Neurobiology: feeding the brain. Nature. 2004;431:137–138. doi: 10.1038/431137a. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plato CF, Garvin JL. Nitric oxide, endothelin and nephron transport: potential interactions. Clin Exp Pharmacol Physiol. 1999;26:262–268. doi: 10.1046/j.1440-1681.1999.03028.x. [DOI] [PubMed] [Google Scholar]
- Plato CF, Pollock DM, Garvin JL. Endothelin inhibits thick ascending limb chloride flux via ET(B) receptor-mediated NO release. Am J Physiol Renal Physiol. 2000;279:F326–F333. doi: 10.1152/ajprenal.2000.279.2.F326. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Frokiaer J, Leipziger J. Transepithelial pressure pulses induce nucleotide release in polarized MDCK cells. Am J Physiol Renal Physiol. 2005;288:F133–F141. doi: 10.1152/ajprenal.00238.2004. [DOI] [PubMed] [Google Scholar]
- Puro DG. Physiology and pathobiology of the pericyte-containing retinal microvasculature: new developments. Microcirculation. 2007;14:1–10. doi: 10.1080/10739680601072099. [DOI] [PubMed] [Google Scholar]
- Rajapakse NW, Mattson DL. Role of L-arginine uptake mechanisms in renal blood flow responses to angiotensin II in rats. Acta Physiol (Oxf) 2011;203:391–400. doi: 10.1111/j.1748-1716.2011.02330.x. [DOI] [PubMed] [Google Scholar]
- Roman RJ, Cowley AW, Jr, Garcia-Estan J, Lombard JH. Pressure-diuresis in volume-expanded rats. Cortical and medullary hemodynamics. Hypertension. 1988;12:168–176. doi: 10.1161/01.hyp.12.2.168. [DOI] [PubMed] [Google Scholar]
- Rosen S, Epstein FH, Brezis M. Determinants of intrarenal oxygenation: factors in acute renal failure. Ren Fail. 1992;14:321–325. doi: 10.3109/08860229209106636. [DOI] [PubMed] [Google Scholar]
- Rouget C. Sur la contractilité des capillaries sanguins. CR Acad Sci. 1879;88:916–918. [Google Scholar]
- Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Rucker HK, Wynder HJ, Thomas WE. Cellular mechanisms of CNS pericytes. Brain Res Bull. 2000;51:363–369. doi: 10.1016/s0361-9230(99)00260-9. [DOI] [PubMed] [Google Scholar]
- Sakagami K, Wu DM, Puro DG. Physiology of rat retinal pericytes: modulation of ion channel activity by serum-derived molecules. J Physiol. 1999;521(Pt 3):637–650. doi: 10.1111/j.1469-7793.1999.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension. 1998;32:59–64. doi: 10.1161/01.hyp.32.1.59. [DOI] [PubMed] [Google Scholar]
- Schrimpf C, Duffield JS. Mechanisms of fibrosis: the role of the pericyte. Curr Opin Nephrol Hypertens. 2011;20:297–305. doi: 10.1097/MNH.0b013e328344c3d4. [DOI] [PubMed] [Google Scholar]
- Schrimpf C, Xin C, Campanholle G, Gill SE, Stallcup W, Lin SL, Davis GE, Gharib SA, Humphreys BD, Duffield JS. Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Soc Nephrol. 2012;23:868–883. doi: 10.1681/ASN.2011080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin DW, Geibisch G. The Kidney: Physiology and Pathophysiology. Elsevier; 2008. [Google Scholar]
- Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- Silldorff EP, Pallone TL. Adenosine signaling in outer medullary descending vasa recta. Am J Physiol Regul Integr Comp Physiol. 2001;280:R854–R861. doi: 10.1152/ajpregu.2001.280.3.R854. [DOI] [PubMed] [Google Scholar]
- Silldorff EP, Yang S, Pallone TL. Prostaglandin E2 abrogates endothelin-induced vasoconstriction in renal outer medullary descending vasa recta of the rat. J Clin Invest. 1995;95:2734–2740. doi: 10.1172/JCI117976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silldorff EP, Kreisberg MS, Pallone TL. Adenosine modulates vasomotor tone in outer medullary descending vasa recta of the rat. J Clin Invest. 1996;98:18–23. doi: 10.1172/JCI118764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims DE. The pericyte–a review. Tissue Cell. 1986;18:153–174. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- Sims DE. Diversity within pericytes. Clin Exp Pharmacol Physiol. 2000;27:842–846. doi: 10.1046/j.1440-1681.2000.03343.x. [DOI] [PubMed] [Google Scholar]
- Smith SW, Chand S, Savage CO. Biology of the renal pericyte. Nephrol Dial Transplant. 2012;27:2149–2155. doi: 10.1093/ndt/gfs134. [DOI] [PubMed] [Google Scholar]
- Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol. 1996;271:H2717–H2722. doi: 10.1152/ajpheart.1996.271.6.H2717. [DOI] [PubMed] [Google Scholar]
- Stern MD, Bowen PD, Parma R, Osgood RW, Bowman RL, Stein JH. Measurement of renal cortical and medullary blood flow by laser-Doppler spectroscopy in the rat. Am J Physiol. 1979;236:F80–F87. doi: 10.1152/ajprenal.1979.236.1.F80. [DOI] [PubMed] [Google Scholar]
- Strick DM, Fiksen-Olsen MJ, Lockhart JC, Roman RJ, Romero JC. Direct measurement of renal medullary blood flow in the dog. Am J Physiol. 1994;267:R253–R259. doi: 10.1152/ajpregu.1994.267.1.R253. [DOI] [PubMed] [Google Scholar]
- Strutz F, Zeisberg M. Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol. 2006;17:2992–2998. doi: 10.1681/ASN.2006050420. [DOI] [PubMed] [Google Scholar]
- Takenaka T, Harrison-Bernard LM, Inscho EW, Carmines PK, Navar LG. Autoregulation of afferent arteriolar blood flow in juxtamedullary nephrons. Am J Physiol. 1994;267:F879–F887. doi: 10.1152/ajprenal.1994.267.5.F879. [DOI] [PubMed] [Google Scholar]
- Takezawa K, Cowley AW, Jr, Skelton M, Roman RJ. Atriopeptin III alters renal medullary hemodynamics and the pressure-diuresis response in rats. Am J Physiol. 1987;252:F992–F1002. doi: 10.1152/ajprenal.1987.252.6.F992. [DOI] [PubMed] [Google Scholar]
- Terada N, Ohno N, Murata S, Katoh R, Stallcup WB, Ohno S. Immunohistochemical study of NG2 chondroitin sulfate proteoglycan expression in the small and large intestines. Histochem Cell Biol. 2006;126:483–490. doi: 10.1007/s00418-006-0184-3. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- Touyz RM. Reactive oxygen species in vascular biology: role in arterial hypertension. Expert Rev Cardiovasc Ther. 2003;1:91–106. doi: 10.1586/14779072.1.1.91. [DOI] [PubMed] [Google Scholar]
- Tu Z, Li Y, Smith DS, Sheibani N, Huang S, Kern T, Lin F. Retinal pericytes inhibit activated T cell proliferation. Invest Ophthalmol Vis Sci. 2011;52:9005–9010. doi: 10.1167/iovs.11-8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MR, Pallone TL. Vasopressin constricts outer medullary descending vasa recta isolated from rat kidneys. Am J Physiol. 1997;272:F147–F151. doi: 10.1152/ajprenal.1997.272.1.F147. [DOI] [PubMed] [Google Scholar]
- Unwin RJ, Bailey MA, Burnstock G. Purinergic signaling along the renal tubule: the current state of play. News Physiol Sci. 2003;18:237–241. doi: 10.1152/nips.01436.2003. [DOI] [PubMed] [Google Scholar]
- Virgintino D, Girolamo F, Errede M, Capobianco C, Robertson D, Stallcup WB, Perris R, Roncali L. An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis. 2007;10:35–45. doi: 10.1007/s10456-006-9061-x. [DOI] [PubMed] [Google Scholar]
- Wada T, Sakai N, Matsushima K, Kaneko S. Fibrocytes: a new insight into kidney fibrosis. Kidney Int. 2007;72:269–273. doi: 10.1038/sj.ki.5002325. [DOI] [PubMed] [Google Scholar]
- Wildman SS, King BF. P2X receptors: epithelial ion channels and regulators of salt and water transport. Nephron Physiol. 2008;108:60–67. doi: 10.1159/000122028. [DOI] [PubMed] [Google Scholar]
- Wu F, Park F, Cowley AW, Jr, Mattson DL. Quantification of nitric oxide synthase activity in microdissected segments of the rat kidney. Am J Physiol. 1999;276:F874–F881. doi: 10.1152/ajprenal.1999.276.6.F874. [DOI] [PubMed] [Google Scholar]
- Wu DM, Kawamura H, Sakagami K, Kobayashi M, Puro DG. Cholinergic regulation of pericyte-containing retinal microvessels. Am J Physiol Heart Circ Physiol. 2003;284:H2083–H2090. doi: 10.1152/ajpheart.01007.2002. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Imaizumi T. Pericyte biology and diseases. Int J Tissue React. 2005;27:125–135. [PubMed] [Google Scholar]
- Yang S, Silldorff EP, Pallone TL. Effect of norepinephrine and acetylcholine on outer medullary descending vasa recta. Am J Physiol. 1995;269:H710–H716. doi: 10.1152/ajpheart.1995.269.2.H710. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Duffield JS. Resolved: EMT produces fibroblasts in the kidney. J Am Soc Nephrol. 2010;21:1247–1253. doi: 10.1681/ASN.2010060616. [DOI] [PubMed] [Google Scholar]
- Zhang W, Edwards A. A model of nitric oxide tubulovascular cross talk in a renal outer medullary cross section. Am J Physiol Renal Physiol. 2007;292:F711–F722. doi: 10.1152/ajprenal.00208.2006. [DOI] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- Zhuo J, Alcorn D, Allen AM, Mendelsohn FA. High resolution localization of angiotensin II receptors in rat renal medulla. Kidney Int. 1992;42:1372–1380. doi: 10.1038/ki.1992.429. [DOI] [PubMed] [Google Scholar]
- Zou AP, Nithipatikom K, Li PL, Cowley AW., Jr Role of renal medullary adenosine in the control of blood flow and sodium excretion. Am J Physiol. 1999;276:R790–R798. doi: 10.1152/ajpregu.1999.276.3.R790. [DOI] [PubMed] [Google Scholar]
- Zou AP, Li N, Cowley AW., Jr Production and actions of superoxide in the renal medulla. Hypertension. 2001;37:547–553. doi: 10.1161/01.hyp.37.2.547. [DOI] [PubMed] [Google Scholar]


